Key Points
Question
Is in-office suprachoroidal viscopexy feasible for the treatment of rhegmatogenous retinal detachment (RRD)?
Findings
In this single-case report, the injection of suprachoroidal sodium hyaluronate, 1%, in the region of the break in a fovea-off RRD led to macular reattachment in less than 24 hours with excellent microstructural recovery. Laser retinopexy was performed, and the patient was able to resume normal activity immediately with no restrictions.
Meaning
In-office suprachoroidal viscopexy is a minimally invasive technique for RRD repair that requires no incisions, no tamponade agent, and no positioning.
This single-case report describes the use of in-office suprachoroidal viscopexy for the repair of rhegmatogenous retinal detachment.
Abstract
Importance
In-office suprachoroidal viscopexy (SCVEXY) is a minimally invasive technique for rhegmatogenous retinal detachment (RRD) repair that can be performed with no incision, no tamponade agent, and no positioning requirements. This technique has the potential to be a step forward in the armamentarium to treat RRDs.
Objective
To describe in-office SCVEXY for RRD repair.
Design, Setting, and Participant
In this single-case report with short follow-up, a man in his 50s with pseudophakia and recent visual loss presented to St Michael’s Hospital, Unity Health Toronto, with a fovea-off RRD in the right eye, extending from 6 to 10 o’clock, with no visible causative retinal break.
Exposure
Injection of suprachoroidal sodium hyaluronate, 1%, in the region of the suspected break, using a 30-gauge needle with a custom-made guard that exposed only 1 mm of the needle.
Main Outcome and Measures
Ability to perform in-office SCVEXY and to obtain a visible choroidal indentation.
Results
After the procedure, a dome-shaped choroidal convexity was present in the superior temporal area. The patient achieved macular reattachment in less than 24 hours with no postoperative abnormalities, such as outer retinal folds, residual subfoveal fluid, or retinal displacement, with rapid recovery of the outer retinal bands on optical coherence tomography. The optical coherence tomography scans acquired in the area of the choroidal convexity demonstrated the location of the viscoelastic material in the suprachoroidal space. Laser retinopexy was applied in the suspected region of the retinal tear, and the patient was able to resume normal activity immediately after the procedure with no restrictions.
Conclusions and Relevance
Suprachoroidal viscopexy is feasible as an in-office technique to create a temporary choroidal buckle for RRD repair. It is a minimally invasive procedure with the potential to maximize anatomical outcomes of integrity and postoperative functional outcomes in RRD because its mechanism of action does not require drainage of subretinal fluid or intraocular gas tamponade. Nevertheless, this was a single-case report with short follow-up, which limits the ability to determine the procedure’s benefits, potential adverse events, failure rates, and best-case selection. Further work is required to refine the procedure and assess its efficacy and safety.
Introduction
Rhegmatogenous retinal detachment (RRD) repair has evolved tremendously during the past century. Although scleral buckle (SB) was the dominant technique for many decades,1 since the early 21st century, pars plana vitrectomy (PPV) has been the treatment of choice for most surgeons.2 However, the functional outcomes after PPV have been reported to be inferior to SB3 and pneumatic retinopexy.4 Advances in multimodal imaging have demonstrated a high risk of unwanted structural abnormalities after PPV.5,6,7,8 Recent evidence suggests that additional procedures, such as subretinal fluid drainage,9 use of heavy liquids,10 and large gas tamponades,11 may be harmful in some cases. This knowledge has led surgeons to modify techniques to not only achieve single-operation reattachment but also maximize the integrity of reattachment. We describe a minimally invasive, in-office treatment option for RRD repair that does not require an intravitreal tamponade or patient positioning and has similar principles as SB.
