Abstract
BACKGROUND
Teclistamab is a T-cell–redirecting bispecific antibody that targets both CD3 expressed on the surface of T cells and B-cell maturation antigen expressed on the surface of myeloma cells. In the phase 1 dose-defining portion of the study, teclistamab showed promising efficacy in patients with relapsed or refractory multiple myeloma.
METHODS
In this phase 1–2 study, we enrolled patients who had relapsed or refractory myeloma after at least three therapy lines, including triple-class exposure to an immunomodulatory drug, a proteasome inhibitor, and an anti-CD38 antibody. Patients received a weekly subcutaneous injection of teclistamab (at a dose of 1.5 mg per kilogram of body weight) after receiving step-up doses of 0.06 mg and 0.3 mg per kilogram. The primary end point was the overall response (partial response or better).
RESULTS
Among 165 patients who received teclistamab, 77.6% had triple-class refractory disease (median, five previous therapy lines). With a median follow-up of 14.1 months, the overall response rate was 63.0%, with 65 patients (39.4%) having a complete response or better. A total of 44 patients (26.7%) were found to have no minimal residual disease (MRD); the MRD-negativity rate among the patients with a complete response or better was 46%. The median duration of response was 18.4 months (95% confidence interval [CI], 14.9 to not estimable). The median duration of progression-free survival was 11.3 months (95% CI, 8.8 to 17.1). Common adverse events included cytokine release syndrome (in 72.1% of the patients; grade 3, 0.6%; no grade 4), neutropenia (in 70.9%; grade 3 or 4, 64.2%), anemia (in 52.1%; grade 3 or 4, 37.0%), and thrombocytopenia (in 40.0%; grade 3 or 4, 21.2%). Infections were frequent (in 76.4%; grade 3 or 4, 44.8%). Neurotoxic events occurred in 24 patients (14.5%), including immune effector cell–associated neurotoxicity syndrome in 5 patients (3.0%; all grade 1 or 2).
CONCLUSIONS
Teclistamab resulted in a high rate of deep and durable response in patients with triple-class–exposed relapsed or refractory multiple myeloma. Cytopenias and infections were common; toxic effects that were consistent with T-cell redirection were mostly grade 1 or 2. (Funded by Janssen Research and Development; MajesTEC-1 ClinicalTrials.gov numbers, NCT03145181 and NCT04557098.)
STANDARD TREATMENT OF MULTIPLE MYeloma includes the administration of immunomodulatory agents, proteasome inhibitors, and anti-CD38 antibodies. However, available therapies for patients who have had disease progression after receiving these agents are limited, and outcomes are generally poor.1-4 B-cell maturation antigen (BCMA) represents a promising new target for myeloma therapy. Three BCMA-directed therapies have been approved for the treatment of patients with myeloma who have received immunomodulatory agents, proteasome inhibitors, and anti-CD38 antibodies. These approved therapies are belantamab mafodotin, an antibody-drug conjugate, and two chimeric antigen receptor T-cell (CAR-T) therapies, idecabtagene vicleucel and ciltacabtagene autoleucel. In this heavily pretreated population, the overall response rate with belantamab mafodotin is approximately 31%.5 Response rates are 67% for idecabtagene vicleucel and 83% for ciltacabtagene autoleucel in patients who have undergone apheresis; however, CAR-T therapy has limitations regarding patient eligibility, safety, and access to treatment.6-8
Teclistamab (JNJ-64007957, Janssen) is a bispecific antibody that targets both CD3 expressed on the surface of T cells and BCMA expressed on the surface of myeloma cells, thus mediating T-cell activation and subsequent lysis of BCMA-expressing myeloma cells. In phase 1 of the multicohort Study of Teclistamab in Participants with Relapsed or Refractory Multiple Myeloma (MajesTEC-1),9 investigators identified the recommended phase 2 dose of teclistamab as a weekly subcutaneous injection of 1.5 mg per kilogram of body weight. At this dose level, teclistamab showed promising efficacy in 40 patients, with 65% of patients having a partial response or better. Here, we report the efficacy and safety results from the pivotal phase 1–2 portion of MajesTEC-1 involving 165 patients with triple-class–exposed relapsed or refractory myeloma. Included in this report is an assessment of pharmacokinetics, immunogenicity, and biomarkers of response and progression.
METHODS
PATIENTS AND TREATMENT
Details regarding the phase 1 study design and methods have been reported previously.9 In brief, eligible patients were 18 years of age or older and had a documented diagnosis of relapsed or refractory myeloma according to the criteria of the International Myeloma Working Group.10 Patients must have previously received at least three lines of therapy (including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody) and have had progressive, measurable disease at screening. Previous treatment with a BCMA-targeted therapy was not allowed. Eligible patients had a score of 0 or 1 on the Eastern Cooperative Oncology Group performance-status scale (which ranges from 0 to 5, with higher scores indicating greater disability). Full eligibility criteria are provided in the protocol, which is available with the full text of this article at NEJM.org.
Patients received once-weekly subcutaneous teclistamab at a dose of 1.5 mg per kilogram, which had been preceded by step-up doses of 0.06 and 0.3 mg per kilogram.9 The step-up doses were separated by 2 to 4 days and were completed 2 to 4 days before the administration of the first full teclistamab dose. Hospitalization and pre-medication with dexamethasone (16 mg), acetaminophen, and diphenhydramine were required for each step-up dose and for the first full dose of teclistamab. The cycle duration was 21 days in phase 1 and 28 days in phase 2. Patients continued to receive teclistamab until the occurrence of disease progression, unacceptable toxicity, withdrawal of consent, death, or the end of the study (defined as 2 years after the administration of the first dose of teclistamab in the last enrolled patient).
STUDY OVERSIGHT
The study was sponsored and designed by Janssen Research and Development in collaboration with the academic authors. The study was conducted in accordance with the principles of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Council for Harmonisation. The study protocol, amendments, and relevant documents were approved by the local independent ethics committee or institutional review board at each study site. All the patients provided written informed consent.
