Abstract
Introduction
Impairment of the type I interferon (IFN–I) signaling pathway is associated with increased severity of COVID-19 disease. Here we have undertaken a systematic review and meta = analysis on the association between the severity of COVID-19 and IFN-1 autoantibodies (AAbs; aIFN-1, aIFN-α, aIFN-ω, and aIFN-β).
Methods
Four databases, including Medline [PubMed], Embase, Web of Science, and Scopus, were systematically searched to find papers on the role of aIFN-1 and its subtype AAbs in the severity of COVID-19 infection. Data on the prevalence of aIFN-1, aIFN-α, aIFN-ω, and aIFN-β were pooled using random- or fixed-effects models. Subgroup analysis was performed based on disease severity. Odds ratios (OR) for COVID-19 disease outcome, including length of hospital stay, ICU admission and death, were calculated in relation to positive or negative plasma IFN-1 AAbs.
Results
A total of 33 studies with 13023 patients were included. The overall prevalence of circulating aIFN-1, aIFN-α, and aIFN-ω AAbs was 17.8 % [13.8, 22.8], 7.2 % [4.7, 10.9], and 4.4 % [2.1, 8.6], respectively, and the overall prevalence of neutralizing aIFN-1, aIFN-α, aIFN-ω, and aIFN-β AAbs was 7.1 % [4.9, 10.1], 7.5 % [5.9, 9.5], 8.0 % [5.7, 11.1] and 1.2 % [0.4, 3.5], respectively. Circulating aIFN-α (OR = 4.537 [2.247, 9.158]), neutralizing aIFN-α (O = 17.482 [8.899, 34.342]), and neutralizing aIFN-ω (OR = 12.529 [7.397, 21.222]) were significantly more frequent in critical/severe patients than in moderate/mild patients (p < 0.001 for all). Anti–IFN–1 was more common in male subjects (OR = 2.248 [1.366, 3.699], p = 0.001) and two COVID-19 outcomes including ICU admission (OR = 2.485 [1.409, 4.385], p = 0.002) and death (OR = 2.593 [1.199, 5.604], p = 0.015) occurred more frequently in patients with positive anti–IFN–1.
Conclusion: aIFN-1 and its subtypes AAbs are associated with severe and critical COVID-19 disease and may be a predictive marker for a poor prognosis, particularly in men.
Keywords: Interferon, COVID-19, Autoantibody
Highlights
-
•
Autoantibodies against IFN-1 and its subtypes increase in COVID-19 patients.
-
•
The presence of autoantibodies against IFN-1 and its subtypes are associated with more severe COVID -19 disease.
-
•
Autoantibodies against IFN-1 and its subtypes may predict worse COVID-19 disease outcomes.
-
•
Autoantibodies against IFN-1 were more prevalent among male patients.
1. Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) began spreading in Wuhan, China, in late December 2019 and has caused nearly 7 million deaths by May 4, 2023 [1]. SARS-CoV-2 infection has shown a broad spectrum of clinical presentations, ranging from asymptomatic to severe disease associated with multiple organ failure and death [2,3]. Impairment of antiviral mechanisms such as interferon type 1 (IFN-1) has been associated with the severity of several life-threatening respiratory infections [4], including influenza pneumonia [5] and COVID-19 [6]. IFN-1 belongs to a family of cytokines that trigger important innate immune defense mechanisms during viral infection [7]. IFN-1 comprises several subtypes: IFN-α, IFN-β, IFN-ω, IFN-ε, and several other subtypes that are structurally related and have a variety of biological functions, including protection against pathogens, immune-regulation, differentiation, and development [8]. Their deficiency can lead to viral spread, and their overproduction can cause excessive inflammation, leading to a poor clinical outcome. Impairment of the IFN-1 pathway can be caused either by inherited genetic defects or by the production of autoantibodies (AAbs) directed against IFN-1 [9]. Several studies have shown that SARS-CoV-2 induces a low and delayed IFN response in severe and critical patients [2,10,11]. Casanova and Anderson showed that inherited or autoimmune deficiencies of IFN-1 are responsible for 15 %–20 % of all critical COVID-19 patients [12]. Recently, Goncalves detected anti–IFN–1 (aIFN-1) AAbs in 18 % of critically ill COVID-19 patients, whereas these AAbs were not detected in any of the patients with mild disease [9].
Detection of IFN-1 AAbs in the population infected with the SARS-CoV-2 virus has been proposed as a predictive marker for unfavourable COVID-19 outcomes; therefore, to test this observation, we systematically reviewed the available work on the association between COVID-19 severity and IFN-1 AAbs in the serum of COVID -19 patients.
2. Material and methods
2.1. Search strategy and study selection
This systematic review and meta-analysis were conducted in compliance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) protocols [13]. The initial search strategy was developed in PubMed and PubMed's Medical Subject Headings (MeSH) and applied to each database searched. We identified articles by searching PubMed, Scopus, Embase, and Web of Science through May 10, 2023, using the following search terms: “COVID-19″ and “autoantibody”. A complete list of search strategies for all databases is provided in Table S1. Studies reporting aIFN AAbs in patients with COVID-19 were extracted. We also searched Google Scholar and the reference lists of the included articles to identify further studies. We included only studies in English.
2.2. Inclusion and exclusion criteria
All included papers met the following criteria [1]: subjects being diagnosed COVID-19 based on a reverse transcription polymerase chain reaction (RT-PCR), serological tests or typical symptoms [2] at least one of the aIFN-1 or its subtypes AAbs had to have been measured. We excluded all case reports [14].
2.3. Eligibility and quality assessment
Four reviewers (A.A., M.A., N.N., and Z.S.) participated in selecting studies based on the articles' title, abstract and full text. In cases where agreement could not be reached, a fifth reviewer (A.S.) reviewed the eligibility to determine final inclusion. Two reviewers (M.A. and N.N.) independently reviewed included articles for quality. Included studies were assessed using the Joanna Briggs Institute (JBI) assessment tools, which provide an assessment tool for most study types, including observational studies [15]. Disagreements were resolved by discussion between the investigators or a third reviewer (A.S.).
2.4. Data extraction
Study characteristics were extracted from the included articles, including first author's last name, study conduct date, title, study design, study location (country), patient demographics, COVID-19 confirmatory method, IFN autoantibody assay, and history of systemic autoimmune rheumatic disease. The number of individuals in whom circulating or neutralizing aIFN-1/α/ω/β was detected was also recorded. In addition, characteristics of COVID-19 patients in studies comparing seropositive patients with seronegative patients to IFN-1 or its subtypes, including duration of admission, demographics, intensive care unit (ICU) admission, and mortality rate, were extracted. Data were stored in a Microsoft Excel spreadsheet (Redmond, WA). Data was collected on the plasma AABs levels in COVID-19 and non-COVID-19 patients or within COVID-19 patients according to disease severity.
2.5. Statistical analysis
The weighted pooled prevalence and 95 % confidence intervals (CIs) of aIFN-1, aIFN-α, aIFN-ω, and aIFN-β were calculated. The magnitudes of the estimated effects of AAbs on COVID-19 outcomes and the prevalence of AAbs in male patients were expressed as odds ratios (OR), with corresponding 95 % CIs in brackets. The prevalence of AAbs in critical/severe or moderate/mild groups was compared using OR and 95 % CIs. Sensitivity analysis was done after removing a study conducted on children [16]. Median values were converted to means using a formula described by Wan et al. [17]. p < 0.05 was considered statistically significant. Heterogeneity was calculated quantitatively using the I2 statistical test. If heterogeneity was high (Cochran's Q < 0.05), the random-effects model was used; otherwise, the fixed-effects model was used. Potential publication bias was examined using funnel plots [18]. All statistical analyses were done using Comprehensive Meta-Analysis Software (CMA v.3, Biostat Inc., Englewood, New Jersey, USA).
3. Results
After removing 1809 duplicate papers, a total of 2343 papers were identified (Fig. 1). Finally, 33 studies were included in the present systematic review and meta-analysis. Four studies were suspected to be the same patients and were removed [[19], [20], [21], [22]]. The selected studies and their characteristics are listed in Table 1. Seventeen studies were conducted in European countries (France = 7 [9,[23], [24], [25], [26], [27], [28]], Spain = 3 [11,29,30], Italy = 3 [6,10,31], Netherlands = 2 [32,33], Belgium = 1 [34] and Switzerland = 1 [35]), six of the selected studies were conducted in North America (United States [7,[36], [37], [38], [39], [40]]) and one in South America (Colombia [41]), three in Asia (Iran = 1 [42], Russia = 1 [43], and Japan = 1 [44]) and six studies were multicenter studies [16,19,22,[45], [46], [47]]. Details of the quality assessment are reported in Table S2.
Fig. 1.
PRISMA flow diagram.
Table 1.