Methods
A man in his 50s with pseudophakia presented to St Michael’s Hospital, Unity Health Toronto, with a reduced Snellen best-corrected visual acuity of 20/50 OD. Scleral-depressed examination identified a fovea-involving inferotemporal RRD from 6 to 10 o’clock, with no definitive causative break (Figure 1A). Based on the rules by Lincoff and Gieser,12 the break was assumed to be temporal or superior temporal. After discussing treatment options and obtaining written informed consent, sodium hyaluronate, 1% (Provisc; Alcon), was injected in the superior temporal quadrant with the patient under subconjunctival anesthesia, using a 30-gauge needle with a custom-made guard that exposed 1 mm of the needle (eFigure 1 in Supplement 1). The custom guard was made using intravenous tubing (Med-RX; Canadian Hospital Specialties Ltd). The injection site in the location of the suspected tear was verified internally with indirect ophthalmoscopy (confirming that the needle was not too deep), and 0.4 mL of viscoelastic was slowly injected transconjunctivally under direct visualization (Video 1) while a dome-shaped choroidal elevation formed. During the injection, the patient had an initial pressure feeling (as did the injecting assistant) as the choroidal bleb was initiated, which lessened and was tolerable during the rest of the procedure as the bleb propagated. Anterior chamber paracentesis was not required because central retinal artery perfusion was confirmed. Although a video of the choroidal convexity forming inside the eye was not available for the in-office procedure, a similar technique was performed intraoperatively in another 2 patients as combined PPV and suprachoroidal viscopexy (SCVEXY) (1 with the assistant injecting and 1 using the viscous fluid control setting of the vitrectomy machine), which was used for additional support of inferior and temporal retinal breaks (Figure 2, Video 2, and Video 3). The reporting guideline for case series by Kempen13 was followed.
Figure 1. Longitudinal Progression of the Retinal Detachment on Ultrawide-Field Photographs.
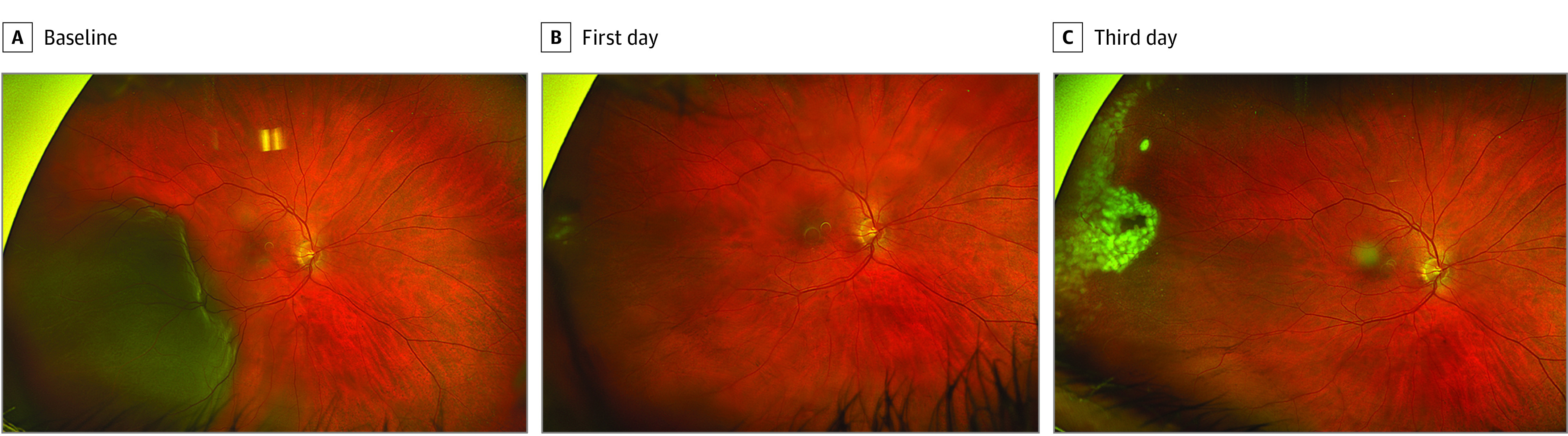
Longitudinal ultrawide-field photographs of a man in his 50s with pseudophakia presenting with a rhegmatogenous retinal detachment (RRD) in the right eye. A, Baseline image demonstrating a fovea-involving inferotemporal RRD from 6 to 10 o’clock, with no definitive causative retinal break. B, First-day post–suprachoroidal viscopexy (SCVEXY) demonstrating substantial resolution of the retinal detachment with some initial spots of laser retinopexy that were applied to the temporal periphery. A small localized temporal hemorrhage was noted near the injection site. C, Third-day post-SCVEXY demonstrating complete laser retinopexy barricade in the suspected region of the causative retinal break.
