Abstract
The majority of clinical isolates of Moraxella catarrhalis produce β-lactamase. The role of this enzyme in the phenomenon of indirect pathogenicity, in which a true pathogen such as Streptococcus pneumoniae is protected from the action of certain β-lactam antibiotics, is well recognized. By using a simple continuous-culture biofilm system, it has been shown that the pneumococcus attains high titers in excess of 1012 CFU/biofilm; furthermore, the penicillin-sensitive pneumococcus used remained susceptible to a range of β-lactam antibiotics in these biofilms (R. K. Budhani and J. K. Struthers, J. Antimicrob. Chemother. 40:601–602, 1997). This system was used to characterize the antibiotic susceptibility of this isolate when grown with β-lactamase-negative or -positive moraxellae. When grown with β-lactamase-producing moraxellae in the presence of either benzylpenicillin or amoxicillin, the pneumococcus was protected in the range of the antibiotic concentrations to which it would be considered resistant. With amoxicillin-clavulanic acid the titers of the two organisms collapsed at the antibiotic concentration at which moraxellae became susceptible. The levels of β-lactamase activity in cell-free supernatants of broth culture, in biofilm, and in biofilm effluent revealed distinct differences in this activity; levels in biofilm were significantly lower than those in broth culture supernatants. The system appears suitable for studying organisms under antibiotic stress and for investigating the interactions of bacteria under such conditions.
Moraxella catarrhalis is a gram-negative diplococcus which is often isolated from respiratory tract secretions sent to microbiology laboratories; a survey conducted in the United Kingdom in 1991 showed that M. catarrhalis was the third most common organism isolated after Streptococcus pneumoniae and Haemophilus influenzae (16). An important feature of M. catarrhalis is that at least 90% of isolates produce a β-lactamase with activity against β-lactams such as penicillin and the amino penicillins (7). Based on isoelectric focusing patterns, the β-lactamases of M. catarrhalis can be designated as BRO-1 or BRO-2. The former type is more common, and levels of expression of the BRO-1 enzyme are higher than those of BRO-2 (5, 7). While the self-protective role of the enzyme is evident, the phenomenon of indirect pathogenicity in mixed infections, in which a true pathogen such as a pneumococcus is also protected by the enzyme, is well recognized (1, 18). It is known that the sputa of patients being treated with penicillin, from which both pneumococci and β-lactamase-producing moraxellae have been isolated, do not contain detectable amounts of the antibiotic (18). This protective effect has been recently demonstrated in vivo, with a mouse model (10), but the details of this interaction in vitro have not been clearly defined. Kassim and Williams used broth cultures in their investigations and have demonstrated a degree of protection of pneumococci by β-lactamase-positive moraxellae in the presence of antibiotics such as penicillin (12, 13). This system was limited by the fact that it does not involve a continuous culture and thus variations in the growth rates of the organisms can occur.
A more reproducible and continuous culture system would be important in defining the relationship between S. pneumoniae and M. catarrhalis. The simple Sorbarod continuous-culture biofilm system appears ideal for such an investigation. We have shown recently that S. pneumoniae attains consistently high concentrations (>1012 CFU/biofilm) for a period of at least 96 h in Sorbarod filters (2); these filters consist of a concertina of cellulose fibers in a paper sleeve. Here we characterize the growth of pneumococci on these biofilms in the presence of β-lactamase-positive or β-lactamase-negative moraxellae and of different concentrations of three β-lactam antibiotics. The characteristics of the protective effect of moraxella BRO-1 β-lactamase in a biofilm system are described.
MATERIALS AND METHODS
Bacteria.
S. pneumoniae ATCC 671310 and the clinical isolate (serotype 19) described previously were used (2). M. catarrhalis ATCC 25238 (β-lactamase negative), β-lactamase-positive M. catarrhalis (BRO-1 β-lactamase), and penicillin-intermediate isolates of S. pneumoniae were obtained from departmental teaching stocks. Isolates of S. pneumoniae resistant to penicillin were obtained from the Antibiotic Reference Laboratory, Public Health Laboratory Service, Colindale, United Kingdom.
Antibiotics.
