Abstract
It has recently become apparent that overwhelming inflammatory reactions contribute to the high mortality rate associated with pneumococcal infection in immunocompetent hosts. Cefodizime (CEF) is an antibiotic that seems to be endowed with immunomodulating properties. To investigate the influence of CEF on the pulmonary inflammatory response induced by Streptococcus pneumoniae, we infected mice with repeated intranasal inoculations of 107 CFU of heat-killed fluorescein isothiocyanate-labeled bacteria, which are insensitive to the killing properties of the drug. CEF downregulated but did not abolish the strong polymorphonuclear leukocyte (PMN) recruitment induced by S. pneumoniae. PMN recruitment was not primarily mediated by leukotriene B4 in this model. The drug did not interfere with intrinsic mechanisms of phagocytosis by PMNs and alveolar macrophages. CEF totally abrogated the pneumococcus-induced tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) secretion in bronchoalveolar lavage fluid. The drug also prevented IL-6 release in lung homogenates and partly inhibited TNF-α, but it did not interfere with IL-1α secretion in the lungs of infected mice. The fractional and selective downregulation of inflammatory cells and cytokines by CEF suggests cell-specific and intracellular specific mechanisms of interaction of the drug. The immunomodulatory properties of CEF may help restrain excessive inflammatory reactions, thus contributing to the reported good clinical efficacy of the drug against lower respiratory tract infections.
Pneumococcal pneumonia is still a leading cause of mortality throughout the world, mainly as a result of inappropriate immune responses to virulent strains. Encapsulated bacteria resist phagocytosis by alveolar macrophages, which then secrete chemotactic factors for polymorphonuclear cell (PMN) recruitment. The excessive release of proinflammatory cytokines, enzymes, and oxygen radicals by both macrophages and PMNs thus initiates a cascade of inflammatory reactions that contributes to tissue injury and death (7, 8, 18, 29, 47, 54, 58). The development of antibiotics which can interact with the immune system has been an expanding field of research over the last decade and still remains an area of intense investigation (24, 34, 43, 56). Cefodizime (CEF), an expanded-spectrum cephalosporin, appears to have such immunomodifying properties: in vitro, the drug has been reported to exert negative (46), neutral (32), or positive (30) effects on PMN chemotaxis; no effect (32, 46) or positive effects (26, 36) on phagocytosis; downregulation of tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and IL-6 release by stimulated human monocytes (31, 43); no effect on IL-1 release (32); and upregulation of release of IL-8 (31) and granulocyte-macrophage colony-stimulating factor (38) from monocytes and bronchial epithelial cells, respectively. Ex vivo, CEF showed either neutral (12, 28) or positive (12, 32, 59, 60) effects on chemotaxis and phagocytosis by PMNs and monocytes, and it restored IL-1 and interferon production in immunocompromised patients and animals (22). In vivo, CEF enhanced phagocytosis and survival of mice infected with CEF-resistant pathogens (Candida albicans and Toxoplasma gondii) (20, 22, 23, 27). The discrepancies among data acquired in vitro, ex vivo, and in vivo support the hypothesis that CEF interacts with the release or activity of inflammatory mediators.
Despite the reported good clinical efficacy of CEF against acute lower respiratory tract infections (11, 39, 41, 49), and despite the fact that it compares favorably in vivo to other cephalosporins (e.g., cefotaxime) even when worse in vitro MICs are observed (45), there is a paucity of information regarding the potential immunomodulatory role of the drug during in vivo pneumococcal pneumonia, and there is no published information regarding cytokine measurement in this context. Moreover, any reported change in the immune function during antibiotic therapy could have been attributed to bacterial clearance by CEF. To our knowledge we are the first group to investigate the direct in vivo interaction of CEF with the pulmonary inflammatory response in a model of Streptococcus pneumoniae-induced pneumonia that excludes the potential influence of CEF on bacterial clearance and which includes at the same time chemotaxis, phagocytosis, and cytokine data. Heat-killed bacteria that cannot be destroyed by the drug were used to avoid downregulation of inflammation through bacterial clearance. Bacteria were labeled with fluorescein isothiocyanate (FITC) to detect engulfment by phagocytes through flow cytometry techniques. We analyzed the influence of CEF on the recruitment and phagocytosis efficacy of PMNs and alveolar macrophages and on the release of TNF-α, IL-1α, and IL-6. Leukotriene B4 (LTB4) was measured as a potential candidate for mediation of PMN chemotaxis.
(The data were presented in part at the fourth International Congress on Biological Response Modifiers [7a]).
MATERIALS AND METHODS
Preparation of FITC-labeled bacteria.
Cells of S. pneumoniae serotype 3 were grown in brain heart infusion broth supplemented with 5% horse serum in the presence of 5% CO2. They were inactivated by heating at 60°C for 2 h and were labeled with FITC (F-7250; Sigma, Oakville, Ontario, Canada) by stirring 108 CFU/ml in 0.5 M carbonate-bicarbonate buffer (pH 9.5) containing 0.2 mg of FITC per ml for 2 h at room temperature. Bacteria were then washed and resuspended in phosphate-buffered saline (PBS) for inoculation into animals. This encapsulated clinical strain isolated by blood culture was previously shown to induce greater phagocytosis by PMNs than by alveolar macrophages and to provoke strong pulmonary inflammation in fatal pneumonia after intranasal inoculation of 107 CFU of live bacteria into CD1 mice (7). The present model of inoculation with repeated injections of 107 CFU of heat-killed bacteria, although less potent for inducing inflammation than infection with live bacteria, seems suitable for the study of interactions of CEF with the immune response, as any change after treatment could be related to the “immunomodulatory” rather than the “antibiotic” properties of the drug. Such models with heat-killed pneumococci do induce cytokine release (48). The labeling of bacteria with FITC allowed us to measure both the percentage and mean fluorescence of phagocytosing macrophages and PMNs (described below).
