Abstract
Background and aims
Current evidence for the use of intra-articular injections for thumb base osteoarthritis (TBOA) is equivocal. This study aims to investigate the efficacy of intra-articular corticosteroids, hyaluronic acid and platelet-rich plasma.
Methods
A Frequentist network meta-analysis was conducted comparing outcomes at short (≤3 months) and medium-term (>3–12 months) time points.
Results
Data from 7 RCTs and 1 non-RCT (446 patients) were collected, consisting of corticosteroids (n = 7), hyaluronic acid (n = 7), platelet-rich plasma (n = 2) and placebo (n = 2). At the short-term time point, no intra-articular injection demonstrated superiority over placebo at reducing pain. At the medium-term time point, superiority of platelet-rich plasma at reducing pain over placebo and corticosteroids was seen following sensitivity analysis (RCTs only) (SMD -1.48 95 % CI -2.71; −0.25). No injection proved superior at improving function at short or medium-term time points.
Conclusions
Overall, despite the promising result for platelet-rich plasma, the evidence quality was limited to two studies only justifying the need for further and larger methodologically robust trials investigating corticosteroids, hyaluronic acid and platelet-rich plasma vs each other and placebo.
Keywords: PRP, Hyaluronic acid, Steroid, Thumb base, Osteoarthritis, Meta-analysis
1. Introduction
The base of the thumb is the second most commonly affected joint by osteoarthritis in the hand, following the distal interphalangeal joints.1 This condition typically affects individuals in the fifth or sixth decade of life, with a higher incidence in postmenopausal women.2 Additional risk factors include connective tissue disorders, ethnicity, and obesity.3 The resulting joint degeneration significantly impairs quality of life, as up to 50 % of hand function depends on the thumb, making simple tasks such as turning keys and opening jars extremely painful.2 First-line treatment options for symptomatic thumb base osteoarthritis (TBOA) typically involve non-surgical options, such as splinting, physiotherapy, ergonomic education, and exercise. Pharmacological options include both oral and topical non-steroidal anti-inflammatory drugs (NSAIDs), although their efficacy can vary, particularly with transdermal NSAID infiltration rates.4 Chronic NSAID use carries an increased risk of acute kidney injury, peptic ulcer disease, myocardial infarction and stroke.5 Consequently, intra-articular injections represent an alternative therapeutic strategy, with corticosteroids (CS) being the most frequently used agent in the United Kingdom (UK), although other options such as hyaluronic acid (HA) and platelet-rich plasma (PRP) are also available.6 The UK's National Institute for Health and Care Excellence (NICE) guidelines on the management of osteoarthritis recommend the use of CS for pain relief in the short term and discourage the use of HA or any other injectable agents. However, this guidance is non-specific to TBOA. The 2018 updated European League Against Rheumatism (EULAR) recommendations for hand osteoarthritis do not endorse the use of CS, HA or any other agent citing a lack of evidence.7 In contrast, HA remains a popular treatment option for knee osteoarthritis, with favourable outcomes reported in large network meta-analyses, supporting its efficacy.8 More recently, there has been an interest in PRP, which is derived from a patient's own blood that is then centrifuged and a concentrated suspension of platelets in plasma is subsequently extracted. Platelets stimulate chondrocyte proliferation thereby causing cartilage repair through the action of autologous growth factors.9 The literature thus far has demonstrated PRP's regenerative potential for chondrocytes along with its anti-inflammatory action. However, its use has been generally confined to knee osteoarthritis, albeit with promising results, with limited data for other joints published.10,11 Whilst previous studies compared CS and HA for TBOA, the emergence of new data on PRP enables comparison between PRP vs CS, HA and placebo.12,13
This is the first study to compare the efficacy of CS, HA and PRP injections in patients with TBOA. A Frequentist network meta-analysis (NMA) at short-term (≤3 months) and medium-term (>3–12 months) time points was conducted. Outcomes of interest were a reduction in pain and an improvement in hand function.
2. Methods
The NMA was conducted in accordance with the Preferred Reporting Items for Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions14 and was registered on the PROSPERO database (CRD42023404461).
For the selection of studies, we utilized the following PICO framework:
Population (P): Adult patients who have been diagnosed with symptomatic TBOA using clinical diagnosis with or without radiographic evidence. Patients of all severity according to recognised scoring systems (e.g., Eaton and Littler or Kellgren-Lawrence) were included.
Intervention (I) and Comparison (C): Included studies should involve one intra-articular injection of the following injectables – corticosteroids (CS), hyaluronic acid (HA), and platelet-rich plasma (PRP). The comparator could be (one or more) injection therapy or placebo.
Outcome (O): Included studies had to report the primary outcomes of improvement in pain and/or function (as measured by a recognised global measure) with a follow-up of at least 2 weeks after intervention.
2.1. Data sources and searches
Trials were identified through searches from inception to Jan 1, 2023 on the following databases: Embase, MEDLINE, CENTRAL and CINAHL via EBSCO. We also manually searched the reference lists of retrieved systematic reviews.
Two independent reviewers (AT, SCS) conducted a thorough evaluation of each study. Initial selection was based on the relevance of the title and abstracts, followed by a full paper screening process. Disagreements between reviewers were resolved through discussion or consultation with a senior author (NJ or JJD).