The sponsor established a safety evaluation team to monitor patient safety throughout the phase 1 study. In phase 2, safety was monitored through continuous review by the sponsor. All the authors affirm that the trial was conducted in accordance with the study protocol and vouch for the accuracy and completeness of the data. Professional medical writers who were funded by the sponsor prepared the first draft of the manuscript in accordance with Good Publication Practice guidelines. All the authors reviewed and revised the manuscript and made the decision to submit it for publication.
END POINTS AND ASSESSMENTS
The primary end point of the phase 2 study was the overall response rate, which was defined as a partial response or better according to the criteria of the International Myeloma Working Group, as assessed by an independent review committee (Table S1 in the Supplementary Appendix, available at NEJM.org).10,11 Key secondary end points included the duration of response; a very good partial response or better; a complete response or better; the time until response; progression-free and overall survival; status with respect to minimal residual disease; and safety, pharmacokinetics, and immunogenicity. Negativity for minimal residual disease was assessed by next-generation sequencing of DNA obtained from bone marrow aspirate (clonoSEQ assay, version 2.0 [Adaptive Biotechnologies]) with a threshold of 10−5 cells. Exploratory end points included levels of soluble BCMA, cytokines, and T-cell activation markers. Additional information regarding all end points is provided in the Supplementary Appendix.
Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03), except for cytokine release syndrome (Table S2) and immune effector cell–associated neurotoxicity syndrome, which were graded according to the criteria of the American Society for Transplantation and Cellular Therapy.12
STATISTICAL ANALYSIS
For phase 2, we determined that a sample size of 100 patients would provide a power of at least 85% to establish an overall response rate of more than 30% at a one-sided significance level of 0.025, assuming an overall response rate of at least 45%. The safety and efficacy populations for this analysis included all the patients who had received at least one dose of teclistamab at the recommended phase 2 dose in phase 1 or phase 2 as of September 7, 2021. The data-cutoff date was March 16, 2022, for the safety and efficacy analyses. The overall response rate and associated two-sided 95% confidence intervals were calculated. Response rates were also compared across various prespecified subgroups, including those defined according to cytogenetic risk, refractory status, and number of previous lines of therapy. We used Kaplan–Meier methods to estimate time-to-event end points (duration of response, progression-free survival, and overall survival).
RESULTS
PATIENTS
From March 3, 2020, to August 13, 2021, a total of 165 patients were enrolled at 35 sites in nine countries to receive teclistamab at the recommended phase 2 dose of 1.5 mg per kilogram. Of these patients, 40 were enrolled in phase 1 and 125 in phase 2. As of March 16, 2022, 70 patients (42.4%) were continuing to receive treatment (Fig. S1), with a median treatment duration of 8.5 months (range, 0.2 to 24.4). A total of 98 patients (59.4%) received at least 6 months of teclistamab treatment, and 79 patients (47.9%) received at least 9 months of treatment. The median relative dose intensity (the ratio of the dose administered to the planned dose) for all treatment cycles, including step-up doses, was 93.7%.
The characteristics of the patients at baseline were similar in phases 1 and 2 (Table 1). In the overall population, the median age was 64 years (range, 33 to 84). The median time between diagnosis and the first dose was 6 years (range, 0.8 to 22.7). Extramedullary disease (defined as the presence of one or more extramedullary soft-tissue lesions) was present in 28 patients (17.0%). Among the 148 patients with available cytogenetic data, 38 (25.7%) had at least one high-risk cytogenetic abnormality, which was defined as del(17p), t(4;14), or t(14;16). Stage III disease was present in 20 of 162 patients (12.3%) who had available International Staging System data. Patients had received a median of 5 previous lines of therapy (range, 2 to 14), and 116 (70.3%) had received at least two immunomodulatory agents, at least two proteasome inhibitors, and at least one anti-CD38 antibody (penta-drug exposure). Before study entry, 148 patients (89.7%) had resistance to the previous line of therapy, 128 (77.6%) had triple-class refractory disease, and 50 (30.3%) had penta-drug refractory disease.
Table 1.
Characteristics of the Patients at Baseline.
| Characteristic | Phase 1 (N = 40) |
Phase 2 (N = 125) |
Total (N = 165) |
|---|---|---|---|
| Age | |||
| Median (range) — yr | 62.5 (39.0–84.0) | 64.0 (33.0–83.0) | 64.0 (33.0–84.0) |
| ≥75 yr — no. (%) | 5 (12.5) | 19 (15.2) | 24 (14.5) |
| Sex — no. (%) | |||
| Male | 26 (65.0) | 70 (56.0) | 96 (58.2) |
| Female | 14 (35.0) | 55 (44.0) | 69 (41.8) |
| Race — no. (%)* | |||
| White | 34 (85.0) | 100 (80.0) | 134 (81.2) |
| Black | 1 (2.5) | 20 (16.0) | 21 (12.7) |
| Asian | 0 | 3 (2.4) | 3 (1.8) |
| Other | 5 (12.5) | 2 (1.6) | 7 (4.2) |
| Median time since diagnosis (range) — yr | 5.6 (0.8–17.4) | 6.2 (0.9–22.7) | 6.0 (0.8–22.7) |
| ≥1 Extramedullary plasmacytoma — no. (%)† | 8 (20.0) | 20 (16.0) | 28 (17.0) |
| ≥60% Plasma cells in bone marrow — no./total no. (%) | 3/38 (7.9) | 15/122 (12.3) | 18/160 (11.2) |
| ECOG performance-status score — no. (%)‡ | |||
| 0 | 17 (42.5) | 38 (30.4) | 55 (33.3) |
| ≥1 | 23 (57.5) | 87 (69.6) | 110 (66.7) |
| International Staging System class — no./total no. (%) | |||
| I | 24/39 (61.5) | 61/123 (49.6) | 85/162 (52.5) |
| II | 11/39 (28.2) | 46/123 (37.4) | 57/162 (35.2) |
| III | 4/39 (10.3) | 16/123 (13.0) | 20/162 (12.3) |
| High-risk cytogenetic profile — no./total no. (%) | 12/37 (32.4) | 26/111 (23.4) | 38/148 (25.7) |
| del(17p) | 9/37 (24.3) | 14/111 (12.6) | 23/148 (15.5) |
| t(4:14) | 4/37 (10.8) | 12/111 (10.8) | 16/148 (10.8) |
| t(14;16) | 1/37 (2.7) | 3/111 (2.7) | 4/148 (2.7) |
| Median no. of lines of previous therapy (range) | 5 (2–11) | 5 (2–14) | 5 (2–14) |
| Previous stem-cell transplantation — no. (%) | 34 (85.0) | 101 (80.8) | 135 (81.8) |
| Previous therapy exposure — no. (%) | |||
| Triple-class§ | 40 (100.0) | 125 (100.0) | 165 (100.0) |
| Penta-drug¶ | 26 (65.0) | 90 (72.0) | 116 (70.3) |
| Refractory status — no. (%) | |||
| Immunomodulatory agent║ | 38 (95.0) | 114 (91.2) | 152 (92.1) |
| Proteasome inhibitor** | 34 (85.0) | 108 (86.4) | 142 (86.1) |
| Anti-CD38 monoclonal antibody†† | 39 (97.5) | 109 (87.2) | 148 (89.7) |
| Triple-class§ | 32 (80.0) | 96 (76.8) | 128 (77.6) |
| Penta-drug¶ | 16 (40.0) | 34 (27.2) | 50 (30.3) |
| Refractory to last line of therapy | 33 (82.5) | 115 (92.0) | 148 (89.7) |
Race was reported by the patients. Included in the category of “other” are 4 patients who did not report race, 2 who reported their race as other, and 1 who reported multiple races.
