Highlights
-
•
A two-center study showed that deep learning radiomics analysis of pre- and post-nCRT CT images could improve the pCR prediction of patients with ESCC.
-
•
The combined model was superior to the clinical and radiomics models in predicting pCR in locally advanced ESCC and the LR classifier performed best in the current study.
-
•
Decision curves demonstrated that the novel predictive model based on deep learning and handcrafted radiomics features combined with hematological parameters has great clinical utility.
Keywords: Esophageal squamous cell carcinoma, Neoadjuvant chemoradiotherapy, Pathological complete response, Radiomics, Machine learning, Computed tomography
Abstract
Purpose
To evaluate and validate CT-based models using pre- and posttreatment deep learning radiomics features and hematological biomarkers for assessing esophageal squamous cell carcinoma (ESCC) pathological complete response (pCR) after neoadjuvant chemoradiotherapy (nCRT).
Material and methods
This retrospective study recruited patients with biopsy-proven ESCC who underwent nCRT from two Chinese hospitals between May 2017 and May 2022, divided into a training set (hospital I, 111 cases), an internal validation set (hospital I, 47 cases), and an external validation set (hospital II, 33 cases). We used minimum redundancy maximum relevance (mRMR) and least absolute shrinkage and selection operator (LASSO) as feature selection methods and three classifiers as model construction methods. The assessment of models was performed using area under the receiver operating characteristic (ROC) curve (AUC) and decision curve analysis (DCA).
Results
A total 190 patients were included in our study (60.8 ± 7.08 years, 133 men), and seventy-seven of them (40.5 %) achieved pCR. The logistic regression (LR)-based combined model incorporating neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio, albumin, and radscores performed well both in the internal and external validation sets with AUCs of 0.875 and 0.857 (95 % CI, 0.776–0.964; 0.731–0.984, P <0.05), respectively. DCA demonstrated that nomogram was useful for pCR prediction and produced clinical net benefits.
Conclusion
The incorporation of radscores and hematological biomarkers into LR-based model improved pCR prediction after nCRT in ESCC. Enhanced pCR predictability may improve patients selection before surgery, providing clinical application value for the use of active surveillance.
Introduction
Esophageal carcinoma is one of the most common malignant tumors, and it ranks seventh and sixth in cancer-related incidence and mortality globally, respectively. More than half of the annual new cases occur in China, and most patients are diagnosed with esophageal squamous cell carcinoma (ESCC) [1,2]. The CROSS trial confirmed that compared with surgery alone, neoadjuvant chemoradiotherapy (nCRT) added to surgery in patients with locally advanced ESCC was still associated with an absolute overall survival benefit of 13 % at 10 years [3]. According to the CROSS trial, the pathological complete response (pCR) rate achieved following nCRT was reported to be as high as one-third (49 % for squamous cell carcinoma and 23 % for adenocarcinoma). However, after a median follow-up of 84.1 months, distant progression occurred in 39 % of patients who underwent nCRT [3,4]. These findings raise the question of whether surgery should be reserved only for patients receiving nCRT with residual disease after surgery and without distant metastasis. Recently, numerous studies have reported findings of nCRT combined with active surveillance in ESCC patients [5,6]. Active surveillance of patients with ESCC who have achieved clinical complete response (cCR) is being examined in the Dutch SANO trial and the French ESOSTRATE trial according to the CROSS regimen [7]. However, considering the disease's poor prognosis, an accurate assessment of the treatment response is needed to make a decision to forego surgery. Currently, treatment response assessment for ESCC is mainly based on pathological evaluation of endoscopic biopsy and imageological assessment, including CT, MRI, and PET/CT [8], [9], [10], [11]. However, regular invasive manipulation for response assessment is a physical and psychological burden. Developing a noninvasive measurement to accurately predict treatment response remains a major challenge.
Radiomics captures unique imaging features at levels beyond the reach of the naked eye, such as intensity, shape, and texture features. Tumor phenotype differences can be visualized noninvasively by using a quantitative radiomics approach, which can be a potential biomarker for the prediction of noninvasiveness [12]. Convolutional neural networks (CNNs) have been applied to tasks in medical imaging classification and tumor detection and staging [13], [14], [15]. A typical CNN is made up of an input and an output layer, with multiple hidden layers in between. These layers may be convolutional, pooling, or fully connected. Deep learning features extracted using a pretrained network may contain extensive abstract information from the hidden layers. The method of using pretrained CNNs as feature extractors has been shown to be an effective way of using deep learning in many cases [16], [17], [18]. Moreover, the combination of deep learning features obtained by CNNs showed excellent performance in predicting treatment response in different cancers [19,20]. Hu and colleagues demonstrated that the model using a pretrained CNN to extract deep learning features for predicting pCR after nCRT in ESCC showed a more satisfactory predictive performance (area under the curve (AUC) of 0.805 (95 % CI, 0.696–0.913)) than that constructed using handcrafted radiomics features [21]. Nevertheless, due to the high heterogeneity of tumors and the effects of nCRT, the phenotypes captured at different time points may be distinctive. The primary tumor characteristics may be present in pretreatment CT images, while the response status may be reflected in posttreatment CT images. It may be beneficial to incorporate pre- and post-treatment CT data to construct a radiomics model. Moreover, to achieve routine clinical application, more meaningful biomarkers need to be combined into the radiomics model, as these biomarkers could further optimize and improve the model and improve the prediction accuracy. Increasing evidence has shown that nutrition and inflammation have a great influence on the prognosis of patients with a variety of tumors [22,23]. Systemic immunoinflammation response biomarkers, such as neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and lymphocyte to monocyte ratio (LMR), have been used to predict the prognosis of various malignancies, including esophageal cancer [24], [25], [26].
Therefore, our study aimed to build deep learning radiomics models based on pre- and post-treatment CT images incorporating hematological biomarkers for preoperatively assessing ESCC pCR after nCRT, providing clinical application value for the use of active surveillance.