2.2. Outcomes of interest
Excel was used for data extraction. The proforma was tested a priori on a sample of three RCTs. From the studies selected, AT and SCS extracted study characteristics such as design and duration; participant characteristics such as age, male/female number, intervention, and comparator characteristics such as type, duration, regime, dose and technique; type of outcome (pain or function or both), measurement of outcome data for each time points of interest for each outcome and adverse effects.
For pain, results of the Visual Analogical Scale (VAS) were extracted. For function, when measured using different scales, we first extracted data on the Michigan Hand Outcomes Questionnaire (MHQ) or Australian/Canadian Hand Osteoarthritis Index (AUSCAN) preferentially, followed by other globally recognised functional scales. Both VAS and MHQ/AUSCAN are recommended as the preferred assessments of pain and physical function in hand osteoarthritis by the Outcome Measures in Rheumatology (OMERACT).
Hand OA Working Group.15
2.3. Eligibility criteria
To ensure maximal data for the network meta-analysis (NMA), we included both randomized controlled trials (RCTs) and non-RCTs, including semi-randomized/quasi-randomized and comparative case series. Review articles, case studies and conference abstracts were excluded. Non-English studies were translated wherever feasible. We excluded studies involving patients under 18 years of age or treatment for rheumatoid arthritis. Studies using other types of injections besides the injections of interest were also excluded, as were studies utilizing additive substances with corticosteroid (CS), hyaluronic acid (HA), or platelet-rich plasma (PRP), studies using mixed infiltration (e.g., CS + HA), and studies comparing different types of CS or HA.
2.4. Risk of bias
Two reviewers, AT and SCS, utilized the RoB 2.0 tool to assess the risk of bias in RCTs. The RoB 2.0 tool assesses six domains of bias, including the randomization process, deviations from intended interventions, missing outcome data, measurement of outcome, blinding of patients, and selection of reported results.16 For non-RCTs, the reviewers employed the ROBINS-I tool to evaluate the following domains of bias: confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of reported results.17 Disputes in bias assessment were resolved through discussions between the two reviewers, and a third senior author (either NJ or JJD) was consulted if necessary.
2.5. Statistical analysis
Baseline characteristics and comparability between patient groups were analysed using ANOVA with a satisfactory p value considered as < 0.05. A Frequentist network meta-analysis was performed using MetaInsight, with a random-effects model applied at both short-term (≤3 months) and medium-term (>3–12 months) time points.18 Continuous data were extracted as mean and standard deviation or calculated from median and interquartile ranges (IQR).19 Inverse-variance weighting with standardised mean difference was used for all continuous data. Network plots were generated to display the connected evidence. Placebo was the reference treatment for network comparisons, and forest plots were created to show pooled effect estimates with 95 % confidence intervals. A league table was used to rank treatments from best to worst based on relative treatment effects. Consistency tables were used to assess agreement between direct and indirect evidence, with p values < 0.05 considered inconsistent. Publication bias was not assessed due to the small number of studies. Sensitivity analysis was performed by excluding studies with calculated data (e.g., from medians or standard error) and non-RCTs.
3. Results
3.1. Search results
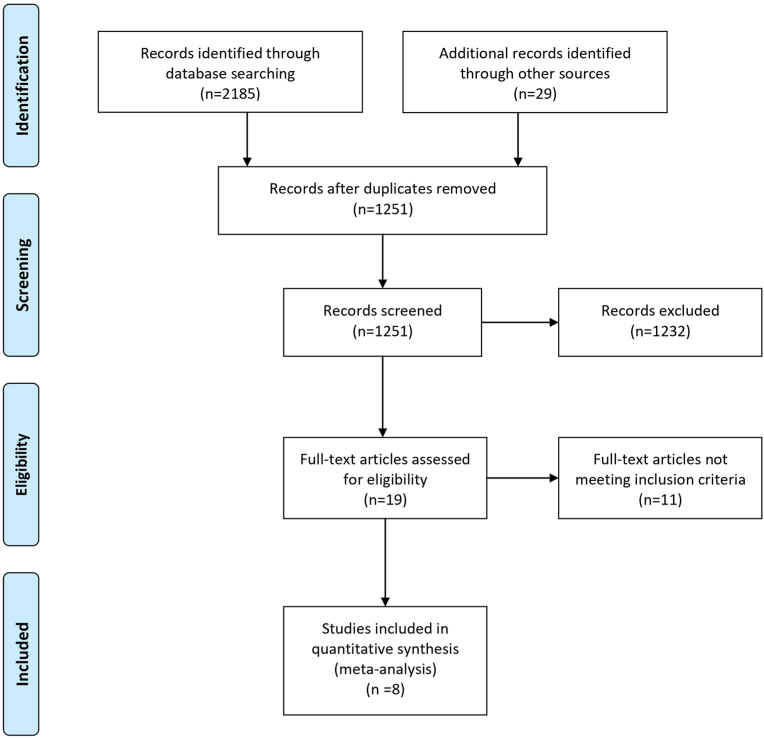
The initial search resulted in 2185 studies with a further 29 identified through reference list searching. After removing duplicates, 1251 studies remained. After reviewing titles and abstracts, 1232 studies were excluded and the remaining 19 studies were included for full-text review, of which eight met the inclusion criteria for inclusion in the NMA. The PRISMA flow diagram of study selection is shown in Fig. 1.
