Abstract
Introduction
Transvaginal radiofrequency ablation is a relatively noninvasive approach for the treatment of fibroids in patients who do not wish to undergo conventional surgery. Information on potential complications of this novel technique is very scarce.
Methods
Retrospective, descriptive, epidemiological study of 115 patients who underwent transvaginal radiofrequency ablation of fibroids and for whom complications were recorded.
Results
We performed 115 transvaginal radiofrequency ablation procedures, we recorded a total of 11 complications (9.6%; 95% CI, 3.8–14.8). Of these, 8 (7.0%) were classified as Clavien-Dindo type I, 1 (0.9%,) as type II, and 2 (1.7%) as type IIIb (severe). No other complications were recorded in a year follow-up.
Conclusion
Transvaginal radiofrequency ablation is a treatment option that makes it possible to treat fibroids that are difficult to manage using other techniques. Few associated complications have been described, and most of them are mild.
Keywords: Complications, Leiomyoma, Radiofrequency ablation, Transvaginal radiofrequency ablation, Fibroid
Highlights
-
•
Transvaginal radiofrequency ablation is a treatment option for fibroids.
-
•
Few associated complications have been described most of them mild.
-
•
It is advisable to follow some rules to ensure the safety of the technique.
Introduction
Uterine fibroids are one of the most frequent causes of consultation in gynecology [1], [2]. Surgical management of fibroids includes hysterectomy, myomectomy or uterine artery embolization for symptomatic fibroids. In the last few decades, there is an increasing number of patients who decide to postpone gestation until a later age, which means that the demand for other non-aggressive surgical techniques, such as Radiofrequency ablation (RFA), that preserve the uterus for future pregnancies, is increased [3], [4]. RFA is a new technique for reducing the fibroids´ volume and its associated symptoms by producing a coagulative necrosis within the fibroid [5].
RFA can be applied by different accesses such as laparoscopic, hysteroscopic or transvaginal [6]. Transvaginal RFA (TRFA) can be an alternative that does not require hospital admission, is very well tolerated and has a short recovery period after treatment. Evidence related to TRFA in relation to clinical practice is consistent, but follow-up periods are short to detect long-term complications [7], [8], [9], [10].
The objective of the present study was to describe the complications recorded after 115 cases of transvaginal radiofrequency ablation of fibroids during the last 5 years of experience.
Material and methods
Type of study
We performed a retrospective, descriptive, epidemiologic study of 115 patients who underwent transvaginal radiofrequency ablation of fibroids at Hospital Universitario Virgen de las Nieves, Granada, Spain between June 2018 and March 2022.
Study population
The study population comprised patients who experienced complications after TRFA of myomas. The inclusion criteria were as follows: International Federation of Gynecology and Obstetrics (FIGO) [11] type 0–4 symptomatic fibroids up to 7 cm that could not be treated using another surgical technique, the patient refused to undergo surgery or if medical treatment was unable to control symptoms; FIGO type 0–4 fibroids in patients undergoing assisted reproduction techniques and hysteroscopic myomectomy was not considered feasible (group II and III fibroids of the STEPW classification of Lasmar et al.) [12]. Exclusion criteria were more than 3 fibroids, FIGO type 5 or 6, > 7 cm, suspicion of malignancy, and conditions that contraindicated general or epidural anesthesia.
Description of the procedure
A VIVA RF System (STARmed Co., JJP Hospitalaria S.L., Seville, Spain) radiofrequency generator was used with a 17 G (STARmed) RF fixed coagulation needle electrode (reference number 17 −35s30F) measuring 35 cm in length with a 1-cm active tip with a continuous infusion pump to cool the circuit. The generator operates at 480 kHz and heats the electrode at the tip of the needle to between 60°C and 90°C, causing coagulation necrosis of proteins within the fibroid (Fig. 1). The maximum power of the generator was 200 W; a maximum power of 150 W was used for all procedures in our study. An intraoperative biopsy specimen was obtained for pathology studies using a Primecut 2 biopsy needle (16 G, 200 mm long; code number PRIM 162002; Tokyo Seimitsu Kan Co., Ltd., Tokyo, Japan).
Fig. 1.
Radiofrequency generator and coagulation needle electrode.
Preoperative studies included clinical evaluation of the symptoms and ultrasound examination to determine the number of fibroids, position, size, and distance between the fibroid pseudocapsule and uterine serosa. Blood count, coagulation assay, and preoperative evaluation were also included. All women were informed in detail about the efficacy, risks, and benefits of the radiofrequency technique, and they all provided written informed consent.
