Abstract
Background
Oblique lumbar interbody fusion (OLIF) offers indirect decompression of stenotic lesions of the spinal canal and foramen through immediate disc height restoration. Only a few studies have reported the effect of cage position and associated intraoperatively modifiable factors for successful immediate indirect decompression following OLIF surgery. This study aimed to investigate the intraoperatively modifiable factors for successful radiological outcomes of OLIF.
Methods
This study included 46 patients with 80 surgical levels who underwent OLIF without direct posterior decompression. Preoperative and postoperative radiological parameters were evaluated and intraoperatively modifiable radiologic parameters for successful immediate radiologic decompression on magnetic resonance image (MRI) were determined. Radiologic parameters were preoperative and postoperative radiological parameters including anterior disc height (ADH), posterior disc height (PDH) lumbar lordotic angle (LLA), segmental lordotic angle (SLA), foraminal height (FH), cage position, cross-sectional area (CSA) of the thecal sac, cross-sectional foraminal area (CSF), facet distance (FD)
Results
All radiologic outcomes significantly improved. Comparing preoperative and postoperative values, mean CSA increased from 99.63±40.21 mm2 to 125.02±45.90 mm2 (p<.0001), and mean left CSF increased from 44.54±12.90 mm2 to 69.91±10.80 mm2 (p<.0001). FD also increased from 1.40±0.44 to 1.92±0.71 mm (p<.0001). FH increased from 16.31±3.3 to 18.84±3.47 mm (p<.0001). ADH and PDH also significantly increased (p<.0001). Immediate postoperative CSF and FH improvement rate (%) were significantly correlated with posterior disc height restoration rate (%) (p=.0443, and p=.0234, respectively). In addition, the patients with a cage positioned in the middle of the vertebral body experienced a greater FH improvement rate (%) compared to the patients with a cage positioned anteriorly. Finally, Visual analogue scale (VAS) for leg pain was improved immediately.
Conclusions
OLIF provided satisfactory immediate indirect decompression in central and foraminal spinal stenosis. Moreover, intraoperative surgical technique for successful radiologic CSF and FH improvement included restoration of the PDH and placement of the cage in the middle.
Keywords: Cage position, Foraminal height restoration, Indirect decompression, Oblique lateral interbody fusion
Introduction
Oblique lumbar interbody fusion (OLIF) is a minimally invasive surgical technique for various degenerative lumbar spine diseases with its advantages being minimal trauma and quick recovery over the more invasive conventional posterior lumbar interbody fusion surgery [1]. The key characteristics of OLIF surgery compared to conventional open surgery is an indirect neural decompression of stenotic lesions with neural patency and prevention of potential iatrogenic complications such as an unintended dural tear or nerve root injury [2,3].
In OLIF surgery, implantation of a large cage into the intervertebral disc space can restore disc height and distract the posterior element of the vertebral column, and these provide not only expansion of the spinal canal and intervertebral foraminal area but also unbuckling of the ligamentum flavum for immediate decompression of stenotic lesions [4], [5], [6]. Moreover, several long-term follow-up studies have reported the regression of the thickened ligamentum flavum at the surgical level [4,7,8]. These immediate and long-term surgical and radiological results bring a favorable long-term clinical outcome following OLIF surgery in degenerative lumbar spine diseases, such as degenerative disc disease, spondylolisthesis, and spinal stenosis [2,9,10].
We observed the immediate postoperative magnetic resonance images (MRI) of the patients who underwent OLIF surgery and found that early favorable clinical outcomes were related to early radiological outcomes, especially in sufficient distraction of posterior vertebral column. We assume that the effect of intraoperatively modifiable factors, such as cage position, improvement of anterior disc height (ADH), posterior disc height (PDH), and restoration of segmental lordosis (SL), is related to the distraction of posterior vertebral elements [11]. However, information on how these factors could affect the immediate successful distraction of the posterior vertebral column is lacking.
Therefore, the purpose of this study was to evaluate the preoperative and postoperative radiological parameters in patients who underwent 1- or 2-level OLIF, and to investigate the favorable intraoperatively modifiable factors affecting successful radiological outcomes of OLIF surgery for indirect decompression.