Fig. 1.
PRISMA flow diagram of study selection.
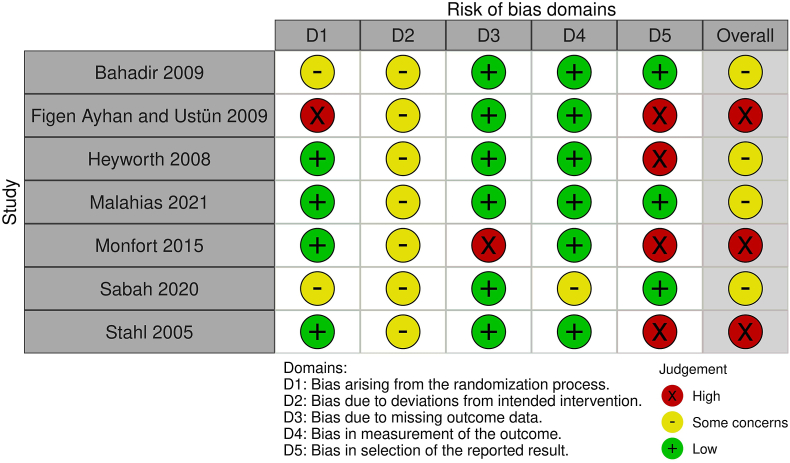
3.2. Methodological quality assessment
The risk of bias is shown in Fig. 2a, Fig. 2ba and b. Among the RCTs, one study was deemed to be at high risk of bias arising from the randomization process, based on the patient's hospital number.20 For domain 3, there was a high risk of bias in one study due to the loss of patients at follow-up in the steroid injection arm and the subsequent missing outcome data.21 Multiple studies were deemed high risk of bias for domain 5 for selective reporting of results.
Fig. 2a.
Risk of bias summary for RCTs.
Fig. 2b.
Risk of bias summary for comparative case series.
The retrospective study was considered critical for domain 1 due to statistically significant differences in baseline characteristics between the two arms, including variables such as smoking and diabetes, which are recognised confounders for osteoarthritis.22
3.3. Study characteristics
All included studies were published between 2005 and 2021. Seven studies were RCTs whilst one was a retrospective comparative study. The inclusion and exclusion criteria were highly variable across the studies, with some studies basing their inclusion criteria on age, sex, radiographic evidence of TBOA, classification using Eaton and Littler or Kellgren- Lawrence, minimum duration of symptoms, minimum amount of pain, hand dominance, failed conservative therapy or all combined. CS and HA were used in seven studies and PRP and placebo in two studies. CS vs HA were the most assessed injections (n = 7). Two studies compared three interventions and five studies compared two interventions. The regime, dose and technique of injections was also varied amongst the included studies. Three studies, with their respective arms, compared injections that were given to participants at baseline only whilst 6 studies compared injections that were given more than once over weekly intervals. Injection techniques varied depending on hand position of supine, prone of semi-prone. Ultrasound was used to assist injections in 2 studies. Table 1 summarises the characteristics of the selected studies, the inclusion and exclusion criteria, interventions and comparators and their respective regimes, doses and technique of injection.
Table 1.
Summary of selected studies.
| Author | Country | Inclusion | Exclusion | Intervention | Regime and dose | Comparator | Regime and dose | Comparator 2 | Regime and dose | Injection technique |
|---|---|---|---|---|---|---|---|---|---|---|
| Bahadir 2009 | Turkey | Grade 2 or 3 Eaton & Littler | Inflammatory arthritis, trauma, carpal tunnel, previous injection | Triamcinolone acetonide | One injection of 20 mg/0.5 mL of triamcinolone acetatonide the start of the trial | Hyaluronic acid | Weekly injections of 5mg/0.5 mL of sodium hyaluronate for 3 weeks | n/a | n/a | Needle inserted just proximal to the radial base of the first metacarpal bone, volar to the extensor pollicis brevis tendon. Hand supine |
| Figen Ayhan and Ustün 2009 | Turkey | Bilateral symptoms with failed prior treatment, VAS >40/100, radiographic evidence of grade 1–4 Eaton & Littler | Previous injection, trauma, inflammatory arthritis, joint infection | Hyaluronic acid | One injection of 1 mL of Hylan G-F20 at the beginning of the study | Saline | One injection of 1 mL saline at the beginning of the study | n/a | n/a | Needle inserted in the dorsal aspect of the radial side of the 1st carpometacarpal joint by holding the thumb in slight flexion. Hand prone |
| Heyworth 2008 | USA | Symptomatic osteoarthritis | Multiple previous injections, pregnancy, previous surgery, trauma to joint, inflammatory arthritis | Betamethasone acetate | Two injections, first saline 1 mL at baseline followed by 1 mL sodium betamethasone a week later | Hyaluronic acid | Two 8 mg injections of hylan G-F 20, first at baseline and second a week later | Saline | Two injections of 1 mL saline, first at baseline and second a week later | nm |