Methods
Patients and treatment
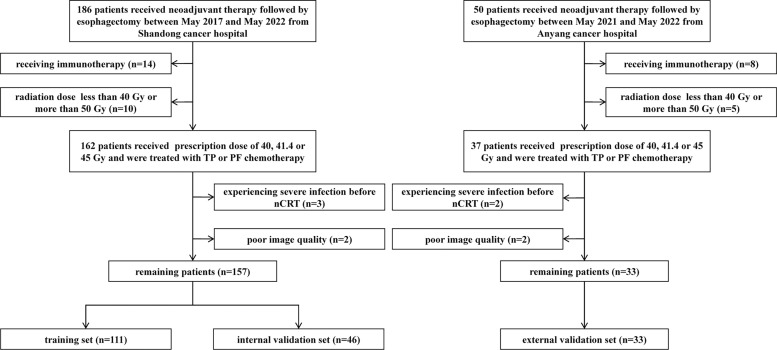
This study was approved by the Institutional Review Board of the Shandong Cancer Hospital and Anyang Tumor Hospital, and the requirement to obtain informed consent from each participant was waived. The training set (n = 111) and internal validation set (n = 46) were retrospectively recruited from Shandong Cancer Hospital at a ratio of approximately 7:3 in sequential chronological order between May 2017 and May 2022. The external set (n = 33) was retrospectively recruited from Anyang Cancer Hospital for the period May 2021 to May 2022. The inclusion criteria were as follows: (1) histologically confirmed locally advanced (T2–4aN+/-M0) ESCC (according to the eighth edition of the American Joint Committee on Cancer (AJCC) staging system); (2) the treatment of all patients included nCRT followed by esophagectomy and postoperative pathology; (3) the availability of pre- and posttreatment CT scans for radiomics analysis; and (4) the availability of complete documentation of baseline laboratory tests. The exclusion criteria were as follows: (1) patients received the treatment of immunotherapy during nCRT; (2) patients failed to complete therapy (radiation dose less than 40 Gy) or received concurrent chemoradiotherapy followed by esophagectomy (radiation dose more than 50 Gy); (3) patients experienced severe infection before nCRT that might influence peripheral blood cell counts; and (4) poor image quality, such as significant motion artifact. The patient recruitment flowchart is displayed in Fig. 1. In our study, all patients were treated with the intensity-modulated radiation therapy (IMRT) technique at a prescription dose of 40, 41.4, or 45 Gy and were treated with cisplatin/taxane (TP) or cisplatin/fluorouracil (PF) chemotherapy during radiotherapy. Surgery was performed within 4 to 8 weeks after the completion of nCRT.
Fig. 1.
Recruitment flowchart for the patients in this study. nCRT, neoadjuvant chemoradiotherapy; PF, cisplatin/fluorouracil; TP, cisplatin/taxane.
Clinical data collection and pathological assessment
We collected age, gender, smoking history, alcohol history, family history, tumor location, TNM stage, and peripheral blood parameters prior to nCRT, including white blood cell count (WBC), neutrophil count (NEU), monocyte count (MO), absolute lymphocyte count (ALC), platelet count (PLT), hemoglobin (HGB), and albumin (ALB). The NLR was defined as the NEU divided by the ALC. The LMR was calculated as the ALC divided by the MO. The PLR was calculated by dividing PLT by ALC.
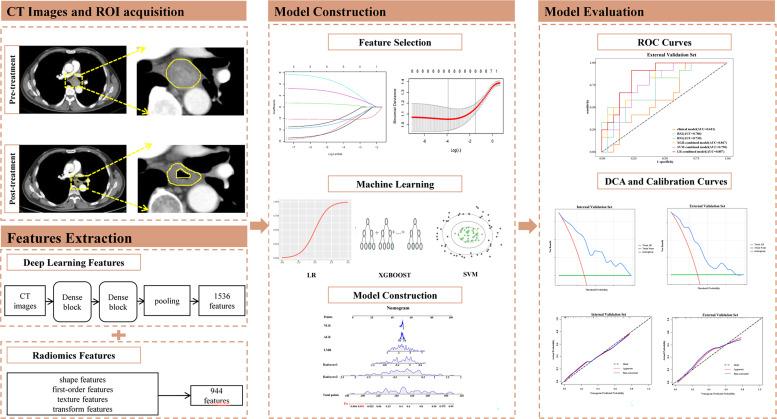
Surgical specimens were evaluated by two pathologists specializing in ESCC who were blinded to the clinical information and CT images. All patients underwent postoperative restaging according to the 8th AJCC staging system. pCR was defined as the absence of residual invasive disease and positive lymph nodes in all layers of the esophagus (ypT0N0). Our study workflow is shown in Fig. 2.
Fig. 2.
Workflow of our study. CT, Computed Tomography; ROI, regions of interest; SVM, support vector machine; LR, logistic regression; ROC, receiver operating characteristic; DCA, decision curve analysis.
CT image acquisition and ROI delineation
All patients underwent standard contrast-enhanced CT scanning from the neck to the abdomen with a SIEMENS CT scanner before and after nCRT. The scanning protocol was as follows: helical scanning mode, HFS patient position, 480 mA tube current, 120 kV tube voltage, 5 mm slice thickness, and 0.7363 × 0.7363 mm/pixel in-plane resolution. The regions of interest (ROIs) of the primary tumor were manually delineated on CT images by two experienced radiation oncologists with 8 and 15 years of clinical experience using the MIM Maestro Workstation (version 7.1.2, MIM Software Inc.) with help from esophagoscopy and 18FDG-PET/CT. We further selected 30 CT images from the training set in a blinded manner, which were randomly assigned equally to two experienced radiation oncologists, and then evaluated the intraobserver and interobserver reproducibility using the CT extracted features of 30 patients.
In the pretreatment CT images, ROIs (ROI1) were drawn along the contour of the primary esophageal tumor. In the posttreatment CT images, we used image registration to delineate ROIs (ROI2) that were defined as areas of esophageal tissues where there was still visible tumor, and if there was no residual tumor after nCRT, ROIs were located at the site of the primary tumor bed. We first registered the post-treatment image with the pre-treatment image by projection transformation and the contour of the pre-treatment CT images’ ROI was projected onto the post-treatment CT images. Then, two radiation oncologists made manual adjustments to compensate for the surrounding tumor shrinkage after treatment and to maintain the consistency of the cranial-to-caudal anatomical range. We first took the largest cross-sectional area of the tumor lesions as the center slice and then selected two slices upward and three slices downward, totaling six consecutive slices. The radiation oncologists were unaware of the patients’ clinicopathologic results.
Radiomics feature extraction and selection
The handcrafted radiomics features were extracted automatically on AccuContour V3.0 (http://www.manteiatech.com/index_en.html), which can preprocess medical imaging data in a standardized manner and extract radiomics features from different image types. A total of 944 radiomics features were extracted from each contoured lesion, including 18 first-order features, 14 shape features, 93 laplacian of gaussian (LoG) features, 744 wavelet-based features, and 75 texture features derived from gray level cooccurence matrix (GLCM), gray level size zone matrix (GLSZM), gray level dependence matrix (GLDM), gray level run length matrix (GLRLM), and neighboring gray tone difference matrix (NGTDM). The details of radiomics feature extraction are shown in Supplementary material S1 and Table S1. The study used the pretrained model InceptionResNetV2 on large ImageNet datasets for deep learning feature extraction. We removed the last fully connected layer and output the features of the last convolutional layer, extracting 1536 features. The details of deep learning feature extraction are shown in Supplementary material S2.
We selected features with intraclass and interclass correlation coefficients (ICC) > 0.75 for further analysis and then used minimum redundancy maximum relevance (mRMR) and least absolute shrinkage and selection operator (LASSO) to select the features from ROI 1 and ROI 2. The mRMR algorithm was applied to identify the features with the highest relevance to tumor treatment response and least redundancy but with a minimum correlation with other features in order to reduce overfitting of the model. The LASSO method was used to select features with nonzero coefficients. And fusion features based on the pre- and post-treatment CT images were built using the respective remaining optimized features, respectively. The feature selection process using the LASSO algorithm is shown in Supplementary material Fig. S1. Then, the selected features were weighted by their coefficients and summed to calculate the radscore. The radscore calculation formula is shown in the Supplementary material S3.
