Abstract
Goals
To determine whether an 18 single nucleotide polymorphisms (SNPs) polygenic risk score (PRS18) improves breast cancer (BC) risk prediction for women at above-average risk of BC, aged 40–49, in a Central European population with BC incidence below EU average.
Methods
502 women aged 40–49 years at the time of BC diagnosis completed a questionnaire on BC risk factors (as per Tyrer-Cuzick algorithm) with data known at age 40 and before BC diagnosis. Blood samples were collected for DNA isolation. 250 DNA samples from healthy women aged 50 served as a control cohort. 18 BC-associated SNPs were genotyped in both groups and PRS18 was calculated. The predictive power of PRS18 to detect BC was evaluated using a ROC curve. 10-year BC risk was calculated using the Tyrer-Cuzick algorithm adapted to the Slovenian incidence rate (S-IBIS): first based on questionnaire-based risk factors and, second, including PRS18.
Results
The AUC for PRS18 was 0.613 (95 % CI 0.570–0.657). 83.3 % of women were classified at above-average risk for BC with S-IBIS without PRS18 and 80.7 % when PRS18 was included.
Conclusion
BC risk prediction models and SNPs panels should not be automatically used in clinical practice in different populations without prior population-based validation. In our population the addition of an 18SNPs PRS to questionnaire-based risk factors in the Tyrer-Cuzick algorithm in general did not improve BC risk stratification, however, some improvements were observed at higher BC risk scores and could be valuable in distinguishing women at intermediate and high risk of BC.
Keywords: Early breast cancer, Risk prediction, Polygenic risk score, Tyrer Cuzick algorithm
Highlights
-
•
S-IBIS risk prediction tool classified 83 % of our cases at above-average BC risk.
-
•
Including 18 SNPs PRS in a Tyrer-Cuzick model did not improve BC risk prediction.
-
•
A well-chosen small SNP panel can perform comparably to larger SNP cohorts.
-
•
BC risk prediction tools should be validated in each population before clinical implementation.
1. Introduction
Female breast cancer (BC) is the most commonly diagnosed cancer worldwide and the fourth leading cause of all cancer deaths [1].
In countries with a higher Human Development Index (HDI), the incidence of BC is higher and the mortality rate lower than in countries with a lower HDI [[1], [2], [3]]. A significant decrease in mortality is directly related to organised BC screening programmes offered in most high HDI countries [[4], [5], [6], [7]].
Most BC screening guidelines are age-dependent and target women aged 50 and older, thus failing to detect the disease at an early stage in younger women at above-average risk of BC [8,9]. Although the European BC guidelines were recently updated, recommending mammographic screening for asymptomatic women starting at age 45, how to detect early BC in young women at above-average BC risk remains a challenge [10].
Several BC risk prediction models calculate individualised BC risk based on known risk factors as age, family history of BC and ovarian cancer, age at menarche, parity, body mass index and use of hormone replacement therapy [[11], [12], [13]]. The Tyrer-Cuzick or International Breast Intervention Study (IBIS) risk prediction model is commonly used in clinical practice and it includes all the aforementioned risk factors [11,13,14]. It was developed in the United Kingdom and adapted separately for BC incidence rates in the Slovenian population (S-IBIS) [15,16]. An exploratory evaluation of S-IBIS performance has been done in a cohort of Slovenian women at above-average risk of BC [17].
About 15 %–30 % of BC are estimated to be familial or hereditary and genetic-risk based screening is offered to individuals with known pathogenic and likely pathogenic variants in high- and moderate-penetrance BC susceptibility genes such as BRCA1, BRCA2, PALB2,TP53, ATM, CHEK2, RAD51C and RAD51D [[18], [19], [20]]. Yet, such pathogenic variants account for only about 30 % of BC heritability [21,22]. Genome wide association studies (GWAS) have discovered more than 300 single nucleotide polymorphisms (SNPs) associated with BC risk with varying degrees of penetrance and prevalence in the population. Individually, they confer a small overall risk but cumulatively explain 30–40 % of the heritability of BC [14,19,[23], [24], [25], [26], [27]]. BC-associated SNPs can be included in risk prediction algorithms and are extensively studied in different populations [13,14,28]. The combined effect of multiple SNPs is expressed by polygenic risk scores (PRS) and women with PRS in the highest percentiles have a higher incidence rate of BC, increased lifetime risk of BC, and earlier onset of the disease compared with women at average risk [26,29]. Over the years, various PRSs were researched, which contained an increasing number of SNPs. Their predictive power was mostly assessed by area under the curve (AUC) statistics, with AUC values generally around 0.60 (0.58–0.65) [14,28,30].
Higher mammographic density also proved to be associated with higher BC risk, with extremely dense breast tissue being associated with a one-to six-fold increased BC risk [31,32]. Age and BMI are the most important confounders among various factors affecting mammographic density [[33], [34], [35]]. More than 50 % of women younger than 50 have mammographically dense breasts, whereas women in the screening age groups have predominantly breasts with scattered density. Nevertheless, in 2022 the European Society of Breast Imaging recommended that women aged 50–70 years with extremely dense breasts should be offered biennial breast MRI screening [[36], [37], [38]]. Inclusion of mammographic density in risk prediction tools improved performance, both alone and in combination with SNP panels [31,39].
In the UK population the IBIS risk prediction model with the addition of an 18 SNPs PRS and mammographic density accurately divided women into 10-year risk groups [39,40]. The 18 SNPs panel performed well overall, even when compared with panels with larger SNPs cohorts [14,41,42]. However, risk stratification is not transferable from one population to another and extrapolation may lead to both over- and underestimation of risk [14,43,44].
