Abstract
Background
The extent of short-acting Beta-2-agonist (β2-agonist) (SABA) use across Asian countries is not well documented. As part of the SABA use IN Asthma (SABINA) III study, we assessed SABA prescriptions and clinical outcomes in patients with asthma from Asia.
Methods
This cross-sectional study recruited patients (aged ≥12 years) with asthma from 8 Asian countries. Data on disease characteristics and asthma treatments were collected using electronic case report forms. Patients were classified by practice type (primary or specialist care) and investigator-defined asthma severity (per Global Initiative for Asthma [GINA] 2017 recommendations). The association of SABA prescriptions with clinical outcomes was analyzed using multivariable regression models.
Results
Overall, 3066 patients were analyzed, with a mean (standard deviation) age of 51.8 (16.7) years; of these patients, 2116 (69%) were female, 2517 (82.1%) had moderate-to-severe asthma and 2498 (81.5%) and 559 (18.2%) were treated in specialist and primary care, respectively. In total, 1423 (46.4%) patients had partly controlled/uncontrolled asthma, with 1149 (37.5%) patients experiencing ≥1 severe asthma exacerbation in the previous year. Overall, 800 (26.7%) patients were prescribed ≥3 SABA canisters in the previous year, which is regarded as overprescription and was associated with a significantly decreased odds of at least partly controlled asthma and increased incidence rates of severe exacerbations (P < 0.01 for both associations).
Conclusion
The findings from this cohort of predominantly specialist-treated patients with asthma indicate SABA overprescription in at least 1 in every 4 patients, and this overprescription is associated with poor clinical outcomes. These data highlight the need for adherence to recently updated asthma treatment recommendations in Asia.
Keywords: Asia, Asthma, Prescriptions, Public health, Adrenergic beta-2 receptor agonists
Background
Asthma, primarily driven by airway inflammation, is a major chronic respiratory disease in Asia and globally.1,2 Although the prevalence of asthma is lower in the Asia-Pacific region (<5%) than in Western countries (≥20%), an upward trend is being observed, potentially as a result of increased urbanization.1,3 Despite the comparatively lower prevalence observed in Asia, the burden of asthma in this region is substantial and amplified due to underdiagnosis, undertreatment, and inaccessible and/or unaffordable healthcare.1,4, 5, 6
Although inhaled corticosteroids (ICS) remain the preferred maintenance medication for patients with mild persistent and more severe asthma,4,7 short-acting Beta-2-agonist (β2-agonists) (SABAs) have been used for rapid symptom relief. Preclinical and clinical studies have shown that SABAs do not address the underlying airway inflammation8 but rather increase airway inflammation;9 therefore, frequent and inappropriate SABA use is associated with increased morbidity, including exacerbations.10, 11, 12, 13 Results from the REcognise Asthma and LInk to Symptoms and Experience (REALISE) Asia, Asthma Insights and Management (AIM), and Asthma Insights and Reality (AIR) surveys reported that less than one-third of patients14, 15, 16 in the Asia-Pacific region used daily maintenance medication, while more than two-thirds of patients14 used only reliever medication. Overuse of reliever medications and underuse of maintenance ICS medications may thus explain the poor asthma control observed in Asia.6,14,15,17 Based on growing safety concerns, the latest Global Initiative for Asthma (GINA) recommendations no longer recommend treatment with as-needed SABA without concomitant ICS for symptom relief in patients ≥12 years of age. Instead, low-dose ICS-formoterol is now recommended as the preferred as-needed reliever for adults and adolescents with mild asthma and for those with moderate-to-severe asthma who are prescribed ICS-formoterol maintenance therapy to treat the underlying inflammation and minimize the risk of exacerbations.4 This recommendation was based on the efficacy of the combination of a low-dose ICS with formoterol, a long-acting β2-agonist (LABA) with a rapid onset of action in reducing severe exacerbations when compared with SABA monotherapy18 and ICS maintenance therapy in patients with mild asthma.19
Assessment of SABA overuse and its local consequences may help clinicians understand the extent of SABA overuse and its associated risks and advocate for changes in clinical practice in alignment with GINA recommendations. Several studies have established an association between SABA overuse and poor clinical outcomes, in addition to increased healthcare resource utilization.10,13,20, 21, 22 However, similar studies conducted in Asian populations are lacking, with the absence of large healthcare databases acting as a barrier to the conduct of such studies in many Asian countries.
The SABA use IN Asthma (SABINA) program was initiated to describe asthma treatment prescription patterns, the extent of SABA use, and its subsequent impact on asthma-related clinical outcomes through a series of large observational cohort studies using a harmonized approach to data collection, evaluation, and interpretation.23 Due to the diversity in healthcare systems, the SABINA program comprises 3 main pillars, which share a common objective and design principles from a granular core protocol to ensure scientific alignment: (i) SABINA I, a retrospective, observational database study conducted in the United Kingdom (UK);24 (ii) SABINA II, a distributed harmonized set of multi-country, retrospective, observational database studies in Canada, France, Germany, Italy, Israel, the Netherlands, Spain, and Sweden;25, 26, 27, 28, 29, 30, 31 and (iii) SABINA III, a multicenter, observational, cross-sectional study in 8351 patients from 24 countries across the Asia-Pacific region,32, 33, 34, 35, 36, 37 Africa,38, 39, 40 the Middle East,41, 42, 43, 44 Latin America,45, 46, 47, 48 and Russia,49 which used electronic case report forms (eCRFs) to record data from individual patients (patient-level data) and from healthcare providers (HCPs).50 Overall, results from the United Kingdom and Europe reported that SABA overprescription (or possession of ≥3 canisters/year) is common and associated with poor clinical outcomes.24,51 Similarly, findings from the SABINA International study (SABINA III) indicated that ≥3 SABA prescriptions per year (vs 1−2 SABA prescriptions) were associated with increasingly lower odds of controlled or partly controlled asthma and higher rates of severe exacerbations across treatment steps and clinical care settings.50 Here, we report trends in SABA prescriptions in 8 Asian countries, as a subset of the SABINA III study.
Methods
Study design
The methodology for SABINA III has been described previously.50 In brief, this cross-sectional, multi-country, multicenter, observational study was conducted in Malaysia, India, South Korea, Thailand, Taiwan, the Philippines, Indonesia, and Singapore, with patient recruitment from March 2019 to January 2020. Study sites were selected using purposive sampling with the aim of obtaining a sample representative of asthma management within each participating country by a national coordinator. The national coordinator also provided advice on the different types of centers (different types of hospitals and geographical distribution) and facilitated the selection of the investigators. The primary objective was to analyze aggregated data from these 8 countries to describe trends in SABA prescriptions in the asthma patient population. The secondary objectives were to determine the associations between SABA prescriptions and health outcomes. Retrospective baseline data were obtained from existing medical records, while patient data were collected during a single study visit and entered in the eCRF. Physicians entered data on exacerbation history, comorbidities, and information of medication prescriptions for asthma in the eCRF based on patient medical records. Physicians also enquired whether patients had experienced any additional exacerbations that were not documented in their medical records. All study site investigators were trained for using the eCRF system. The study was compliant with the study protocol, local ethics committees, and the Declaration of Helsinki; signed informed consent was obtained from all patients or their legal guardians.
Study population
Patients aged ≥12 years under HCP care who met the following criteria were eligible for enrollment: (i) a documented physician diagnosis of asthma in their medical records; (ii) ≥3 prior HCP consultations with the same HCP or HCP practice; and (iii) medical records containing data for ≥12 months before the study visit. Patients with other chronic respiratory diseases, such as chronic obstructive pulmonary disease, were excluded. Investigators, who were HCPs, were required to select and enroll patients under their care who met the inclusion criteria.
Study variables and outcomes
As described previously,50 patients were categorized by their SABA canister prescriptions during the 12 months before the study visit. SABA prescriptions were categorized as 0, 1–2, 3–5, 6–9, 10–12, and ≥13 canisters, and overprescription was defined as a prescription of ≥3 SABA canisters in the year prior to the study visit.23 ICS canister prescriptions were recorded by average daily dose as low, medium, or high based on GINA 2017 recommendations.7
Secondary variables included practice type (primary or specialist care), investigator-classified asthma severity (guided by GINA 2017;7 patients at GINA treatment steps 1–2 were categorized as having mild asthma and patients at steps 3–5 as having moderate-to-severe asthma), asthma treatments in the previous 12 months, and asthma duration. Other variables included healthcare insurance (not reimbursed, partially reimbursed, or fully reimbursed), education level (primary and secondary school, high school, or university and/or post-graduate education), body mass index (BMI), number of comorbidities, and tobacco smoking status. In addition, data for SABA over-the counter (OTC) purchase, which was based on patient recall, was obtained directly from patients at the study visit and entered in the eCRF by the investigator.
