Highlights
-
•
Vulvar Paget's diseae (VPD) is usually treated by wide local excision.
-
•
Patients with VPD frequently require repeat excisions due to involved surgical margins.
-
•
Fluorescein mapping provides improved estimation of disease extent in VPD.
Keywords: Fluorescein Mapping, Vulvar Paget’s disease
Abstract
Background
Vulvar Paget disease (VPD) is a rare neoplastic condition exhibiting extensive multifocal involvement. It is clinically difficult to distinguish the margins of VPD from normal skin resulting in involved surgical margins leading to frequent lesion persistence and repeated excisions. Recently, fluorescein mapping has shown promise in providing accurate surgical margins in VPD. However, utilization of this technique after previous resection has not been explored.
Case
A 63-year-old female underwent wide local excision of a large microinvasive VPD with involved resection margins. Two months later, the patient underwent additional surgery to excise the involved margins and for sentinel inguinal lymph nodes evaluation. With gross visualization, the vulvar skin appeared normal. However, after intravenous fluorescein sodium injection and Wood's lamp illumination, residual satellite pathological area was observed and resected, revealing more microinvasive tumor.
Conclusion
Fluorescein mapping directly highlights sites of involvement in VPD and provides an improved estimation of disease extent which is otherwise not clinically visible.
1. Introduction
Extramammary Paget disease (EMPD) of the vulva is a rare condition characterized by the presence of intraepithelial nests of cluster of Paget's cells in the skin of the vulva. It predominantly affects postmenopausal women, presents as erythematous, eczematous patches, and may be misdiagnosed as other dermatological conditions (Ishizuki and Nakamura, 2021)(Caruso et al., 2023). A timely and accurate histological diagnosis of Vulvar Paget disease (VPD) is crucial for appropriate management and prognosis. Surgery, usually wide local excision (WLE), is the mainstay of treatment for primary VPD. Characteristically there is microscopic multifocality and extension of the Paget's cells beyond the clinically visible margins resulting in high rate of positive margins occurring in 40–75% of patients (Nitecki et al., 2018) and local recurrences, ranging from 20 to 70% (Caruso et al., 2023). Thus, many patients require multiple excisions which are limited by the anatomy of the vulva and result in significant deformity and morbidity. In order to improve the surgical results, Mohs micrographic surgery (MMS) technique has gained popularity in the evaluation of the surgical margins for EMPD. Specifically peripheral MMS, in which the periphery of the tumor is marked and excised until a clear margin is achieved (O’Connor et al., 2012)(Bae et al., 2013). Recently, fluorescein mapping was suggested as a safe method to improve resection accuracy and to identify occult satellite lesions (Wagar et al., 2023). This non-invasive method utilizes the fluorescent properties of fluorescein dye, which selectively accumulates in areas with disrupted epithelial barriers or neoplastic changes (Schupper et al., 2021).
Primary EMPD is subdivided into intraepithelial cutaneous Paget disease in situ as the usual type, intraepithelial cutaneous Paget disease with invasion, and intraepithelial cutaneous Paget disease as a manifestation of underlying skin-appendage adenocarcinoma (Wilkinson and Brown, 2002). It has been reported that invasive EMPD represents less than 2% of all vulvar malignancies (Nitecki et al., 2018)(van der Linden et al., 2016). Dermal invasion is frequently associated with regional lymph node metastases and recurrence (Ishizuki and Nakamura, 2021) (van der Linden et al., 2016).
Herein, we present a case of invasive EMPD re-operated for positive surgical margins and lymphvascular space invasion (LVSI) using fluorescein mapping and peripheral MOHS to enhance the diagnostic accuracy of the residual tumor.
2. Case Description
A 63-year-old female with overall good health sought medical attention due to the presence of a painful and pruritic vulvar lesion. The woman suffered from the lesion for 2 years and failed to show improvement after initial treatment with clobetasol propionate ointment and intradermal steroid injections. Gynecological examination revealed a lesion measuring 4 × 3 cm, with surface leucoplakia located on the right labia majora (Fig. 1) with an intact vagina and no palpable inguinal lymph nodes.
