Abstract
BACKGROUND
Use of extracorporeal membrane oxygenation (ECMO) as bridge to lung transplant has increased. However, little is known about patients placed on ECMO who die while on the waiting list. Using a national lung transplant data set, we investigated variables associated with waitlist mortality of patients bridged to lung transplant.
METHODS
All patients supported on ECMO at time of listing were identified using the United Network for Organ Sharing database. Univariable analyses were performed using bias-reduced logistic regression. Cause-specific hazard models were used to determine the effect of variables of interest on hazard of outcomes.
RESULTS
From April 2016 to December 2021, 634 patients met inclusion criteria. Of these, 445 (70%) were successfully bridged to transplant, 148 (23%) died on the waitlist, and 41 (6.5%) were removed for other reasons. Univariable analysis found associations between waitlist mortality and blood group, age, body mass index, serum creatinine, lung allocation score, days on waitlist, United Network for Organ Sharing region, and being listed at a lower-volume center. Cause-specific hazard models demonstrated that patients at high-volume centers were 24% more likely to survive to transplant and 44% less likely to die on the waitlist. Among patients who were successfully bridged to transplant, there was no difference in survival between low- and high-volume centers.
CONCLUSIONS
ECMO is an appropriate strategy to bridge selected high-risk patients to lung transplant. Of those placed on ECMO with intent to transplant, about one quarter may not survive to transplantation. High-risk patients requiring advanced support strategies may be more likely to survive to transplant when bridged at a high-volume center.
Lung transplant remains the singular durable strategy for survival in critically patients with end-stage lung disease. Of those patients who are successfully transplanted, one quarter will be hospitalized pre-transplant, with nearly 14% requiring support in the intensive care unit.1 Previously, mechanical ventilation was the only support strategy available leading to higher mortality rates compared with nonventilated patients.2 The use of extracorporeal membrane oxygenation (ECMO) to bridge these patients to transplant has increased significantly over the past two decades3,4 with equivalent survival to those supported with mechanical ventilation.5 Further evidence of acceptable outcomes using ECMO as a bridge to transplant (BTT) has resulted in increased implementation of its use.6-8 Little is known, however, about the characteristics of patients placed on ECMO who die while on the waiting list rather than undergoing successful transplantation. Using a national lung transplant data set, we investigated patient variables associated with waitlist mortality while on ECMO and compared with those who were successfully bridged to transplant.
PATIENTS AND METHODS
National data were collected from the United Network for Organ Sharing (UNOS) standard transplant analysis and research file based on Organ Procurement and Transplantation Network data as of March 31, 2022. All patients in the present study were activated on the waitlist between April 2016 (the period when UNOS began consistently collecting ECMO data) through December 31, 2021. This data set consisted of a prospectively collected open cohort using informed consent. Inclusion criteria for this study comprised all patients on ECMO listed for lung transplant at age ≥18 years at time of listing. Exclusion criteria included multiorgan transplants or previous lung transplant. Waitlist removal codes (eg, death, too sick to transplant, or removed due to transplant) were used to determine the outcomes. Patients who were successfully bridged to transplant and those who died on ECMO were compared. The study was approved through the University of Pittsburgh institutional review board protocol 20050181 (approval date: June 15, 2020).
Univariable analyses were performed using Mann-Whitney U tests for bivariate continuous variables and Kruskal-Wallis tests for analyses with 3 groups. Chi-square tests were used for categorical variables with post-hoc comparisons performed by analysis of the adjusted standardized residuals. Competing risk models were utilized to determine the effect of variables of interest on the hazard of outcomes.9 Analyses were conducted in R (v. 4.1.2; The R Project for Statistical Computing) with packages gtsummary, survival, and tidycmprsk. Transplant center volume was defined as the number of lung transplants performed at a center during a particular year. Center volume was divided into 2 categories a priori based on previous lung transplant literature, with 34 or fewer annual transplants defining a “lower” volume center, and 35 or more defining “high” volume.10-12 Adjusted cause-specific hazard models were performed using variables that were significantly different between high- and low-volume centers as nuisance covariates. Kaplan-Meier analyses were conducted to evaluate the relationship between center volume and posttransplant survival in patients who survived to transplant. A P < .05 was considered statistically significant.