The assessed asthma-related health outcomes included asthma symptom control (using the GINA 2017 assessment of asthma control and categorized as well controlled, partly controlled, and uncontrolled) and number of severe asthma exacerbations (defined based on American Thoracic Society/European Respiratory Society recommendations52 as a deterioration in asthma resulting in hospitalization, emergency room treatment, or the need for intravenous or oral corticosteroids [OCS] for ≥3 days or a single intramuscular corticosteroid dose) in the year before the study.
Statistical analysis
Patient-level analyses are presented as country-aggregated descriptive statistics. A logistic multivariable regression model and a negative binomial regression model were used to analyze the associations of SABA prescriptions with at least partly controlled asthma (partly controlled plus well-controlled asthma, with uncontrolled asthma as the reference) and severe exacerbation incidence rates, respectively. All regression models used a complete-case analysis and were adjusted for prespecified variables (country, age, sex, and smoking status) and potential confounders (asthma severity as classified by investigators, healthcare insurance, education level, comorbidities, duration of asthma, and BMI). Patients with 0 SABA prescriptions were excluded from the secondary analyses because alternative relievers used by such patients were not recorded. The Kendall correlation test was used to assess the correlation between prescriptions of ICS and prescriptions of SABAs in addition to maintenance therapy. All statistical tests were 2-sided at a 5% level of significance and performed using R statistical software (version 3.6.0).
Results
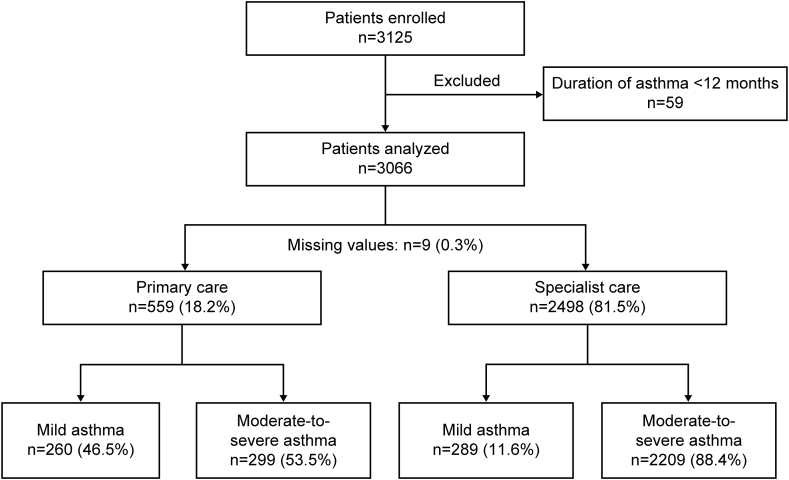
Of the 3125 patients enrolled, 59 were excluded because their asthma duration was <12 months (Fig. 1). Most patients were recruited from Malaysia (n = 732; 23.9%), followed by India (n = 510; 16.6%) and South Korea (n = 476; 15.5%; Fig. 2).
Fig. 1.
Patient disposition and study population by practice type and investigator-classified asthma severity
Fig. 2.
Patient enrollment across countries in the SABINA III Asian cohort
Most patients were treated by specialists (81.5%), whereas 18.2% of patients were treated in primary care (Fig. 1). Overall, 82.1% of patients had moderate-to-severe asthma (GINA steps 3–5). Patients had a mean (standard deviation [SD]) age of 51.8 (16.7) years, and most were female (69.0%; Table 1). Over half of all patients were obese (n = 1702 [55.5%]) according to the Asia-Pacific body mass index classification,53 and most had no history of smoking (n = 2591 [84.5%]). Over one-fourth of all patients had received primary/secondary school education (37.9%) and university and/or post-graduate education (30.2%). Similar findings were observed in both primary and specialist care. Additionally, almost one-fourth (21.4%) of patients did not have reimbursed healthcare. Most patients had 0 (32.0%) or 1–2 (49.2%) comorbidities. Patients experienced a mean (SD) of 0.84 (1.82) severe asthma exacerbations in the year before the study, and 37.5% experienced ≥1 severe asthma exacerbation (Table 2). Over half of all patients (53.6%) had well-controlled asthma, 29.4% had partly controlled asthma, and 17.1% had uncontrolled asthma across severities.
Table 1.
Demographic and lifestyle characteristics by investigator-classified asthma severity and practice type.
| Demographic and lifestyle characteristics | All (N = 3066)a | Primary care (n = 559) |
Specialists (n = 2498) |
||||
|---|---|---|---|---|---|---|---|
| Investigator-classified mild asthma (n = 260) | Investigator-classified moderate-to-severe asthma (n = 299) | All (n = 559) | Investigator-classified mild asthma (n = 289) | Investigator-classified moderate-to-severe asthma (n = 2209) | All (n = 2498) | ||
| Age (years) | |||||||
| Mean (SD) | 51.8 (16.7) | 46.2 (16.8) | 50.1 (16.3) | 48.3 (16.6) | 45.5 (19.9) | 53.5 (15.9) | 52.6 (16.6) |
| Range | 12.0–92.0 | 12.0–81.0 | 15.0–91.0 | 12.0–91.0 | 12.0–88.0 | 12.0–92.0 | 12.0–92.0 |
| Age groups (years) | |||||||
| 12–17 | 87 (2.8) | 24 (9.2) | 3 (1.0) | 27 (4.8) | 40 (13.8) | 20 (0.9) | 60 (2.4) |
| 18–54 | 1515 (49.4) | 137 (52.7) | 180 (60.2) | 317 (56.7) | 141 (48.8) | 1052 (47.6) | 1193 (47.8) |
| ≥55 | 1464 (47.7) | 99 (38.1) | 116 (38.8) | 215 (38.5) | 108 (37.4) | 1137 (51.5) | 1245 (49.8) |
| Sex | |||||||
| Female | 2116 (69.0) | 191 (73.5) | 211 (70.6) | 402 (71.9) | 191 (66.1) | 1516 (68.6) | 1707 (68.3) |
| Male | 950 (31.0) | 69 (26.5) | 88 (29.4) | 157 (28.1) | 98 (33.9) | 693 (31.4) | 791 (31.7) |
| BMI (kg/m2) | |||||||
| Mean (SD) | 26.3 (5.6) | 27.2 (6.0) | 26.5 (6.1) | 26.8 (6.1) | 25.6 (5.7) | 26.3 (5.4) | 26.2 (5.5) |
| Median (min, max) | 25.5 (12.6, 71.3) | 26.5 (12.6, 49.7) | 25.4 (16.6, 52.5) | 25.8 (12.6, 52.5) | 25.0 (14.9, 58.7) | 25.5 (14.2, 71.3) | 25.5 (14.2, 71.3) |
| BMI groupsb(kg/m2) | |||||||
| <18.5 | 127 (4.1) | NA | NA | NA | NA | NA | NA |
| 18.5–22.9 | 716 (23.4) | NA | NA | NA | NA | NA | NA |
| 23–24.9 | 521 (17.0) | NA | NA | NA | NA | NA | NA |
| ≥25 | 1702 (55.5) | NA | NA | NA | NA | NA | NA |
| Education level | |||||||
| Not established | 302 (9.8) | 8 (3.1) | 28 (9.4) | 36 (6.4) | 19 (6.6) | 245 (11.1) | 264 (10.6) |
| Primary or secondary school | 1162 (37.9) | 149 (57.3) | 116 (38.8) | 265 (47.4) | 96 (33.2) | 798 (36.1) | 894 (35.8) |
| High school | 675 (22.0) | 51 (19.6) | 51 (17.1) | 102 (18.2) | 90 (31.1) | 481 (21.8) | 571 (22.9) |
| University and/or post-graduate education | 927 (30.2) | 52 (20.0) | 104 (34.8) | 156 (27.9) | 84 (29.1) | 685 (31.0) | 769 (30.8) |
| Healthcare insurance/medication funding | |||||||
| Not reimbursed | 655 (21.4) | 42 (16.2) | 53 (17.7) | 95 (17.0) | 78 (27.0) | 481 (21.8) | 559 (22.4) |
| Partially reimbursed | 775 (25.3) | 17 (6.5) | 107 (35.8) | 124 (22.2) | 78 (27.0) | 568 (25.7) | 646 (25.9) |
| Fully reimbursed | 1469 (47.9) | 194 (74.6) | 118 (39.5) | 312 (55.8) | 127 (43.9) | 1027 (46.5) | 1154 (46.2) |
| Unknown | 167 (5.4) | 8 (3.1) | 28 (9.4) | 36 (6.4) | 19 (6.6) | 245 (11.1) | 264 (10.6) |
| Smoking status | |||||||
| Active smoker | 126 (4.1) | 11 (4.2) | 20 (6.7) | 31 (5.5) | 7 (2.4) | 88 (4.0) | 95 (3.8) |
| Former smoker | 349 (11.4) | 23 (8.8) | 36 (12.0) | 59 (10.6) | 25 (8.7) | 265 (12.0) | 290 (11.6) |
| Never smoker | 2591 (84.5) | 226 (86.9) | 243 (81.3) | 469 (83.9) | 257 (88.9) | 1856 (84.0) | 2113 (84.6) |
| Number of comorbidities | |||||||
| 0 | 981 (32.0) | 94 (36.2) | 112 (37.5) | 206 (36.9) | 103 (35.6) | 670 (30.3) | 773 (30.9) |
| 1–2 | 1508 (49.2) | 126 (48.5) | 111 (37.1) | 237 (42.4) | 147 (50.9) | 1118 (50.6) | 1265 (50.6) |
| 3–4 | 455 (14.8) | 39 (15.0) | 60 (20.1) | 99 (17.7) | 33 (11.4) | 323 (14.6) | 356 (14.3) |
| ≥5 | 122 (4.0) | 1 (0.4) | 16 (5.4) | 17 (3.0) | 6 (2.1) | 98 (4.4) | 104 (4.2) |
BMI, body mass index; max, maximum; min, minimum; NA, not available; SD, standard deviation, WHO, World Health Organization. Data are presented as n (%) unless otherwise specified.