The following antibiotics were used: benzylpenicillin (Britannia, Redhill, United Kingdom), amoxicillin and amoxicillin-clavulanic acid (SmithKline Beecham, Worthing, United Kingdom), vancomycin (Lilly, Basingstoke, United Kingdom), and aztreonam (Bristol-Myers Squibb, Hounslow, United Kingdom). The ratio of amoxicillin to clavulanic acid used was 5:1.
Tube MIC and MBC and the E test.
Tube MIC and minimal-bactericidal-concentration (MBC) tests were done by a recognized procedure using brain heart infusion (BHI) broth (Oxoid, Unipath, Basingstoke, United Kingdom); the concentration of bacteria at the beginning of each experiment was approximately 105 CFU/ml (11). The penicillin E test was performed with direct sensitivity test agar (Oxoid, Unipath), according to the manufacturer’s instructions (AB Biodisk, Solna, Sweden).
Sorbarod biofilms.
The method described previously was used (2, 9, 14). For each experiment, 12 20- by 10-mm Sorbarod filters (Ilacon, Tonbridge, Kent, United Kingdom) were prepared in order to have a control antibiotic-free biofilm and enough biofilms to cover twofold dilutions of an antibiotic in concentrations ranging from 0.004 to 16 mg/liter. Individual filters were inoculated with 3 ml of an exponential-phase broth culture of an organism or with the same volume of both bacteria. For broth culture and the biofilm “feeding,” BHI was used. BHI was delivered at a rate of 0.1 ml/min by means of a 12-channel 205U peristaltic pump (Watson Marlow, Falmouth, United Kingdom). After 24 h, when steady-state growth had been obtained (2), individual biofilms were exposed to a single concentration of an antibiotic for 18 h, using the same broth (BHI) and flow rate. After this period, effluent was collected for 15 min in order to determine the concentration of planktonic bacteria, and the biofilm was then disintegrated in 5 ml of BHI broth with a vortex mixer and the titers of the bacteria were determined by a recognized method (11). All biofilm titers were multiplied by a factor of 6.57 to take into account the 5 ml of broth added and the volume of the filter itself (14). The titers of effluent and biofilm were determined in triplicate on Columbia blood agar (Oxoid). When titrating mixtures of the two organisms, Columbia blood agar containing 2 μg of vancomycin per ml or 10 μg of aztreonam per ml was used as the selective medium for M. catarrhalis or S. pneumoniae, respectively. The biofilm eradicating concentration (BEC), the lowest concentration of an antibiotic that eradicated the organisms from biofilm, was determined as described previously (2, 14).
In order to ensure that bacteria were not being killed by any β-lactam antibiotics during vortex mixing of the biofilm and the titration steps, a broad-spectrum β-lactamase preparation (Bacillus cereus β-lactamases I and II; Oxoid) was included at these stages in several experiments. The final concentration of the added β-lactamase was approximately 104 mU/ml.
Determination of moraxella β-lactamase activity.
The method used was essentially the same as that described previously (3, 12). Supernatants of broth culture, biofilm after vortex mixing, and biofilm effluent were collected following centrifugation at 2,000 × g for 10 min. One-hundred-microliter volumes of the supernatant were mixed with equal volumes of nitrocefin (Oxoid), at a concentration of 100 μg/ml, in the wells of 96-well microtiter plates (Sterilin, Stone, Staffordshire, United Kingdom). Plates were incubated in the dark at 37°C for 30 min and were then read immediately at 492 nm in a Multiscan MCC/340 MKII plate reader. Readings were converted to milliunits per milliliter by using a standard curve prepared under the same conditions with B. cereus type I β-lactamase (Sigma, Poole, Dorset, United Kingdom) of known specific activity. Readings were converted to nanomoles of nitrocefin hydrolyzed per minute.
RESULTS
The results of the tube MIC and MBC tests, E test, and BEC test for the various isolates of S. pneumoniae are shown in Table 1. Using the tube MIC and MBC test and E test as controls, it is evident that the BECs of the various penicillin-sensitive, -intermediate, and -resistant isolates of pneumococcus tested showed that irrespective of penicillin susceptibility, there was no protection of the organism in this biofilm mode of growth. We were therefore confident that any survival of the pneumococcal isolate serotype 19 in the presence of antibiotic would be due to M. catarrhalis BRO-1 β-lactamase.