“Infection” and treatment.
Lightly anesthetized female CD1 Swiss mice (20 to 22 g) were inoculated intranasally with 50 μl of PBS containing 107 bacteria every 12 h until five doses were administered. Control mice received intranasal PBS. To facilitate the migration of the inoculum to the alveoli and to ensure infectivity in 100% of the mice, animals were held in a vertical position for at least 2 min. CEF was dissolved in saline and administered subcutaneously at 30 mg/kg of body weight/dose at 12-h intervals, starting 96 h before the first inhalation of bacteria and ending at the time of the last bacterial inoculation. Control animals received saline. All mice had free access to mouse chow and water and were exposed to alternate standardized light and dark periods of 14 and 10 h, respectively, each day. These schedules of inoculation and treatment were based on previously observed bacterial counts during pneumonia (7) and on reported antibiotic effects (27, 60).
Experimental protocol.
Four groups of 12 animals received either bacteria alone, CEF alone, bacteria plus CEF, or the appropriate control diluent. Four hours after the last injection of bacteria and/or CEF, animals were killed by cervical dislocation, and two series of procedures were performed with each of these four groups. Half of the animals in each group were sampled at the retro-orbital sinus of the left eye for detection of TNF-α, IL-1α, IL-6, and LTB4 in serum, and then bronchoalveolar lavage (BAL) was performed to monitor leukocyte recruitment and phagocytosis of bacteria and to quantify cytokines and leukotrienes; the other six mice in each group were weighed, and the lungs were removed for assessment of lung weight, PMN infiltration in tissue through measurement of myeloperoxidase (MPO), release of inflammatory mediators, and histopathology. The time of sacrifice was based on previous determination of maximal cell recruitment and cytokine release in animals exposed to multiple inoculations with heat-killed bacteria.
Inflammatory cells in BAL fluid.
Leukocyte recruitment in alveoli was monitored by harvesting a total of 3 ml of BAL fluid in cold PBS. After centrifugation at 3,400 × g for 10 min, supernatants were used to detect inflammatory mediators (as described below) and protein content through the Bradford method (21); cells in the pellet were quantified with a hemacytometer, and the ratio of PMNs to macrophages was obtained from Diff-Quick-stained cytospin preparations (B4132-1; Baxter, Pointe-Claire, Quebec, Canada). A fraction of the BAL fluid was fixed in 1% paraformaldehyde-PBS and analyzed with an Epics 753 flow cytometer (Coulter Electronics) for phagocytosis. Therefore, phagocytosis data reflected in vivo rather than ex vivo phagocytosis of bacteria.
Phagocytosis assays.
After stimulation of cells at 488 nm (argon laser), the green fluorescence (525 nm; log scale), the forward angle light scatter (FALS), and the side scatter (SS) were recorded. The populations of macrophages and PMNs in BAL fluid had different FALSs and/or SSs. By selecting each population on the FALS-versus-SS histogram, we could determine the percentages of macrophages and PMNs that had a green fluorescence intensity greater than those for control cells, thus obtaining the percentages of cells actively involved in phagocytosis. The numbers of phagocytosing cells in BAL fluid were then derived from the total cell counts determined as described above. The mean fluorescence (intensity of fluorescence) reflected the number of bacteria ingested per phagocyte. Both the number of phagocytosing cells and the mean fluorescence were indicators of the intrinsic phagocytic efficacy of both cell populations.
Processing of lung tissue.
The lungs and heart were removed together, and blood was removed with sterile saline infusion through the right ventricle until the effluent was clear. The right lungs were then homogenized with a Potter homogenizer at 1 g/10 ml in potassium phosphate buffer (50 mM; pH 6.5). To 600 μl of homogenate was added 600 μl of phosphate buffer containing aprotinin (20 U) and CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} (0.2%) for measurement of cytokines. To 100 μl of homogenate was added 100 μl of hexadecyltrimethylammonium bromide (to achieve a final concentration of 0.5%) for measurement of MPO. Part of the crude homogenate was also used for measurement of LTB4, without addition of any detergent. The left lungs were processed for light microscopy and electron microscopy as described below.
Cytokine and LTB4 assays.
TNF-α, IL-1α, and IL-6 levels in the supernatant of BAL fluid, in the supernatant of lung homogenates (after centrifugation at 3,000 × g for 30 min at 4°C in a microcentrifuge), and in serum were measured with commercially available enzyme-linked immunosorbent assay kits (80-2802-00, 1900-01, and 80-3748-01, respectively; Genzyme Corp., Cambridge, Mass.). LTB4 was quantified with a radioimmunoassay (8-6020; Cedarlane, Hornby, Ontario, Canada).
MPO assay.
PMN infiltration in lung tissue was quantified through the measurement of MPO as previously described (7). Briefly, blood-free lung homogenates were sonicated and centrifuged at 3,000 × g for 30 min at 4°C. MPO was evaluated by adding 150 μl of the supernatant to a mixture of 825 μl of phosphate buffer, 75 μl of a o-dianisidine solution at a concentration of 1.25 mg/ml in distilled water, and 75 μl of hydrogen peroxide at 0.05%. The enzymatic reaction was stopped after 15 min by addition of 75 μl of 1% sodium azide, and absorbance was read at 450 nm against a standard curve made with commercially available MPO (M-6908; Sigma).
Histology.