Characteristics of the included studies.
| First author (year/country) | Study design | Sample size (number and critical situation) | Age (mean ± SD) | Male | Auto-antibody (assay) | History of systemic autoimmune rheumatic disease | PCR for SARS-CoV-2 |
|---|---|---|---|---|---|---|---|
| Goncalves et al. (2020/France) | Prospective cohort | 84 critical (admitted to ICU) and 10 mild* | 65.4 ± 11.6 | 67 | aIFN-α2, aIFN-ω, IFN-β (ELISA) | 9 autoimmune disease | Yes |
| Wijst et al. (2020/United States) | Prospective cohort | 284 (26 critical, 102 severe, 156 moderate)* | 51.9 ± 16.2 | 195 | aIFN-α2 (radioligand binding assay) | ND | Yes |
| Solanich et al. (2021/Spain) | Retrospective | 275 severe admitted to ICU* | 63.3 ± 11.9 | 211 | aIFN-α2, aIFN-ω (ELISA) | No | Yes |
| Chauvineau-Grenier (2020/France) | Prospective cohort | 139 critical* | 64.4 ± 15.8 | 86 | aIFN-α2, aIFN-ω, aIFN-β (luciferase reporter assay, ELISA) | No | ND |
| Bastard et al. (2020/Multicenter) | Prospective cohort | 987 critical, 663 asymptomatic or mild* | ND | 761 males among critically ill patients | aIFN-α2, aIFN-ω (ELISA) | No | Yes |
| Bastard et al. (2020/Multicenter) | Prospective cohort | 3595 critical, 623 severe, 1639 asymptomatic or paucisymptomatic* | ND | ND | aIFN-α2, aIFN-ω, aIFN-β (Multiplex particle-based assay, ELISA) | No | Yes (PCR and/or serological test and/or displaying typical symptom) |
| Matuozzo et al. (2023/Multicenter) | Cohort | 928 | ND | ND | aIFN-1 | ND | Yes |
| Akbil et al. (2021/Multicenter) | Prospective cohort | 430 (237 critical)* | 62 ± 15.6 | 312 | aIFN-α, aIFN- ω (ELISA) | 14 autoimmune diseases | Yes |
| Abers et al. (2020/Italy) | Prospective cohort | 218 (135 critical, 44 severe, 39 mild/moderate) | ND | ND | aIFN-α, aIFN-ω, aIFN-β (ELISA) | ND | Yes |
| Troya et al. (2020/Spain) | Cohort | 31 severe and 16 critical | 68.5 ± 4 | 28 | aIFN-α2, aIFN-ω (luciferase reporter assays, ELISA) | No | Yes |
| Frasca et al. (2020/Italy) | Cohort | 360* | 62.25 ± 12.5 | 249 | aIFN‐α, aIFN-ω, aIFN‐β (ELISA) | No | Yes |
| Lopez et al. (2021/France) | Prospective cohort | 26 critical, 44 mild* | 44.9 ± 10.0 | 24 | aINF-α2 (ELISA) | ND | Yes |
| Troya et al. (2022/Spain) | Retrospective cohort | 178 critical* | 73.8 ± 3.8 | 115 | aINF-α2, aIFN-ω, aIFN-β | 19 autoimmune diseases | ND |
| Koning et al. (2020/Netherlands) | Prospective cohort | 210 severe to critical | 64.2 ± 5.1 | 133 | aIFN-1 (multiplex particle-based assay, ELISA) | ND | Yes |
| Eto et al. (2022/Japanese) | Prospective cohort | 627 (170 critical, 235 severe, 112 moderate, 105 mild, 5 asymptomatic)* |
60.0 ± 20.1 | 440 | aINF-α2, aIFN-ω (ELISA and ISRE reporter assays) | ND | ND |
| Raadsen et al. (2020/Netherlands) | Prospective cohort | 282 (100 mild, 43 moderate, 97 severe, 38 critical)* | 54.4 ± 15.7 | 229 | aINF-α2 (ELISA and a pseudo virus–based neutralization assay) | ND | Yes |
| Chang et al. (2021/United States) | Prospective cohort | 147* | 57.4 ± 15.7 | 56 | aIFN-1 (ELISA) | ND | Yes |
| Wang et al. (2020/United States) | Prospective cohort | 194 (55 severe, 103 moderate, 7 mild, 29 asymptomatic)* | 60.4 ± 18.7 | 87 | aIFN-1 ELISA) | ND | Yes |
| Vazquez et al. (2020/United States) | Retrospective cohort | 116 hospitalized* | ND | ND | aINF-α2 (radioligand binding assay), aIFN-ω (cell-based assay) | ND | Yes |
| Manry et al. (2022/France) | Cohort | 1261 died | 70.7 ± 13.0 | 821 | aINF-α, aIFN-ω, aIFN-β (ELISA) | ND | ND |
| Savvateeva et al. (2021/Russia) | Cohort | 86 critical* | ND | ND | aINF-α, aIFN-ω (microarray-based assay) | ND | Yes |
| Acosta-Ampudia et al. (2020/Colombia) | Cohort | 19 recovered, 18 severe | 50.7 ± 8.7 | 10 | aINF-α2 (ELISA) | ND | Yes |
| Yee et al. (2021/United States) | Prospective cohort | 103 inpatients and 24 outpatients | 55.2 ± 15.2 | 86 | aIFN-α, aIFN-ω | ND | ND |
| Steels et al. (2022/Belgium) | Prospective cohort | 52 severe* | 65.7 ± 11.8 | 38 | aIFN-α2 (Luminex bead-based assay) | ND | Yes |
| Ziegler et al. (2022/United States) | Prospective cohort | 8 severe and 12 mild or moderate* | ND | ND | aIFN-α2, aIFN-ω (microarray-based platform) | ND | Yes |
| Soltani-Zangbar et al. (2022/Iran) | Cross-sectional | 50 severe and 50 mild* | 46.7 ± 9.0 | 54 | aIFN-α2 (ELISA) | No | Yes |
| Scordio et al. (2022/Italy) | Cohort | 3 critical, 3 moderate, and 2 mild* | 57.5 ± 13.2 | 7 | aINF-α, aIFN-ω, aIFN-β (bioassay) | ND | Yes |
| Busnadiego et al. (2021/Switzerland) | Prospective cohort | 103 critical* | 64.3 ± 11.3 | 80 | aINF-α2, aIFN-ω, aIFN-β (bead-based serological assay) | No | Yes |
| Mathian et al. (2022/France) | Retrospective cohort | 5 | ND | ND | aINF-α2, aIFN-ω, aIFN-β (ELISA) | 5 systemic lupus erythematosus | ND |
| Smith et al. (2022/Multicenter) | Cohort | 126* | ND | ND | aINF-α2, aIFN-ω (ELISA) | ND | Yes |
| Arrestier et al. (2022/France) | Prospective cohort | 925 critical | 61.6 ± 12.4 | 652 | aINF-α2, aIFN-ω, aIFN-β (luciferase reporter assay) | ND | Yes |
| Bodansky et al. (2023/Multicenter) | Cohort | 168 severe and 45 mild | 11.1 ± 8.3 | 107 | aINF-α2 (radioligand binding assay) | ND | Yes |
| Carapito et al. (2022/France) | Prospective cohort | 47 critical and 25 non-critical | 39.6 ± 9.8 | 53 | aIFN-1 (ELISA) | No | Yes |
aIFN: Anti-Interferon, ND: Not determined, ELISA: Enzyme-linked immunosorbent assay, PCR: Polymerase chain reaction*The study has healthy control group.