Construction and validation of radiomics signatures
Radiomics signatures were constructed using three common machine learning classifiers based on the selected features including support vector machine (SVM), extreme gradient boosting (XGBoost), and logistic regression (LR) to screen the optimal classifier to construct the radiomics signature1 (RS1) and radiomics signature2 (RS2), which represented the features of the pre- and post-treatment tumors, respectively, and the validation method was used to improve the effectiveness of the model. In the training process, grid search and ten-fold cross-validation were used to select the best hyperparameters. The details of classifiers are shown in Supplementary material S4. Receiver operating characteristic (ROC) analysis and the area under the ROC curve (AUC) were used to evaluate each model's predictive performance. Additionally, we calculated each model's sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV).
Combined and clinical model construction and validation
To screen out the predictors of pCR (p<0.05), we utilized univariate analysis to choose statistically significant clinicopathological characteristics, based on which a clinical model was developed and a combined model was developed by combining the radscores. The AUC values were used to evaluate the predictable performance of different models. To assess the performance of different models, the integrated discrimination improvement (IDI) and net reclassification index (NRI) were adopted. In addition, a nomogram combining significant hematological parameters and radscores was developed to provide a visualized outcome measure. The Hosmer-Lemeshow goodness-of-fit test was used to evaluate the nomogram calibration performance. By calculating the net benefits, decision curve analysis (DCA) was performed to assess the utility of the nomogram.
Statistical analysis
SPSS (Version 26.0, IBM), R (Version 4.2.1), and Python software (Version 3.7.0) were used for all analyses. The differences in continuous variables between the two sets were compared using the Mann-Whitney U test or independent-samples T test. The differences in categorical variables between the two sets were compared using the chi-square test or Fisher's exact test. Each P value was determined using a two-sided test, and a P value <0.05 was considered statistically significant.
Results
Clinical characteristics
The clinical characteristics and hematological parameters of 190 ESCC patients are summarized in Tables 1 and 2. There were no significant differences in pCR among the three sets. No significant differences were observed in age, gender, family history, and chemotherapy between the pCR and non-pCR groups (P>0.05). Moreover, regarding hematological parameters, monocytes, NLR, LMR, and ALB showed significant differences in the training set with P<0.05. ALB was significantly different in the internal validation set with P<0.05.
Table 1.
Baseline characteristics of the patients.
| Characteristic |
Training Set (111) |
Internal Validation Set (46) |
External Validation Set (33) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-pCR (61) | pCR (50) | P1 | Non-pCR (30) | pCR (16) | P2 | Non-pCR (21) | pCR (12) | P3 | |
| Age | 62.00 (54.00, 66.00) |
61.00 (54.00, 66.00) |
0.981 | 60.50 (54.25, 65.00) |
61.50 (58.00, 65.25) |
0.234 | 65.00 (62.00, 69.00) |
61.50 (54.75, 67.25) |
0.230 |
| Gender | 0.465 | 0.462 | 0.710 | ||||||
| Male | 52 (85.2) | 40 (80.0) | 26 (86.7) | 15 (93.8) | 14 (66.7) | 9 (75.0) | |||
| Female | 9 (14.8) | 10 (20.0) | 4 (13.3) | 1 (6.2) | 7 (33.3) | 3 (25.0) | |||
| Smoking history | 0.396 | 0.777 | 1.000 | ||||||
| No | 28 (45.9) | 27 (54.0) | 10 (33.3) | 6 (37.5) | 10 (47.6) | 6 (50.0) | |||
| Yes | 33 (54.1) | 23 (46.0) | 20 (66.7) | 10 (62.5) | 11 (52.4) | 6 (50.0) | |||
| Alcohol history | 0.191 | 0.639 | 0.721 | ||||||
| No | 29 (47.5) | 30 (60.0) | 11 (36.7) | 7 (43.8) | 11 (52.4) | 5 (41.7) | |||
| Yes | 32 (52.5) | 20 (40.0) | 19 (63.3) | 9 (56.2) | 10 (47.6) | 7 (58.3) | |||
| Family history | 0.252 | ||||||||
| No | 52 (85.2) | 43 (86.0) | 0.674 | 21 (70.0) | 12 (75.0) | 0.762 | 20 (95.2) | 10 (83.3) | |
| Esophageal cancer | 2 (3.3) | 3 (6.0) | 4 (13.3) | 1 (6.2) | 0 (0.0) | 1 (8.3) | |||
| Other cancer | 7 (11.5) | 4 (8.0) | 5 (16.7) | 3 (18.8) | 1 (4.8) | 1 (8.3) | |||
| Tumor location | 0.418 | 0.678 | 0.511 | ||||||
| Upper | 4 (6.6) | 5 (10.0) | 1 (3.3) | 1 (6.2) | 0 (0.0) | 1 (8.3) | |||
| Middle | 19 (31.1) | 20 (40.0) | 12 (40.0) | 8 (50.0) | 11 (52.4) | 6 (50.0) | |||
| Distal | 38 (62.3) | 25 (50.0) | 17 (56.7) | 7 (43.8) | 10 (47.6) | 5 (41.7) | |||
| Clinical T stage | 0.704 | 0.487 | 0.252 | ||||||
| T2 | 2 (3.3) | 2 (4.0) | 1 (3.3) | 2 (12.5) | 0 (0.0) | 1 (8.3) | |||
| T3 | 56 (91.8) | 47 (94.0) | 27 (90.0) | 13 (81.2) | 20 (95.2) | 10 (83.3) | |||
| T4 | 3 (4.9) | 1 (2.0) | 2 (6.7) | 1 (6.2) | 1 (4.8) | 1 (8.3) | |||
| Clinical N stage | 0.911 | 0.527 | 0.121 | ||||||
| N0 | 18 (29.5) | 16 (32.0) | 10 (33.3) | 8 (50.0) | 6 (28.6) | 7 (58.3) | |||
| N1 | 33 (54.1) | 25 (50.0) | 14 (46.7) | 6 (37.5) | 12 (57.1) | 5 (41.7) | |||
| N2 | 10 (16.4) | 9 (18.0) | 6 (20.0) | 2 (12.5) | 3 (14.3) | 0 (0.0) | |||
| Radiation dose (Gy) | 0.688 | 0.423 | 0.349 | ||||||
| 40/20 | 7 (11.5) | 4 (8.0) | 1 (3.3) | 2 (12.5) | 4 (19.0) | 0 (0.0) | |||
| 41.4/23 | 51 (83.6) | 42 (84.0) | 28 (93.3) | 13 (81.2) | 17 (81.0) | 10 (83.3) | |||
| 45/25 | 3 (4.9) | 4 (8.0) | 1 (3.3) | 1 (6.2) | 0 (0.0) | 2 (16.7) | |||
| Chemotherapy | 0.673 | 0.626 | 1.000 | ||||||
| PF regimen | 9 (14.8) | 6 (12.0) | 4 (13.3) | 3 (18.8) | 2 (9.5) | 0 (0.0) | |||
| TP regimen | 52 (85.2) | 44 (88.0) | 26 (86.7) | 13 (81.2) | 19 (90.5) | 11 (100.0) | |||
| Radscore1 | −0.38 (−0.49, −0.07) |
0.01 (−0.17,0.14) |
<0.001 | −0.40 (−0.68, −0.14) |
−0.05 (−0.26, 0.19) |
<0.001 | −0.35 (−0.59, −0.10) |
0.03 (−0.19, 0.25) |
0.018 |
| Radscore2 | −0.53 (−0.93, −0.15) |
0.19 (−0.15,0.46) |
<0.001 | −0.48 (−1.45, −0.19) |
0.10 (−0.05, 0.31) |
<0.001 | −0.56 (−1.44, −0.10) |
0.08 (−0.13, 0.46) |
0.001 |
Values are presented as number (%) or median (IQR). IQR, interquartile range; pCR, pathologic complete response. P value is calculated from chi-square test for categorized variables and two-sample t-test/Mann-Whitney U test for continues variables, which represents the univariate association test of subgroups. P1 Between responders and non-responders in the training set. P2 Between responders and non-responders in the internal validation set. P3 Between responders and non-responders in the external validation set.