The average BC incidence in Slovenia is 1454 new cases per year with an age-adjusted standardized incidence rate (ASR) of 107.2/100 000 which is below the European ASR of 142.8/100 000 [45,46].
The Slovenian national BC screening programme offers biennial mammography to all women aged 50–69, but approximately 15 % of BC patients in Slovenia are 40–49 years old at the time of diagnosis [46,47]. They usually present with palpable tumours and have an overall higher stage of disease than women of BC screening age. This underscores an unmet clinical need for a risk prediction model that would allow early detection of these patients [46].
The goal of our study was to determine whether the addition of an 18 SNPs PRS to the S-IBIS prediction model would improve the model's accuracy and detection of women aged 40–49 years at above-average risk of BC who would be eligible for early individualised screening in a population with BC incidence below European average.
2. Patients and methods
2.1. Participants
Our case study group included 502 female BC patients aged 40–49 years at the time of diagnosis, treated at the Institute of Oncology Ljubljana between 2018 and 2020. All patients underwent genetic counselling in our Cancer Genetics Clinic and were referred to germline genetic testing as per our protocol [48]. Patients who tested negative for a moderate- and high-penetrance BC susceptibility gene panel were included and completed a questionnaire on risk factors for BC as per the Tyrer-Cuzick algorithm (Table 1) with data known at age 40 and prior to BC diagnosis.
Table 1.
Breast cancer risk factors used for 10-year breast cancer risk calculation with the S-IBIS software. The “age at baseline” for all of our participants was 40 years old.
| Risk factor |
|---|
| Age at baseline |
| Height |
| Weight |
| Age at menarche |
| Age at first childbirth |
| Menopausal status |
| Hormone replacement therapy use |
| Prior benign breast disease |
| Family history of breast and/or ovarian cancer - first- and second-degree relatives (age at diagnosis and current age or age at death) |
Blood samples for DNA isolation were collected either during treatment or at follow-up. 250 DNA samples from healthy women aged 50 years with no previous BC diagnosis were used as a control group.
All participants provided written informed consent. The present study was approved by the Institutional Review Board of the Institute of Oncology Ljubljana and the National Medical Ethics Committee of the Republic of Slovenia and the procedures used met the ethical standards of these bodies.
2.2. Genotyping
DNA was isolated from blood samples as previously published [49]. 18 SNPs associated with BC risk, previously identified via GWAS and validated in the UK population by Evans et al., were genotyped using the allelic discrimination method (Table 2) [42]. Genotyping was performed on the ABI7900 (Thermo Fisher Scientific, Applied Biosystems, Waltham, MA, USA) using the TaqMan SNP Genotyping Assays, TaqMan Genotyping Master Mix (both Thermo Fisher Scientific) and 18 ng of DNA input according to the manufacturer's instructions. Validation using Sanger sequencing was performed on randomly selected samples to ensure the accuracy of TaqMan SNP genotyping. Sanger sequencing was performed as previously described by our group [49].
Table 2.
Panel of chosen 18 single nucleotide polymorphisms (SNP); OR=Odds ratio for the high-risk allele versus the low-risk allele. Adapted after Evans et al.
| SNP | Gene/Locus | Chromosome | Position | Risk allele | OR | TaqMan SNP Genotyping Assay ID |
|---|---|---|---|---|---|---|
| rs614367 | 11q13 | 11 | 69328764 | T | 1.21 | C_591893_10 |
| rs704010 | ZMIZ1 | 10 | 80841148 | T | 1.08 | C_7430570_10 |
| rs713588 | 10q | 10 | 5886962 | A | 0.99 | C_11318810_10 |
| rs889312 | MAP3K | 5 | 56031884 | C | 1.12 | C_8886795_10 |
| rs909116 | LSP1 | 11 | 1941946 | T | 1.17 | C_ 8693148_10 |
| rs1011970 | CDKN2A | 9 | 22062134 | T | 1.07 | C_ 8766774_10 |
| rs1156287 | COX11 | 17 | 53076799 | A | 1.07 | C_ 1229857_10 |
| rs1562430 | 8q24 | 8 | 128387852 | T | 1.17 | C_ 1332306_20 |
| rs2981579 | FGFR2 | 10 | 123337335 | A | 1.27 | C 15885469_10_ |
| rs3757318 | ESR1 | 6 | 151914113 | A | 1.16 | C_ 27475058_20 |
| rs3803662 | TOX3 | 16 | 52586341 | A | 1.24 | C_ 25968567_10 |
| rs4973768 | SLC4A7 | 3 | 27416013 | T | 1.1 | C_ 11561768_10 |
| rs8009944 | RAD51L1 | 14 | 69039588 | C | 1.08 | C_ 2564858_10 |
| rs9790879 | 5p12 | 5 | 44899885 | C | 1.1 | C_ 404998_10 |
| rs10995190 | ZNF365 | 10 | 64278682 | G | 1.16 | C_ 31346611_10 |
| rs11249433 | NOTCH | 1 | 121280613 | G | 1.11 | C_ 31617470_30 |
| rs13387042 | 2q | 2 | 217905832 | A | 1.36 | C_ 32048042_10 |
| rs10931936 | CASP8 | 2 | 202143928 | T | 1.08 | C_ 2960444_10 |
2.3. Breast cancer risk calculation with S-IBIS
10-year BC risk was calculated using the Tyrer-Cuzick BC risk assessment algorithm adapted to Slovenian BC incidence rate (S-IBIS). For each woman we calculated 10-year BC risk in two ways: first, using only the classical (questionnaire-based) risk factors and second, adding PRS18. We considered the lower threshold of 1.3 % for above-average BC risk, as previously determined when adapting the Tyrer-Cuzick BC risk assessment algorithm to Slovenian incidence rate [16].