Practice type was not recorded for 9 patients.
According to Asia-Pacific BMI classification. The Asia-Pacific BMI classification data were available for the broad BMI categories; however, the stratification by practice type was only available as per the WHO classification. Therefore, all fields under practice type are marked as NA
Table 2.
Asthma-related clinical characteristics.
| Asthma-related clinical characteristics | All (N = 3066)a | Primary care (n = 559) |
Specialists (n = 2498) |
||||
|---|---|---|---|---|---|---|---|
| Investigator-classified mild asthma (n = 260) | Investigator-classified moderate-to-severe asthma (n = 299) | All (n = 559) | Investigator-classified mild asthma (n = 289) | Investigator-classified moderate-to-severe asthma (n = 2209) | All (n = 2498) | ||
| Asthma duration (years) | |||||||
| Mean (SD) | 14.1 (14.5) | 21.46 (16.0) | 15.82 (14.5) | 18.44 (15.5) | 12.43 (13.6) | 13.23 (14.2) | 13.13 (14.1) |
| Median (min, max) | 9.0 (1.0, 83.0) | 17.0 (1.0, 68.0) | 11.0 (1.0, 65.0) | 13.0 (1.0, 68.0) | 8.0 (1.0, 65.0) | 8.0 (1.0, 83.0) | 8.0 (1.0, 83.0) |
| Number of severe asthma exacerbations 12 months before the study visit | |||||||
| Mean (SD) | 0.84 (1.8) | 0.93 (2.2) | 0.86 (1.6) | 0.89 (1.9) | 0.38 (1.2) | 0.89 (1.9) | 0.83 (1.8) |
| Number of severe asthma exacerbations 12 months before the study visit by group | |||||||
| 0 | 1917 (62.5) | 156 (60.0) | 186 (62.2) | 342 (61.2) | 237 (82.0) | 1333 (60.3) | 1570 (62.9) |
| 1 | 585 (19.1) | 52 (20.0) | 54 (18.1) | 106 (19.0) | 26 (9.0) | 452 (20.5) | 478 (19.1) |
| 2 | 241 (7.9) | 19 (7.3) | 27 (9.0) | 46 (8.2) | 12 (4.2) | 181 (8.2) | 193 (7.7) |
| 3 | 151 (4.9) | 21 (8.1) | 14 (4.7) | 35 (6.3) | 8 (2.8) | 108 (4.9) | 116 (4.6) |
| >3 | 172 (5.6) | 12 (4.6) | 18 (6.0) | 30 (5.4) | 6 (2.1) | 135 (6.1) | 141 (5.6) |
| GINA classification | |||||||
| Step 1 | 205 (6.7) | 98 (37.7) | 0 (0.0) | 98 (17.5) | 107 (37.0) | 0 (0.0) | 107 (4.3) |
| Step 2 | 344 (11.2) | 162 (62.3) | 0 (0.0) | 162 (29.0) | 182 (63.0) | 0 (0.0) | 182 (7.3) |
| Step 3 | 1003 (32.7) | 0 (0.0) | 166 (55.5) | 166 (29.7) | 0 (0.0) | 834 (37.8) | 834 (33.4) |
| Step 4 | 1234 (40.2) | 0 (0.0) | 119 (39.8) | 119 (21.3) | 0 (0.0) | 1109 (50.2) | 1109 (44.4) |
| Step 5 | 280 (9.1) | 0 (0.0) | 14 (4.7) | 14 (2.5) | 0 (0.0) | 266 (12.0) | 266 (10.6) |
| Level of asthma control | |||||||
| Well controlled | 1643 (53.6) | 146 (56.2) | 134 (44.8) | 280 (50.1) | 203 (70.2) | 1156 (52.3) | 1359 (54.4) |
| Partly controlled | 900 (29.4) | 67 (25.8) | 121 (40.5) | 188 (33.6) | 63 (21.8) | 646 (29.2) | 709 (28.4) |
| Uncontrolled | 523 (17.1) | 47 (18.1) | 44 (14.7) | 91 (16.3) | 23 (8.0) | 407 (18.4) | 430 (17.2) |
GINA, Global Initiative for Asthma; max, maximum; min, minimum; SD, standard deviation. Data are presented as n (%) unless otherwise specified.
Practice type was not recorded for 9 patients
Asthma treatment in the 12 months before the study visit
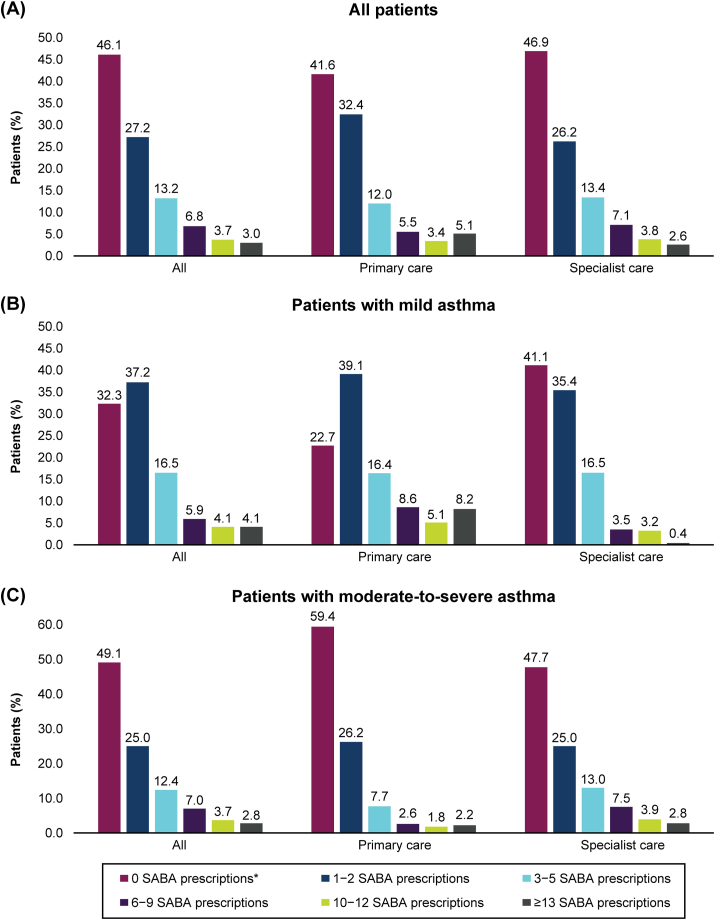
Overall, 26.7% of patients were prescribed ≥3 SABA canisters in the previous 12 months (Fig. 3). Similar results were observed across severities, with 30.5% and 25.8% of patients with mild and moderate-to-severe asthma, respectively, being prescribed ≥3 canisters.