Included in this category are patients with soft-tissue plasmacytomas that were not associated with bone.
Scores on the Eastern Cooperative Oncology Group (ECOG) performance-status scale range from 0 to 5, with higher scores indicating greater disability. A total of 23 patients in phase 1 and 86 patients in phase 2 had an ECOG score of 1; 1 patient in phase 2 had a performance score of 3.
Triple-class exposure includes at least one immunomodulatory drug, at least one proteasome inhibitor, and at least one anti-CD38 antibody.
Penta-drug exposure includes at least two immunomodulatory drugs, at least two proteasome inhibitors, and at least one anti-CD38 antibody.
Immunomodulatory agents include thalidomide, lenalidomide, and pomalidomide.
Proteasome inhibitors include bortezomib, carfilzomib, and ixazomib.
Anti-CD38 monoclonal antibodies include daratumumab and isatuximab.
EFFICACY
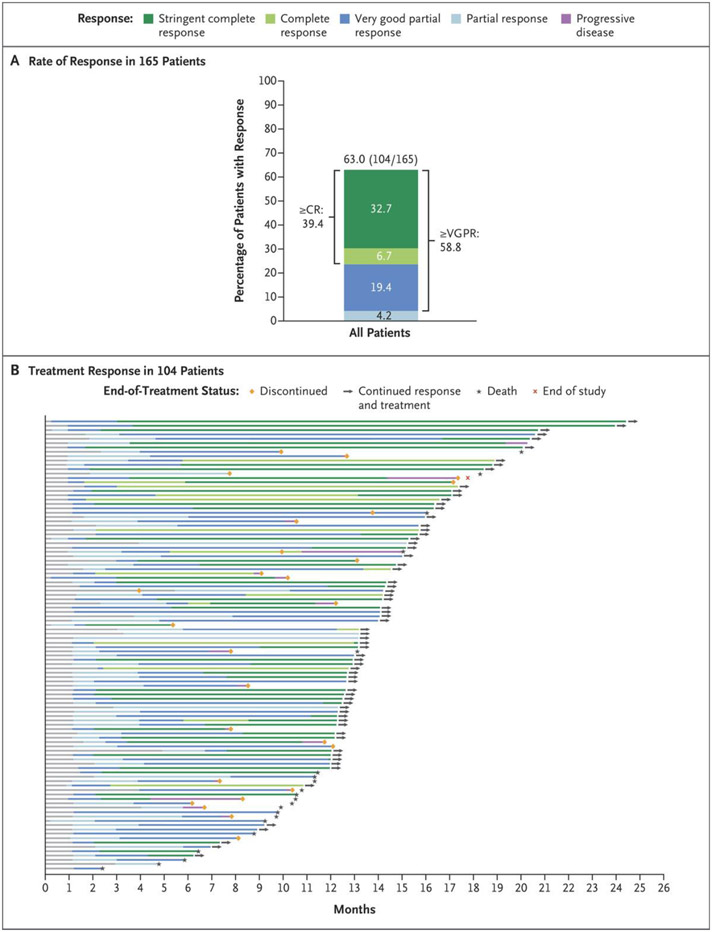
The median follow-up was 14.1 months (range, 0.3 to 24.4). Responses occurred in 104 of 165 patients (63.0%; 95% confidence interval [CI], 55.2 to 70.4). A very good partial response or better occurred in 97 patients (58.8%), and a complete response or better occurred in 65 (39.4%) (Fig. 1A and Table S4). The median time until the first response was 1.2 months (range, 0.2 to 5.5), and the median time until a best response was 3.8 months (range, 1.1 to 16.8). Negativity for minimal residual disease (at a threshold of 10−5) was reported in 44 patients (26.7%; 95% CI, 20.1 to 34.1). Among the 65 patients who had a complete response or better, 30 (46%) had no minimal residual disease (Table S4). Response rates were lower in patients with extramedullary disease, stage III disease, and at least 60% marrow replacement by plasma cells and were higher in those who had received no more than three previous lines of therapy. Otherwise, response rates were consistent across most clinically relevant subgroups, including patients with high-risk cytogenetic abnormalities and those with penta-drug refractory disease (Fig. S2).
Figure 1. Response to Teclistamab in Patients with Relapsed or Refractory Multiple Myeloma.