Fig. 1.
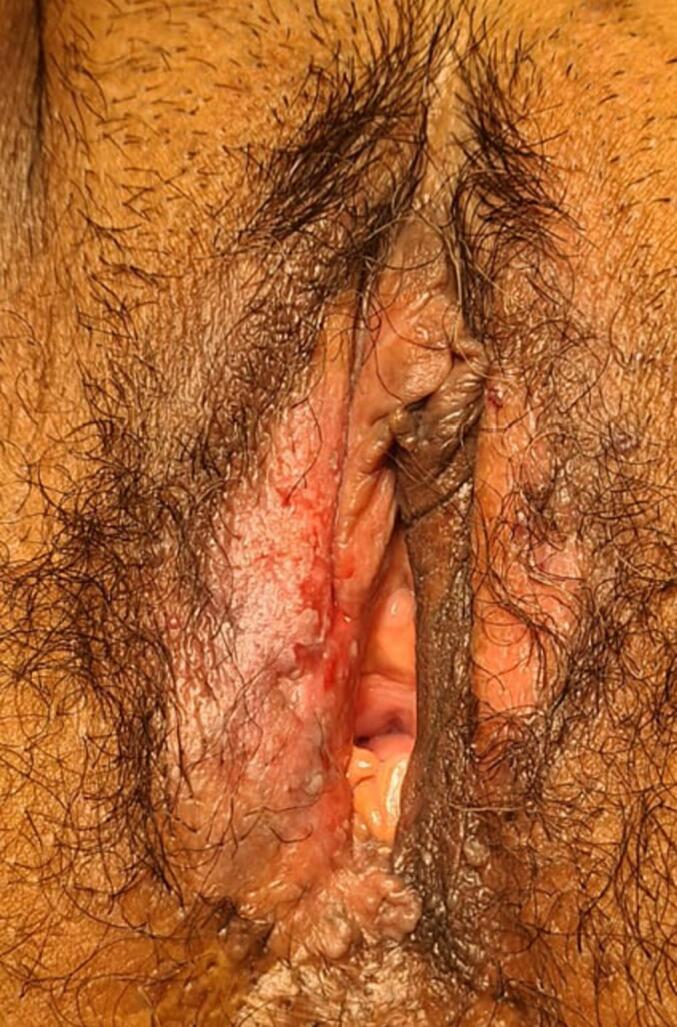
Vulvar Paget's lesion of the right labia majora prior to primary excision.
A biopsy taken from the lesion confirmed a diagnosis of EMPD. The woman had an unremarkable colonoscopy and mammography from the preceding year as well as normal cervical pap smear, negative for HPV.
The patient underwent a wide local excision of the vulvar tumor. The pathological examination revealed invasive EMPD measuring 6.5 cm, with evidence of microinvasion to 0.65 mm and focal lymphatic channel spread with no perineural or vascular involvement. During the operation the specimen was sent for frozen section to assess the surgical margins. Involvement was observed at 12–1o'clock and 6o'clock and more tissue was removed from the upper and lower areas of the incision. In the final pathology the additional margins that were removed from the 12–1o'clock were still involved with tumor. Although the invasion was microscopic to only 0.65 mm there was evidence of lymphatic channel involvement. Six weeks after the operation, the patient underwent PET CT that showed pathological FDG uptake in the right vulva and weak but pathological uptake in a small right inguinal lymph node.
Consequently, the patient underwent additional surgery to excise the involved margins and for sentinel inguinal lymph node (SLN) evaluation. During the operation, 5 ml of 10% fluorescein sodium were injected intravenously and after 30 s the vulva was illuminated with Wood's lamp to identify and mark the vulvar margins of the lesion (Fig. 2A) which was invisible without the dye (Fig. 2B). As can be seen in Fig. 2, the green area is above the scar of the previous operation representing the area of the remaining prior positive margins.