2.4. Calculation of PRS18
PRS18 was calculated based on published estimates disease odds ratio (OR) for the high-risk allele versus the low-risk allele. We used a previously validated formula in which based on a log-additive risk model, the three genotypes (“non-risk homozygote”, “heterozygote” and “risk homozygote”) have relative risk values of 1, OR, and OR2 for each SNP. We adjusted the risk values to 1/μ, OR/μ, and OR2/μ, where μ is the unscaled population average relative risk, μ = (1- p)2 + 2p (1- p) OR + p2 OR2, with p being the risk allele population frequency. Missing genotypes were assigned a relative risk of 1 [50]. We included effect allele frequencies (EAF) to adjust PRS scores to our population and calibrated the mean PRS to a mean of 1.0 as described in the literature [43,51]. We considered the OR values as reported in the most recent GWAS publications on BC associated SNPs in populations of European ancestry [27,28,52].
2.5. Statistical methods
The predictive power of PRS18 to detect BC was evaluated using a Receiver Operator Characteristic (ROC) curve.
Various regression/classification methods were used to assess whether the data can be used to predict BC. Specifically, four methods were considered: LASSO regression, RIDGE regression, simple logistic regression and random forests. The models were validated using leave-one-out cross-validation (CV), splitting the dataset into a training set from which the model was built, and a testing set which was used to evaluate the model. The training set was used to develop a model in which the 18 SNPs predicted BC incidence as accurately as possible by assigning a regression coefficient to each SNP. We used an additional CV loop to optimize the penalty parameter in LASSO and RIDGE regression minimizing the (cross-validated) deviance based on 10-fold CV.
A Mann-Whitney U test was performed to determine whether there was a difference between the control and case groups for each SNP. In addition, the performance of each SNP as a predictor of BC was assessed by calculating the area under the ROC curve (AUC) for each SNP.
Exact McNemar's test was used to compare sensitivities of risk classification of S-IBIS with and without PRS18.
A possible correlation between the S-IBIS score and/or PRS18 and tumor aggressiveness was analysed by calculating the Pearson coefficient considering locoregional advanced disease and distant metastases at presentation.
2.6. Mammographic density
Mammographic density was not regularly reported at our Institute in the earlier years evaluated in the study. Therefore, only women with a complete mammographic report were included in the analysis of the impact of mammographic density on risk prediction. Either mammographic imaging done prior to diagnosis or contralateral breast mammography was used to assess density. Mammographic density was classified using the BI-RADS 5th edition reporting system, which defines four categories of breast density: extremely fatty (A), scattered density (B), heterogeneous density (C) and extremely dense (D) [53]. 10-year BC risk score was calculated using S-IBIS with the inclusion of mammographic density and a separate analysis comparing S-IBIS with and without mammographic density was performed.
The analysis was performed using R language for statistical computing (R version 3.6.0) [54].
3. Results
Based on the calculation of 10-year BC risk score with S-IBIS using only classical BC risk factors, 83.3 % of cases were classified at above-average risk, with the median value being 1.7 % and the IQR 1.4–2.3 % (min. 0.8 % and max 11.5 %).
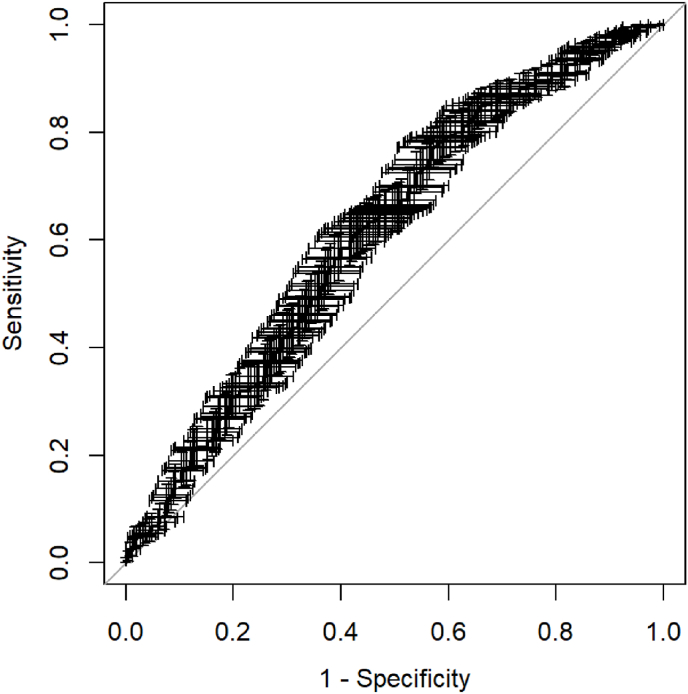
Polygenic risk score based on 18 SNPs was higher in BC patients (mean 1.17, IQR 0.86–1.42, max 3.9) than controls (mean 1.00, IQR 0.67–1.28, max 2.91). The AUC for PRS18 was 0.613 (95 % confidence interval (CI) 0.570–0.657) (Fig. 1).
Fig. 1.
ROC curve for PRS18 as a predictor of breast cancer.The graphical presentation of the error represents the 95 % confidence intervals for both sensitivity and specificity for each individual probability threshold. PRS18 – polygenic risk score based on 18 SNPs.
The AUCs for the 18 SNPs as predictors of BC, with the four regression/classification approaches, were: Lasso regression: 0.588 (95 % CI 0.544–0.631), Ridge regression: 0.591 (95 % CI 0.548–0.635), simple logistic regression: 0.596 (95 % CI 0.552–0.639), random forest: 0.582 (95 % CI 0.538–0.626).
