Abstract
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs) are enzymes that generate superoxide anion (O2•-) and hydrogen peroxide (H2O2), and that are widely distributed in mammalian tissues. Many bioactives, especially plant (poly)phenols are being studied for their capacity to regulate NOXs. The modulation of these enzymes are of central relevance to maintain redox homeostasis and regulate cell signaling.
In in vitro and ex vivo assays, and in experimental animal models, different (poly)phenols are able to modulate NOX-dependent generation of O2•- and H2O2. Mechanistically, most of the known effects of (poly)phenols and of their metabolites on NOX1, NOX2, and NOX4, include the modulation of: i) the expression of the different constituent subunits, and/or ii) posttranslational modifications involved in the assembly and translocation of the protein complexes. Very limited evidence is available on a direct action of (poly)phenols on NOX active site (electron-transferring protein). Moreover, it is suggested that the regulation by (poly)phenols of systemic events, e.g. inflammation, is frequently associated with their capacity to regulate NOX activation. Although of physiological significance, more studies are needed to understand the specific targets/mechanisms of NOX regulation by (poly)phenols, and the (poly)phenol chemical structures and moieties directly involved in the observed effects. It should be kept in mind the difficulties of NOX's studies associated with the complexity of NOXs biochemistry and the methodological limitations of O2•- and H2O2 the determinations. Studies relating human ingestion of specific (poly)phenols, with NOX activity and disease conditions, are guaranteed to better understand the health importance of (poly)phenol consumption and the involvement of NOXs as biological targets.
Keywords: Flavonoid, Antioxidant, Oxidative stress, Inflammation, Microbiota
Graphical abstract
Abbreviations:
- LPS
lipopolysaccharide
- NOX
NADPH-oxidase
- PBMC
peripheral blood mononuclear cell
- RFM
ring fission metabolites
- SRPM
structurally related polyphenol metabolites
- TNFα
tumor necrosis factor alpha
1. Introduction
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, or NADPH-oxidases (NOXs) are enzymes that have attracted extensive research because of their ability to generate superoxide anion (O2•-) and hydrogen peroxide (H2O2), and of their widespread distribution in mammalian tissues. The modulation of these enzymes can be of relevance to maintain redox homeostasis and regulate cell signaling. Many plant bioactives consumed as part of human diets are studied for their capacity to mitigate different pathophysiological conditions, largely as negative regulators of NOXs. This review summarizes and discusses current information associating the consumption of a class of bioactives, i.e. (poly)phenols, with the regulation of NOXs and their potential health effects. Besides studies in humans and experimental animal, we include select in vitro studies when results address mechanistic concepts of physiological relevance. Overall, the gathered information intends to give support to the possibility that NOX regulation could occur because of the ingestion of (poly)phenols.
2. Bioactives and plant (poly)phenols
Bioactives is a term that intends to group substances that the animals could need to optimize its function and sustain health. In humans, a deficiency of bioactives is not linked to known disease states. Bioactives can be ingested as components of foods, as dietary supplements or as part of pharmacological formulas. Other commonly used terms equivalent to ‘bioactive’ are food factor, nutraceutical, or, in many cases phytonutrient [1].
Based on the above definition thousands of compounds can be considered bioactives, including those from different origin, mechanism of action, and organ/tissue specificity. This heterogeneity results in myriads of potential biological actions. To narrow down this immense matrix, this review is focused on plant (poly)phenols, i.e., polyphenols (molecules with multiple phenolic rings) and phenolics (molecules with a single phenolic ring). Plant (poly)phenols are secondary metabolites mostly involved in plant structure and responses to environmental stimuli. Thousands of chemical structures are synthetized by plants and a few hundred are part of plants consumed in human diets [2]. The chemical structures of representative plant (poly)phenols are depicted in Supplemental Material.
Within dietary (poly)phenols, we focus on those compounds that would be of physiological relevance because: i) have been reported to exert significant effects in defined organs/systems; and ii) are molecules which absorption, distribution, metabolism, and excretion (ADME) are known. Additionally, we select data from studies using purified (poly)phenols or their metabolites, and/or plant extracts well characterized in terms of (poly)phenol composition.
3. Oxidants, antioxidants, redox homeostasis and oxidative stress
Extensive research during the last decades has allowed to establish the relevance of redox reactions in biological systems. Thus, the constant consumption of oxygen in aerobic organisms, leads to the production of chemical species that result from the partial reduction of oxygen, i.e., O2•-, H2O2 and hydroxyl radical, which are generically termed oxidants. These species can interact chemically with biological targets, e.g. proteins, lipids, nucleic acids, and carbohydrates, causing their oxidation and potential loss of their biological function. In the case of proteins, their reversible oxidation can lead to redox events, including modulation of redox signaling [3].
Antioxidant defenses protect the body from the undesired oxidation of cellular components, and from oxidant-mediated changes in cellular functions. Antioxidant strategies include enzymes able to metabolize oxidants, e.g. superoxide dismutase, catalase, and peroxidases, enzymes that repair the damage, and non-enzymatic antioxidant defenses [4]. The non-enzymatic defenses can act by: i) directly reacting with oxidants, through redox reactions, and then decreasing or cancelling oxidant reactivity; ii) directly sequestering metal transition ions involved in catalyzing oxidant production; iii) indirectly inhibiting oxidant production through their interaction with oxidant-producing enzymes; or iv) modulating pathways that lead to increased oxidant production, e.g. NF-κB pathway [5].
The maintenance of redox homeostasis is normally associated with reversible changes in oxidant production, and/or with increases in antioxidant defenses. When the capacity of the biological system to respond to the oxidant insult is overwhelmed, an irreversible damage can occur with undesirable consequences. This condition is defined as oxidative stress, and more specifically, oxidative distress [6].
4. (Poly)phenols, and (poly)phenol metabolites as biological antioxidants
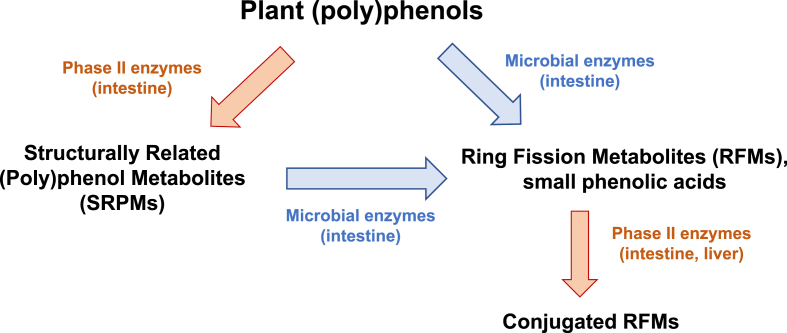
(Poly)phenols can act as direct or indirect antioxidants. During decades, and still in many laboratories, the ‘antioxidant capacity’ of these bioactives has been studied as a mechanism to explain their health benefits [7]. The faulty interpretation of these studies is that (poly)phenols exert beneficial health effects because of their direct reaction with oxidants, i.e., direct antioxidant effects. This assumption neglects the fact that a chemical reaction between an oxidant and a (poly)phenol needs to occur at a rate that is physiologically significant for a biological system. Although thermodynamically feasible, this direct antioxidant reaction will be physiologically relevant only when a large-enough amount of the (poly)phenol is available at the proper place to generate a physiological change [8,9]. In mammals, most of the direct antioxidant actions of (poly)phenols would be only relevant in the upper digestive tract given that, once in the intestine ingested (poly)phenols are extensively metabolized (Fig. 1) [10]. Such metabolism is basically mediated by: i) phase II enzymes in enterocytes, generating structurally related (poly)phenol metabolites (SRPM); or ii) by the resident microbiota which enzymatically generates both, ring fission metabolites (RFM) and small phenolic acids. Moreover, these microbiota metabolites can be subject to additional biotransformation by phase II enzymes (Fig. 1). In brief, either the ingested (poly)phenols or these metabolites can be responsible for the indirect antioxidant actions of (poly)phenols.