3.1. Circulating and neutralizing aIFN-1/α/ω/β
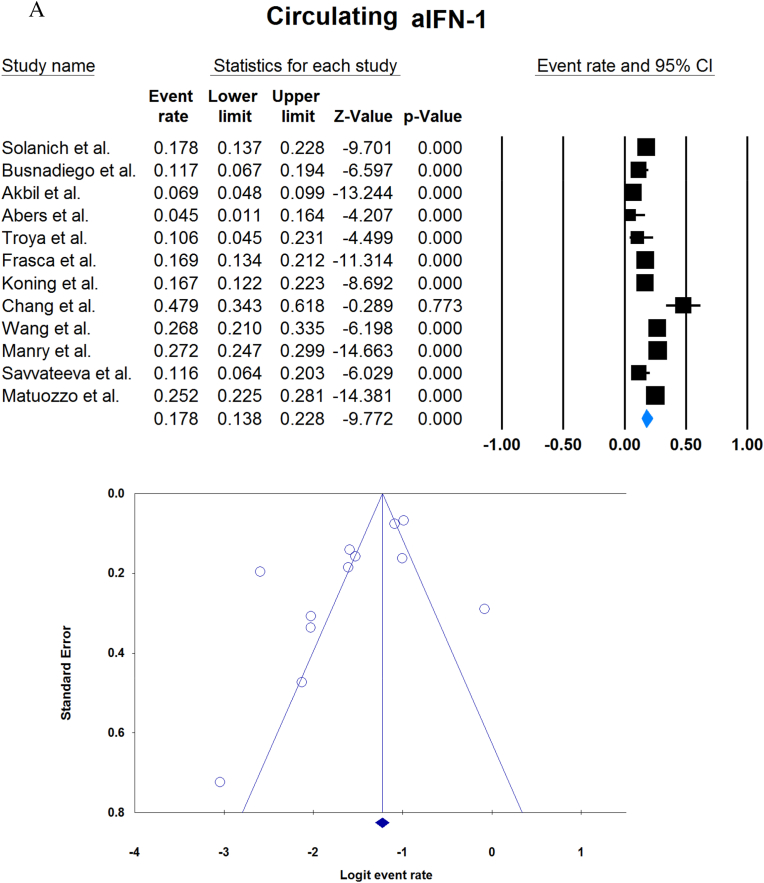
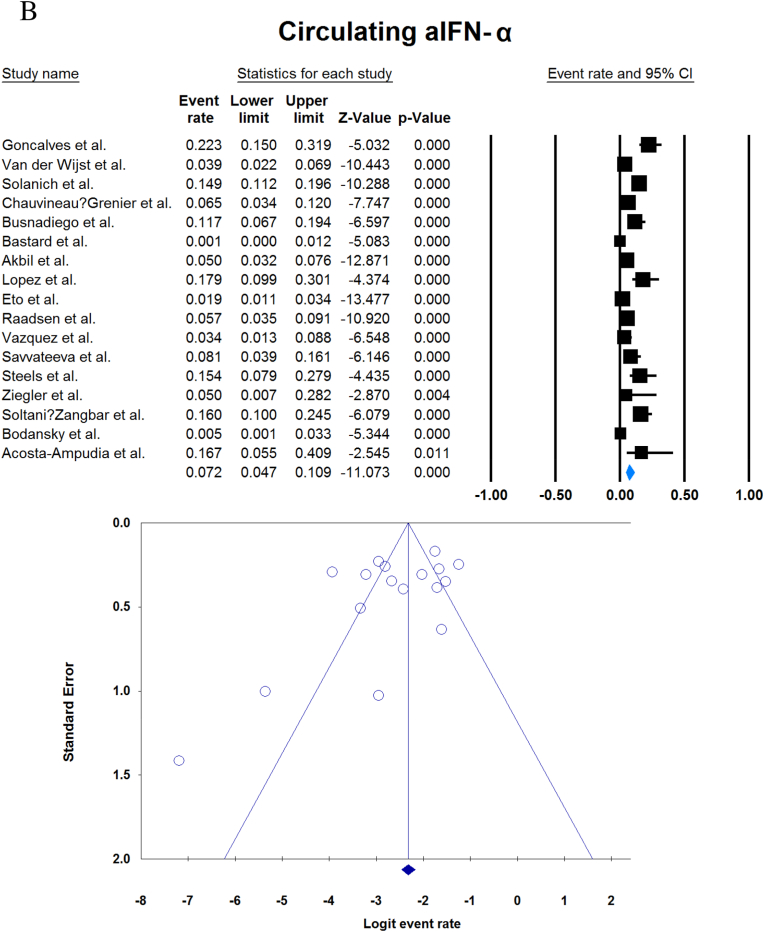
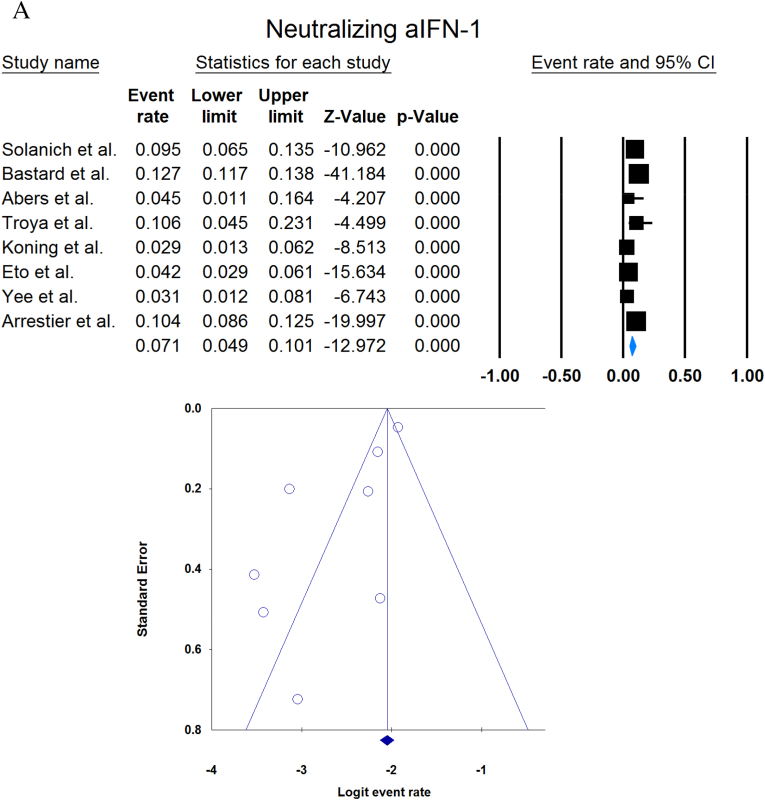
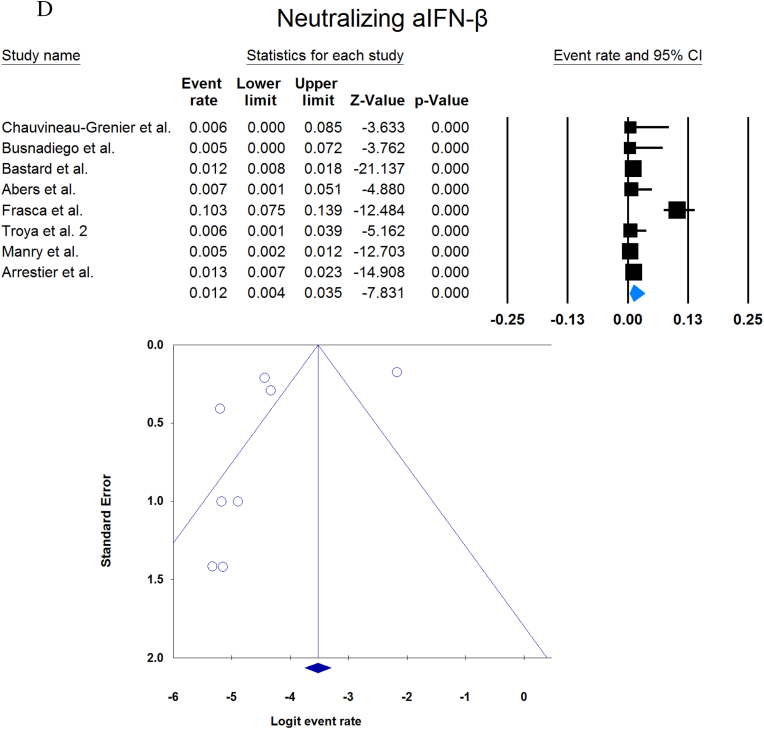
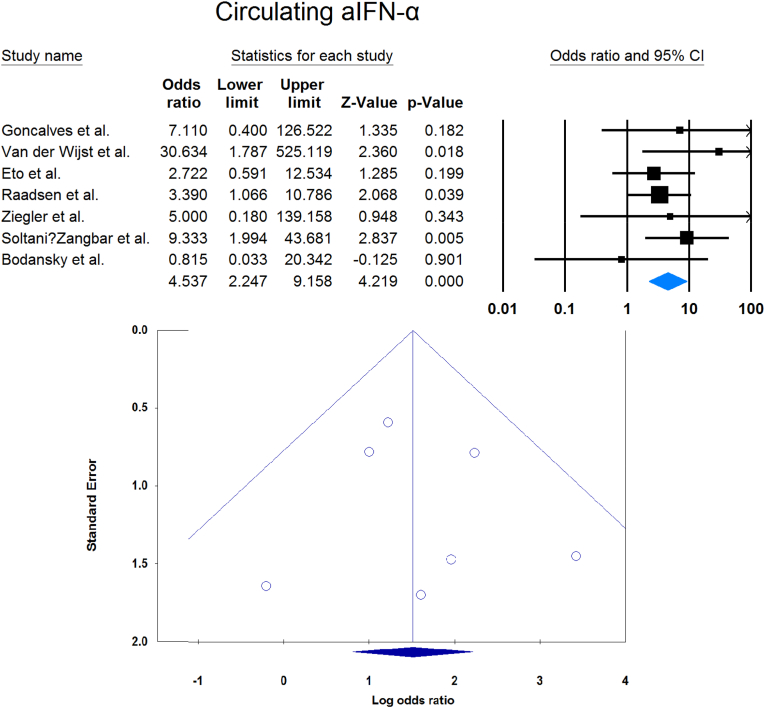
A total of 13023 patients (from 33 studies) diagnosed with COVID-19 and had at least one of the AAbs targeting IFN-1, IFN-α, IFN-ω, or IFN-β were included in this analysis. Seropositivity of patients with different stages of disease against IFN-1 and its subtypes is detailed in Table 2. Forest plots and funnel plots for each analysis are shown in Fig. 2, Fig. 3. The overall prevalence of circulating aIFN-1, aIFN-α, and aIFN-ω AAbs was 17.8 % [13.8, 22.8], 7.2 % [4.7, 10.9], and 4.4 % [2.1, 8.6], respectively, among COVID-19 patients, regardless of disease severity (Fig. 5). Sensitivity analysis by removing the study by Bodansky et al. showed 7.9 % [5.2, 11.8] prevalence of circulating aIFN-α. Also, the overall prevalence of neutralizing aIFN-1, aIFN-α, aIFN-ω, and aIFN-β AAbs was 7.1 % [4.9, 10.1], 7.5 % [5.9, 9.5], 8.0 % [5.7, 11.1], and 1.2 % [0.4, 3.5], respectively, regardless of disease severity (Fig. 6).