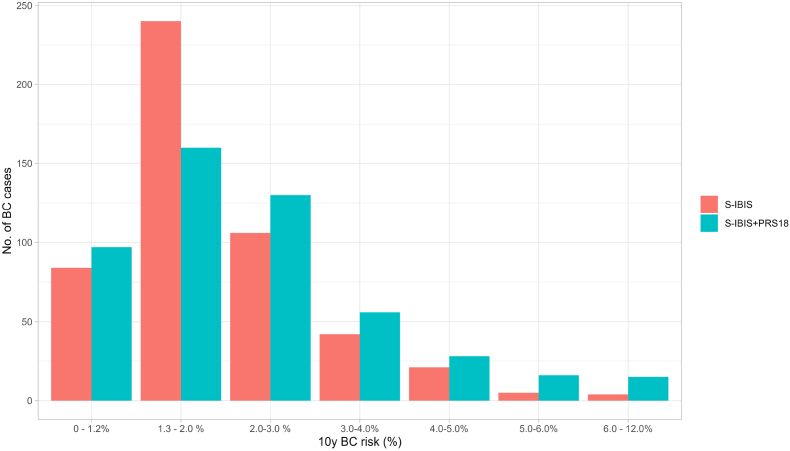
When PRS18 was included in the S-IBIS calculation, the distribution in different risk categories widened, with a minimum and a maximum value of 0.5 % and 12.7 %, respectively, compared with 0.8 % and 11.5 % for S-IBIS alone. With the addition of PRS18 80.7 % of cases were classified at above average risk, with a median value of 2.0 % and an IQR of 1.3%–2.9 %.
The distribution of participants across risk intervals is shown in Fig. 2.
Fig. 2.
Distribution of 10y breast cancer risk at age 40 calculated with S-IBIS with and without PRS18; S-IBIS: 10-year breast cancer (BC) risk score (%) calculated with the S-IBIS tool without PRS18; S-IBIS + PRS18: 10-year BC risk score (%) calculated wih the S-IBIS tool including PRS18. PRS18 – polygenic risk score based on 18 SNPs.
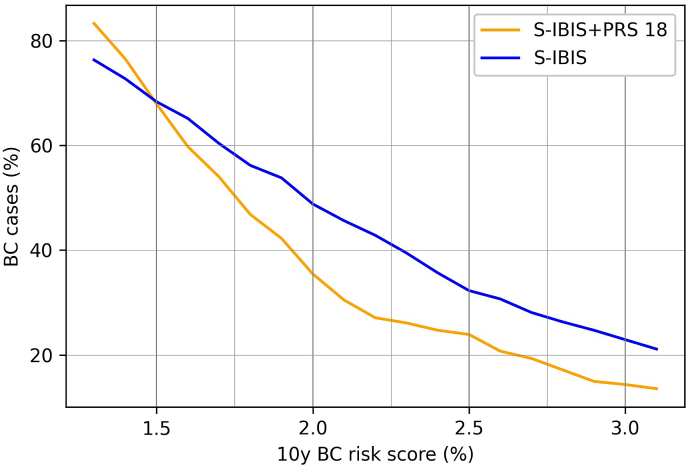
The difference in sensitivity for classification into above-average risk categories between S-IBIS with and without inclusion of PRS18 was statistically significant (p < 0.05). The curves of sensitivity for S-IBIS without PRS18 and with added PRS18 are shown in Fig. 3.
Fig. 3.
Sensitivity of S-IBIS with and without PRS18; S-IBIS: 10-year breast cancer (BC) risk score (%) calculated wih the S-IBIS tool without PRS18 at age 40; S-IBIS + PRS18: 10- year BC risk score (%) calculated wih the S-IBIS tool including PRS18. PRS18 – polygenic risk score based on 18 SNPs.
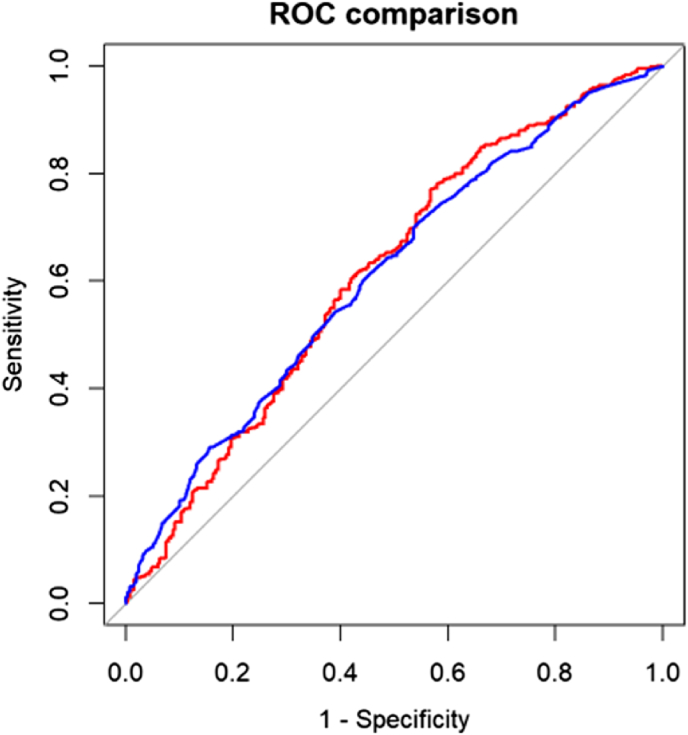
Evaluation of each SNP showed that 5 SNPs were significantly different between the control and case groups as revealed by Mann Whitney U tests (p < 0.1 after Benjamini-Hochberg adjustment): rs889312, rs2981579, rs3803662, rs13387042 and rs3757318. PRS was recalculated using only the selected SNPs and then another ROC curve was generated to evaluate the predictive power of the 5-SNPs PRS to detect BC. The AUC for the 5 SNPs model was 0.611 (95 % CI 0.568–0.654). The comparison between the ROC curve for PRS18 and PRS5 is shown in Fig. 4.
