This nonrandomized controlled trial investigates whether the addition of pembrolizumab to perioperative chemotherapy in patients with locally advanced, resectable gastric and gastroesophageal junction adenocarcinoma increases pathologic complete response and whether the addition of maintenance pembrolizumab improves disease-free survival.
Key Points
Question
Is treatment with the combination of perioperative chemotherapy and immune checkpoint blockade active and safe in patients with locally advanced, resectable gastric or gastroesophageal junction adenocarcinoma?
Findings
This multicenter, phase 2 nonrandomized controlled trial of perioperative capecitabine, oxaliplatin, and pembrolizumab, followed by maintenance pembrolizumab, included 34 evaluable patients and resulted in a pathologic complete response rate of 20.6% and a 2-year disease-free survival rate of 67.8%. With the exception of diarrhea, this regimen did not result in increased treatment-related adverse events.
Meaning
These findings suggest that treatment with perioperative capecitabine, oxaliplatin, and pembrolizumab has encouraging activity and acceptable toxicity and warrants further investigation.
Abstract
Importance
Combining immune checkpoint blockade (ICB) with chemotherapy improves outcomes in patients with metastatic gastric and gastroesophageal junction (G/GEJ) adenocarcinoma; however, whether this combination has activity in the perioperative setting remains unknown.
Objective
To evaluate the safety and preliminary activity of perioperative chemotherapy and ICB followed by maintenance ICB in resectable G/GEJ adenocarcinoma.
Design, Setting, and Participants
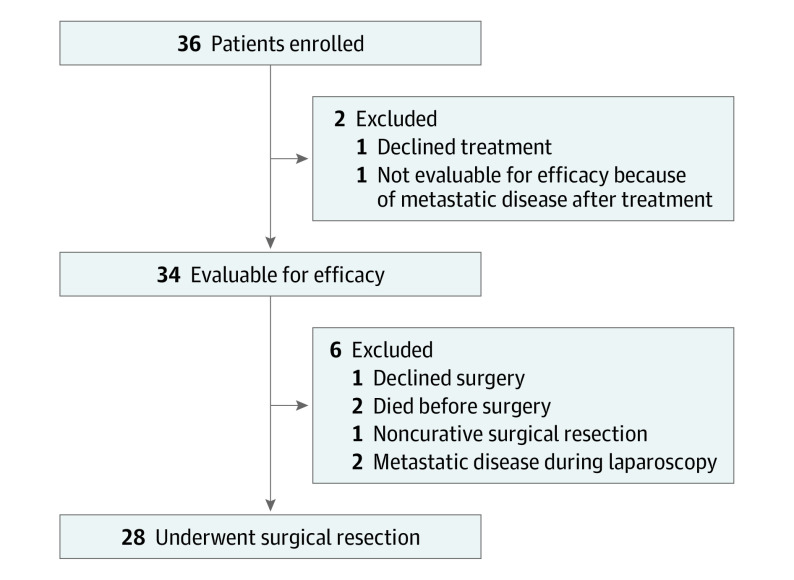
This investigator-initiated, multicenter, open-label, single-stage, phase 2 nonrandomized controlled trial screened 49 patients and enrolled 36 patients with resectable G/GEJ adenocarcinoma from February 10, 2017, to June 17, 2021, with a median (range) follow-up of 35.2 (17.4-73.0) months. Thirty-four patients were deemed evaluable for efficacy analysis, with 28 (82.4%) undergoing curative resection. This study was performed at 4 referral institutions in the US.
Interventions
Patients received 3 cycles of capecitabine, 625 mg/m2, orally twice daily for 21 days; oxaliplatin, 130 mg/m2, intravenously and pembrolizumab, 200 mg, intravenously with optional epirubicin, 50 mg/m2, every 3 weeks before and after surgery with an additional cycle of pembrolizumab before surgery. Patients received 14 additional doses of maintenance pembrolizumab.
Main Outcomes and Measures
The primary end point was pathologic complete response (pCR) rate. Secondary end points included overall response rate, disease-free survival (DFS), overall survival (OS), and safety.