Whole lungs were fixed in glutaraldehyde, embedded in paraffin, and processed for light microscopy. Tissue sections were fixed in glutaraldehyde followed by osmium tetroxide and then processed for light microscopy and electron microscopy according to standard methods (7).
Statistical analysis.
All statistical analyses were performed on StatView SE+ graphics (Abacus Concepts, Inc., Berkeley, Calif.). Differences between groups were evaluated with analysis of variance by a least-squares method. If the F test indicated a difference (P < 0.05), group comparisons were performed with Fisher’s protected least significant difference test and a P value of <0.05 was considered significant. All data are presented as means ± standard errors of the means (SEMs).
RESULTS
Inflammatory cells and phagocytosis.
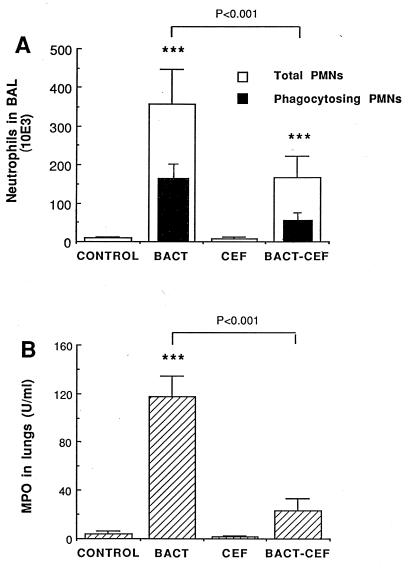
“Infection” with S. pneumoniae stimulated PMN recruitment in BAL fluid (Fig. 1A; P < 0.001 for the difference between control and infected mice) without altering the macrophage, lymphocyte, or eosinophil count. Treatment with CEF significantly reduced, but did not abolish the total PMN recruitment (P < 0.001 for the difference between infected-treated and infected mice and P < 0.001 for that between infected-treated and control mice). Control animals that received PBS showed no PMN recruitment, thus confirming the absence of bacterial contamination from the upper airways, as already assessed in previous experiments (7). The number of PMNs that actively phagocytized bacteria, as detected by flow cytometry (Fig. 1A), also fell, from 155 × 103 in infected mice to 57 × 103 in infected-treated mice (P < 0.05). This fall in the number of phagocytosing PMNs coincided, to the same order of magnitude, with the fall in the total number of recruited PMNs, thus conserving a similar percent phagocytosing cells (ratio of fluorescent PMNs to total PMNs), which indicated a reduction in chemotaxis rather than defective intrinsic phagocytic efficacy. Similarly, the percent phagocytosing macrophages (of the total macrophage count) remained stable, at 64 and 56% in infected and infected-treated mice, respectively. Moreover, the mean fluorescence of neither PMNs nor macrophages was altered by CEF, indicating that those cells which were active in the phagocytic process ingested the same amount of bacteria per cell in treated animals as in untreated infected animals. PMN recruitment in lung tissue of infected mice, detected through MPO elevation, was clearly inhibited by CEF, as shown in Fig. 1B. The MPO level fell from 117 U/ml of lung homogenate supernatant for infected animals to 23 U/ml for infected-treated mice (P < 0.001). Values comparable to those for uninfected controls were obtained for the latter group.
FIG. 1.
Mean (plus SEM) neutrophil and phagocytosing neutrophil counts in BAL fluid (A) and MPO levels in lung homogenates (B) of mice 4 h after the last injection of S. pneumoniae (BACT), CEF, S. pneumoniae plus CEF (BACT-CEF), or the appropriate diluent (CONTROL). ∗∗∗, P < 0.001 compared with counts in control mice.
Inflammatory mediators and other host factors.
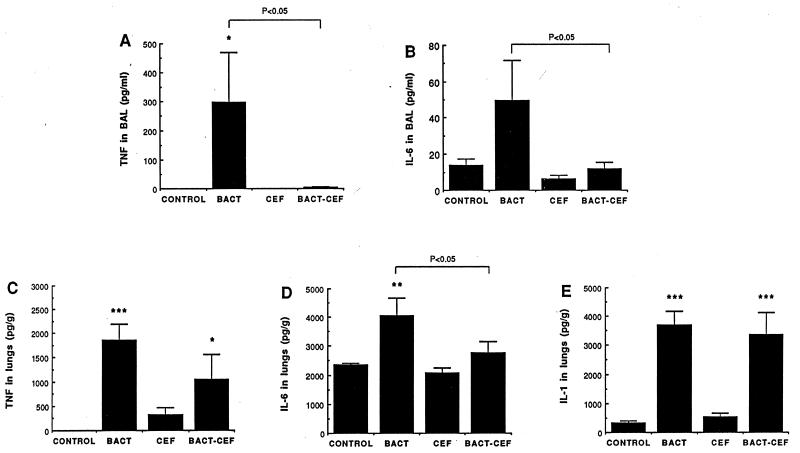
The most abundant cytokine in cell-free BAL fluid after S. pneumoniae infection was TNF-α (300 pg/ml) (Fig. 2A). IL-1α was undetectable in BAL fluid, while IL-6 was weakly secreted (50 pg/ml) (Fig. 2B). As CEF abrogated TNF-α and IL-6 in BAL fluid, values comparable to those for uninfected controls were obtained. TNF-α, IL-6, and IL-1 levels in lung tissue homogenate were significantly increased after infection (Fig. 2C to E). CEF selectively affected these cytokines in lung tissue, by reducing IL-6 to normal levels (Fig. 2D) and partly reducing the TNF-α level (Fig. 2C) without altering IL-1 (Fig. 2E). No cytokine could be detected in blood in this model, which does not manifest bacteremia. No significant release of LTB4 could be demonstrated for the infected animals at the time of measurement, either in BAL fluid, lung tissue, or serum. The amount of proteins recovered in cell-free BAL fluid was increased significantly after infection (338 ± 14 versus 216 ± 39 μg/ml in control mice; P < 0.05) but CEF totally prevented the effect of infection (162 ± 34 μg/ml; P < 0.01 compared to infected mice). Normal values were obtained after CEF was administered to uninfected mice (213 ± 42 μg/ml).