Table 2.
Hematological parameters of the patients.
| Characteristic |
Training Set (111) |
Internal Validation Set (46) |
External Validation Set (33) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-pCR (61) | pCR (50) | P1 | Non-pCR (30) | pCR (16) | P2 | Non-pCR (22) | pCR (11) | P3 | |
| WBC (×109/L) | 6.84 (5.28, 8.82) |
6.11 (5.12, 7.57) |
0.132 | 6.67 (5.70, 7.64) |
6.66 (5.60, 7.00) |
0.492 | 5.23 (3.90, 7.26) |
5.22 (4.62, 5.97) |
0.924 |
| NEU (×109/L) | 4.41 (3.28, 5.63) |
3.93 (3.16, 4.82) |
0.123 | 4.34 (3.50, 5.13) |
4.34 (3.60, 4.56) |
0.827 | 2.98 (2.08, 4.50) |
3.03 (2.61, 3.24) |
0.909 |
| MO (×109/L) | 0.53 (0.41, 0.67) |
0.43 (0.36, 0.53) |
0.015 | 0.46 (0.42, 0.58) |
0.42 (0.36, 0.49) |
0.217 | 0.42 (0.29, 0.50) |
0.41 (0.24, 0.50) |
0.593 |
| ALC (×109/L) | 1.57 (1.26, 2.03) |
1.62 (1.37, 2.12) |
0.321 | 1.48 (1.28, 1.77) |
1.42 (1.26, 1.85) |
0.972 | 1.65 (1.26, 2.08) |
1.90 (1.39, 2.12) |
0.390 |
| PLT (×109/L) | 261.00 (217.00, 295.00) |
252.50 (223.25, 290.50) |
0.693 | 249.50 (206.25, 304.50) |
241.50 (215.25, 292.00) |
0.947 | 218.50 (182.25, 282.00) |
231.00 (169.00, 314.00) |
0.606 |
| HGB(g/L) | 146.00 (132.00, 154.00) |
144.00 (134.25, 151.00) |
0.272 | 138.00 (131.50, 148.00) |
147.00 (142.25, 151.00) |
0.072 | 136.00 (127.25, 144.00) |
140.00 (136.50, 150.00) |
0.285 |
| NLR | 2.70 (1.93, 3.72) |
2.33 (1.86, 2.83) |
0.039 | 2.85 (2.13, 3.50) |
3.03 (2.44, 3.14) |
0.899 | 1.98 (1.40, 2.95) |
1.63 (1.42, 2.05) |
0.302 |
| PLR | 162.63 (118.95, 205.22) |
149.71 (120.83, 178.48) |
0.364 | 171.15 (119.09, 212.27) |
160.46 (135.13, 215.65) |
0.718 | 134.67 (107.46, 181.09) |
137.80 (125.49, 144.90) |
0.879 |
| LMR | 3.27 (2.42, 3.89) |
3.69 (3.08, 4.99) |
0.001 | 3.49 (2.70, 4.33) |
3.59 (3.11, 4.12) |
0.480 | 0.51 (0.34, 0.72) |
0.64 (0.57, 0.76) |
0.127 |
| ALB(g/L) | 44.20 (41.60, 46.20) |
45.95 (43.37, 48.68) |
0.042 | 43.30 (40.40, 46.48) |
46.80 (42.60, 48.82) |
0.034 | 41.85 (40.30, 45.75) |
43.80 (39.85, 47.10) |
0.593 |
Values are presented as median (IQR).
WBC, white blood cell; NEU, neutrophil count; MO, monocyte count; ALC, absolute lymphocyte count; PLT, platelet count; HGB, hemoglobin; ALC, absolute lymphocyte count; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; LMR, lymphocyte to monocyte ratio. P value is calculated from two-sample t-test/Mann-Whitney U test for continues variables, which represents the univariate association test of subgroups. P1 Between responders and non-responders in the training set. P2 Between responders and non-responders in the internal validation set. P3 Between responders and non-responders in the external validation set.
Feature selection
1862 and 2015 radiomics features from ROI1 and ROI2, respectively, with high reliability and reproductivity, which had intraclass correlation coefficients of > 0.75 and were selected for further analysis. Next, we used mRMR and lasso method to screen sixteen features as the most predictive features from ROI1 (n = 8) and ROI2 (n = 8) that were used for radiomics signature models construction. The rad-scores based on fusion feasures showed statistically significant differences between the pCR and non-pCR groups in the training set (Mann-Whitney U test, P<0.001), internal validation set (Mann-Whitney U test, P<0.001), and the validation set (Mann-Whitney U test, P<0.05). Furthermore, these selected imaging features, which are poorly linked or uncorrelated, as seen in the heatmaps, were not redundant. The details of the heatmaps are shown in Fig. S2.
Assessment of the performance of radiomics signatures
The performance metrics of radiomics signatures of the three machine learning classifiers based on the cut-off value are shown in Tables 3 and 4. In the RS1, the AUC values based on SVM, XGBoost, and LR were 0.708, 0.719, and 0.808 in the internal validation set, and 0.718, 0.730, and 0.722 in the external validation set. In the internal validation set, although the RS1-based LR model achieved a relatively high AUC, the sensitivity and specificity were quite different (1.000 vs 0.500), which was not conducive to the generalization of the model. The performance of the XGBoost model achieved a higher AUC than the SVM model (AUC: 0.718 vs 0.708), and the sensitivity and specificity of the XGBoost model were more balanced than the SVM model (sensitivity:specificity 0.750:0.633 vs 1.000:0.433). In the external validation set, the RS1-based XGBoost classifier performed well, with an AUC of 0.730 and accuracy of 0.667, respectively. The sensitivity of XGBoost was markedly high in the two validation cohorts (0.750–0.917), whereas the specificity remained moderate (0.524–0.633). The NPV and PPV of XGBoost exceeded 0.8 and 0.5, respectively. In the RS2, the AUC values based on SVM, XGBoost, and LR were 0.827, 0.835, and 0.808 in the internal validation set and 0.726, 0.780, and 0.746 in the external validation set. The RS2 based on the XGBoost classifier performed well in both the internal validation set and the external validation set. In the internal validation set, the RS2-based XGBoost model achieved a relatively high AUC compared to other models, and the sensitivity and specificity were quite balanced (0.875 vs 0.700), so that the performance of the model is more balanced. In the external validation set, the sensitivity and specificity of the RS2-based SVM model and LR model were quite different (sensitivity:specificity:1.000:0.571 vs. 1.000:0.429), which was not conducive to the generalization of the model. The RS2-based XGBoost model can make the sensitivity, specificity, and AUC of the model tend to be at the same level. Therefore, we chose the XGBoost classifier as the machine learning algorithm for constructing the radiomics signatures in this study. In addition, a comparison of the different model's ROC curves of the three machine learning classifiers in internal and external validation sets is shown in Fig. 3.