Panel A shows the rates of stringent complete response, complete response (CR), very good partial response (VGPR), and partial response in 165 patients who were treated with teclistamab. A stringent complete response was defined as a complete response with a normal serum free light-chain ratio and an absence of clonal plasma cells on immunohistochemical analysis or negative two-color to four-color flow cytometry. Differences in percentage totals are due to rounding. Responses were assessed by an independent review committee with a cutoff date of March 16, 2022. Panel B shows responses over time in the 104 patients who had an overall response (partial response or better) among the 165 patients treated at the recommended phase 2 dose.
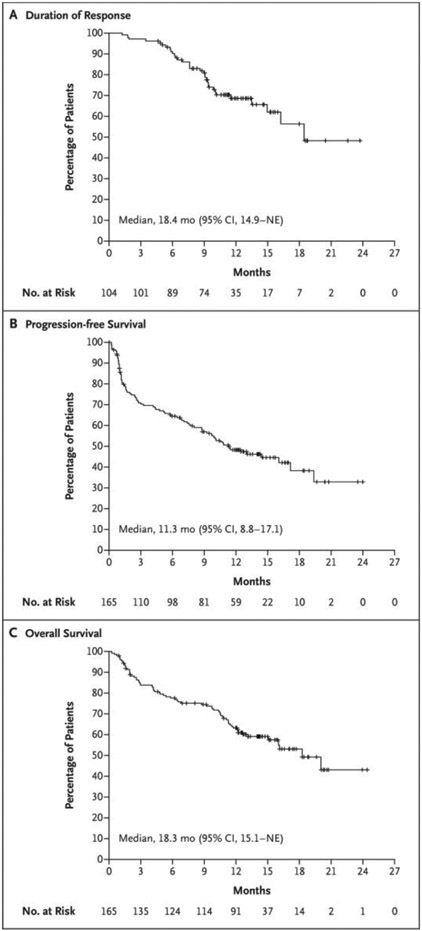
Responses were durable and deepened over time (Fig. 1B). The median duration of response was 18.4 months (95% CI, 14.9 to not estimable) and was not yet mature at the time of this report after censoring of data for 71 patients (68.3%) (Fig. 2A). The Kaplan–Meier estimate of maintenance of response for at least 12 months was 68.5% (95% CI, 57.7 to 77.1). The median duration of progression-free survival was 11.3 months (95% CI, 8.8 to 17.1) (Fig. 2B). The median duration of overall survival was 18.3 months (95% CI, 15.1 to not estimable) and was not mature after censoring of data for 97 patients (58.8%) (Fig. 2C).
Figure 2. Kaplan–Meier Analysis of Response Duration and of Progression-free and Overall Survival.
Panel A shows the duration of response to teclistamab therapy in the 104 patients who had an overall response (partial response or better) among the 165 patients who received the recommended phase 2 weekly dose of 1.5 mg per kilogram of body weight. Panel B shows progression-free survival in the overall population. Disease progression was assessed by an independent review committee on the basis of the criteria of the International Myeloma Working Group. Panel C shows overall survival among the 165 patients. Tick marks indicate censored data. NE denotes not estimable.
SAFETY
All 165 patients reported having an adverse event (Table 2 and Table S5), which were grade 3 or 4 in 156 patients (94.5%). One patient had a dose reduction during cycle 21 because of recurrent neutropenia, and 104 patients (63.0%) skipped a dose because of adverse events. Two patients discontinued teclistamab because of adverse events (grade 3 adenoviral pneumonia and grade 4 progressive multifocal leukoencephalopathy). The most common adverse events were hematologic, including neutropenia (in 117 patients [70.9%]), anemia (in 86 [52.1%]), and thrombocytopenia (in 66 [40.0%]). Of the 117 patients in whom neutropenia developed, 91 received granulocyte colony-stimulating factor therapy at the physician’s discretion. Infections occurred in 126 patients (76.4%); 74 patients (44.8%) had grade 3 or 4 infections. Hypogammaglobulinemia occurred in 123 patients (74.5%) as determined by means of adverse-event reporting, laboratory analyses (IgG level, <500 mg per deciliter), or both; of these patients, 65 received intravenous immunoglobulin at the physician’s discretion. Injection-site reactions were reported in 60 patients (36.4%); all such events were grade 1 or 2.
Table 2.
Adverse Events in 165 Patients (Safety Population).*
| Event | Any Grade | Grade 3 or 4 |
|---|---|---|
| no. of patients (%) | ||
| Any adverse event | 165 (100) | 156 (94.5) |
| Hematologic | ||
| Neutropenia | 117 (70.9) | 106 (64.2) |
| Anemia | 86 (52.1) | 61 (37.0) |
| Thrombocytopenia | 66 (40.0) | 35 (21.2) |
| Lymphopenia | 57 (34.5) | 54 (32.7) |
| Leukopenia | 29 (17.6) | 12 (7.3) |
| Nonhematologic | ||
| Diarrhea | 47 (28.5) | 6 (3.6) |
| Fatigue | 46 (27.9) | 4 (2.4) |
| Nausea | 45 (27.3) | 1 (0.6) |
| Injection-site erythema | 43 (26.1) | 0 |
| Pyrexia | 45 (27.3) | 1 (0.6) |
| Headache | 39 (23.6) | 1 (0.6) |
| Arthralgia | 36 (21.8) | 1 (0.6) |
| Constipation | 34 (20.6) | 0 |
| Cough | 33 (20.0) | 0 |
| Pneumonia | 30 (18.2) | 21 (12.7) |
| Covid-19 | 29 (17.6) | 20 (12.1) |
| Bone pain | 29 (17.6) | 6 (3.6) |
| Back pain | 27 (16.4) | 4 (2.4) |
| Cytokine release syndrome† | 119 (72.1) | 1 (0.6) |
| Neurotoxic event | 24 (14.5) | 1 (0.6) |
Listed are adverse events of any grade that were reported in at least 15% of the patients, as well as neurotoxic events. Covid-19 denotes coronavirus disease 2019.
In this analysis, events associated with cytokine release syndrome were graded according to the criteria of the American Society for Transplantation and Cellular Therapy.