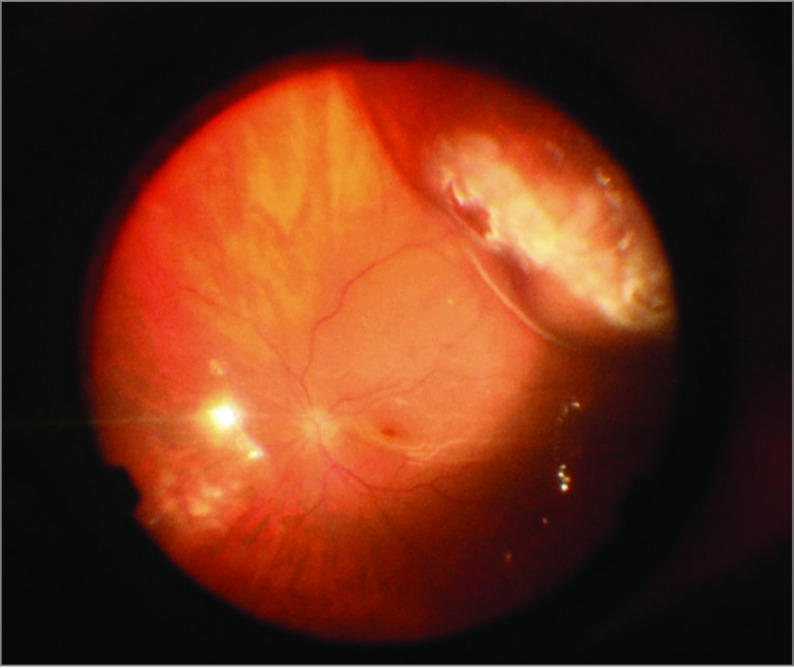
Figure 2. Choroidal Indentation After Intraoperative Suprachoroidal Viscopexy (SCVEXY).
Final appearance of the choroidal convexity formed after SCVEXY.
Video 1. In-Office Suprachoroidal Viscopexy (SCVEXY).
SCVEXY performed in the office setting under subconjunctival anesthesia and direct visualization with indirect ophthalmoscopy.
Video 2. Custom Needle Setup and Choroidal Indentation Following Combined Vitrectomy and Intraoperative Suprachoroidal Viscopexy (SCVEXY) Using Viscous Fluid Control Injection.
Dome-shaped convexity forming intraoperatively following SCEVXY (using viscous fluid control injection). This was a combined pars plana vitrectomy and SCVEXY performed to provide additional support to temporal retinal breaks. The setup of the custom needle and syringe is demonstrated.
Video 3. Choroidal Indentation Following Combined Vitrectomy and Manual Intraoperative Suprachoroidal Viscopexy (SCVEXY).
Dome-shaped choroidal convexity forming intraoperatively following SCEVXY (manual injection of viscoelastic by assistant into the suprachoroidal space while the surgeon stabilizes the syringe/needle). This was a combined pars plana vitrectomy and SCVEXY performed to provide additional support for a large temporal retinal break.
Results
The patient was able to resume normal activity with no restrictions the day after the procedure. On the first day after SCVEXY, the macula was completely attached (Figure 1B). Longitudinal swept-source optical coherence tomography (SS-OCT) demonstrated reattachment with rapid recovery of foveal external limiting membrane and ellipsoid zone integrity (eFigure 2 in Supplement 1). No retinal displacement was observed on fundus autofluorescence imaging (eFigure 3 in Supplement 1). The SS-OCT scans in the location of the choroidal convexity demonstrated a hyporeflective gap between the sclera and choroid, indicating the location of the viscoelastic in the suprachoroidal space (SCS) (eFigure 4 in Supplement 1). Laser retinopexy was applied in the suspected region of the tear on the first postoperative days (Figure 1B and C). Mild residual subretinal fluid was observed in the inferior periphery (eFigure 5 in Supplement 1) that slowly improved with no open breaks. The choroidal convexity reduced in size during the first week (Figure 3) and had completely resolved by 2 weeks. The extent of the suprachoroidal viscoelastic was appreciated with a 12 × 12-mm volume cube performed in the temporal midperiphery (Video 4). By postoperative day 5, best-corrected visual acuity was 20/25, which remained stable during the first month of follow-up.