TABLE 1.
Susceptibilities of six isolates of S. pneumoniae to benzylpenicillina
| Isolate | Susceptibility | Tube MIC and MBC | E test result | BEC |
|---|---|---|---|---|
| ATCC 671310 | Sensitive | 0.008, 0.008 | 0.008 | ND |
| Serotype 19 | Sensitive | 0.016, 0.016 | 0.016 | 0.032 |
| R1819 | Intermediate | 1.0, 1.0 | 0.5 | 1.0 |
| R1855 | Intermediate | 1.0, 1.0 | 0.38 | 1.0 |
| 2312 | Resistant | 4.0, 8.0 | 6.0 | 8.0 |
| 2323 | Resistant | 4.0, 8.0 | 4.0 | 4.0 |
All values are expressed in milligrams per liter. ND, not done.
In order to determine if the initial biofilm inoculum concentrations had any effect on the ability of the two organisms to establish themselves together on a biofilm, the following experiment was done. Undiluted broth cultures of either S. pneumoniae or M. catarrhalis were mixed with a 10-fold dilution of the other organism; this mixture was used to inoculate a Sorbarod filter. After 24 h, effluent was collected, the biofilm was sacrificed, and the titers of the two organisms were determined on the selective media. The results in Table 2 show that after the 24-h period, the two organisms achieved similar titers within the biofilms, on the order of 1013 CFU/biofilm. Fluctuations in the titers of the organisms in the biofilm effluent were noted; on occasion, they were in excess of 10-fold, as shown in Table 2. It is important to note that the effluent titers were at least 4 log values lower than those in the biofilms.
TABLE 2.
Biofilm and effluent organism titers in mixed Sorbarod biofilm cultures of S. pneumoniae serotype 19 and M. catarrhalis ATCC 25238
| Inoculum ratio (pneumococcus/ moraxella) | Pneumococcus titer
|
Moraxella titer
|
||
|---|---|---|---|---|
| Biofilm | Effluent | Biofilm | Effluent | |
| 100:100 | 13.74 | 9.47 | 12.99 | 8.60 |
| 100:10−1 | 13.77 | 9.69 | 13.12 | 8.47 |
| 100:10−2 | 13.75 | 9.77 | 13.23 | 8.44 |
| 100:10−4 | 13.54 | 9.34 | 13.60 | 8.43 |
| Pneumococcus control | 13.77 | 9.47 | ||
| 100:100 | 13.74 | 8.78 | 13.80 | 10.69 |
| 10−1:100 | 13.72 | 8.94 | 13.77 | 9.84 |
| 10−4:100 | 13.59 | 8.68 | 13.78 | 9.85 |
| Moraxella control | 13.92 | 9.84 | ||
An undiluted broth culture of one organism was mixed with an equal volume of a dilution of the other organism and this mixture was used to inoculate a biofilm. After 24 h effluent was collected, the biofilm was harvested, and the organism titers were determined on selective media as described in the text. Control biofilms were inoculated with only one organism. Titers in biofilm (log10) are expressed as recoverable CFU per filter; titers in effluent are expressed as CFU per milliliter.