RESULTS
A total of 634 patients on ECMO met initial inclusion criteria: 445 (70.2%) who were transplanted, 148 (23.3%) who died on the waitlist, and 41 (6.5%) who were removed for other reasons (eg, condition improved). Univariable comparisons between the 593 patients who were transplanted or died on the waitlist are displayed in Table 1. Patients who died on the waitlist were older (54 vs 47 years, P < .001), on the waitlist for longer duration (14 vs 9 days, P < .001), were more likely to have blood type AB, and were listed with lower lung allocation scores (LAS) and higher creatinine relative to patients who survived to transplant. Patients listed at a high-volume center were more likely to survive to transplant. There was a significant association between geographical region and waitlist outcome (P = .014). Patients on ECMO listed in the southwestern region (AZ/CA/NV/NM/UT) were more likely to live to transplant rather than die on the waitlist, whereas patients listed in the northeast regions (both NY/VT and DE/DC/MD/NJ/PA/WV/VA) were more likely to die on the waitlist.
TABLE 1.
Univariable Comparison of Patient Characteristics Between Waitlist Outcomes
| Variable | n | Overall (N = 593) |
Died on WL (n = 148) |
Transplanted (n = 445) |
P Valuea |
|---|---|---|---|---|---|
| Age at listing, y | 593 | 49 (37-57) | 54 (44-62) | 47 (36-56) | <.001 |
| High-volume center (>34/y) | 593 | <.001 | |||
| High-volume | 412 (69) | 86 (58) | 326 (73) | ||
| Lower-volume | 181 (31) | 62 (42) | 119 (27) | ||
| Diagnosis group | 593 | .070 | |||
| Obstructive | 12 (2.0) | 3 (2.0) | 9 (2.0) | ||
| Pulmonary hypertension | 43 (7.3) | 17 (11) | 26 (5.8) | ||
| Restrictive | 488 (82) | 120 (81) | 368 (83) | ||
| Suppurative | 50 (8.4) | 8 (5.4) | 42 (9.4) | ||
| Sex | 593 | .21 | |||
| Female | 223 (38) | 62 (42) | 161 (36) | ||
| Male | 370 (62) | 86 (58) | 284 (64) | ||
| Blood type | 593 | .003 | |||
| A | 192 (32) | 35 (24) | 157 (35) | ||
| AB | 29 (4.9) | 14 (9.5)b | 15 (3.4)b | ||
| B | 74 (12) | 20 (14) | 54 (12) | ||
| O | 298 (50) | 79 (53) | 219 (49) | ||
| Prostaglandin infusions at listing | 593 | 14 (2.4) | 5 (3.4) | 9 (2.0) | .35 |
| Resistant infection | 569 | 29 (5.1) | 7 (5.1) | 22 (5.1) | .98 |
| Diabetes | 591 | 165 (28) | 43 (29) | 122 (28) | .72 |
| Ventilator at listing | 593 | 322 (54) | 75 (51) | 247 (56) | .31 |
| Body mass index, kg/m2 | 592 | 26.5 (22.9-29.8) | 26.9 (23.9-30.4) | 26.2 (22.7-29.5) | .031 |
| Pulmonary arterial mean pressure, mmHg | 391 | 29 (23-40) | 31 (23-41) | 28 (22-39) | .27 |
| Cardiac output, L/min | 353 | 5.4 (4.3-6.9) | 5.1 (4.2-6.4) | 5.5 (4.4-7.2) | .14 |
| Creatinine, mg/dL | 593 | 0.60 (0.47-0.81) | 0.68 (0.52-0.90) | 0.59 (0.45-0.80) | <.001 |
| Days on waitlist | 593 | 9 (5-21) | 14 (7-31) | 9 (4-18) | <.001 |
| LAS at listing | 593 | 88 (85-90) | 87 (83-89) | 88 (86-90) | <.001 |
| Center volume, annual | 593 | 54 (29-78) | 43 (24-71) | 60 (33-89) | <.001 |
| Region | 593 | .014 | |||
| AL/AR/FL/GA/LA/MS/PR | 67 (11) | 16 (11) | 51 (11) | ||
| AZ/CA/NV/NM/UT | 126 (21) | 20 (14)b | 106 (24)b | ||
| CO/IA/KS/MI/NE/WY | 16 (2.7) | 2 (1.4) | 14 (3.1) | ||
| CT/ME/MA/NH/RI/VT | 9 (1.5) | 3 (2.0) | 6 (1.3) | ||
| DE/DC/MD/NJ/PA/WV/VA | 69 (12) | 25 (17)b | 44 (9.9)b | ||
| IL/MN/ND/SD/WI | 74 (12) | 21 (14) | 53 (12) | ||
| IN/MI/OH | 87 (15) | 25 (17) | 62 (14) | ||
| KY/NC/SC/TN/VI | 58 (9.8) | 9 (6.1) | 49 (11) | ||
| NY/VT | 28 (4.7) | 12 (8.1)b | 16 (3.6)b | ||
| OK/TX | 59 (9.9) | 15 (10) | 44 (9.9) |
Wilcoxon rank sum test; Pearson’s χ test; Fisher’s exact test
P < .05 post hoc. Values are presented as median (interquartile range) or n (%). LAS, lung allocation score; WL ,waitlist.