Table 3.
Performance of RS1 model based on the cut-off value.
| Matrix | Training Set |
Internal Validation Set |
External Validation Set |
||||||
|---|---|---|---|---|---|---|---|---|---|
| SVM | XGBOOST | LR | SVM | XGBoost | LR | SVM | XGBoost | LR | |
| AUC (95 %CI) |
0.849 (0.774–0.923) |
0.843 (0.771–0.915) |
0.787 (0.701–0.873) |
0.708 (0.560–0.857) |
0.719 (0.568–0.869) |
0.808 (0.684–0.933) |
0.718 (0.540–0.897) |
0.730 (0.577–0.915) |
0.722 (0.539–0.906) |
| Sensitivity | 0.840 | 0.920 | 0.880 | 1.000 | 0.750 | 1.000 | 0.750 | 0.917 | 0.917 |
| Specificity | 0.738 | 0.639 | 0.623 | 0.433 | 0.633 | 0.500 | 0.619 | 0.524 | 0.619 |
| Accuracy | 0.784 | 0.766 | 0.739 | 0.630 | 0.674 | 0.674 | 0.667 | 0.667 | 0.727 |
| PPV | 0.724 | 0.676 | 0.657 | 0.485 | 0.522 | 0.516 | 0.529 | 0.524 | 0.579 |
| NPV | 0.849 | 0.907 | 0.864 | 1.000 | 0.826 | 1.000 | 0.812 | 0.917 | 0.929 |
RS1, radiomics signature 1; AUC, area under the curve; 95 % CI, 95 % confidence interval; PPV, positive predictive value; NPV, negative predictive value; SVM, support vector machine; LR, logistic regression.
Table 4.
Performance of RS2 model based on the cut-off value.
| Matrix | Training Set |
Internal Validation Set |
External Validation Set |
||||||
|---|---|---|---|---|---|---|---|---|---|
| SVM | XGBoost | LR | SVM | XGBoost | LR | SVM | XGBoost | LR | |
| AUC (95 %CI) |
0.847 (0.774–0.921) |
0.875 (0.809–0.940) |
0.853 (0.784–0.923) |
0.827 (0.700–0.954) |
0.835 (0.718–0.953) |
0.808 (0.673–0.943) |
0.726 (0.551–0.902) |
0.786 (0.616–0.951) |
0.746 (0.572–0.920) |
| Sensitivity | 0.660 | 0.880 | 0.880 | 0.750 | 0.875 | 0.750 | 1.000 | 0.750 | 1.000 |
| Specificity | 0.918 | 0.754 | 0.738 | 0.800 | 0.700 | 0.833 | 0.571 | 0.714 | 0.429 |
| Accuracy | 0.802 | 0.811 | 0.802 | 0.783 | 0.761 | 0.804 | 0.727 | 0.727 | 0.636 |
| PPV | 0.868 | 0.746 | 0.733 | 0.667 | 0.609 | 0.706 | 0.571 | 0.600 | 0.500 |
| NPV | 0.767 | 0.885 | 0.882 | 0.857 | 0.913 | 0.862 | 1.000 | 0.833 | 1.000 |
RS2, radiomics signature 2; AUC, area under the curve; 95 % CI, 95 % confidence interval; PPV, positive predictive value; NPV, negative predictive value; SVM, support vector machine; LR, logistic regression.
Fig. 3.
Comparison of radiomics signatures’ ROC curves of the three machine learning classifiers. ROC curves of radiomics signature1 based on SVM (support vector machine), LR (logistic regression) and XGBoost (extreme gradient boosting) in the training (a), internal (b), and external validation set (c), respectively. ROC curves of radiomics signature2 based on SVM (support vector machine), LR (logistic regression), and XGBoost in the training (d), internal (e), and external validation set (f), respectively.
Combined and clinical model construction and comparison
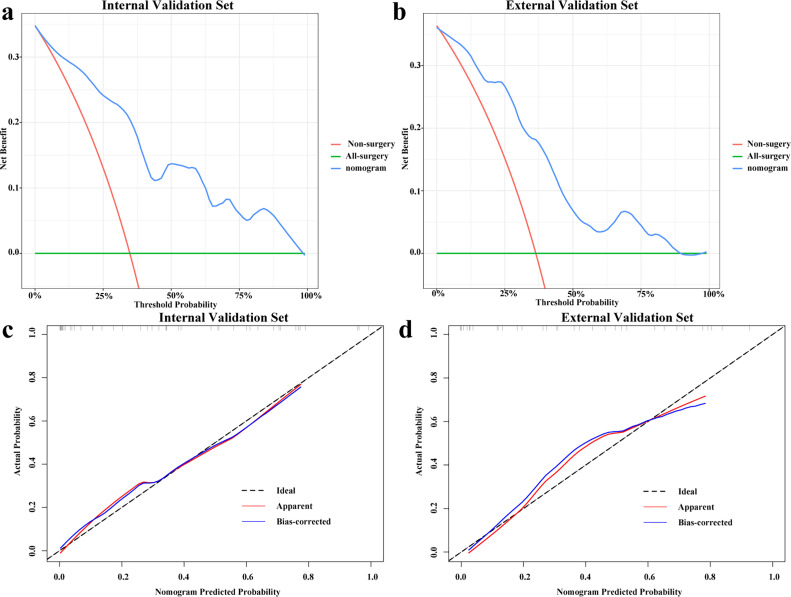
As shown in Fig. 4, NLR (p = 0.039), LMR (p = 0.001), ALB (p = 0.042), radscore1 (p<0.001) and radscore2 (p<0.001) were selected using univariate logistic regression analysis as independent predictors and used to construct the combined model and develop a visual nomogram. The ROC curves demonstrated that the LR-based combined model was superior to radiomics signatures or the clinical model in predicting pCR in the internal validation set (AUC=0.875; 95 % CI, 0.776–0.964, P<0.05) and the external validation set (AUC=0.857; 95 % CI, 0.731–0.984, P<0.05). IDI and continuous NRI showed significant improvement in the accuracy of the LR-based combined model when radiomics features were added to the established clinicopathological features in the three sets (IDI=0.2647–0.2823; continuous NRI=0.9583–1.1667; categorical NRI=0.2292–0.5833, all P<0.01). In addition, the Hosmer-Lemeshow test demonstrated adequate calibration of nomogram for predicting pCR in the internal validation set (P = 0.309) and external validation set (P = 0.263). As shown in Fig. 5, the calibration curves demonstrated good agreement between the predictions by the nomogram and the actual observations of pCR in both the internal and external validation sets. DCA revealed that the nomogram model had a better net benefit than the all-surgery and non-surgery strategies when the threshold probability of the internal validation set was within 0.98 and the threshold probability of the external validation set was within 0.78.
