Abstract
Family poverty has been associated with altered brain structure, function, and connectivity in youth. However, few studies have examined how disadvantage within the broader neighborhood may influence functional brain network organization. The present study leveraged a longitudinal community sample of 538 twins living in low-income neighborhoods to evaluate the prospective association between exposure to neighborhood poverty during childhood (6–10 y) with functional network architecture during adolescence (8–19 y). Using resting-state and task-based fMRI, we generated two latent measures that captured intrinsic brain organization across the whole-brain and network levels – network segregation and network segregation-integration balance. While age was positively associated with network segregation and network balance overall across the sample, these associations were moderated by exposure to neighborhood poverty. Specifically, these positive associations were observed only in youth from more, but not less, disadvantaged neighborhoods. Moreover, greater exposure to neighborhood poverty predicted reduced network segregation and network balance in early, but not middle or late, adolescence. These effects were detected both across the whole-brain system as well as specific functional networks, including fronto-parietal, default mode, salience, and subcortical systems. These findings indicate that where children live may exert long-reaching effects on the organization and development of the adolescent brain.
Keywords: Neighborhood poverty, Network organization, Brain development, Graph theory, Adolescence, Functional connectivity
1. Introduction
Over 15 million children in the United States grow up in poverty (Children Incorporated, 2022). Childhood poverty is associated with heightened risk for cognitive and socioemotional difficulties, academic under-achievement, and poorer physical and mental health (Evans and Kim, 2013, McLoyd, 1998). The last decade has witnessed a burgeoning literature demonstrating that disadvantage may “get under the skin” by influencing brain development (Farah, 2018, Hyde et al., 2020). Until recently, neuroimaging research in this area has largely focused on household disadvantage, such as family income and education. Nevertheless, studies indicate that economic resources within the household versus the broader neighborhood uniquely influence development (Carroll et al., 2023, Evans, 2004, Hyde et al., 2020, Leventhal and Brooks-Gunn, 2000). Since disadvantaged neighborhoods expose youth to unique risk factors outside the home (e.g., community violence, neurotoxicants) (Hyde et al., 2020), additional research is required to delineate the mechanisms underlying risk and resilience following neighborhood disadvantage.
A growing literature has begun to directly relate neighborhood disadvantage to brain development, above and beyond household-level disadvantage (Hyde et al., 2022, Rakesh and Whittle, 2021). Neighborhood disadvantage has been uniquely associated with altered structure and function in cortical and subcortical regions underlying cognitive and socioemotional processing (Gard et al., 2021, Mullins et al., 2020, Rakesh et al., 2022, Tomlinson et al., 2020, Whittle et al., 2017). Beyond these regional effects, neighborhood disadvantage may further impact functional connectivity patterns among brain systems supporting cognitive and affective regulation, including cortico-limbic, fronto-parietal, and default mode systems (Ip et al., 2022, Marshall et al., 2018, Rakesh et al., 2021a, Rakesh et al., 2021b).
Although previous studies describing the neurobiological embedding of neighborhood disadvantage focus on connectivity strength, computational approaches such as graph theory capture the broader organization of the brain and its constituent networks (Bassett and Sporns, 2017). Important properties of network architecture include the degree to which the brain organizes into distinct, functionally specialized networks (network segregation) and the efficiency of information flow (network integration) (Sporns, 2013). High segregation and high integration generate a “small-world” system, which efficiently coordinates specialized cognitive functions at low wiring and energy costs (Bassett and Bullmore, 2006). Neighborhood disadvantage has recently been associated with lower integration of a salience emotion network (Gellci et al., 2019) and with weaker age-related variation in the segregation of whole-brain, somatomotor, ventral attention, and limbic networks (Tooley et al., 2020).
Emergent translational evidence suggests that context modulates not only the outcome, but also the pace, of brain development (Tooley et al., 2021). Life history (Ellis and Del Giudice, 2019) and stress acceleration theories (Callaghan and Tottenham, 2016) postulate that adversity accelerates neurodevelopment. Though such acceleration may confer short-term benefits, it may also abbreviate periods of peak neuroplasticity, limiting the capacity for subsequent adaptation and increasing risk for negative long-term outcomes (Gee, 2021). Conversely, other theories posit that in disadvantaged environments, fewer resources (e.g., education, nutrition) may delay neurodevelopment (Johnson et al., 2016). Although such delays may increase risk for negative outcomes, they may also prolong plasticity and adaptability to varied contexts. Several cross-sectional and longitudinal studies report alterations in brain structure, function, and connectivity following exposure to adversity and disadvantage, in directions interpreted as both accelerated (e.g., more negative cortico-limbic coupling; Brieant et al., 2021; Gee et al., 2013a; McLaughlin et al., 2019) and delayed neurodevelopment (e.g., decelerating gaps between brain-predicted versus chronological age; McLaughlin et al., 2019; Rakesh et al., 2021c; Rakesh et al., 2021d).
To understand how disadvantage modulates network maturation, “normative” patterns of age-related variation in functional network architecture must first be considered. Cross-sectional studies suggest higher network segregation with age, although these patterns differ by network and cognitive context (Grayson and Fair, 2017, Gu et al., 2015, Keller et al., 2022, Tooley et al., 2022, Wig, 2017). Nonetheless, these findings rely on small samples or specific cohorts, report conflicting conclusions on whether network segregation continues to refine across adolescence, and have not examined age-related variation in small-worldness throughout adolescence.
When considering contextual influences on age-related variation in network topology, one cross-sectional study simulating network development reported delayed peaks in network segregation in disadvantaged youth (7–13 y) (Siugzdaite et al., 2022). Conversely, another cross-sectional study found weaker positive associations between age (8–22 y) and network segregation following neighborhood disadvantage, a pattern interpreted as accelerated development (Tooley et al., 2020). Therefore, this limited literature does not paint a clear picture of how neighborhood disadvantage moderates associations between age and network topology. Moreover, current work has exclusively focused on cortical network segregation; thus, the overall and age-related effects of neighborhood disadvantage on other important topological properties (e.g., small-worldness, subcortical architecture) remain unknown.
Thus, the present study characterized how exposure to neighborhood disadvantage was associated with functional network architecture in a longitudinal population-based sample of twin youth recruited from neighborhoods with above-average poverty levels, resulting in strong representation of lower-income families that have been historically excluded from neuroimaging research. As youth begin to spend more time in the neighborhood during childhood, while brain organization rapidly remodels during adolescence to specialize to contextual demands (Grayson and Fair, 2017), we examined prospective associations between neighborhood poverty during childhood and network topology during adolescence. We interrogated network topology across the entire brain and specific networks implicated in adversity and psychopathology, including fronto-parietal, default mode, salience, and subcortical systems (Hyde et al., 2022, Menon, 2011). To increase reliability and capture the interrelatedness of different topological properties, we extracted two latent variables indexing network segregation and network segregation-integration balance. First, we probed the relationship between neighborhood poverty and network topology. Given findings suggesting reduced segregation following disadvantage across the lifespan (Chan et al., 2018, Rakesh et al., 2021a), we hypothesized that greater neighborhood disadvantage would predict reduced network segregation and network balance. Second, we examined the cross-sectional association between age and network topology, expecting positive associations between age with network segregation and balance. Lastly, we tested whether neighborhood poverty moderated cross-sectional links between age and network topology. As studies suggest both accelerated and delayed neurodevelopment following disadvantage, we did not specify directional hypotheses for this aim.
2. Materials and methods
2.1. Participants
As described in Suarez et al. (2022), participants were part of the Michigan Twins Neurogenetics Study (MTwiNS), recruited from the Twin Study of Behavioral and Emotional Development – Child (TBED-C), a project within the broader Michigan State Twin Registry (Burt and Klump, 2013). Using birth records, the TBED-C identified twin families living within 120 miles of East Lansing, MI, including urban (e.g., Detroit, Flint, Lansing), suburban, and rural areas. The study included both a population-based sample (528 twin families) with children aged 6–10 years, and an “at-risk” sample (502 twin families) from the same geographic region, but only recruited from neighborhoods with above-median levels of poverty (>10.5 % of families in the neighborhood living below the poverty line, the median at study onset; Burt and Klump, 2019). In an ongoing follow-up neuroimaging study, MTwiNS recruited families from the “at-risk” sample, as well as those in the population-based sample that would have met criteria for the at-risk sample (i.e., living in neighborhoods with above-median levels of poverty). Although TBED-C and MTwiNS recruited twins to parse genetic versus environmental influences on brain development, risk, and resilience, twins are broadly representative of singletons in the population (Willemsen et al., 2021); thus, this dataset is useful for interrogating context-dependent brain organization at the population level without leveraging the genetically informed design.