Methods
Study population
A cohort of 46 patients who underwent OLIF surgery by a single orthopedic surgeon (corresponding author) at a tertiary hospital between 2015 and 2021 were retrospectively assessed. Inclusion criteria included patients who were (1) diagnosed with degenerative lumbar stenosis, (2) underwent 1- or 2-level OLIF surgery, (3) without direct posterior decompression, and (4) provided appropriate radiologic data, including immediate postoperative MRI prior to the staged percutaneous posterior fixation. The exclusion criteria included patients who underwent concomitant open laminectomy and decompression with OLIF, and those who were operated on at the sacral or thoracic level. All surgeries were performed using a left-sided retroperitoneal approach, followed by staged percutaneous posterior fixation.
This study was approved by the Institutional Review Boards of Korea University Ansan Hospital and was conducted in accordance with the approved study protocol (IRB No. 2021AS0325).
Radiologic evaluation
Plain radiographs and MRI preoperatively and within 1 week after the OLIF surgery before staged percutaneous posterior fixation were obtained for all patients. On the basis of plain radiographs, ADH, PDH, foraminal height (FH), segmental lordotic angle (SLA), and lumbar lordortic angle (LLA) were evaluated preoperatively and postoperatively. ADH was defined as the distance from the superior to the inferior endplate of the surgical level at the anterior edge. PDH was defined as the distance from the superior to the inferior endplate of the surgical level at the posterior edge. FH was defined as the maximum distance from the inferior pedicle border of the upper vertebrae and the superior pedicle border of the lower vertebrae. SLA was defined as the Cobb angle between the superior endplate of the upper vertebrae and the inferior endplate of the lower vertebrae at surgical level. LLA was defined as the Cobb angle between the superior endplate of L1 and S1 vertebrae [11]. Postoperative cage position was also evaluated on the plain radiographs. We divided the patients into 2 groups based on the position of the cage: anterior third of the inferior endplate (anterior group) versus middle third of the inferior endplate (posterior group) (Fig. 1).
Fig. 1.
(A) Anterior disc height (ADH), posterior disc height (PDH). (B) Lumbar lordosis (LL). (C) Segmental lordotic angle (SLA) were evaluated on lateral radiographs. (D) Cage position was evaluated on lateral radiographs and divided into 2 groups: anterior cage position and middle cage position.
The cross-sectional area (CSA) of the thecal sac was measured on T2-weighted axial MRI at the disc level preoperatively and postoperatively within a week after surgery. The facet distance (FD) was measured by drawing a line perpendicular to facet joint, at the point where it is the largest distance between superior and inferior facet on T2-weighted axial MRI [12]. The cross-sectional area of the left and right intervertebral foramen (CSF) was measured on T2-weighted sagittal MRI preoperatively and postoperatively (Fig. 2).
Fig. 2.
(A) Cross-sectional area (CSA) of the thecal sac. (B) Facet distance (FD) were evaluated on T2 weighted axial MRI. (C) Cross-sectional area of intervertebral foramen (CSF) was evaluated on T2 weighted sagittal MRI.
The improvement rate for each parameter was calculated using the following equation: postoperative value/preoperative value × 100.
Statistical analysis
Data are presented as the mean ± standard deviation. Radiographic measurements were obtained by an orthopedic spine surgeon (first author) who was not involved in the surgery. Preoperative and postoperative ADH, PDH, FH, CSA, CSF, LL, and SLA were compared using a paired t-test. The relationship between the improvement rate of ADH, PDH and cage position, and CSA, CSF, and FH was analyzed using the Pearson's correlation coefficient. All statistical analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC, USA); p values <.05 were considered statistically significant.
Results
A total of 46 patients (33 females, 13 males) with 80 surgical levels were assessed in this study. The mean age of the patients at the time of surgery was 68.7 years, with a range of 42 to 88 years. Table 1 presents the baseline characteristics analyzed in this study, including age, sex, and surgical levels. Among the 80 surgical levels, 40% (n=32) was involved at L3–4 and 35% (n=28) was involved at L4–5. Twelve patients underwent 1-level OLIF surgery whereas the remaining 34 patients underwent 2-level OLIF surgery.