Results
A total of 34 patients (median [range] age, 65.5 [25-90] years; 23 [67.6%] male) were evaluable for efficacy. Of these patients, 28 (82.4%) underwent curative resection, 7 (20.6%; 95% CI, 10.1%-100%) achieved pCR, and 6 (17.6%) achieved a pathologic near-complete response. Of the 28 patients who underwent resection, 4 (14.3%) experienced disease recurrence. The median DFS and OS were not reached. The 2-year DFS was 67.8% (95% CI, 0.53%-0.87%) and the OS was 80.6% (95% CI, 0.68%-0.96%). Treatment-related grade 3 or higher adverse events for evaluable patients occurred in 20 patients (57.1%), and 12 (34.3%) experienced immune-related grade 3 or higher adverse events.
Conclusion and Relevance
In this trial of unselected patients with resectable G/GEJ adenocarcinoma, capecitabine, oxaliplatin, and pembrolizumab resulted in a pCR rate of 20.6% and was well tolerated. This trial met its primary end point and supports the development of checkpoint inhibition in combination with perioperative chemotherapy in locally advanced G/GEJ adenocarcinoma.
Trial Registration
ClinicalTrials.gov Identifier: NCT02918162
Introduction
Perioperative platinum-fluoropyrimidine chemotherapy is the current standard treatment for patients with locally advanced, resectable gastric and gastroesophageal junction (G/GEJ) adenocarcinoma.1,2 Immune checkpoint blockade has established efficacy in the treatment of gastroesophageal cancers, especially in cases of mismatch repair deficiency (dMMR) and elevated programmed cell death ligand 1 (PD-L1) expression.3,4 We hypothesized that the addition of pembrolizumab to perioperative chemotherapy in unselected patients with locally advanced G/GEJ adenocarcinoma would result in increased pathologic complete responses (pCRs) and that the addition of maintenance pembrolizumab would further improve disease-free survival (DFS). Here, we present the results of our investigator-initiated phase 2 trial testing perioperative capecitabine, oxaliplatin, and optional epirubicin with pembrolizumab (COP) with maintenance pembrolizumab in patients with resectable G/GEJ adenocarcinoma.
Methods
Study Design and Procedures
The primary objective of this phase 2 nonrandomized controlled trial was to evaluate the pCR rate of perioperative COP in patients with locally advanced (T2 or greater or lymph node positive), resectable G/GEJ adenocarcinoma. Secondary end points included DFS, overall survival (OS), and safety. Forty-nine patients were screened and 36 patients were enrolled between February 10, 2017, and June 17, 2021. One patient declined treatment, and another had omental disease on imaging after treatment initiation. Therefore, 34 patients were deemed evaluable for efficacy analysis, with 28 (82.4%) undergoing curative resection (Figure 1). Race and ethnicity were patient reported and determined during screening (as part of the census on eligibility checklist). Patients received 3 cycles of chemoimmunotherapy (capecitabine, 625 mg/m2, orally twice daily for 21 days; oxaliplatin, 130 mg/m2, intravenously and pembrolizumab, 200 mg, intravenously with optional epirubicin, 50 mg/m2) before and after surgery with an additional cycle of pembrolizumab before surgery. Additionally, 14 doses of pembrolizumab were given after postoperative chemoimmunotherapy. The PD-L1 combined positive scoring (CPS) and correlative exploratory methods are described in the eMethods in Supplement 1. Tumor response was assessed by Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. All patients provided written informed consent. This study was approved by the institutional review boards at all institutions. The study followed the Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guideline. The amended trial protocol is available in Supplement 2.
Figure 1. CONSORT Diagram.
Statistical Analysis
The primary end point was pCR, defined as no invasive disease within an entirely submitted and evaluated gross lesion, and histologically negative nodes. Disease-free survival was defined as the time from treatment initiation to the date of disease progression, recurrence, or death. At the time of study activation, the reported pCR rate with doublet chemotherapy was 3%.5 With a sample size of 28 patients, the study had 80% power to detect an increase in pCR rate from 3% to 15% based on a 1-sided binomial test with actual α = .051. Because of lower-than-expected accruals during the COVID-19 pandemic and the changing treatment landscape, the initial primary end point of DFS was changed to pCR on November 24, 2020 (at which time no formal data analysis had been performed on the end points). All patients who met eligibility criteria and received at least 1 dose of study treatment were considered evaluable for efficacy. All patients who received at least 1 dose of study treatment were evaluable for safety (eMethods in Supplement 1). Analyses were conducted using R software, version 4.2.1 (R Foundation for Statistical Computing). The Survival package was used to calculate progression-free survival and OS, and the KMunicate package was used to draw the risk table for Kaplan-Meier plots.