Cytokine release syndrome occurred in 119 patients (72.1%). Most events occurred after step-up and cycle 1 doses, with 6 patients (3.6%) having cytokine release syndrome in cycle 2 or later. Most events of cytokine release syndrome were grade 1 or 2 in severity and fully resolved, except for one grade 3 event, which occurred in a patient with concurrent pneumonia and resolved in 2 days (Table S6). No patients discontinued teclistamab owing to the development of cytokine release syndrome. The median time until the onset of cytokine release syndrome was 2 days (range, 1 to 6) after the most recent dose, and the median duration was 2 days (range, 1 to 9). Supportive measures for the treatment of cytokine release syndrome were provided to 110 patients (66.7%); these treatments included the administration of tocilizumab (in 60 patients [36.4%]), low-flow oxygen by nasal cannula (in 21 [12.7%]), and glucocorticoids (in 14 [8.5%]) (Table S6). A single vasopressor was administered in 1 patient (0.6%).
Investigator-assessed neurotoxic events, including immune effector cell–associated neurotoxicity syndrome, were reported in 24 patients (14.5%). Most events were grade 1 or 2, except for one grade 4 seizure event that occurred in a patient with bacterial meningitis during cycle 7. No patients discontinued therapy because of neurotoxic events.
Headache, which was the most frequent of the neurotoxic events that were deemed by the investigator to be related to teclistamab, was reported in 14 patients (8.5%) (Table S7). Five patients had a total of 9 events of immune effector cell–associated neurotoxicity syndrome, all either grade 1 or 2. Of these 9 events, 7 were concurrent with cytokine release syndrome, and all 9 events resolved without discontinuation or dose reduction. Supportive treatment for neurotoxic events was provided in 14 patients (8.5%). Of these patients, 4 received supportive treatment for immune effector cell–associated neurotoxicity syndrome. Supportive measures included the administration of tocilizumab (in 3 patients), dexamethasone (in 3), levetiracetam (in 2), and gabapentin (in 1). Serious adverse events are reported in Table S8.
A total of 68 patients (41.2%) died, with most deaths (41) attributed to progressive disease (Table S9). Nineteen patients died from adverse events, including 12 deaths from coronavirus disease 2019 (Covid-19). Five deaths were considered by investigators to be related to teclistamab: in 1 patient who had discontinued teclistamab due to progressive multifocal leukoencephalopathy, in 2 patients who had contracted Covid-19, in 1 patient who had hepatic failure, and in 1 patient who had streptococcal pneumonia.
PHARMACOKINETICS, IMMUNOGENICITY, AND BIOMARKERS
Teclistamab exposure was sustained over the predetermined target level (6 μg per milliliter, based on the upper boundary of an experimentally determined range of the 90% maximal effective concentration) with a low peak-to-trough ratio (Fig. S3). Of the 146 patients who had received the recommended phase 2 dose and could be evaluated for immunogenicity, none were found to have antibodies against teclistamab.
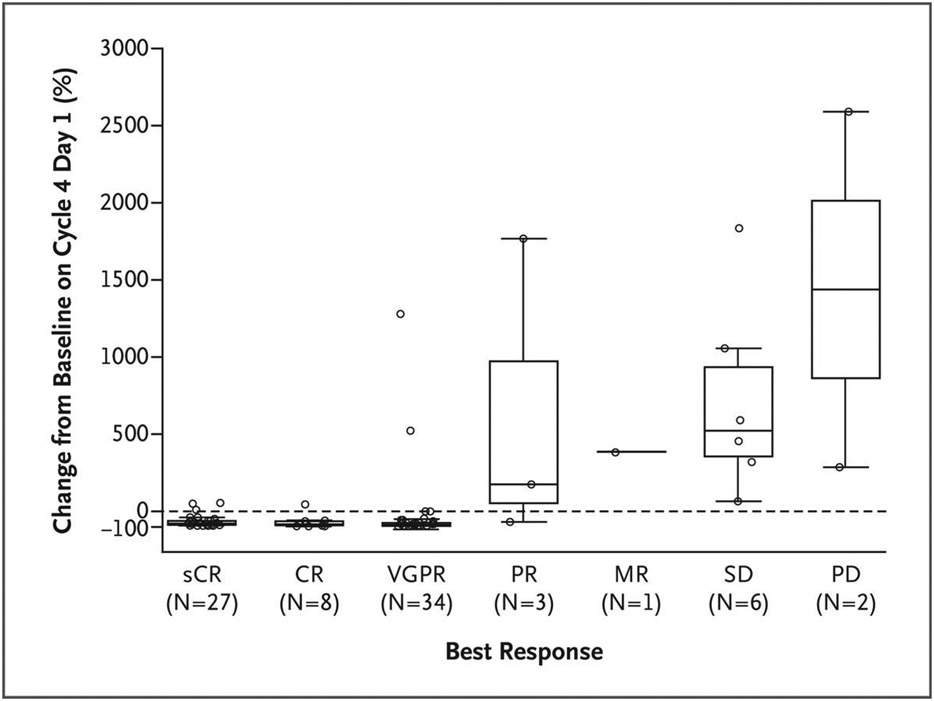
Serum levels of soluble BCMA were assessed as a potential marker of tumor burden and response.13 Rapid decreases in total levels of soluble BCMA occurred in 40 of 59 evaluable patients (68%) who had a partial response or better within the first month of treatment. In contrast, increased soluble BCMA levels occurred in 27 of 28 patients (96%) who did not have a response to teclistamab during cycle 1. In addition, reductions in soluble BCMA levels occurred during the first 4 cycles of treatment in 63 of 72 evaluable patients (88%) who had a partial response or better, whereas all the evaluable patients who did not have a response (9 of 9 patients) had increased levels of soluble BCMA. Reductions in soluble BCMA levels were greater in patients with a deeper response (Fig. 3).
Figure 3. Changes in Levels of Soluble B-Cell Maturation Antigen (BCMA) after Teclistamab Therapy, According to Tumor Response.