Table 1.
Baseline characteristics of 46 patients (80 surgical levels) who underwent oblique lumbar interbody fusion with indirect decompression.
| Characteristics | |
|---|---|
| Age (years) | 68.67 ± 8.95 |
| Sex | |
| Male | 13 |
| Female | 33 |
| Fused levels | |
| 1 level | 10 |
| 2 level | 26 |
| Surgical levels | Total 80 levels |
| L1–2 | 1 (1.25%) |
| L2–3 | 13 (16.25%) |
| L3–4 | 32 (40%) |
| L4–5 | 28 (35%) |
| L5–S1 | 6 (7.5%) |
As described previously, all patients were diagnosed with degenerative lumbar disease, including central spinal stenosis and foraminal stenosis.
Radiologic outcome
Indirect decompression was successfully achieved radiologically based on postoperative MRI. All radiological measurements including CSA, CSF, LLA, SLA, ADH, PDH, FD, and FH had significant difference, preoperatively and postoperatively (all p-values <.0001 except for SLA, p=.029).
The mean CSA increased from 99.63±40.21 mm2 preoperatively to 125.02±45.90 mm2 postoperatively (p<0.0001). The mean change in CSA was 25.39 mm2. The mean left CSF increased from 44.54±12.90 mm2 preoperatively to 69.91±10.80 mm2 postoperatively (p<.0001). The mean right CSF increased from 36.56±13.26 mm2 preoperatively to 58.29±17.58 mm2 postoperatively. ADH increased from 9.21±3.65 mm preoperatively to 14.50±3.37 mm postoperatively, and PDH also increased from 5.22±1.45 mm preoperatively to 8.07±2.00 mm postoperatively (p<.0001 and p<.0001, respectively). The mean differences between preoperative and postoperative disc heights were 5.29 mm in ADH and 2.85 mm in PDH. Postoperatively, LL and SLA at the surgical level significantly improved from 29.64±16.80° to 33.73±12.47° (p<.001), and from 8.98±10.13° to 10.68±8.21° (p<.001), respectively. FH on plain radiograph significantly increased from 16.31±3.3 mm to 18.84±3.47 mm (p<.001). On MRI, FD also significantly increased from 1.40±0.44 mm to 1.92±0.71 mm postoperatively (Table 2).
Table 2.
Comparison of radiologic parameters before and after surgery (n=80).
| Parameters | Preoperative Mean±SD | Postoperative Mean±SD | Mean difference Δ | p-value |
|---|---|---|---|---|
| FD | 1.40±0.44 | 1.92±0.71 | 0.52 | <.0001 |
| CSA | 99.63±40.21 | 125.02±45.90 | 25.39 | <.0001 |
| CSF | ||||
| Left | 44.54±12.90 | 69.91±19.80 | 25.37 | <.0001 |
| Right | 36.56±13.26 | 58.29±17.58 | 21.72 | <.0001 |
| LL | 29.64±16.80 | 33.73±12.47 | 4.08 | <.0001 |
| SL | 8.98±10.13 | 10.68±8.21 | 1.70 | .0298 |
| ADH | 9.21±3.65 | 14.50±3.37 | 5.29 | <.0001 |
| PDH | 5.22±1.45 | 8.07±2.00 | 2.85 | <.0001 |
| FH | 16.31±3.3 | 18.84±3.47 | 2.53 | <.0001 |
FD, facet distance (based on axial MR image); CSA, cross-sectional area of spinal canal (based on axial MR image); CSF, cross-sectional area of foramen (based on sagittal MR image); LL, lumbar lordosis (based on lateral radiographic image); SL, segmental lordosis (based on lateral radiographic image); ADH, anterior disc height (based on lateral radiographic image)
PDH, posterior disc height (based on lateral; radiographic image); FH, foraminal height (based on lateral radiographic image).