Fig. 4.
Comparison of ROC curves for PRS18 (red) and PRS5 (blue) as predictors of breast cancer.PRS18 – polygenic risk score based on 18 SNPs; PRS5 – polygenic risk score based on 5 “significant” SNPs. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Mammographic density was reported for 412 patients and was distributed among BIRADS categories as follows: 6.5 % BIRADS A, 31.1 % BIRADS B, 42.7 % BIRADS C and 19.7 % BIRADS D. An increase in predicted risk was observed in the group with BIRADS D (1.25–1.45-fold increased risk) and in women with BIRADS C and BMI>25 (1.06-fold increased risk). Adding mammographic density to S-IBIS did not improve classification in the above-average risk categories overall and significantly decreased sensitivity from 83 % to 62 %.
BC molecular subtypes of the participants were as follows: 14.2 % of patients had HER-2 positive disease, 18.1 % had triple negative disease, 31.1 % had Luminal B BC and 36.6 % had Luminal A BC. More than one third of patients (37.1 %) had node-positive disease at presentation and 4.3 % of patients had distant metastases at presentation. Neither a high S-IBIS score nor a high PRS correlated with more aggressive molecular subtype, locally advanced tumour at presentation or distant metastases (p 0.231).
4. Discussion
The main finding of our study is that the addition of an 18-SNPs panel to the Tyrer-Cuzick algorithm adapted to the Slovenian population (S-IBIS) did not significantly improve BC prediction compared with Evans’ original work on the British population [42,55]. Nevertheless, we observed some improvement at higher BC risk scores, that could be valuable in distinguishing women at intermediate and high risk of BC.
This leads to the general conclusion that risk prediction models should not automatically be used in clinical practice in different populations without prior population-based validation.
Interestingly, the performance of PRS18 in our study was better than in the study by Evans et al. and comparable to the performance in studies with larger SNP panels, suggesting that a larger selection of SNPs may not significantly improve AUC values [14,42,56]. Additionally, we found that the PRS, which was calculated from the 5 SNPs that differed significantly between groups, predicted BC with similar accuracy as PRS18. We could not find any study evaluating a PRS based solely on these five SNPs, nor could we find any other research groups selecting smaller SNPs cohorts from larger panels. However, from a cost-effectiveness perspective, eliminating two thirds of SNPs without potentially sacrificing quality is useful information, and we can consider our result as a proof of concept that could potentially be effective for better SNPs selection in the future.
The sample size calculation was based on published data and a predicted reclassification of approximately 10 % of cases if PRS18 were added to S-IBIS [55]. The main reason for the suboptimal performance of PRS18 when added to S-IBIS in our study is that most of our patients presented with multiple risk factors and were classified as at above-average risk for BC regardless of the inclusion of PRS18. In fact, only 5.4 % of patients with high PRS were classified at below-average risk when risk factors without PRS were included in S-IBIS; 4.4 % were reclassified to above-average risk when PRS was added. Still, the addition of PRS resulted in better discrimination between groups and thus reclassified some patients from above-average to average or below-average risk groups.
The lack of improvement in risk prediction with the addition of mammographic density in the model was likely due to the usual distribution of BIRADS categories in our study group. The predominance of the BIRADS-C category is the norm in the 40-49y age group, so no additional risk could be expected [57]. Additionally, BMI was not known for all patients and previous studies have shown that lack of adjustment for BMI and age may lead to underestimation of risk [35]. Since mammographic density was not the focus of our study, further research with more accurate information about BMI would be more informative about the usefulness of mammographic density in BC screening.
According to our results, neither a high S-IBIS score nor a high PRS were associated with a more aggressive molecular subtype, locally advanced tumour at presentation or distant metastases. Indeed, an inverse association between low IBIS score and high tumour aggressiveness has been previously reported in literature [55,58]. Given that hormone-dependent BC, especially luminal A, is the most common BC subtype, it is known that BC risk-prediction tools tend to have an ER + bias. This leads to a difference in IBIS scores in patients with less common but more aggressive subtypes [56,59]. We could explain our results with a selection bias: participants were recruited either during therapy or at follow-up appointments, with the former being commoner than the latter, resulting in a higher percentage of participants with more aggressive diagnoses.
Finally, we would like to highlight the effort and resources we have put into this study. We were very impressed with the published results of the 18-SNPs panel in the UK and hoped to prove its value in our population. The results presented do not justify clinical use of the panel in our setting and further research will be required.
5. Conclusion
The main message of our study is that BC risk prediction models and SNP panels should not be automatically used in clinical practice in different populations without prior population-based validation. PRS18 performed well in our study compared with the results of other studies for larger SNP panels, but it was still only slightly better than a random classifier. The combination of PRS18 and classical risk factors did not perform better than classical risk factors alone in 10-year prediction of BC risk. Nevertheless, we observed some improvement at higher BC risk scores, that could be valuable in distinguishing women at intermediate and high risk of BC. Further prospective studies on different sets of SNPs are needed to optimize risk stratification in our population and achieve individualised screening for young women at moderate and high risk of BC.