Univariable comparisons between high-volume and lower-volume centers are displayed in Table 2. There was a significant difference in waitlist outcome between patients at high- and low-volume centers (P < .001) with 79% of patients at high-volume centers transplanted vs 66% at low volume centers. There was a significantly higher proportion of patients at high-volume centers supported on mechanical ventilation (60% vs 41%, P < .001) at time of listing, and a trend for differences in LAS (P = .053). All other characteristics including age, diagnosis group, blood type, and comorbidities showed no difference between high- and low-volume centers.
TABLE 2.
Univariable Comparison of Patient Characteristics Between High- and Lower-Volume Centers
| Variable | n | Overall (N = 593) |
High-Volume (n = 412) |
Lower-Volume (n = 181) |
P Valuea |
|---|---|---|---|---|---|
| Age at listing, y | 593 | 49 (37-57) | 49 (37-57) | 51 (37-57) | .39 |
| Diagnosis group | 593 | .54 | |||
| Obstructive | 12 (2.0) | 10 (2.4) | 2 (1.1) | ||
| Pulmonary hypertension | 43 (7.3) | 27 (6.6) | 16 (8.8) | ||
| Restrictive | 488 (82) | 339 (82) | 149 (82) | ||
| Suppurative | 50 (8.4) | 36 (8.7) | 14 (7.7) | ||
| Sex | 593 | .57 | |||
| Female | 223 (38) | 158 (38) | 65 (36) | ||
| Male | 370 (62) | 254 (62) | 116 (64) | ||
| Blood type | 593 | .54 | |||
| A | 192 (32) | 138 (33) | 54 (30) | ||
| AB | 29 (4.9) | 17 (4.1) | 12 (6.6) | ||
| B | 74 (12) | 51 (12) | 23 (13) | ||
| O | 298 (50) | 206 (50) | 92 (51) | ||
| Prostaglandin infusions at listing | 593 | 14 (2.4) | 10 (2.4) | 4 (2.2) | >.99 |
| Resistant infection | 569 | 29 (5.1) | 21 (5.2) | 8 (4.9) | .88 |
| Diabetes | 591 | 165 (28) | 120 (29) | 45 (25) | .27 |
| Ventilator at listing | 593 | 322 (54) | 248 (60) | 74 (41) | <.001 |
| Body mass index, kg/m2 | 592 | 26.5 (22.9-29.8) | 26.4 (22.9-29.6) | 26.6 (23.0-29.9) | .52 |
| Pulmonary arterial mean pressure, mmHg | 391 | 29 (23-40) | 30 (23-40) | 29 (22-41) | .75 |
| Cardiac output, L/min | 353 | 5.4 (4.3-6.9) | 5.5 (4.4-7.1) | 5.2 (4.1-6.6) | .14 |
| Creatinine, mg/dL | 593 | 0.60 (0.47-0.81) | 0.60 (0.46-0.81) | 0.61 (0.50-0.80) | .48 |
| Days on waitlist | 593 | 9 (5-21) | 9 (5-21) | 11 (5-22) | .22 |
| LAS at listing | 593 | 88 (85-90) | 88 (86-90) | 88 (84-90) | .053 |
| Center volume, annual | 593 | 54 (29-78) | 71 (54-95) | 23 (18-27) | <.001 |
| Waitlist outcome | 593 | < .001 | |||
| Died on waitlist | 148 (25) | 86 (21) | 62 (34) | ||
| Transplanted | 445 (75) | 326 (79) | 119 (66) |
Wilcoxon rank sum test; Pearson’s χ2 test; Fisher’s exact test. Values are presented as median (interquartile range) or n (%). LAS, lung allocation score.