TRFA was performed under general or epidural anesthesia, prior administration of prophylaxis with intravenous cefotaxime 2 g in an outpatient setting. The needle electrode was inserted under ultrasonographic visualization via the anterior or posterior fornix of the vagina until the tip was located within the fibroid, 0.5 cm deep to the pseudo capsule. In each of the ablation shots, the power released and the change in tissue impedance were displayed on the radiofrequency generator screen, and a permanent change in the echogenicity of the treated tissue was seen in the ultrasound image (ie, it becomes hyperechoic). After each target area was treated, the needle was moved to an adjacent, untreated part of the fibroid, and the ablation process was repeated. The procedure was considered complete when a change in echogenicity was detected in approximately 80% of the fibroid volume (Fig. 2). Appropriate safety measures were taken to avoid thermal damage (Fig. 3). After the procedure, all women remained under observation for 4 h previous to discharge. They were advised to take NSAIDs on demand.
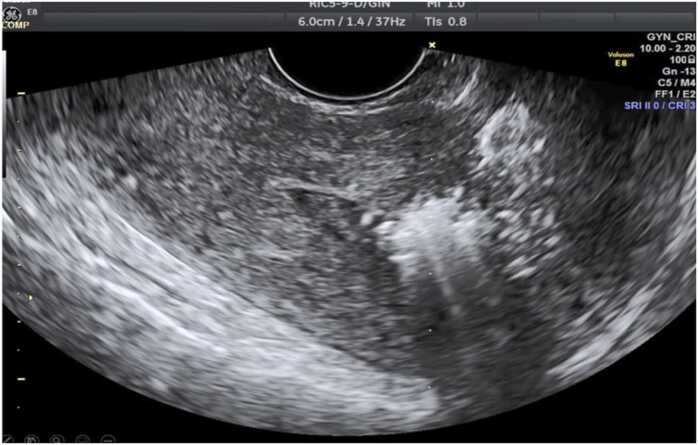
Fig. 2.
Sagital view of the uterus in a transvaginal ultrasound. Notice the chage in ecogenicity after the treatment with Radiofrecuency to the myoma.
Fig. 3.
Measurement of the distance between the myoma capsule and the uterine serous membrane to identify areas at risk of injury (left). Establishing the limits of the myoma with respect to the biopsy line to avoid thermal injury. The radiofrequency ablation traces (in white) start 0.5 cm from the capsule (right).
Approval for the study was provided by the Biomedical Research Ethics Committee of Andalusia (Spain).
Outcome measures
The study variables included patient’s age, main cause for consultation, clinical evaluation of symptoms before the procedure (total number of days of menstrual bleeding), characteristics of the fibroid: volume (cc) and the FIGO type (0−7).
Intraoperative and postoperative complications appearing up to 30 days after the procedure were classified according to the Clavien-Dindo scale [13]. The patients were asked about pain during the postoperative period (Likert-type scale from 1 to 5). Scheduled visits were recorded at 2, 6, and 12 months. The variables collected were: days taking analgesics at home after surgery, days of sick leave, volume of the fibroid by ultrasound, total duration of menstrual bleeding, subjective evaluation of the reduction in menstrual bleeding after treatment, and other adverse effect observed. It was also calculated the relative reduction in volume at 6 months (initial volume–volume at 6 months/initial volume).
Data collection
Data were collected systematically using questionnaires. A protocol was designed to collect sociodemographic and clinical data, as well as variables associated with the fibroid and complications. Information relative to complications was also retrieved from the clinical history. Imaging data for the most severe complications occurring in the study population were also recorded.
Statistical analysis
With all the gathered information, a database was set up for subsequent statistical analysis. A descriptive analysis of the collected variables was conducted, using a frequency distribution for qualitative variables and means and standard deviations for quantitative ones. Furthermore, two comparison groups were formed based on whether the patients had experienced complications or not. For the comparison of both groups, the chi-square test or Fisher's test was used for qualitative variables, and the Student's t-test or the Mann-Whitney U-test for quantitative variables. A significance level of p < 0.05 was established for all the performed statistical tests. Additionally, we conducted an individual analysis for each patient with complications, creating a table containing data concerning the type and size of the myoma, along with a description of each complication and the corresponding treatment.