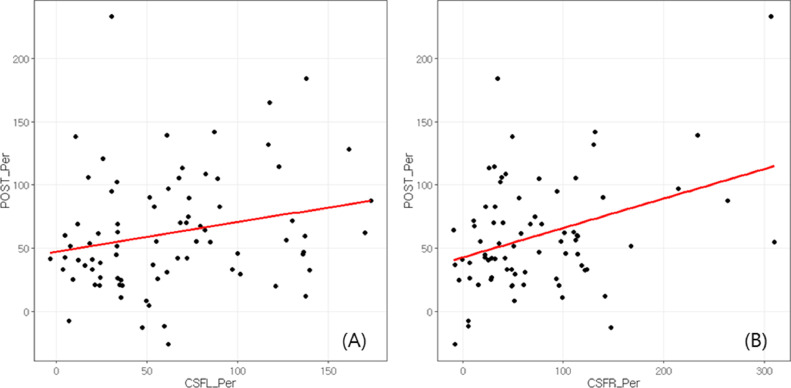
In Table 3, the correlation between the % change of ADH and PDH, and the improvement rate in CSA, CSF for left and right foramen, FH, and FD are summarized. Correlations between the postoperative CSF and FH improvement rate, and between the CSF and PDH improvement rate (p=.0443 and p=.0234 (left) and p=.002 (right), respectively) were statistically significant (Fig. 3). In addition, ADH improvement rate also correlated with postoperative FH improvement rate (p=.0334). ADH improvement rate was not correlated with postoperative improvement rate of CSF (p=.1513) though.
Table 3.
Correlation of disc height increment with CSA and CSF and FD increment.
| % Ant disc height increment |
% Post disc height increment |
|||
|---|---|---|---|---|
| Pearson correlation | p value | Pearson correlation | p value | |
| CSA improvement rate (%); mean | 0.1888 | .0935 | 0.1943 | .0841 |
| CSF improvement rate (%); mean | ||||
| Left | 0.1619 | .1513 | 0.2255 | .0443 |
| Right | 0.1242 | .2851 | 0.3589 | .002 |
| FD improvement rate (%); mean | 0.0487 | .6679 | -0.1259 | .266 |
| FH improvement rate (%); mean | 0.2706 | .0334 | 0.2876 | .0234 |
CSA, cross-sectional area of spinal canal (based on axial MR image); CSF, cross-sectional area of foramen (based on sagittal MR image); FD, facet distance (based on axial MR image); FH, foraminal height (based on sagittal radiographic image).
Fig. 3.
(A) Correlation of CSF improvement rate and PDH improvement rate for left foramen; (B) Correlation of CSF improvement rate and PDH improvement rate for right foramen; CSF, cross-sectional area of intervertebral foramen; PDH, posterior disc height.
Table 4 shows the comparison of the change of preoperative and postoperative radiologic parameters based on the cage position. Postoperative FH improvement rate was significantly higher in patients with the cage located in the middle of the inferior vertebral body than those patients with cage located anteriorly (p<.001). Improvement rates of spatial radiologic parameters such as CSA and CSF as well as FD also tended to be higher in patients with the cage located in the middle of the inferior vertebral body, although they were not statistically significant.
Table 4.
Comparison of radiologic parameters based on the distribution of cage location before and after surgery.
| Parameters | Cage located at anterior (n=39) | Cage located at middle (n=41) | p-value |
|---|---|---|---|
| FD (mm) | |||
| Preoperative | 1.4 (1.11, 1.69) | 1.27 (1.05, 1.48) | .2859 |
| Postoperative | 1.99 (1.57, 2.34) | 1.69 (1.48, 1.99) | .2956 |
| % Facet distance change | 29.66 (4.89, 81.03) | 30.3 (6.08, 70.91) | .8378 |
| CSA (mm2) | |||
| Preoperative | 105.58±38.63 | 104.84±45.88 | .9455 |
| Postoperative | 118.2 (96.07, 157.68) | 127.76 (94.96, 161.74) | .7262 |
| % CSA improvement | 19.35 (8.28, 35.81) | 22.76 (11.56, 48.56) | .4405 |
| CSF (mm2), Left | |||
| Preoperative | 46.85±14.08 | 40.96±11 | .07 |
| Postoperative | 70.63±18.41 | 70.57±24.67 | >.99 |
| % CSF improvement | 51.6 (24.47, 72.93) | 67.73 (30.7, 130.13) | .1471 |
| FH (mm) | |||
| Preoperative | 16.7±2.41 | 15.86±4.08 | .3396 |
| Postoperative | 18.2±2.46 | 19.57±4.28 | .1374 |
| % FH improvement | 6.64 (0.82, 17.24) | 20.16 (13.28, 38.08) | <.001 |
FD, facet distance (based on axial MR image); CSA, cross-sectional area of spinal canal (based on axial MR image); CSF, cross-sectional area of foramen (based on sagittal MR image); FH, foraminal height (based on sagittal radiographic image).