Fig. 2.
Residual vulvar Paget's lesion illuminated with Wood's lamp after fluorescein sodium injection (2A) and demarcated for planned excision (2B). The residual lesion was not evident by gross visualization without the fluorescein dye.
Additionally, a dye mixture of methylene blue and ICG was injected into the skin surrounding the lesion and two sentinel inguinal lymph nodes were detected on the right side. Wide local excision of the lesion was performed, guided by the borders indicated by fluorescein. The specimen was sent for frozen section analysis. Peripheral Mohs micrography revealed marginal involvement at 9–13o’clock, prompting an expansion of the resection area. A 5 mm width strip of skin with dermal tissue was removed.
The final pathology report confirmed clear surgical margins and no involvement of the resected inguinal lymph nodes. There was still a tiny focus of microinvasion, less than 1 mm in the specimen. Deep surgical margins were free of tumor. There were no metastases in the sentinel inguinal lymph nodes.
Following the procedure, the patient experienced chylous discharge from the inguinal incision, which was successfully managed with a low-fat diet, local pressure, and the administration of tranexamic acid, leading to improvement in her condition.
3. Discussion
VPD is a rare vulvar skin disease accounting for approximately 1–2% of all vulvar neoplasms, typically occurring in elderly women (Caruso et al., 2023). While primary EMPD remains confined to the epidermis invasive VPD is a rare subset of EMPD, extending beyond the basement membrane and invading into the dermal layers, potentially leading to metastasis. Early-stage disease (limited to the epidermis and superficial dermis, i.e. ≤ 1 mm) has a more favorable prognosis, while deeper invasion and lymph node involvement are associated with worse outcomes (van der Linden et al., 2016)(Preti et al., 2021). Van Linden et al showed in a large cohort study of 113 women with VPD that most, 77% had non– invasive VPD while 23% of cases were invasive at initial diagnosis, and in 2.7% of cases, the disease had already metastasized. Survival was dependent on depth of invasion. The 5 year free-disease survival was 98% in non-invasive and microinvasive VPD and 50% in invasive VPD (van der Linden, 2019). Similar results were recently published by Preti et al (Preti et al., 2021). Out of 122 women with VPD, 87% were diagnosed with intraepithelial VPD, 18% with microinvasive and 13% with invasive VPD to more than 1 mm. At 120 months, the cancer-specific survival was 100% for intraepithelial and microinvasive VPD versus 31% for invasive disease (p = less than0.0001).
Our patient represented a specific treatment dilemma. On the one hand she had microinvasion to less than 1 mm which carries a good prognosis. On the other hand, the pathological findings showed positive surgical margins and lymphatic channel involvement which might increase the risk of groin lymph node metastasis. VPD carries high rates of surgical margin involvement occurring in as frequently as 92–97% of cases in some series (Nitecki et al., 2018)(Preti et al., 2021)(van der Linden, 2019) due to the presence of Paget cells extending beyond the visible lesion. Most studies determined that margin status has no impact on recurrence rate and survival (Nitecki et al., 2018)(Preti et al., 2021)(van der Linden, 2019)(Onaiwu et al., 2017). Nevertheless, the presence of invasion and lymphatic channel involvement prompted our team to reoperate on the patient and to evaluate the groin lymph nodes. Previous studies showed that SLN biopsy is an accurate method to evaluate inguinal lymph node metastases because they have good correlation with prognosis and survival. Ogata et al (Ogata D, Kiyohara Y, Yoshikawa S, 2016) found that SLN positivity was related to the level of invasion: 0% for intraepithelial lesions, 4.1% for microinvasion, and 42.8% for dermal invasion. Invasion level and presence of lymphvascular invasion were shown to be an independent predictive factor for metastases in the SLNs and for recurrence-free and overall survival (Maeda et al., 2023). Our patient had microinvasion and lymphvascular space invasion suggesting increased risk for lymph node involvement. PET CT done for primary metastatic evaluation showed weak suspicious pathological uptake in a small right inguinal lymph node. Two sentinel lymph nodes were removed – with no metastasis in the final pathology so further lymphadenectomy was not performed.