FIG. 2.
Mean (plus SEM) levels of TNF-α (A) and IL-6 (B) in BAL fluid and of TNF-α (C), IL-6 (D), and IL-1α (E) in lung homogenates 4 h after the last injection of S. pneumoniae (BACT), CEF, S. pneumoniae plus CEF (BACT-CEF), or the appropriate diluent (CONTROL). ∗, ∗∗, and ∗∗∗, P < 0.05, P < 0.01, and P < 0.001 compared with control value, respectively.
Histopathology.
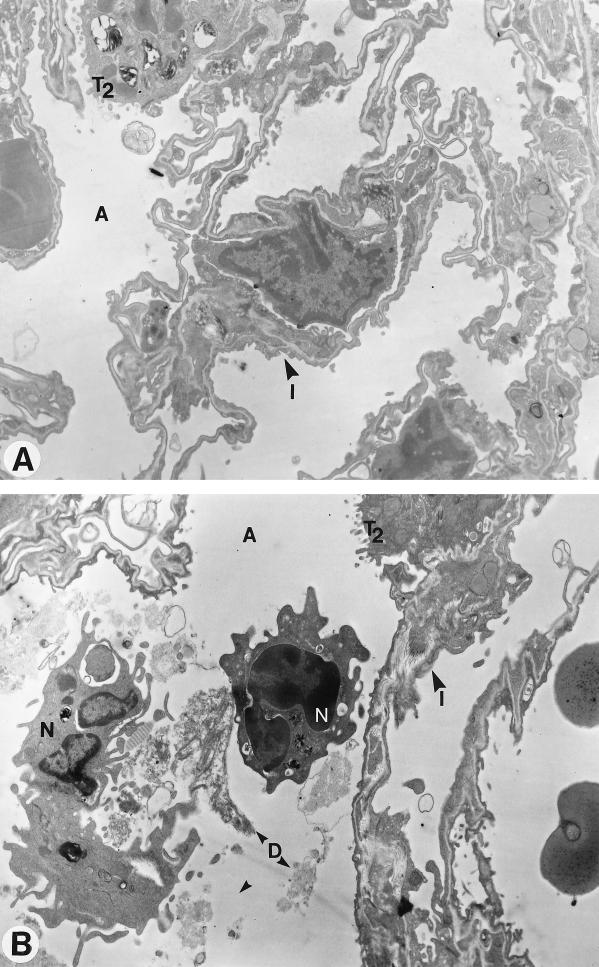
Electron microscopy confirmed the inhibiting influence of CEF on recruitment of PMNs, which were mainly localized near bronchoalveolar areas in infected animals (Fig. 3). Tissue damage was moderate after inoculation of heat-killed bacteria, in contrast with that observed after inoculation of living organisms (7), but less debris was seen after treatment with CEF.
FIG. 3.
Electron microscopy of lung architecture of mice infected with heat-killed S. pneumoniae and treated with CEF (A) or control diluent (B). Tissue is well preserved and few neutrophils are seen after therapy, while in the untreated infected mouse the tissue contains cell debris and numerous neutrophils. T2, type 2 pneumocytes; A, alveolus; I, interstitium; N, neutrophils; D, debris. Magnification, ×8,300.
DISCUSSION
In addition to the interactions between antibiotics and bacteria and between the immune system and bacteria, antibiotics interact with the immune system. In a recent literature review by Van Vlem et al. (56), 670 statements concerning positive, neutral, or negative effects of 153 antibiotics on the immune system are listed. Of the 115 statements obtained from reports on in vivo studies, only 30 concerned models in infected animals, as any change in the immune function could be the consequence of the mere disappearance of the infection rather than an intrinsic effect of the antibiotic per se. The model of pulmonary inflammation due to FITC-labeled heat-killed S. pneumoniae that we developed allowed us to confirm a downmodulatory role for CEF which does not result from bacterial clearance by the drug. CEF modified the inflammatory response by disturbing the cytokine cascade and the recruitment of PMNs to the site of infection. Despite reduction in chemotaxis of PMNs, the intrinsic phagocytic activity of alveolar macrophages and PMNs remained unaltered, as evaluated by the percentage and mean fluorescence of phagocytosing cells. The drug selectively inhibited the release of TNF-α and IL-6, which have been associated with cell recruitment and tissue injury in several infectious diseases, including pneumonia (47, 54). Overall, our results demonstrate that CEF reduces inflammation, in addition to its intrinsic antibiotic properties, and suggest that some immunological protection afforded to the host might contribute to the reported good clinical efficacy of the drug against acute lower respiratory tract infections (11, 39, 41, 45, 49), especially when living bacteria induce strong inflammation.