We have assessed 708 twins from 354 families for MTwiNS. Of these, 559 twins met fMRI eligibility criteria (see Table S1). To facilitate model estimation and convergence, participants with missing data on any variable (i.e., neighborhood poverty, family income) were excluded from all analyses (n = 21). Therefore, the current sample included 538 twins from 306 families (53.9 % boys; 79.7 % White, 11.0 % Black, 9.3 % other racial/ethnic group membership). Youth were between 8 and 19 y, although 95.9 % of the sample was between 10 and 18 y (M = 14.74 y, SD = 2.05 y). At the time of data collection, the mean neighborhood poverty level for families in this study was 20 % (i.e., 20 % of families’ neighbors were living below the poverty line), ranging from 0 % to 93 %. Participants’ guardians provided informed consent and participants provided assent in compliance with Institutional Review Board policies and APA ethical standards in the treatment of human participants.
2.2. Neighborhood poverty
We quantified neighborhood poverty by geocoding family addresses during the childhood TBED-C wave (6–10 y) and calculating the proportion of residents living below the poverty line within the family’s census tract, the variable used to recruit participants (Tomlinson et al., 2020). The distribution of neighborhood poverty values is shown in Fig. S1.
2.3. MRI acquisition and processing
2.3.1. Pseudo-rest compilation
During the adolescent MTwiNS wave (8–19 y), participants completed one 7-minute resting-state scan (eyes open, black fixation cross) and three task-based scans, including a reward task (Peckins et al., 2022), a socioemotional face processing task (Suarez et al., 2022), and a cognitive control task (Tomlinson et al., 2020). The reliability of functional connectivity data increases with greater scan length and concatenation of multiple shorter scans across contexts (Birn et al., 2013, Cho et al., 2021). Thus, consistent with previous work (Fair et al., 2007, Kraus et al., 2021), we concatenated participants’ resting-state and task-based scans (with task effects regressed out) to generate approximately 20 min of “pseudo-rest” scan data per participant.
2.3.2. Neuroimaging procedures
As described in Suarez et al. (2022), each participant was scanned with one of two research-dedicated GE Discovery MR750 3T scanners located at the University of Michigan Functional MRI Laboratory. To take advantage of improvements in MRI data acquisition and harmonize our protocol with the Adolescent Brain Cognitive Development Study (Casey et al., 2018), we altered our acquisition protocol after the first 140 families. For the first 140 families (280 twins), blood oxygenation level-dependent (BOLD) functional images were acquired via an 8-channel head coil and a reverse spiral sequence (TR/TE = 2000/30 ms, flip angle = 90°, FOV = 22 cm), which covered 43 interleaved oblique slices of 3 mm thickness. High-resolution T1-weighted SPGR images (156, 1 mm-thick slices) were aligned with the AC-PC plane, and later used during normalization of the functional images. For the remaining 214 families (428 twins), BOLD functional images were acquired with a 32-channel head coil and a gradient-echo sequence with multiband acquisition (TR/TE = 800/30 ms, flip angle = 52°, FOV = 21.6 cm), which covered 742 interleaved axial slices of 2.4 mm thickness. High-resolution T1-weighted SPGR images (208, 1 mm-thick slices) were aligned with the AC-PC plane and used during normalization of the functional images. For both acquisition sequences, BOLD functional images encompassed the entire cerebrum and most of the cerebellum to maximize coverage of limbic structures. Functional data was preprocessed and analyzed using Statistical Parametric Mapping version 12 (SPM12; Wellcome Trust Centre, London, United Kingdom) via standard procedures (see Supplementary Information for details about MRI data collection and processing and for associations between acquisition sequence and network topology).
2.3.3. Motion and denoising correction strategy
A conservative, multistep procedure was used to correct for motion artifacts combining multiple correction strategies (Parkes et al., 2018). First, data from each scanner session were motion-scrubbed to identify and remove motion artifacts from the fMRI time series, using a mean framewise displacement cut-off value of 0.5 mm (Power et al., 2012). Scanner sessions where > 20 % of the session was identified as motion artifact were excluded from subsequent analyses. Participants who did not have at least two independent usable scanner sessions due to motion artifact after scrubbing were removed from the sample. Secondly, ICA-AROMA was applied at the subject-level to remove motion-related artifacts (Pruim et al., 2015a, Pruim et al., 2015b), prior to the construction of subject-level connectivity matrices and networks.
2.4. Graph theoretical analysis
2.4.1. Node identification
We parcellated the brain into 286 cortical regions of interest (ROIs) from a commonly used functional atlas (Gordon et al., 2016) and augmented this cortical atlas with 54 subcortical ROIs from a separate functional atlas (Tian et al., 2020). Cortical ROIs unassigned to a specific network were not included due to insufficient coverage. Connectivity analyses were run on the preprocessed resting-state data and residualized task-based fMRI data using CONN toolbox’s ROI-to-ROI connectivity analysis procedure (see Supplementary Information for full details).
2.4.2. Graph construction
All graph theoretical analyses were conducted using the Brain Connectivity Toolbox (2019.03.03) in Matlab (version 2022a; Rubinov and Sporns, 2010). Consistent with other graph theoretical investigations, we set all negative connections within each whole-brain connectivity matrix to zero and then Fisher r-to-z transformed each matrix (Hallquist and Hillary, 2018, Rubinov and Sporns, 2010). We retained all connections without additional thresholding given controversies around gold-standard thresholding approaches and the cognitive relevance of weak connections (Civier et al., 2019, Hallquist and Hillary, 2018, Santarnecchi et al., 2014). We used these matrices to construct weighted, undirected whole-brain graphs. Specifically, the strength of each connection was retained rather than binarized because, compared to unweighted graphs, weighted graphs have closer resemblance to biological systems and generate more robust metrics of network topology (Good et al., 2010, Hallquist and Hillary, 2018, Santarnecchi et al., 2014).
2.4.3. Network statistics
Following whole-brain graph construction, we extracted measures of functional network architecture at: (a) the whole-brain level (i.e., across all 340 ROIs in the graph) and (b) the level of individual networks (i.e., averaging across the ROIs of each network within the whole-brain graph). Given their functional similarities, we integrated fronto-parietal and cingulo-opercular nodes into one network (hereafter referred to as the “fronto-parietal network” for simplicity), and salience and ventral attention nodes into one network (hereafter referred to as “salience network” for simplicity). This method maximized the number of ROIs within networks of interest to boost the reliability of our network-level metrics. Integrating these networks allowed us to maintain consistency with the network structure of other common atlases (Glasser et al., 2016, Yeo et al., 2011) and studies of neighborhood disadvantage (Tooley et al., 2020) to facilitate comparisons with alternate parcellation schemes and empirical studies. Lastly, subcortical ROIs were labeled as the “subcortical network/system” for parsimony. Overall, this approach generated 340 ROIs organized a priori into 11 large-scale networks.
At the whole-brain level, we characterized measures of network segregation (system segregation, modularity), network integration (global efficiency), and small-worldness (small-world propensity). First, system segregation and modularity probe the extent to which the brain organizes into distinct networks, involving stronger within- and weaker between-network connectivity (Bullmore and Bassett, 2011, Chan et al., 2014). System segregation characterizes macro-scale segregation by comparing the relative strength of within-network versus between-network connectivity, using the network affiliations defined a priori from the atlases (Chan et al., 2014). Modularity measures meso-scale segregation by comparing the observed within-network connectivity against that estimated from a network partition that maximizes modularity and is therefore independent from the parcellation-defined network affiliations (Newman, 2006). We examined both system segregation and modularity because we were interested in network segregation globally across the brain, captured by distinct spatial scales. Third, global efficiency quantifies how efficiently information flows across the brain, computed as the average inverse shortest path length across all brain nodes (Latora and Marchiori, 2001). Finally, small-world propensity is a recently developed metric optimized for weighted graphs that compares the relative segregation and integration observed against respective lattice and random networks (Bassett and Bullmore, 2017, Muldoon et al., 2016). Higher values on these metrics reflect greater network segregation, integration, and small-worldness, respectively.
For network-level analyses, we derived a measure of network segregation (participation coefficient), focusing on four networks that have been implicated in disadvantage and mental health: the fronto-parietal, default mode, and salience networks, and the subcortical system (Hyde et al., 2022, Menon, 2011), although see Table S4 for exploratory analyses with the remaining seven networks in the system. We calculated participation coefficient for each brain region and averaged across all regions within each network to describe network-level topology. Participation coefficient compares how strongly a node communicates with nodes from the same or different networks, defined from the applied atlases (Guimerà and Nunes Amaral, 2005). We focused specifically on this network-level metric of functional organization over others given our interest in the relative segregation versus integration of networks at a more global, rather than local, scale. Higher participation coefficient values reflect greater between-network integration, whereas lower values reflect greater segregation.