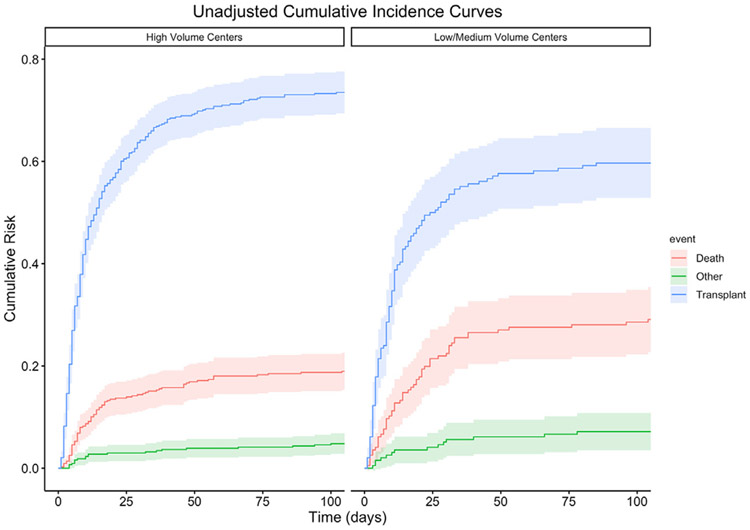
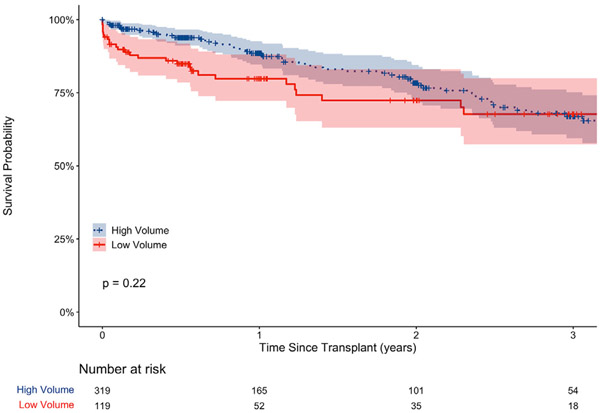
To investigate the relationship between center volume and outcomes, cause-specific hazard models were conducted with center volume (high/lower) as the predictor of interest. Patients at high-volume centers were 24% more likely to survive to transplant (χ2 (1) = 10.43, P = .001) relative to lower-volume centers and were 44% less likely to die on the waitlist (χ2 (1) = 10.49, P = .001). There was no difference between high- and lower-volume centers for removal for other reasons (χ2 (1) = 0.67, P = .42). An unadjusted cumulative incidence plot is displayed in Figure 1. Differences in waitlist outcomes remained following adjustment for region, ventilator rates at listing, and LAS, with patients at lower-volume centers less likely to be transplanted (hazard ratio 0.71, 95% CI 0.57-0.89, P = .003) and more likely to die on the waitlist (hazard ratio 1.54, 95% CI 1.09-2.17, P = .015) relative to high volume centers (Table 3). For transplanted patients, the median follow-up time was 364.5 days. Kaplan-Meier analysis found no difference in posttransplant survival between high- and low-volume centers (Figure 2, P = .22).
FIGURE 1.
Cumulative risk of waitlist outcome of patients supported on extracorporeal membrane oxygenation at high- vs low-volume centers.
TABLE 3.