Fig. 4.
Comparison of ROC curve analyses in combined model based on three machine learning classifiers and nomogram of the combined model. ROC curves of the clinical model (orange curve), RS1 radiomics signature 1 (blue curve), RS2 radiomics signature 2 (green curve), XGB (extreme gradient boosting)-combined model (yellow curve), SVM (support vector machine)-combined model (purple curve), LR (logistic regression)-combined model (red curve) in the internal set (a) and external validation set (b), respectively. The visual nomogram in the combined model (d).
Fig. 5.
Decision and Calibration curve analysis for the nomogram. The y-axis measures the net benefit. The blue line represents the nomogram model. The red line represents the assumption that none of patients have surgery. The green line represents the assumption that all patients have surgery. The decision curve showed that if the threshold probability of a patient is within 98 % and 78 % in the internal set (a) and external validation set (b), respectively, using the nomogram model in the current study to predict the pCR of patients treated with active surveillance adds more benefit than either the all-sugery scheme or the non-sugery. Calibration curves of the nomogram in the internal set (c) and external validation set (d).
Discussion
In the present study, we compared pre- and post-treatment CT-based radiomics and deep learning features for predicting pCR in patients with ESCC receiving nCRT using three machine learning classifiers and found that the XGBoost-based radiomics signature performed well. In addition, the LR-based combined model yielded significantly improved prediction performance in predicting pCR.
Accumulating data have demonstrated that pCR following nCRT in ESCC significantly improves overall and disease-free survival and is also related to long-term survival [27]. However, in the CROSS trial, 39 % of the patients still developed distant progression after nCRT and surgery after a median follow-up of 84.1 months. This suggests that many patients may have already developed micrometastases at the time of diagnosis. Therefore, standard esophagectomy might be of no benefit for these patients. Meanwhile, patients who are anticipated to achieve a pCR may not benefit from esophagectomy because of the postoperative complications and high mortality, as well as the deterioration in quality of life brought on by symptoms such as reflux and weight loss [28]. Therefore, the issue of organ preservation for locally advanced ESCC after nCRT has aroused widespread concern and heated discussion. And a few studies found that, compared with standard esophagectomy, active surveillance after nCRT may benefit patients, and the overall survival rate was not significantly different [29,30]. Hence, an accurate strategy for treatment response evaluation plays an important role in the active surveillance of ESCC following nCRT. Currently, treatment response is assessed by biopsy or imaging examination. Biopsy is an additional invasive procedure that may affect residual tumor lesions and lead to adverse outcomes. Moreover, patients may suffer from anxiety and psychological burden. However, whether imaging evaluation is subjective and can be used to accurately evaluate the treatment response remains to be further discussed [31,32]. A large number of previous studies have demonstrated the application of pretreatment CT-based radiomic features for the treatment response prediction of nCRT in ESCC, with AUCs of 0.70−0.79 [33], [34], [35], [36]. Moreover, Li and colleagues studied pre- and post-CT imaging handcrafted radiomics features to predict the probability of pCR following nCRT in ESCC, and the radiomics+clinical model achieved an AUC of 0.84 for pCR prediction [37]. Based on the optimal cutoff value of 60 %, 13 % of patients could consider active surveillance by the radiomics+clinical model. In terms of deep learning, Hu et al. found that the optimal model built using CT-based deep learning features prior to nCRT administration extracted from ResNet50 for predicting pCR after nCRT in ESCC achieved an AUC and accuracy of 0.805 (95 % CI, 0.696−0.913) and 77.1 % (65.6 %−86.3 %), respectively, showing better predictive performance than the radiomics model of 0.725 (0.605−0.846) and 67.1 % (54.9 %−77.9 %), respectively [21]. Hence, our study has an obvious predominance compared to theirs. First, we innovatively incorporated handcrafted radiomics features and deep learning features based on pre- and post-treatment CT images. To the best of our knowledge, no study has tried this pattern. In addition, we used three classifiers to choose the most appropriate machine learning methods, which is one of the critical issues in radiomics research. In our study, the XGBoost algorithms exhibited high and stable predictive capability in both the internal and external validation sets, which is selected as the best machine learning algorithm for constructing the radiomics signatures. The XGBoost algorithm is based on the gradient enhanced decision tree and uses second-order Taylor expansion to calculate the loss function. It has good performance in computation speed and prediction accuracy [38].
Furthermore, for routine clinical application, systemic immunoinflammation response biomarkers were also added to the model. There are increasing data showing that systemic inflammation is closely related to the promotion, progression, and metastasis of tumors [39,40]. Neutrophils promote angiogenesis in the early stages of tumor progression. Moreover, neutrophils stimulate transcription factors that increase the synthesis of inflammatory mediators, which may help cancer cells evade immune surveillance [41,42]. Antigen-specific immune responses are carried out by lymphocytes, which may indirectly control tumor angiogenesis by modulating myeloid cell activation [41]. Proangiogenic substances, including vascular endothelial growth factor A (VEGFA), platelet-derived growth factors (PDGFs), and fibroblast growth factor 2 (FGF2), which can promote angiogenesis and invasion, are abundant in activated platelets [41,43]. Therefore, the NLR and PLR may reveal some relationship between inflammation and immune responses in peripheral blood and help to determine the host inflammatory state. Meanwhile, during the progression of malignant tumors, the release of many inflammatory cytokines can lead to malnutrition, which is reflected by hypoalbuminemia and low lymphocyte counts, and cachexia, reflected by sarcopenia caused by an increase in protein degradation in skeletal muscle [25]. In addition, while radiotherapy can deliver high-energy radiation to destroy tumor cells, it can also damage healthy tissue in the surrounding area, including the muscles [44]. A worsening nutritional status may lead to tumor progression through the suppression of tumor immunity [45]. Our results were in keeping with previously published studies [46,47]. In our study, the results demonstrated that NLR, PLR, and ALB were independent hematological parameter predictors of pCR in ESCC. Therefore, the integration of parameters related to systemic immunoinflammation response, malnutrition, and sarcopenia analysis into clinical practice can not only have the potential to enhance the accuracy of tumor treatment response assessment but also enable timely optimization of treatment plans and implement interventions to improve nutritional status and overall outcome. An area that could be explored in future research on this basis is utilizing artificial intelligence, such as machine learning and deep learning, to measure total muscle volume according to MRI or CT imaging, which could help physicians with better risk stratification for therapeutic or nutritional interventions to improve clinical outcomes in patients with ESCC [44]. More importantly, when hematological biomarkers were added, we found the LR-based combined model had superior performance. These results suggested that different features were suitable for different models. The incorporation of radiomics signature models and systemic inflammation significantly improved the NRI and IDI. Therefore, for pCR prediction, combining pre- and post-treatment CT data could achieve a more accurate prediction result. Meanwhile, the performance was significantly superior in RS2 than in RS1, which to a certain extent indicates the important predictive value of posttreatment CT data. For clinical application, the DCA proved that the threshold probability within 0.98 and 0.78 of the internal and external validation sets, using the nomogram was above the two reference lines, indicating that it had clinical benefits. The threshold probability is where the expected benefit of treatment is equal to the expected benefit of avoiding treatment. For example, if the possibility of pCR of a patient is over the threshold probability, then a treatment strategy for active surveillance may be adopted. In other words, at the given threshold cutoff, the correct pCR prediction of the nomogram can help avoid surgeries and provides more clinical benefit than both the all-surgery and non-surgery schemes. Meanwhile, the combined model possessed good clinical applicability, and the visual nomogram can be used as a convenient and accurate tool to stratify high-risk patients for individualized treatment strategies.