2.5. Dimensionality reduction
To account for shared variance, and reduce the dimensionality, across descriptors of both whole-brain and network-level topology, we conducted a principal component analysis using the psych package in R (Revelle, 2015; see Table S3 for analyses with individual metrics). A Kaiser-Meyer-Olkin test indicated that ∼80 % of the variance in each graph metric could be explained by other graph metrics, corroborating the utility of dimensionality reduction.
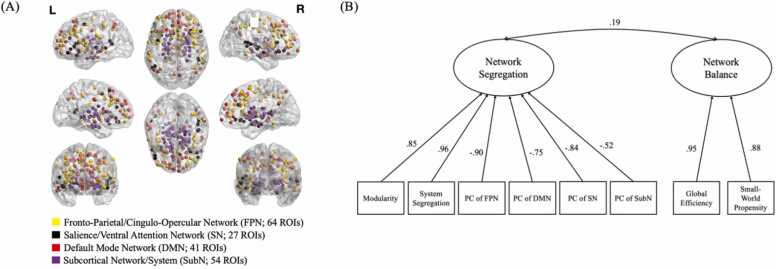
To establish the number of components to extract, we applied the n_components function in the parameters package in R (Lüdecke et al., 2020). The most optimal solution extracted two components and was supported by eight methods, including parallel analysis, Velicer’s minimum average partial criteria, optimal coordinates, Kaiser’s criterion, Bentler’s criterion, very simple structure complexity 1, and the scree plot (based on both standard errors and variance explained; see Fig. S2). We next repeated the principal component analysis with an oblimin rotation and extracted two components (see Fig. 1). The first principal component was labeled “network segregation” (variance explained = 50 %). The second principal component was labeled “network balance” to capture the mutual balance between segregation and integration (variance explained = 22 %). We extracted standardized component scores, with higher scores representing greater network segregation and network balance, respectively.
Fig. 1.
Definition of functional brain network architecture. A) Brain networks of interest. Networks were parcellated using Gordon (cortical) and Tian (subcortical) functional atlases, and visualized using BrainNetViewer (Xia et al., 2013). B) Principal components of functional brain network architecture. Only loadings stronger than .30 are depicted. Loadings on the first component were reverse-scored to represent network segregation rather than network integration, consistent with other studies of neighborhood disadvantage (Tooley et al., 2020). Modularity, system segregation, global efficiency, and small-world propensity are whole-brain measures calculated across all 340 ROIs in the graph. Participation coefficient is a network-level measure calculated by averaging across ROIs within each network of interest in the whole-brain graph. PC = participation coefficient.
2.6. Analyses
To evaluate the association of functional brain network architecture with neighborhood poverty (Aim 1) and age (Aim 2), we estimated multiple regression models in Mplus (version 1.8.8). We used maximum likelihood estimation with robust standard errors to address potential violations of distributional assumptions. Network segregation and network balance were simultaneously entered as outcome variables in the model to account for their covariance and decrease the number of tested models. To probe whether neighborhood poverty moderated the association between age and functional network architecture (Aim 3), we repeated our models after adding an interaction term between neighborhood poverty and age (mean-centered).
To account for the nesting of twins within families, we use the TYPE = COMPLEX command to cluster twins by household, consistent with other phenotypic and neuroimaging twin studies (South et al., 2017, Suarez et al., 2022). According to simulations, this approach corrects for statistical data dependencies to produce unbiased model estimates (Rebollo et al., 2006).
All models controlled for sex, race, family income, parental education, scanner sequence (multiband versus spiral), head motion (mean framewise displacement), and mean functional connectivity. We controlled for race (0 = Minoritized, 1 = Non-Hispanic White), a socially constructed category, to account for differences in exposure to structural and personal racism, discrimination, and unequal experiences of poverty, stress, and opportunity among people of color (Pager and Shepherd, 2008, Roberts and Rizzo, 2021), consistent with other work (Suarez et al., 2022). Because most racial/ethnic groups were small (Asian = 4, Black = 59, Latino = 6, Native American = 3, Other = 31, Pacific Islander = 6), and because each group faces an array of structural and personal racism, we binarized this race/ethnicity variable (even though greater representation of these groups would have been ideal to represent the diversity of adversity exposures among them). Further, controlling for mean functional connectivity ensured that our findings reflect variation in network topology rather than overall connectivity strength (Hallquist and Hillary, 2018, van Wijk et al., 2010). To disentangle whether the effects of neighborhood poverty were specific to neighborhood resources, we additionally controlled for concurrent family income and parental education during the neuroimaging wave in adolescence (8–19 y). Family income was measured as primary caregiver-reported ranges of monthly household gross income, including additional sources outside of employment such as government assistance and child support. Parental education was quantified as the highest level of education completed by the primary caregiver. Compared to participants excluded from this study (e.g., inability to scan, missing data), included participants did not differ in terms of sex, race, family income, parental education, and neighborhood poverty (all p’s > .136) but were significantly older by an average of six months (p = .006).
Previous studies suggest that neurochemical changes induced by puberty drive structural and functional neurodevelopment, perhaps to a greater extent than chronological age (Blakemore et al., 2010, Gracia-Tabuenca et al., 2021). We therefore conducted supplementary analyses after replacing the main and interactive effects of age with puberty (continuous Pubertal Development Scale scores), age after regressing out puberty, and puberty after regressing out age. We found that age-related variation in network topology as a function of neighborhood poverty was more closely tied to chronological age rather than pubertal maturation (see Table S5).
3. Results
3.1. Preliminary analyses
We explored associations among variables of interest using zero-order correlations (see Table S2). Notably, neighborhood poverty was significantly, though modestly, negatively correlated with family income (r = –.34) and parental education (r = –.20), as expected.
3.2. How does neighborhood poverty during childhood relate to functional brain network organization during adolescence?
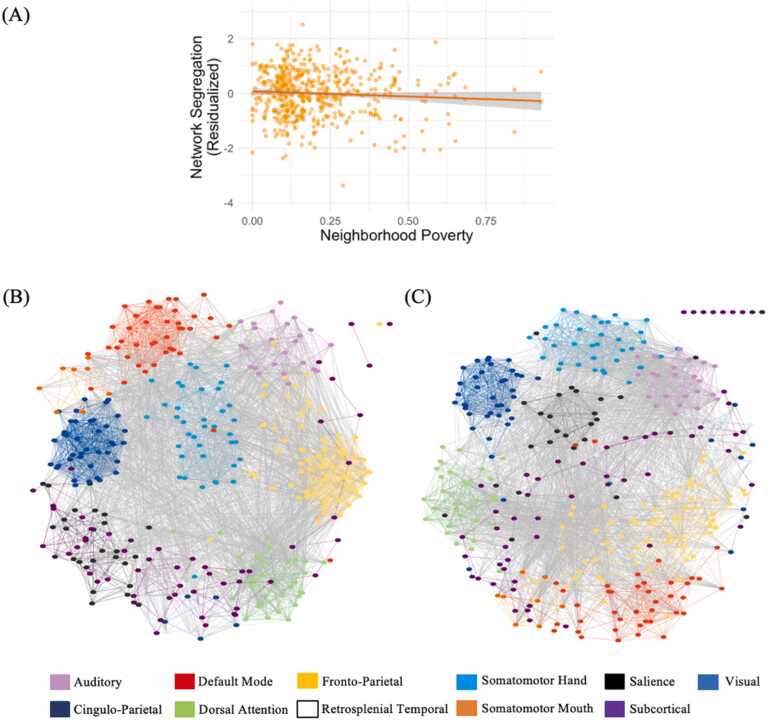
After adjusting for demographic factors, neuroimaging covariates, and household-level disadvantage, we found that neighborhood poverty was significantly negatively associated with the principal component of network segregation (b* = –0.10, p = .035, 95 % CI [–0.19, –0.01]; Fig. 2), which captures segregation across the whole brain (system segregation, modularity) and individual networks (participation coefficient of the four networks examined). Neighborhood poverty was not significantly associated with the principal component of network balance (b* = –0.02, p = .245, 95 % CI [–0.06, 0.02]), which represents global efficiency and small-world propensity across the whole brain. Furthermore, family income and education were unrelated to either network segregation or network balance (all p’s > .381), indicating the unique effects of neighborhood resources on functional network architecture.
Fig. 2.