Serum levels of soluble BCMA were assessed as a potential marker of tumor burden and response. As expected, reductions in soluble BCMA levels were greater in patients with a better response than in those with a poor response. Shown is the percent change from baseline in serum soluble BCMA levels on day 1 of cycle 4 according to the best response in 81 patients with plasma samples that could be evaluated as of August 9, 2021 (data-cutoff date for pharmacokinetic analyses). The median percent change in soluble BCMA levels was a reduction of 82% in 27 patients with a stringent complete response (sCR), a reduction of 87% in the 8 patients with a complete response (CR), a reduction of 90% in 34 patients with a very good partial response (VGPR), an increase of 175% among the 3 patients with a partial response (PR), an increase of 381% in the 1 patient with a minimal response (MR), an increase of 522% in the 6 patients with stable disease (SD), and an increase of 1437% in the 2 patients with progressive disease (PD). The boxes represent interquartile ranges, the horizontal line in each box represents the median, and the whiskers show the minimum and maximum values after the exclusion of outliers (circles) that were more than 1.5 times the values represented at each end of the box.
Pharmacodynamic induction of cytokines and T-cell activation were observed after the administration of teclistamab. No significant associations were observed between treatment response and maximum change in cytokine levels or T-cell activation; however, patients who had response to treatment had higher levels of interferon-γ, interleukin-6, interleukin-10, and interleukin-2 receptor α than those without a response (Fig. S4); they also had higher cell-surface levels of CD38 or T-cell immunoglobulin and mucin domain–containing protein 3 on CD8+ T cells, which suggests that immune activation that occurred early after treatment initiation may be a predictor of clinical response (Fig. S5).
DISCUSSION
In this phase 1–2 study, we found that at a median follow-up of 14.1 months, a once-weekly subcutaneous dose (1.5 mg per kilogram after step-up dosing) of teclistamab induced deep and durable responses in patients with triple-class–exposed relapsed or refractory multiple myeloma, findings that are consistent with results of the phase 1 study.9 Responses occurred in 63.0% of the patients, with 39.4% having a complete response or better, despite a history of extensive previous treatment and disease that was refractory to currently available therapies. The overall depth of response was highlighted by the observation that 26.7% of the patients were found to have no minimal residual disease at a sensitivity level of 10−5; these patients made up 82% of those in whom minimal residual disease could be evaluated. Such absence of minimal residual disease has been associated with improved overall survival and progression-free survival in patients with myeloma.14-16 In this study, progression-free survival was 11.3 months. At the time of this report, the median duration of response to teclistamab was 18.4 months. Most of the patients with this duration of response were still having a response by the data-cutoff date.
Responses were consistent across clinically relevant subgroups, including patients with high-risk cytogenetic abnormalities or penta-drug refractory disease with the exceptions of those with extramedullary disease, stage III disease, and the presence of at least 60% plasma cells in the marrow. The latter two subgroups were small and had results with wide confidence intervals (i.e., the upper boundaries were similar to or exceeded the response rate among all treated patients). The lower response rate in patients with extramedullary plasmacytomas probably reflects the poor prognosis in this population, which is historically challenging to treat.17 In addition, subgroup analyses suggest that patients who have received no more than three previous therapy lines may have a better response rate than those who have received more than three therapies, a finding that supports the use of teclistamab in earlier therapy lines (although the confidence intervals for this finding are wide).
The mechanism of action of teclistamab is distinct from that of other available therapies in these patients. Teclistamab is a bispecific antibody with dual binding sites targeting both CD3 expressed on the surface of T cells and BCMA expressed on the surface of myeloma cells. Teclistamab redirects CD3+ T cells to BCMA+ myeloma cells, resulting in T-cell activation and subsequent lysis and death of BCMA+ cells.18 This effect occurs regardless of T-cell–receptor specificity or major histocompatibility complex class I molecules on the surface of myeloma cells.18 Although clinical proof of concept has been obtained for other CD3-redirection platforms in other indications,19-21 no CD3-redirecting therapy has been approved for the treatment of myeloma.
It is important to put the results of this study in context with new and emerging therapies for multiple myeloma. Although cross-trial comparisons are challenging to interpret owing to differences in study design and patient populations, the response rates observed with teclistamab (63%) compare favorably with those of belantamab mafodotin, which has an overall response rate of 31% in patients with triple-class refractory disease.5 In a matching-adjusted indirect comparison of data from the DREAMM-2 study, teclistamab provided a substantial efficacy benefit over belantamab mafodotin in patients with relapsed or refractory multiple myeloma who had received at least three previous lines of therapy.22 Overall response rates of 67 to 83% have been observed with approved CAR-T therapies in patients who have undergone apheresis.6,8 CAR-T therapy requires that patients have access to specialized care centers and can wait the minimum 4-week period for production, which can result in attrition rates of approximately 10 to 15%,6-8 factors that underscore the need for effective off-the-shelf treatment options for patients with relapsed or refractory myeloma. Teclistamab is readily available and has been associated with rapid onset of response after a median of approximately 1 month of treatment.
Adverse events in this phase 1–2 trial were common and included low-grade cytokine release syndrome and grade 3 or 4 cytopenia and infection in a patient population that has an increased susceptibility to infection due to the immunodeficiency associated with myeloma and immunosuppressive effects of previous myeloma therapies.23 Of the 19 deaths due to adverse events in our study, 12 were attributed to Covid-19, which was consistent with the higher mortality observed from Covid-19 in patients with hematologic cancers during this time frame.24 The median relative dose intensity was high, and dose reductions and discontinuations owing to adverse events were infrequent. Events associated with cytokine release syndrome were generally mild, as evidenced by the rarity of grade 3 events and the lack of grade 4 events. In contrast, higher-grade cytokine release syndrome and neurotoxic events have been described after CAR-T administration in patients with multiple myeloma.6,8 Furthermore, the lower-grade profile of cytokine release syndrome with teclistamab supports the potential for outpatient administration.
In this phase 1–2 study, teclistamab had substantial clinical activity that compares favorably with that of existing therapies for patients with heavily pretreated relapsed or refractory multiple myeloma. The high rate of deep and durable responses in this population indicates the potential for teclistamab to provide substantial clinical benefit to a broader population of patients. Toxic effects were also common but were mainly of low grade and reversible.