Fig. 1.
Metabolism of plant (poly)phenols ingested by humans and rodents. Depending on their chemical structures, (poly)phenols can be metabolized by: i) intestinal phase II enzymes to produce metabolites that maintain most of the original structure: Structurally Related (Poly)phenol metabolites (SRPMs), e.g. glucuronidated, sulfated, and methylated conjugates of the ingested (poly)phenols; ii) microbial enzymes that are able to breakdown the benzene rings to produce Ring Fission Metabolites (RFMs), e.g. small phenolic acids and other small molecules (γ-valerolactones, C6–C3 phenylpropanoic acids, C6–C2 phenylacetic acids, C6–C1 benzoic acids, hydroxyhippuric acid, etc.). These RFMs can be substrates for intestinal phase II enzymes producing their conjugated derivatives resulting in a diverse and complex mixture of compounds, including from larger (e.g. flavonoid conjugates) to smaller molecules (e.g. hydroxyhippuric acid).
In this scenario, indirect antioxidant actions can explain the observed systemic physiological effects of many (poly)phenols. For example, the actions of (poly)phenols regulating NOXs, would explain many of their beneficial health effects. The lower concentrations of (poly)phenols (and their metabolites) necessary to exert such indirect antioxidant actions are compatible with those found in cells and tissues [8,9]. In summary, the regulation of NOXs by (poly)phenols is a relevant example of their indirect antioxidant effects that are of potential relevance for human physiology.
5. NADPH-oxidase (NOX) activity
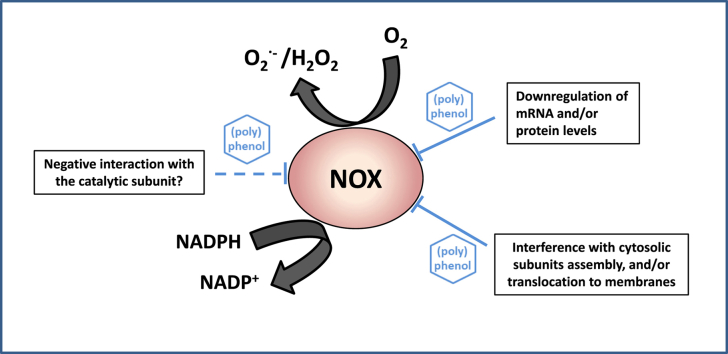
NOX are oxidoreductases that catalyse the one-electron reduction of O2 to O2•- and the oxidation of NADPH (Fig. 2). The NOX active site (electron-transferring protein) is composed by a C-terminal cytoplasmic region that incorporates the NADPH, and N-terminal transmembrane segments containing two heme-groups that release O2•- [11].
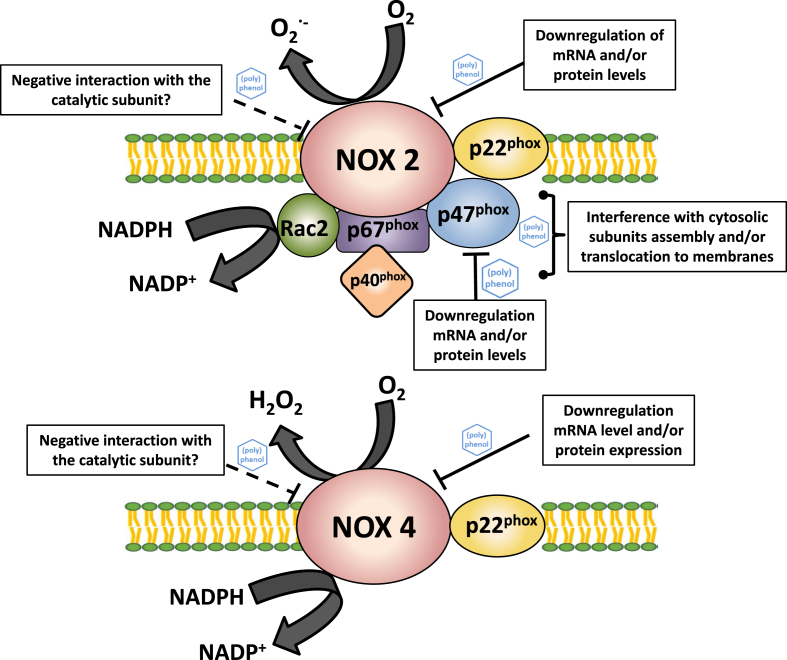
Fig. 2.
Schematic structures of NOX2 and NOX4 and potential sites for their regulation by (poly)phenols. For NOX1 regulation, interactions are similar to those on NOX2 (considering differences in the respective subunits).
The first NOX activity studied was that present in polymorphonuclear phagocytes, which is involved in cell bactericidal actions [12,13]. Subsequent investigations demonstrated the ubiquitous presence of NOXs in non-phagocytic cells. The NOX family known so far is constituted by seven isoforms: NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1 and DUOX2 [14]. These enzymes have been shown to participate in numerous physiological and pathological processes, beyond their well-known contribution to the immune response [15].
A way of organizing NOX isoforms is according to their activation mechanisms:
-
i)
NOX1, NOX2, and NOX3 are activated through the assembly of several subunits in a protein complex, and its translocation to the cell membrane. Essentially, each isoform has a catalytic transmembrane subunit (NOX1, NOX2 -also called gp91phox-, or NOX3), a stabilizer membrane subunit (p22phox), and organizer/activator cytosolic subunits, e.g. NOXO1 and NOXA1 for NOX1; p47phox and p67phox for NOX2; NOXO1, NOXA1, and p67phox for NOX3. The activation of these isoforms requires the phosphorylation of the organizer subunits, and NOX2 in leucocytes requires an extra subunit, p40phox. Finally, an additional subunit, G-protein Rac, binds to the NOX complex to complete the activation of these isoforms to be able to produce O2•- [14].
-
ii)
NOX4 is a constitutive isoform with no requirement of cytosolic subunits, being its activity solely determined by the expression of the catalytic transmembrane proteins NOX4 and p22phox [16]. To note, NOX4 releases H2O2 because its special topology facilitates the dismutation of the O2•- generated in the active site [17].
-
iii)
NOX5, DUOX1 and DUOX2 are activated by calcium and protein phosphorylation [18,19], with no requirement of cytosolic subunits. NOX5 produces exclusively O2•-, and DUOX1 and DUOX2 release both O2•- and H2O2 [14].
NOX1, NOX2 and NOX4 are the most studied isoforms in physiological and pathological conditions given that they are the most widely distributed NOXs in mammalian cells and tissues. Intracellularly, NOX1 and NOX2 are located at the cell membrane, whereas NOX4 has been detected in several alternative locations, as the nuclear membrane, endoplasmic reticulum, and mitochondria [20,21].