| Malahias 2021 | Greece | Clinical and radiographic evidence of TBOA, grades 1 to 3 Eaton & Littler | Systemic rheumatic disease, self-reported comorbid hand conditions, history of gout or pseudogout, bleeding predisposition, previous surgery to the affected thumb, previous injection, grade 4 Eaton & Littler | Methylprednisolone succinate | Two injections of 125mg/2 mL methylprednisolone sodium succinate 15 days apart | Platelet rich plasma | Two injections of platelet rich plasma injections 15 days apart. Platelet rich plasma mannually prepared | n/a | n/a | Ultrasound guided injection using free hand one man's technique |
| Monfort 2015 | Spain | Symptoms lasting >90 days requiring analgesic relief, at least grade 1 Kellgren-Lawrence | Pregnancy, severe renal/liver disease, injection within last 3 months, previous thumb surgery, previous physical therapy | Betamethasone acetate | Once weekly injections of 0.5 mL betamethasone disodium phosphate 1.5 mg and betamethasone acetate 1.5 mg for 3 weeks | Hyaluronic acid | Once weekly injections of 0.5 mL of hyaluronic acid 5 mg/mL 500–1000 kDa for 3 weeks | n/a | n/a | Ultrasound guided injection inserted lateral to the abductor pollicis longus tendon using free hand one man's technique. Hand semi-prone |
| Sabah 2020 | Egypt | Failed conservative treatment of at least 4 weeks | TBOA with failed conservative treatment of at least 4 weeks | Betamethasone acetate | One injection of 1 mL betamethasone acetate the start of the trial | Hyaluronic acid | One injection of containing 730,000 Da of hyaluronic acid at the start of the trial | Platelet Rich Plasma | One injection of 20 mL of platelet rich plasma at the start of trial. Platelet rich plasma produced by commercial kit | Needle inserted just proximal to the radial base of the first metacarpal bone, volar to the extensor pollicis brevis tendon. Hand supine |
| Stahl 2005 | Israel | Symptomatic CMCJ OA grade 2 Eaton Littler classification | nm | Methylprednisolone acetate | One injection of 40 mg methylprednisolone acetate at beginning of study | Hyaluronic acid | One injection of 15 mg of hyaluronic acid at beginning of study | n/a | n/a | Needle inserted in the dorsal aspect of the radial side of the 1st carpometacarpal joint by holding the thumb in slight flexion. Hand prone |
| Comparative Case Series | ||||||||||
| Tenti 2017 | Italy | Age 45–75, symptoms >3 months, VAS >30/100, grade 2 or 3 Kellgren-Lawrence | Inflammatory joint disease, septic arthritis, major trauma, prior surgery of upper limb, coagulation disorders, severe comorbidity, therapy with chondroitin sulfate, glucosamine, diacerein and steroids | Triamcinolone acetonide | Two injections of 0.5 mL of triamcinolone acetonide 20 mg/mL at baseline and at 15 days | Hyaluronic acid | Two injections of 1 mL of Sinovial H-L 3.2 % 16 mg + 16 mg/mL baseline and 15 days | n/a | n/a | Needle inserted lateral to the abductor pollicis longus tendon using free hand one man's technique. Hand semi-prone |
n/a – not applicable.
nm – not mentioned.
3.4. Patient demographics
Data from 446 patients were collected. In the CS group, a total of 184 injections were analysed. The mean age of patients was 61.2 ± 3.89, 83 % were female and the dominant hand was injected in 75 % of patients. In the HA group, a total of 213 injections were analysed. The mean age of patients was 62 ± 4.57, 87 % were female and the dominant hand was injected in 70 % of patients. In the PRP group, a total of 31 injections were analysed. The mean age of patients was 57.6 ± 5.18, and 81 % were female. In the Placebo group, a total of 47 injections were analysed. The mean age of patients was 63.3 ± 0.7, 94 % were female and the dominant hand was injected in 44 % of patients. Baseline comparability was found among age (p = 0.6) but not gender (p = 0.2). There was insufficient data to calculate ANOVA for hand dominance. Patient demographics are shown in Table 2.
Table 2.
Patient demographics.
| Author | Mean age intervention (years) | Mean age comparator one (years) | Mean age comparator two (years) | Male/Female Total |
|---|---|---|---|---|
| Parallel group RCT | ||||
| Bahadir 2009 | 62.9 | 60.8 | n/a | 0/40 |
| Figen Ayhan and Ustün 2009 | 62.6 | 62.6 | n/a | 0/29 |
| Heyworth 2008 | 60 | 65 | 64 | 8/52 |
| Malahias 2021 | 63 | 62.8 | n/a | 6/26 |
| Monfort 2015 | 62.8 | 62.8 | n/a | 11/77 |
| Sabah 2020 | 52.45 | 52.45 | 52.45 | 6/39 |
| Stahl 2005 | 62 | 62 | n/a | 6/46 |
| Retrospective comparative study | ||||
| Tenti 2017 | 65.5 | 68.6 | n/a | 32/68 |
n/a – not applicable.