Results
Antitumor Activity
A total of 34 patients (median [range] age, 65.5 [25-90] years; 23 [67.6%] male and 11 [32.4%] female; 5 [14.7%] Asian, 5 [14.7%] Black or African American, 22 [64.7%] White, and 2 [5.9%] with unknown race or ethnicity) were evaluable for efficacy, with 28 (82.4%) undergoing curative resection. Baseline characteristics are presented in eTable 1 in Supplement 1. The PD-L1 CPS was available for 24 patients (70.6%). Of the 28 patients (82.4%) who had MMR or microsatellite instability (MSI) testing performed, 3 were classified as having dMMR/MSI-high.
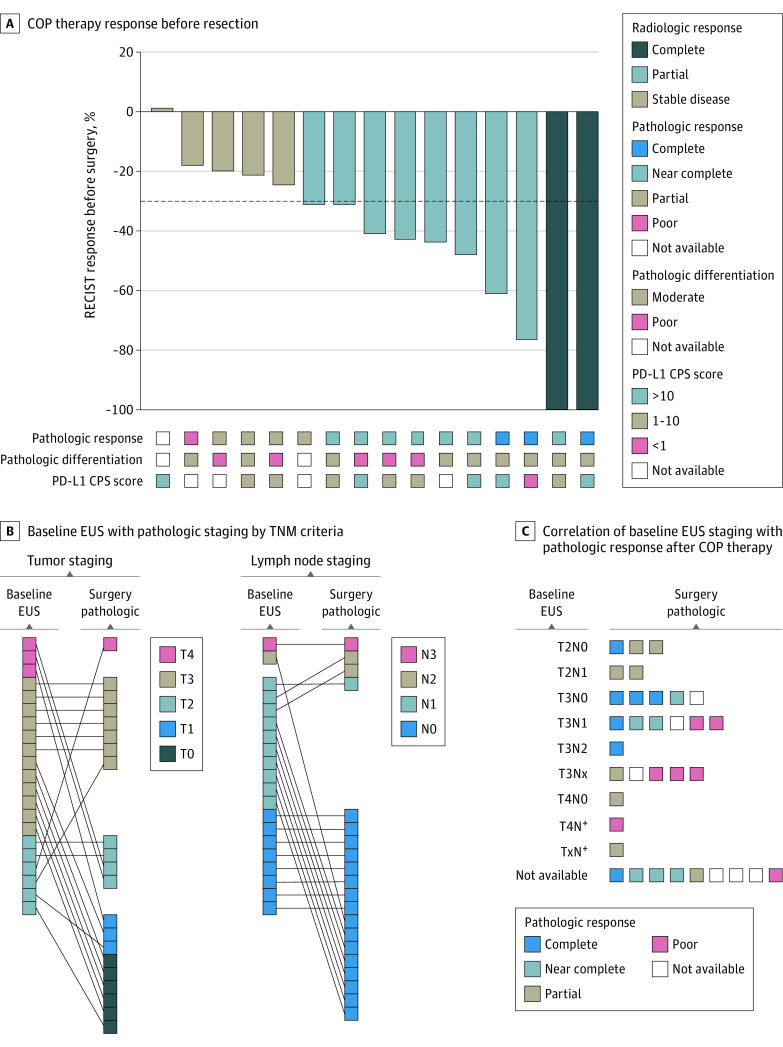
Of the evaluable patients, 7 (20.6%; 95% CI, 10.1%-100%) achieved a pCR. According to College of American Pathology classification, 6 patients (17.6%) had a pathologic near-complete response and 8 (23.5%) had a partial response (eTable 2 in Supplement 1). In patients with PD-L1 CPS scores of 1 or higher (21 patients), the pCR rate was 23.8%, and in patients with scores of 10 or higher (12 patients) at baseline, the pCR rate was 33.3%. One of the 4 patients (25.0%) with a PD-L1 CPS score of less than 1 and 1 of the 3 (33.3%) who were classified as dMMR/MSI-high achieved a pCR. Sixteen of 34 evaluable patients had RECIST measurable disease at baseline, of whom 10 (62.5%) exhibited a radiologic response, including 2 complete responses (Figure 2A). Of the 25 patients who underwent baseline endoscopic ultrasonography, 9 (36.0%) had node-negative disease. Four of these 9 patients (44.4%) with node-negative disease on baseline endoscopic ultrasonography achieved a pCR, compared with only 2 of the 16 patients (12.0%) with initial node-positive disease (Figure 2B and C). At the time of resection, 22 (78.6%) had pathologic node-negative disease (Figure 2B and C). Overall, 27 patients (79.4%) completed neoadjuvant COP and underwent surgery (eTable 4 in Supplement 1). The R0 resection rate, including those with pCR, was 96.4%.