Adjusted Cumulative Risk Regression of Waitlist Outcomes
| Characteristic | N | Transplanted | Died on Waitlist | ||
|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Center volume | |||||
| High-volume | 438 | … | … | ||
| Lower-volume | 196 | 0.71 (0.57-0.89) | .003 | 1.54 (1.09-2.17) | .015 |
| Region | |||||
| AL/AR/FL/GA/LA/MS/PR | 73 | … | … | ||
| AZ/CA/NV/NM/UT | 137 | 1.13 (0.83-1.55) | .43 | 0.77 (0.40-1.47) | .43 |
| CO/IA/KS/MI/NE/WY | 16 | 1.94 (1.04-3.63) | .038 | 0.51 (0.12-2.25) | .38 |
| CT/ME/MA/NH/RI/VT | 9 | 0.72 (0.35-1.48) | .38 | 1.56 (0.52-4.70) | .43 |
| DE/DC/MD/NJ/PA/WV/VA | 72 | 0.85 (0.57-1.25) | .41 | 1.70 (0.92-3.14) | .088 |
| IL/MN/ND/SD/WI | 79 | 0.88 (0.61-1.25) | .47 | 1.30 (0.70-2.43) | .41 |
| IN/MI/OH | 89 | 1.17 (0.80-1.70) | .42 | 1.50 (0.81-2.77) | .20 |
| KY/NC/SC/TN/VI | 61 | 1.44 (0.98-2.10) | .062 | 0.71 (0.31-1.61) | .41 |
| NY/VT | 31 | 0.63 (0.37-1.10) | .10 | 1.92 (0.95-3.88) | .069 |
| OK/TX | 67 | 0.83 (0.57-1.21) | .33 | 1.18 (0.59-2.37) | .63 |
| Ventilator at listing | 634 | 0.98 (0.81-1.19) | .85 | 1.03 (0.75-1.42) | .86 |
| LAS at listing | 634 | 1.01 (1.00-1.01) | .040 | 1.00 (0.99-1.01) | .45 |
Data for “Other” outcome not shown. HR, hazard ratio; LAS, lung allocation score.
FIGURE 2.
Posttransplant survival of patients bridged on extracorporeal membrane oxygenation at high- and low-volume centers.
An analysis of donor characteristics for the 445 patients who were transplanted is reported in Table 4. No differences in donor age or use of extended criteria donors were demonstrated between high- and low-volume centers, though donor type (donation after cardiac death [DCD] vs donation after brain death) was significantly associated with center volume (p= 0.019). DCD donors constituted only 6.1% of all transplanted lungs, but their use was highly concentrated at high-volume centers.
TABLE 4.
Donor Characteristics
| Variable | N | Overall (N = 445) |
High-Volume (n = 326) |
Lower-Volume (n = 119) |
P Valuea |
|---|---|---|---|---|---|
| Age, y | 445 | 33 (24-44) | 33 (24-43) | 33 (24-47) | .55 |
| Race | 445 | .13 | |||
| Black | 66 (15) | 43 (13) | 23 (19) | ||
| Hispanic | 97 (22) | 78 (24) | 19 (16) | ||
| Other | 21 (4.7) | 17 (5.2) | 4 (3.4) | ||
| White | 261 (59) | 188 (58) | 73 (61) | ||
| Donor type | 445 | .019 | |||
| DBD | 418 (94) | 301 (92) | 117 (98) | ||
| DCD | 27 (6.1) | 25 (7.7) | 2 (1.7) | ||
| Perfused | 383 | 17 (4.4) | 12 (4.3) | 5 (4.9) | .78 |
| Total ischemic time, h | 435 | 5.85 (4.90-7.13) | 5.95 (5.08-7.13) | 5.63 (4.45-7.12) | .045 |
| Distance from donor to Tx center, mi | 445 | 167 (67-293) | 172 (70-309) | 158 (56-226) | .15 |
| Transplant type | 445 | .35 | |||
| Double | 432 (97) | 318 (98) | 114 (96) | ||
| Single | 13 (2.9) | 8 (2.5) | 5 (4.2) | ||
| Age >55 y | 445 | 39 (8.8) | 28 (8.6) | 11 (9.2) | .83 |
| PO2 < 300 mm Hg | 443 | 104 (23) | 82 (25) | 22 (18) | .13 |
| Diabetes | 440 | 30 (6.8) | 21 (6.5) | 9 (7.8) | .64 |
| Purulent bronchoscopy | 445 | 91 (20) | 66 (20) | 25 (21) | .86 |
| Abnormal x-ray film | 441 | 326 (74) | 246 (76) | 80 (67) | .052 |
| 1+ extended criteria | 445 | 381 (86) | 283 (87) | 98 (82) | .24 |
| 2+ extended criteria | 445 | 195 (44) | 149 (46) | 46 (39) | .18 |
| 3+ extended criteria | 445 | 57 (13) | 39 (12) | 18 (15) | .38 |
Wilcoxon rank sum test; Pearson’s χ2 test; Fisher’s exact test. Values are presented as median (interquartile range) or n (%). DBD, donation after brain death; DCD, donation after cardiac death; Tx, transplantation.