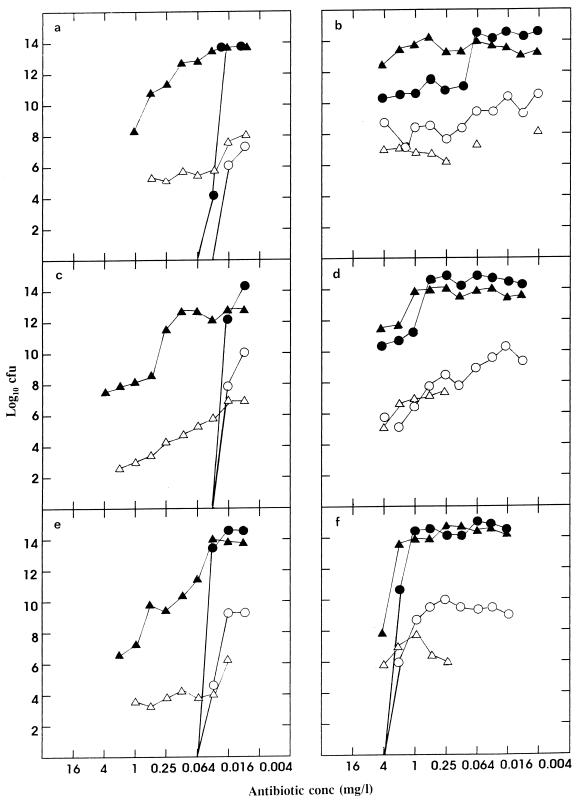
The results of the experiments combining the pneumococcus with either β-lactamase-negative or -positive M. catarrhalis in the presence of various concentrations of antibiotics are shown in Fig. 1. Clearly, the β-lactamase-negative moraxella was unable to protect the pneumococcus; the BEC obtained for the antibiotics used with serotype 19 was essentially the same as that shown in Table 1. In contrast, the presence of β-lactamase-positive moraxellae had a definite protective effect. In repeated experiments with benzylpenicillin and amoxicillin, there was an approximate 3- to 4-log value collapse of the pneumococcal titer at concentrations between 0.064 and 2.0 mg/liter. The surviving pneumococcal population, on the order of 1010 CFU/biofilm, was protected at concentrations of both penicillin and amoxicillin of above 2 mg/liter. With amoxicillin-clavulanic acid, the titer of the pneumococcus was essentially maintained until the collapse of the moraxella population at 1 to 2 mg/liter; in these experiments, the pneumococcal population was then eliminated. Despite the massive collapse in the moraxella population in the presence of amoxicillin-clavulanic acid, a surviving population of this organism, with a biofilm titer in the region of 107 CFU/filter, was maintained up to at least 16 mg/liter (data not shown). The results of the pneumococcal experiments with benzylpenicillin and amoxicillin in which B. cereus β-lactamase was included in biofilm vortex mixing and in all the subsequent titration steps are shown in Tables 3 and 4. These results show that the addition of the B. cereus β-lactamase did not significantly affect the titers.
FIG. 1.
Biofilm and effluent titers of S. pneumoniae (• and ○, respectively) and M. catarrhalis (▴ and ▵, respectively) (β-lactamase negative [a, c, and e] and β-lactamase positive [BRO1] [b, d, and f]) grown together on Sorbarod biofilms in the presence of benzylpenicillin (a and b), amoxicillin (c and d), and amoxicillin-clavulanic acid (e and f). Individual filters were inoculated with mixtures of the two organisms and, after 24 h, were exposed to a single concentration of the antibiotic for 18 h. Effluent was collected for 15 min, the biofilm was sacrificed, and the titers of pneumococci and moraxellae were determined on selective agar. Titers in biofilm are expressed as total recoverable CFU per biofilm. Titers in effluent are expressed as CFU per milliliter.
TABLE 3.
Pneumococcal titers from biofilms and biofilm effluents after vortex mixing and subsequent titration in the presence and absence of B. cereus β-lactamasea
| Antibiotic concn (mg/liter) | Pneumococcal titer after exposure to:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Penicillin
|
Amoxicillin
|
|||||||
| BHI
|
BHI with β-lactamase
|
BHI
|
BHI with β-lactamase
|
|||||
| Biofilm | Effluent | Biofilm | Effluent | Biofilm | Effluent | Biofilm | Effluent | |
| 4.0 | NG | NG | NG | NG | NG | NG | NG | NG |
| 1.0 | NG | NG | NG | NG | NG | NG | NG | NG |
| 0.25 | NG | NG | NG | NG | NG | NG | NG | NG |
| 0.064 | NG | NG | NG | NG | NG | NG | NG | NG |
| 0.016 | 11.77 | 6.17 | 11.64 | 6.07 | 10.67 | 5.30 | 10.51 | 5.14 |
| 0.004 | 14.49 | 8.30 | 14.50 | 9.20 | 14.23 | 8.14 | 14.41 | 8.36 |
Individual biofilms were inoculated with mixtures of S. pneumoniae serotype 19 and M. catarrhalis ATCC 25238 and, after 24 h, were exposed to a single concentration of an antibiotic for 18 h; effluent was then collected for 15 min. Individual biofilms were sacrificed in BHI or BHI containing 104 mU of B. cereus β-lactamase per ml. Titrations of the biofilm and effluent were then performed in the absence or presence of the B. cereus enzyme. Titers in biofilm (log10) are expressed as recoverable CFU per biofilm; titers in effluent are expressed as CFU per milliliter. NG, no growth.