Contributions
Oblak Tjaša: design of the study, writing of the manuscript. Škerl Petra: laboratory work, writing of the manuscript. Narang Benjamin J.: statistical analysis. Blagus Rok: design of the study, statistical analysis, writing of the manuscript. Krajc Mateja: design of the study, writing of the manuscript. Novaković Srdjan: design of the study, laboratory work, writing of the manuscript. Žgajnar Janez: design of the study, writing of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The study was supported by the research program of the Slovenian Research Agency (ARRS) P3-0352.
Contributor Information
Tjaša Oblak, Email: toblak@onko-i.si.
Petra Škerl, Email: pskerl@onko-i.si.
Benjamin J. Narang, Email: benjamin.narang@ijs.si.
Rok Blagus, Email: rok.blagus@mf.uni-lj.si.
Mateja Krajc, Email: mkrajc@onko-i.si.
Srdjan Novaković, Email: snovakovic@onko-i.si.
Janez Žgajnar, Email: jzgajnar@onko-i.si.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021 May;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.European Cancer Information System https://ecis.jrc.ec.europa.eu/
- 3.Arnold M., Morgan E., Rumgay H., Mafra A., Singh D., Laversanne M., et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022 Dec;66:15–23. doi: 10.1016/j.breast.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dibden A., Offman J., Duffy S.W., Gabe R. Worldwide review and meta-analysis of cohort studies measuring the effect of mammography screening programmes on incidence-based breast cancer mortality. Cancers. 2020 Apr 15;12(4):976. doi: 10.3390/cancers12040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Koning H.J. Mammographic screening: evidence from randomised controlled trials. Ann Oncol. 2003 Aug;14(8):1185–1189. doi: 10.1093/annonc/mdg319. [DOI] [PubMed] [Google Scholar]
- 6.Duffy S.W., Tabár L., Yen A.M.F., Dean P.B., Smith R.A., Jonsson H., et al. Beneficial effect of consecutive screening mammography examinations on mortality from breast cancer: a prospective study. Radiology. 2021 Jun;299(3):541–547. doi: 10.1148/radiol.2021203935. [DOI] [PubMed] [Google Scholar]
- 7.Duffy S.W., Tabár L., Yen A.M., Dean P.B., Smith R.A., Jonsson H., et al. Mammography screening reduces rates of advanced and fatal breast cancers: results in 549,091 women. Cancer. 2020 Jul;126(13):2971–2979. doi: 10.1002/cncr.32859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Commission Initiative on Breast and Colorectal cancer https://healthcare-quality.jrc.ec.europa.eu
- 9.Clift A.K., Dodwell D., Lord S., Petrou S., Brady S.M., Collins G.S., et al. The current status of risk-stratified breast screening. Br J Cancer. 2022 Mar 9;126(4):533–550. doi: 10.1038/s41416-021-01550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Commission. European Breast Cancer Guidelines: Screening Ages and Frequencies [Internet]. Available from: https://healthcare-quality.jrc.ec.europa.eu/ecibc/european-breast-cancer-guidelines.
- 11.Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. [DOI] [PubMed]
- 12.Gail M.H.B.L., Byar D.P.G.S., Corle D.K.S.C., Mulhvill J.J. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989 Dec 20;81(24):1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 13.Pal Choudhury P., Brook M.N., Hurson A.N., Lee A., Mulder C.V., Coulson P., et al. Comparative validation of the BOADICEA and Tyrer-Cuzick breast cancer risk models incorporating classical risk factors and polygenic risk in a population-based prospective cohort of women of European ancestry. Breast Cancer Res. 2021 Dec;23(1):22. doi: 10.1186/s13058-021-01399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts E., Howell S., Evans D.G. Polygenic risk scores and breast cancer risk prediction. Breast. 2023 Feb;67:71–77. doi: 10.1016/j.breast.2023.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krajc M., Gareth Evans D., Blatnik A., Lokar K., Žagar T., Tomšič S., et al. Screening strategy modification based on personalized breast cancer risk stratification and its implementation in the national guidelines – pilot study. Slovenian J Public Health. 2020 Oct 18;59(4):211–218. doi: 10.2478/sjph-2020-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zadnik V., Krajc M. Development and implementation of personalised breast cancer risk evaluation tool for Slovenian population. Onkologija. 2018 Dec;XXII(2):6–10. [Google Scholar]
- 17.Oblak T., Zadnik V., Krajc M., Lokar K., Zgajnar J. Breast cancer risk based on adapted IBIS prediction model in Slovenian women aged 40–49 years - could it be better? Radiol Oncol. 2020 Jul 2;54(3):335–340. doi: 10.2478/raon-2020-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apostolou P., Fostira F. Hereditary breast cancer: the era of new susceptibility genes. BioMed Res Int. 2013:1–11. doi: 10.1155/2013/747318. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mueller S.H., Lai A.G., Valkovskaya M., Michailidou K., Bolla M.K., Wang Q., et al. Aggregation tests identify new gene associations with breast cancer in populations with diverse ancestry. Genome Med. 2023 Jan 26;15(1):7. doi: 10.1186/s13073-022-01152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiovitz S., Korde L.A. Genetics of breast cancer: a topic in evolution. Ann Oncol. 2015 Jul;26(7):1291–1299. doi: 10.1093/annonc/mdv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antoniou A.C., Easton D.F. Models of genetic susceptibility to breast cancer. Oncogene. 2006 Sep 25;25(43):5898–5905. doi: 10.1038/sj.onc.1209879. [DOI] [PubMed] [Google Scholar]