Greater exposure to neighborhood poverty during childhood is associated with reduced functional network segregation during adolescence. A) Neighborhood poverty is negatively associated with the principal component of network segregation. Network segregation is residualized against age, sex, race, head motion, scan type, mean functional connectivity, family income, and parental education. B-C) Individual graphs from a participant with the lowest (0 %; Panel B) and highest (93 %; Panel C) levels of neighborhood poverty in the sample. These graphs provide a qualitative illustration of the association between neighborhood poverty and network segregation across the sample. Each color represents a brain network, each colored line represents a within-network connection, and each gray line represents a between-network connection. For visualization purposes, only connections stronger than .30 are depicted (isolate nodes at the top right corner represent nodes without connections stronger than .30). Nodes with stronger connectivity are closer together. Overall, functional networks are more segregated (i.e., distance is shorter among nodes within networks and greater among nodes between networks) at low (Panel B) compared to high (Panel C) levels of neighborhood poverty. Spring graphs generated in Cytoscape (Shannon et al., 2003).
We further conducted supplemental analyses with the individual graph metrics that make up the principal components of network topology (see Table S3). We found that neighborhood poverty significantly predicted reduced segregation at the whole-brain level (system segregation, modularity), but was not associated with the remaining properties examined (global efficiency, small-world propensity, participation coefficient of the four networks of interest).
3.3. How does functional brain network organization vary across adolescence?
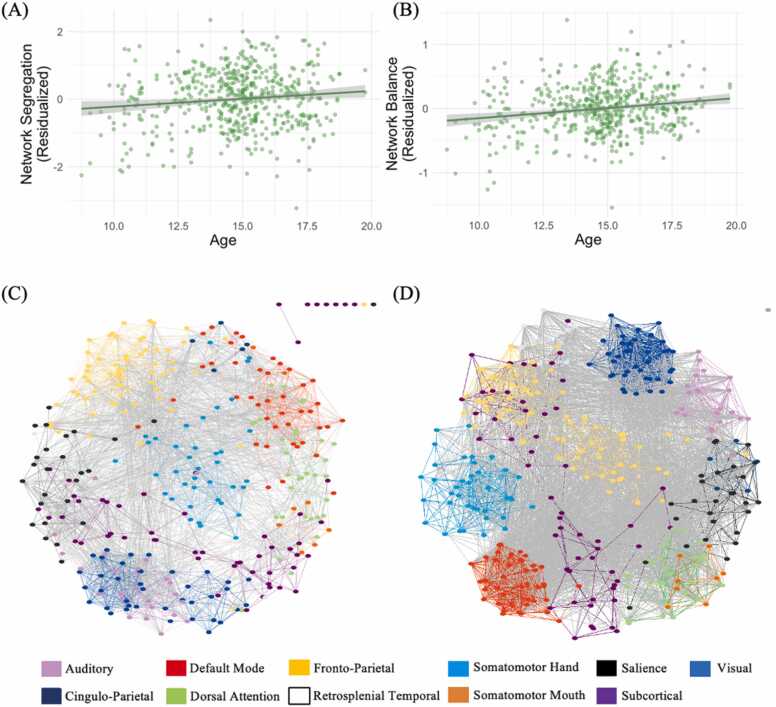
We found that age was significantly positively associated with both network segregation (b* = 0.12, p = .010, 95 % CI [0.03, 0.20]) and network balance (b* = 0.08, p < .001, 95 % CI [0.04, 0.12]) across adolescence (see Fig. 3). Supplemental analyses with individual graph metrics (see Table S3) demonstrated that the observed pattern with network segregation was evident at the level of meso-scale segregation (modularity) and the level of individual networks (participation coefficients of the fronto-parietal network and subcortical system). The pattern with network balance was only found for the small-world propensity indicator of this latent factor. Age was unrelated to other measures of network topology (system segregation, global efficiency, participation coefficient of salience and default mode networks).
Fig. 3.
Older age is associated with greater functional network segregation and network balance across adolescence. A-B) Age is positively associated with the principal components of network segregation (Panel A) and network balance (Panel B). Principal components are residualized against neighborhood poverty, sex, race, head motion, scan type, mean functional connectivity, family income, and parental education. C-D) Individual graphs from the youngest (8.75 y; Panel C) and oldest (19.75 y; Panel D) participant in the sample. These graphs provide a qualitative illustration of the association between age and network segregation across the sample. Each color represents a brain network, each colored line represents a within-network connection, and each gray line represents a between-network connection. For visualization purposes, only connections stronger than .30 are depicted (isolate nodes at the top right corner represent nodes without connections stronger than .30). Nodes with stronger connectivity are closer together. Overall, functional networks are more segregated (i.e., distance is shorter among nodes within networks and greater among nodes between networks) in older (Panel D) compared to younger (Panel C) youth. Spring graphs generated in Cytoscape (Shannon et al., 2003).
3.4. Does neighborhood poverty during childhood moderate age-related variation in functional brain network organization across adolescence?
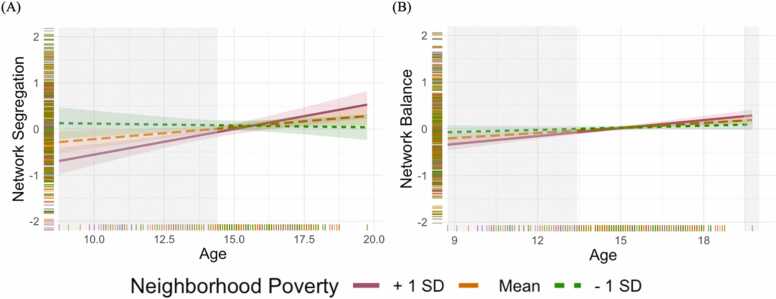
Neighborhood poverty was found to significantly moderate the association between age and network segregation (b* = 0.13, p = .006, 95 % CI [0.04, 0.22]; see Fig. 4). Simple slopes analyses revealed that age was positively associated with network segregation at high (+1 SD; p < .001) and mean (p = .015), but not low (–1 SD; p = .775), levels of neighborhood poverty. We subsequently conducted region of significance analyses to identify the exact neighborhood poverty levels at which the relationship between age and network organization was significant (Preacher et al., 2006). We found that the association between age and network segregation was significant when neighborhood poverty was above average (percentage of neighbors living below the poverty line >18 %; n = 233). Lastly, we switched the predictor and moderator variables to calculate the age range when neighborhood poverty was significantly associated with network architecture. We found that neighborhood poverty was associated with network segregation only in younger youth (<14.40 y; n = 187).
Fig. 4.
Neighborhood poverty during childhood moderates the association between age and functional brain network architecture across adolescence. A-B) Age is positively associated with the principal component of network segregation (Panel A) and network balance (Panel B) at high and mean, but not low, levels of neighborhood poverty. Interaction effects are significant within regions shaded in gray, such that greater neighborhood poverty levels mainly predict lower network segregation and network balance in younger, but not older, youth. Plots were generated using the interactions package in R. For visualization purposes, only values of network segregation and network balance within two SD’s from the mean are depicted to illustrate the observed effects more clearly (Network Segregation: n = 9 above two SD’s from mean, n = 20 below two SD’s from mean; Network Balance: n = 14 above two SD’s from mean, n = 5 below two SD’s from mean). See Fig. S3 for the full, non-truncated figure.
We also observed a significant interaction effect between neighborhood poverty and age for network balance (b* = 0.05, p = .008, 95 % CI [0.01, 0.08]; see Fig. 4), such that age was positively associated with network balance at high (p < .001) and mean (p < .001), but not low (p = .268), levels of neighborhood poverty. Specifically, the association between age and network balance was significant when neighborhood poverty was above low-average (percentage of residents living below the poverty line >10 %; n = 397). Additionally, neighborhood poverty was only associated with network balance in younger (<13.43 y; n = 126) youth, with some indication of an association among older (>19.45 y; n = 2) youth. However, as only two participants in the sample were older than 19.45 y, the latter results should be interpreted with caution.
Supplemental analyses interrogating individual graph metrics (see Table S3) revealed that the interaction pattern found with network segregation was evident for all indicators of this latent factor, including macro-scale (system segregation) and meso-scale (modularity) whole-brain segregation, and segregation of the four main networks of interest (participation coefficient of fronto-parietal, salience, default mode, and subcortical systems). In contrast, the interaction pattern found with network balance was only observed for the small-world propensity indicator of this latent factor (but not for global efficiency).
3.5. How do neighborhood poverty and age relate to the topology of alternate networks?
To parse the specificity versus globality of associations between neighborhood poverty and age with the topology of large-scale functional networks, we conducted exploratory analyses with the participation coefficient of the remaining seven networks that were not the focus of this study (see Table S4). We found that greater neighborhood poverty predicted significantly higher participation coefficient of auditory, somatomotor hand, and somatomotor mouth networks. Age was positively related to the participation coefficient of the auditory network and negatively related to the participation coefficient of the dorsal attention and visual networks. Finally, neighborhood poverty only moderated the association between age and the participation coefficient of dorsal attention and somatomotor mouth networks.