TABLE 4.
Pneumococcal titers from biofilms and biofilm effluents after vortex mixing and subsequent titration in the presence and absence of B. cereus β-lactamasea
| Antibiotic concn (mg/liter) | Pneumococcal titer after exposure to:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Benzylpenicillin
|
Amoxicillin
|
|||||||
| BHI
|
BHI with β-lactamase
|
BHI
|
BHI with β-lactamase
|
|||||
| Biofilm | Effluent | Biofilm | Effluent | Biofilm | Effluent | Biofilm | Effluent | |
| 16.0 | 11.95 | 8.44 | 11.81 | 7.04 | 10.32 | 5.25 | 10.23 | 5.04 |
| 4.0 | 13.43 | 9.28 | 13.56 | 8.68 | 10.27 | 6.06 | 10.49 | 6.34 |
| 1.0 | 14.41 | 9.36 | 14.39 | 9.53 | 14.43 | 9.46 | 14.36 | 9.59 |
| 0.25 | 14.51 | 10.30 | 14.50 | 10.17 | 14.60 | 9.47 | 14.64 | 9.43 |
| 0.064 | 14.59 | 10.44 | 14.55 | 10.34 | 14.49 | 9.20 | 14.27 | 9.20 |
| 0.016 | 14.56 | 10.70 | 14.41 | 10.68 | ND | ND | ND | ND |
Individual biofilms were inoculated with mixtures of S. pneumoniae serotype 19 and β-lactamase-positive M. catarrhalis and, after 24 h, were exposed to a single concentration of an antibiotic for 18 h; effluent was then collected for 15 min. Individual biofilms were sacrificed in BHI or BHI containing 104 mU of B. cereus β-lactamase per ml. Titrations of the biofilm and effluent were then performed in the absence or presence of the B. cereus enzyme. Titers in biofilm (log10) are expressed as recoverable CFU per biofilm; titers in effluent are expressed as CFU per milliliter. ND, not done.
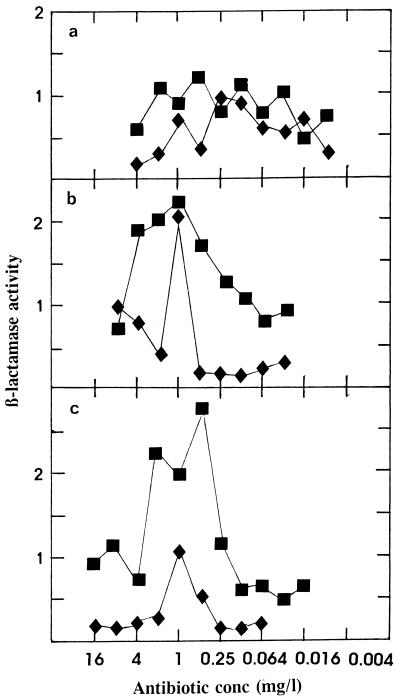
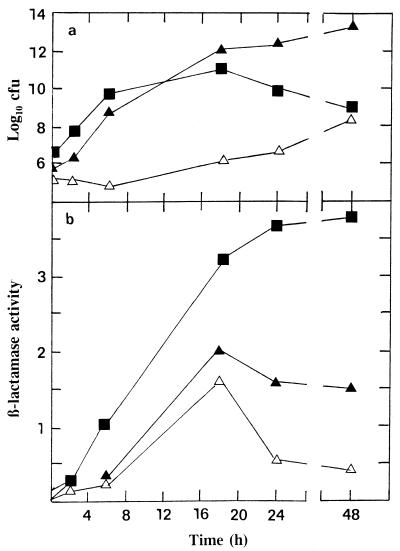
The β-lactamase activities in biofilm and effluent supernatants are shown in Fig. 2. For penicillin there was a random level of β-lactamase activity, but for both amoxicillin and amoxicillin-clavulanic acid an increase in the biofilm β-lactamase activity was noted at antibiotic concentrations between 0.125 and 8 mg/liter. This increase was matched to a lesser extent in the biofilm effluents. An increase in cell-free β-lactamase activity was also observed, for example, when moraxella strain BRO1 alone was exposed to amoxicillin under the same biofilm conditions (data not shown). It was considered important to determine the level of cell-free β-lactamase activity during the growth of strain BRO1 in broth culture and during the establishment of the organism on biofilms, in order to determine the relative degree of β-lactamase activity under the two different growth conditions. The results in Fig. 3 show that β-lactamase activity was always lower in the biofilms. Even at the very high organism numbers achieved in biofilm after 24 h, the rate of activity was always lower than that of the broth culture cell-free fraction.