Other isoforms have received less attention because of their highly specific localization, e.g. NOX3 is restricted to the inner ear and some fetal tissues [[22], [23], [24]], and DUOX1/2 to the thyroid and lungs. NOX5, although present in human tissues, has been less studied because its absence in rodents [25] limits experimental studies.
6. NADPH-oxidase (NOX) regulation
The assessment of NOXs activity is not simple, essentially because of their ubiquitous and highly reactive substrates and products. In addition, the identification of NOX regulators faces other challenges: i) the diversity of isoforms and the complexity of the enzyme structure; and ii) the experimental strategies used for measuring NOX products.
As explained above, in terms of enzyme structure and activation, NOX1 and NOX2 are substantially different from NOX4. The regulation (activation or inhibition) of NOX1 and NOX2 can occur at several levels including: i) the expression of transmembrane and/or cytosolic subunits; ii) disruption of the assembly/translocation process and of posttranslational modifications; and iii) a direct action on the protein complex (enzyme already assembled), i.e. the regulator directly acting on the protein structure and/or on the active center. In the case of NOX4, its regulation does not involve an assembly process, being only dependent on the amount of protein and/or on a direct action of the regulator on the enzyme [26].
In terms of methodologies, most studies evaluating NOXs activities use chemical probes that react with O2•- and/or H2O2 generating colorimetric, fluorometric, or chemiluminescent products [27]. These probes do not always present specificity for O2•- and H2O2, and any assayed compound can compete with the probes for their reactions with O2•- or H2O2. In both situations, there will be a misestimation of NOX activity that would provide unreliable information about the regulation of the enzyme. Such methodological limitations can be minimized. For example, in in vitro conditions, controls with superoxide dismutase and/or catalase can be included to specifically remove O2•- and H2O2. Additionally, working with the assayed compounds at concentrations below the range of their oxidant scavenging capacity will prevent the artifactual estimation of NOX activity [28].
From a practical point of view, measuring decreases in O2•- and H2O2 generation will not allow to establish how NOX activity is modified. To evaluate such mechanisms of activation/inactivation it is necessary the concurrent determination of other parameters including protein expression, activation of subunits, translocation/assembly ratio, and posttranslational modifications.
In addition, other open questions in the knowledge of NOX physiology include: i) if inhibiting NOXs is beneficial or damaging in a specific physiological condition; and ii) if the different NOXs are regulated differentially in terms of their time course of activation/inhibition, the inhibition/activation degree, and the overall coordination between inhibition/activation of the different isoforms expressed in a cell type.
7. NOX regulation by (poly)phenols
In this section we will summarize current evidence on how (poly)phenols can act on NOXs modifying their expression, and/or their capacity to generate O2•- or H2O2. These oxidants, when are present in excess, cause changes in redox homeostasis and oxidative damage to biomolecules, events that are closely associated with inflammation. Therefore, oxidants and inflammation are cause and consequence of multiple pathologies including for example, cardiovascular, kidney and intestinal diseases, diabetes and obesity, and neurodegeneration. Thus, by decreasing excessive production of oxidants, (poly)phenols would be mitigating pathological conditions. However, it also has to be considered that negative regulation of NOXs can have undesirable side effects, e.g. the inhibition of intestinal NOX1 was associated with increased susceptibility to intestinal infection [29].
Additionally, extreme care should be taken when interpreting results addressing the regulation of NOX by (poly)phenols outside studies in animals or humans. It must be considered that being (poly)phenols extremely reactive compounds, their presence can give rise to experimental artifacts when assayed in cell cultures and in in vitro enzyme activity determinations.
7.1. (Poly)phenols and NOX1
The regulation of NOX1 involves several steps including protein expression and enzyme assembly (Fig. 2). We will mainly focus on the effect of (poly)phenols on intestinal NOX1 given the abundance of NOX1 in this tissue, particularly in the colon.
Caco-2 (human intestinal epithelial) cells are used to mimic the intestinal epithelium exposed to food and xenobiotic substances. Treatment of Caco-2 cells, differentiated into mature enterocytes, with tumor necrosis factor-alpha (TNFα), lipopolysaccharide (LPS), bile acids, cholesterol and cholesterol oxidation products induce NOX1 gene, and/or protein expression, and/or oxidant production associated to NOX1 activity [[30], [31], [32], [33], [34], [35], [36]]. Several (poly)phenols attenuate NOX1 upregulation and the associated increase in oxidant production. Such effects were observed for select wine (poly)phenols, e.g. (−)-epicatechin and caffeic acid [31], anthocyanins (cyanidin-3-O-glucoside, delphinidin-3-O-glucoside, peonidin-3-O-glucoside) and for their RFM, protocatechuic acid [32]. Also for flavanols, e.g. (−)-epicatechin [33] and epigallocatechin-3-gallate [34]; ellagic acid [35]; and curcumin [36]. In a cell model of colorectal cancer, (−)-epicatechin gallate and (−)-epigallocatechin gallate dimers not only decreased epidermal growth factor-induced NOX1 transcription, not affecting mRNA stability, but decreased the enzyme activity [37]. Molecular modeling supports that the interaction of both dimers with NOX1 flavin adenine dinucleotide (FAD)-binding pockets through hydrogen bonds and hydrophobic interactions, can be mediating NOX1 inhibition [37].
Increases in NOX1 mRNA and NOX1 protein expression triggered by LPS were suppressed in Caco-2 cells pretreated with trans-resveratrol [38]. In Caco-2 cell monolayers, the bile acid deoxycholic acid (DCA) causes a rapid increase in oxidant production, probably associated to NOX1 activation, although NOX1 expression is not affected [39]. NOX1 activation was decreased by (−)-epicatechin [39] and by a series of procyanidins [40,41].
In mice, long-term consumption of a high fat diet causes an increased expression of the NOX1 catalytic subunit and supplementation with both (−)-epicatechin [42], and an anthocyanin (cyanidin and delphinidin)-rich extract [32,36] mitigates the upregulation of NOX1 in the colon and/or the ileum.
Regarding the cytosolic subunits NOXO1 and NOXA1, there are no reports on their regulation by (poly)phenols in the intestine. One report showed that cyanidin-3-glucoside, at a supra-physiological concentration (50 μM), mitigated NOXA1 overexpression induced by TNFα in vascular smooth muscle cells [43]. By contrast, other report found that (−)-epicatechin administration did not affect NOXO1 expression in kidneys from N(ω)-nitro-l-arginine methyl ester (l-NAME)-hypertensive rats [44].
In summary, NOX1 activity and expression can be modulated by different (poly)phenols. This is particularly relevant for intestinal health, given that its epithelium is exposed to large amounts of both, non-metabolized (poly)phenols and their phase II- and microbiota-generated metabolites. In addition, the potential associations among (poly)phenols presence, NOX1 and intestinal barrier permeability, makes the modulation of this enzyme of high relevance for both, intestinal physiology and pathological conditions, e.g. colorectal cancer. However, this idea is mainly based on in vitro and rodent studies, with no experimental evidence reported in humans.