3.6. Does the timing of exposure to neighborhood poverty matter?
Although we focus on neighborhood poverty during childhood (6–10 y), we conducted supplemental analyses with neighborhood poverty during adolescence (8–19 y) to characterize potential timing-related specificity in associations with functional network architecture. We observed very similar associations between network topology with neighborhood poverty, age, and their interaction for the two developmental periods (see Supplementary Information).
4. Discussion
This study evaluated the prospective associations between neighborhood poverty during childhood and functional brain network organization during adolescence in youth residing in neighborhoods with above-average poverty levels. Using latent factors that clustered interrelated measures of brain organization across the whole-brain and network level, we found that greater exposure to neighborhood poverty predicted reduced network segregation, but not segregation-integration balance. Nevertheless, this association was not uniform across adolescence. Though measures of network segregation and network balance were higher in older youth, neighborhood poverty moderated these effects, such that age was positively related to network segregation and balance at high and mean, but not low, levels of neighborhood poverty. Importantly, given our sampling frame, average levels of neighborhood poverty in our sample still represent relatively concentrated disadvantage. Lastly, neighborhood poverty predicted reduced network segregation and network balance in early, but not middle or late, adolescence.
The present study builds upon a growing literature identifying environmental adversities outside the household as critical influences on the developing brain. Neighborhood disadvantage has been previously associated with differential patterns of functional activation and topology in specific brain regions, as well as functional connectivity patterns across specific brain networks, which underlie cognitive and socioemotional functioning (Gellci et al., 2019, Hyde et al., 2022, Sripada et al., 2021). Our results extend this literature by showing that exposure to neighborhood poverty in childhood is associated with reduced functional segregation across the brain during adolescence. These findings are consistent with other research linking disadvantage to weaker within-network connectivity, a marker of reduced segregation (Brody et al., 2019, Rakesh et al., 2021a, Sripada et al., 2014). Segregated systems consist of clearly demarcated networks, creating a neural infrastructure for networks to perform more differentiated computations that support specialized cognitive functions. While youth from disadvantaged neighborhoods exhibited a less segregated functional architecture, neighborhood poverty was overall not associated with the balance between segregation and integration (i.e., small-worldness) across the entire sample. Notably, our results were observed while statistically controlling for family income and parental education, indicating that limited socioeconomic resources within the broader neighborhood may uniquely influence functional brain network organization.
Beyond environmental influences on network topology, we further assessed how network segregation and network balance varied with age across adolescence cross-sectionally to inform future longitudinal work explicitly characterizing network development. The current study is the first to our knowledge to directly examine age-related variation in small-worldness across adolescence. We found that age was positively associated with network balance, suggesting a potentially late developmental emergence of an “optimized” small-world architecture. Age was also positively associated with network segregation, both across the brain (i.e., modularity) and in networks underlying cognitive control and emotion processing (i.e., participation coefficient of fronto-parietal and subcortical systems) (Delgado, 2007, Dosenbach et al., 2008, LeDoux, 2003). Supplemental analyses further suggested that age was positively related to the segregation of dorsal attention and visual networks, and negatively related to the segregation of the auditory network. These results converge with studies reporting overall age-related decreases in between-network connectivity and increases in within-network connectivity and segregation across adolescence, particularly among association networks (Keller et al., 2022). Such topological refinements may undergird developmental changes in top-down regulatory behavior and bottom-up emotional reactivity characteristic of adolescence (Bassett and Bullmore, 2006, Casey et al., 2019, Luna et al., 2015, Wig, 2017).
Though our overall age-related findings are consistent with some studies, other research suggests that functional network segregation and small-worldness across the entire brain may be predominantly established prior to adolescence (Fair et al., 2009, Marek et al., 2015). Discrepant findings about adolescent network remodeling may stem from socioeconomic differences across samples. Given our sampling frame, our findings more specifically represent youth from lower-income neighborhoods. Indeed, we found that the association between age and network topology was moderated by neighborhood poverty levels in childhood, such that network segregation and balance were positively associated with age, but only among youth from more disadvantaged neighborhoods. Supplemental analyses indicated that these results were specific to chronological age rather than pubertal physiology. Exploratory analyses further suggested that neighborhood poverty during childhood and adolescence showed similar associations with functional network architecture overall and as a function of age. However, this finding should be interpreted cautiously since neighborhood poverty was highly stable across waves, consistent with prior work (Vanderbilt-Adriance and Shaw, 2008), challenging our ability to precisely parse timing-dependent effects.
Given disagreements about normative and context-dependent trajectories of functional network maturation, our findings are challenging to interpret. As overall network topology may largely develop before adolescence, continual age-related variation in network segregation and balance in adolescents from disadvantaged neighborhoods could indicate a slightly protracted pace of functional network development. While longitudinal designs are required to explicate this hypothesis, this interpretation is consistent with other cross-sectional and longitudinal studies suggesting delayed structural and functional brain development following adversity and disadvantage (Hair et al., 2015, Hanson et al., 2013, Keding et al., 2021, Rakesh et al., 2021c, Rakesh et al., 2021d, Rao et al., 2010, Siugzdaite et al., 2022, Whittle et al., 2017). Multiple hardships present within under-resourced environments may delay neurodevelopment, such as lower access to high-quality nutrition and educational resources (Johnson et al., 2016). However, in our data, network segregation and balance were statistically similar by mid-adolescence irrespective of neighborhood resources, potentially reflecting a neurodevelopmental “catch-up” in disadvantaged youth. Alternatively, differential associations between age and network topology following neighborhood disadvantage may reflect a developmental trajectory that is entirely unique to current environmental demands, as opposed to one that is accelerated or delayed relative to the “norm” (Rakesh et al., 2023).
Contrary to our interpretation of delayed functional network maturation in disadvantaged youth, another recent study found the opposite pattern of potentially accelerated development of functional network segregation following exposure to neighborhood disadvantage (Tooley et al., 2020). These conflicting conclusions epitomize ongoing debates regarding whether adversity and disadvantage may accelerate or delay neurodevelopment (Callaghan and Tottenham, 2016, Ellis and Del Giudice, 2019, Johnson et al., 2016, Rakesh et al., 2023, Roubinov et al., 2021, Tooley et al., 2021). While demographic, methodological, and conceptual differences across studies may underlie these discrepancies, delineating the boundary conditions under which neighborhood poverty differentially modulates neuroplasticity constitutes an important goal for future research.
In supplemental analyses with individual networks, we found that in youth from more, but not less, disadvantaged neighborhoods, age was negatively associated with the integration of fronto-parietal, default mode, salience, and subcortical systems. Our findings are consistent with studies that consistently link neighborhood resources to the structure, function, and connectivity of higher-order cortical networks (Hyde et al., 2022, Rakesh et al., 2021a) supporting the dynamic regulation, engagement, and disengagement of cognitive and attentional processes that underlie goal-directed behavior (Menon, 2011). These results also converge with extensive evidence that links adversity exposure to structural and functional alterations in subcortical regions underlying emotion processing (Hyde et al., 2022). As functional network architecture demonstrates greater segregation with age, particularly for association and subcortical systems (Fareri et al., 2015, Gee et al., 2013b, Keller et al., 2022), the observed pattern of findings in this study may indicate disadvantage-related delays in the developmental decoupling of functional networks underlying cognitive and socioemotional processing and regulation, although future longitudinal work is needed to explicitly test this hypothesis. Exploratory analyses with the remaining seven networks in the system suggested that these findings were mostly specific to higher-order association networks (with the exception of the somatomotor mouth network).
Though the current study has several strengths including a large, well-sampled cohort of families living in low-income neighborhoods and rigorous processing and analytic techniques to characterize functional network architecture, some limitations warrant consideration. First, while our study was prospective, the brain data was cross-sectional throughout adolescence. Relatedly, given our wide age range (8–19 y), our sample may differ in multiple ways other than poverty and age (e.g., cohort effects), though we sought to mitigate this limitation through multiple statistical covariates. However, longitudinal research is necessary to truly characterize normative and context-dependent trajectories of functional brain network development. Second, since most neuroimaging studies involve limited socioeconomic, ethnoracial, and geographic diversity, care is needed when considering which neurodevelopmental trajectories are “normative” and interpreting the meaning of “deviations” from such trajectories. Despite our strong sampling frame, this sample does not have high representation of wealthy neighborhoods and thus our results may not generalize across the entire population. Moreover, though representative, given our single-site design within a single Midwestern state, the ethnoracial diversity of the sample is relatively low, indicating the need to replicate our results among cohorts with greater ethnoracial diversity and across multiple regions worldwide. Finally, additional research should identify the proximal mechanisms through which neighborhood disadvantage reconfigures network topology to inform priorities for policy reform. Candidate “active ingredients” include toxicants (e.g., particulate matter, lead), stressors (e.g., community violence, low neighborhood social cohesion), and less stimulating learning contexts (e.g., school quality, resource access) (Hyde et al., 2020, Leventhal and Brooks-Gunn, 2000).