FIG. 2.
β-Lactamase activities in the supernatant fractions of biofilm (■) and effluent (⧫) preparations (Fig. 1) with the mixtures of the pneumococcus and strain BRO1. The supernatant was obtained by centrifuging both biofilms after vortex mixing and effluent at 3,500 rpm for 10 min. β-Lactamase activity (expressed as nanomoles of nitrocefin hydrolyzed per minute) was determined as described in the text. (a, benzylpenicillin; b, amoxicillin; c, amoxicillin-clavulanic acid).
FIG. 3.
(a) Titers of strain BRO1 grown in broth culture and biofilm (plus biofilm effluent) collected at various times after inoculation. Titers in biofilm are expressed as recoverable CFU per biofilm; titers in effluent are expressed as CFU per milliliter. (b) β-Lactamase activity (nanomoles of nitrocefin hydrolyzed per minute) in the supernatants of the same specimens. ■, broth; ▴, biofilm; ▵, effluent.
DISCUSSION
The work presented here shows that the Sorbarod continuous-culture biofilm system is useful for studying the effect of antibiotics on organisms grown together. By defining the system with strains of pneumococcus with different susceptibilities to penicillin we were able to show that this biofilm mode of growth did not provide any protection for this organism. This is in contrast to the results of previous studies, on the basis of which the biofilm mode of growth has been considered protective for microorganisms (4, 8, 15). When the two bacteria were grown together on a biofilm, in the absence of antibiotics, they reached and maintained the same titers as the organisms grown separately, that is, in excess of 1012 CFU/biofilm. We were therefore confident that in the absence of antibiotics, neither organism could establish and maintain itself on biofilm to the detriment of the other. The BEC for the pneumococcus in the presence of the β-lactamase-negative moraxella was the same as that published previously (2). When the pneumococcus was grown with the β-lactamase-positive moraxella in the presence of benzylpenicillin or amoxicillin, an approximately 4-log value collapse of the pneumococcus population was observed. The surviving pneumococcal population, on the order of 1010 CFU/biofilm, was protected in the antibiotic concentration at which the organism is considered to be highly resistant to penicillin, that is, in excess of 2 mg/liter (6). With amoxicillin-clavulanic acid the protective effect existed up to the collapse of the moraxella population at concentrations of 1 to 2 mg/liter and above, showing the positive effect of clavulanic acid in abolishing the indirect pathogenic effect of β-lactamase-producing M. catarrhalis; however, the pneumococcus would still be regarded as resistant according to the criteria used (6). Thus, there appeared to be two identifiable pneumococcal populations observed in the experiments with penicillin and amoxicillin, one in the sensitive to intermediate range and another that remained resistant to these antibiotics due to the protective effect of the moraxella β-lactamase.