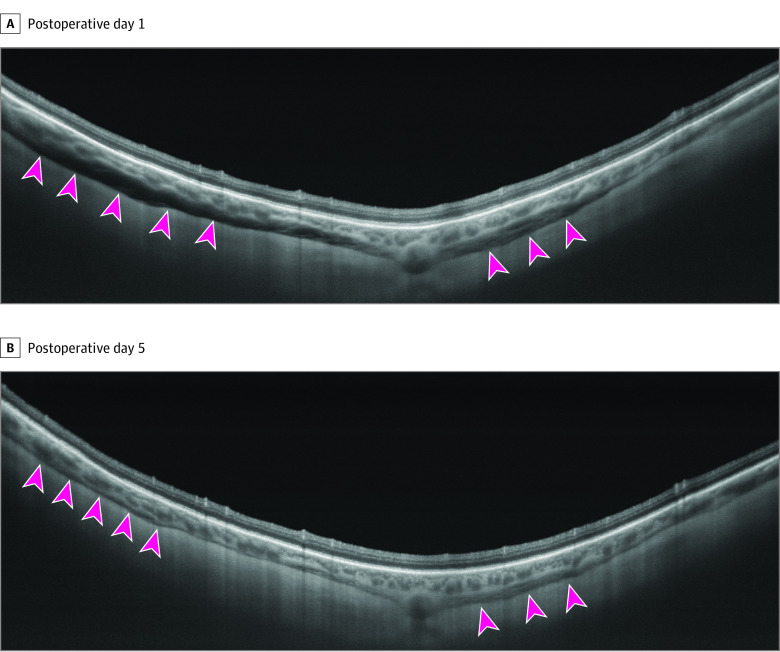
Figure 3. Longitudinal Reabsorption of the Suprachoroidal Viscoelastic.
Longitudinal vertical swept-source optical coherence tomography was performed at the viscopexy injection site, demonstrating the progressive reabsorption of the suprachoroidal viscoelastic (hyporeflective space between the choroid and sclera indicated by the arrowheads) from postoperative day 1 (A) to postoperative day 5 (B).
Video 4. Identification of Suprachoroidal Viscoelastic With Swept-Source Optical Coherence Tomography.
Swept-source optical coherence tomography volume cube demonstrates the location of suprachoroidal viscoelastic following suprachoroidal viscopexy.
Discussion
Suprachoroidal implantation in RRD repair was first described in 1986 by Poole and Surdasky.14 They created a sclerotomy anterior to the retinal tears, through which the SCS would be entered with a cyclodialysis spatula and hyaluronic acid injected into the SCS. In 2013, El Rayes and Elborgy15 described a modified technique in which they created a pocket in the SCS through which a light-guided catheter would be advanced to localize the region of the break. The concept of a temporary buckle was advanced by Kreissig et al16 in 1989 using the Lincoff parabulbar balloon.
SCVEXY can be performed in the office and seems relatively less invasive compared with prior approaches of SCS viscoelastic injection, although there are risks of choroidal hemorrhage, infection, and inadvertent intraocular injection or retinal perforation. Our procedure was performed with a custom 30-gauge needle guard using intravenous tubing that exposed 1 mm of the needle and allowed injection of viscoelastic into the SCS. During the injection, if the needle is intrascleral, there is significant resistance that is overcome as the needle is slightly advanced into the SCS with additional pressure.