Fig. 3.
SABA prescriptions according to investigator-classified asthma severity and practice type. SABA, short-acting β2-agonist
SABA monotherapy
Only 2.9% of patients were prescribed SABA monotherapy, with a mean (SD) of 4.0 (4.6) canisters (Table 3). Among these patients, 41.2% were prescribed ≥3 canisters, and 15.3% were prescribed ≥10 canisters. Among patients with mild asthma who were prescribed SABA monotherapy, 42.9% and 38.1% were prescribed ≥3 SABA canisters under primary and specialist care, respectively.
Table 3.
SABA prescriptions in the 12 months before the study visit.
| SABA prescriptions in the previous 12 months | All (N = 3066)a | Primary care (n = 559) |
Specialists (n = 2498) |
||||
|---|---|---|---|---|---|---|---|
| Investigator-classified mild asthma (n = 260) | Investigator-classified moderate-to-severe asthma (n = 299) | All (n = 559) | Investigator-classified mild asthma (n = 289) | Investigator-classified moderate-to-severe asthma (n = 2209) | All (n = 2498) | ||
| Number of patients prescribed inhaled SABA monotherapy | |||||||
| Yes | 89 (2.9) | 45 (17.3) | 0 (0.0) | 45 (8.1) | 43 (14.9) | 1 (0.0) | 44 (1.8) |
| No | 2977 (97.1) | 215 (82.7) | 299 (100) | 514 (91.9) | 246 (85.1) | 2208 (100) | 2454 (98.2) |
| Number of canisters/inhalers prescribed per patient 12 months before the study visit | |||||||
| n | 85 | 42 | NA | 42 | 42 | 1 | 43 |
| Mean (SD) | 4.0 (4.6) | 5.0 (5.6) | NA | 5.0 (5.6) | 3.1 (3.1) | 3.0 (NA) | 3.1 (3.0) |
| Median (min, max) | 2.0 (1.0, 16.0) | 2.0 (1.0, 16.0) | NA | 2.0 (1.0, 16.0) | 2.0 (1.0, 12.0) | 3.0 (3.0, 3.0) | 2.0 (1.0, 12.0) |
| Missing data | 4 (4.5) | 3 (6.7) | NA | 3 (6.7) | 1 (2.3) | 0 (0.0) | 1 (2.3) |
| Number of canisters/inhalers prescribed per patient 12 months before the study visit by category | |||||||
| 1–2 | 50 (58.8) | 24 (57.1) | NA | 24 (57.1) | 26 (61.9) | 0 (0.0) | 26 (60.5) |
| 3–5 | 18 (21.2) | 5 (11.9) | NA | 5 (11.9) | 12 (28.6) | 1 (100) | 13 (30.2) |
| 6–9 | 4 (4.7) | 4 (9.5) | NA | 4 (9.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 10–12 | 6 (7.1) | 2 (4.8) | NA | 2 (4.8) | 4 (9.5) | 0 (0.0) | 4 (9.3) |
| ≥13 | 7 (8.2) | 7 (16.7) | NA | 7 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing data (n) | 4 | 3 | NA | 3 | 1 | 0 | 1 |
| Total | 85 | 42 | NA | 42 | 42 | 1 | 43 |
| Number of patients prescribed inhaled SABA in addition to maintenance therapy | |||||||
| Yes | 1594 (52.0) | 157 (60.4) | 138 (46.2) | 295 (52.8) | 129 (44.6) | 1169 (52.9) | 1298 (52) |
| No | 1472 (48.0) | 103 (39.6) | 161 (53.8) | 264 (47.2) | 160 (55.4) | 1040 (47.1) | 1200 (48) |
| Number of canisters/inhalers prescribed per patient 12 months before the study visit | |||||||
| n | 1531 | 156 | 110 | 266 | 126 | 1138 | 1264 |
| Mean (SD) | 5.1 (10.6) | 4.6 (4.7) | 5.1 (20.1) | 4.8 (13.4) | 2.9 (2.8) | 5.4 (10.4) | 5.1 (10.0) |
| Median (min, max) | 2.0 (1.0, 210.0) | 3.0 (1.0, 18.0) | 2.0 (1.0, 210.0) | 2.0 (1.0, 210.0) | 2.0 (1.0, 16.0) | 3.0 (1.0, 196.0) | 3.0 (1.0, 196.0) |
| Missing data | 63 (4.0) | 1 (0.6) | 28 (20.3) | 29 (9.8) | 3 (2.3) | 31 (2.7) | 34 (2.6) |
| Number of canisters/inhalers prescribed per patient 12 months before the study visit by category | |||||||
| 1–2 | 766 (50.0) | 76 (48.7) | 71 (64.5) | 147 (55.3) | 75 (59.5) | 544 (47.8) | 619 (49.0) |
| 3–5 | 377 (24.6) | 37 (23.7) | 21 (19.1) | 58 (21.8) | 35 (27.8) | 283 (24.9) | 318 (25.2) |
| 6–9 | 199 (13.0) | 18 (11.5) | 7 (6.4) | 25 (9.4) | 10 (7.9) | 164 (14.4) | 174 (13.8) |
| 10–12 | 106 (6.9) | 11 (7.1) | 5 (4.5) | 16 (6.0) | 5 (4.0) | 85 (7.5) | 90 (7.1) |
| ≥13 | 83 (5.4) | 14 (9.0) | 6 (5.5) | 20 (7.5) | 1 (0.8) | 62 (5.4) | 63 (5.0) |
| Missing data (n) | 63 | 1 | 28 | 29 | 3 | 31 | 34 |
| Total | 1531 | 156 | 110 | 266 | 126 | 1138 | 1264 |
| Number of patients prescribed oral SABA | |||||||
| Yes | 83 (2.7) | 13 (5.0) | 12 (4.0) | 25 (4.5) | 5 (1.7) | 53 (2.4) | 58 (2.3) |
| No | 2983 (97.3) | 247 (95.0) | 287 (96.0) | 534 (95.5) | 284 (98.3) | 2156 (97.6) | 2440 (97.7) |
| Number of patients prescribed nebulized SABA | |||||||
| Yes | 224 (7.3) | 80 (30.8) | 51 (17.1) | 131 (23.4) | 20 (6.9) | 73 (3.3) | 93 (3.7) |
| No | 2842 (92.7) | 180 (69.2) | 248 (82.9) | 428 (76.6) | 269 (93.1) | 2136 (96.7) | 2405 (96.3) |
Max, maximum; min, minimum; NA, not available; SABA, short-acting β2-agonist; SD, standard deviation. Data are presented as n (%) unless otherwise specified. Missing data are not included in the calculation of percentages.
Practice type was not recorded for 9 patients
SABA plus maintenance therapy
Over half (52.0%) of all patients were prescribed SABA in addition to maintenance therapy, with a mean (SD) of 5.1 (10.6) canisters (Table 3). Overall, 50% of patients who were prescribed SABA in addition to maintenance therapy were prescribed ≥3 SABA canisters, and 12.3% were prescribed ≥10 SABA canisters. A similar proportion of patients were prescribed ≥3 canisters in primary and specialist care (44.7% and 51.0%, respectively) across severities.
Other SABA prescriptions
Altogether, 2.7% and 7.3% of all patients were prescribed oral and nebulized forms of SABA, respectively (Table 3).
Over-the-counter SABA
Overall, 7.7% of patients purchased SABA OTC (Supplementary Table 1) of whom 32.8% purchased ≥3 canisters. Patients treated in primary care had greater SABA purchases than patients in specialist care across severities (12.3% vs 6.7%). Notably, 33.9% and 38.6% of patients who purchased SABA OTC had partly controlled and uncontrolled asthma, respectively (Supplementary Table 2).
Other prescriptions of asthma medications in the 12 months before the study visit
Overall, 14.0% of patients were prescribed maintenance ICS monotherapy, with a mean (SD) of 5.1 (11.5) ICS canisters (Supplementary Table 3) Most patients were prescribed low-dose (43.7%) or medium-dose (50.8%) ICS. Almost one-third (32.4%) of patients were prescribed ICS monotherapy in primary care, 86.2% of whom had mild asthma. In contrast, only 9.9% of patients in specialist care were prescribed ICS monotherapy.