Figure 2. Tumor Response.
A, Waterfall plot depicting Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 response to capecitabine, oxaliplatin, and pembrolizumab (COP) therapy before resection (n = 16). Corresponding pathologic response by College of American Pathologists (CAP) criteria, pathologic differentiation, and baseline programmed cell death ligand 1 combined positive scoring (PD-L1 CPS) score are shown. B, Comparison of baseline endoscopic ultrasonography (EUS) with pathologic staging by TNM criteria (T stage, left; N stage, right). C, Correlation of baseline EUS staging with pathologic response after COP therapy by CAP criteria.
By February 28, 2023, the median (range) follow-up time was 35.2 (17.4-73.0) months for censored patients. During this time, 12 patients had events, including 3 deaths without progression (2 before surgery and 1 due to surgical complication), 2 deaths after treatment discontinuation, and 7 progressions or recurrences (3 of whom have died). Five patients progressed at or before surgery. Of the patients who underwent resection, 4 (14.3%) experienced disease recurrence. Median DFS and OS have not been reached (Figure 3A and B). The 2-year survival rates for evaluable patients were 67.8% (95% CI, 0.53%-0.87%) for DFS and 80.6% (95% CI, 0.68%-0.96%) for OS.
Figure 3. Disease-Free and Overall Survival.
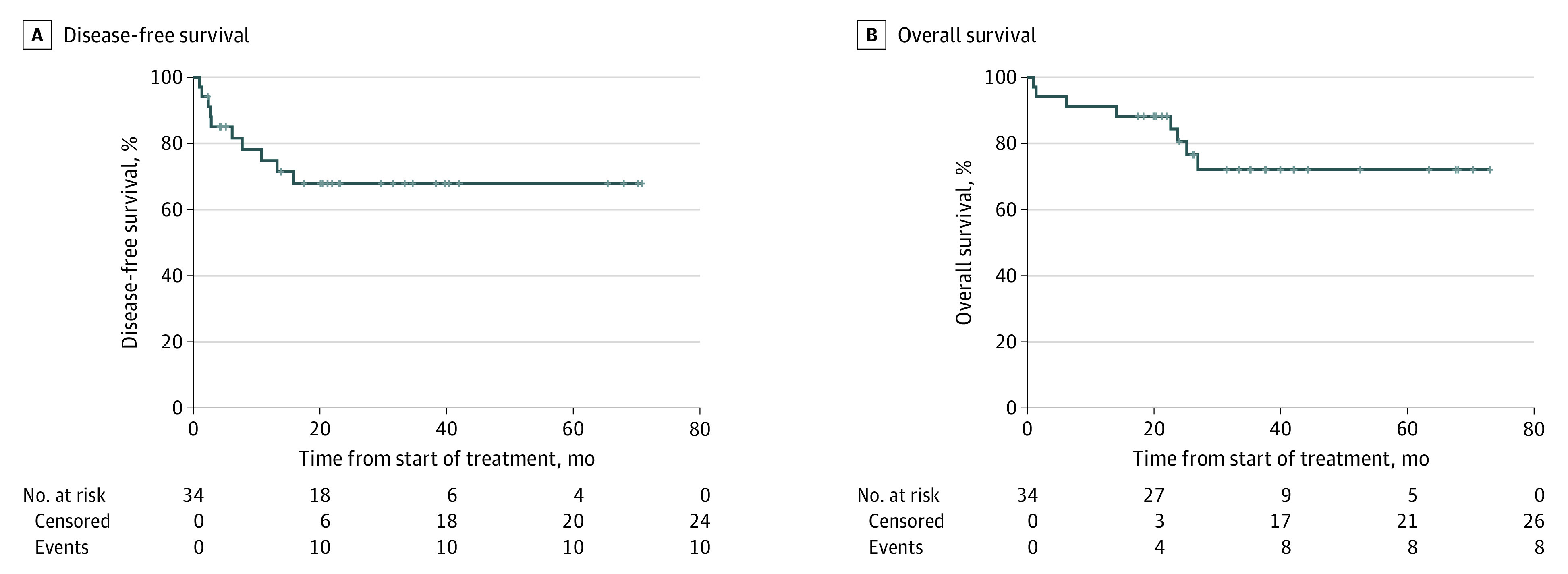
Kaplan-Meier curve for disease-free survival and overall survival for the 34 evaluable patients. Median overall survival and disease-free survival were not reached.