COMMENT
The implementation of ECMO as a BTT for decompensated patients has evolved from initially being considered a contraindication to transplant into a critically important strategy.13 Its use has increased considerably in the last decade and constitutes as much as 5.2% of lung transplant patients as recently as 2017.14 Though these patients may exhibit increased perioperative and in-hospital mortality rates,15 posttransplant survival may not be impacted. Comparisons of patients bridged to transplant with ECMO to patients who are not bridged have demonstrated equivalent posttransplant survival at 1 and 3 years in both adult7,15-17 and pediatric18 populations.
Several factors, however, seem to attenuate comparable survival in ECMO BTT patients. Extended duration of ECMO support17,19,20 as well as ECMO support in redo lung transplant patients21 have negative effects on posttransplant survival. Center volume may also have notable impact on posttransplant survival of patients supported preoperatively with ECMO.5,22 Multiple studies using both institutional outcomes and national datasets have reported on increased survival after ECMO BTT at higher-volume centers. Hayes and colleagues,22 in a retrospective UNOS data set analysis, demonstrated that low center volume was associated with significant reduction in posttransplant survival for patients preoperatively bridged with ECMO. The impact of center volume on survival outcomes was not demonstrated in patients who were not bridged with ECMO. Our study evaluated the impact of center volume on outcomes of patients supported on ECMO at the time of listing for transplant to evaluate the outcome of either death or successful transplant using a cut point of 35 transplants a year to stratify medium/high- vs low-volume transplant centers.
Though we did not find a difference in posttransplant survival, we did note a significant difference in waitlist mortality when stratified by center volume. Patients supported on ECMO at the time of listing at centers with >35 transplants per year were significantly more likely to survive to transplant rather than suffer an ECMO mortality prior to transplant. The observed increase in waitlist mortality at low-volume transplant centers is likely multifactorial. High-volume transplant centers may be associated with advanced critical care programs, as the effect of center volume on mortality is also seen in hospitalized patients who are not bridged.23 Similarly, larger centers may also be associated with robust ECMO programs, in which high volume also leads to improved mortality rates.24 Larger transplant centers may be also more aggressive in their donor selection and include variances from standard criteria (such as an abnormal radiograph). An evaluation of donor characteristics provides additional insight into the transplantation trends of bridged patients at high vs low volume hospitals. In this cohort, the use of DCD donors was overwhelmingly confined to large volume centers. Though long-term survival outcomes with DCD donors are comparable,25 DCD donors make up a very small percentage of transplanted lung in the United States. The lack of its widespread adoption is multifactorial,26 though higher-volume centers may be more equipped to consider DCD offers in their donor pool, thereby contributing to increased likelihood of successfully bridging patients to transplant.
The impact of UNOS transplant region on ECMO waitlist mortality is another significant finding in our study. During the span of the waitlist data analyzed in this data set (2016-2021), two important factors have contributed to regional differences in lung transplant outcome. First is the transition in 2017 from lung allocation confinement within the Donation Service Area to a distance 250 nautical miles, allowing for a broader distribution of donor organs. Prior to this change, incidence of lung transplant was impacted more by Donation Service Area than LAS or blood type,27 with significant variations in local lung availability contributing to differences in regional outcomes.28 These impacts are likely reflected in the regional differences in successful transplant outcome for patients listed on ECMO in our analysis. Second, the impact of the COVID-19 pandemic on transplant practice and organ availability during this time period should be considered. The outset of the COVID-19 pandemic created volume shifts across the country, with reduction in lung transplant volume in some UNOS regions and increase in others.29
Our analysis additionally reports on several characteristics known to be associated with poor posttransplant survival and are noted in this study to be associated with pretransplant mortality on ECMO, including advanced age, blood type AB, increased serum creatinine, and greater number of days on the wait list. The clinical impact of minor differences in patient characteristics such as body mass index on waitlist outcomes is unclear and statistical significance may be attributable to the large sample size of our analysis. While LAS score comprises several patient comorbidities and risk factors, lower LAS score was associated with waitlist mortality in bridged patients. The relationship between LAS score on ECMO run duration here is unclear, though greater number of days on the waitlist may have contributed to the increased mortality in this group.