Overall, 84.2% of patients were prescribed ICS/LABA fixed-dose combinations, with 39.0% receiving low-dose ICS and 49.8% receiving medium-dose ICS (Supplementary Table 3). Most patients with moderate-to-severe asthma in primary and specialist care were prescribed ICS/LABA; however, 5.8% and 26.6% of patients with mild asthma in primary and specialist care, respectively, also had ICS/LABA prescriptions.
OCS bursts were prescribed to 29.7% of patients, with comparable findings in primary and specialist care (Supplementary Table 3). In addition, 7.8% of patients were prescribed OCS maintenance doses and 19.1% were prescribed antibiotics for their asthma (Supplementary Table 4).
Overall, 25.8%, 42.9%, and 38.6% of patients who were prescribed SABA monotherapy, ICS, and ICS/LABA fixed-dose combinations, respectively, had experienced ≥1 severe asthma exacerbation (Table 4). Notably, 20.8% of patients who were prescribed short-course OCS had never experienced a severe asthma exacerbation.
Table 4.
Number of severe exacerbations and treatments in the 12 months before the study visit.
| All (N = 3066) | SABA mono (n = 89) | SABA as an add-on (n = 1594) | ICS mono (n = 429) | ICS/LABA (fixed dose) (n = 2581) |
OCS short course (n = 910) | |
|---|---|---|---|---|---|---|
| Number of severe exacerbations 12 months before the study visit, n (%) | ||||||
| 0 | 1917 (62.5) | 66 (74.2) | 869 (54.5) | 245 (57.1) | 1584 (61.4) | 189 (20.8) |
| 1 | 585 (19.1) | 14 (15.7) | 364 (22.8) | 81 (18.9) | 514 (19.9) | 333 (36.6) |
| 2 | 241 (7.9) | 2 (2.2) | 162 (10.2) | 48 (11.2) | 209 (8.1) | 145 (15.9) |
| 3 | 151 (4.9) | 5 (5.6) | 81 (5.1) | 32 (7.5) | 120 (4.6) | 110 (12.1) |
| 4 | 66 (2.2) | 1 (1.1) | 38 (2.4) | 8 (1.9) | 60 (2.3) | 50 (5.5) |
| 5 | 40 (1.3) | 1 (1.1) | 26 (1.6) | 3 (0.7) | 36 (1.4) | 28 (3.1) |
| >5 | 66 (2.2) | 0 (0.0) | 54 (3.4) | 12 (2.8) | 58 (2.2) | 55 (6.0) |
| Total (n) | 3066 | 89 | 1594 | 429 | 2581 | 910 |
ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; mono, monotherapy; OCS, oral corticosteroids; SABA, short-acting β2-agonist
Factors associated with SABA overprescription
In a post hoc analysis conducted to determine the association between ICS and SABA prescriptions in addition to maintenance therapy, patients with a high number of ICS prescriptions received significantly more SABA prescriptions (data not shown; Kendall correlation coefficient, 0.18; P < 0.001).
Association of SABA prescriptions with asthma-related outcomes
In prespecified regression analyses (Supplementary Figure 1), prescriptions of ≥3 SABA canisters (vs 1–2 canisters) were associated with an increase in the incidence rate of severe exacerbations (P < 0.05; Fig. 4). Additionally, higher SABA prescriptions (≥3 canisters) were associated with a significantly decreased odds of having at least partly controlled asthma vs 1–2 canisters (P < 0.01; Fig. 4).
Fig. 4.
Association of SABA prescriptions with (A) severe exacerbations and (B) level of asthma symptom control. BMI, body mass index; CI, confidence interval; GINA, Global Initiative for Asthma; IRR, incidence rate ratio; OR, odds ratio; SABA, short-acting β2-agonist
Comparison of results between SABINA Asia and SABINA III
A comparison of data on sociodemographic and clinical characteristics, asthma treatments, and asthma-related clinical outcomes in the previous 12 months between the SABINA Asia cohort and the overall SABINA III population is summarized in Supplementary Table 5. The key differences are highlighted in the Discussion section.
Discussion
This Pan-Asian study conducted in >3000 patients with asthma demonstrated that ≥3 SABA canisters were prescribed to 26% of patients in the 12 months before the study visit. Most patients (98.2%) were prescribed maintenance therapy in the form of either ICS or ICS/LABA fixed-dose combination therapy; furthermore, OCS burst treatment was prescribed to 29.7% of patients, potentially for the management of worsening asthma symptoms and/or to treat severe exacerbations.4 Notably, SABA overprescription significantly increased the odds of uncontrolled asthma and severe exacerbation incidence rates.
The patient and disease characteristics and key results of this study are generally consistent with the global trends observed in the SABINA III study.50 However, compared with the overall SABINA III population (mean age, 49.4 years),50 patients in this Asian cohort were slightly older (mean age, 51.8 years); this is in line with previous studies that have reported increasing asthma prevalence with age in Asian populations, including those from South Korea,54 China,55 Taiwan,56 and India.57,58 Moreover, in accordance with increasing evidence that older females with a high BMI represent a distinct asthma phenotype,59,60 the majority of patients in this Asian cohort were female (69%) and classified as obese (55%; BMI ≥25 kg/m2). Interestingly, despite older age and high BMI being associated with poorer disease control,61,62 compared with the overall SABINA III population, a higher proportion of patients from Asia had well-controlled asthma (53.6% vs 43.3%), with a lower proportion experiencing ≥1 severe asthma exacerbation in the preceding 12 months (37.5% vs 45.4%).50 Such findings may be attributable to the fact that a lower proportion of patients in this Asian cohort were overprescribed SABA treatments compared with the SABINA III study (26.0% vs 38.0%).50 Moreover, most patients in this Pan-Asian study were treated by specialists who are likely more familiar with current asthma treatment guidelines.
Overall, findings from Asia are also consistent with those from the SABINA I/II studies, where over one-third of patients were overprescribed SABA across 5 countries in Europe.51 However, only 7.7% of patients in this Asian cohort purchased SABA OTC, potentially reflecting the fact that SABA purchase is illegal in Taiwan and strictly regulated in Singapore. A higher proportion of patients with OTC SABA purchases had partly controlled or uncontrolled asthma compared with those in the overall SABINA Asia cohort (72.5% vs 17.1%), suggesting that poor asthma control resulted in SABA purchase. However, 27.5% of patients with OTC SABA purchases had well-controlled asthma, potentially indicating unnecessary SABA use in some patients. Cumulatively, our results suggest that high SABA prescriptions are a potential public health risk across Asia and should be closely monitored, together with OTC SABA purchase, to identify patients at risk of poor asthma-related outcomes. In our study, almost half of all patients were not prescribed any inhaled SABA. While a proportion of these patients may have had well-controlled asthma or purchased SABA OTC, over 20% of patients used oral or nebulized SABA as a reliever. For instance, in the Philippines, oral/nebulized SABA is commonly used and available for purchase even without prescriptions. Oral SABA use in low-resource settings in Asian countries can be attributed to a preference for oral medications, together with treatment affordability issues.63, 64, 65, 66 While the use of low-dose ICS-formoterol as maintenance and reliever therapy (MART) was not recorded in the study, the use of MART by patients with moderate-to-severe asthma cannot be discounted.
Among patients with mild asthma who had received ICS maintenance therapy, >60% were prescribed medium-to-high doses of ICS in the previous 12 months. While ICS/LABA could have been prescribed to some patients with mild asthma as anti-inflammatory reliever therapy for as-needed use in alignment with GINA recommendations, some prescriptions in primary care could also be attributed to the unfamiliarity of primary care physicians with GINA recommendations67 or local guidelines recommending low-dose ICS/LABA instead of ICS monotherapy for mild asthma, such as in the Philippines. Additionally, results of the Kendall correlation test demonstrated that patients with a high number of ICS prescriptions received significantly more SABA prescriptions (correlation coefficient, 0.18; P < 0.001). This is consistent with findings from a study analyzing administrative claims data from 38,538 patients with persistent asthma, which also reported that patients receiving ICS monotherapy were more likely to use SABAs, compared with those prescribed ICS/LABA fixed-dose combination therapy.68 These observations may be explained by the fact that ICS/LABA fixed-dose combination therapy has been shown to be more effective than ICS monotherapy in relieving asthma symptoms, improving lung function and reducing SABA use.69 Indeed, in this Asian cohort, a higher proportion of patients prescribed ICS monotherapy compared with those prescribed an ICS/LABA fixed-dose combination experienced ≥1 severe asthma exacerbation (42.9% vs 38.6%).