Table 2.
Seropositivity of patients with different severity of disease regarding aIFN-1 and its subtypes AAbs.
| First author | Sample size | aIFN-l- Circulating/Neutralizing | Circulating aIFN-α2/ω/β |
Neutralizing activity against aIFN-α2/ω/β |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Critical | Severe | Moderate | Mild | Critical | Severe | Moderate | Mild | |||
| Goncalves et al. | 94 | −/− | 21 of 84/10 of 21*/1 of 21* | −/−/- | −/−/- | 0 of 10/−/− | 15 of 84/10 of 21*/1 of 21* | −/−/- | −/−/- | 0 of 10/−/− |
| Van der Wijst et al. | 284 | −/− | 5 of 26/−/− | 6 of 102/−/− | 0 of 156/−/− | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- |
| Solanich et al. | 275 | 49/26 | −/−/- | 41 of 275/30 of 275/- | −/−/- | −/−/- | −/−/- | 25 of 275/22 of 275/- | −/−/- | −/−/- |
| Chauvineau-Grenier et al. | 139 | −/− | −/−/- | 9 of 139/-/0 of 86 | −/−/- | −/−/- | −/−/- | 6 of 139/-/0 of 86 | −/−/- | −/−/- |
| Busnadiego et al. | 103 | 12/- | 12 of 103/8 of 103/0 of 103 | −/−/- | −/−/- | −/−/- | 11 of 103/-/0 of 103 | −/−/- | −/−/- | −/−/- |
| Bastard et al. (Duplicate) | 1650 | −/− | −/−/- | −/−/- | −/−/- | 0 of 663/0 of 663/- | 88 of 987/65 of 987/- | −/−/- | −/−/- | 0 of 663/0 of 663/- |
| Bastard et al. | 4117 | -/523 | −/−/- | −/−/- | −/−/- | −/−/- | 360 of 3595/385 of 3595/23 of 1773 | 32 of 522/20 of 522/0 of 187 | −/−/- | 5 of 1639/13 of 1639/- |
| Akbil et al. | 403 | 28/- | 20 of 403/17 of 403/- | 18 of 237/11 of 237/- | −/−/- | −/−/- | −/−/- | |||
| Abers et al. | 44 | 2/2 | −/−/- | −/−/- | −/−/- | −/−/- | −/−/1 of 135 | 0 of 44/2 of 44/- | −/−/- | −/−/- |
| Troya et al. | 47 | 5/5 | −/−/- | −/−/- | −/−/- | −/−/- | 3 of 16/3 of 16/- | 2 of 31/2 of 31/- | −/−/- | −/−/- |
| Frasca et al. | 360 | 61/- | −/−/- | −/−/- | −/−/- | −/−/- | 13 of 360/9 of 360/37 of 360 | |||
| Lopez et al. | 56 | −/− | 10 of 56/−/− | −/−/- | −/−/- | −/−/- | −/−/- | |||
| Troya et al. | 178 | −/− | −/−/- | −/−/- | −/−/- | −/−/- | 27 of 178/26 of 178/1 of 178 | −/−/- | −/−/- | −/−/- |
| Koning et al. | 210 | 35/6 | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- |
| Eto et al. | 622 | -/26 | 8 of 170/6 of 170/- | 2 of 235/2 of 235/- | 1 of 112/0 of 112/- | 1 of 105/3 of 105/- | 12 of 170/17 of 170/- | 6 of 235/3 of 235/- | 1 of 112/1 of 112/- | 1 of 105/0 of 105/- |
| Raadsen et al. | 282 | −/− | 5 of 38/−/− | 7 of 97/−/− | 0 of 43/−/− | 4 of 100/−/− | 5 of 38/−/− | 7 of 97/−/− | 0 of 43/−/− | 1 of 100/−/− |
| Chang et al. | 48 | 23/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- |
| Wang et al. | 194 | 52/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- |
| Vazquez et al. | 116 | −/− | 4 of 116/−/− | 2 of 116/−/− | ||||||
| Manry et al. | 1121 | 305/- | −/−/- | −/−/- | −/−/- | −/−/- | 140 of 1121/165 of 1121/6 of 1094 | −/−/- | −/−/- | −/−/- |
| Savvateeva et al. | 86 | 10/- | 7 of 86/6 of 86/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- |
| Yee et al. | 127 | -/4 | −/−/- | −/−/- | −/−/- | −/−/- | 4 of 103 (IFN-α or IFN-ω) | 0 of 24 (IFN-α or IFN-ω) | ||
| Steels et al. | 52 | −/− | −/−/- | 8 of 52/−/− | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- |
| Ziegler et al. | 20 | −/− | −/−/- | 1 of 8/1 of 8/- | 0 of 12/0 of 12/- | −/−/- | −/−/- | −/−/- | −/−/- | |
| Soltani-Zangbar et al. | 100 | −/− | −/−/- | 14 of 50/−/− | −/−/- | 2 of 50/−/− | −/−/- | −/−/- | −/−/- | −/−/- |
| Scordio et al. | 8 | −/− | −/−/- | −/−/- | −/−/- | −/−/- | 2 of 3/2 of 3/- | −/−/- | 1 of 3/2 of 3/- | 0 of 2/1 of 2/- |
| Mathian et al. | 5 | −/− | −/−/- | −/−/- | −/−/- | −/−/- | 4 of 5/4 of 5/2 of 5 | −/−/- | −/−/- | |
| Smith et al. | 126 | −/− | −/−/- | −/−/- | −/−/- | −/−/- | 4 of 126/−/− | |||
| Arrestier et al. | 925 | -/96 | −/−/- | −/−/- | −/−/- | −/−/- | 74 of 925/71 of 925/12 of 925 | −/−/- | −/−/- | −/−/- |
| Bodansky et al. | 213 | −/− | −/−/- | 1 of 168/−/− | −/−/- | 0 of 45/−/− | −/−/- | −/−/- | −/−/- | −/−/- |
| Matuozzo et al. (Duplicate) | 928 | 234/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- |
| Acosta-Ampudia et al. | 18 | −/− | −/−/- | 3 of 18/−/− | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- |
| Carapito et al. | 72 | 2/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- | −/−/- |
| Total | 13023 | 818/688 | 12.2 % [7.0, 20.4]/4.9 % [2.8, 8.5]/0 % | 8.4 % [4.8, 14.3]/5.0 % [0.8, 24.7]/0 % | 0.7 % [0.2, 2.9]/0 %/- | 2.5 % [1.3, 4.8]/1.7 % [0.6, 4.7]/- | 10.6 % [8.7, 12.8]/10.8 % [8.4, 13.9]/1.1 % [0.8, 1.5] | 6.3 % [5.1, 7.8]/4.4 % [2.4, 7.9]/0 % | 3.5 % [0.3, 32.8]/11.4 % [0.1, 96.2]/- | 1.2 % [0.3, 4.7]/2.8 % [0.2, 32.5]/- |
aIFN: Anti-Interferon*Among patients who were positive for aIFN-α2.
Fig. 2.
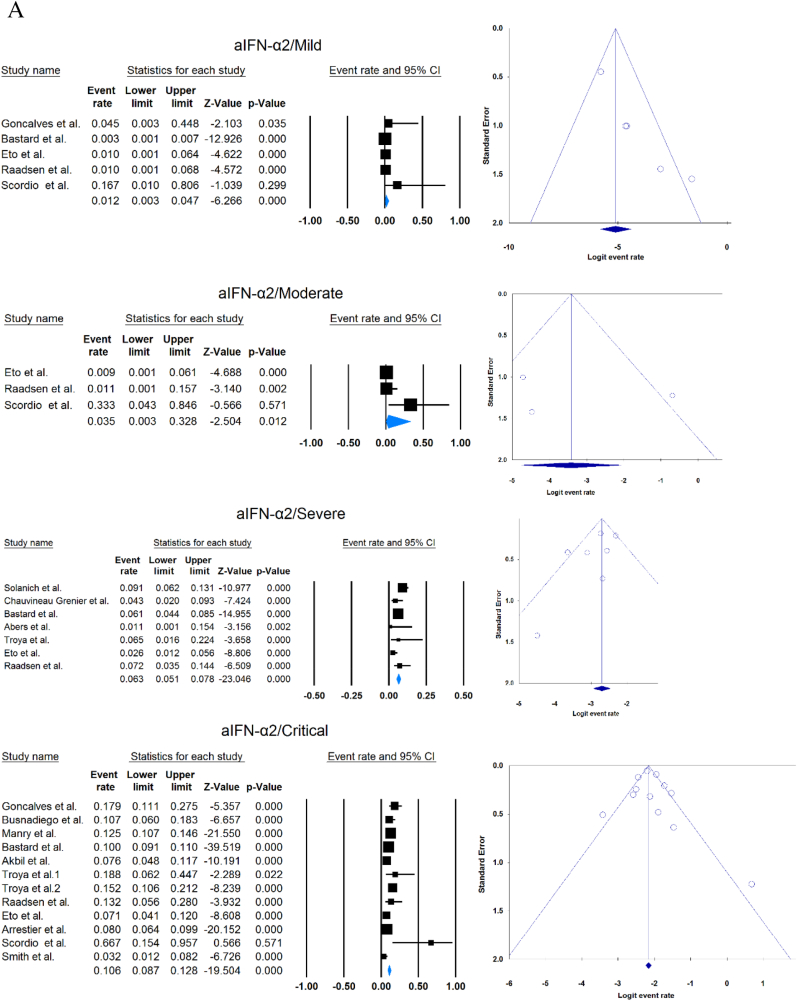
Prevalence of circulating autoantibodies against interferon α2 (A) and ω (B) in COVID -19 patients with different disease severity.