Clinical outcome
We evaluated VAS for leg pain at rest for all patients preoperatively and at postoperative 1 week just before staged posterior fixation. Mean preoperative VAS for leg pain at rest was 7.61±1.19 and mean 1-week postoperative VAS for leg pain was 4.16±5.29.
Discussion
In this study, significant improvements in ADH, PDH, LLA, SLA, CSA, CSF, FH, and FD were observed in the immediate postoperative period. PDH improvement correlated with the postoperative CSF improvement rate as well as FH improvement rate. Postoperative FH improvement rate was significantly greater in the group with the cage located in the middle. These results have the potential to provide insight into the surgical outcomes of OLIF surgery.
Indications of OLIF surgery based on clinical symptoms
Typically, surgical indications for OLIF surgery are believed to be efficacious for the treatment of mechanical low back pain resulting from disc degeneration, segmental instability, and degenerative scoliosis. Leg pain with neurogenic claudication accompanied by mild spinal canal stenosis is also considered for OLIF surgery. However, OLIF has been thought to be insufficient for radicular symptoms at rest, and thus posterior direct decompression with or without fusion is usually recommended for radicular symptoms. In our study, VAS for leg pain improved immediately within a week following OLIF surgery. With the radiologic improvement of CSA and CSF as well the immediate improvement of radicular symptoms, we suggest that utilization of OLIF surgery may offer an advantage in the treatment of radicular pain as well as neurogenic claudication due to spinal canal stenosis.
Radiologic indications and contraindications of OLIF surgery
The success of indirect decompression through OLIF surgery depends on many factors. [11,[13], [14], [15]] The most important factor for successful OLIF surgery is to identify the appropriate surgical indication. In OLIF surgery, a large cage can distract the interbody disc space as well as the posterior vertebral element, including the facet joint, which allows the restoration of disc and foraminal height [2,5,10,15]. In our study, the restoration of disc height and the distraction of facet joint relieved the buckled ligamentum flavum immediately, resulting in increased CSA. The increase of foraminal height can also immediately relieve the compression of neural elements. Based on these findings, we can conclude that sufficient indirect decompression after OLIF surgery can be accomplished in patients with intervertebral disc space narrowing, which can be fully opened and restored by insertion of a large cage.
A recent retrospective review of 45 patients (101 levels) reported that bony stenosis at the lateral recess is a predictor of failure of indirect decompression [16]. Although indirect decompression using OLIF can stretch the soft tissue elements that compress the neural element, there may be a limitation of indirect decompression in relieving bony stenosis. Another study reported a revision surgery for direct decompression after OLIF surgery in patients with bony lateral stenosis [17]. Bony stenosis at the lateral recess can be observed in patients with a severe hypertrophied facet joint. Severe facet joint arthropathy may be related to insufficient facet joint distraction, causing inconsequential relief of stenosis through indirect decompression. Given these assumptions, patients with severe facet joint arthropathy and resulting radicular symptoms may be better suited for direct decompression than indirect decompression.