Results
After 115 initial transvaginal radiofrequency ablation sessions, we recorded 11 complications (9.6% [95% CI, 3.8%−14.8%]). Table 1 compares the characteristics of women with and without complications after the procedure. There were no differences in mean age or in the presenting symptoms, the most frequent of which was bleeding. Similarly, no significant differences between the 2 groups were recorded related to days of bleeding before the technique. The mean presurgical fibroid volume was higher in patients with complications (68.3 cc vs 34.8 cc; p < 0.05). Volume decreased 6 months after surgery in both groups, but the final volume was lower in patients who did not experience complications (p < 0.05). No differences were found between the groups for the relative reduction in volume at 6 months (53.1% vs 63.5%), or in the subjective perception of the reduction in bleeding at 6 months (48.2% vs 52.3%) in those with and without complications respectively, although the number of days of bleeding was significantly greater in women who did not experience complications.
Table 1.
General patient characteristics and outcome of transvaginal radiofrequency ablation of myomas.
| No complications |
Complications |
||
|---|---|---|---|
| n = 104 | n = 11 | p | |
| Mean age, mean (SD) | 42.4 (5.4) | 42.5 (7.3) | NS |
| Reason for consultation: | |||
|
88 (84.6%) | 10 (90.9%) | NS |
|
16 (15.4%) | 1 (9.1%) | |
| Previous total days of menstrual bleeding, mean (SD): | 9.2 (4.8) | 7.6 (2.2) | NS. |
|
5.8 (2.1) | 4.7 (1.4) | < 0.05 |
| Percentage of subjective reduction in bleeding, mean (SD) | 52.3 (24.3) | 48.2 (29.9) | NS |
| Initial mean myoma volume (cc), mean (SD) | 34.8 (34.6) | 68.3 (50.2) | 0.05 |
|
12.7 (24.2) | 32 (51.5) | 0.03 |
|
63.5% | 53.1% | NS |
| FIGO type: | |||
| 0 | 3 (2.9%) | 1 (9.1%) | NS |
| 1 | 3 (2.9%) | 0 (0%) | |
| 2 | 36 (34.6%) | 5 (45.5%) | |
| 3 | 51 (49.0%) | 3 (27.3%) | |
| 4 | 11 (10.6%) | 2 (18.2%) | |
| Pain after radiofrequency ablation (scale, 1–5) | 2.1 (1.2) | 2.8 (1.3) | NS |
| Time with analgesic treatment (days), mean (SD) | 4.1 (5.1) | 9.3 (6.5) | 0.03 |
| Sick leave (days), mean (SD). | 6.3 (7.0) | 9.3 (6.5) | NS |
The mean pain score for patients with complications was 2.8/5 compared with 2.1/5 in patients without complications (p: ns). The number of days of sick leave and the average number of days with analgesic treatment was greater in patients who experienced complications (p < 0.03).
Table 2 summarizes the characteristics of patients who developed complications. Of the 11 cases affected, 8 involved mild complications (Clavien-Dindo type I). Two patients developed fever, 2 expelled necrotic debris, 1 had diarrhea, 1 experienced a complication associated with the epidural anesthetic, and 3 complained of low back pain.
Table 2.
General characteristics of myomas and complications in patients who underwent transvaginal radiofrequency ablation.
| Case | Type of myoma (FIGO) | Previous volume (cc) | Volume at 6 months (cc) | Clavien-Dindo | Type of complication | Time to onset | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | 4 | 172 | 84 | I | Fever | 2 days | Antipyretics |
| 2 | 0 | 33 | 0 | I | Discharge of debris | 7 days | None |
| 3 | 2 | 26 | 14 | I | Diarrhea | 2 days | Diet |
| 4 | 2 | 20 | 24 | IIIb | Intestinal perforation | 3 days | Surgery. Intestinal resection |
| 5 | 3 | 15 | 3 | I | Dural puncture (wet tap) | Immediate | Analgesics and corticosteroids |
| 6 | 2 | 94 | 19 | I | Low back pain | Immediate | Analgesics |
| 7 | 2 | 72 | 1,6 | I | Discharge of debris | 25 days | None |
| 8 | 3 | 73 | 0 | II | Urinary tract infection + fever | 7 days | Antibiotics and antipyretics |
| 9 | 2 | 136 | 167 | I | Low back pain | Immediate | Analgesics |
| 10 | 3 | 37 | 0 | I | Low back pain | Immediate | Analgesics |
| 11 | 4 | 73 | 41 | IIIb | Hemoperitoneum | Immediate | Laparoscopic surgery |
One urinary tract infection was detected (Clavien-Dindo type II) requiring oral antibiotic therapy. There were two severe complications (Clavien-Dindo type IIIb) that had to be resolved with surgical treatment. One case involved an intestinal perforation during the procedure that required further surgery and intestinal resection (Fig. 4) and other involved hemoperitoneum immediately after surgery for bleeding of the uterine serosa that needed laparoscopy-guided local coagulation (Fig. 5). No other complications were recorded during the monitoring period (12 months).