Supplementary Material
Acknowledgments
Supported by Janssen Research and Development. Dr. Usmani is supported by a career development award from the Leukemia and Lymphoma Society. Dr. Popat is supported by the Biomedical Research Centre of the National Institute for Health Research and University College London Hospitals.
We thank the patients who participated in the study and their families and caregivers; the physicians and nurses who cared for patients and supported this clinical trial; the staff members at the study sites and those involved in data collection and analyses; and Valerie Kinchen, Ph.D., and Linda Wychowski, Ph.D., of Eloquent Scientific Solutions, who provided medical writing support.
APPENDIX
The authors’ full names and academic degrees are as follows: Philippe Moreau, M.D., Alfred L. Garfall, M.D., Niels W.C.J. van de Donk, M.D., Ph.D., Hareth Nahi, M.D., Ph.D., Jesús F. San-Miguel, M.D., Ph.D., Albert Oriol, M.D., Ph.D., Ajay K. Nooka, M.D., Thomas Martin, M.D., Laura Rosinol, M.D., Ajai Chari, M.D., Lionel Karlin, M.D., Lotfi Benboubker, M.D., Maria-Victoria Mateos, M.D., Ph.D., Nizar Bahlis, M.D., Rakesh Popat, M.D., Ph.D., Britta Besemer, M.D., Joaquín Martínez-López, M.D., Ph.D, Surbhi Sidana, M.D., Michel Delforge, M.D., Ph.D., Lixia Pei, Ph.D., Danielle Trancucci, M.Sc., Raluca Verona, Ph.D., Suzette Girgis, Ph.D., Shun X.W. Lin, Ph.D., Yunsi Olyslager, M.Sc., Mindy Jaffe, M.S.N., Clarissa Uhlar, Ph.D., Tara Stephenson, Ph.D., Rian Van Rampelbergh, M.D., Arnob Banerjee, M.D., Ph.D., Jenna D. Goldberg, M.D., Rachel Kobos, M.D., Amrita Krishnan, M.D., and Saad Z. Usmani, M.D.
The authors’ affiliations are as follows: the Hematology Clinic, University Hospital Hôtel-Dieu, Nantes (P.M.), Service d’Hématologie Clinique, Centre Hospitalier Lyon Sud, Pierre-Bénite (L.K.), and Service d’Hématologie et Thérapie Cellulaire, Hôpital Bretonneau, Centre Hospitalier Régional Universitaire, Tours (L.B.) — all in France; Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, Philadelphia (A.L.G.), and Janssen Research and Development, Spring House (R.V., S.G., S.X.W.L., C.U., T.S., A.B.) — both in Pennsylvania; the Department of Hematology, Amsterdam University Medical Center, Vrije Universiteit Amsterdam, Cancer Center Amsterdam, Amsterdam (N.W.C.J.D.); Karolinska University Hospital at Huddinge, Stockholm (H.N.); Clínica Universidad de Navarra, Centro de Investigación Médica Aplicada, Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), Instituto de Investigación Sanitaria de Navarra, Pamplona (J.F.S.-M.), Institut Català d’Oncologia and Institut Josep Carreras, Hospital Germans Trias i Pujol, Badalona (A.O.), Hospital Clínic, August Pi i Sunyer Biomedical Research Institute, University of Barcelona, Barcelona (L.R.), University Hospital of Salamanca, Instituto de Investigación Biomédica de Salamanca, Centro del Investigación del Cáncer, CIBERONC, Salamanca (M.-V.M.), and Hematological Malignancies Clinical Research Unit, Hospital 12 de Octubre Universidad Complutense, Centro Nacional de Investigaciones Oncológicas, CIBERONC, Madrid (J.M.-L.) — all in Spain; Winship Cancer Institute, Emory University, Atlanta (A.K.N.); the University of California, San Francisco, San Francisco (T.M.), Stanford University School of Medicine, Stanford (S.S.), and City of Hope Comprehensive Cancer Center, Duarte (A.K.) — all in California; Mount Sinai School of Medicine (A.C.) and Memorial Sloan Kettering Cancer Center (S.Z.U.) — both in New York; Arnie Charbonneau Cancer Institute, University of Calgary, Calgary, AB, Canada (N.B.); Clinical Research Facility, National Institute for Health Research University College London Hospitals, NHS Foundation Trust, London (R.P.); the Department of Hematology, Oncology, and Immunology, University of Tübingen, Tübingen, Germany (B.B.); the University of Leuven, Leuven (M.D.), and Janssen Research and Development, Antwerp (Y.O., R.V.R.) — both in Belgium; Janssen Research and Development, Raritan, NJ (L.P., D.T., M.J., J.D.G., R.K.); and Levine Cancer Institute–Atrium Health, Charlotte, NC (S.Z.U.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
The authors’ full names, academic degrees, and affiliations are listed in the Appendix.