Fig. 3.
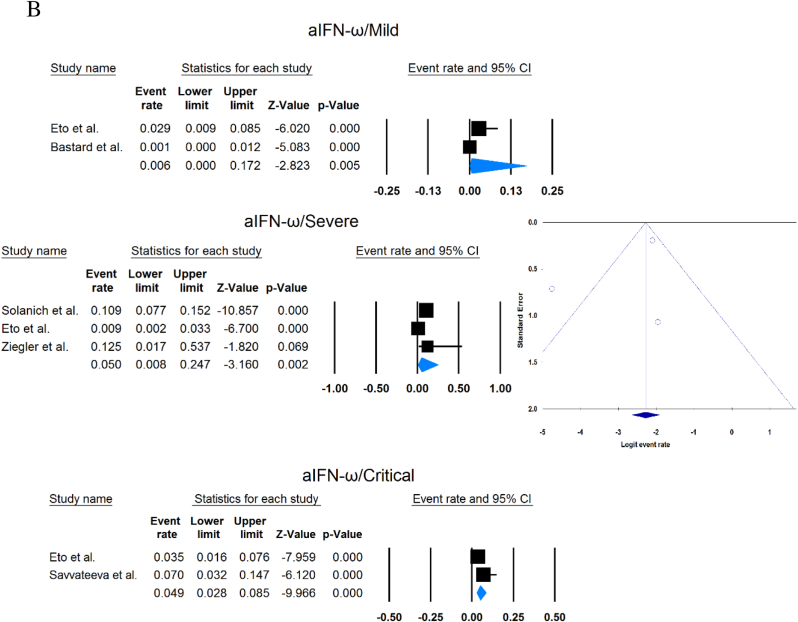
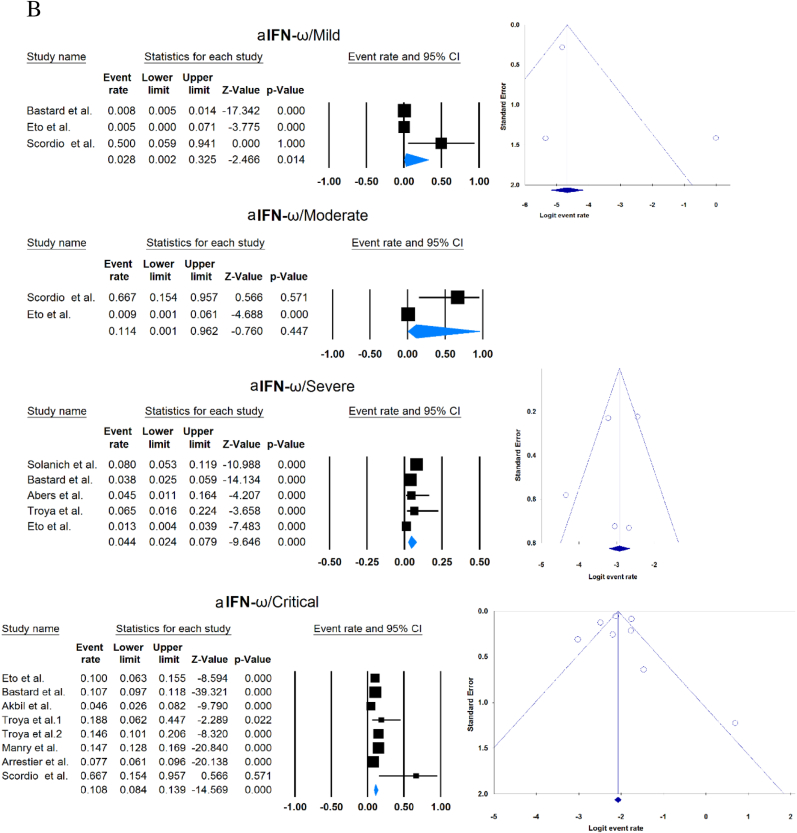
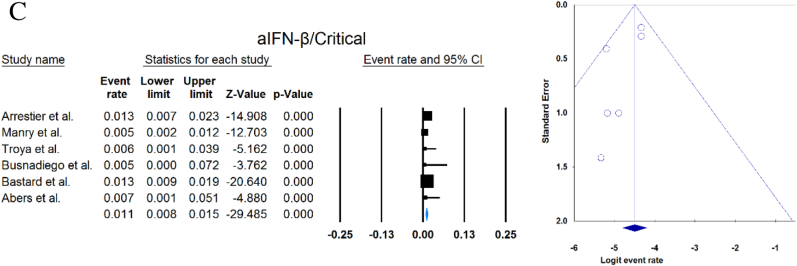
Prevalence of neutralizing autoantibodies against interferon α2 (A), ω (B), and β (C) among COVID -19 patients with different disease severity.
Fig. 5.
Overall prevalence of circulating aIFN-1 (A), aIFN-α (B), and aIFN-ω (C) autoantibodies regardless of disease severity.
Fig. 6.
Overall prevalence of neutralizing aIFN-1 (A), aIFN-α (B), aIFN-ω (C), and aIFN-β (D) autoantibodies regardless of disease severity.
Outcomes of COVID-19 in positive versus negative aIFN-1/α patients.
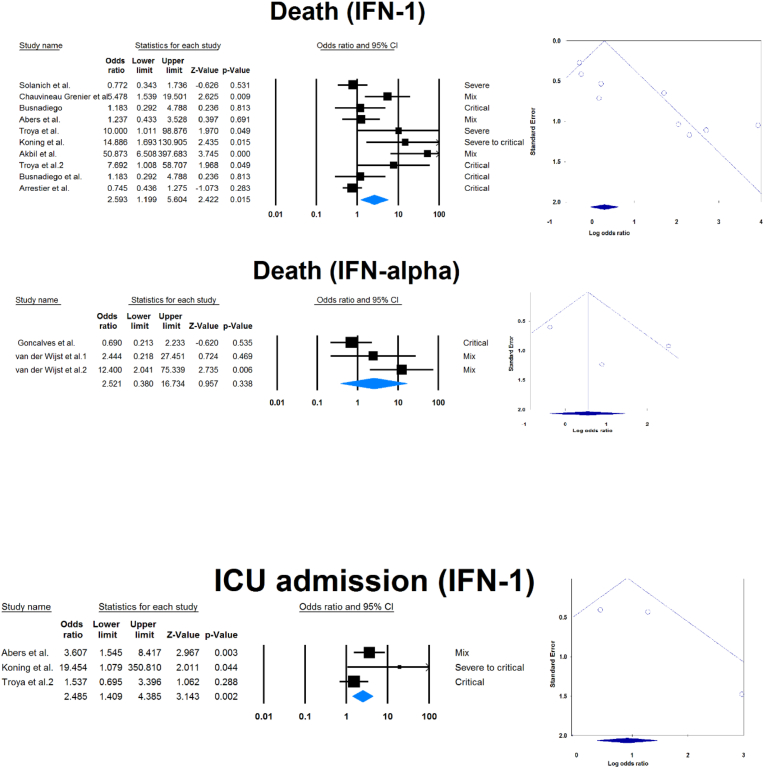
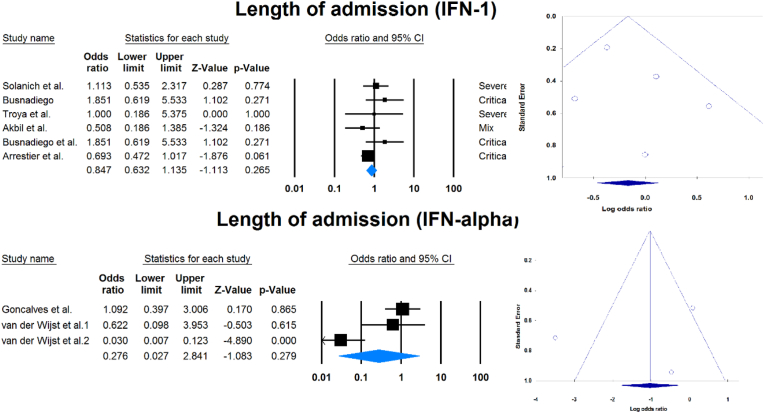
Fourteen studies compared patients with positive and negative AAbs to IFN-1 or IFN-α. Table 3 and Fig. 4 show the association between sex, death, ICU admission, and length of hospital stay with positive serum AAbs against IFN-1 or IFN-α.