To date, only a few studies have been conducted in patients with asthma in Asia.14,16,70 Moreover, no study has focused specifically on SABA prescriptions. To the best of our knowledge, this is the first study assessing trends in SABA overprescription and their implications for patient health status in Asia. The results are in agreement with those of global studies demonstrating an association between SABA overuse and frequent exacerbations12,24,28,71 and poor asthma symptom control.20 Taken together, our study underscores the need for educational initiatives targeting patients, pharmacists, and physicians to reduce SABA overreliance and improve outcomes through adherence to local and international recommendations.
More than 80% of patients in our cohort had moderate-to-severe asthma (GINA steps 3–5), probably because most patients were treated by specialists. Restricted accessibility to primary care clinics and country-specific regulatory roadblocks limited recruitment by primary care physicians, which would have provided a more complete overview of real-world practice in Asian countries.72 Based on medical records, >98% of patients in SABINA Asia received maintenance medication. In contrast, self-reported use of daily asthma controller medication ranged from 13.9% in the REALISE Asia study15 to 32% in the AIM survey in the Asia-Pacific region, indicating suboptimal use of maintenance medication.14 The level of asthma symptom control was also greater in our study, as >80% of patients had either well-controlled or partly controlled asthma vs 50.3% in the REALISE Asia study.15 Over 60% of patients in our cohort did not experience any severe exacerbation in the previous 12 months, whereas >60% of patients in the Asia-Pacific region in the AIR survey reported severe exacerbations.16 In addition, over two-thirds of patients had partial or full healthcare reimbursement, which may not necessarily reflect the true healthcare landscape in Asia. For example, Taiwan offers fully reimbursed healthcare,73 while healthcare expenditures are largely out-of-pocket in India.74 Collectively, this Asian cohort may represent a “better-case scenario” with respect to disease characteristics, sociodemographic parameters, and access to healthcare in these countries. Nonetheless, excessive SABA prescriptions were common, suggesting that the clinical scenario could be considerably worse in these Asian countries than that observed in our study. However, overall, the results should be interpreted in the context of country-specific clinical practices and regulations, which will be discussed in separate publications describing country-specific findings.
Some limitations of this multi-country study should be considered. For instance, as data input into the eCRF relied on physicians, misinterpretation of instructions and possible erroneous classification of asthma severity or practice type may have affected the findings. The use of prescription data may not always reflect actual use, and predominantly specialist-acquired data from Asia may have limited generalizability. Additionally, asthma severity is a strong independent risk factor for future exacerbations;75 therefore, the fact that over 80% of patients in this study were classified with moderate-to-severe asthma, may have increased exacerbation rates, thereby further limiting the generalizability of our findings. This study also used purposive sampling which may be prone to research bias. Finally, this study only recorded the number of comorbidities (categorized as 0, 1–2, 3–4, and ≥5) in the eCRF, while data on the type and rate of comorbidities, which may have impacted the patient outcomes, were not collected. However, aggregated data from these 8 Asian countries enabled the analysis of a large patient population reflective of real-world diagnosis, treatment, and follow-up practices across Asia. Moreover, the standardized threshold used for determining SABA overprescription allowed for a direct comparison with global data.
Conclusions
This large observational study conducted across 8 Asian countries identified SABA overprescription in more than one-quarter of patients with asthma in this predominantly specialist-treated cohort. Considering the safety concerns associated with SABA overuse, these results highlight a potential public health concern, indicating the need for HCPs and policymakers to work together to ensure that clinical practices in Asia are aligned with the latest evidence-based treatment guidelines.
Abbreviations
AIM, Asia Asthma Insights and Management; AIR, Asthma Insights and Reality; BMI, body mass index; CI, confidence interval; eCRF, electronic case report form; GINA, Global Initiative for Asthma; IRR, incidence rate ratio; HCP, healthcare provider; ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; MART, maintenance and reliever therapy; NA, not available; OCS, oral corticosteroids; OR, odds ratio; OTC, over the counter; REALISE, REcognise Asthma and LInk to Symptoms and Experience; SABINA, SABA use IN Asthma; SD, standard deviation; SABA, short-acting β2-agonist.
Acknowledgements
AstraZeneca funded all SABINA studies and was involved in designing the studies, developing the study protocol, conducting the studies, and performing the analyses. AstraZeneca was given the opportunity to review the manuscript before submission and funded medical writing support.
Writing and editorial support was provided by Saurabh Gagangras of Cactus Life Sciences (part of Cactus Communications, Mumbai, India) in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3) and fully funded by AstraZeneca.
Funding
This study was funded by AstraZeneca.
Availability of data and materials
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Authors' contributions
MJHIB contributed to conceptualization, methodology, supervision, and visualization efforts. LY supported supervision efforts. HC-W, SD, LS, TT, HFL, HFY, and ABYL contributed equally to investigation efforts. All authors contributed equally to writing, review, and editing of the manuscript.
Ethics approval and consent to participate
The study was compliant with the study protocol, local ethics committees, and the Declaration of Helsinki; signed informed consent was obtained from all patients or their legal guardians. Because patients were only queried as aggregates and no protected, identifiable health information was available for queries, no institutional review board approval was required for the use of this database or the completion of this study.
Authors’ consent for publication
All authors agree to publish this work. All authors confirm and agree to the editorial policy. Authors confirm that their manuscript is original, has not been published before, is not currently being considered for publication elsewhere, and has not been posted to a preprint server.
Competing interest
H-CW, LS, TT, HFL, and ABY-L report no disclosures. SD has received honoraria for educational activities from and served on the advisory boards of AstraZeneca, Novartis, Boehringer Ingelheim, and Zambon. KHY is a member of the advisory boards of GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis Healthcare, Takeda Healthcare, Nycomed, Teva, MSD, Mundipharma, Hyundai, and Ankook. DVD is a member of the Advisory Boards and/or Speakers’ Bureau and has received honoraria/lecture fees from AstraZeneca, Boehringer-Ingelheim, Cathay Drug Co. Inc., Getz Pharma, GlaxoSmithKline, Glenmark, Johnson & Johnson, Multicare Pharmaceuticals, Mundipharma, Novartis Healthcare, Orient EuroPharma, Pfizer, Takeda Healthcare, Unilab, and Westmont Pharmaceuticals, Inc.; DVD has also received honoraria as Principal Investigator in clinical trials sponsored by AstraZeneca, GlaxoSmithKline, Johnson & Johnson, Novartis Healthcare, and Takeda Healthcare. MJHIB was an employee of AstraZeneca at the time of study conduct and manuscript development. LY is an employee of AstraZeneca.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2023.100823.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Global Asthma Network (GAN) 2018. The Global Asthma Report.http://www.globalasthmareport.org [Google Scholar]