3.5. Outcome of interest: pain
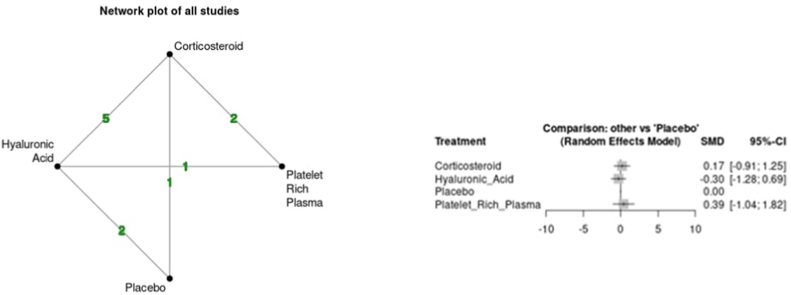
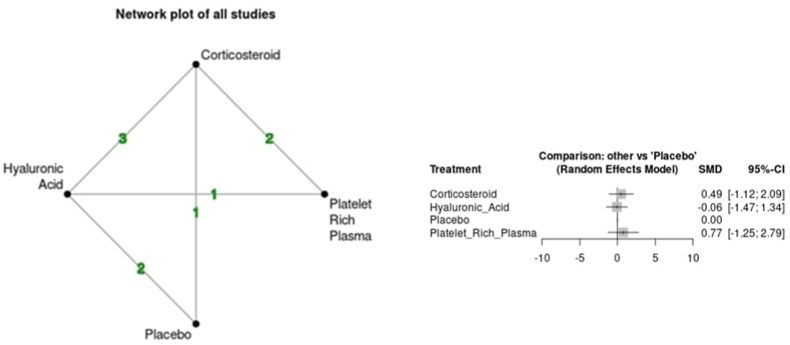
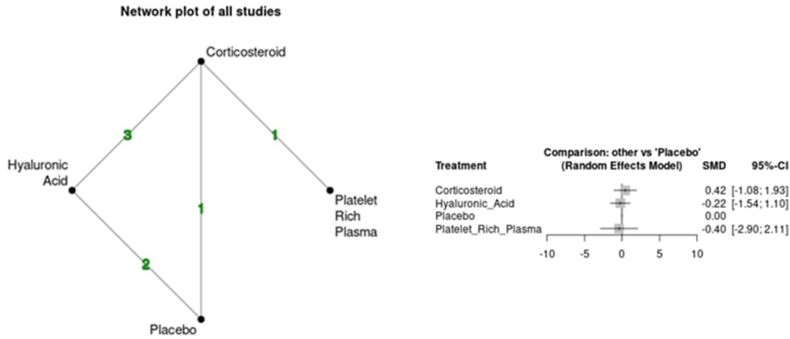
Variability was seen between the results of each intra-articular injection and pain. The CS group demonstrated an increase in VAS at the short-term time point (SMD 0.17 95 % CI -0.91; 1.25) but a reduction at the medium-term time point (SMD -0.04 95 % CI -1.08; 1.00). The HA group demonstrated a reduction in VAS vs placebo at both short (SMD -0.30 95 % CI -1.28; 0.69) and medium-term (SMD -0.45 95 % CI -1.40; 0.51) time points. Lastly, the PRP group demonstrated an increase in VAS at the short-term time point (SMD 0.39 95 % CI -1.04; 1.82) but a reduction at the medium-term time point (SMD -1.28 95 % CI -3.11; 0.55). Overall, all comparisons had large 95 % CI and did not support any statistically significant improvement or worsening of pain vs placebo at any time point. No evidence of inconsistency was found in indirect comparisons over the short and medium-term follow-up (p > 0.05). Fig. 3a, Fig. 3ba and b shows the network plots for each time point and comparison of outcomes based on forest plots for VAS.
Fig. 3a.
Network plot and forest plot of pain scores (VAS) for short term time point.
Fig. 3b.
Network plot and forest plot of pain scores (VAS) for medium-term time point.
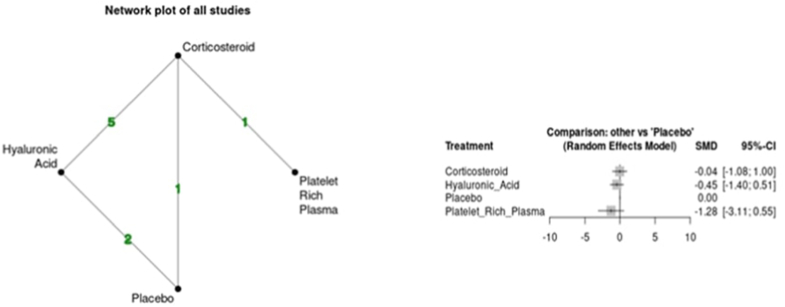
Sensitivity analysis at the short-term time point by excluding the study in which data was derived using statistical calculations did not alter the results of the NMA at this time point.23 Sensitivity analysis at the medium-term time point, by excluding the retrospective study demonstrated statistically significant superiority of the PRP group (SMD -1.48 95 % CI -2.71; −0.25) in reducing VAS at this time point vs placebo (Fig. 4).22 Furthermore, league table analysis also demonstrated superiority of PRP over CS and placebo but not HA (Table S1).
Fig. 4.
Forest plot for sensitivity analysis at the medium-term time point by excluding Tenti et al. (2017).