Table 3.
Comparison of COVID -19 results in seropositive patients and serunegative patients.
| Variable | aIFN | Sample size (positive/negative) | OR [95 % CI] | P-value |
|---|---|---|---|---|
| Male | 1 | 365/3917 | 2.248 [1.366, 3.699] | 0.001 |
| α | 39/612 | 0.737 [0.326, 1.668] | 0.464 | |
| Death | 1 | 238/2316 | 2.593 [1.199, 5.604] | 0.015 |
| α | 26/343 | 2.521 [0.380, 16.734] | 0.338 | |
| ICU admission | 1 | 63/514 | 2.485 [1.409, 4.385] | 0.002 |
| Length of admission | 1 | 164/1692 | 0.847 [0.632, 1.135] | 0.265 |
| α | 26/343 | 0.276 [0.027, 2.841] | 0.279 |
ICU: Intensive care unit, IFN: Interferon, OR: Odds ratio, CI: confidence interval.
Fig. 4.
Association between sex, death, ICU admission, and length of hospital stay with positive serum autoantibodies against IFN-1 or IFN-α.
3.2. Moderate/mild versus critical/severe in terms of aIFN-1/α/ω
Meta-analysis showed that circulating aIFN-α was significantly more prevalent in critical/severe patients than in moderate/mild patients (OR = 4.537 [2.247, 9.158], p < 0.001, I2 = 0 %) (Fig. 7). Also, the prevalence of neutralizing aIFN-α was significantly higher in critical/severe patients than moderate/mild patients (OR = 17.482 [8.899, 34.342], p < 0.001, I2 = 34 %) (Fig. 8). The prevalence of neutralizing aIFN-ω was significantly higher in critical/severe than moderate/mild patients (OR = 12.529 [7.397, 21.222], p < 0.001, I2 = 11 %) (Fig. 9).
Fig. 7.
Circulating aIFN-α prevalence in critical/severe patients compared with moderate/mild patients.
Fig. 8.
Neutralizing aIFN-α prevalence in critical/severe patients compared with moderate/mild patients.
Fig. 9.
Circulating aIFN-ω prevalence in critical/severe patients compared with moderate/mild patients.
3.3. Qualitative report of comparison of plasma aIFN-α/ω levels
Soltani-Zangbar et al. reported higher aIFN-α2 levels in severe than mild patients [42], though three other studies showed no difference [33,47,48]. Three studies reported higher levels of aIFN-α2 among COVID-19 patients than in the control group [33,42,49], whereas two studies found no difference [47,48]. Furthermore, Chen et al. demonstrated higher aIFN-ω levels in severe patients than in mild patients [49], whereas Smith found no difference [47]. Only one study found no difference between aIFN-ω levels in COVID-19 patients and the control group [47]. Table 1 provides information on the characteristics of the studies reported.
3.4. Publication bias
Publication bias was assessed by visual inspection of the asymmetry of the funnel plot. The figures show no evidence of publication bias; however, for some subgroup analyses, there were insufficient studies to present proper funnel plots. Funnel plots are provided for all forest plots.
4. Discussion
This meta-analysis of 33 studies analyzed data from 13023 COVID-19 patients with respect to aIFN-1 AAbs. We estimated the pooled rate of seropositivity of circulating aIFN-1, aIFN-α, and aIFN-ω AAbs among COVID-19 patients to be approximately 17.8 % [13.8, 22.8], 7.2 % [4.7, 10.9], and 4.4 % [2.1, 8.6], and the rates of neutralizing aIFN-1, aIFN-α, aIFN-ω, and aIFN-β AAbs were 7.1 % [4.9, 10.1], 7.5 % [5.9, 9.5], 8.0 % [5.7, 11.1], and 1.2 % [0.4, 3.5], respectively. In contrast, the aIFN-α and aIFN-ω subtypes are present in about 0.3 % of the normal population [50]. The discrepancies in seropositivity rates reported by different studies can be attributed to the stage and severity of the disease, the measurement method and the characteristics of the patients. We performed a subgroup analysis in which patients were divided by COVID-19 disease severity that revealed significantly higher rates of aIFN-1, aIFN-α, and aIFN-ω in severe patients. A subsequent analysis provided further confirmation when patients were divided into severe/critical group or mild/moderate group, showed that the odds ratio of being positive for aIFN-α was significantly higher in the critical/severe group than in the mild/moderate group. One explanation for the high aIFN rate in severe patients is that IFN blockade provides an optimal environment for viral replication and disease progression [51]. Furthermore, patients with more severe disease are more prone to ICU admission and death, which we demonstrated that higher rates were associated with patients positive for aIFN-1 AAbs, though the association of IFN-1 AAbs with ICU admission and death may just be an epiphenomenon for disease severity. Our findings are supported by large cohorts and previous meta-analyses on this topic [50,52]. It has been suggested that aIFN-1, aIFN-α, and aIFN-ω AAbs may be appropriate biomarkers of disease severity and that their elimination by plasma exchange may improve clinical status [53]. However, IFN therapy has not been shown to improve mortality or length of hospital stay in COVID-19 patients [54]. Furthermore, length of hospital stay was not associated with aIFN-1 AAbs seropositivity, with no difference between positive and negative groups for IFN-α AAbs. However, the small size of the available sample may have led to a type 2 statistical error and a false negative result. The analysis also showed that aIFN-1 AAbs were more frequent in male subjects, which may explain previous findings of an increased incidence of severe disease in male subjects [55,56]. The recent meta-analysis by Wang et al. on this topic included 8 studies and concluded that AAbs against IFN-1 accumulate in 5 % of the total population and reach 10 % in severe patients. However, the prevalence of neutralizing and circulating AAbs was not reported separately [52]. In agreement with them, we found that aIFN-I AAbs were more prevalent in male subjects. Furthermore, although AAbs can decrease circulating levels of IFN-1 and its subtypes, a meta-analysis by Pires et al. showed that there were no significant differences in peripheral IFN-α in patients with different disease severity [57]. However, a significant difference was found between healthy control subjects and COVID -19 patients with mild disease.
Regarding the biomarker potential of aIFN AAbs, Beck et al. [58] suggested that an impaired IFN-1 response could be the hallmark of severe COVID-19. Zhang et al. also reported an association between SARS-CoV-2 infection and defects in genes regulating IFN-1 using extensive genetic sequencing [59]. There are two main hypotheses regarding the cause of an elevated aIFN-1. Firstly, AAbs facilitate viral replication because the host response system is neutralized by AAbs [19]. In support of this, Trouillet-Assant et al. [60] reported that a decreased plasma concentration of the IFN-1 cytokine predicts a poor prognosis and that patients with the lowest IFN-1 concentration have longer durations of admission. Secondly, viral evasion leads to the increased production of IFN-1, which contributes to the cytokine storm in COVID-19 disease [61], TNF-mediated inflammation [62], and high IFN-1 secretion impairs antibody production [63]. Massive production of IFN-1 leads to tissue damage, and autoantibodies may provide impaired protection against the cytokine storm [64]. In accordance with the second hypothesis, single-cell RNA-sequencing studies show hyperactivation of the IFN-1 pathway in severe patients, disrupting immune function, leading to overreaction and eventually resulting in organ damage [65,66]. Post-mortem analyzes of COVID-19 patients reveal a high concentration of IFN-1 in lung tissue [67]. Menezes et al. also demonstrated that higher levels of IFNB1 transcripts are found in the nasal mucosa of COVID-19 patients and predict a poor outcome [68].
IFN-1 are critical for controlling SARS-CoV-2 infection, which may explain the increased susceptibility of older adults with a reduced ability to secrete IFN-1 [59]. The Bodansky et al. study showed a low frequency of positive AAbs against IFN-α2, 0.4 % in children and adolescents [16], and sensitivity analysis on removing this young population in the Bodansky study showed a rate of 7.9 % [5.2, 11.8], indicating that aIFN-α2-AAbs mainly occur in older patients.
Our study had some limitations. Firstly, limited analysis of IFN-ω or aIFN-β levels could be performed as few studies reported them, increasing the risk of a false negative result. Secondly, another limitation is the low sensitivity of the assays used in some studies to measure aIFN-1 and its subtypes, which may have led to underreporting. Assessment of aIFN-1 in the post-COVID-19 phase may provide insights into the role of aIFN-1 AAbs in patients with long-COVID-19 and post-COVID-19 syndrome, and future studies should also investigate aIFN-γ AAbs [48].
5. Conclusion
In conclusion, aIFN-1 and its subtypes AAbs are associated with severe and critical COVID-19 disease and may be a predictive marker for a poor prognosis, particularly in men.
Credit author statement
Conceptualization: AA, AS. Investigation: AA, AH, MA, MMM, ZS, TJ, AS. Writing - Original Draft: AA, AH, MA, NNN. Writing - Review & Editing: TJ, AS.