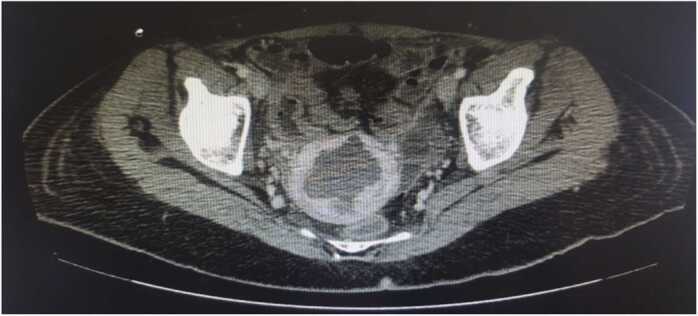
Fig. 4.
CT scan showing the extension of the thermal lesion beyond the serous membrane leading to an intestinal lesion.
Fig. 5.
Laparoscopy image showing foci of peritoneal and uterine bleeding after radiofrequency ablation of myomas.
Discussion
Our experience shows that TRFA is an effective approach for the management fibroids that are difficult to treat using other techniques with few complications recorded, most of them mild in comparison with other techniques such as surgical myomectomy and uterine artery embolization [14], [15]. However, less cumulative experience is available, the total number of cases treated is lower and therefore, our data must be interpreted with caution until confirmed in studies with larger samples.
Successful implementation of a new treatment technique depends on a knowledge of potential post procedure complications and outcomes, and while radiofrequency is widely used to treat tumors, little information is available on potential complications. Most publications on treatment of fibroids focus on radiofrequency administered via high-intensity focused ultrasound–guided (HIFU), magnetic resonance–guided [16], [17] or laparoscopic RFA [18], [19], [20] but few on TRFA in the treatment of fibroids [9], [17]. In 2017, Rey et al. [10] reported 2 cases of a fibroid remaining in the uterine cavity as the only complication (out of 205 procedures), resolved by hysterectomy at 30 and 45 days after the initial intervention.
In a series comprising 60 TRFA, our group [9] reported one intestinal perforation requiring surgery and intestinal resection. Other authors recently published a case of intestinal perforation managed in the same way [21].
Most of the complications in our series (epidural anesthetic–related, diarrhea, urinary tract infection, and low back pain) may not be specifically associated with the radiofrequency technique, as they are common in any surgical procedure.
The overall frequency of adverse events following epidural anesthesia ranges from 0.2% to 2.68% [22], [23]. One patient experienced complication associated with epidural anesthesia (vomiting and headache) that resolved spontaneously with oral treatment. Three patients reported low back pain during the days following the procedure. In all three cases the pain was managed with oral analgesics administered at home. The possible causes of low back pain include neurologic or muscular involvement (20–30%) resulting from the lithotomy position [24], associated with epidural anesthesia [25], [26], and with the coagulative necrosis of the fibroid as a consequence of TRFA. Low back pain was more frequent in women with large fibroids (37.4 cc, 94.4 cc, and 136 cc) suggesting an association with necrosis of the fibroid. Nevertheless, this should be further investigated in larger scale studies.
Two patients developed fever 2–3 days after treatment. One women took oral antipyretics at home and the other had mild leukocytosis with normal physical examination, abdominal ultrasound, and urine culture and resolved in a few days. In similar procedures, such as HIFU [27] or uterine artery embolization [28], there have been reports of Systemic Inflammatory Response Syndrome secondary to massive necrosis of the fibroid tissue with fever and leukocytosis, not reported to date in TRFA. The risk factors for this complication include high volume of fibroids treated (172 cc and 73 cc respectively). Our group does not recommend treating fibroids measuring more than 110 cc or more than 3 fibroids during the same procedure to avoid this complication. Patients with such large fibroids could probably benefit from other techniques, such as surgery or uterine artery embolization. Some authors recommend NSAIDs or corticosteroids for prevention and treatment of this syndrome [29], [30], [31] but their use in TRFA has not been evaluated.