3.6. Outcome of interest: function
Variability was seen between the results of each intra-articular injection and function. The CS group demonstrated worsening of function at short (SMD 0.49 95 % −1.12; 2.09) and medium-term (SMD 0.42 95 % −1.08; 1.93) time points. The HA group demonstrated an improvement of function at short (SMD -0.06 95 % CI -1.47; 1.34) and medium-term (SMD -0.22 95 % CI -1.54; 1.10) time points. Lastly, there was inconsistency within the PRP group with worsening of function demonstrated at the short-term (SMD 0.77 95 % CI -1.25; 2.75) time point but improvement at the medium-term (SMD -0.40 95 % CI -2.90; 2.11) time point. As with pain, all comparisons had large 95 % CI and did not support any statistically significant improvement or worsening of function vs placebo at any time point. No evidence of inconsistency was found in indirect comparisons over the short and medium-term follow-up (p > 0.05). Fig. 5a, Fig. 5ba and b shows the network plots for each time point and comparison of outcomes based on forest plots for function.
Fig. 5a.
Network plot and forest plot of global functional assessment at the short-term time point.
Fig. 5b.
Network plot and forest plot of global functional assessment at the medium-term time point.
Sensitivity analysis by excluding the study in which data was derived using statistical calculations at the short-term time point did not alter the results of the NMA.23 Sensitivity analysis by excluding the retrospective study at the medium-term time point also did not alter the results of the NMA.22
4. Discussion
According to the main findings of the present NMA, no significant differences were found between any intra-articular injections compared to placebo at improving pain at the short-term time point. At the medium-term time point, only following sensitivity analysis, intra-articular PRP injections resulted in significant reduction of VAS vs placebo (SMD -1.48 95 % CI -2.71; −0.25). Furthermore, league table analysis demonstrates PRP's superiority over CS and reducing VAS at this time point (SMD -1.23 95 % CI -2.26 to −0.21). However, despite this being a novel finding, this was based on two studies only of variable methodological quality. No significant differences were found between any intra-articular injections vs placebo at improving function either at the short or medium-term time point.
PRP is an emerging therapy for knee osteoarthritis, with several studies indicating better outcomes when compared to HA and CS.24,25 The intra-articular use of PRP for osteoarthritis is based on its rich source of bioactive molecules such as cytokines and growth factors found within platelets. Upon degradation, these molecules are released into the joint space and bind to specific receptors on collagen, osteoclasts, and chondrocytes, thereby inducing chondroprotection.26 PRP's anti-inflammatory properties are thought to occur via the recruitment of resident stem cells to the site of injury, where they secrete additional anti-inflammatory cytokines through their paracrine and trophic effects.27 In contrast, the anti-inflammatory effects of CS are primarily due to its direct suppression of inflammatory pathways.28 It should be noted, however, that no in vitro study has directly compared the anti-inflammatory effects of PRP and CS.
Very few in vivo studies have investigated PRP injections for hand osteoarthritis specifically. Loibl et al. evaluated 10 patients with TBOA treated with two intra-articular PRP injections four weeks apart and reported decreased pain (VAS) after six months from 6.2 to 5.4 (p < 0.05).29 No change in function was demonstrated. Another more recent case series by Swärd and Wilcke evaluated 21 patients.30 Similar to Loibl et al. patients were given two intra-articular injections four weeks apart, however no significant reduction in pain was demonstrated (median numerical ratings from 2 to 1). No improvement in function was also demonstrated. The heterogeneity of results between the two-case series is also reflected within our cohort of studies in which PRP was used as an intervention. Abdelsabor Sabaah et al. randomised 45 patients between PRP, HA and CS groups but could not conclude any benefit of PRP over HA and CS in reducing pain and improving function after three months. Conversely, Malahias et al. reported an improvement in pain and function at a 12-month follow-up in their randomised study of 33 subjects (PRP n = 16); median VAS 75 to 20 (p = 0.005) and DASH 50.4 to 20.4 (p = 0.002). Furthermore, PRP was superior at reducing pain and improving function vs CS at 12 months (p = 0.015 and p = 0.025 respectively). This correlates with the findings of this NMA in the improvement of pain with PRP at the medium-term time point (SMD -1.48 95 % CI -2.71; −0.25) and superiority over CS in league table analysis (SMD -1.23 95 % CI -2.26 to −0.21) after sensitivity analysis.