The β-lactamase levels in biofilm and effluent supernatants (Fig. 2) showed that there was no discernible difference across the range of penicillin concentrations used. However, peaks were noted in the biofilm supernatants in the amoxicillin and amoxicillin-clavulanic acid experiments; a rise in β-lactamase activity in the effluents was less distinct. This rise in the cell-free β-lactamase activity in biofilm may represent a subpopulation of strain BRO1 that undergoes lysis as a result of antibiotic activity, releasing the enzyme into the cell-free environment. Similar results were obtained with strain BRO1 alone when it was exposed to amoxicillin under the same biofilm conditions (data not shown). Figure 3 shows the titers of bacteria and β-lactamase levels in cell-free supernatants both of broth culture and during the establishment of strain BRO1 on biofilms. Despite the significantly higher bacterial titer in biofilm than in broth, the level of enzyme activity in biofilm supernatants was always lower. Comparing the maximum broth titer at 18 h with the maximum biofilm titer at 48 h and the enzyme activities at these times showed that the relative activity of cell-free enzyme in biofilm was on the order of 200-fold lower (Fig. 3). The population of moraxellae collected over 15 min in the effluent must reflect the presence of free planktonic bacteria which are eluted from the biofilm. As shown in Fig. 3, this effluent population is at least 3 log values less than the equivalent established biofilm population of the moraxella. Examining the levels of cell-free enzyme activity in the established biofilm and effluent populations showed that, based on relative organism numbers, the activity in biofilm is at least 3 to 4 log values lower than that in the effluent. Production of β-lactamase by M. catarrhalis is considered to be constitutive (17); however, some regulatory system may exist at the high organism numbers achieved in this biofilm system.
Of particular interest in this study was the absence of strain BRO1 bacteria in certain effluents of mixtures of the two organisms. While this was seen on occasion when the pneumococcus and the moraxella were grown on Sorbarod filters in the absence of antibiotics, this phenomenon was regularly observed in mixed infections in the presence of lower concentrations of the three antibiotics used. This is evident in the graphs presented in Fig. 1, which show that the moraxella was absent from 13 of 15 effluents at antibiotic concentrations below 0.25 mg/liter. This phenomenon was observed in repeated experiments with penicillin (two of three experiments), amoxicillin (two of three experiments), and amoxicillin-clavulanic acid (two of two experiments). The effluent curves of strain BRO1 in Fig. 1 show that when it is present, titers were on the order of 106 to 108 CFU/ml, at least 4 log values lower than the titer of the equivalent biofilm population at a particular antibiotic concentration. Despite this significant difference in the biofilm and effluent moraxella titers, the repeated absence of the β-lactamase-positive moraxella could indicate a type of interspecies signalling system whereby a pneumococcus population stressed by an antibiotic attempts to keep an ally in the biofilm environment itself. The indirect pathogenic role of β-lactamase-producing M. catarrhalis that has been proposed (1, 18) may not therefore involve a passive interaction. The Sorbarod biofilm system thus appears to have a role in elucidating the interaction between bacteria when they are exposed to antibiotics.
ACKNOWLEDGMENTS
R. K. Budhani is a predoctoral student funded by a scholarship from the Shah Latif University, Khairpur, Pakistan.
We thank A. Johnson of the Public Health Laboratory Service for providing the resistant pneumococcal isolates.