Modifiable factors for successful radiologic outcome following OLIF surgery
Previous studies on the indirect decompression of OLIF have reported a significant increase in CSA, CSF, and FH postoperatively, which is consistent with the result of the current study [5,14,15,17]. Even though regression of the ligamentum flavum has been reported with its significant role in CSA expansion after long-term follow-up, our results indicate that indirect decompression can also be successfully accomplished immediately with a significant improvement in spatial radiological parameters [4,8]. In our study, there were statistically significant increments in ADH, PDH, FH, CSA, CSF, and FD in immediate postoperative MRI. This result indicates that OLIF is sufficient to immediately provide good radiological outcomes in patients with degenerative lumbar stenosis.
In our study, the improvement rate of PDH correlated with the improvement rate of CSF and FH. However, the improvement rate ADH did not correlate with the improvement rate of CSF and FH. We assume that ADH restoration increases segmental lordosis, which creates foraminal stenosis. The effect of indirect decompression by anterior disc height restoration of OLIF surgery might be altered by potential foraminal stenosis created by increasing segmental lordosis.
In addition, cages located in the middle of the inferior vertebral body had a greater effect on improvement of FH. Greater improvements in CSF and increase in FD were observed in patients with the cage located in the middle than in those with the cage located anteriorly. These results are consistent with previously reported results that showed that FH or CSF increased as the cage was placed more posteriorly and with a smaller cage angle. Since we used only a 6-degree lordotic cage with various cage heights, the influence of cage angle on FH or CSF change was minimal. Therefore, the factors that significantly affect the improvement of CSF and FH are the posterior disc height restoration as well as more posterior location of cage. These results support our hypothesis that the effect of indirect decompression through OLIF surgery might occur through the distraction of the middle and posterior vertebral column. With these results, we provide a strong rationale to place the cage in the middle or posteriorly to increase the amount of posterior disc height restoration and the amount of facet distraction, which brings better immediate radiological outcomes after OLIF surgery.
Limitations
Our study has some limitations. First, this is a small-sized retrospective study with the wide range of patient's age. This makes it difficult to accurately reflect the radiologic outcome with respect of the degree of degenerative change. Second, even though we concluded that there was significant improvement in CSA, CSF, and foraminal height postoperatively, we did not evaluate immediate postoperative Oswestry disability index (ODI) scores, which are as significant as radiologic outcomes. We only evaluated difference between preoperative and immediate postoperative VAS scores for leg pain at rest, not for back pain. Further research is required to clarify the minimal clinically important difference in each radiologic parameter for both successful radiological and clinical outcomes following OLIF surgery.
Conclusions
Indirect decompression through OLIF helps relieve symptoms related to degenerative lumbar disease through disc height restoration and foraminal area restoration. For successful immediate radiological postoperative outcomes including increased cross-sectional canal area and to relieve buckling of the ligamentum flavum, restoration of posterior disc height is more important than the anterior disc height, and cage location should be carefully designed.
Declaration of Competing Interest
No potential conflict of interest relevant to this article were reported for all authors.
Acknowledgments
This work was supported by the Korea University Grant.
Footnotes
FDA device/drug status: Not applicable.
Author disclosures: JP: Nothing to disclose. S-MP: Nothing to disclose. SH: Nothing to disclose. YJ: Nothing to disclose. J-YH: Nothing to disclose.