Several reasons could explain the general inconsistency of results for PRP between RCTs included in our NMA. Most notably, concerns regarding the methodological quality (reflected in risk of bias table Fig. 2a, Fig. 2b) which render the studies prone to selection bias. For example, differences in baseline characteristics including age of patients and stage of osteoarthritis. Patients with all stages of TBOA (I-IV Eaton and Littler) were included in Abdelsabor Sabah et al. On the contrary, Malahias et al. excluded patients with stage 4 TBOA (Eaton and Littler), which represents the most severe pathology of TBOA radiologically, with complete loss of articular cartilage. Given the main mechanism of action of PRP is thought to involve chondroprotection,26 excluding patients with stage 4 TBOA could explain the difference in results between Malahias et al. and Abdelsabor Sabah et al. Currently, there is no consensus regarding the optimal technique for producing PRP for clinical use. In our NMA, Malahias et al. PRP was manually prepared at a local hospital whilst Abdelsabor Sabah et al. used a commercial kit to prepare the PRP. Both techniques can produce different concentration of platelets.30 Furthermore, the optimal number of PRP injections required to achieve significant clinical outcomes is still undetermined due to a lack of standardization. Consequently, the most effective treatment regimen remains unclear. Two injections were given to patients in Malahias et al. whilst only one was given in Abdelsabor Sabaah et al. which could explain the positive results demonstrated in the former study. When analysing data for knee osteoarthritis, several RCTs report that a course of PRP injections result in improved outcomes than a single injection alone.31
The strength of this study is that it is the first to compare CS, HA, PRP and placebo as a treatment for TBOA, assessing outcomes of pain and function in an NMA, providing limited but novel evidence. Previously, CS and HA have been compared in a recent meta-analysis which demonstrated an improvement in pain in the medium term (3–6 months) in the CS group (MD −1.32, 95 % CI −2.23 to −0.41).13 No improvement in function was demonstrated. The result for pain was based on two studies comprising 92 patients only. Our NMA comprised 446 patients in total with 184 receiving CS and 213 receiving HA, a significantly larger patient cohort for comparison. Furthermore, Riley et al. included studies published from 2004 to 2015. Our NMA also includes three further studies published from 2017 to 2021, updating the evidence base for injection therapy for TBOA.22,23,32 Unlike Riley et al. we also broadened our inclusion criteria by including comparative case series in our present study to add to the data set, given the small sample size of studies included in their meta-analysis. Lastly, whilst Riley et al. undertook a pairwise meta-analysis, due to limited data, this was performed for VAS when assessing pain and grip/pinch strength when assessing function (not enough data was collected on global functional assessment scores e.g., FIHOA, AUSCAN). Our extra data allowed us to perform a NMA of global functional assessment scales, which determines functional deficit during day-to-day activities which is arguably more clinically relevant and a key methodological difference between our study and Riley et al.
The present network meta-analysis (NMA) is associated with various limitations. Firstly, the limited number of studies that fulfilled the eligibility criteria, which encompassed fewer than 500 participants and only two studies investigated PRP injections, comprising just 31 patients. Consequently, the sample size of patients treated with PRP represents the most critical limitation of this study, raising questions about the external validity of the outcome favouring PRP at the medium-term time point. Moreover, this outcome is based on just two studies, of which 15 showed no response to PRP, and 16 demonstrated a favourable response. Thus, the result based on 16/31 patients suggests possible investigator preference, conduct or reporting bias, or strong confounding effects. Secondly, the inadequate methodology of the included studies limited the review, with five studies considered to have a high risk of bias. Lastly, all the comparisons showed wide confidence intervals (CI), indicating that the distribution of the probability of obtaining a specific estimated effect for pain and function comparisons is unlikely. Consequently, the positive findings from the present NMA should be interpreted with caution.
5. Conclusion
No intra-articular injection (corticosteroids, hyaluronic acid or platelet-rich plasma) demonstrated superiority over placebo at reducing pain at the short-term time point. At the medium-term time point, superiority of platelet-rich plasma at reducing pain over placebo and corticosteroids was seen following sensitivity analysis, however this was based on two studies with the result unlikely to have clinical significance. No injection proved superior at improving function at the short or medium-term time points. Given this NMA's limitations, there is a need for further robust multicentre RCTs with larger patient sample sizes, investigating corticosteroids, hyaluronic acid and platelet-rich plasma vs each other and placebo.
Author statement
Contributorship: Study searches, data extraction and risk of bias assessments were conducted by AT and SCS. Disagreements were discussed with NJ and JJD. AT and SCS wrote the first draft of the manuscript. NJ and JJD reviewed the draft manuscript providing corrections. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval declaration
N/a.
Informed consent declaration
N/a.
Conflict of interest
None.
Declaration of competing interest
None.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jor.2023.09.017.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Li Y., White C. Five things to know about…carpometacarpal osteoarthritis of the thumb. CMAJ (Can Med Assoc J) 2013;185:149. doi: 10.1503/cmaj.111444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillis J., Calder K., Williams J. Review of thumb carpometacarpal arthritis classification, treatment and outcomes. Can J Plast Surg. 2011;19 doi: 10.1177/229255031101900409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spaans A., van Minnen L., Kon M., Schuurman A., Schreuders A., Vermeulen G. Conservative treatment of thumb base osteoarthritis: a systematic review. J Hand Surg Am. 2015;40:1–6. doi: 10.1016/j.jhsa.2014.08.047. [DOI] [PubMed] [Google Scholar]