Funding
No external fund was received for conducting this study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Y Renaudineau
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtauto.2023.100219.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- 1.Organization W.H. 2023. COVID-19 Weekly Epidemiological Update. [141:[ [Google Scholar]
- 2.Galani I.-E., Rovina N., Lampropoulou V., Triantafyllia V., Manioudaki M., Pavlos E., et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat. Immunol. 2021;22(1):32–40. doi: 10.1038/s41590-020-00840-x. [DOI] [PubMed] [Google Scholar]
- 3.Tajbakhsh A., Gheibi Hayat S.M., Taghizadeh H., Akbari A., Inabadi M., Savardashtaki A., et al. COVID-19 and cardiac injury: clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev. Anti-infect. Ther. 2021;19(3):345–357. doi: 10.1080/14787210.2020.1822737. [DOI] [PubMed] [Google Scholar]
- 4.Ghale R., Spottiswoode N., Anderson M.S., Mitchell A., Wang G., Calfee C.S., et al. Prevalence of type-1 interferon autoantibodies in adults with non-COVID-19 acute respiratory failure. Respir. Res. 2022;23(1):354. doi: 10.1186/s12931-022-02283-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Q., Pizzorno A., Miorin L., Bastard P., Gervais A., Le Voyer T., et al. Autoantibodies against type I IFNs in patients with critical influenza pneumonia. J. Exp. Med. 2022;219(11) doi: 10.1084/jem.20220514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abers M.S., Rosen L.B., Delmonte O.M., Shaw E., Bastard P., Imberti L., et al. Neutralizing type-I interferon autoantibodies are associated with delayed viral clearance and intensive care unit admission in patients with COVID-19. Immunol. Cell Biol. 2021;99(9):917–921. doi: 10.1111/imcb.12495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yee D., Tso M., Shaw E., Rosen L., Samuels E., Bastard P., et al., editors. Type I Interferon Autoantibodies Are Detected in Those with Critical COVID-19, Including a Young Female Patient. vol. 450. Open Forum Infectious Diseases; 2021. [Google Scholar]
- 8.Bekisz J., Schmeisser H., Hernandez J., Goldman N.D., Zoon K.C. Mini ReviewHuman interferons alpha, beta and omega. Growth Factors. 2004;22(4):243–251. doi: 10.1080/08977190400000833. [DOI] [PubMed] [Google Scholar]
- 9.Goncalves D., Mezidi M., Bastard P., Perret M., Saker K., Fabien N., et al. Antibodies against type I interferon: detection and association with severe clinical outcome in COVID-19 patients. Clinical & translational immunology. 2021;10(8):e1327. doi: 10.1002/cti2.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frasca F., Scordio M., Santinelli L., Gabriele L., Gandini O., Criniti A., et al. Anti-IFN-α/-ω neutralizing antibodies from COVID-19 patients correlate with downregulation of IFN response and laboratory biomarkers of disease severity. Eur. J. Immunol. 2022;52(7):1120–1128. doi: 10.1002/eji.202249824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Troya J., Bastard P., Casanova J.-L., Abel L., Pujol A. Low lymphocytes and IFN-neutralizing autoantibodies as biomarkers of COVID-19 mortality. J. Clin. Immunol. 2022;42(4):738–741. doi: 10.1007/s10875-022-01241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casanova J.L., Anderson M.S. Unlocking life-threatening COVID-19 through two types of inborn errors of type I IFNs. J. Clin. Invest. 2023;133(3) doi: 10.1172/JCI166283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemarquis A., Campbell T., Aranda-Guillén M., Hennings V., Brodin P., Kämpe O., et al. Severe COVID-19 in an APS1 patient with interferon autoantibodies treated with plasmapheresis. J. Allergy Clin. Immunol. 2021;148(1):96–98. doi: 10.1016/j.jaci.2021.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., et al. vol. 5. Joanna briggs institute reviewer's manual The Joanna Briggs Institute; 2017. (Chapter 7: Systematic Reviews of Etiology and Risk). [Google Scholar]
- 16.Bodansky A., Vazquez S.E., Chou J., Novak T., Al-Musa A., Young C., et al. NFKB2 haploinsufficiency identified via screening for IFN-α2 autoantibodies in children and adolescents hospitalized with SARS-CoV-2-related complications. J. Allergy Clin. Immunol. 2023;151(4) doi: 10.1016/j.jaci.2022.11.020. 926-30.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14(1):135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed) 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., Zhang Y., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science (New York, NY) 2020;370(6515) doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaw E.R., Rosen L.B., Cheng A., Dobbs K., Delmonte O.M., Ferré E.M.N., et al. Temporal dynamics of anti–type 1 interferon autoantibodies in patients with coronavirus disease 2019. Clin. Infect. Dis. 2022;75(1):e1192–e1194. doi: 10.1093/cid/ciab1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Wijst M.G.P., Vazquez S.E., Hartoularos G.C., Bastard P., Grant T., Bueno R., et al. 2021. Longitudinal Single-Cell Epitope and RNA-Sequencing Reveals the Immunological Impact of Type 1 Interferon Autoantibodies in Critical COVID-19. bioRxiv : the Preprint Server for Biology. [Google Scholar]
- 22.Matuozzo D., Talouarn E., Marchal A., Zhang P., Manry J., Seeleuthner Y., et al. Rare predicted loss-of-function variants of type I IFN immunity genes are associated with life-threatening COVID-19. Genome Med. 2023;15(1):22. doi: 10.1186/s13073-023-01173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chauvineau-Grenier A., Bastard P., Servajean A., Gervais A., Rosain J., Jouanguy E., et al. Autoantibodies neutralizing type I interferons in 20% of COVID-19 deaths in a French hospital. J. Clin. Immunol. 2022;42(3):459–470. doi: 10.1007/s10875-021-01203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez J., Mommert M., Mouton W., Pizzorno A., Brengel-Pesce K., Mezidi M., et al. Early nasal type I IFN immunity against SARS-CoV-2 is compromised in patients with autoantibodies against type I IFNs. J. Exp. Med. 2021;(10):218. doi: 10.1084/jem.20211211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manry J., Bastard P., Gervais A., Le Voyer T., Rosain J., Philippot Q., et al. The risk of COVID-19 death is much greater and age dependent with type I IFN autoantibodies. Proc. Natl. Acad. Sci. USA. 2022;119(21) doi: 10.1073/pnas.2200413119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carapito R., Li R., Helms J., Carapito C., Gujja S., Rolli V., et al. Identification of driver genes for critical forms of COVID-19 in a deeply phenotyped young patient cohort. Sci. Transl. Med. 2022;14(628) doi: 10.1126/scitranslmed.abj7521. [DOI] [PubMed] [Google Scholar]
- 27.Mathian A., Breillat P., Dorgham K., Bastard P., Charre C., Lhote R., et al. Lower disease activity but higher risk of severe COVID-19 and herpes zoster in patients with systemic lupus erythematosus with pre-existing autoantibodies neutralising IFN-α. Ann. Rheum. Dis. 2022;81(12):1695–1703. doi: 10.1136/ard-2022-222549. [DOI] [PubMed] [Google Scholar]
- 28.Arrestier R., Bastard P., Belmondo T., Voiriot G., Urbina T., Luyt C.-E., et al. Auto-antibodies against type I IFNs in > 10% of critically ill COVID-19 patients: a prospective multicentre study. Ann. Intensive Care. 2022;12(1):121. doi: 10.1186/s13613-022-01095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solanich X., Rigo-Bonnin R., Gumucio V.D., Bastard P., Rosain J., Philippot Q., et al. Pre-existing autoantibodies neutralizing high concentrations of type I interferons in almost 10% of COVID-19 patients admitted to intensive care in barcelona. J. Clin. Immunol. 2021;41(8):1733–1744. doi: 10.1007/s10875-021-01136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Troya J., Bastard P., Planas-Serra L., Ryan P., Ruiz M., de Carranza M., et al. Neutralizing autoantibodies to type I IFNs in >10% of patients with severe COVID-19 pneumonia hospitalized in Madrid, Spain. J. Clin. Immunol. 2021;41(5):914–922. doi: 10.1007/s10875-021-01036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scordio M., Frasca F., Santinelli L., Sorrentino L., Pierangeli A., Turriziani O., et al. High frequency of neutralizing antibodies to type I Interferon in HIV-1 patients hospitalized for COVID-19. Clin. Immunol. 2022;241 doi: 10.1016/j.clim.2022.109068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koning R., Bastard P., Casanova J.-L., Brouwer M.C., van de Beek D., van Agtmael M., et al. Autoantibodies against type I interferons are associated with multi-organ failure in COVID-19 patients. Intensive Care Med. 2021;47(6):704–706. doi: 10.1007/s00134-021-06392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raadsen M.P., Gharbharan A., Jordans C.C.E., Mykytyn A.Z., Lamers M.M., van den Doel P.B., et al. Interferon-α2 auto-antibodies in convalescent plasma therapy for COVID-19. J. Clin. Immunol. 2022;42(2):232–239. doi: 10.1007/s10875-021-01168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steels S., Van Elslande J., Cockx M., Frans G., Imbrechts M., Dillaerts D., et al. Transient increase of pre-existing anti-IFN-α2 antibodies induced by SARS-CoV-2 infection. J. Clin. Immunol. 2022;42(4):742–745. doi: 10.1007/s10875-022-01235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Busnadiego I., Abela I.A., Frey P.M., Hofmaenner D.A., Scheier T.C., Schuepbach R.A., et al. Critically ill COVID-19 patients with neutralizing autoantibodies against type I interferons have increased risk of herpesvirus disease. PLoS Biol. 2022;20(7) doi: 10.1371/journal.pbio.3001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Wijst M.G.P., Vazquez S.E., Hartoularos G.C., Bastard P., Grant T., Bueno R., et al. Type I interferon autoantibodies are associated with systemic immune alterations in patients with COVID-19. Sci. Transl. Med. 2021;13(612) doi: 10.1126/scitranslmed.abh2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang S.E., Feng A., Meng W., Apostolidis S.A., Mack E., Artandi M., et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat. Commun. 2021;12(1):5417. doi: 10.1038/s41467-021-25509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang E.Y., Mao T., Klein J., Dai Y., Huck J.D., Jaycox J.R., et al. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595(7866):283–288. doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]