One patient reported a history of mild diarrhea 48 h after the procedure. We do not know if this was associated with the treatment itself or with the anesthetic, antibiotics, or analgesics administered during treatment.
Two patients reported transvaginal discharge of fibroid debris during the days following the procedure (10–12 days) similar to what is found in uterine artery embolization [32], [33] or hysteroscopy [34], in which the fibroid is only partially removed and the debris is composed of the necrotic fragments. In both cases, the fibroid had a major submucosal component (FIGO type 0 of 33 cc and type 1 of 72 cc).
We recorded 2 severe complications. One patient had a thermal lesion of the intestine that required surgery and resection (Fig. 4). This type of complication has been reported in patients undergoing radiofrequency treatment for other conditions [35], [36], [37], [38], and HIFU-guided RFA [39] and one case for TRFA [17]. The intestinal lesion may be due to direct puncture, with immediate onset of symptoms, or, more commonly, to a thermal lesion, which appears later. In their review of 9988 cases treated with HIFU-guided radiofrequency, Chen et al. [40] reported this complication in 0.06% of patients. Intestinal lesion is operator-dependent, since it relies on the ultrasound image and the positioning of the needle during treatment, considering patient-dependent factors such as obesity, previous scars, and the presence of multiple fibroids, where shadows can prevent identifying its capsule. It is therefore important to use a high-quality device that provides a clear ultrasound image, to identify the limits of the fibroid and the uterus, measuring the distance to the serosa in the various ultrasound planes to identify the areas of greatest risk of heat damage (Fig. 3), sparing a 0.5-cm margin with respect to the pseudocapsule, and considering the reference limits of the fibroid in relation to the on-screen biopsy line. Furthermore, a conservative approach should be followed, since only 80% myoma ablation is needed for the technique to be considered complete [9]. As the procedure of thermal ablation advances, the image becomes more difficult to interpret because new hypeprecogenic areas appear, so we recommend starting treating the areas closest to the serosa when the ultrasound image is clearer.
The second severe complication was recorded in a patient who required surgery to control abdominal bleeding 5 h after the procedure. Laparoscopy revealed 4 areas of bleeding in the uterine serosa and parietal peritoneum (Fig. 5) that required bipolar coagulation. TRFA of fibroids involves multiple punctures of the uterine serosa and peritoneum in order to reach the fibroid. We estimate that the procedure used to treat a fibroid measuring 40 cc generally requires 30–40 punctures. Potential coagulation disorder was ruled out. The only specific circumstance reported by the surgeon was that the patient moved continuously during the procedure owing to insufficient sedation. We postulate that one of the movements could have increased the size of the opening created by the needle in the serosa and peritoneum causing bleeding.
The procedure takes 15 min on average and causes little pain. It can be performed under general (conscious sedation) or epidural anesthesia. Even though there are no reported data to suggest that one is better than the other, it's essential that the patient's pelvis and lower limbs remain immobile during the technique. Since in our series there were more cases of complications in patients who received epidural anaesthesia, we prefer to use general anaesthesia, although the final choice depends on the preferences of the anaesthesiologist based on the patient's clinical characteristics.
Risk factors that may be associated with complications include: the choice of fibroids to be treated that do not maintain an appropriate safety margin with respect to the uterine serosa (indicated treatment of fibroids types 0–4), the application of radiofrequency in areas close to the serosa (leave a safety distance to the serous layer of at least 1 cm), large fibroids projecting shadows, poor ultrasound transmission, or circumstances that prevent correct visualisation of the intracavitary needle at all times (e.g. if the patient moves during the procedure).
In our experience, the key points to avoid significant complications with vaginal radiofrequency are: to ensure a clear ultrasound image of the fibroid, the capsule and the relationship to the uterine serosa; maintain a conservative approach during the procedure, respect the established safety margins and recommended size of the fibroid; and provide appropriate anaesthesia to prevent patient movement.
When introducing a new technique, knowledge of potential associated complications and how to prevent them is as valuable as the effectiveness of the technique. This article is the first to specifically address the complications of TRFA for the treatment of fibroids. Our study is limited by the number of cases treated. Compared with other series of patients undergoing TRFA, the 115 cases we report comprise a relatively large sample. Therefore, our findings should be confirmed in larger samples with longer follow-up periods. Our study is also limited by the fact that all the procedures were performed by the same surgeon. Future studies should further investigate the mechanisms underlying the complications, risk factors associated and ways to prevent them ensuring that the technique is as safe as possible.