7.2. (Poly)phenols and NOX2
Given that NOX2 was the first NOX isoform described. and being abundant in phagocytic cells, early studies on the effects of (poly)phenols on NOX2 were mostly carried out during the phagocyte respiratory burst by measuring either O2•- production or O2 consumption [45]. In experiments exposing phagocytic cells to in vitro stimuli, some (poly)phenols, i.e. quercetin, morin, and rutin, diminish O2•- production. However, given that (poly)phenols were tested at high concentrations (10–500 μM), their O2•- scavenging capacity complicates the interpretation of the results [46,47]. Findings that, in human polymorphonuclear leukocytes, quercetin (100 μM) inhibited the respiratory burst when evaluated as O2 consumption, suggest an effect on NOX2 activity beyond the potential O2•- scavenging action. Authors proposed that quercetin exerts a generalized effect on the cell membrane [48], as was also proposed for (−)-epicatechin and procyanidins [49].
In terms of the molecular mechanisms involved in the regulation of phagocytic NOX2 by (poly)phenols (Fig. 2), it was described that: i) luteolin decreases gp91phox mRNA levels and p47phox translocation to the membrane in monocytes differentiated into macrophages [50]; ii) eupalin decreases p47phox membrane translocation in human fibroblast [51]; and iii) morin decreases gp91phox and p47phox protein levels, and p47phox phosphorylation in microglial cells [52].
The effects of (poly)phenols on NOX2 were also studied in non-phagocytic cells. We will focus on endothelial cells to describe potential mechanisms of action for circulating (poly)phenols that can also be operative in other cell types. Using human umbilical vein endothelial cells (HUVEC) incubated in the presence of either (−)-epicatechin or its phase-II metabolites, 3'- and 4'-O-methyl epicatechin, the SRPM but not the non-metabolized compound, inhibited NOX2. Such inhibition was mechanistically paralleled with that afforded by apocynin [53,54]. These pioneer studies suggested that a specific chemical structure of SRPMs, i.e. the methoxy-group, is relevant for their capacity to inhibit NOX2 activity as measured by NADPH consumption.
In addition, further studies on the molecular mechanisms involved in the regulation of non-phagocytic NOX2 by (poly)phenols showed that puerarin decreases p47phox expression in vascular smooth muscle cells [55]; and that quercetin decreases gp91phox mRNA and protein levels [56].
In experimental animals, the effects of (poly)phenols on NOX2 have been mostly studied in rodent models in which variations in the expression of NOX2 subunits were triggered by unhealthy challenges. For example, in the heart and blood vessels of hypertensive rodents it was observed that: i) puerarin [57], vaccarin [58], and nobiletin [59] decreased the expression of gp91phox; and ii) curcumin [60], (−)-epicatechin [61,62] and naringin [63] decreased p47phox expression. Similar actions decreasing NOX2 subunits (gp91phox and p47phox) expression were observed for other (poly)phenols in different tissues and conditions, e.g. nobiletin in liver from high fat diet-fed rats [64] and (−)-epicatechin in kidney from high fructose diet-fed rats [65] and LPS-treated rats [66].
Results from clinical studies in healthy populations do not show clear in vivo effects of (poly)phenols or (poly)phenol-rich foods on phagocytic NOX2. Most published studies were carried out supplementing healthy volunteers with (poly)phenol-enriched supplements or pure (poly)phenols, isolating phagocytic cells from blood, and measuring the respiratory burst stimulated in vitro. Consumption of a blueberry extract by healthy individuals reduced NOX2 activity in peripheral blood mononuclear cell (PBMCs), 1–6 h after consumption [67]. The administration of soluble mate tea during 8 d decreased protein levels of p47phox in non-stimulated immune cells [68]. By contrast, studies reported that quercetin [[69], [70], [71]] or red wine consumption [72,73] do not modify the respiratory burst in phagocytes from healthy volunteers. A cyanidin- and delphinidin-rich extract provided together with a high-fat meal do not modify NOX2 (gp91phox) expression in PBMCs [74]. In individuals under hemodialysis, supplementation with a concentrated red grape juice (and vitamin E) reduced PBMCs NOX2 activity [75].
Acute administration of dark chocolate to smokers [76], and to patients with peripheral arterial diseases [77], decreased the serum level of a soluble derived peptide from NOX2, i.e. sNOX2‐dp. However, the determination of sNOX2‐dp as indicative of NOX2 activation is not widely used, which reduces the significance of these results.
In summary, the presented studies suggest the regulation of NOX2 subunits expression, and/or of the assembly of the NOX2 complex, as the main mechanisms involved in NOX2 regulation by (poly)phenols in both, phagocytic and non-phagocytic cells.
7.3. (Poly)phenols and NOX4
NOX4 is NOX isoform present in different mammalian cells. Its activity only depends on gene expression and/or protein levels of the different subunits, since NOX4 does not need an assembly process for activation (Fig. 2). We will focus our analysis on the effects of (poly)phenols on NOX4 in kidneys due to its abundance in this tissue.
In renal cells, different (poly)phenols prevented the increase in NOX4 protein levels triggered by exposure to high glucose concentrations: i) (−)-epicatechin and one of its RFM, 2,3-dihydroxybenzoic acid in proximal tubular cells [78]; ii) morin in rat glomerular mesangial cells [79]; and iii) naringin in rat podocytes [80]. However, in the last two studies supraphysiological concentrations (10–80 μΜ) of polyphenols were used. In terms of the effects of RFM, it was observed that hydroxyhippuric acid, benzoic acid-4-sulfate, isovanillic acid-3-sulfate, and vanillic acid- 4-sulfate, but not the non-metabolized anthocyanidins decreased the levels of NOX4 mRNA in human aortic endothelial cells (HAEC) treated with palmitate [81].
In animal models, regulation of renal NOX4 was reported for: i) puerarin, punicalagin, and naringenin in diabetic nephropathy in mice [80,82,83]; ii) puerarin, and icariin in fibrosis induced by unilateral ureteral obstruction in mice [84,85]; iii) nobiletin, galangin, and genistein in models of two-kidney, one-clip hypertension in rats [[86], [87], [88]]; iv) (−)-epicatechin in hypertensive rats [44,89] and rats subjected to acute endotoxemia [66]; and v) procyanidin B2 in adriamycin-induced nephrotic syndrome in mice.
To the best of our knowledge, no clinical studies are reported on the effects of (poly)phenols on renal NOX4. On the other hand, the reduction of NOX4 mRNA levels in PBMCs was reported in humans consuming an extract rich in cyanidin and delphinidin [74].
In summary, the analyzed' studies suggest the capacity of select (poly)phenols to down-regulate the expression of NOX4 subunits resulting in a decreased generation of O2•- and H2O2.
8. Conclusions
-
-
Either in in vitro and ex vivo assays, or in experimental animal models, treatments with different (poly)phenols are associated with a decreased NOX-dependent generation of O2•- and/or H2O2.
-
-
Most of the observed effects of (poly)phenols and their metabolites on NOXs appear related to the modulation of the expression of the different constituent subunits, and/or to posttranslational modifications needed for the assembly, translocation and consequent activation of the protein complex. Very limited evidence is available for a direct interaction of (poly)phenols and their metabolites with NOX proteins which could lead to enzyme regulation.
-
-
The relationship among NOXs, (poly)phenols and health, has to be framed within the capacity of (poly)phenols to regulate cell signaling and maintain redox homeostasis. This associated with their capacity to mitigate disease and pathologies, e.g. those associated with the chronic inflammation that underlies, among others, cardiovascular, kidney and intestinal diseases, diabetes and obesity, and neurodegeneration. It should be considered that not always the inhibition of NOXs is positive for health, given the known physiological events regulated by NOXs, e.g. bactericidal actions of NOXs present in phagocytic cells, enhancing the activation of signaling cascades.