- 2.Song W.J., Kang M.G., Chang Y.S., et al. Epidemiology of adult asthma in Asia: toward a better understanding. Asia Pac Allergy. 2014;4:75–85. doi: 10.5415/apallergy.2014.4.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong G.W., Leung T.F., Ko F.W. Changing prevalence of allergic diseases in the Asia-pacific region. Allergy Asthma Immunol Res. 2013;5:251–257. doi: 10.4168/aair.2013.5.5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Initiative for Asthma (GINA) 2023. Global Strategy for Asthma Management and Prevention.https://ginasthma.org/2023-gina-main-report/ [Google Scholar]
- 5.Gold L.S., Thompson P., Salvi S., et al. Level of asthma control and health care utilization in Asia-Pacific countries. Respir Med. 2014;108:271–277. doi: 10.1016/j.rmed.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Thompson P.J., Salvi S., Lin J., et al. Insights, attitudes and perceptions about asthma and its treatment: findings from a multinational survey of patients from 8 Asia-Pacific countries and Hong Kong. Respirology. 2013;18:957–967. doi: 10.1111/resp.12137. [DOI] [PubMed] [Google Scholar]
- 7.Global Initiative for Asthma (GINA) 2017. Global Strategy for Asthma Management and Prevention.http://ginasthma.org/ [Google Scholar]
- 8.Kaplan A., Mitchell P.D., Cave A.J., et al. Effective asthma management: is it time to let the AIR out of SABA? J Clin Med. 2020;9:921. doi: 10.3390/jcm9040921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao H., Li R., Lv Y., et al. Albuterol inhalation increases FeNO level in steroid-naive asthmatics but not COPD patients with reversibility. Clin Res J. 2017;11:328–336. doi: 10.1111/crj.12340. [DOI] [PubMed] [Google Scholar]
- 10.FitzGerald J.M., Tavakoli H., Lynd L.D., et al. The impact of inappropriate use of short acting beta agonists in asthma. Respir Med. 2017;131:135–140. doi: 10.1016/j.rmed.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Silver H.S., Blanchette C.M., Kamble S., et al. Relationship between short-acting beta2-adrenergic agonist use and healthcare costs. Am J Manag Care. 2011;17:19–27. [PubMed] [Google Scholar]
- 12.Stanford R.H., Shah M.B., D'Souza A.O., et al. Short-acting beta-agonist use and its ability to predict future asthma-related outcomes. Ann Allergy Asthma Immunol. 2012;109:403–407. doi: 10.1016/j.anai.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Davidsen J.R. Drug utilization and asthma control among young Danish adults with asthma. Analyses of trends and determinants. Dan Med J. 2012;59:B4501. [PubMed] [Google Scholar]
- 14.Nathan R.A., Thompson P.J., Price D., et al. Taking aim at asthma around the world: global results of the asthma insight and management survey in the Asia-Pacific region, Latin America, Europe, Canada, and the United States. J Allergy Clin Immunol Pract. 2015;3:734–742 e5. doi: 10.1016/j.jaip.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Price D., David-Wang A., Cho S.H., et al. Time for a new language for asthma control: results from REALISE Asia. J Asthma Allergy. 2015;8:93–103. doi: 10.2147/JAA.S82633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabe K.F., Adachi M., Lai C.K., et al. Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol. 2004;114:40–47. doi: 10.1016/j.jaci.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 17.Rabe K.F., Vermeire P.A., Soriano J.B., et al. Clinical management of asthma in 1999: the asthma insights and reality in Europe (AIRE) study. Eur Respir J. 2000;16:802–807. doi: 10.1183/09031936.00.16580200. [DOI] [PubMed] [Google Scholar]
- 18.O'Byrne P.M., FitzGerald J.M., Bateman E.D., et al. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med. 2018;378:1865–1876. doi: 10.1056/NEJMoa1715274. [DOI] [PubMed] [Google Scholar]
- 19.Bateman E.D., Reddel H.K., O'Byrne P.M., et al. As-needed budesonide-formoterol versus maintenance budesonide in mild asthma. N Engl J Med. 2018;378:1877–1887. doi: 10.1056/NEJMoa1715275. [DOI] [PubMed] [Google Scholar]
- 20.Azzi E.A., Kritikos V., Peters M.J., et al. Understanding reliever overuse in patients purchasing over-the-counter short-acting beta(2) agonists: an Australian community pharmacy-based survey. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-028995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hull S.A., McKibben S., Homer K., et al. Asthma prescribing, ethnicity and risk of hospital admission: an analysis of 35,864 linked primary and secondary care records in East London. NPJ Prim Care Respir Med. 2016;26 doi: 10.1038/npjpcrm.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadatsafavi M., Tavakoli H., Lynd L., et al. Has asthma medication use caught up with the evidence?: a 12-year population-based study of trends. Chest. 2017;151:612–618. doi: 10.1016/j.chest.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 23.Cabrera C.S., Nan C., Lindarck N., et al. SABINA: global programme to evaluate prescriptions and clinical outcomes related to short-acting beta(2)-agonist use in asthma. Eur Respir J. 2020;55 doi: 10.1183/13993003.01858-2019. [DOI] [PubMed] [Google Scholar]
- 24.Bloom C.I., Cabrera C., Arnetorp S., et al. Asthma-related health outcomes associated with ahort-Acting beta(2)-Agonist inhaler use: an observational UK study as part of the SABINA global program. Adv Ther. 2020;37:4190–4208. doi: 10.1007/s12325-020-01444-5. [DOI] [PubMed] [Google Scholar]
- 25.Di Marco F., D'Amato M., Lombardo F.P., et al. The burden of short-acting beta(2)-agonist use in asthma: is there an Italian case? an update from SABINA program. Adv Ther. 2021;38:3816–3830. doi: 10.1007/s12325-021-01772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molina J., Plaza V., Nuevo N., et al. Clinical consequences of the qveruse of short-acting β2-adrenergic agonists (SABA) in the treatment of asthma in Spain: the SABINA study. Open Respir Arch. 2023;5 doi: 10.1016/j.opresp.2023.100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noorduyn S.G., Qian C., Johnston K.M., et al. SABA use as an indicator for asthma exacerbation risk: an observational cohort study (SABINA Canada) ERJ Open Res. 2022;8:140–2022. doi: 10.1183/23120541.00140-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nwaru B.I., Ekstrom M., Hasvold P., et al. Overuse of short-acting beta(2)-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55 doi: 10.1183/13993003.01872-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raherison-Semjen C., Izadifar A., Russier M., et al. Self-reported asthma prevalence and management in adults in France in 2018: ASTHMAPOP survey. Respir Med Res. 2021;80 doi: 10.1016/j.resmer.2021.100864. [DOI] [PubMed] [Google Scholar]
- 30.Vervloet M., van Dijk L., Weesie Y.M., et al. Understanding relationships between asthma medication use and outcomes in a SABINA primary care database study. NPJ Prim Care Respir Med. 2022;32:43. doi: 10.1038/s41533-022-00310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Worth H., Criée C.P., Vogelmeier C.F., et al. Prevalence of overuse of short-acting beta-2 agonists (SABA) and associated factors among patients with asthma in Germany. Resir Res. 2021;108 doi: 10.1186/s12931-021-01701-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz DV, Nicodemus LA, Parena-Santiago EL, et al. Short-acting β2-agonist prescription patterns in patients with asthma in the Philippines: results from SABINA III Acta Med Philipp [Internet]. https://actamedicaphilippinaupmeduph/indexphp/acta/article/view/4816. 2022.