Several limitations in this study should be considered, including the retrospective nature of the analysis and limited data reportable within a large national data set. Our analysis is also limited to two timepoints: ECMO status at listing and status at removal. Thus, we are unable to capture adverse outcomes for patients who may have been placed on ECMO BTT after listing and did not live to transplant. Similarly, the ECMO run data begin at the point of listing and we are unable to determine duration of ECMO support prior to listing. While comparable outcomes in bridged patients on venovenous vs venoarterial ECMO have been reported,16 we are unable to report on cannulation strategy or patient acuity including whether patients were awake, ambulatory, or participating in physical therapy (one of several characteristics known to improve posttransplant survival).7 In addition, we are unable to evaluate whether there exists a volume outcome relationship with both ECMO center volume and transplant center volume. In the future, single-center analyses may be designed to prospectively capture outcomes of all bridged patients, including those who may have been cannulated after listing. These findings reveal the need to investigate factors associated with waitlist mortality on patients bridged with ECMO including consideration of whether patients in need of advanced support strategies should be transferred to a higher-volume program for greater odds of survival to transplant. Further study is needed to determine practice patterns that may be enacted by lower-volume centers to lead to improved outcomes in this cohort of patients.
ECMO has evolved as an important strategy in the armamentarium of lung transplant surgeons as a method to bridge endstage pulmonary patients to lung transplantation. Using a large national database, we report several factors associated with BTT ECMO waitlist mortality, including waitlist duration, transplant center volume, and UNOS region. As we continue to hone strategies to support critical patients, it is important to continue to identify and mitigate risks of preoperative ECMO mortality to provide patients with the greatest opportunity to survive to transplant.
FUNDING SOURCES
This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C.
Footnotes
DISCLOSURES
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2018 annual data report: lung. Am J Transplant. 2020;20s1:427–508. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton BCS, Dincheva GR, Matthay MA,et al. Improved survival after lung transplantation for adult requiring preoperative invasive mechanical ventilation: a national cohort study. J Thorac Cardiovasc Surg. 2020;160:1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayanga AJ, Aboagye J, Esper S, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation in the United States: an evolving strategy in the management of rapidly advancing pulmonary disease. J Thorac Cardiovasc Surg. 2015;149:291–296. [DOI] [PubMed] [Google Scholar]
- 4.Stokes JW, Gannon WD, Bacchetta M. Extracorporeal membrane oxygenation as a bridge to lung transplant. Semin Respir Crit Care Med. 2021;42:380–391. [DOI] [PubMed] [Google Scholar]
- 5.Hayanga JWA, Hayanga HK, Holmes SD, et al. Mechanical ventilation and extracorporeal membrane oxygenation as a bridge to lung transplantation: closing the gap. J Heart Lung Transplant. 2019;38:1104–1111. [DOI] [PubMed] [Google Scholar]
- 6.Hoopes CW, Kukreja J, Golden J, Davenport DL, Diaz-Guzman E, Zwischenberger JB. Extracorporeal membrane oxygenation as a bridge to pulmonary transplantation. J Thorac Cardiovasc Surg. 2013;145:862–867 [discussion: 867-868]. [DOI] [PubMed] [Google Scholar]
- 7.Tipograf Y, Salna M, Minko E, et al. Outcomes of extracorporeal membrane oxygenation as a bridge to lung transplantation. Ann Thorac Surg. 2019;107:1456–1463. [DOI] [PubMed] [Google Scholar]
- 8.Toyoda Y, Bhama JK, Shigemura N, et al. Efficacy of extracorporeal membrane oxygenation as a bridge to lung transplantation. J Thorac Cardiovasc Surg. 2013;145:1065–1071. [DOI] [PubMed] [Google Scholar]
- 9.Virani SS, Alonso A, Aparicio HJ, et al. Heart disease ANA stroke statistics-2021 update, a report from the American Heart Association. Circulation. 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 10.Kilic A, George TJ, Beaty CA, Merlo CA, Conte JV, Shah AS. The effect of center volume on the incidence of postoperative complications and their impact on survival after lung transplantation. J Thorac Cardiovasc Surg. 2012;144:1502–1509. [DOI] [PubMed] [Google Scholar]