5. Conclusion
TRFA is a treatment option for fibroids with few associated complications most of them mild. Prospective studies involving larger numbers of patients and a variety of surgeons are needed to confirm it. It is important to remember the safety precautions including visualisation of the intra-abdominal needle and to maintain the safety margins of the fibroid when performing radiofrequency to ensure that the technique is safe.
Disclosures section
Authors have no conflicts of interest or financial ties to disclose.
CRediT authorship contribution statement
Conceptualization: Ángel Santalla-Hernández. Methodology: Ángel Santalla-Hernández, Mariña Naveiro-Fuentes. Rebeca Benito-Villegas, Jesus Villegas-Alcazar, María Setefilla López-Criado , Ana Lara-Serrano, Jorge Fernández Parra. Formal analysis: Ángel Santalla-Hernández, Mariña Naveiro-Fuentes, Rebeca Benito-Villegas, Jesus Villegas-Alcazar, María Setefilla López-Criado, Ana Lara-Serrano, Jorge Fernández Parra. Investigation: Ángel Santalla-Hernández, Mariña Naveiro-Fuentes, Rebeca Benito-Villegas, Jesus Villegas-Alcazar, María Setefilla López-Criado, Ana Lara-Serrano, Jorge Fernández Parra. Writing – original draft preparation: Ángel Santalla-Hernández, Irene Pelayo-Delgado. Writing – review & editing: Ángel Santalla-Hernández, Irene Pelayo-Delgado, Juan Luis Alcázar. All authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Acknowledgments
We thank PhD Roi Naveiro (statistician) for supervising the statistics and Thomas O′Boyle for translating the manuscript into English.
References
- 1.Baird D.D., Dunson D.B., Hill M.C., Cousins D., Schectman J.M. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann A., Bernuit D., Gerlinger C., Schaefers M., Geppert K. Prevalence symptoms and management of uterine fibroids: an international internet-based survey of 21,746 women. BMC Women’s Health. 2012;12:6. doi: 10.1186/1472-6874-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pérez-López F.R., Ornat L., Ceausu I., Depypere H., Erel C.T., Lambrinoudaki I., et al. EMAS position statement: management of uterine fibroids. Maturitas. 2014;79(1):106–116. doi: 10.1016/j.maturitas.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Havryliuk Y., Setton R., Carlow J.J., Shaktman B.D. Symptomatic fibroid management: systematic review of the literature. JSLS. 2017;21(3) doi: 10.4293/JSLS.2017.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo X., Shen Y., Song W.X., Chen P. wen, Xie X. mei, Wang X. yu. Pathologic evaluation of uterine leiomyoma treated with radiofrequency ablation. Int J Gynaecol Obstet. 2007;99(1):9–13. doi: 10.1016/j.ijgo.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 6.Bradley L.D., Pasic R.P., Miller L.E. Clinical performance of radiofrequency ablation for treatment of uterine fibroids: systematic review and meta-analysis of prospective studies. J Laparoendosc Adv Surg Tech A. 2019;29(12):1507–1517. doi: 10.1089/lap.2019.0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang X., Thapa A., Lu J., Bhujohory V.S., Liu Y., Qiao S. Ultrasound-guided transvaginal radiofrequency myolysis for symptomatic uterine myomas. Eur J Obstet Gynecol Reprod Biol. 2014;177:38–43. doi: 10.1016/j.ejogrb.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Jones S., O’Donovan P., Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int. 2012;2012 doi: 10.1155/2012/194839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santalla-Hernández Á., Naveiro-Fuentes M., Benito-Villena R., López-Criado M.S., González-Paredes A., Fernández-Parra J. Efficacy complications and factors predictive of response to treatment with transvaginal radiofrequency ablation for symptomatic uterine myomas. J Minim Invasive Gynecol. 2022;29(6):743–752. doi: 10.1016/j.jmig.2022.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Rey V.E., Labrador R., Falcon M., Garcia-Benitez J.L. Transvaginal radiofrequency ablation of myomas: technique outcomes and complications. J Laparoendosc Adv Surg Tech A. 2019;29(1):24–28. doi: 10.1089/lap.2018.0293. [DOI] [PubMed] [Google Scholar]