Our study has several limitations. First, the study is limited by its retrospective nature. A larger subgroup of patients from more institutions is needed. In addition, this algorithm can be used for routine clinical applications, but we need to confirm this finding in a larger cohort. Our collaboration with other cancer centers would help further confirm this result in a large sample cohort. Second, the modalities of neoadjuvant treatment regimens were not all the same, which reflects clinical reality. Third, the model also needs to be improved by the comprehensive integration of other data, such as genomic and transcriptomic results, as well as combined MR and PET imaging features that we will further investigate in the future.
In conclusion, The LR-based combined model incorporating handcrafted radiomics and deep learning features and hematological parameters performed better than the other classification models, which showed favorable performance in pCR prediction after nCRT in ESCC, facilitating stratification of high-risk patients and providing clinical application value for the use of active surveillance in patients who have a sufficient response to treatment.
Consent
This study was approved by the Institutional Review Board of Shandong Cancer Hospital and Anyang Tumor Hospital, and written informed consent was waived.
Author statement
Conception and design: Meng Zhang, Zhenjiang Li, and Yong Yin; Administrative support: Zhenjiang Li, Yong Yin; Provision of study materials or patients: Meng Zhang, Hongfu Sun, and Zhenjiang Li; Collection and assembly of data: Meng Zhang, Yukun Lu, Hongfu Sun, Zhenjiang Li, and Chuanke Hou; Data analysis and interpretation: Meng Zhang, Zhenjiang Li, Zichun Zhou, Qichao Zhou, and Xiao Liu; Manuscript writing: Meng Zhang; Final approval of the manuscript: All authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82102173, 82072094, and 12275162) and 2021 Shandong Medical Association Clinical Research Fund -- Qilu Special Project (Grant No. YXH2022ZX02198).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2023.101804.
Contributor Information
Zhenjiang Li, Email: zhenjli1987@163.com.
Yong Yin, Email: yinyongsd@126.com.
Appendix. Supplementary materials
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Kocarnik J.M., Compton K., Dean F.E., et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022;8(3):420–444. doi: 10.1001/jamaoncol.2021.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro J., Van Lanschot J.J.B., Hulshof M., et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 4.Eyck B.M., Van Lanschot J.J.B., Hulshof M., et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J. Clin. Oncol. 2021;39(18):1995–2004. doi: 10.1200/JCO.20.03614. [DOI] [PubMed] [Google Scholar]
- 5.Castoro C., Scarpa M., Cagol M., et al. Complete clinical response after neoadjuvant chemoradiotherapy for squamous cell cancer of the thoracic oesophagus: is surgery always necessary? J. Gastrointest. Surg. 2013;17(8):1375–1381. doi: 10.1007/s11605-013-2269-3. [DOI] [PubMed] [Google Scholar]
- 6.Taketa T., Xiao L., Sudo K., et al. Propensity-based matching between esophagogastric cancer patients who had surgery and who declined surgery after preoperative chemoradiation. Oncology. 2013;85(2):95–99. doi: 10.1159/000351999. [DOI] [PubMed] [Google Scholar]
- 7.Noordman B.J., Wijnhoven B.P.L., Lagarde S.M., et al. Neoadjuvant chemoradiotherapy plus surgery versus active surveillance for oesophageal cancer: a stepped-wedge cluster randomised trial. BMC Cancer. 2018;18:142. doi: 10.1186/s12885-018-4034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noordman B.J., Spaander M.C.W., Valkema R., et al. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol. 2018;19(7):965–974. doi: 10.1016/S1470-2045(18)30201-8. [DOI] [PubMed] [Google Scholar]
- 9.van der Wilk B.J., Eyck B.M., Doukas M., et al. Residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer: locations undetected by endoscopic biopsies in the preSANO trial. Br. J. Surg. 2020;107(13):1791–1800. doi: 10.1002/bjs.11760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borggreve A.S., Heethuis S.E., Boekhoff M.R., et al. Optimal timing for prediction of pathologic complete response to neoadjuvant chemoradiotherapy with diffusion-weighted MRI in patients with esophageal cancer. Eur. Radiol. 2020;30(4):1896–1907. doi: 10.1007/s00330-019-06513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valkema M.J., Noordman B.J., Wijnhoven B.P.L., et al. Accuracy of 18F-FDG PET/CT in predicting residual disease after neoadjuvant chemoradiotherapy for esophageal cancer. J. Nucl. Med. 2019;60(11):1553–1559. doi: 10.2967/jnumed.118.224196. [DOI] [PubMed] [Google Scholar]
- 12.Mayerhoefer M.E., Materka A., Langs G., et al. Introduction to radiomics. J. Nucl. Med. 2020;61(4):488–495. doi: 10.2967/jnumed.118.222893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y., Hu Y., Zhang X., et al. Two-stage selective ensemble of CNN via deep tree training for medical image classification. IEEE Trans. Cybern. 2022;52(9):9194–9207. doi: 10.1109/TCYB.2021.3061147. [DOI] [PubMed] [Google Scholar]
- 14.Shin S.Y., Lee S., Yun I.D., et al. Joint weakly and semi-supervised deep learning for localization and classification of masses in breast ultrasound images. IEEE Trans. Med. Imaging. 2019 Mar;38(3):762–774. doi: 10.1109/TMI.2018.2872031. [DOI] [PubMed] [Google Scholar]
- 15.Wu J., Li C., Gensheimer M., et al. Radiological tumor classification across imaging modality and histology. Nat. Mach. Intell. 2021 Sep;3:787–798. doi: 10.1038/s42256-021-00377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhosale Y.H., Patnaik K.S. PulDi-COVID: chronic obstructive pulmonary (lung) diseases with COVID-19 classification using ensemble deep convolutional neural network from chest X-ray images to minimize severity and mortality rates. Biomed. Signal Process. Control. 2023 Mar;81 doi: 10.1016/j.bspc.2022.104445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitney H.M., Li H., Ji Y., et al. Comparison of breast MRI tumor classification using human-engineered radiomics, transfer learning from deep convolutional neural networks, and fusion methods. Proc IEEE Inst. Electr. Electron. Eng. 2020;108(1):163–177. doi: 10.1109/jproc.2019.2950187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raghu S., Sriraam N., Temel Y., et al. EEG based multi-class seizure type classification using convolutional neural network and transfer learning. Neural Netw. 2020 Apr;124:202–212. doi: 10.1016/j.neunet.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Cui Y., Zhang J., Li Z., et al. A CT-based deep learning radiomics nomogram for predicting the response to neoadjuvant chemotherapy in patients with locally advanced gastric cancer: a multicenter cohort study. EClinicalMedicine. 