REFERENCES
- 1.Brook I. The concept of indirect pathogenicity by β-lactamase production, especially in ear, nose and throat infection. J Antimicrob Chemother. 1989;24:63–72. doi: 10.1093/jac/24.suppl_b.63. [DOI] [PubMed] [Google Scholar]
- 2.Budhani R K, Struthers J K. The use of Sorbarod biofilms to determine the antimicrobial susceptibilities of a strain of Streptococcus pneumoniae. J Antimicrob Chemother. 1997;40:601–602. doi: 10.1093/jac/40.4.601. [DOI] [PubMed] [Google Scholar]
- 3.Dragicevic P, Hill S L, Burnett D, Merrikin D, Stockley R A. Activities and sources of β-lactamase in sputum from patients with bronchiectasis. J Clin Microbiol. 1989;27:1055–1061. doi: 10.1128/jcm.27.5.1055-1061.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duguid I G, Evans E, Brown M R W, Gilbert P. Effect of biofilm culture on the susceptibility of Staphylococcus epidermidis to tobramycin. J Antimicrob Chemother. 1992;30:803–810. doi: 10.1093/jac/30.6.803. [DOI] [PubMed] [Google Scholar]
- 5.Farmer T, Reading C. β-Lactamases of Branhamella catarrhalis and their inhibition by clavulanic acid. Antimicrob Agents Chemother. 1982;21:506–508. doi: 10.1128/aac.21.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedland I R, McCracken G H. Management of infections caused by antibiotic-resistant Streptococcus pneumoniae. N Engl J Med. 1994;331:377–382. doi: 10.1056/NEJM199408113310607. [DOI] [PubMed] [Google Scholar]
- 7.Fung C P, Yeo S F, Livermore D M. Susceptibility of Moraxella catarrhalis isolates to β-lactam antibiotics in relation to β-lactamase pattern. J Antimicrob Chemother. 1994;33:215–222. doi: 10.1093/jac/33.2.215. [DOI] [PubMed] [Google Scholar]
- 8.Gander S. Bacterial biofilms: resistance to antimicrobial agents. J Antimicrob Chemother. 1996;37:1047–1050. doi: 10.1093/jac/37.6.1047. [DOI] [PubMed] [Google Scholar]
- 9.Hodgson A E, Nelson S M, Brown M R W, Gilbert P. A simple in-vitro model for growth control of bacterial biofilms. J Appl Bacteriol. 1995;79:87–93. doi: 10.1111/j.1365-2672.1995.tb03128.x. [DOI] [PubMed] [Google Scholar]
- 10.Hol H, van Dijke E E M, Verduin C M, Verhoef J, van Dijk H. Experimental evidence for Moraxella-induced neutralisation in pneumococcal pneumonia. J Infect Dis. 1994;170:1613–1616. doi: 10.1093/infdis/170.6.1613. [DOI] [PubMed] [Google Scholar]
- 11.Holt A, Brown D. Antimicrobial susceptibility testing. In: Hawkey P M, Lewis D A, editors. Medical bacteriology, a practical approach. Oxford, United Kingdom: IRL Press; 1989. pp. 167–194. [Google Scholar]
- 12.Kassim M. The in-vitro studies of Branhamella catarrhalis BRO1: epidemiology and antimicrobial susceptibilities with emphasis on the importance of the β-lactamase enzyme. Ph.D. thesis. London, United Kingdom: Queen Mary’s College, University of London; 1994. [Google Scholar]
- 13.Kassim M, Williams J D. Forum of Evaluation of Anti-Infective Therapy, Antibiotics and the Respiratory Tract. 1992. In-vitro studies of the indirect pathogenic role of Branhamella (Moraxella) catarrhalis BRO1, abstr. 5. [Google Scholar]
- 14.Muli F, Struthers J K. Use of a continuous-culture biofilm system to study the antimicrobial susceptibilities of Gardnerella vaginalis and Lactobacillus acidophilus. Antimicrob Agents Chemother. 1998;42:1428–1432. doi: 10.1128/aac.42.6.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nichols W W. Biofilms, antibiotics and penetration. Rev Med Microbiol. 1991;2:177–181. [Google Scholar]
- 16.Powell M, Mcvey D, Kassim M H, Chen H Y, Williams J D. Antimicrobial susceptibility of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella (Branhamella) catarrhalis isolated in the United Kingdom from sputa. J Antimicrob Chemother. 1991;28:249–259. doi: 10.1093/jac/28.2.249. [DOI] [PubMed] [Google Scholar]
- 17.Stobberingh E E, Van Eck H J, Houben A W, Van Boven C P A. Analysis of the relationship between ampicillin resistance and β-lactamase production in Branhamella catarrhalis. Drugs. 1986;31:23–27. doi: 10.2165/00003495-198600313-00007. [DOI] [PubMed] [Google Scholar]
- 18.Wardle J R. Branhamella catarrhalis as an indirect pathogen. Drugs. 1986;31:93–96. doi: 10.2165/00003495-198600313-00020. [DOI] [PubMed] [Google Scholar]