- 22.Sokolova A., Johnstone K.J., McCart Reed A.E., Simpson P.T., Lakhani S.R. Hereditary breast cancer: syndromes, tumour pathology and molecular testing. Histopathology. 2023 Jan;82(1):70–82. doi: 10.1111/his.14808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torabi Dalivandan S., Plummer J., Gayther S.A. Risks and function of breast cancer susceptibility alleles. Cancers. 2021 Aug 5;13(16):3953. doi: 10.3390/cancers13163953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skol A.D., Sasaki M.M., Onel K. The genetics of breast cancer risk in the post-genome era: thoughts on study design to move past BRCA and towards clinical relevance. Breast Cancer Res. 2016 Dec;18(1):99. doi: 10.1186/s13058-016-0759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins A. The genetics of breast cancer: risk factors for disease. Appl Clin Genet. 2011 Jan:11. doi: 10.2147/TACG.S13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mars N., Koskela J.T., Ripatti P., Ttj Kiiskinen, Havulinna A.S., Lindbohm J.V., et al. Polygenic and clinical risk scores and their impact on age at onset and prediction of cardiometabolic diseases and common cancers. Nat Med. 2020 Apr;26(4):549–557. doi: 10.1038/s41591-020-0800-0. [DOI] [PubMed] [Google Scholar]
- 27.Michailidou K., Lindström S., Dennis J., Beesley J., Hui S., Kar S., et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017 Nov 2;551(7678):92–94. doi: 10.1038/nature24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mavaddat N., Michailidou K., Dennis J., Lush M., Fachal L., Lee A., et al. Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am J Hum Genet. 2019 Jan;104(1):21–34. doi: 10.1016/j.ajhg.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pharoah P.D.P., Antoniou A., Bobrow M., Zimmern R.L., Easton D.F., Ponder B.A.J. Polygenic susceptibility to breast cancer and implications for prevention. Nat Genet. 2002 May;31(1):33–36. doi: 10.1038/ng853. [DOI] [PubMed] [Google Scholar]
- 30.Chatterjee N., Shi J., García-Closas M. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat Rev Genet. 2016 Jul;17(7):392–406. doi: 10.1038/nrg.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eriksson M., Czene K., Vachon C., Conant E.F., Hall P. Long-term performance of an image-based short-term risk model for breast cancer. J Clin Oncol. 2023 Mar 17;22 doi: 10.1200/JCO.22.01564. JCO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darabi H., Czene K., Zhao W., Liu J., Hall P., Humphreys K. Breast cancer risk prediction and individualised screening based on common genetic variation and breast density measurement. Breast Cancer Res. 2012 Feb;14(1):R25. doi: 10.1186/bcr3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Razzaghi H., Troester M.A., Gierach G.L., Olshan A.F., Yankaskas B.C., Millikan R.C. Mammographic density and breast cancer risk in White and African American Women. Breast Cancer Res Treat. 2012 Sep;135(2):571–580. doi: 10.1007/s10549-012-2185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Checka C.M., Chun J.E., Schnabel F.R., Lee J., Toth H. The relationship of mammographic density and age: implications for breast cancer screening. Am J Roentgenol. 2012 Mar;198(3):W292–W295. doi: 10.2214/AJR.10.6049. [DOI] [PubMed] [Google Scholar]
- 35.Bodewes F.T.H., Van Asselt A.A., Dorrius M.D., Greuter M.J.W., De Bock G.H. Mammographic breast density and the risk of breast cancer: a systematic review and meta-analysis. Breast. 2022 Dec;66:62–68. doi: 10.1016/j.breast.2022.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sprague B.L., Gangnon R.E., Burt V., Trentham-Dietz A., Hampton J.M., Wellman R.D., et al. Prevalence of mammographically dense breasts in the United States. JNCI J Natl Cancer Inst. 2014;106(10) doi: 10.1093/jnci/dju255. https://academic.oup.com/jnci/article-lookup/doi/10.1093/jnci/dju255 2023 May 2. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mann R.M., Athanasiou A., Baltzer P.A.T., Camps-Herrero J., Clauser P., Fallenberg E.M., et al. Breast cancer screening in women with extremely dense breasts recommendations of the European Society of Breast Imaging (EUSOBI) Eur Radiol. 2022 Jun;32(6):4036–4045. doi: 10.1007/s00330-022-08617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wanders J.O.P., Holland K., Karssemeijer N., Peeters P.H.M., Veldhuis W.B., Mann R.M., et al. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: a cohort study. Breast Cancer Res. 2017 Dec;19(1):67. doi: 10.1186/s13058-017-0859-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Veen E.M., Brentnall A.R., Byers H., Harkness E.F., Astley S.M., Sampson S., et al. Use of single-nucleotide polymorphisms and mammographic density plus classic risk factors for breast cancer risk prediction. JAMA Oncol. 2018 Apr 1;4(4):476. doi: 10.1001/jamaoncol.2017.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans D.G.R., Harkness E.F., Brentnall A.R., van Veen E.M., Astley S.M., Byers H., et al. Breast cancer pathology and stage are better predicted by risk stratification models that include mammographic density and common genetic variants. Breast Cancer Res Treat. 2019 Jul;176(1):141–148. doi: 10.1007/s10549-019-05210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brentnall A.R., Evans D.G., Cuzick J. Distribution of breast cancer risk from SNPs and classical risk factors in women of routine screening age in the UK. Br J Cancer. 2014 Feb;110(3):827–828. doi: 10.1038/bjc.2013.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans D.G., Brentnall A., Byers H., Harkness E., Stavrinos P., Howell A., et al. The impact of a panel of 18 SNPs on breast cancer risk in women attending a UK familial screening clinic: a case–control study. J Med Genet. 2017 Feb;54(2):111–113. doi: 10.1136/jmedgenet-2016-104125. [DOI] [PubMed] [Google Scholar]
