Abstract
The 4,378-bp putative tetracycline resistance transposon Tn5706 of Pasteurella multocida consists of an internal tet(H)-tetR resistance gene region which is flanked by almost identical insertion elements, IS1596 and IS1597. Two reading frames for proteins of 70 and 228 amino acids were detected in each of these insertion sequence elements. The 228-amino-acid protein revealed homology to transposase proteins of gram-positive bacteria.
Bacteria of the genus Pasteurella represent nonmotile, facultatively anaerobic, gram-negative rods (3). Isolates of P. multocida and P. haemolytica cause economically important diseases in food-producing animals (3). Tetracyclines have been used for the treatment and the prevention of diseases in which pasteurellae are involved (3). Surveys of tetracycline resistance in P. multocida and P. haemolytica revealed high percentages of tetracycline-resistant isolates (9, 15). Tetracycline resistance determinants of the classes B, D, H, and M have been detected in members of the genus Pasteurella (2, 4, 7, 8, 11). The tet(H) gene, which seems to be indigenous to pasteurellae, was detected on plasmids but was also detected in the chromosomal DNA (7, 8), suggesting the involvement of a transposable element.
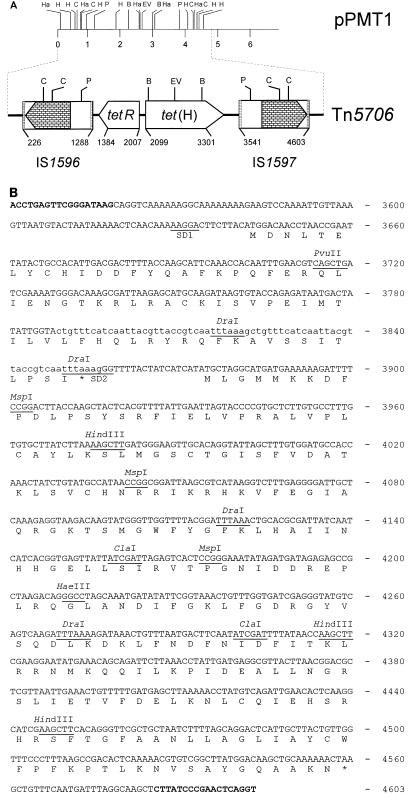
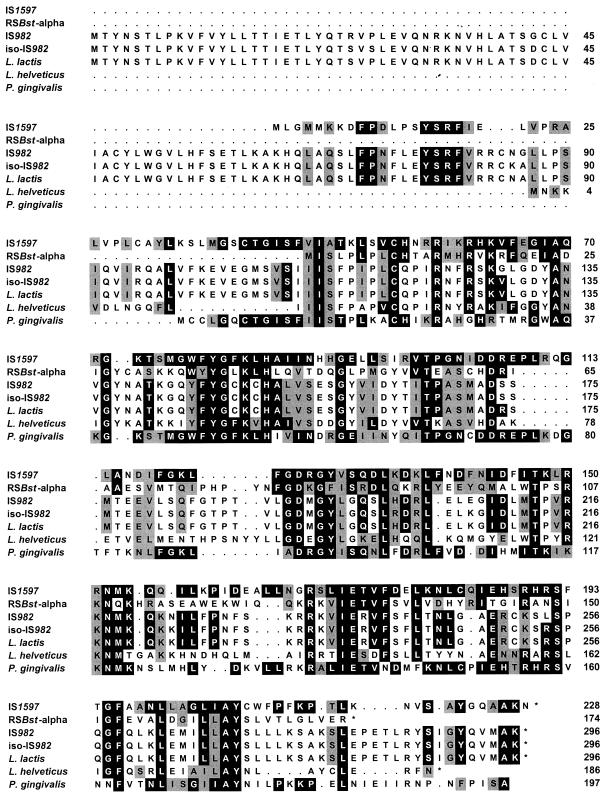
In this study, the 6.8-kbp plasmid pPMT1 was isolated from a multiresistant P. multocida isolate obtained from a clinical case of pneumonia in cattle. This plasmid conferred tetracycline resistance to its new host when it was transformed into Escherichia coli JM107 (Stratagene, Heidelberg, Germany). PCR analysis with tet(H)-specific primers (7) and purified plasmid pPMT1 DNA resulted in the amplification of the expected ca. 1.08-kbp fragment. BclI digestion of this amplification product yielded two fragments whose sizes of 0.26 and 0.81 kbp corresponded closely to the fragment sizes calculated from the tet(H) sequence (8). Sequence analysis of the 3.49-kbp ClaI fragment of plasmid pPMT1 confirmed the presence of two open reading frames which proved to be highly homologous to those representing the tet(H) structural gene and its repressor gene tetR of pVM111 (8). The tetR gene of pPMT1 (positions 2007 to 1384; Fig. 1A) specified a protein of 207 amino acids (aa) which differed from the TetR repressor protein of pVM111 by three amino acid exchanges: TetR of pPMT1 has Asn-12, Lys-29, and Leu-30, whereas TetR of pVM111 has Asp-12, Asn-29, and Val-30 (8). The tet(H) structural gene (positions 2099 to 3301; Fig. 1A) coded for a protein of 400 aa, one of which (Ala-137) differed from the corresponding amino acid (Val-137) in the Tet(H) protein of pVM111 (8). Analysis of the regions upstream of the tetR gene and downstream of the tet(H) gene revealed the presence of closely related sequences. A structural element which resembled an insertion element (Fig. 1A) was located about 250 bp downstream of the tet(H) gene. This element, designated IS1597 (positions 3541 to 4603; Fig. 1A), consisted of 1,063 bp and carried inverted repeated sequences of 18 bp at its ends. Two open reading frames which coded for putative proteins of 70 and 228 aa were detected (Fig. 1B). A similar element, designated IS1596 (positions 1288 to 226; Fig. 1A), was detected about 100 bp upstream of the tetR gene. IS1596 showed the same structural properties and differed from IS1597 by 1 bp (an A at position 500 in IS1596 compared to a T at position 4329 in IS1597). This single-base-pair exchange, however, caused an amino acid exchange in the 228-aa proteins (Lys-152 in IS1596 compared to Asn-152 in IS1597). The reading frame for the 70-aa protein showed a tandem duplication of 34 bp at its end (Fig. 