The present study provides evidence that growing up in disadvantaged neighborhoods is associated with differences in the functional architecture of large-scale brain networks. We found that neighborhood poverty moderated the association between age and network segregation and segregation-integration balance. These results could be suggestive of slightly delayed network development in early adolescence, followed by a developmental catch-up by mid-adolescence. These results indicate that where children live early in life might have long-reaching effects on the organization and development of the adolescent brain. Importantly, neighborhood resources are governed by policies and systems that concentrate poverty to specific locations, particularly in marginalized communities (Riley, 2018, Slopen and Heard-Garris, 2021). Taken together, this study suggests that policy and structural interventions aimed at systems, rather than families, may be particularly meaningful for promoting positive neurobehavioral development in youth.
Funding
Research reports in this publication related to MTwiNS was supported by the National Institutes of Mental Health (NIMH) and the Office of the Director National Institute of Health (OD), under Award Number UG3MH114249 and the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD093334 to SAB and LWH. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
CRediT authorship contribution statement
Cleanthis Michael: Conceptualization, Methodology, Formal analysis, Writing – original draft, Visualization. Scott Tillem: Conceptualization, Methodology, Resources, Data curation, Writing – review & editing. Chandra S. Sripada: Methodology, Writing – review & editing. S. Alexandra Burt: Conceptualization, Writing – review & editing, Project administration, Funding acquisition. Kelly L. Klump: Methodology, Writing – review & editing, Project administration, Funding acquisition. Luke W. Hyde: Conceptualization, Methodology, Writing – review & editing, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank the staff of the TBED-C and MTwiNS studies for their hard work, and we thank the families who participated in TBED-C and MTwiNS for sharing their lives with us.
Data statement
All data analyzed in this study are shared via the NIMH Data Archive (https://nda.nih.gov/edit_collection.html?id=2818). Syntax to reproduce analyses is available upon request through the first author.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2023.101316.
Appendix A. Supplementary material
Supplementary material
.
Data Availability
Data will be made available on request.
References
- Bassett D.S., Bullmore E. Small-world brain networks. Neuroscientist. 2006;12(6):512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Bassett D.S., Bullmore E.T. Small-world brain networks revisited. Neuroscientist. 2017;12(3):499–516. doi: 10.1177/1073858416667720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett D.S., Sporns O. Network neuroscience. Nat. Neurosci. 2017;20(3):353–364. doi: 10.1038/nn.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn R.M., Molloy E.K., Patriat R., Parker T., Meier T.B., Kirk G.R., Nair V.A., Meyerand M.E., Prabhakaran V. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. NeuroImage. 2013;83:550–558. doi: 10.1016/j.neuroimage.2013.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.J., Burnett S., Dahl R.E. The role of puberty in the developing adolescent brain. Hum. Brain Mapp. 2010;31(6):926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieant A.E., Sisk L.M., Gee D.G. Associations among negative life events, changes in cortico-limbic connectivity, and psychopathology in the ABCD Study. Dev. Cogn. Neurosci. 2021;52 doi: 10.1016/j.dcn.2021.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G.H., Yu T., Nusslock R., Barton A.W., Miller G.E., Chen E., Holmes C., McCormick M., Sweet L.H. The protective effects of supportive parenting on the relationship between adolescent poverty and resting-state functional brain connectivity during adulthood. Psychol. Sci. 2019;30(7):1040–1049. doi: 10.1177/0956797619847989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E.T., Bassett D.S. Brain graphs: graphical models of the human brain connectome. Annu. Rev. Clin. Psychol. 2011;7:113–140. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- Burt S.A., Klump K.L. The Michigan State University Twin Registry (MSUTR): an update. Twin Res. Hum. Genet. 2013;16(1):344–350. doi: 10.1017/thg.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt S.A., Klump K.L. The Michigan State University Twin Registry (MSUTR): 15 years of twin and family research. Twin Res. Hum. Genet. 2019;22(6):741–745. doi: 10.1017/thg.2019.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B.L., Tottenham N. The stress acceleration hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci. 2016;7:76–81. doi: 10.1016/j.cobeha.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S.L., Shewark E.A., Mikhail M.E., Thaler D.J., Pearson A.L., Klump K.L., Burt S.A. Identifying the ‘active ingredients’ of socioeconomic disadvantage for youth outcomes in middle childhood. Dev. Psychopathol. 2023:1–9. doi: 10.1017/S0954579423000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Cannonier T., Conley M.I., Cohen A.O., Barch D.M., Heitzeg M.M., Soules M.E., Teslovich T., Dellarco D.V., Garavan H., Orr C.A., Wager T.D., Banich M.T., Speer N.K., Sutherland M.T., Riedel M.C., Dick A.S., Bjork J.M., Thomas K.M.…Dale A.M. The Adolescent Brain Cognitive Development (ABCD) study: imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Heller A.S., Gee D.G., Cohen A.O. Development of the emotional brain. Neurosci. Lett. 2019;693:29–34. doi: 10.1016/j.neulet.2017.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M.Y., Park D.C., Savalia N.K., Petersen S.E., Wig G.S. Decreased segregation of brain systems across the healthy adult lifespan. Proc. Natl. Acad. Sci. U. S. A. 2014;111(46):E4997–E5006. doi: 10.1073/pnas.1415122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M.Y., Na J., Agres P.F., Savalia N.K., Park D.C., Wig G.S. Socioeconomic status moderates age-related differences in the brain’s functional network organization and anatomy across the adult lifespan. Proc. Natl. Acad. Sci. U. S. A. 2018;115(22):E5144–E5153. doi: 10.1073/pnas.1714021115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Children Incorporated (2022). Understanding child poverty: Facts and statistics. Retrieved from 〈https://childrenincorporated.org/understanding-child-poverty-facts-and-statistics/〉.
- Cho J.W., Korchmaros A., Vogelstein J.T., Milham M.P., Xu T. Impact of concatenating fMRI data on reliability for functional connectomics. NeuroImage. 2021;226 doi: 10.1016/j.neuroimage.2020.117549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civier O., Smith R.E., Yeh C.H., Connelly A., Calamante F. Is removal of weak connections necessary for graph-theoretical analysis of dense weighted structural connectomes from diffusion MRI? NeuroImage. 2019;194:68–81. doi: 10.1016/j.neuroimage.2019.02.039. [DOI] [PubMed] [Google Scholar]
- Delgado M.R. Reward-related responses in the human striatum. Ann. N. Y. Acad. Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. A dual-networks architecture of top-down control. Trends Cogn. Sci. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis B.J., Del Giudice M. Developmental adaptation to stress: an evolutionary perspective. Annu. Rev. Psychol. 2019;70:111–139. doi: 10.1146/annurev-psych-122216. [DOI] [PubMed] [Google Scholar]
- Evans G.W. The environment of childhood poverty. Am. Psychol. 2004;59(2):77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Evans G.W., Kim P. Childhood poverty, chronic stress, self-regulation, and coping. Child Dev. Perspect. 2013;7(1):43–48. doi: 10.1111/cdep.12013. [DOI] [Google Scholar]
- Fair D.A., Schlaggar B.L., Cohen A.L., Miezin F.M., Dosenbach N.U.F., Wenger K.K., Fox M.D., Snyder A.Z., Raichle M.E., Petersen S.E. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. NeuroImage. 2007;35(1):396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Power J.D., Dosenbach N.U.F., Church J.A., Miezin F.M., Schlaggar B.L., Petersen S.E. Functional brain networks develop from a “local to distributed” organization. PLoS Comput. Biol. 2009;5(5) doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah M.J. Socioeconomic status and the brain: prospects for neuroscience-informed policy. Nat. Rev. Neurosci. 2018;19(7):428–438. doi: 10.1038/s41583-018-0023-2. [DOI] [PubMed] [Google Scholar]