It is thought that, during bacterial pneumonia, PMNs migrate from the bloodstream to the site of infection under stimulation with chemotactic factors, such as the C5a fraction of complement, granulocyte colony-stimulating factor, macrophage inflammatory protein (MIP-2, the murine homologue of IL-8 in humans), GRO-alpha, LTB4, and platelet-activating factor, through mechanisms both dependent on and independent of the CD18 family of leukocyte adhesion molecules (13, 14, 16, 33, 47, 50, 51, 54). With our model we showed significant PMN recruitment after infection without significant activation of LTB4, suggesting that LTB4 is not required or at least is not the primary chemotactic mediator for PMN recruitment against S. pneumoniae. CEF may have contributed to the partial reduction in PMN counts by altering release of chemokines (such as MIP-2) by alveolar macrophages and other cells. Although the chemokines have not been detected in pneumococcal pneumonia in CD1 mice, it is probable that CEF acted upon many immune and nonimmune cells. Reduction in calcium ion concentration in PMNs (46, 52), interaction of CEF with membrane glycoproteins (10), or interaction with the expression of adhesion molecules (19, 37) may also have contributed to the alteration of PMN chemotaxis and functions, as with other antibiotics. However, reduction in PMN chemotaxis through inhibition of phosphoinositide metabolism, as occurs with aminoglycosides (57), appears to be unlikely to occur with CEF. Leukopenia or direct cytotoxicity for PMNs should also be excluded, as they were not reported to occur after CEF treatment.
The consequences of limiting PMN recruitment depend on the extent of inhibition: the high mortality rate from pneumococcal infection in neutropenic subjects actually provides evidence that a minimal number of PMNs is necessary for the host to resist bacterial invasion; on the other hand, high PMN recruitment is also associated with fatal outcome, as phagocytes release toxic components that contribute to tissue injury, edema, hypoxemia, and death (reviewed in references 7 and 58). Partial blockade by CEF of the excessive PMN recruitment in infected animals thus appears likely to produce a healthy equilibrium beneficial to the host. In addition, CEF did not alter PMN or macrophage phagocytic activity. Moreover, CEF as an antibiotic can restrain bacterial growth in the lungs during infection with live bacteria, despite limited PMN recruitment, thus providing protection through both its antibiotic effects and its immunomodulatory properties.
Interactions of antibiotics with cytokine release have been reported in numerous in vitro studies but in few in vivo studies (25, 34, 56). Our results support the in vitro observation made by Meloni et al. (31) that CEF downregulates TNF-α and IL-6 secretion by monocytes exposed to an inflammatory stimulus. In fact, alveolar and interstitial macrophages as well as blood monocytes are well-recognized potential sources for TNF-α, IL-1, and IL-6, but epithelial cells, fibroblasts, endothelial cells, and PMNs may also participate in cytokine release and inflammation (9, 47). The uptake of CEF and interaction with CEF of immune and nonimmune cells in our model resulted in complete inhibition of cytokines in BAL fluid but selective and fractional inhibition of TNF-α, IL-6, and IL-1 in lung homogenate supernatants. IL-1α, evaluated in our experiment, exerts its biologic activity in a membrane-associated form (in contrast to IL-1β, TNF-α, or IL-6) (1), which possibly explains why this cytokine could be recovered only in homogenized tissues and was not released into cell-free BAL fluid. Our data thus indicate that cell-specific and intracellular specific sites of interaction of the drug resulted in selective inhibitory mechanisms. It is unlikely that binding of CEF to penicillin-binding proteins in inactivated bacteria altered peptidoglycan structure and inflammation. In fact, the selective inhibition of TNF-α and IL-6 in lung tissue without reduction in IL-1 level suggests that immune system components rather than bacterial components were altered by CEF. Moreover, pneumolysin, teichoic acid, and capsule components are all likely to induce inflammation (reviewed in reference 7). Other investigators (5, 6, 31, 34) reported differential modulation of cytokine production by antibiotics, but no mechanism was evoked. Pefloxacin, ciprofloxacin, and ofloxacin reduced TNF-α, IL-1β, and IL-6 secretion from human adherent mononuclear leukocytes stimulated in vitro with bacterial lipopolysaccharide without inhibiting IL-1α (3–5, 44). The reduction in TNF-α correlated with abnormal intracellular levels of cyclic AMP, and the differential modulation suggested a reduction in the levels of cytokines that play a systemic role rather than those which act mostly through local intercellular contact. The reduction of proinflammatory cytokines through the stimulation (by clarithromycin) of the anti-inflammatory cytokine IL-10 has also been evoked (34). Additional potential mechanisms might include direct or indirect alteration of mRNA expression (15, 17). Since the mechanisms are likely to be complex, protein binding studies as well as immunocytochemistry and mRNA hybridization studies need first to be performed to identify which particular cell types are affected by infection and treatment.
Other reports support our hypothesis that partial and selective blockade of cell recruitment and inflammatory mediator release constitutes a useful therapeutic approach to pulmonary infections, as the secretion of TNF-α, IL-1, and IL-6 has been associated both with the pathogenesis and with the protective immune mechanisms in a number of pulmonary disorders, including pneumococcal pneumonia: TNF-α and IL-1 at low levels elevate nonspecific antibacterial resistance (53, 55), but their excessive release, or their combination, also induces synergistic toxicity to host cells (2, 40, 54). The role of each cytokine and the consequences of selective inhibition for the pathophysiology of pneumonia cannot be fully determined from the present experiment. Since TNF-α, IL-1, and IL-6 in BAL fluid have already been identified as being associated with severe pneumonia in humans and IL-6 appears to reflect the severity of stress, whether of infective or noninfective origin (35, 42), CEF possibly protects patients from multiple adverse reactions and contributes in various ways to the successful outcome of pneumonia. Interestingly, CEF demonstrated downmodulation properties despite sustained bacterial challenge, and the drug deserves to be fully investigated from the perspective of therapy for pneumonia against gram-positive and gram-negative microorganisms. Studies with cell wall components and living microorganisms are warranted, as in vivo treatments with antibiotics contribute to lysis of bacteria and release of toxins which may participate in inflammatory responses.