-
-
The association between (poly)phenols and NOXs, although of physiological significance, still lacks many answers due to the complexity of NOXs biochemistry, including: i) the number of structurally and functionally different NOX isoforms; ii) the tissue-specific NOXs expression patterns; iii) NOXs cellular localization; and iv) the different mechanisms of NOXs regulation among the NOX family members.
-
-
The high number and the diversity of (poly)phenols that affect NOXs expression and function, suggest that (poly)phenols can regulate NOXs by modulating systemic events, like inflammation or calcium homeostasis that are the underlying cause of NOXs upregulation.
-
-
The use and development of appropriate methodologies to evaluate NOX activity is crucial to establish the importance of NOX regulation by (poly)phenols on their health-promoting actions.
-
-
Further studies are needed to better define the underlined mechanisms of action, including both, the specific molecular targets for (poly)phenol actions, and the chemical structures or moieties involved in the observed effects on NOXs.
Declaration of competing interest
All authors declare that there are no conflicts of interest to be disclosed.
Acknowledgements
This work was supported by the following grants: UBACYT 20020190100157BA (CGF), UBACYT 20020170100586BA (MG); PICT-2021-CAT-I-00082 (MG) and PICT-2021-CAT-II-00024 (CGF), and USDA CA-D-NTR-7244-H 360 (PIO).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102927.
Appendix A. Supplementary data
The following is the supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- 1.Lupton J.R., Atkinson S.A., Chang N., Fraga C.G., Levy J., Messina M., Richardson D.P., van Ommen B., Yang Y., Griffiths J.C., Hathcock J. Exploring the benefits and challenges of establishing a DRI-like process for bioactives. Eur. J. Nutr. 2014;53(Suppl 1):1–9. doi: 10.1007/s00394-014-0666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 3.Sies H. Oxidative eustress: on constant alert for redox homeostasis. Redox Biol. 2021;41 doi: 10.1016/j.redox.2021.101867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forman H.J., Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021;20:689–709. doi: 10.1038/s41573-021-00233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraga C.G., Oteiza P.I., Galleano M. Plant bioactives and redox signaling: (–)-Epicatechin as a paradigm. Mol. Aspect. Med. 2018;61:31–40. doi: 10.1016/j.mam.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraga C.G., Oteiza P.I., Galleano M. In vitro measurements and interpretation of total antioxidant capacity. Biochim. Biophys. Acta. 2014;1840:931–934. doi: 10.1016/j.bbagen.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 8.Fraga C.G., Galleano M., Verstraeten S.V., Oteiza P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Aspect. Med. 2010;31:435–445. doi: 10.1016/j.mam.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Galleano M., Verstraeten S.V., Oteiza P.I., Fraga C.G. Antioxidant actions of flavonoids: thermodynamic and kinetic analysis. Arch. Biochem. Biophys. 2010;501:23–30. doi: 10.1016/j.abb.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Ottaviani J.I., Borges G., Momma T.Y., Spencer J.P.E., Keen C.L., Crozier A., Schroeter H. The metabolome of [2-(14)C](-)-epicatechin in humans: implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives. Sci. Rep. 2016;6 doi: 10.1038/srep29034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bedard K., Krause K.-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 12.Rossi F., Zatti M. Biochemical aspects of phagocytosis in polymorphonuclear leucocytes. NADH and NADPH oxidation by the granules of resting and phagocytizing cells. Experientia. 1964;20:21–23. doi: 10.1007/BF02146019. [DOI] [PubMed] [Google Scholar]
- 13.Cagan RH, Karnovsky ML. Enzymatic basis of the respiratory stimulation during phagocytosis. Nature (London, U. K.) 1964;204:255–257. doi: 10.1038/204255a0. [DOI] [PubMed] [Google Scholar]
- 14.Schröder K. NADPH oxidases: current aspects and tools. Redox Biol. 2020;34 doi: 10.1016/j.redox.2020.101512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor J.P., Tse H.M. The role of NADPH oxidases in infectious and inflammatory diseases. Redox Biol. 2021;48 doi: 10.1016/j.redox.2021.102159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prior K.K., Leisegang M.S., Josipovic I., Löwe O., Shah A.M., Weissmann N., Schröder K., Brandes R.P. CRISPR/Cas9-mediated knockout of p22phox leads to loss of Nox1 and Nox4, but not Nox5 activity. Redox Biol. 2016;9:287–295. doi: 10.1016/j.redox.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takac I., Schröder K., Zhang L., Lardy B., Anilkumar N., Lambeth J.D., Shah A.M., Morel F., Brandes R.P. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jagnandan D., Chruch J.E., Banfi B., Stuehr D.J., Marrero M.B., Fulton D.J.R. Novel mechanism of activation of NADPH oxidase 5. calcium sensitization via phosphorylation. J. Biol. Chem. 2007;282(9):6494–6507. doi: 10.1074/jbc.M608966200. [DOI] [PubMed] [Google Scholar]
- 19.Rigutto S., Hoste C., Grasberger H., Milenkovic M., Communi D., Dumont J.E., Corvilain B., Miot F., De Deken X. Activation of dual oxidases Duox1 and Duox2: differential regulation mediated by camp-dependent protein kinase and protein kinase C-dependent phosphorylation. J. Biol. Chem. 2009;284(11):6725–6734. doi: 10.1074/jbc.M806893200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L., Nguyen M.V.C., Lardy B., Jesaitis A.J., Grichine A., Rousset F., Talbot M., Paclet M.-H., Qian G.X., Morel F. New insight into the Nox4 subcellular localization in HEK293 cells: first monoclonal antibodies against Nox4. Biochimie. 2011;93(3):457–468. doi: 10.1016/j.biochi.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Block K., Gorin Y., Abboud H.E. Subcellular localization of Nox4 and regulation in diabetes. Proc. Natl. Acad. Sci. U.S.A. 2009;106:14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bánfi B., Malgrange B., Knisz J., Steger K., Dubois-Dauphin M., Krause K.H. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- 23.Ris-Stalpers C. Physiology and pathophysiology of the DUOXes. Antioxidants Redox Signal. 2006;8:1563–1572. doi: 10.1089/ars.2006.8.1563. [DOI] [PubMed] [Google Scholar]