- Fareri D.S., Gabard-Durnam L., Goff B., Flannery J., Gee D.G., Lumian D.S., Caldera C., Tottenham N. Normative development of ventral striatal resting state connectivity in humans. NeuroImage. 2015;118:422–437. doi: 10.1016/j.neuroimage.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard A.M., Maxwell A.M., Shaw D.S., Mitchell C., Brooks-Gunn J., McLanahan S.S., Forbes E.E., Monk C.S., Hyde L.W. Beyond family-level adversities: exploring the developmental timing of neighborhood disadvantage effects on the brain. Dev. Sci. 2021;24(1) doi: 10.1111/desc.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G. Early adversity and development: parsing heterogeneity and identifying pathways of risk and resilience. Am. J. Psychiatry. 2021;178(11):998–1013. doi: 10.1176/appi.ajp.2021.21090944. [DOI] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H., Hare T.A., Bookheimer S.Y., Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U. S. A. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., Goff B., Telzer E.H., Shapiro M., Hare T.A., Bookheimer S.Y., Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J. Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellci K., Marusak H.A., Peters C., Elrahal F., Iadipaolo A.S., Rabinak C.A. Community and household-level socioeconomic disadvantage and functional organization of the salience and emotion network in children and adolescents. NeuroImage. 2019;184:729–740. doi: 10.1016/j.neuroimage.2018.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Coalson T.S., Robinson E.C., Hacker C.D., Harwell J., Yacoub E.…Van Essen D.C. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171–178. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good B.H., De Montjoye Y.A., Clauset A. Performance of modularity maximization in practical contexts. Phys. Rev. E. 2010;81(4) doi: 10.1103/PhysRevE.81.046106. [DOI] [PubMed] [Google Scholar]
- Gordon E.M., Laumann T.O., Adeyemo B., Huckins J.F., Kelley W.M., Petersen S.E. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb. Cortex. 2016;26(1):288–303. doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia-Tabuenca Z., Moreno M.B., Barrios F.A., Alcauter S. Development of the brain functional connectome follows puberty-dependent nonlinear trajectories. NeuroImage. 2021;229 doi: 10.1016/j.neuroimage.2021.117769. [DOI] [PubMed] [Google Scholar]
- Grayson D.S., Fair D.A. Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. NeuroImage. 2017;160:15–31. doi: 10.1016/j.neuroimage.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S., Satterthwaite T.D., Medaglia J.D., Yang M., Gur R.E., Gur R.C., Bassett D.S. Emergence of system roles in normative neurodevelopment. Proc. Natl. Acad. Sci. U. S. A. 2015;112(44):13681–13686. doi: 10.1073/pnas.1502829112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimerà R., Nunes Amaral L.A. Functional cartography of complex metabolic networks. Nature. 2005;433(7028):895–900. doi: 10.1038/nature03288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair N.L., Hanson J.L., Wolfe B.L., Pollak S.D. Association of child poverty, brain development, and academic achievement. JAMA Pediatr. 2015;169(9):822–829. doi: 10.1001/jamapediatrics.2015.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist M.N., Hillary F.G. Graph theory approaches to functional network organization in brain disorders: a critique for a brave new small-world. Netw. Neurosci. 2018;3(1):1–26. doi: 10.1162/netn_a_00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Hair N., Shen D.G., Shi F., Gilmore J.H., Wolfe B.L., Pollak S.D. Family poverty affects the rate of human infant brain growth. PLoS ONE. 2013;8(12) doi: 10.1371/journal.pone.0080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde L.W., Gard A.M., Tomlinson R.C., Burt S.A., Mitchell C., Monk C.S. An ecological approach to understanding the developing brain: examples linking poverty, parenting, neighborhoods, and the brain. Am. Psychol. 2020;75(9):1245–1259. doi: 10.1037/amp0000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde L.W., Gard A.M., Tomlinson R.C., Suarez G.L., Westerman H.B. Parents, neighborhoods, and the developing brain. Child Dev. Perspect. 2022;16(3):148–156. doi: 10.1111/cdep.12453. [DOI] [Google Scholar]
- Ip K.I., Sisk L.M., Horien C., Conley M.I., Rapuano K.M., Rosenberg M.D., Greene A.S., Scheinost D., Constable R.T., Casey B.J., Baskin-Sommers A., Gee D.G. Associations among household and neighborhood socioeconomic disadvantages, resting-state frontoamygdala connectivity, and internalizing symptoms in youth. J. Cogn. Neurosci. 2022;34(10):1810–1841. doi: 10.1162/jocn_a_01826. [DOI] [PubMed] [Google Scholar]
- Johnson S.B., Riis J.L., Noble K.G. State of the art review: poverty and the developing brain. Pediatrics. 2016;137(4) doi: 10.1542/peds.2015-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keding T.J., Heyn S.A., Russell J.D., Zhu X., Cisler J., McLaughlin K.A., Herringa R.J. Differential patterns of delayed emotion circuit maturation in abused girls with and without internalizing psychopathology. Am. J. Psychiatry. 2021;178(11):1026–1036. doi: 10.1176/appi.ajp.2021.20081192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A.S., Sydnor V.J., Pines A., Fair D.A., Bassett D.S., Satterthwaite T.D. Hierarchical functional system development supports executive function. Trends Cogn. Sci. 2022;27(2):160–174. doi: 10.1016/j.tics.2022.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus B.T., Perez D., Ladwig Z., Seitzman B.A., Dworetsky A., Petersen S.E., Gratton C. Network variants are similar between task and rest states. NeuroImage. 2021;229 doi: 10.1016/j.neuroimage.2021.117743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latora V., Marchiori M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001;87(19) doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell. Mol. Neurobiol. 2003;23(5):727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal T., Brooks-Gunn J. The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychol. Bull. 2000;126(2):309–337. doi: 10.1037/0033-2909.126.2.309. [DOI] [PubMed] [Google Scholar]
- Lüdecke D., Ben-Shachar M., Patil I., Makowski D. Extracting, computing and exploring the parameters of statistical models using R. J. Open Source Softw. 2020;5(53):2445. doi: 10.21105/joss.02445. [DOI] [Google Scholar]
- Luna B., Marek S., Larsen B., Tervo-Clemmens B., Chahal R. An integrative model of the maturation of cognitive control. Annu. Rev. Neurosci. 2015;38:151–170. doi: 10.1146/annurev-neuro-071714-034054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S., Hwang K., Foran W., Hallquist M.N., Luna B. The contribution of network organization and integration to the development of cognitive control. PLoS Biol. 2015;13(12) doi: 10.1371/journal.pbio.1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall N.A., Marusak H.A., Sala-Hamrick K.J., Crespo L.M., Rabinak C.A., Thomason M.E. Socioeconomic disadvantage and altered corticostriatal circuitry in urban youth. Hum. Brain Mapp. 2018;39(5):1982–1994. doi: 10.1002/hbm.23978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Weissman D., Bitrán D. Childhood adversity and neural development: a systematic review. Annu. Rev. Dev. Psychol. 2019;1:277–312. doi: 10.1146/annurev-devpsych-121318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoyd V.C. Socioeconomic disadvantage and child development. Am. Psychol. 1998;53(2):185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Muldoon S.F., Bridgeford E.W., Bassett D.S. Small-world propensity and weighted brain networks. Sci. Rep. 2016;6 doi: 10.1038/srep22057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins T.S., Campbell E.M., Hogeveen J. Neighborhood deprivation shapes motivational-neurocircuit recruitment in children. Psychol. Sci. 2020;31(7):881–889. doi: 10.1177/0956797620929299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M.E.J. Modularity and community structure in networks. Proc. Natl. Acad. Sci. 2006;103(23):8577–8582. doi: 10.1073/pnas.0601602103. www.pnas.orgcgidoi10.1073pnas.0601602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pager D., Shepherd H. The sociology of discrimination: racial discrimination in employment, housing, credit, and consumer markets. Annu. Rev. Sociol. 2008;34:181–209. doi: 10.1146/annurev.soc.33.040406.131740. 〈https://www.jstor.org/stable/29737787〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes L., Fulcher B., Yücel M., Fornito A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. NeuroImage. 2018;171:415–436. doi: 10.1016/j.neuroimage.2017.12.073. [DOI] [PubMed] [Google Scholar]
- Peckins M.K., Westerman H.B., Burt S.A., Murray L., Alves M., Miller A.L., Gearhardt A.N., Klump K.L., Lumeng J.C., Hyde L.W. A brief child-friendly reward task reliably activates the ventral striatum in two samples of socioeconomically diverse youth. PLoS ONE. 2022;17(2) doi: 10.1371/journal.pone.0263368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher K.J., Curran P.J., Bauer D.J. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J. Educ. Behav. Stat. Winter. 2006;31(3):437–448. 〈http://www.quantpsy.org/〉 [Google Scholar]