Exploratory quantitative multiplex immunofluorescence analysis revealed that COP increased tumor CD3+ and CD8+ T-cell densities and that the degree of pathologic response was associated with increased clustering of pan-CK+ cells to CD3+ cells, suggesting conversion to a more inflamed tumor microenvironment (eResults and eFigure in Supplement 1).
Safety
Three grade 5 events occurred during treatment, 1 due to gastric hemorrhage (90-year-old patient) and 1 due to gastric perforation (81-year-old patient), both possibly related to therapy. The third grade 5 event, cardiac arrest (77-year-old patient), was attributed to a postoperative surgical complication. Of the 35 patients included in the safety analysis, 20 (57.1%) developed grade 3 or higher treatment-related adverse events, and 12 (34.3%) developed grade 3 or higher immune-related adverse events (eTable 3 in Supplement 1). Except for diarrhea, the addition of pembrolizumab to chemotherapy did not result in any new or increased treatment-related adverse events. Treatment tolerability is detailed in the eResults in Supplement 1.
Discussion
Immune checkpoint blockade in combination with chemotherapy improves outcomes in patients with metastatic disease and was recently approved as maintenance therapy following chemoradiotherapy and resection in esophageal and GEJ cancer.3,6,7 We examined the preliminary activity and safety of perioperative COP followed by maintenance pembrolizumab in locally advanced G/GEJ adenocarcinoma.
Having found an estimated pCR rate of 20.6% (95% CI, 10.1%-100%), this study provides some evidence of efficacy compared with historical pCR rates of 3% reported with fluorouracil-cisplatin and 6.3% with capecitabine and oxaliplatin (CAPOX).5,8 For comparison, the FLOT4 trial demonstrated pCR in 15% and 6% of patients treated with fluorouracil plus leucovorin, oxaliplatin and docetaxel (FLOT) and epirubicin, cisplatin, and fluorouracil or capecitabine, respectively.2 We also noted radiologic responses in 10 of the 16 patients (62.5%) who had RECIST measurable disease, similar to that reported with CAPOX (50%) and FLOT (45.6%).8,9 Four of the 9 patients (44.4%) who were node negative on baseline endoscopic ultrasonography achieved a pCR compared with only 2 of the 16 patients (12.0%) who were initially node positive (Figure 2B and C). This finding suggests that patients with bulky disease would likely benefit from additional neoadjuvant therapy if experiencing a radiographic response. The interim results of the DANTE study further support our findings that the addition of immune checkpoint blockade, especially in those with elevated PD-L1 expression, is associated with increased pathologic regression.10
With a median follow-up time of 35.2 months, the median DFS and OS have yet to be reached. The 2-year DFS was 67.8% with COP followed by maintenance pembrolizumab and is comparable to the 2-year DFS of approximately 53% achieved with FLOT.2 Overall, 79.4% of patients completed neoadjuvant COP and underwent surgery, slightly lower than with FLOT (94%).2 Except for diarrhea, the addition of pembrolizumab to chemotherapy did not result in any new or increased treatment-related adverse events. The higher-than-expected observed incidence of grade 5 events may reflect the small sample size and/or advanced age at enrollment of several patients.
Limitations
This study has some limitations. We acknowledge the nonrandomized design, small number of patients, lack of baseline laparoscopic staging, and slower-than-expected accrual as limitations.