1B). The nucleotide sequence of this reading frame did not exhibit homology to any database sequences, the deduced amino acid sequence also did not show similarities to known bacterial proteins. In contrast, the 228-aa protein of IS1597 showed distinct similarities of 47.7% to the 174-aa transposase protein of the insertion sequence RSBst-alpha from Bacillus stearothermophilus (10), of 44.6% to the 186-aa DNA binding protein from Lactobacillus helveticus (13), and of 36.1 to 37.2% to the 296-aa transposase proteins of the insertion elements IS982 from Lactococcus lactis subsp. cremoris (16), iso-IS982 from L. lactis (14), and an IS982-like element from L. lactis biovar diacetylactis (5) (Fig. 2). Moreover, a high degree of amino acid sequence similarity (67.5%) was observed between the 228 amino acid proteins of IS1596 and IS1597 and a not further specified putative protein of Porphyromonas gingivalis (12), of which only part of the reading frame was deposited in the database (Fig. 2). The overall low degree of homology, both on the nucleotide sequence level and in the deduced amino acid sequences, may indicate that IS1596 and IS1597 represent members of a new family of insertion sequence elements. Analysis of the pPMT1 sequences immediately up- and downstream of Tn5706 revealed the duplication of the 7-bp sequence TATGATA at the integration site.
FIG. 1.
(A) Restriction map of plasmid pPMT1 from P. multocida and organization of the integrated transposon Tn5706. Cleavage sites for restriction enzymes are abbreviated as follows: B, BclI; C, ClaI; EV, EcoRV; Ha, HaeIII; H, HindIII; P, PvuII. A distance scale (in kilobase pairs) is displayed below the map of pPMT1; the numbers in Tn5706 refer to the sequence deposited in the database (Y15510). The hatched boxes at the ends of IS1596 and IS1597 indicate the 18-bp inverted repeats, and the stacked brick-like boxes in IS1596 and IS1597 mark the extent and direction of transcription of the putative transposase reading frames. (B) Nucleotide sequence of IS1597. The deduced amino acid sequences of the 70- and 228-aa proteins are displayed in the one-letter code. Asterisks indicate translational stop codons. The 18-bp inverted repeated sequences are shown in boldface type. The internal directly repeated 34-bp sequence is displayed in lowercase letters. Important restriction endonuclease cleavage sites with regard to panel A are indicated. The numbers refer to the Tn5706 sequence deposited in the database.
FIG. 2.
Amino acid alignment of the putative transposase protein of IS1597 from P. multocida with transposase proteins of RSBst-alpha from B. stearothermophilus (10), IS982 from L. lactis subsp. cremoris (16), iso-IS982 from L. lactis (14), an IS982-like element from L. lactis biovar diacetylactis (5), a DNA-binding protein of L. helveticus (13), and part of a not further specified putative protein from P. gingivalis (12). Asterisks mark translational stop codons. Black boxes indicate identical amino acids and gray boxes represent conservative exchanges (1, 6) compared to those present in the 228-aa protein of IS1597.
Although little is known to date about the mobility of Tn5706 and its integration sites, the occurrence of the tet(H) gene on plasmids and in the chromosomal DNA may be due to the movement of Tn5706 or related elements. A recent study on the location of tet(H) genes in P. haemolytica from cattle and P. multocida from turkeys, cattle, and pigs revealed a predominant location on plasmids (7). The fact that a complete tet(H)-encoding transposon-like element resides on a small plasmid such as pPMT1 underlines the role of plasmids as vectors for transposon-encoded resistance genes in the spread of antibiotic resistance.