- 24.Fischer H. Mechanisms and function of DUOX in epithelia of the lung. Antioxidants Redox Signal. 2009;11:2453–2465. doi: 10.1089/ars.2009.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Touyz R.M., Anagnostopoulou A., Rios F., Montezano A.C., Camargo L.L. NOX5: molecular biology and pathophysiology. Exp. Physiol. 2019;104:605–616. doi: 10.1113/EP086204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schröder K., Weissmann N., Brandes R.P. Organizers and activators: cytosolic Nox proteins impacting on vascular function. Free Radic. Biol. Med. 2017;109:22–32. doi: 10.1016/j.freeradbiomed.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Maghzal G.J., Krause K.H., Stocker R., Jaquet V. Detection of reactive oxygen species derived from the family of NOX NADPH oxidases. Free Radic. Biol. Med. 2012;53:1903–1918. doi: 10.1016/j.freeradbiomed.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Reis J., Massari M., Marchese S., Ceccon M., Aalbers F.S., Corana F., Valente S., Mai A., Magnani F., Mattevi A. A closer look into NADPH oxidase inhibitors: validation and insight into their mechanism of action. Redox Biol. 2020;32 doi: 10.1016/j.redox.2020.101466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aviello G., Singh A.K., ÓNeill S., Conroy E., Gallagher W., DÁgostino G., Walker A.W., Bourke B., Scholz D., Knaus U.G. Colitis susceptibility in mice with reactive oxygen species deficiency is mediated by mucus barrier and immune defense defects. Mucosal Inmunol. 2019;12(6):1316–1326. doi: 10.1038/s41385-019-0205-x. [DOI] [PubMed] [Google Scholar]
- 30.Biasi F., Mascia C., Astegiano M., Chiarpotto E., Nano M., Vizio B., Leonarduzzi G., Poli G. Pro-oxidant and proapoptotic effects of cholesterol oxidation products on human colonic epithelial cells: a potential mechanism of inflammatory bowel disease progression. Free Radic. Biol. Med. 2009;47:1731–1741. doi: 10.1016/j.freeradbiomed.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Guina T., Deiana M., Calfapietra S., Cabboi B., Maina M., Tuberoso C.I., Leonarduzzi G., Gamba P., Gargiulo S., Testa G., Poli G., Biasi F. The role of p38 MAPK in the induction of intestinal inflammation by dietary oxysterols: modulation by wine phenolics. Food Funct. 2015;6:1218–1228. doi: 10.1039/c4fo01116c. [DOI] [PubMed] [Google Scholar]
- 32.Cremonini E., Daveri E., Mastaloudis A., Adamo A.M., Mills D., Kalanetra K., Hester S.N., Wood S.M., Fraga C.G., Oteiza P.I. Anthocyanins protect the gastrointestinal tract from high fat diet-induced alterations in redox signaling, barrier integrity and dysbiosis. Redox Biol. 2019;26 doi: 10.1016/j.redox.2019.101269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Contreras T.C., Ricciardi E., Cremonini E., Oteiza P.I. (-)-Epicatechin in the prevention of tumor necrosis alpha-induced loss of Caco-2 cell barrier integrity. Arch. Biochem. Biophys. 2015;573:84–91. doi: 10.1016/j.abb.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 34.Mascia C., Maina M., Chiarpotto E., Leonarduzzi G., Poli G., Biasi F. Proinflammatory effect of cholesterol and its oxidation products on CaCo-2 human enterocyte-like cells: effective protection by epigallocatechin-3-gallate. Free Radic. Biol. Med. 2010;49:2049–2057. doi: 10.1016/j.freeradbiomed.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 35.Iglesias D.E., Cremonini E., Fraga C.G., Oteiza P.I. Ellagic acid protects Caco-2 cell monolayers against inflammation-induced permeabilization. Free Radic. Biol. Med. 2020;152:776–786. doi: 10.1016/j.freeradbiomed.2020.01.022. [DOI] [PubMed] [Google Scholar]
- 36.Iglesias D.E., Cremonini E., Oteiza P.I., Fraga C.G. Curcumin Mitigates TNFα-induced caco-2 cell monolayer permeabilization through modulation of NF-κB, ERK1/2, and JNK pathways. Mol. Nutr. Food Res. 2022;66(21) doi: 10.1002/mnfr.202101033. [DOI] [PubMed] [Google Scholar]
- 37.Zhu W., Oteiza P.I. NADPH oxidase 1: a target in the capacity of dimeric ECG and EGCG procyanidins to inhibit colorectal cancer cell invasion. Redox Biol. 2023;65 doi: 10.1016/j.redox.2023.102827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Etxeberria U., Castilla-Madrigal R., Lostao M.P., Martínez J.A., Milagro F.I. Trans-resveratrol induces a potential anti-lipogenic effect in lipopolysaccharide-stimulated enterocytes. Cell. Mol. Biol. 2015;61:9–16. doi: 10.14715/cmb/2015.61.8.2. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z., Litterio M.C., Müller M., Vauzour D., Oteiza P.I. (-)-Epicatechin and NADPH oxidase inhibitors prevent bile acid-induced Caco-2 monolayer permeabilization through ERK1/2 modulation. Redox Biol. 2020;28 doi: 10.1016/j.redox.2019.101360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erlejman A.G., Fraga C.G., Oteiza P.I. Procyanidins protect Caco-2 cells from bile acid- and oxidant-induced damage. Free Radic. Biol. Med. 2006;41:1247–1256. doi: 10.1016/j.freeradbiomed.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Da Silva M., Jaggers G.K., Verstraeten S.V., Erlejman A.G., Fraga C.G., Oteiza P.I. Large procyanidins prevent bile-acid-induced oxidant production and membrane-initiated ERK1/2, p38, and Akt activation in Caco-2 cells. Free Radic. Biol. Med. 2012;52:151–159. doi: 10.1016/j.freeradbiomed.2011.10.436. [DOI] [PubMed] [Google Scholar]
- 42.Cremonini E., Wang Z., Bettaieb A., Adamo A.M., Daveri E., Mills D.A., Kalanetra K.M., Haj F.G., Karakas S., Oteiza P.I. (-)-Epicatechin protects the intestinal barrier from high fat diet-induced permeabilization: implications for steatosis and insulin resistance. Redox Biol. 2018;14:588–599. doi: 10.1016/j.redox.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo X., Fang S., Xiao Y., Song F., Zou T., Wang M., Xia M., Ling W. Cyanidin-3-glucoside suppresses TNF-α-induced cell proliferation through the repression of Nox activator 1 in mouse vascular smooth muscle cells: involvement of the STAT3 signaling. Mol. Cell. Biochem. 2012;362:211–218. doi: 10.1007/s11010-011-1144-3. [DOI] [PubMed] [Google Scholar]
- 44.Prince P.D., Fraga C.G., Galleano M. (-)-Epicatechin administration protects kidneys against modifications induced by short-term l-NAME treatment in rats. Food Funct. 2020;11:318–327. doi: 10.1039/c9fo02234a. [DOI] [PubMed] [Google Scholar]
- 45.Ciz M., Denev P., Kratchanova M., Vasicek O., Ambrozova G., Lojek A. Flavonoids inhibit the respiratory burst of neutrophils in mammals. Oxid. Med. Cell. Longev. 2012;2012 doi: 10.1155/2012/181295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pagonis C., Tauber A.I., Pavlotsky N., Simons E.R. Flavonoid impairment of neutrophil response. Biochem. Pharmacol. 1986;35:237–245. doi: 10.1016/0006-2952(86)90520-4. [DOI] [PubMed] [Google Scholar]
- 47.Kostyuk V.A., Potapovich A.I., Speransky S.D., Maslova G.T. Protective effect of natural flavonoids on rat peritoneal macrophages injury caused by asbestos fibers. Free Radic. Biol. Med. 1996;21:487–493. doi: 10.1016/0891-5849(96)00117-7. [DOI] [PubMed] [Google Scholar]