From our very early experience, in-office SCVEXY has the potential to be a reasonable approach in select patients. This procedure seems ideal for acute RRDs without proliferative vitreoretinopathy and preferably in cooperative patients with breaks within 1 clock hour. It may be particularly beneficial for inferior breaks where pneumatic retinopexy may be less likely to succeed; however, the procedure could also be performed for superior breaks because the lack of tamponade and positioning requirements are significant advantages. In addition, SCVEXY could be performed in combination with PPV or pneumatic retinopexy for additional support of retinal breaks. Sodium hyaluronate 2.3% (Healon 5; Abbott Medical Optics) seems to be ideal because it remains in the SCS for up to 3 weeks and retains a useful effect for at least 7 to 10 days.
Strengths and Limitations
Benefits of this technique include the complete natural reabsorption of the viscoelastic agent, the reduced invasiveness with the injection through a small-gauge needle, and the fact that these substances are immunologically inert. We can also avoid some of the complications associated with traditional SB surgery.
Nevertheless, with our limited experience, we cannot determine (1) potential concerns about intraocular pressure increase with large volumes of viscoelastic injection, (2) difficulty in controlling the exact direction of viscoelastic expansion, and (3) variations in the time course of choroidal elevation subsidence. Longer-acting viscoelastic agents may be superior. In the event of failure, it is uncertain whether SCVEXY would interfere with subsequent procedures. We do not anticipate issues with pneumatic retinopexy and SB, although with PPV the suprachoroidal bleb could interfere with trocar placement, and viscoelastic may need to be expressed with a scleral incision.
Conclusions
In conclusion, SCVEXY is a technique for the delivery of viscoelastic into the SCS for RRD repair by creating a temporary choroidal buckle. Suprachoroidal viscopexy is designed to be minimally invasive and can be performed in the office. When successful, SCVEXY has the potential to maximize anatomical outcomes of integrity and functional outcomes. However, we stress that this is a description of our early experience with limited follow-up, which limits the ability to determine the procedure’s benefits, potential adverse events, failure rates, and ideal case selection.
eFigure 1. Device Created for the Suprachoroidal Injection Of Viscoelastic (SCVEXY)
eFigure 2. Postoperative Reattachment of the Fovea
eFigure 3. Postoperative Fundus Autofluorescence Imaging
eFigure 4. Longitudinal Swept-Source Optical Coherence Tomography Scans at the Temporal Macula and at the Suprachoroidal Viscopexy Location
eFigure 5. Ultrawide-Field Fundus Swept-Source Optical Coherence Tomography Scans Through the Suprachoroidal Viscopexy Location and Through the Inferior Residual Subretinal Fluid
Data Sharing Statement
References
- 1.Williams GA, Aaberg TA Jr. Techniques of scleral buckling. In: Ryan SJ, Wilkinson CP, eds. Retina . Vol 3. 4th ed. Elsevier Mosby; 2006:2035-2207. [Google Scholar]
- 2.Moinuddin O, Abuzaitoun RO, Hwang MW, et al. Surgical repair of primary non-complex rhegmatogenous retinal detachment in the modern era of small-gauge vitrectomy. BMJ Open Ophthalmol. 2021;6(1):e000651. doi: 10.1136/bmjophth-2020-000651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heimann H, Hellmich M, Bornfeld N, Bartz-Schmidt KU, Hilgers RD, Foerster MH. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment (SPR Study): design issues and implications. SPR Study report no. 1. Graefes Arch Clin Exp Ophthalmol. 2001;239(8):567-574. doi: 10.1007/s004170100319 [DOI] [PubMed] [Google Scholar]
- 4.Hillier RJ, Felfeli T, Berger AR, et al. The Pneumatic Retinopexy versus Vitrectomy for the Management of Primary Rhegmatogenous Retinal Detachment Outcomes Randomized Trial (PIVOT). Ophthalmology. 2019;126(4):531-539. doi: 10.1016/j.ophtha.2018.11.014 [DOI] [PubMed] [Google Scholar]