ACKNOWLEDGMENTS
M.O. is a recipient of the Fonds de Recherche en Santé du Québec (FRSQ) junior II scholarship. This work was supported by a grant from Hoechst Marion Roussel, Romainville, France.
We thank Maurice Dufour for flow cytometry analysis.
REFERENCES
- 1.Abbas A K, Lichtman A H, Pober J S. Effector mechanisms of immune responses. Cytokines. In: Wonsiewicz M J, editor. Cellular and molecular immunology. Philadelphia, Pa: The W. B. Saunders Company; 1991. pp. 232–235. [Google Scholar]
- 2.Amura C R, Fontan P A, Sanjuan N, Sordelli D O. The effect of treatment with interleukin-1 and tumor necrosis factor on Pseudomonas aeruginosa lung infection in a granulocytopenic mouse model. Clin Immunol Immunopathol. 1994;73:261–266. doi: 10.1006/clin.1994.1196. [DOI] [PubMed] [Google Scholar]
- 3.Bailly S, Fay M, Gougerot-Pocidalo M A. Effects of quinolones on tumor necrosis factor production by human monocytes. Int J Immunopharmacol. 1990;12:31–36. doi: 10.1016/0192-0561(90)90065-u. [DOI] [PubMed] [Google Scholar]
- 4.Bailly S, Fay M, Gougerot-Pocidalo M A. Effet des antibiotiques sur la production de cytokines par les monocytes humains. Pathol Biol. 1993;41:838–844. [PubMed] [Google Scholar]
- 5.Bailly S, Mahe Y, Ferrua B, Fay M, Tursz T, Wakasugi H, Gougerot-Pocidalo M A. Quinolone-induced differential modification of IL-1 alpha and IL-1 beta production by LPS-stimulated human monocytes. Cell Immunol. 1990;128:277–288. doi: 10.1016/0008-8749(90)90025-m. [DOI] [PubMed] [Google Scholar]
- 6.Bailly S, Pocidalo J-J, Fay M, Gougerot-Pocidalo M-A. Differential modulation of cytokine production by macrolides: interleukin-6 production is increased by spiramycin and erythromycin. Antimicrob Agents Chemother. 1991;35:2016–2019. doi: 10.1128/aac.35.10.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergeron Y, Ouellet N, Deslauriers A-M, Simard M, Olivier M, Bergeron M G. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect Immun. 1998;66:912–922. doi: 10.1128/iai.66.3.912-922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Bergeron Y, Ouellet N, Simard M, Olivier M, Bergeron M G. Conference program, summaries and abstracts of the Fourth International Congress on Biological Response Modifiers, San Antonio, Tex. 1997. Immunotherapy of pneumococcal pneumonia with cefodizime, abstr. 15. [Google Scholar]
- 8.Boulnois G J. Pneumococcal proteins and the pathogenesis of disease caused by Streptococcus pneumoniae. J Gen Microbiol. 1992;138:249–259. doi: 10.1099/00221287-138-2-249. [DOI] [PubMed] [Google Scholar]
- 9.Cassatella M A. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 10.Fietta A, Bersani C, Bertoletti R, Grassi F M, Gialdroni-Grassi G. In vitro and ex vivo enhancement of nonspecific phagocytosis by cefodizime. Chemotherapy (Basel) 1988;34:430–434. doi: 10.1159/000238603. [DOI] [PubMed] [Google Scholar]
- 11.Gialdroni-Grassi, G. 1990. Cefodizime in clinical use: a review of the clinical trial reports. J. Antimicrob. Chemother. 26(Suppl. C):117–125. [DOI] [PubMed]
- 12.Gialdroni-Grassi, G., and P. M. Shah. 1992. Cefodizime host-defence enhancement: considerations of dose-response relationships in healthy volunteers. Infection 20(Suppl. 1):S51–S53. [DOI] [PubMed]
- 13.Greenberger M J, Streiter R M, Kunkel S L, Danforth J M, Laichalk L L, McGillicudy D C, Standiford T J. Neutralization of MIP-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. J Infect Dis. 1996;173:159–163. doi: 10.1093/infdis/173.1.159. [DOI] [PubMed] [Google Scholar]
- 14.Hebert J C, O’Reilly M, Garnelli R L. Protective effect of recombinant granulocyte colony-stimulating factor against pneumococcal infections in splenectomized mice. Arch Surg. 1990;125:1075–1082. doi: 10.1001/archsurg.1990.01410200141022. [DOI] [PubMed] [Google Scholar]
- 15.Honda, J., A. Keisuke, O. Yasumitu, N. Sin, and O. Kotaro. 1995. Effects of macrolides on cytokine mRNA expression. Can. J. Infect. Dis. 6(Suppl. C):423C. (Abstr. 3195.)