Nucleotide sequence accession number.
The nucleotide sequence of the 4,828-bp region of plasmid pPMT1 including the entire transposon Tn5706 and its adjacent flanking regions has been submitted to the EMBL database and has been assigned accession no. Y15510.
Acknowledgments
C.K. is the recipient of a fellowship of the Graduiertenkolleg “Zell- und Molekularbiologie in der Tiermedizin” (GRK 158/2-96) funded by the Deutsche Forschungsgemeinschaft.
We thank Keith G. H. Dyke for help with the sequence comparisons and Gundula Schulze-Tanzil for helpful discussions prior to the beginning of this study.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Aoki T, Takahashi A. Class D tetracycline resistance determinants of R plasmids from the fish pathogens Aeromonas hydrophila, Edwardsiella tarda, and Pasteurella piscicida. Antimicrob Agents Chemother. 1987;31:1278–1280. doi: 10.1128/aac.31.8.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biberstein E L. Pasteurella. In: Biberstein E L, Zee Y C, editors. Review of veterinary microbiology. Boston, Mass: Blackwell Scientific Publications, Inc.; 1990. pp. 175–180. [Google Scholar]
- 4.Chaslus-Dancla E, Lesage-Descauses M-C, Leroy-Sétrin S, Martel J-L, Lafont J-P. Tetracycline resistance determinants, Tet B and Tet M, detected in Pasteurella haemolytica and Pasteurella multocida from bovine herds. J Antimicrob Chemother. 1995;36:815–819. doi: 10.1093/jac/36.5.815. [DOI] [PubMed] [Google Scholar]
- 5.De Felipe F L, Magni C, de Mendoza D, López P. Citrate utilization gene cluster of the Lactococcus lactis biovar diacetylactis: organization and regulation of expression. Mol Gen Genet. 1995;246:590–599. doi: 10.1007/BF00298965. [DOI] [PubMed] [Google Scholar]
- 6.Galan J E. ‘Avirulence genes’ in animal pathogens? Trends Microbiol. 1998;6:3–6. doi: 10.1016/S0966-842X(97)01183-9. [DOI] [PubMed] [Google Scholar]
- 7.Hansen L M, Blanchard P C, Hirsh D C. Distribution of tet(H) among Pasteurella isolates from the United States and Canada. Antimicrob Agents Chemother. 1996;40:1558–1560. doi: 10.1128/aac.40.6.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen L M, McMurray L M, Levy S B, Hirsh D C. A new tetracycline resistance determinant, Tet H, from Pasteurella multocida specifying active efflux of tetracycline. Antimicrob Agents Chemother. 1993;37:2699–2705. doi: 10.1128/aac.37.12.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hörmansdorfer S, Bauer J. Resistance pattern of bovine pasteurellae. Berl Münch Tierärztl Wochenschr. 1996;109:168–171. [PubMed] [Google Scholar]
- 10.Kiel J A, Boels J M, Ten Berge A M, Venema G. Two putative insertion sequences flank a truncated glycogen branching enzyme gene in the thermophile Bacillus stearothermophilus CU21. DNA Seq. 1993;4:1–9. doi: 10.3109/10425179309015616. [DOI] [PubMed] [Google Scholar]
- 11.Kim E-H, Aoki T. The transposon-like structure of IS26-tetracycline and kanamycin resistance determinant derived from transferable R plasmid of fish pathogen Pasteurella piscicida. Microbiol Immunol. 1994;38:31–38. doi: 10.1111/j.1348-0421.1994.tb01741.x. [DOI] [PubMed] [Google Scholar]
- 12.Pavloff, N., P. A. Pemberton, J. Potempa, W.-C. A. Chen, R. N. Pike, V. Prochazka, M. C. Kiefer, J. Travis, and P. J. Barr. 1996. GenBank accession no. U54691.
- 13.Pridmore, R. D., T. Stefanova, and B. Mollet. 1995. EMBL database accession no. S49426.
- 14.Van Kranenburg R, Marugg J D, van Swam I I, Willem N J, de Vos W M. Molecular characterization of the plasmid-encoded eps gene cluster essential for exopolysaccharide biosynthesis in Lactococcus lactis. Mol Microbiol. 1997;24:387–397. doi: 10.1046/j.1365-2958.1997.3521720.x. [DOI] [PubMed] [Google Scholar]
- 15.Watts J L, Yancey R J, Salmon S A, Case C A. A 4-year-survey of antimicrobial susceptibility trends for isolates from cattle with respiratory disease in North America. J Clin Microbiol. 1994;32:725–731. doi: 10.1128/jcm.32.3.725-731.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu W, Mierau I, Mars A, Johnson E, Dunny G, McKay L L. Novel insertion sequence-like element IS982 in lactococci. Plasmid. 1995;33:218–225. doi: 10.1006/plas.1995.1023. [DOI] [PubMed] [Google Scholar]