- 4.Heyneman C., Lawless-Liday C., Wall G. Oral versus topical NSAIDs in rheumatic diseases: a comparison. Drugs. 2000;60:555–574. doi: 10.2165/00003495-200060030-00004. [DOI] [PubMed] [Google Scholar]
- 5.Marcum Z., Hanlon J. Recognizing the risks of chronic nonsteroidal anti-inflammatory drug use in older adults. Ann Long Term Care. 2010;18:24–27. [PMC free article] [PubMed] [Google Scholar]
- 6.Dean B., Kluzek S., Carr A., et al. Base of thumb osteoarthritis in UK interface services-a cohort and survey-based study to assess current practice. Rheumatology. 2021;60:4094–4102. doi: 10.1093/rheumatology/keaa884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kloppenburg M., Kroon F.P., Blanco F.J., et al. 2019. 2018 Update of the EULAR Recommendations for the Management of Hand Osteoarthritis; pp. 16–24. [DOI] [PubMed] [Google Scholar]
- 8.Li B., Zhang Y., Bi L. Comparative efficacy of treatments for patients with knee osteoarthritis: a network meta-analysis. Eur J Med Res. 2020;25:1–11. doi: 10.1186/s40001-020-00426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie X., Zhang C., Tuan R. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res Ther. 2014;16 doi: 10.1186/ar4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Migliorini F., Driessen A., Quack V., et al. Comparison between intra-articular infiltrations of placebo, steroids, hyaluronic and PRP for knee osteoarthritis: a Bayesian network meta-analysis. Arch Orthop Trauma Surg. 2020;141:1473–1490. doi: 10.1007/s00402-020-03551-y. [DOI] [PubMed] [Google Scholar]
- 11.Singh H., Knapik D.M., Polce E.M., et al. Relative efficacy of intra-articular injections in the treatment of knee osteoarthritis: a systematic review and network meta-analysis. Am J Sports Med. 2021;50:3140–3148. doi: 10.1177/03635465211029659. [DOI] [PubMed] [Google Scholar]
- 12.Kroon F., Rubio R., Schoones J., Kloppenburg M. Intra-articular therapies in the treatment of hand osteoarthritis: a systematic literature review. Drugs Aging. 2016;33 doi: 10.1007/s40266-015-0330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riley N., Vella-Baldacchino M., Thurley N., Hopewell S., Carr A.J., Dean B.J.F. Injection therapy for base of thumb osteoarthritis: a systematic review and meta-analysis. BMJ. 2019;9 doi: 10.1136/bmjopen-2018-027507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutton B., Salanti G., Caldwell D., et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 15.Kroon F.P.B., van der Heijde D., Maxwell L.J., et al. Core outcome measurement instrument selection for physical function in hand osteoarthritis using the OMERACT Filter 2.1 process. Semin Arthritis Rheum. 2021;51:1311–1319. doi: 10.1016/j.semarthrit.2021.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 17.Sterne J.A., Hernán M.A., Reeves B.C., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owen R., Bradbury N., Xin Y., Cooper N., Sutton A. MetaInsight: an interactive web-based tool for analyzing, interrogating, and visualizing network meta-analyses using R-shiny and netmeta. Res Synth Methods. 2019;10:569–581. doi: 10.1002/jrsm.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:1–13. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figen Ayhan F., Ustün N. The evaluation of efficacy and tolerability of Hylan G-F 20 in bilateral thumb base osteoarthritis: 6 months follow-up. Clin Rheumatol. 2009;28 doi: 10.1007/s10067-008-1080-0. [DOI] [PubMed] [Google Scholar]
- 21.Monfort J., Rotés-Sala D., Segalés N., et al. Comparative efficacy of intra-articular hyaluronic acid and corticoid injections in osteoarthritis of the first carpometacarpal joint: results of a 6-month single-masked randomized study. Joint Bone Spine. 2015;82:116–121. doi: 10.1016/j.jbspin.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Tenti S., Pascarelli N., Giannotti S., Galeazzi M., Giordano N., Fioravanti A. Can hybrid hyaluronic acid represent a valid approach to treat rizoarthrosis? A retrospective comparative study. BMC Muscoskel Disord. 2017;18 doi: 10.1186/s12891-017-1809-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdelsabor Sabaah H., El Fattah R., Al Zifzaf D., Saad H. A comparative study for different types of thumb base osteoarthritis injections: a randomized controlled interventional study. Ortop Traumatol Rehabil. 2020;22:447–454. doi: 10.5604/01.3001.0014.6055. [DOI] [PubMed] [Google Scholar]
- 24.Kon E., Buda R., Filardo G., et al. Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc. 2010;18:472–479. doi: 10.1007/s00167-009-0940-8. [DOI] [PubMed] [Google Scholar]
- 25.Shen L., Yuan T., Chen S., Xie X., Zhang C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:1–12. doi: 10.1186/s13018-017-0521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moussa M., Lajeunesse D., Hilal G., et al. Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Exp Cell Res. 2017;352:146–156. doi: 10.1016/j.yexcr.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 27.do Amaral R., da Silva N., Haddad N., et al. Platelet-rich plasma obtained with different anticoagulants and their effect on platelet numbers and mesenchymal stromal cells behavior in vitro. Stem Cell Int. 2016;2016:1–12. doi: 10.1155/2016/7414036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coutinho A., Chapman K. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loibl M., Lang S., Dendl L., et al. Leukocyte-reduced platelet-rich plasma treatment of basal thumb arthritis: a pilot study. BioMed Res Int. 2016;2016 doi: 10.1155/2016/9262909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swärd E., Wilcke M. Effects of intra-articular Platelet-Rich Plasma (PRP) injections on osteoarthritis in the thumb basal joint and scaphoidtrapeziotrapezoidal joint. PLoS One. 2022;17 doi: 10.1371/journal.pone.0264203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Görmeli G., Görmeli C., Ataoglu B., Çolak C., Aslantürk O., Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25:958–965. doi: 10.1007/s00167-015-3705-6. [DOI] [PubMed] [Google Scholar]
- 32.Malahias M., Roumeliotis L., Nikolaou V., Chronopoulos E., Sourlas I., Babis G. Platelet-rich plasma versus corticosteroid intra-articular injections for the treatment of trapeziometacarpal arthritis: a prospective randomized controlled clinical trial. Cartilage. 2021;12:51–61. doi: 10.1177/1947603518805230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.