- Pruim R.H.R., Mennes M., Buitelaar J.K., Beckmann C.F. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. NeuroImage. 2015;112:278–287. doi: 10.1016/j.neuroimage.2015.02.063. [DOI] [PubMed] [Google Scholar]
- Pruim R.H.R., Mennes M., van Rooij D., Llera A., Buitelaar J.K., Beckmann C.F. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage. 2015;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- Rakesh D., Whittle S. Socioeconomic status and the developing brain – a systematic review of neuroimaging findings in youth. Neurosci. Biobehav. Rev. 2021;130:379–407. doi: 10.1016/j.neubiorev.2021.08.027. [DOI] [PubMed] [Google Scholar]
- Rakesh D., Seguin C., Zalesky A., Cropley V., Whittle S. Associations between neighborhood disadvantage, resting-state functional connectivity, and behavior in the adolescent brain cognitive development study: the moderating role of positive family and school environments. Biol. Psychiatry.: Cogn. Neurosci. Neuroimaging. 2021;6(9):877–886. doi: 10.1016/j.bpsc.2021.03.008. [DOI] [PubMed] [Google Scholar]
- Rakesh D., Zalesky A., Whittle S. Similar but distinct-effects of different socioeconomic indicators on resting state functional connectivity: findings from the Adolescent Brain Cognitive Development (ABCD) Study®. Dev. Cogn. Neurosci. 2021;51 doi: 10.1016/j.dcn.2021.101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakesh D., Cropley V., Zalesky A., Vijayakumar N., Allen N.B., Whittle S. Neighborhood disadvantage and longitudinal brain-predicted-age trajectory during adolescence. Dev. Cogn. Neurosci. 2021;51 doi: 10.1016/j.dcn.2021.101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakesh D., Kelly C., Vijayakumar N., Zalesky A., Allen N.B., Whittle S. Unraveling the consequences of childhood maltreatment: deviations from typical functional neurodevelopment mediate the relationship between maltreatment history and depressive symptoms. Biol. Psychiatry.: Cogn. Neurosci. Neuroimaging. 2021;6(3):329–342. doi: 10.1016/j.bpsc.2020.09.016. [DOI] [PubMed] [Google Scholar]
- Rakesh D., Zalesky A., Whittle S. Assessment of parent income and education, neighborhood disadvantage, and child brain structure. JAMA Netw. Open. 2022;5(8) doi: 10.1001/jamanetworkopen.2022.26208. e2226208-e2226208. https://doi.org/10.1001/jamanetworkopen.2022.26208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakesh D., Whittle S., Sheridan M.A., McLaughlin K.A. Childhood socioeconomic status and the pace of structural neurodevelopment: accelerated, delayed, or simply different? Trends Cogn. Sci. 2023 doi: 10.1016/j.tics.2023.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H., Betancourt L., Giannetta J.M., Brodsky N.L., Korczykowski M., Avants B.B., Gee J.C., Wang J., Hurt H., Detre J.A., Farah M.J. Early parental care is important for hippocampal maturation: Evidence from brain morphology in humans. NeuroImage. 2010;49(1):1144–1150. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollo I., de Moor M.H., Dolan C.V., Boomsma D.I. Phenotypic factor analyses of family data: correction of the bias due to dependency. Twin Res. Hum. Genet. 2006;9(3):367–376. doi: 10.1375/twin.9.3.367. [DOI] [PubMed] [Google Scholar]
- Revelle, W. (2015). Package ‘psych’. 〈https://cran.rstudio.org/web/packages/psych/psych.pdf〉.
- Riley A.R. Neighborhood disadvantage, residential segregation, and beyond — lessons for studying structural racism and health. J. Racial Ethn. Health Disparities. 2018;5(2):357–365. doi: 10.1007/s40615-017-0378-5. [DOI] [PubMed] [Google Scholar]
- Roberts S.O., Rizzo M.T. The psychology of American racism. Am. Psychol. 2021;76(3):475–487. doi: 10.1037/amp0000642. [DOI] [PubMed] [Google Scholar]
- Roubinov D., Meaney M.J., Boyce W.T. Change of pace: how developmental tempo varies to accommodate failed provision of early needs. Neurosci. Biobehav. Rev. 2021;131:120–134. doi: 10.1016/j.neubiorev.2021.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Santarnecchi E., Galli G., Polizzotto N.R., Rossi A., Rossi S. Efficiency of weak brain connections support general cognitive functioning. Hum. Brain Mapp. 2014;35(9):4566–4582. doi: 10.1002/hbm.22495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siugzdaite R., Akarca D., Johnson A., Carozza S., Anwyl-Irvine A.L., Uh S., Smith T., Bignardi G., Dalmaijer E., Astle D.E. Socio-economic disadvantage is associated with alterations in brain wiring economy. bioRxiv. 2022 doi: 10.1101/2022.06.08.495247. [DOI] [Google Scholar]
- Slopen N., Heard-Garris N. Structural racism and pediatric health—a call for research to confront the origins of racial disparities in health. JAMA Pediatr. 2021;176(1):13–15. doi: 10.1001/jamapediatrics.2021.3594. https://doi.org/10.1001/jamapediatrics.2021.3594. [DOI] [PubMed] [Google Scholar]
- South S.C., Krueger R.F., Knudsen G.P., Ystrom E., Czajkowski N., Aggen S.H., Reichborn-Kjennerud T. A population based twin study of DSM–5 maladaptive personality domains. Personal. Disord.: Theory Res. Treat. 2017;8(4):366–375. doi: 10.1037/per0000220. [DOI] [PubMed] [Google Scholar]
- Sporns O. Network attributes for segregation and integration in the human brain. Curr. Opin. Neurobiol. 2013;23(2):162–171. doi: 10.1016/j.conb.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Sripada C., Angstadt M., Taxali A., Clark D.A., Greathouse T., Rutherford S., Dickens J.R., Shedden K., Gard A.M., Hyde L.W., Weigard A., Heitzeg M. Brain-wide functional connectivity patterns support general cognitive ability and mediate effects of socioeconomic status in youth. Transl. Psychiatry. 2021;11(1) doi: 10.1038/s41398-021-01704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R.K., Swain J.E., Evans G.W., Welsh R.C., Liberzon I. Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacology. 2014;39(9):2244–2251. doi: 10.1038/npp.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez G.L., Burt S.A., Gard A.M., Burton J., Clark D.A., Klump K.L., Hyde L.W. The impact of neighborhood disadvantage on amygdala reactivity: pathways through neighborhood social processes. Dev. Cogn. Neurosci. 2022;54 doi: 10.1016/j.dcn.2022.101061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Margulies D.S., Breakspear M., Zalesky A. Topographic organization of the human subcortex unveiled with functional connectivity gradients. Nat. Neurosci. 2020;23(11):1421–1432. doi: 10.1038/s41593-020-00711-6. [DOI] [PubMed] [Google Scholar]
- Tomlinson R.C., Burt S.A., Waller R., Jonides J., Miller A.L., Gearhardt A.N., Peltier S.J., Klump K.L., Lumeng J.C., Hyde L.W. Neighborhood poverty predicts altered neural and behavioral response inhibition. NeuroImage. 2020;209 doi: 10.1016/j.neuroimage.2020.116536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley U.A., MacKey A.P., Ciric R., Ruparel K., Moore T.M., Gur R.C., Gur R.E., Satterthwaite T.D., Bassett D.S. Associations between neighborhood SES and functional brain network development. Cereb. Cortex. 2020;30(1):1–19. doi: 10.1093/cercor/bhz066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley U.A., Bassett D.S., Mackey A.P. Environmental influences on the pace of brain development. Nat. Rev. Neurosci. 2021;22(6):372–384. doi: 10.1038/s41583-021-00457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley U.A., Park A.T., Leonard J.A., Boroshok A.L., McDermott C.L., Tisdall M.D., Bassett D.S., Mackey A.P. The age of reason: functional brain network development during childhood. J. Neurosci. 2022;42(44):8237–8251. doi: 10.1523/JNEUROSCI.0511-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderbilt-Adriance E., Shaw D.S. Protective factors and the development of resilience in the context of neighborhood disadvantage. J. Abnorm. Child Psychol. 2008;36:887–901. doi: 10.1007/s10802-008-9220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk B.C.M., Stam C.J., Daffertshofer A. Comparing brain networks of different size and connectivity density using graph theory. PLoS ONE. 2010;5(10) doi: 10.1371/journal.pone.0013701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Vijayakumar N., Simmons J.G., Dennison M., Schwartz O., Pantelis C., Sheeber L., Byrne M.L., Allen N.B. Role of positive parenting in the association between neighborhood social disadvantage and brain development across adolescence. JAMA Psychiatry. 2017;74(8):824–832. doi: 10.1001/jamapsychiatry.2017.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig G.S. Segregated systems of human brain networks. Trends Cogn. Sci. 2017;21(12):981–996. doi: 10.1016/j.tics.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Willemsen G., Odintsova V., de Geus E., Boomsma D.I. Twin-singleton comparisons across multiple domains of life. Twin High. -Order Pregnancies. 2021:51–71. doi: 10.1007/978-3-030-47652-6_4. [DOI] [Google Scholar]
- Xia M., Wang J., He Y. BrainNet viewer: a network visualization tool for human brain connectomics. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M.…Buckner R.L. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.