Fluorescein mapping is an emerging modality for the visualization of VPD (Wagar et al., 2023). Fluorescein sodium injection directly highlights sites of involvement under Wood's lamp, providing a better indication of disease extent through biopsy and subsequent pathology of involved areas (Wagar et al., 2023) (Misas JE, Cold CJ, 1991). Misas et al (Misas JE, Cold CJ, 1991) described fluorescein mapping in 2 patients with VPD with higher histologic predictive values compared to gross visualization. Recently, Wager et al (Wagar et al., 2023) described 8 patients with VPD treated by a 2-step procedure using fluorescein mapping. Primarily, the extent of the lesion was evaluated by the dye and biopsies taken to delineate the lesion circumference. In the second stage again under fluorescein guidance, the excisional procedure was performed. A 1-cm margin was taken around the fluorescein-demarcated area to ensure complete resection. Four patients had satellite lesions identified outside of the grossly visible tumor. Similarly, in our patient, during the second operation, we were unable to see the residual tumor in the vulva since the skin looked intact (Fig. 2A). Only after fluorescein was injected did the pathological area became visible and amenable to outlining and dissection. The demarcated lesion was well above the previous scar and would not have been removed unless seen by the fluorescein dye. In order to preserve vulvar anatomy, we excised the lesion exactly at the demarcated margins. Mohs revealed involved margins and 5 mm of tissue were further removed around the excised area. Mohs microsurgery (MMS) has been proposed to decrease the rate of local recurrence in VPD, as it allows the entire circumference to be inspected. In a pooled analysis of 8 studies (Bae et al., 2013) the overall recurrence rate of EMPD after MMS was 12.2%, with an estimated 5-year tumor-free rate of 83.6%. However, MMS is time consuming, require specially trained personnel and can result in large margin removal. Fluorescein mapping with the accurate location and dimensions of the lesion might serve as an easily applicable and less expensive method while preserving a maximal amount of normal tissue.
In conclusion, vulvar fluorescein mapping allows improved visualizing and highlighting of vulvar Paget's lesion boundaries compared to gross visualization and assists in identification of satellite lesions. This method should be further explored and compared to other surgical and medical treatment modalities for VPD.
“Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.”
CRediT authorship contribution statement
Allison Joyce Siegel: Data curation, Writing – original draft. Malgorzata Budzynska: Data curation, Writing – original draft. Brandon Oleg Litvak: Data curation, Writing – original draft. Ofri Peled: Conceptualization, Methodology, Writing – review & editing. Letizia Schreiber: Methodology, Writing – original draft. Sofia Leytes: Methodology, Writing – review & editing. Tally Levy: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Supervision, Validation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bae J.M., Choi Y.Y., Kim H., Oh B.H., Roh M.R., Nam K., Chung K.Y. Mohs micrographic surgery for extramammary Paget disease: A pooled analysis of individual patient data. J. Am. Acad. Dermatol. 2013;68:632–637. doi: 10.1016/j.jaad.2012.12.960. [DOI] [PubMed] [Google Scholar]