- 48.Long G.D., DeChatelet L.R., O'Flaherty J.T., McCall C.E., Bass D.A., Shirley P.S., Parce J.W. Effects of quercetin on magnesium-dependent adenosine triphosphatase and the metabolism of human polymorphonuclear leukocytes. Blood. 1981;57:561–566. doi: 10.1182/blood.V57.3.561.561. [DOI] [PubMed] [Google Scholar]
- 49.Verstraeten S.V., Fraga C.G., Oteiza P.I. Interactions of flavan-3-ols and procyanidins with membranes: mechanisms and the physiological relevance. Food Funct. 2015;6:32–41. doi: 10.1039/c4fo00647j. [DOI] [PubMed] [Google Scholar]
- 50.Makino J., Nakanishi R., Kamiya T., Hara H., Ninomiya M., Koketsu M., Adachi T. Luteolin suppresses the differentiation of THP-1 cells through the Inhibition of NOX2 mRNA expression and the membrane translocation of p47phox. J. Nat. Prod. 2013;76:1285–1290. doi: 10.1021/np400224w. [DOI] [PubMed] [Google Scholar]
- 51.Tsai M.H., Lin Z.C., Liang C.J., Yen F.L., Chiang Y.C., Lee C.W. Eupafolin inhibits PGE2 production and COX2 expression in LPS-stimulated human dermal fibroblasts by blocking JNK/AP-1 and Nox2/p47(phox) pathway. Toxicol. Appl. Pharmacol. 2014;279:240–251. doi: 10.1016/j.taap.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 52.Jung J.S., Choi M.J., Lee Y.Y., Moon B.I., Park J.S., Kim H.S. Suppression of lipopolysaccharide-induced neuroinflammation by morin via MAPK, PI3K/Akt, and PKA/HO-1 signaling pathway modulation. J. Agric. Food Chem. 2017;65:373–382. doi: 10.1021/acs.jafc.6b05147. [DOI] [PubMed] [Google Scholar]
- 53.Steffen Y., Schewe T., Sies H. (-)-Epicatechin elevates nitric oxide in endothelial cells via inhibition of NADPH oxidase. Biochem. Biophys. Res. Commun. 2007;359:828–833. doi: 10.1016/j.bbrc.2007.05.200. [DOI] [PubMed] [Google Scholar]
- 54.Steffen Y., Gruber C., Schewe T., Sies H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch. Biochem. Biophys. 2008;469:209–219. doi: 10.1016/j.abb.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 55.Zhu L.H., Wang L., Wang D., Jiang H., Tang Q.Z., Yan L., Bian Z.H., Wang X.A., Li H. Puerarin attenuates high-glucose-and diabetes-induced vascular smooth muscle cell proliferation by blocking PKCbeta2/Rac1-dependent signaling. Free Radic. Biol. Med. 2010;48:471–482. doi: 10.1016/j.freeradbiomed.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 56.Sul O.J., Ra S.W. Quercetin prevents LPS-induced oxidative stress and inflammation by modulating NOX2/ROS/NF-kB in lung epithelial cells. Molecules. 2021;26:6949. doi: 10.3390/molecules26226949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li W., Zhao W., Wu Q., Lu Y., Shi J., Chen X. Puerarin improves diabetic aorta injury by inhibiting NADPH oxidase-derived oxidative stress in STZ-induced diabetic rats. J. Diabetes Res. 2016;2016 doi: 10.1155/2016/8541520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu X., Zhou Z., Zhang Q., Cai W., Zhou Y., Sun H., Qiu L. Vaccarin administration ameliorates hypertension and cardiovascular remodeling in renovascular hypertensive rats. J. Cell. Biochem. 2018;119:926–937. doi: 10.1002/jcb.26258. [DOI] [PubMed] [Google Scholar]
- 59.Zhang N., Wei W.Y., Yang Z., Che Y., Jin Y.G., Liao H.H., Wang S.S., Deng W., Tang Q.Z. Nobiletin, a polymethoxy flavonoid, protects against cardiac hypertrophy induced by pressure-overload via inhibition of NAPDH oxidases and endoplasmic reticulum stress. Cell. Physiol. Biochem. 2017;42:1313–1325. doi: 10.1159/000478960. [DOI] [PubMed] [Google Scholar]
- 60.Wongeakin N., Bhattarakosol P., Patumraj S. Molecular mechanisms of curcumin on diabetes-induced endothelial dysfunctions: txnip, ICAM-1, and NOX2 expressions. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/161346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Litterio M.C., Jaggers G., Sagdicoglu Celep G., Adamo A.M., Costa M.A., Oteiza P.I., Fraga C.G., Galleano M. Blood pressure-lowering effect of dietary (-)-epicatechin administration in L-NAME-treated rats is associated with restored nitric oxide levels. Free Radic. Biol. Med. 2012;53:1894–1902. doi: 10.1016/j.freeradbiomed.2012.08.585. [DOI] [PubMed] [Google Scholar]
- 62.Litterio M.C., Vazquez Prieto M.A., Adamo A.M., Elesgaray R., Oteiza P.I., Galleano M., Fraga C.G. (-)-Epicatechin reduces blood pressure increase in high-fructose-fed rats: effects on the determinants of nitric oxide bioavailability. J. Nutr. Biochem. 2015;26:745–751. doi: 10.1016/j.jnutbio.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 63.Pengnet S., Prommaouan S., Sumarithum P., Malakul W. Naringin reverses high-cholesterol diet-induced vascular dysfunction and oxidative stress in rats via regulating LOX-1 and NADPH oxidase subunit expression. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/3708497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bunbupha S., Pakdeechote P., Maneesai P., Prasarttong P. Nobiletin alleviates high-fat diet-induced nonalcoholic fatty liver disease by modulating AdipoR1 and gp91phox expression in rats. J. Nutr. Biochem. 2021;87 doi: 10.1016/j.jnutbio.2020.108526. [DOI] [PubMed] [Google Scholar]
- 65.Prince P.D., Lanzi C.R., Toblli J.E., Elesgaray R., Oteiza P.I., Fraga C.G., Galleano M. Dietary (-)-epicatechin mitigates oxidative stress, NO metabolism alterations, and inflammation in renal cortex from fructose-fed rats. Free Radic. Biol. Med. 2016;90:35–46. doi: 10.1016/j.freeradbiomed.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 66.Prince P.D., Fischerman L., Toblli J.E., Fraga C.G., Galleano M. LPS-induced renal inflammation is prevented by (-)-epicatechin in rats. Redox Biol. 2017;11:342–349. doi: 10.1016/j.redox.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodriguez-Mateos A., Rendeiro C., Bergillos-Meca T., Tabatabaee S., George T.W., Heiss C., Spencer J.P. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: a randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013;98:1179–1191. doi: 10.3945/ajcn.113.066639. [DOI] [PubMed] [Google Scholar]
- 68.Panza V.P., Brunetta H.S., de Oliveira M.V., Nunes E.A., da Silva E.L. Effect of mate tea (Ilex paraguariensis) on the expression of the leukocyte NADPH oxidase subunit p47phox and on circulating inflammatory cytokines in healthy men: a pilot study. Int. J. Food Sci. Nutr. 2019;70:212–221. doi: 10.1080/09637486.2018.1486393. [DOI] [PubMed] [Google Scholar]
- 69.Henson D., Nieman D., Davis J.M., Dumke C., Gross S., Murphy A., Carmichael M., Jenkins D.P., Quindry J., McAnulty S., McAnulty L., Utter A., Mayer E. Post-160-km race illness rates and decreases in granulocyte respiratory burst and salivary IgA output are not countered by quercetin ingestion. Int. J. Sports Med. 2008;29:856–863. doi: 10.1055/s-2007-989424. [DOI] [PubMed] [Google Scholar]