- 11.Munro M.G., Critchley H.O.D., Fraser I.S., FIGO Menstrual Disorders Working Group The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril. 2011;95(7):2204–2208. doi: 10.1016/j.fertnstert.2011.03.079. [DOI] [PubMed] [Google Scholar]
- 12.Lasmar R.B., Xinmei Z., Indman P.D., Celeste R.K., Di Spiezio Sardo A. Feasibility of a new system of classification of submucous myomas: a multicenter study. Fertil Steril. 2011;95(6):2073–2077. doi: 10.1016/j.fertnstert.2011.01.147. [DOI] [PubMed] [Google Scholar]
- 13.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniels J., Middleton L.J., Cheed V., McKinnon W., Rana D., Sirkeci F., et al. Uterine artery embolisation versus myomectomy for premenopausal women with uterine fibroids wishing to avoid hysterectomy: the FEMME RCT. Health Technol Assess. 2022;26(22):1–74. doi: 10.3310/ZDEG6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manyonda I., Belli A.M., Lumsden M.A., Moss J., McKinnon W., Middleton L.J., et al. Uterine-artery embolization or myomectomy for uterine fibroids. N Engl J Med. 2020;383(5):440–451. doi: 10.1056/NEJMoa1914735. [DOI] [PubMed] [Google Scholar]
- 16.Ren X.L., Zhou X.D., Yan R.L., Liu D., Zhang J., He G.B., et al. Sonographically guided extracorporeal ablation of uterine fibroids with high-intensity focused ultrasound: midterm results. J Ultrasound Med. 2009;28(1):100–103. doi: 10.7863/jum.2009.28.1.100. [DOI] [PubMed] [Google Scholar]
- 17.Wu X.J., Guo Q., Cao B.S., Tan L.X., Zhang H.Y., Cai Y.R., et al. Uterine Leiomyomas: safety and efficacy of US-guided suprapubic transvaginal radiofrequency ablation at 1-year follow-up. Radiology. 2016;279(3):952–960. doi: 10.1148/radiol.2015142537. [DOI] [PubMed] [Google Scholar]
- 18.Iversen H., Dueholm M. Radiofrequency thermal ablation for uterine myomas: long-term clinical outcomes and reinterventions. J Minim Invasive Gynecol. 2017;24(6):1020–1028. doi: 10.1016/j.jmig.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Bradley L.D., Pasic R.P., Miller L.E. Clinical performance of radiofrequency ablation for treatment of uterine fibroids: systematic review and meta-analysis of prospective studies. J Laparoendosc Adv Surg Tech A. 2019;29(12):1507–1517. doi: 10.1089/lap.2019.0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee B.B., Yu S.P. Radiofrequency ablation of uterine fibroids: a review. Curr Obstet Gynecol Rep. 2016;5(4):318–324. doi: 10.1007/s13669-016-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caruso A., Rodríguez Pérez A., Cabezas Palacios M.N., Valdés Hernández J., Guadix Martín M.P. Perforación intestinal tras ablación por radiofrecuencia de mioma uterino. Reporte de caso y revisión bibliográfica. Clín e Investig En Ginecol Y Obstet. 2022;49(1) [Google Scholar]
- 22.Moen V., Dahlgren N., Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990-1999. Anesthesiology. 2004;101(4):950–959. doi: 10.1097/00000542-200410000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Auroy Y., Benhamou D., Bargues L., Ecoffey C., Falissard B., Mercier F.J., et al. Major complications of regional anesthesia in France: the SOS regional anesthesia hotline service. Anesthesiology. 2002;97(5):1274–1280. doi: 10.1097/00000542-200211000-00034. [DOI] [PubMed] [Google Scholar]
- 24.Benzon H.T., Asher Y.G., Hartrick C.T. Back pain and neuraxial anesthesia. Anesth Analg. 2016;122(6):2047–2058. doi: 10.1213/ANE.0000000000001270. [DOI] [PubMed] [Google Scholar]
- 25.Flaatten H., Raeder J. Spinal anaesthesia for outpatient surgery. Anaesthesia. 1985;40(11):1108–1111. doi: 10.1111/j.1365-2044.1985.tb10612.x. [DOI] [PubMed] [Google Scholar]