- 5.Brosh K, Francisconi CLM, Qian J, et al. Retinal displacement following pneumatic retinopexy vs pars plana vitrectomy for rhegmatogenous retinal detachment. JAMA Ophthalmol. 2020;138(6):652-659. doi: 10.1001/jamaophthalmol.2020.1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francisconi CLM, Marafon SB, Figueiredo NA, et al. Retinal displacement after pneumatic retinopexy versus vitrectomy for rhegmatogenous retinal detachment (ALIGN). Ophthalmology. 2022;129(4):458-461. doi: 10.1016/j.ophtha.2021.12.007 [DOI] [PubMed] [Google Scholar]
- 7.Lee WW, Bansal A, Sadda SR, et al. Outer retinal folds after pars plana vitrectomy vs. pneumatic retinopexy for retinal detachment repair: post hoc analysis from PIVOT. Ophthalmol Retina. 2022;6(3):234-242. doi: 10.1016/j.oret.2021.09.001 [DOI] [PubMed] [Google Scholar]
- 8.Muni RH, Felfeli T, Sadda SR, et al. Postoperative photoreceptor integrity following pneumatic retinopexy vs pars plana vitrectomy for retinal detachment repair: a post hoc optical coherence tomography analysis from the pneumatic retinopexy versus vitrectomy for the management of primary rhegmatogenous retinal detachment outcomes randomized trial. JAMA Ophthalmol. 2021;139(6):620-627. doi: 10.1001/jamaophthalmol.2021.0803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bansal A, Naidu SC, Marafon SB, et al. Retinal displacement after scleral buckle versus combined buckle and vitrectomy for rhegmatogenous retinal detachment: ALIGN scleral buckle versus pars plana vitrectomy with scleral buckle. Ophthalmol Retina. Published online May 20, 2023. doi: 10.1016/j.oret.2023.05.012 [DOI] [PubMed] [Google Scholar]
- 10.McKay BR, Bansal A, Kryshtalskyj M, Wong DT, Berger A, Muni RH. Evaluation of Subretinal fluid Drainage Techniques During Pars Plana Vitrectomy for Primary Rhegmatogenous Retinal Detachment-ELLIPSOID Study. Am J Ophthalmol. 2022;241:227-237. doi: 10.1016/j.ajo.2022.05.008 [DOI] [PubMed] [Google Scholar]
- 11.Farahvash A, Marafon SB, Juncal VR, Figueiredo N, Ramachandran A, Muni RH. Impact of tamponade agent on retinal displacement following pars plana vitrectomy for rhegmatogenous retinal detachment repair: a computer simulation model. Acta Ophthalmol. 2022;100(7):e1470-e1478. doi: 10.1111/aos.15118 [DOI] [PubMed] [Google Scholar]
- 12.Lincoff H, Gieser R. Finding the retinal hole. Arch Ophthalmol. 1971;85(5):565-569. doi: 10.1001/archopht.1971.00990050567007 [DOI] [PubMed] [Google Scholar]
- 13.Kempen JH. Appropriate use and reporting of uncontrolled case series in the medical literature. Am J Ophthalmol. 2011;151(1):7-10.e1. doi: 10.1016/j.ajo.2010.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poole TA, Sudarsky RD. Suprachoroidal implantation for the treatment of retinal detachment. Ophthalmology. 1986;93(11):1408-1412. doi: 10.1016/S0161-6420(86)33553-X [DOI] [PubMed] [Google Scholar]
- 15.El Rayes EN, Elborgy E. Suprachoroidal buckling: technique and indications. J Ophthalmic Vis Res. 2013;8(4):393-399. [PMC free article] [PubMed] [Google Scholar]
- 16.Kreissig I, Failer J, Lincoff H, Ferrari F. Results of a temporary balloon buckle in the treatment of 500 retinal detachments and a comparison with pneumatic retinopexy. Am J Ophthalmol. 1989;107(4):381-389. doi: 10.1016/0002-9394(89)90661-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Device Created for the Suprachoroidal Injection Of Viscoelastic (SCVEXY)
eFigure 2. Postoperative Reattachment of the Fovea
eFigure 3. Postoperative Fundus Autofluorescence Imaging
eFigure 4. Longitudinal Swept-Source Optical Coherence Tomography Scans at the Temporal Macula and at the Suprachoroidal Viscopexy Location
eFigure 5. Ultrawide-Field Fundus Swept-Source Optical Coherence Tomography Scans Through the Suprachoroidal Viscopexy Location and Through the Inferior Residual Subretinal Fluid
Data Sharing Statement