- 11.Scarborough JE, Bennett KM, Davis RD, et al. Temporal trends in lung transplant center volume and outcomes in the United States. Transplantation. 2010;89:639–643. [DOI] [PubMed] [Google Scholar]
- 12.Mooney JJ, Weill D, Boyd JH, Nicolls MR, Bhattacharya J, Dhillon GS. Effect of transplant center volume on cost and readmissions in medicare lung transplant recipients. Ann Am Thorac Soc. 2016;13:1034–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayanga AJ, Du AL, Joubert K. Mechanical ventilation and extracorporeal membrane oxygenation as a bridging strategy to lung transplantation: significant gains in suvival. Am J Transplant. 2018;18:125–135. [DOI] [PubMed] [Google Scholar]
- 14.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2016 annual data report: lung. Am J Transplant. 2018;18(S1):363–433. [DOI] [PubMed] [Google Scholar]
- 15.Ius F, Natanov R, Salman J, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation may not impact overall mortality risk after transplantation: results from a 7-year single-centre experience. Eur J Cardiothorac Surg. 2018;54:334–340. [DOI] [PubMed] [Google Scholar]
- 16.Xia Y, Ragalie W, Yang EH, et al. Venoarterial versus venovenous extracorporeal membrane oxygenation as bridge to lung transplantation. Ann Thorac Surg. 2022;114:2080–2086. [DOI] [PubMed] [Google Scholar]
- 17.Langer F, Aliyev P, Schafers HJ, et al. Improving outcomes in bridge-to-transplant: extended extracorporeal membrane oxygenation support to obtain optimal donor lungs for marginal recipients. ASAIO J. 2019;65:516–521. [DOI] [PubMed] [Google Scholar]
- 18.Sainathan S, Ryan J, Sharma M, Harano T, Morell V, Sanchez P. Outcome of bridge to lung transplantation with extracorporeal membrane oxygenation in pediatric patients 12 years and older. Ann Thorac Surg. 2021;112:1083–1088. [DOI] [PubMed] [Google Scholar]
- 19.Crotti S, Iotti GA, Lissoni A, et al. Organ allocation waiting time during extracorporeal bridge to lung transplant affects outcomes. Chest. 2013;144: 1018–1025. [DOI] [PubMed] [Google Scholar]
- 20.Oh DK, Hong SB, Shim TS, et al. Effects of the duration of bridge to lung transplantation with extracorporeal membrane oxygenation. PLoS ONE. 2021;16:e0253520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoetzenecker K, Donahoe L, Yeung JC, et al. Extracorporeal life support as a bridge to lung transplantation—experience of a high-volume transplant center. J Thorac Cardiovasc Surg. 2018;155:1316–1328. [DOI] [PubMed] [Google Scholar]
- 22.Hayes D, Tobias JD, Tumin D. Center volume and extracorporeal membrane oxygenation support at lung transplantation in the lung allocation score era. Am J Respir Crit Care Med. 2016;194:317–326. [DOI] [PubMed] [Google Scholar]
- 23.Ranganath NK, Malas J, Chen S, et al. High lung transplant center volume is associated with increased survival in hospitalized patients. Ann Thorac Surg. 2021;111:1652–1658. [DOI] [PubMed] [Google Scholar]
- 24.Muguruma K, Kunisawa S, Fushimi K, Imanaka Y. Epidemiology and volume-outcome relationship of extracorporeal membrane oxygenation for respiratory failure in Japan: a retrospective observational study using a national administrative database. Acute Med Surg. 2020;7:e486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raemdonck DV, Keshavjee S, Levvey B, et al. Donation after circulatory death in lung transplantation- five-year follow-up from ISHLT registry. J Heart Lung Transplant. 2019;38:1235–1245. [DOI] [PubMed] [Google Scholar]
- 26.Siddique A, Urban M, Strah H, et al. Controlled DCD lung transplantation: circumventing imagined and real barriers—time for an international taskforce? J Heart Lung Transplant. 2022;41:1198–1203. [DOI] [PubMed] [Google Scholar]
- 27.Kosztowski M, Zhou S, Bush E, Higgins RL, Segev DL, Gentry SE. Geographic disparities in lung transplant rates. Am J Transplant. 2019;19:1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benvenuto LJ, Anderson DR, Hanyoung PK, et al. Geographic disparities in donor lung supply and lung transplant waitlist outcomes: a cohort study. Am J Transplant. 2019;18:1471–1480. [DOI] [PubMed] [Google Scholar]
- 29.Benvenuto L, Snyder ME, Aversa M, et al. Geographic differences in lung transplant volume and donor availability during the COVID-19 pandemic. Transplantation. 2021;105:861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]