2022;46 doi: 10.1016/j.eclinm.2022.101348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.She Y., He B., Wang F., et al. Deep learning for predicting major pathological response to neoadjuvant chemoimmunotherapy in non-small cell lung cancer: a multicentre study. EBioMedicine. 2022;86 doi: 10.1016/j.ebiom.2022.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Y., Xie C., Yang H., et al. Computed tomography-based deep-learning prediction of neoadjuvant chemoradiotherapy treatment response in esophageal squamous cell carcinoma. Radiother. Oncol. 2021 Jan;154:6–13. doi: 10.1016/j.radonc.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Song M., Zhang Q., Song C., et al. The advanced lung cancer inflammation index is the optimal inflammatory biomarker of overall survival in patients with lung cancer. J. Cachexia Sarcopenia Muscle. 2022;13(5):2504–2514. doi: 10.1002/jcsm.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie H., Ruan G., Ge Y., et al. Inflammatory burden as a prognostic biomarker for cancer. Clin. Nutr. 2022;41(6):1236–1243. doi: 10.1016/j.clnu.2022.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Z., Zhu H., Cai H. Preoperative prognostic nutritional index predict survival in patients with resectable esophageal squamous cell carcinoma. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.824839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H., Shang X., Ren P., et al. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J. Cell. Physiol. 2019;234(2):1794–1802. doi: 10.1002/jcp.27052. [DOI] [PubMed] [Google Scholar]
- 26.Jomrich G., Paireder M., Kristo I., et al. High systemic immune-inflammation index is an adverse prognostic factor for patients with gastroesophageal adenocarcinoma. Ann. Surg. 2021;273(3):532–541. doi: 10.1097/SLA.0000000000003370. [DOI] [PubMed] [Google Scholar]
- 27.Rizvi F.H., Syed A.A., Khattak S., et al. Complete pathological response after neoadjuvant treatment in locally advanced esophageal cancer predicts long term survival: a retrospective cohort study. Int. J. Surg. 2014;12(6):621–625. doi: 10.1016/j.ijsu.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Chen N., Ajani J., Wu A. Nonoperative management of gastrointestinal malignancies in era of neoadjuvant treatment. Chin. J. Cancer Res. 2023;35(1):44–57. doi: 10.21147/j.issn.1000-9604.2023.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Wille B.J., Noordman B.J., Neijenhuis L.K.A., et al. Active surveillance versus immediate surgery in clinically complete responders after neoadjuvant chemoradiotherapy for esophageal cancer: a multicenter propensity matched study. Ann. Surg. 2021;274(6):1009–1016. doi: 10.1097/SLA.0000000000003636. [DOI] [PubMed] [Google Scholar]
- 30.van der Wilk B.J., Eyck B.M., Hofstetter W.L., et al. Chemoradiotherapy followed by active surveillance versus standard esophagectomy for esophageal cancer: a systematic review and individual patient data meta-analysis. Ann. Surg. 2022;275(3):467–476. doi: 10.1097/SLA.0000000000004930. [DOI] [PubMed] [Google Scholar]
- 31.Essink-Bot M.L., Kruijshaar M.E., Bac D.J., et al. Different perceptions of the burden of upper GI endoscopy: an empirical study in three patient groups. Qual. Life Res. 2007 Oct;16(8):1309–1318. doi: 10.1007/s11136-007-9239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters Y., Siersema P.D. Public preferences and predicted uptake for esophageal cancer screening strategies: a labeled discrete choice experiment. Clin. Transl. Gastroenterol. 2020 Nov;11(11):e00260. doi: 10.14309/ctg.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Z., He B., Zhuang X., et al. CT-based radiomic signatures for prediction of pathologic complete response in esophageal squamous cell carcinoma after neoadjuvant chemoradiotherapy. J. Radiat. Res. 2019;60(4):538–545. doi: 10.1093/jrr/rrz027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin X., Zheng X., Chen D., et al. Prediction of response after chemoradiation for esophageal cancer using a combination of dosimetry and CT radiomics. Eur. Radiol. 2019;29(11):6080–6088. doi: 10.1007/s00330-019-06193-w. [DOI] [PubMed] [Google Scholar]
- 35.Hu Y., Xie C., Yang H., et al. Assessment of intratumoral and peritumoral computed tomography radiomics for predicting pathological complete response to neoadjuvant chemoradiation in patients with esophageal squamous cell carcinoma. JAMA Netw. Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.15927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rishi A., Zhang G.G., Yuan Z., et al. Pretreatment CT and 18F-FDG PET-based radiomic model predicting pathological complete response and loco-regional control following neoadjuvant chemoradiation in oesophageal cancer. J. Med. Imaging Radiat. Oncol. 2021;65(1):102–111. doi: 10.1111/1754-9485.13128. [DOI] [PubMed] [Google Scholar]
- 37.Li Y., Liu J., Li H.X., et al. Radiomics signature facilitates organ-saving strategy in patients with esophageal squamous cell cancer receiving neoadjuvant chemoradiotherapy. Front. Oncol. 2021;10 doi: 10.3389/fonc.2020.615167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen T., Guestrin C. XGBoost: a scalable tree boosting system. Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining; San Francisco, California; Association for Computing Machinery; 2016. pp. 785–794. [Google Scholar]
- 39.Greten F.R., Grivennikov S.I. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Denk D., Greten F.R. Inflammation: the incubator of the tumor microenvironment. Trends Cancer. 2022;8(11):901–914. doi: 10.1016/j.trecan.2022.07.002. [DOI] [PubMed] [Google Scholar]
- 41.De Palma M., Biziato D., Petrova T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer. 2017;17(8):457–474. doi: 10.1038/nrc.2017.51. [DOI] [PubMed] [Google Scholar]
- 42.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mantovani A., Allavena P., Sica A., et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 44.Erul E., Guven D.C., Onur M.R., et al. Role of sarcopenia on survival and treatment-related toxicity in head and neck cancer: a narrative review of current evidence and future perspectives. Eur. Arch. Otorhinolaryngol. 2023;280(8):3541–3556. doi: 10.1007/s00405-023-08014-9. [DOI] [PubMed] [Google Scholar]
- 45.Yeom E., Yu K. Understanding the molecular basis of anorexia and tissue wasting in cancer cachexia. Exp. Mol. Med. 2022;54(4):426–432. doi: 10.1038/s12276-022-00752-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abe T., Oshikiri T., Goto H., et al. Albumin-derived NLR score is a novel prognostic marker for esophageal squamous cell carcinoma. Ann. Surg. Oncol. 2022;29(4):2663–2671. doi: 10.1245/s10434-021-11012-y. [DOI] [PubMed] [Google Scholar]
- 47.Wu Y., Chen J., Zhao L., et al. Prediction of pathologic response to neoadjuvant chemoradiotherapy in patients with esophageal squamous cell carcinoma incorporating hematological biomarkers. Cancer Res. Treat. 2021;53(1):172–183. doi: 10.4143/crt.2020.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.