- 26.Greensmith J.E., Murray W.B. Complications of regional anesthesia. Curr Opin Anaesthesiol. 2006;19(5):531–537. doi: 10.1097/01.aco.0000245280.99786.a3. [DOI] [PubMed] [Google Scholar]
- 27.Liu L., Wang T., Lei B. High-intensity focused ultrasound (HIFU) ablation versus surgical interventions for the treatment of symptomatic uterine fibroids: a meta-analysis. Eur Radio. 2022;32(2):1195–1204. doi: 10.1007/s00330-021-08156-6. [DOI] [PubMed] [Google Scholar]
- 28.Schirf B.E., Vogelzang R.L., Chrisman H.B. Complications of uterine fibroid embolization. Semin Interv Radio. 2006;23(2):143–149. doi: 10.1055/s-2006-941444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bilhim T., Pisco J.M. The role of nonsteroidal anti-inflammatory drugs (NSAIDs) in the management of the post-embolization symptoms after uterine artery embolization. Pharmaceuticals. 2010;3(6):1729–1738. doi: 10.3390/ph3061729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackburn H., West S. Management of postembolization syndrome following hepatic transarterial chemoembolization for primary or metastatic liver cancer. Cancer Nurs. 2016;39(5):E1–E18. doi: 10.1097/NCC.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 31.Bissler J.J., Racadio J., Donnelly L.F., Johnson N.D. Reduction of postembolization syndrome after ablation of renal angiomyolipoma. Am J Kidney Dis. 2002;39(5):966–971. doi: 10.1053/ajkd.2002.32770. [DOI] [PubMed] [Google Scholar]
- 32.Kitamura Y., Ascher S.M., Cooper C., Allison S.J., Jha R.C., Flick P.A., et al. Imaging manifestations of complications associated with uterine artery embolization. Radiographics. 2005;25(Suppl 1):S119–S132. doi: 10.1148/rg.25si055518. [DOI] [PubMed] [Google Scholar]
- 33.Martin J., Bhanot K., Athreya S. Complications and reinterventions in uterine artery embolization for symptomatic uterine fibroids: a literature review and meta analysis. Cardiovasc Interv Radio. 2013;36(2):395–402. doi: 10.1007/s00270-012-0505-y. [DOI] [PubMed] [Google Scholar]
- 34.Ciebiera M., Łoziński T., Wojtyła C., Rawski W., Jakiel G. Complications in modern hysteroscopic myomectomy. Ginekol Pol. 2018;89(7):398–404. doi: 10.5603/GP.a2018.0068. [DOI] [PubMed] [Google Scholar]
- 35.Liu E., Shehata M., Liu T., Amorn A., Cingolani E., Kannarkat V., et al. Prevention of esophageal thermal injury during radiofrequency ablation for atrial fibrillation. J Inter Card Electro. 2012;35(1):35–44. doi: 10.1007/s10840-011-9655-0. [DOI] [PubMed] [Google Scholar]
- 36.Halm U., Gaspar T., Zachäus M., Sack S., Arya A., Piorkowski C., et al. Thermal esophageal lesions after radiofrequency catheter ablation of left atrial arrhythmias. Am J Gastroenterol. 2010;105(3):551–556. doi: 10.1038/ajg.2009.625. [DOI] [PubMed] [Google Scholar]
- 37.Rhim H., Dodd G.D., Chintapalli K.N., Wood B.J., Dupuy D.E., Hvizda J.L., et al. Radiofrequency thermal ablation of abdominal tumors: lessons learned from complications. Radiographics. 2004;24(1):41–52. doi: 10.1148/rg.241025144. [DOI] [PubMed] [Google Scholar]
- 38.Giddins G., Shewring D., Downing N. Articular cartilage and soft tissue damage from radiofrequency thermal ablation wands at wrist arthroscopy. J Hand Surg Eur Vol. 2021;46(6):632–636. doi: 10.1177/1753193420980347. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L., Zhang W., Orsi F., Chen W., Wang Z. Ultrasound-guided high intensity focused ultrasound for the treatment of gynaecological diseases: a review of safety and efficacy. Int J Hyperth. 2015;31(3):280–284. doi: 10.3109/02656736.2014.996790. [DOI] [PubMed] [Google Scholar]
- 40.Chen J., Chen W., Zhang L., Li K., Peng S., He M., et al. Safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: a review of 9988 cases. Ultrason Sonochem. 2015;27:671–676. doi: 10.1016/j.ultsonch.2015.05.031. [DOI] [PubMed] [Google Scholar]