Contributor Information
P. Moreau, Hematology Clinic, University Hospital Hôtel-Dieu, Nantes, France
A.L. Garfall, Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania
N.W.C.J. van de Donk, Department of Hematology, Amsterdam University Medical Center, Vrije Universiteit Amsterdam, Cancer Center Amsterdam, Amsterdam
H. Nahi, Karolinska University Hospital at Huddinge, Stockholm
J.F. San-Miguel, Clinica Universidad de Navarra, Centro de Investigación Médica Aplicada, Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), Instituto de Investigación Sanitaria de Navarra, Pamplona, Spain
A. Oriol, Institut Català d’Oncologia and Institut Josep Carreras, Hospital Germans Trias i Pujol, Badalona, Spain
A.K. Nooka, Winship Cancer Institute, Emory University, Atlanta
T. Martin, University of California, San Francisco, San Francisco, California
L. Rosinol, Hospital Clínic, August Pi i Sunyer Biomedical Research Institute, University of Barcelona, Barcelona, Spain
A. Chari, Mount Sinai School of Medicine, New York
L. Karlin, Service d’Hematologie Clinique, Centre Hospitalier Lyon Sud, Pierre-Bénite, France
L. Benboubker, Service d’Hématologie et Thérapie Cellulaire, Hôpital Bretonneau, Centre Hospitalier Régional Universitaire, Tours, France
M.-V. Mateos, University Hospital of Salamanca, Instituto de Investigación Biomédica de Salamanca, Centro del Investigación del Cáncer, CIBERONC, Salamanca, Spain
N. Bahlis, Arnie Charbonneau Cancer Institute, University of Calgary, Calgary, AB, Canada
R. Popat, Clinical Research Facility, National Institute for Health Research University College London Hospitals, NHS Foundation Trust, London
B. Besemer, Department of Hematology, Oncology, and Immunology, University of Tübingen, Tübingen, Germany
J. Martínez-López, Hematological Malignancies Clinical Research Unit, Hospital 12 de Octubre Universidad Complutense, Centro Nacional de Investigaciones Oncológicas, CIBERONC, Madrid, Spain
S. Sidana, Stanford University School of Medicine, Stanford
M. Delforge, University of Leuven, Leuven, Belgium
L. Pei, Janssen Research and Development, Raritan, NJ
D. Trancucci, Janssen Research and Development, Raritan, NJ
R. Verona, Janssen Research and Development, Spring House, Pennsylvania
S. Girgis, Janssen Research and Development, Spring House, Pennsylvania
S.X.W. Lin, Janssen Research and Development, Spring House, Pennsylvania
Y. Olyslager, Janssen Research and Development, Antwerp, Belgium
M. Jaffe, Janssen Research and Development, Raritan, NJ
C. Uhlar, Janssen Research and Development, Spring House, Pennsylvania
T. Stephenson, Janssen Research and Development, Spring House, Pennsylvania
R. Van Rampelbergh, Janssen Research and Development, Antwerp, Belgium
A. Banerjee, Janssen Research and Development, Spring House, Pennsylvania
J.D. Goldberg, Janssen Research and Development, Raritan, NJ
R. Kobos, Janssen Research and Development, Raritan, NJ
A. Krishnan, City of Hope Comprehensive Cancer Center, Duarte, California
S.Z. Usmani, Memorial Sloan Kettering Cancer Center, New York; Levine Cancer Institute-Atrium Health, Charlotte, NC
REFERENCES
- 1.Yong K, Delforge M, Driessen C, et al. Multiple myeloma: patient outcomes in real-world practice. Br J Haematol 2016;175:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimopoulos MA, Moreau P, Terpos E, et al. Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Hemasphere 2021;5(2):e528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SK, Callander NS, Adekola K, et al. Multiple myeloma, version 3.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2020;18:1685–717. [DOI] [PubMed] [Google Scholar]
- 4.Mateos M-V, Weisel K, De Stefano V, et al. LocoMMotion: a prospective, non-interventional, multinational study of real-life current standards of care in patients with relapsed and/or refractory multiple myeloma. Leukemia 2022;36:1371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lonial S, Lee HC, Badros A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol 2020;21:207–21. [DOI] [PubMed] [Google Scholar]
- 6.Munshi NC, Anderson LD Jr, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med 2021;384:705–16. [DOI] [PubMed] [Google Scholar]
- 7.Shah N, Chari A, Scott E, Mezzi K, Usmani SZ. B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia 2020;34:985–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 2021;398:314–24. [DOI] [PubMed] [Google Scholar]
- 9.Usmani SZ, Garfall AL, van de Donk NWCJ, et al. Teclistamab, a B-cell maturation antigen χ CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MajesTEC-1): a multicentre, open-label, single-arm, phase 1 study. Lancet 2021;398:665–74. [DOI] [PubMed] [Google Scholar]
- 10.Rajkumar SV, Harousseau J-L, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood 2011;117:4691–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 2016;17(8):e328–e346. [DOI] [PubMed] [Google Scholar]
- 12.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 2019;25:625–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girgis S, Lin SXW, Pillarisetti K, et al. Teclistamab and talquetamab modulate levels of soluble B-cell maturation antigen in patients with relapsed and/or refractory multiple myeloma. J Clin Oncol 2021;39:Suppl:8047. abstract. [Google Scholar]
- 14.Munshi NC, Avet-Loiseau H, Anderson KC, et al. A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma. Blood Adv 2020;4:5988–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.San-Miguel J, Avet-Loiseau H, Paiva B, et al. Sustained minimal residual disease negativity in newly diagnosed multiple myeloma and the impact of daratumumab in MAIA and ALCYONE. Blood 2022;139:492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiss S, Gede N, Hegyi P, et al. Addition of daratumumab to multiple myeloma backbone regimens significantly improves clinical outcomes: a systematic review and meta-analysis of randomised controlled trials. Sci Rep 2021;11:21916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosiñol L, Beksac M, Zamagni E, et al. Expert review on soft-tissue plasmacytomas in multiple myeloma: definition, disease assessment and treatment consid-erations. Br J Haematol 2021;194:496–507. [DOI] [PubMed] [Google Scholar]
- 18.Pillarisetti K, Powers G, Luistro L, et al. Teclistamab is an active T cell-redirecting bispecific antibody against B-cell maturation antigen for multiple myeloma. Blood Adv 2020;4:4538–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huehls AM, Coupet TA, Sentman CL. Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol 2015;93:290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strohl WR, Naso M. Bispecific T-cell redirection versus chimeric antigen receptor (CAR)-T cells as approaches to kill cancer cells. Antibodies (Basel) 2019;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchings M, Mous R, Clausen MR, et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an open-label, phase 1/2 study. Lancet 2021;398:1157–69. [DOI] [PubMed] [Google Scholar]
- 22.Moreau P, Usmani SZ, van de Donk NWCJ, et al. Matching-adjusted indirect treatment comparison (MAIC) of teclistamab (tec) versus belantamab mafodotin (belamaf) for the treatment of patients (pts) with triple-class exposed (TCE) relapsed/refractory multiple myeloma (RRMM). J Clin Oncol 2022;40:8035. abstract. [Google Scholar]
- 23.Nucci M, Anaissie E. Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin Infect Dis 2009;49:1211–25. [DOI] [PubMed] [Google Scholar]
- 24.Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood 2020;136:2881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.