- 39.Vazquez S.E., Bastard P., Kelly K., Gervais A., Norris P.J., Dumont L.J., et al. Neutralizing autoantibodies to type I interferons in COVID-19 convalescent donor plasma. J. Clin. Immunol. 2021;41(6):1169–1171. doi: 10.1007/s10875-021-01060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ziegler C.G.K., Miao V.N., Owings A.H., Navia A.W., Tang Y., Bromley J.D., et al. Impaired local intrinsic immunity to SARS-CoV-2 infection in severe COVID-19. Cell. 2021;184(18) doi: 10.1016/j.cell.2021.07.023. 4713-33.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Acosta-Ampudia Y., Monsalve D.M., Rojas M., Rodríguez Y., Gallo J.E., Salazar-Uribe J.C., et al. COVID-19 convalescent plasma composition and immunological effects in severe patients. J. Autoimmun. 2021;118 doi: 10.1016/j.jaut.2021.102598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soltani-Zangbar M.S., Parhizkar F., Ghaedi E., Tarbiat A., Motavalli R., Alizadegan A., et al. A comprehensive evaluation of the immune system response and type-I Interferon signaling pathway in hospitalized COVID-19 patients. Cell Commun. Signal. 2022;20(1):106. doi: 10.1186/s12964-022-00903-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savvateeva E., Filippova M., Valuev-Elliston V., Nuralieva N., Yukina M., Troshina E., et al. Microarray-based detection of antibodies against SARS-CoV-2 proteins, common respiratory viruses and type I interferons. Viruses. 2021;13(12):2553. doi: 10.3390/v13122553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eto S., Nukui Y., Tsumura M., Nakagama Y., Kashimada K., Mizoguchi Y., et al. Neutralizing type I interferon autoantibodies in Japanese patients with severe COVID-19. J. Clin. Immunol. 2022;42(7):1360–1370. doi: 10.1007/s10875-022-01308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akbil B., Meyer T., Stubbemann P., Thibeault C., Staudacher O., Niemeyer D., et al. Early and rapid identification of COVID-19 patients with neutralizing type I interferon auto-antibodies. J. Clin. Immunol. 2022;42(6):1111–1129. doi: 10.1007/s10875-022-01252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bastard P., Gervais A., Le Voyer T., Rosain J., Philippot Q., Manry J., et al. Autoantibodies neutralizing type I IFNs are present in ∼4% of uninfected individuals over 70 years old and account for ∼20% of COVID-19 deaths. Science immunology. 2021;6(62) doi: 10.1126/sciimmunol.abl4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith N., Possémé C., Bondet V., Sugrue J., Townsend L., Charbit B., et al. Defective activation and regulation of type I interferon immunity is associated with increasing COVID-19 severity. Nat. Commun. 2022;13(1):7254. doi: 10.1038/s41467-022-34895-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen P.K., Yeo K.J., Chang S.H., Liao T.L., Chou C.H., Lan J.L., et al. The detectable anti-interferon-γ autoantibodies in COVID-19 patients may be associated with disease severity. Virol. J. 2023;20(1):33. doi: 10.1186/s12985-023-01989-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simula E.R., Manca M.A., Noli M., Jasemi S., Ruberto S., Uzzau S., et al. Increased presence of antibodies against type I interferons and human endogenous retrovirus W in intensive care unit COVID-19 patients. Microbiol. Spectr. 2022;10(4) doi: 10.1128/spectrum.01280-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bastard P., Gervais A., Le Voyer T., Rosain J., Philippot Q., Manry J., et al. Autoantibodies neutralizing type I IFNs are present in ∼4% of uninfected individuals over 70 years old and account for ∼20% of COVID-19 deaths. Science immunology. 2021;6(62) doi: 10.1126/sciimmunol.abl4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fajgenbaum D.C., Hayday A.C., Rogers A.J., Towers G.J., Wack A., Zanoni I. Anti-type I interferon antibodies as a cause of severe COVID-19. Faculty reviews. 2022;11:15. doi: 10.12703/r-01-0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X., Tang Q., Li H., Jiang H., Xu J., Bergquist R., et al. Autoantibodies against type I interferons in COVID-19 infection: a systematic review and meta-analysis. Int. J. Infect. Dis. 2023;130:147–152. doi: 10.1016/j.ijid.2023.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Prost N., Bastard P., Arrestier R., Fourati S., Mahévas M., Burrel S., et al. Plasma exchange to rescue patients with autoantibodies against type I interferons and life-threatening COVID-19 pneumonia. J. Clin. Immunol. 2021;41(3):536–544. doi: 10.1007/s10875-021-00994-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akbari A., Razmi M., Sedaghat A., Alavi Dana S.M.M., Amiri M., Halvani A.M., et al. Comparative effectiveness of pharmacological interventions on mortality and the average length of hospital stay of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. Expert Rev. Anti-infect. Ther. 2022;20(4):585–609. doi: 10.1080/14787210.2022.1997587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akbari A., Fathabadi A., Razmi M., Zarifian A., Amiri M., Ghodsi A., et al. Characteristics, risk factors, and outcomes associated with readmission in COVID-19 patients: a systematic review and meta-analysis. Am. J. Emerg. Med. 2022;52:166–173. doi: 10.1016/j.ajem.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peckham H., de Gruijter N.M., Raine C., Radziszewska A., Ciurtin C., Wedderburn L.R., et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020;11(1):6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.da Silva R.P., Gonçalves J.I.B., Zanin R.F., Schuch F.B., de Souza A.P.D. Circulating type I interferon levels and COVID-19 severity: a systematic review and meta-analysis. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.657363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beck D.B., Aksentijevich I. Susceptibility to severe COVID-19. Science (New York, NY) 2020;370(6515):404–405. doi: 10.1126/science.abe7591. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science (New York, NY) 2020;370(6515) doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trouillet-Assant S., Viel S., Gaymard A., Pons S., Richard J.C., Perret M., et al. Type I IFN immunoprofiling in COVID-19 patients. J. Allergy Clin. Immunol. 2020;146(1) doi: 10.1016/j.jaci.2020.04.029. 206-8.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nile S.H., Nile A., Qiu J., Li L., Jia X., Kai G. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huys L., Van Hauwermeiren F., Dejager L., Dejonckheere E., Lienenklaus S., Weiss S., et al. Type I interferon drives tumor necrosis factor-induced lethal shock. J. Exp. Med. 2009;206(9):1873–1882. doi: 10.1084/jem.20090213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Costa Silva R.C.M., Bandeira-Melo C., Paula Neto H.A., Vale A.M., Travassos L.H. COVID-19 diverse outcomes: aggravated reinfection, type I interferons and antibodies. Med. Hypotheses. 2022;167 doi: 10.1016/j.mehy.2022.110943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ku C.-L., Chi C.-Y., von Bernuth H., Doffinger R. Autoantibodies against cytokines: phenocopies of primary immunodeficiencies? Hum. Genet. 2020;139(6):783–794. doi: 10.1007/s00439-020-02180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J.-Y., Wang X.-M., Xing X., Xu Z., Zhang C., Song J.-W., et al. Single-cell landscape of immunological responses in patients with COVID-19. Nat. Immunol. 2020;21(9):1107–1118. doi: 10.1038/s41590-020-0762-x. [DOI] [PubMed] [Google Scholar]
- 66.Lulin H., Yi S., Bo G., Li J., Xiaoqi L., Jialiang Y., et al. Blood single cell immune profiling reveals the interferon-MAPK pathway mediated adaptive immune response for COVID-19. medRxiv. 2020;2020 03.15.20033472. [Google Scholar]
- 67.Lee J.S., Park S., Jeong H.W., Ahn J.Y., Choi S.J., Lee H., et al. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Science immunology. 2020;5(49) doi: 10.1126/sciimmunol.abd1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Menezes S.M., Braz M., Llorens-Rico V., Wauters J., Van Weyenbergh J. Endogenous IFNβ expression predicts outcome in critical patients with COVID-19. The Lancet Microbe. 2021;2(6):e235–e236. doi: 10.1016/S2666-5247(21)00063-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.