- 43.Evans D.G., Veen E.M., Byers H., Roberts E., Howell A., Howell S.J., et al. The importance of ethnicity: are breast cancer polygenic risk scores ready for women who are not of White European origin? Int J Cancer. 2022 Jan;150(1):73–79. doi: 10.1002/ijc.33782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang S., Qian F., Zheng Y., Ogundiran T., Ojengbede O., Zheng W., et al. Genetic variants demonstrating flip-flop phenomenon and breast cancer risk prediction among women of African ancestry. Breast Cancer Res Treat. 2018 Apr;168(3):703–712. doi: 10.1007/s10549-017-4638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.European Cancer Information System (ECIS) Estimates of Breast Cancer Incidence and Mortality [Internet]. Chart "Estimated incidence by cancer, EU 27, Female, All cancers". https://ecis.jrc.ec.europa.eu/explorer.php?$0-0$1-AE27$2-All$4-2$3-All$6-0,85$5-2020,2020$7-7$CEstByCancer$X0_8-3$CEstRelativeCanc$X1_8-3$X1_9-AE27$CEstBySexByCancer$X2_8-3$X2_-1-1 Available from:
- 46.Epidemiology and Cancer Registry . Institute of Oncology Ljubljana; 2022. Slovenian cancer registry. Cancer in Slovenia 2019.https://www.onko-i.si/fileadmin/onko/datoteke/rrs/lp/Letno_porocilo_2019.pdf Available from: [Google Scholar]
- 47.Jarm K., Kadivec M., Šval C., Hertl K., Primic Žakelj M., Dean P.B., et al. In: Quality assured implementation of the Slovenian breast cancer screening programme. Mahumud R.A., editor. vol. 16. PLOS ONE; 2021 Oct 8. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dragoš V.Š., Strojnik K., Klančar G., Škerl P., Stegel V., Blatnik A., et al. Identification of spliceogenic variants beyond canonical GT-AG splice sites in hereditary cancer genes. Int J Mol Sci. 2022 Jul 4;23(13):7446. doi: 10.3390/ijms23137446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stegel V., Krajc M., Žgajnar J., Teugels E., De Grève J., Hočevar M., et al. The occurrence of germline BRCA1 and BRCA2sequence alterations in Slovenian population. BMC Med Genet. 2011 Dec;12(1):9. doi: 10.1186/1471-2350-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mealiffe M.E., Stokowski R.P., Rhees B.K., Prentice R.L., Pettinger M., Hinds D.A. Assessment of clinical validity of a breast cancer risk model combining genetic and clinical information. JNCI J Natl Cancer Inst. 2010 Nov 3;102(21):1618–1627. doi: 10.1093/jnci/djq388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts E., Van Veen E.M., Byers H., Barnett-Griness O., Gronich N., Lejbkowicz F., et al. Breast cancer polygenic risk scores derived in White European populations are not calibrated for women of Ashkenazi Jewish descent. Genet Med. 2023 Sep;25(9) doi: 10.1016/j.gim.2023.100846. [DOI] [PubMed] [Google Scholar]
- 52.Mavaddat N., Pharoah P.D.P., Michailidou K., Tyrer J., Brook M.N., Bolla M.K., et al. Prediction of breast cancer risk based on profiling with common genetic variants. JNCI J Natl Cancer Inst. 2015 May;107(5) doi: 10.1093/jnci/djv036. https://academic.oup.com/jnci/article-lookup/doi/10.1093/jnci/djv036 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sickles E., D'Orsi C., Basset L. ACR BI-RADS® atlas, breast imaging report-ing and data system. American College of Radiology; Reston, VA: 2013. ACR BI-RADS® mammography. [Google Scholar]
- 54.R Core Team. R . R Foundation for Statistical Computing; Vienna, Austria: 2021. A language and environment for statistical computing.https://www.R-project.org/ Available from: [Google Scholar]
- 55.Van Veen E.M., Brentnall A.R., Byers H., Harkness E.F., Astley S.M., Sampson S., et al. Use of single-nucleotide polymorphisms and mammographic density plus classic risk factors for breast cancer risk prediction. JAMA Oncol. 2018 Apr 1;4(4):476. doi: 10.1001/jamaoncol.2017.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brane A., Behring M., Halilova K.I., Norian L. Comments on “Association between breast cancer risk and disease aggressiveness: characterizing underlying gene expression patterns.”. Int J Cancer. 2021 Sep 15;149(6) doi: 10.1002/ijc.33605. 1398–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohmaru A., Maeda K., Ono H., Kamimura S., Iwasaki K., Mori K., et al. Age-related change in mammographic breast density of women without history of breast cancer over a 10-year retrospective study. PeerJ. 2023 Feb 14;11 doi: 10.7717/peerj.14836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ugalde‐Morales E., Grassmann F., Humphreys K., Li J., Eriksson M., Tobin N.P., et al. Association between breast cancer risk and disease aggressiveness: characterizing underlying gene expression patterns. Int J Cancer. 2021 Feb 15;148(4):884–894. doi: 10.1002/ijc.33270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holm J., Li J., Darabi H., Eklund M., Eriksson M., Humphreys K., et al. Associations of breast cancer risk prediction tools with tumor characteristics and metastasis. J Clin Oncol. 2016 Jan 20;34(3):251–258. doi: 10.1200/JCO.2015.63.0624. [DOI] [PubMed] [Google Scholar]