- 33.Shen S.Y., Chen C.W., Liu T.C., et al. SABA prescriptions and asthma management practices in patients treated by specialists in Taiwan: results from the SABINA III study. J Formos Med Assoc. 2022;121:2527–2537. doi: 10.1016/j.jfma.2022.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Theerakittikul T., Nakwan N., Niyompattama A., et al. Short-acting beta(2)-agonist prescription patterns in patients with asthma treated by specialists in Thailand: results from SABINA III. J Asthma. 2023 doi: 10.1080/02770903.2023.2228895. [DOI] [PubMed] [Google Scholar]
- 35.Ban A.Y., Vengadasalam P., Taher S.W., et al. Short-acting beta(2)-agonist prescription patterns and clinical outcomes in Malaysia: a nationwide cohort of the SABINA III study. Malays Fam Physician. 2023;18:32. doi: 10.51866/oa.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Modi M., Mody K., Jhawar P., et al. Short-acting beta2-agonists over-prescription in patients with asthma: an Indian subset analysis of international SABINA III study. J Asthma. 2023;60:1347–1358. doi: 10.1080/02770903.2022.2147079. [DOI] [PubMed] [Google Scholar]
- 37.Wiyono W.H., Amin M., Djajalaksana S., et al. An evaluation of short-acting β2-agonist prescriptions and associated clinical outcomes in asthma management in Indonesia – the SABINA Indonesia study. J Respirol Indones. 2022;42:121–128. [Google Scholar]
- 38.Khattab A., Madkour A., Ambaram A., et al. Over-prescription of short-acting beta(2)-agonists is associated with poor asthma outcomes: results from the African cohort of the SABINA III study. Curr Med Res Opin. 2022;38:1983–1995. doi: 10.1080/03007995.2022.2100649. [DOI] [PubMed] [Google Scholar]
- 39.Smith C., Ambaram A., Mitha E., et al. Over-prescription of short-acting beta(2)-agonists for asthma in South Africa: results from the SABINA III study. Afr J Thorac Crit Care Med. 2022;28:172–180. doi: 10.7196/AJTCCM.2022.v28i4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakaya J., Mecha J., Beekman M. Over-prescription of short-acting beta(2)-agonists remains a serious health concern in Kenya: results from the SABINA III study. BMC Prim Care. 2023;24:141. doi: 10.1186/s12875-023-02030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al Zaabi A., Busaidi N., Al Mutairy S., et al. Overprescription of short-acting beta(2)-agonists is associated with poor asthma symptom control: results from five Middle Eastern countries included in the SABINA International (III) study. Expet Rev Respir Med. 2022;16:833–847. doi: 10.1080/17476348.2022.2099841. [DOI] [PubMed] [Google Scholar]
- 42.Yorgancioglu A., Aksu K., Nayci S.A., et al. Short-acting beta(2)-agonist prescription patterns in patients with asthma in Turkey: results from SABINA III. BMC Pulm Med. 2022;22:216. doi: 10.1186/s12890-022-02008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Jahdali H., Wali S., Albanna A.S., et al. Overprescription of short-acting beta(2) -agonists among patients with asthma in Saudi Arabia: results from the SABINA III cohort study. Clin Res J. 2022;16:812–825. doi: 10.1111/crj.13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al Zaabi A., Al Busaidi N., Pradhan R., et al. Over-prescription of short-acting beta(2)-agonists and asthma management in the Gulf region: a multicountry observational study. Asthma Res Pract. 2022;8:3. doi: 10.1186/s40733-022-00085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montero-Arias F., Garcia J.C.H., Gallego M.P., et al. Over-prescription of short-acting beta(2)-agonists is associated with poor asthma outcomes: results from the Latin American cohort of the SABINA III study. J Asthma. 2023;60:574–587. doi: 10.1080/02770903.2022.2082305. [DOI] [PubMed] [Google Scholar]
- 46.Herrera-Garcia J.C., Barriga-Acevedo R.M., Saavedra-Sanchez S.B., et al. Over-prescription of short-acting β2-agonists in Mexico: results from the SABINA III study Biomed. J Sci Tech Res. 2022;45:36236–36247. https://biomedres.us/fulltexts/BJSTR.MS.ID.007167.php [Google Scholar]
- 47.Pedrozo-Pupo J.C., Pacheco Gallego M.C., Banos Alvarez I.J., et al. A cross-sectional study on prescription patterns of short-acting beta(2)-agonists in patients with asthma: results from the SABINA III Colombia cohort. J Asthma Allergy. 2022;15:1167–1178. doi: 10.2147/JAA.S365009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mattarucco W., Altieri H., Baldasaria R., et al. Prescription pattern of short-acting beta-2 agonists and potential effects on asthma control: Argentine cohort of the SABINA III study. Rev Argentina de Med. 2022;10:116–123. [Google Scholar]
- 49.Avdeev S., Voznesenskiy N., Boldina M., et al. SABA overuse in Russia - burden and possible causes: an analysis of the Russian population in the SABINA III (SABA use IN Asthma) study. J Asthma Allergy. 2022;15:371–379. doi: 10.2147/JAA.S350393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bateman E.D., Price D.B., Wang H.C., et al. Short-acting beta(2)-agonist prescriptions are associated with poor clinical outcomes of asthma: the multi-country, cross-sectional SABINA III study. Eur Respir J. 2022;59 doi: 10.1183/13993003.01402-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janson C., Menzies-Gow A., Nan C., et al. SABINA: an overview of short-acting beta(2)-agonist use in asthma in European countries. Adv Ther. 2020;37:1124–1135. doi: 10.1007/s12325-020-01233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reddel H.K., Taylor D.R., Bateman E.D., et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 53.Consultation W.H.O.E. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 54.Kim Y.K., Kim S.H., Tak Y.J., et al. High prevalence of current asthma and active smoking effect among the elderly. Clin Exp Allergy. 2002;32:1706–1712. doi: 10.1046/j.1365-2222.2002.01524.x. [DOI] [PubMed] [Google Scholar]
- 55.Chan-Yeung M., Zhan L.X., Tu D.H., et al. The prevalence of asthma and asthma-like symptoms among adults in rural Beijing, China. Eur Respir J. 2002;19:853–858. doi: 10.1183/09031936.02.00250602. [DOI] [PubMed] [Google Scholar]
- 56.Jan I.S., Chou W.H., Wang J.D., et al. Prevalence of and major risk factors for adult bronchial asthma in Taipei City. J Formos Med Assoc. 2004;103:259–263. [PubMed] [Google Scholar]
- 57.Aggarwal A.N., Chaudhry K., Chhabra S.K., et al. Prevalence and risk factors for bronchial asthma in Indian adults: a multicentre study. Indian J Chest Dis Allied Sci. 2006;48:13–22. [PubMed] [Google Scholar]
- 58.Jindal S.K., Aggarwal A.N., Gupta D., et al. Indian study on epidemiology of asthma, respiratory symptoms and chronic bronchitis in adults (INSEARCH) Int J Tubercul Lung Dis. 2012;16:1270–1277. doi: 10.5588/ijtld.12.0005. [DOI] [PubMed] [Google Scholar]
- 59.Haldar P., Pavord I.D., Shaw D.E., et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore W.C., Meyers D.A., Wenzel S.E., et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lv N., Xiao L., Camargo C.A., Jr., et al. Abdominal and general adiposity and level of asthma control in adults with uncontrolled asthma. Ann Am Thorac Soc. 2014;11:1218–1224. doi: 10.1513/AnnalsATS.201405-214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tupper O.D., Ulrik C.S. Long-term predictors of severe exacerbations and mortality in a cohort of well-characterised adults with asthma. Respir Res. 2021;22:269. doi: 10.1186/s12931-021-01864-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chin M.C., Sivasampu S., Khoo E.M. Prescription of oral short-acting beta 2-agonist for asthma in non-resource poor settings: a national study in Malaysia. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Loh L.C., Wong P.S. Asthma prescribing practices of government and private doctors in Malaysia--a nationwide questionnaire survey. Asian Pac J Allergy Immunol. 2005;23:7–17. [PubMed] [Google Scholar]
- 65.Sun H.L., Kao Y.H., Chou M.C., et al. Differences in the prescription patterns of anti-asthmatic medications for children by pediatricians, family physicians and physicians of other specialties. J Formos Med Assoc. 2006;105:277–283. doi: 10.1016/S0929-6646(09)60118-2. [DOI] [PubMed] [Google Scholar]
- 66.Tan N.C., Tay I.H., Ngoh A., et al. Factors influencing family physicians' drug prescribing behaviour in asthma management in primary care. Singapore Med J. 2009;50:312–319. [PubMed] [Google Scholar]
- 67.Chapman K.R., Hinds D., Piazza P., et al. Physician perspectives on the burden and management of asthma in six countries: the Global Asthma Physician Survey (GAPS) BMC Pulm Med. 2017;17:153. doi: 10.1186/s12890-017-0492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Broder M.S., Gutierrez B., Chang E., et al. Ratio of controller to total asthma medications: determinants of the measure. Am J Manag Care. 2010;16:170–178. [PubMed] [Google Scholar]
- 69.Greenstone I.R., Ni Chroinin M.N., Masse V., et al. Combination of inhaled long-acting beta2-agonists and inhaled steroids versus higher dose of inhaled steroids in children and adults with persistent asthma. Cochrane Database Syst Rev. 2005;4:CD005533. doi: 10.1002/14651858.CD005533. [DOI] [PubMed] [Google Scholar]
- 70.Price D., Fletcher M., van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24 doi: 10.1038/npjpcrm.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang J.F., Chaudhuri R., Thomson N.C., et al. Insights into frequent asthma exacerbations from a primary care perspective and the implications of UK National Review of Asthma Deaths recommendations. NPJ Prim Care Respir Med. 2018;28:35. doi: 10.1038/s41533-018-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alemayehu C., Mitchell G., Nikles J. Barriers for conducting clinical trials in developing countries- a systematic review. Int J Equity Health. 2018;17:37. doi: 10.1186/s12939-018-0748-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hsu J.C., Lu C.Y. The evolution of Taiwan's National Health Insurance drug reimbursement scheme. Daru. 2015;23:15. doi: 10.1186/s40199-014-0080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahlin T., Nichter M., Pillai G. Health insurance in India: what do we know and why is ethnographic research needed. Anthropol Med. 2016;23:102–124. doi: 10.1080/13648470.2015.1135787. [DOI] [PubMed] [Google Scholar]
- 75.Nakwan N. Impact of asthma severity as risk factor to future exacerbations in patients admitted for asthma exacerbation. Multidiscip Respir Med. 2021;16:780. doi: 10.4081/mrm.2021.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.