- Caruso, G., Barcellini, A., Mazzeo, R., Gallo, R., Vitale, M.G., Passarelli, A., Mangili, G., Pignata, S., Palaia, I., 2023. Vulvar Paget’s Disease: A Systematic Review of the MITO Rare Cancer Group. Cancers (Basel). 15, 1–47. https://doi.org/10.3390/cancers15061803. [DOI] [PMC free article] [PubMed]
- Ishizuki S., Nakamura Y. Extramammary paget’s disease: Diagnosis, pathogenesis, and treatment with focus on recent developments. Curr. Oncol. 2021;28:2969–2986. doi: 10.3390/curroncol28040260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Nagai K., Uehara J., Toyoshima R., Nakagawa T., Yoshino K. Sentinel lymph node biopsy in extramammary Paget disease: A 13-year institutional experience. J. Dermatol. 2023;50:57–63. doi: 10.1111/1346-8138.16602. [DOI] [PubMed] [Google Scholar]
- Misas JE, Cold CJ, H.F., 1991. Vulvar Paget disease: fluorescein-aided visualization of margins. Obs. Gynecol 77, 156–9. [PubMed]
- Nitecki R., Davis M., Watkins J.C., Wu Y.E., Vitonis A.F., Muto M.G., Berkowitz R.S., Horowitz N.S., Feltmate C.M. Extramammary Paget Disease of the Vulva: A Case Series Examining Treatment, Recurrence, and Malignant Transformation. Int. J. Gynecol. Cancer. 2018;28:632–638. doi: 10.1097/IGC.0000000000001189. [DOI] [PubMed] [Google Scholar]
- O’Connor E.A., Hettinger P.C., Neuburg M., Dzwierzynski W.W. Extramammary paget’s disease: A novel approach to treatment using a modification of peripheral mohs micrographic surgery. Ann. Plast. Surg. 2012;68:616–620. doi: 10.1097/SAP.0b013e31821b6c7b. [DOI] [PubMed] [Google Scholar]
- Ogata D, Kiyohara Y, Yoshikawa S, T.T., 2016. Usefulness of sentinel lymph node biopsy for prognostic prediction in extramammary Paget’s disease. Eur J Dermatol 26, 254–9. https://doi.org/10.1684/ejd.2016.2744. [DOI] [PubMed]
- Onaiwu C.O., Salcedo M.P., Pessini S.A., Munsell M.F., Euscher E.E., Reed K.E., Schmeler K.M. Paget’s disease of the vulva: A review of 89 cases. Gynecol. Oncol. Reports. 2017;19:46–49. doi: 10.1016/j.gore.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preti M., Micheletti L., Borella F., Cosma S., Marrazzu A., Gallio N., Privitera S., Tancredi A., Bevilacqua F., Benedetto C. Vulvar Paget’s disease and stromal invasion: Clinico-pathological features and survival outcomes. Surg. Oncol. 2021;38:1–6. doi: 10.1016/j.suronc.2021.101581. [DOI] [PubMed] [Google Scholar]
- Schupper A.J., Rao M., Mohammadi N., Baron R., Lee J.Y.K., Acerbi F., Hadjipanayis C.G. Fluorescence-Guided Surgery: A Review on Timing and Use in Brain Tumor Surgery. Front. Neurol. 2021;12:1–14. doi: 10.3389/fneur.2021.682151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden M, Oonk MHM, van Doorn HC, Bulten J, van Dorst EBL, Fons G, Lok CAR, van Poelgeest MIE, Slangen BMF, Massuger LFAG, de H.J., 2019. Vulvar Paget disease: A national retrospective cohort study. J Am Acad Dermatol 81, 956–962. https://doi.org/10.1016/j.jaad.2018.11.016. [DOI] [PubMed]
- van der Linden M., Meeuwis K.A.P., Bulten J., Bosse T., van Poelgeest M.I.E., de Hullu J.A. Paget disease of the vulva. Crit. Rev. Oncol. Hematol. 2016;101:60–74. doi: 10.1016/j.critrevonc.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Wagar M.K., Zhang R.C., Weisman P., Spencer R.J., Kushner D.M. Fluorescein Mapping in Vulvar Paget Disease. Obstet. Gynecol. 2023;141:608–612. doi: 10.1097/AOG.0000000000005084. [DOI] [PubMed] [Google Scholar]
- Wilkinson E.J., Brown H.M. Vulvar Paget disease of urothelial origin: A report of three cases and a proposed classification of vulvar Paget disease. Hum. Pathol. 2002;33:549–554. doi: 10.1053/hupa.2002.124788. [DOI] [PubMed] [Google Scholar]