- 16.Hopkins H, Stull T, Von-Essen S, Robbins R A, Rennard S I. Neutrophil chemotactic factors in bacterial pneumonia. Chest. 1989;95:1021–1027. doi: 10.1378/chest.95.5.1021. [DOI] [PubMed] [Google Scholar]
- 17.Howard M, Frizzell R A, Bedwell D M. Aminoglycoside antibiotics restore CFTR function by overcoming premature stop mutations. Nature. 1996;2:467–469. doi: 10.1038/nm0496-467. [DOI] [PubMed] [Google Scholar]
- 18.Johnston, R. B. J. 1991. Pathogenesis of pneumococcal pneumonia. Rev. Infect. Dis. 13(Suppl. 6):S509–S517. [DOI] [PubMed]
- 19.Kadota, J. I., S. Kusano, R. Shirai, K. Kawakami, K. Iida, et al. 1995. Effect of roxithromycin on peripheral neutrophil adhesion molecules in patients with chronic lower respiratory disease. Can. J. Infect. Dis. 6(Suppl. C):423C. (Abstr. 3196.) [DOI] [PubMed]
- 20.Klesel N, Limbert M, Seibert G, Winkler I, Schrinner E. Cefodizime, an aminothiazolyl cephalosporin. III. Therapeutic activity against experimentally induced pneumonia in mice. J Antibiot. 1984;37:1712–1718. doi: 10.7164/antibiotics.37.1712. [DOI] [PubMed] [Google Scholar]
- 21.Kruger N J. The Bradford method for protein quantitation. Methods Mol Biol. 1994;32:9–15. doi: 10.1385/0-89603-268-X:9. [DOI] [PubMed] [Google Scholar]
- 22.Labro, M. T. 1990. Cefodizime as a biological response modifier: a review of its in vivo, ex vivo, and in vitro immunomodulatory properties. J. Antimicrob. Chemother. 26(Suppl. C):37–47. [DOI] [PubMed]
- 23.Labro, M. T. 1992. Immunological evaluation of cefodizime: a unique molecule among cephalosporins. Infection 20(Suppl. 1):S45–S47. [DOI] [PubMed]
- 24.Labro, M. T. 1994. Experimental evaluation of antibiotics as immunomodulators. J. Chemother. 6(Suppl. 3):11–15. [PubMed]
- 25.Labro M T. The prohost effect of antimicrobial agents as a predictor of clinical outcome. J Chemother. 1997;9:100–108. [PubMed] [Google Scholar]
- 26.Labro M T, Amit N, Babin-Chevaye C, Hakim J. Cefodizime (HR 221) potentiation of human neutrophil oxygen-independent bactericidal activity. J Antimicrob Chemother. 1987;19:331–341. doi: 10.1093/jac/19.3.331. [DOI] [PubMed] [Google Scholar]
- 27.Limbert M, Bartlett R R, Dickneite G, Klesel N, Schorlemmer H U, Seibert G, Winkler I, Schrinner E. Cefodizime, an aminothiazolyl cephalosporin. IV. Influence on the immune system. J Antibiot. 1984;37:1719–1726. doi: 10.7164/antibiotics.37.1719. [DOI] [PubMed] [Google Scholar]
- 28.Limbert, M., H. Mullner, and P. M. Shah. 1992. Influence of cefodizime on the reagibility of human leukocytes. Infection 20(Suppl. 1):S48–S50. [DOI] [PubMed]
- 29.Lukacs N W, Ward P A. Inflammatory mediators, cytokines, and adhesion molecules in pulmonary inflammation and injury. Adv Immunol. 1996;62:257–291. doi: 10.1016/s0065-2776(08)60432-0. [DOI] [PubMed] [Google Scholar]
- 30.McCafferty A C, McGregor E, Jones M, Henderson J S, Cree I A. The effect of cefodizime on phagocyte function in non-patient volunteers and patients with chronic renal failure. In vitro and ex vivo studies. Int J Clin Lab Res. 1996;26:229–235. doi: 10.1007/BF02602954. [DOI] [PubMed] [Google Scholar]
- 31.Meloni F, Ballabio P, Bianchi L, Grassi F A, Gialdroni-Grassi G G. Cefodizime modulates in vitro tumor necrosis factor-alpha, interleukin-6 and interleukin-8 release from human peripheral monocytes. Chemotherapy (Basel) 1995;41:289–295. doi: 10.1159/000239358. [DOI] [PubMed] [Google Scholar]
- 32.Meroni, P. L., F. Capsoni, M. O. Borghi, W. Barcellini, F. Minonzio, et al. 1992. Immunopharmacological activity of cefodizime in young and elderly subjects: in vitro and ex vivo studies. Infection 20(Suppl. 1):S61–S63. [DOI] [PubMed]
- 33.Mizgerd J P, Meek B B, Kutkoski G J, Biullard D C, Beaudet A L, Doerschuk C M. Selectins and neutrophil traffic: margination and Streptococcus pneumoniae-induced emigration in murine lungs. J Exp Med. 1996;184:639–645. doi: 10.1084/jem.184.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morikawa K, Watabe H, Araake M, Morikawa S. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob Agents Chemother. 1996;40:1366–1370. doi: 10.1128/aac.40.6.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moussa K, Michie H J, Cree I A, McCafferty A C, Winter J H, Dhillon D P, Stephens S, Brown R A. Phagocyte function and cytokine production in community-acquired pneumonia. Thorax. 1994;49:107–111. doi: 10.1136/thx.49.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oishi K, Matsumoto K, Yamamoto M, Morito T, Yoshida T. Stimulatory effect of cefodizime on macrophage-mediated phagocytosis. J Antibiot (Tokyo) 1989;42:989–992. doi: 10.7164/antibiotics.42.989. [DOI] [PubMed] [Google Scholar]
- 37.Okubo, Y., J. Honda, K. Arikawa, and K. Oizumi. 1995. Macrolides reduce the expression of surface MAC-1 molecule on neutrophil. Can. J. Infect. Dis. 6(Suppl. C):424C. (Abstr. 3200.)