- 70.Heinz S.A., Henson D.A., Nieman D.C., Austin M.D., Jin F. A 12-week supplementation with quercetin does not affect natural killer cell activity, granulocyte oxidative burst activity or granulocyte phagocytosis in female human subjects. Br. J. Nutr. 2010;104:849–857. doi: 10.1017/S000711451000156X. [DOI] [PubMed] [Google Scholar]
- 71.Nieman D.C., Henson D.A., Gross S.J., Jenkins D.P., Davis J.M., Murphy E.A., Carmichael M.D., Dumke C.L., Utter A.C., McAnulty S.R., McAnulty L.S. Quercetin reduces illness but not immune perturbations after intensive exercise. Med. Sci. Sports Exerc. 2007;39:1561–1569. doi: 10.1249/mss.0b013e318076b566. [DOI] [PubMed] [Google Scholar]
- 72.Watzl B., Bub A., Pretzer G., Roser S., Barth S.W., Rechkemmer G. Daily moderate amounts of red wine or alcohol have no effect on the immune system of healthy men. Eur. J. Clin. Nutr. 2004;58:40–45. doi: 10.1038/sj.ejcn.1601742. [DOI] [PubMed] [Google Scholar]
- 73.Ellinger S., Arendt B.M., Fimmers R., Stehle P., Spengler U., Goerlich R. Bolus ingestion but not regular consumption of native or dealcoholized red wine modulates selected immunological functions of leukocytes in healthy volunteers. Ann. Nutr. Metab. 2008;52:288–295. doi: 10.1159/000146275. [DOI] [PubMed] [Google Scholar]
- 74.Cremonini E., Daveri E., Iglesias D.E., Kang J., Wang Z., Gray R., Mastaloudis A., Kay C.D., Hester S.N., Wood S.M., Fraga C.G., Oteiza P.I. A randomized placebo-controlled cross-over study on the effects of anthocyanins on inflammatory and metabolic responses to a high-fat meal in healthy subjects. Redox Biol. 2022;51 doi: 10.1016/j.redox.2022.102273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Castilla P., Dávalos A., Teruel J.L., Cerrato F., Fernández-Lucas M., Merino J.L., Sánchez-Martín C.C., Ortuño J., Lasunción M.A. Comparative effects of dietary supplementation with red grape juice and vitamin E on production of superoxide by circulating neutrophil NADPH oxidase in hemodialysis patients. Am. J. Clin. Nutr. 2008;87:1053–1061. doi: 10.1093/ajcn/87.4.1053. [DOI] [PubMed] [Google Scholar]
- 76.Loffredo L., Carnevale R., Perri L., Catasca E., Augelletti T., Cangemi R., Albanese F., Piccheri C., Nocella C., Pignatelli P., Violi F. NOX2-mediated arterial dysfunction in smokers: acute effect of dark chocolate. Heart. 2011;97:1776–1781. doi: 10.1136/heartjnl-2011-300304. [DOI] [PubMed] [Google Scholar]
- 77.Loffredo L., Perri L., Catasca E., Pignatelli P., Brancorsini M., Nocella C., De Falco E., Bartimoccia S., Frati G., Carnevale R., Violi F. Dark chocolate acutely improves walking autonomy in patients with peripheral artery disease. J. Am. Heart Assoc. 2014;3(4) doi: 10.1161/JAHA.114.001072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Álvarez Cilleros D., López-Oliva M.E., Martín M.Á., Ramos S. (-)-Epicatechin and the colonic metabolite 2,3-dihydroxybenzoic acid protect against high glucose and lipopolysaccharide-induced inflammation in renal proximal tubular cells through NOX-4/p38 signalling. Food Funct. 2020;11:8811–8824. doi: 10.1039/d0fo01805h. [DOI] [PubMed] [Google Scholar]
- 79.Ke Y.Q., Liu C., Hao J.B., Lu L., Lu N.N., Wu Z.K., Zhu S.S., Chen X.L. Morin inhibits cell proliferation and fibronectin accumulation in rat glomerular mesangial cells cultured under high glucose condition. Biomed. Pharmacother. 2016;84:622–627. doi: 10.1016/j.biopha.2016.09.088. [DOI] [PubMed] [Google Scholar]
- 80.Zhang J., Yang S., Li H., Chen F., Shi J. Naringin ameliorates diabetic nephropathy by inhibiting NADPH oxidase 4. Eur. J. Pharmacol. 2017;804:1–6. doi: 10.1016/j.ejphar.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 81.Bharat D., Cavalcanti R.R.M., Petersen C., Begaye N., Cutler B.R., Costa M.M.A., Ramos R.K.L.G., Ferreira M.R., Li Y., Bharath L.P., Toolson E., Sebahar P., Looper R.E., Jalili T., Rajasekaran N.S., Jia Z., Symons J.D., Anandh Babu P.V. Blueberry metabolites attenuate lipotoxicity-induced endothelial dysfunction. Mol. Nutr. Food Res. 2018;62(2) doi: 10.1002/mnfr.201700601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li X., Cai W., Lee K., Liu B., Deng Y., Chen Y., Zhang X., He J.C., Zhong Y. Puerarin attenuates diabetic kidney injury through the suppression of NOX4 expression in podocytes. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-14906-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.An X., Zhang Y., Cao Y., Chen J., Qin H., Yang L. Punicalagin protects diabetic nephropathy by inhibiting pyroptosis based on TXNIP/NLRP3 pathway. Nutrients. 2020;12(5):1516. doi: 10.3390/nu12051516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou X., Bai C., Sun X., Gong X., Yang Y., Chen C., Shan G., Yao Q. Puerarin attenuates renal fibrosis by reducing oxidative stress induced-epithelial cell apoptosis via MAPK signal pathways in vivo and in vitro. Ren. Fail. 2017;39:423–431. doi: 10.1080/0886022X.2017.1305409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen H.A., Chen C.M., Guan S.S., Chiang C.K., Wu C.T., Liu S.H. The antifibrotic and anti-inflammatory effects of icariin on the kidney in a unilateral ureteral obstruction mouse model. Phytomedicine. 2019;59 doi: 10.1016/j.phymed.2019.152917. [DOI] [PubMed] [Google Scholar]
- 86.Iampanichakul M., Poasakate A., Potue P., Rattanakanokchai S., Maneesai P., Prachaney P., Settheetham-Ishida W., Pakdeechote P. Nobiletin resolves left ventricular and renal changes in 2K-1C hypertensive rats. Sci. Rep. 2022;12(1):9289. doi: 10.1038/s41598-022-13513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chaihongsa N., Maneesai P., Sangartit W., Rattanakanokchai S., Potue P., Khamseekaew J., Bunbupha S., Pakdeechote P. Cardiorenal dysfunction and hypertrophy induced by renal artery occlusion are normalized by galangin treatment in rats. Biomed. Pharmacother. 2022;152 doi: 10.1016/j.biopha.2022.113231. [DOI] [PubMed] [Google Scholar]
- 88.Poasakate A., Maneesai P., Potue P., Bunbupha S., Tong-Un T., Settheetham-Ishida W., Khamseekaew J., Pakdeechote P. Genistein alleviates renin-angiotensin system mediated vascular and kidney alterations in renovascular hypertensive rats. Biomed. Pharmacother. 2022;146 doi: 10.1016/j.biopha.2021.112601. [DOI] [PubMed] [Google Scholar]
- 89.Prince P.D., Rodríguez Lanzi C., Fraga C.G., Galleano M. Dietary (-)-epicatechin affects NF-κB activation and NADPH oxidases in the kidney cortex of high-fructose-fed rats. Food Funct. 2019;10:26–32. doi: 10.1039/c8fo02230e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.