Conclusions
To our knowledge, this is the first completed trial demonstrating encouraging activity with perioperative CAPOX and pembrolizumab with maintenance pembrolizumab in unselected patients with resectable G/GEJ adenocarcinoma.11,12,13 Additional studies on this treatment are warranted.
eMethods. Supplemental Methods
eTable 1. Patient Characteristics
eTable 2. Pathological Responses
eTable 3. Adverse Events
eTable 4. Treatment Completion
eResults. Supplemental Results
eFigure. Change in Tumor Immune Microenvironment
eReferences
Trial Protocol
Data Sharing Statement
References
- 1.Cunningham D, Allum WH, Stenning SP, et al. ; MAGIC Trial Participants . Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11-20. doi: 10.1056/NEJMoa055531 [DOI] [PubMed] [Google Scholar]
- 2.Al-Batran SE, Homann N, Pauligk C, et al. ; FLOT4-AIO Investigators . Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948-1957. doi: 10.1016/S0140-6736(18)32557-1 [DOI] [PubMed] [Google Scholar]
- 3.Shitara K, Van Cutsem E, Bang Y-J, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571-1580. doi: 10.1001/jamaoncol.2020.3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013-e180013. doi: 10.1001/jamaoncol.2018.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715-1721. doi: 10.1200/JCO.2010.33.0597 [DOI] [PubMed] [Google Scholar]
- 6.Kelly RJ, Ajani JA, Kuzdzal J, et al. ; CheckMate 577 Investigators . Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384(13):1191-1203. doi: 10.1056/NEJMoa2032125 [DOI] [PubMed] [Google Scholar]
- 7.Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27-40. doi: 10.1016/S0140-6736(21)00797-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu Y, Fang Y, Shen Z, et al. Oxaliplatin plus capecitabine in the perioperative treatment of locally advanced gastric adenocarcinoma in combination with D2 gastrectomy: NEO-CLASSIC study. Oncologist. 2019;24(10):1311-e989. doi: 10.1634/theoncologist.2019-0416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giommoni E, Lavacchi D, Tirino G, et al. Results of the observational prospective RealFLOT study. BMC Cancer. 2021;21(1):1086. doi: 10.1186/s12885-021-08768-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Batran S-E, Lorenzen S, Thuss-Patience PC, et al. Surgical and pathological outcome, and pathological regression, in patients receiving perioperative atezolizumab in combination with FLOT chemotherapy versus FLOT alone for resectable esophagogastric adenocarcinoma: interim results from DANTE, a randomized, multicenter, phase IIb trial of the FLOT-AIO German Gastric Cancer Group and Swiss SAKK. J Clin Oncol. 2022;40(16)(suppl):4003. doi: 10.1200/JCO.2022.40.16_suppl.4003 [DOI] [Google Scholar]
- 11.Ding P, Guo H, Sun C, et al. Combined systemic immune-inflammatory index (SII) and prognostic nutritional index (PNI) predicts chemotherapy response and prognosis in locally advanced gastric cancer patients receiving neoadjuvant chemotherapy with PD-1 antibody sintilimab and XELOX: a prospective study. BMC Gastroenterol. 2022;22(1):121. doi: 10.1186/s12876-022-02199-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang H, Yu X, Li N, et al. Efficacy and safety of neoadjuvant sintilimab, oxaliplatin and capecitabine in patients with locally advanced, resectable gastric or gastroesophageal junction adenocarcinoma: early results of a phase 2 study. J Immunother Cancer. 2022;10(3):e003635. doi: 10.1136/jitc-2021-003635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Han G, Li H, et al. Camrelizumab combined with FLOFOX as neoadjuvant therapy for resectable locally advanced gastric and gastroesophageal junction adenocarcinoma: updated results of efficacy and safety. J Clin Oncol. 2021;39(15)(suppl):4036. doi: 10.1200/JCO.2021.39.15_suppl.4036 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods
eTable 1. Patient Characteristics
eTable 2. Pathological Responses
eTable 3. Adverse Events
eTable 4. Treatment Completion
eResults. Supplemental Results
eFigure. Change in Tumor Immune Microenvironment
eReferences
Trial Protocol
Data Sharing Statement