- 38.Pacheco Y, Hosni R, Dagrosa E E, Gormand F, Guibert B, Chabannes B, Lagarde M, Perrin-Fayolle M. Antibiotics and production of granulocyte-macrophage colony-stimulating factor by human bronchial epithelial cells in vitro. A comparison of cefodizime and ceftriaxone. Drug Res. 1994;44:559–563. [PubMed] [Google Scholar]
- 39.Pauwels, R. A. 1992. Review of effectiveness of cefodizime in the treatment of lower respiratory tract infections with parenchymal involvement. Infection 20(Suppl. 1):S26–S30. [DOI] [PubMed]
- 40.Pietsch K, Ehlers S, Jacobs E. Cytokine gene expression in the lungs of BALB/c mice during primary and secondary intranasal infection with Mycoplasma pneumoniae. Microbiology (Reading) 1994;140:2043–2048. doi: 10.1099/13500872-140-8-2043. [DOI] [PubMed] [Google Scholar]
- 41.Piovano C F, Palombini B C, Dagrosa E E, Mendoza F, Facco E B. Cefodizime once daily in the treatment of lower respiratory tract infections. Arzneim-forsch. 1997;47:674–677. [PubMed] [Google Scholar]
- 42.Puren A J, Feldman C, Savage N, Becker P J, Smith C. Patterns of cytokine expression in community-acquired pneumonia. Chest. 1995;107:1342–1349. doi: 10.1378/chest.107.5.1342. [DOI] [PubMed] [Google Scholar]
- 43.Ritts, R. E. 1990. Antibiotics as biological response modifiers. Chemotherapy (Basel) 26:(Suppl. C):31–36. [DOI] [PubMed]
- 44.Roche Y, Fay M, Gougerot-Pocidalo M A. Interleukin-1 production by antibiotic-treated human monocytes. J Antimicrob Chemother. 1988;21:597–607. doi: 10.1093/jac/21.5.597. [DOI] [PubMed] [Google Scholar]
- 45.Shah, P. M., and H. Knothe. 1992. In vivo activity of cefodizime. Infection 20(Suppl. 1):S9–S13. [DOI] [PubMed]
- 46.Shaio M F, Chang F Y. Influence of cefodizime on chemotaxis and the respiratory burst in neutrophils from diabetics. J Antimicrob Chemother. 1990;26:55–59. doi: 10.1093/jac/26.1.55. [DOI] [PubMed] [Google Scholar]
- 47.Simon R H, Paine R. Participation of pulmonary alveolar epithelial cells in lung inflammation. J Lab Clin Med. 1995;126:108–118. [PubMed] [Google Scholar]
- 48.Simpson S Q, Singh R, Bice D E. Heat-killed pneumococci and pneumococcal capsular polysaccharides stimulate tumor necrosis factor-alpha production by murine macrophages. Am J Respir Cell Mol Biol. 1994;10:284–289. doi: 10.1165/ajrcmb.10.3.8117447. [DOI] [PubMed] [Google Scholar]
- 49.Sollet, J. P. 1990. An open multicentre study of the efficacy and tolerance of cefodizime 1 g bd intravenously or intramuscularly in lower respiratory tract infections. J. Antimicrob. Chemother. 26(Suppl. C):103–110. [DOI] [PubMed]
- 50.Standiford T J, Kunkel S L, Greenberger M J, Laichalk L L, Strieter R M. Expression and regulation of chemokines in bacterial pneumonia. J Leukoc Biol. 1996;59:24–28. doi: 10.1002/jlb.59.1.24. [DOI] [PubMed] [Google Scholar]
- 51.Sugawara T, Miyamoto M, Takayama S, Kato M. Separation of neutrophils from blood in human and laboratory animals and comparison of the chemotaxis. J Pharmacol Toxicol Methods. 1995;33:91–100. doi: 10.1016/1056-8719(94)00062-9. [DOI] [PubMed] [Google Scholar]
- 52.Sugita, K., and T. Nishimura. 1995. Effects of antimicrobial agents on chemotaxis of human polymorphonuclear neutrophils. Can. J. Infect. Dis. 6(Suppl. C):424C. (Abstr. 3203.)
- 53.Takashima K, Tateda K, Matsumoto T, Iizawa Y, Nakao M, Yamaguchi K. Role of tumor necrosis factor alpha in pathogenesis of pneumococcal pneumonia in mice. Infect Immun. 1997;65:257–260. doi: 10.1128/iai.65.1.257-260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tuomanen E I, Austrian R, Masure H R. Pathogenesis of pneumococcal infection. N Engl J Med. 1995;332:1280–1284. doi: 10.1056/NEJM199505113321907. [DOI] [PubMed] [Google Scholar]
- 55.Van der Poll T, Keogh C V, Buurman W A, Lowry S F. Passive immunization against tumor necrosis factor-alpha impairs host defense during pneumococcal pneumonia in mice. Am J Respir Crit Care Med. 1997;155:603–608. doi: 10.1164/ajrccm.155.2.9032201. [DOI] [PubMed] [Google Scholar]
- 56.Van Vlem B, Vanholder R, De Paepe P, Vogelaers D, Ringoir S. Immunomodulating effects of antibiotics: literature review. Infection. 1996;24:275–291. doi: 10.1007/BF01743360. [DOI] [PubMed] [Google Scholar]
- 57.Venezio F R, DiVincenzo C A. Effects of aminoglycoside antibiotics on polymorphonuclear leukocyte function in vivo. Antimicrob Agents Chemother. 1985;27:712–714. doi: 10.1128/aac.27.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiss S J. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 59.Wenisch C, Bartunek A, Zedtwitz-Liebenstein K, Hiesmayr M, Parschalk B, Pernerstorfer T. Prospective randomized comparison of cefodizime versus cefuroxime for perioperative prophylaxis in patients undergoing coronary artery bypass grafting. Antimicrob Agents Chemother. 1997;41:1584–1588. doi: 10.1128/aac.41.7.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wenisch C, Parschalk B, Hasenhündl M, Wiesinger E, Graninger W. Effect of cefodizime and ceftriaxone on phagocytic function in patients with severe infections. Antimicrob Agents Chemother. 1995;39:672–676. doi: 10.1128/AAC.39.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]