References
- 1.Park J, Ham DW, Kwon BT, Park SM, Kim HJ, Yeom JS. Minimally invasive spine surgery: techniques, technologies, and indications. Asian Spine J. 2020;14(5):694–701. doi: 10.31616/asj.2020.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg. 2015;1(1):2–18. doi: 10.3978/j.issn.2414-469X.2015.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehren C, Mayer HM, Zandanell C, Siepe CJ, Korge A. The oblique anterolateral approach to the lumbar spine provides access to the lumbar spine with few early complications. Clin Orthop Relat Res. 2016;474(9):2020–2027. doi: 10.1007/s11999-016-4883-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Limthongkul W, Tanasansomboon T, Yingsakmongkol W, Tanaviriyachai T, Radcliff K, Singhatanadgige W. Indirect decompression effect to Central canal and ligamentum flavum after extreme lateral lumbar interbody fusion and oblique lumbar interbody fusion. Spine (Phila Pa 1976) 2020;45(17):E1077–E1E84. doi: 10.1097/BRS.0000000000003521. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu T, Fujibayashi S, Otsuki B, Murata K, Matsuda S. Indirect decompression via oblique lateral interbody fusion for severe degenerative lumbar spinal stenosis: a comparative study with direct decompression transforaminal/posterior lumbar interbody fusion. Spine J. 2021;21(6):963–971. doi: 10.1016/j.spinee.2021.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Jain D, Ray WZ, Vaccaro AR. Advances in techniques and technology in minimally invasive lumbar interbody spinal fusion. JBJS reviews. 2020;8(4):e0171. doi: 10.2106/JBJS.RVW.19.00171. [DOI] [PubMed] [Google Scholar]
- 7.Ohtori S, Orita S, Yamauchi K, et al. Change of lumbar ligamentum flavum after indirect decompression using anterior lumbar interbody fusion. Asian Spine J. 2017;11(1):105–112. doi: 10.4184/asj.2017.11.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahatthanatrakul A, Kim HS, Lin GX, Kim JS. Decreasing thickness and remodeling of ligamentum flavum after oblique lumbar interbody fusion. Neuroradiology. 2020;62(8):971–978. doi: 10.1007/s00234-020-02414-y. [DOI] [PubMed] [Google Scholar]
- 9.Li HM, Zhang RJ, Shen CL. Differences in radiographic and clinical outcomes of oblique lateral interbody fusion and lateral lumbar interbody fusion for degenerative lumbar disease: a meta-analysis. BMC Musculoskelet Disord. 2019;20(1):582. doi: 10.1186/s12891-019-2972-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phan K, Maharaj M, Assem Y, Mobbs RJ. Review of early clinical results and complications associated with oblique lumbar interbody fusion (OLIF) J Clin Neurosci. 2016;31:23–29. doi: 10.1016/j.jocn.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 11.Lin GX, Rui G, Sharma S, Mahatthanatrakul A, Kim JS. The correlation of intraoperative distraction of intervertebral disc with the postoperative canal and foramen expansion following oblique lumbar interbody fusion. Eur Spine J. 2021;30(1):151–163. doi: 10.1007/s00586-020-06604-3. [DOI] [PubMed] [Google Scholar]
- 12.Cho IY, Park SY, Park JH, Suh SW, Lee SH. MRI findings of lumbar spine instability in degenerative spondylolisthesis. J Orthop Surg (Hong Kong) 2017;25(2) doi: 10.1177/2309499017718907. [DOI] [PubMed] [Google Scholar]
- 13.Kepler CK, Huang RC, Sharma AK, et al. Factors influencing segmental lumbar lordosis after lateral transpsoas interbody fusion. Orthop Surg. 2012;4(2):71–75. doi: 10.1111/j.1757-7861.2012.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park SJ, Lee CS, Chung SS, Kang SS, Park HJ, Kim SH. The ideal cage position for achieving both indirect neural decompression and segmental angle restoration in lateral lumbar interbody fusion (LLIF) Clin Spine Surg. 2017;30(6):E784–Ee90. doi: 10.1097/BSD.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 15.Sato J, Ohtori S, Orita S, et al. Radiographic evaluation of indirect decompression of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for degenerated lumbar spondylolisthesis. Eur Spine J. 2017;26(3):671–678. doi: 10.1007/s00586-015-4170-0. [DOI] [PubMed] [Google Scholar]
- 16.Wang TY, Nayar G, Brown CR, Pimenta L, Karikari IO, Isaacs RE. Bony lateral recess stenosis and other radiographic predictors of failed indirect decompression via extreme lateral interbody fusion: multi-institutional analysis of 101 consecutive spinal levels. World Neurosurg. 2017;106:819–826. doi: 10.1016/j.wneu.2017.07.045. [DOI] [PubMed] [Google Scholar]
- 17.Malham GM, Parker RM, Goss B, Blecher CM. Clinical results and limitations of indirect decompression in spinal stenosis with laterally implanted interbody cages: results from a prospective cohort study. Eur Spine J. 2015;24(Suppl 3):339–345. doi: 10.1007/s00586-015-3807-3. [DOI] [PubMed] [Google Scholar]