Abstract
The current model for the synchronization of GnRH neural activity driving GnRH and LH pulses proposes that a set of arcuate (ARC) neurons that contain kisspeptin, neurokinin B, and dynorphin (KNDy neurons) is the GnRH pulse generator. This study tested the functional role of ovine KNDy neurons in pulse generation and explored the roles of nearby Kiss1 receptor (Kiss1R)-containing cells using lesions produced with saporin (SAP) conjugates. Injection of NK3-SAP ablated over 90% of the KNDy cells, while Kiss-SAP (saporin conjugated to kisspeptin-54) lesioned about two-thirds of the Kiss1R population without affecting KNDy or GnRH cell number. Both lesions produced a dramatic decrease in LH pulse amplitude but had different effects on LH pulse patterns. NK3-SAP increased interpulse interval, but Kiss-SAP did not. In contrast, Kiss-SAP disrupted the regular hourly occurrence of LH pulses, but NK3-SAP did not. Because Kiss1R is not expressed in KNDy cells, HiPlex RNAScope was used to assess the colocalization of 8 neurotransmitters and 3 receptors in ARC Kiss1R-containing cells. Kiss1R cells primarily contained transcript markers for GABA (68%), glutamate (28%), ESR1 (estrogen receptor-α) mRNA, and OPRK1 (kappa opioid receptor) mRNA. These data support the conclusion that KNDy neurons are essential for GnRH pulses in ewes, whereas ARC Kiss1R cells are not but do maintain the amplitude and regularity of GnRH pulses. We thus propose that in sheep, ARC Kiss1R neurons form part of a positive feedback circuit that reinforces the activity of the KNDy neural network, with GABA or glutamate likely being involved.
Keywords: kisspeptin, KNDy, Kiss1R, pulse generator, neurokinin B, NK3R
It has been known for almost half a century that the episodic release of GnRH drives the corresponding pulsatile pattern of LH (1, 2), and that this pattern is essential for normal secretion of gonadotropins from the pituitary and thus fertility (3). However, identification of the neural mechanisms responsible for episodic GnRH secretion (known as the GnRH pulse generator) only became possible after the discovery that kisspeptin (4, 5) and neurokinin B (NKB) (6) were critical for fertility in humans. The current model for this mechanism proposes that a network of KNDy neurons located in the arcuate nucleus (ARC), and contain kisspeptin, NKB, and dynorphin (7) are the GnRH pulse generator (8-10). According to this hypothesis, NKB and dynorphin act within the KNDy network as start and stop signals to initiate and terminate each pulse, respectively, whereas kisspeptin is the output signal driving GnRH secretion during a pulse. There is now convincing evidence for this hypothesis based on work in rodents and ruminants (11-14), but whether it applies to humans and other primates remains unclear (15, 16).
Early anatomical work on the location of receptors for kisspeptin (Kiss1R), NKB (NK3R), and dynorphin (KOR), and the effects of agonists and antagonists to these receptors on LH secretion (8, 14, 17) were critical to the development of this hypothesis. Subsequent work with selective lesions and stimulation of KNDy neurons using transgenic mice (18-21) has provided critical tests of it and led to its general acceptance.
One key tenant of the KNDy model for the GnRH pulse generator is that kisspeptin is the output signal of this network but does not have any direct effects on its activity. This is supported by the absence of Kiss1R in KNDy neurons of rats (22) and sheep (23) and the inability of kisspeptin to increase electrical activity of KNDy neurons in slices of the murine hypothalamus (24). However, studies have reported that kisspeptin infusions increase LH pulse frequency in men (25, 26) and women (27). Moreover, a similar effect of kisspeptin is seen in patients with an inactivating mutation of NK3R (28). The report that an IV injection of kiss-10 reset the timing of the next LH pulse in men (29) also argues for a possible role of kisspeptin beyond just being the output signal from KNDy cells. These observations have led to the proposal that the pulsatile pattern of GnRH secretion arises not from the KNDy network but from neural elements downstream from the KNDy neurons (30). Another hypothesis is that the non-KNDy Kiss1R-containing neurons found in the ARC (22, 23) could provide important input to nearby KNDy cells. Reports that local administration of a Kiss1R antagonist into the ARC-inhibited LH pulse frequency in rats indicate that kisspeptin can indeed act in this nucleus to modulate LH pulse frequency (31).
Studies in sheep and goats, including the discovery of KNDy neurons (7) and the identification of these cells as the origin of bursts of multiunit electrical activity correlating with LH pulses (32), played key roles in the development of the KNDy hypothesis. Subsequent results describing the effects of agonists and antagonists to receptors for each KNDy peptide on LH pulse patterns and the associated burst of multiunit electrical activity provided strong support for this hypothesis in ruminants (10, 14, 33). Comparison of these data with similar studies in rodents (11, 14) also identified differences in the specificity of NKB actions (24, 34-36) and the role of dynorphin (24, 37, 38) between ruminants and rodents, indicating species differences that may be relevant to the generation of GnRH pulses in humans (39). However, there have been no direct tests of the role of KNDy neurons that selectively lesioned these cells in either sheep or goats. There is also evidence that ARC Kiss1R neurons may play a role in GnRH pulse generation in sheep. Specifically, local administration of a Kiss1R antagonist into the ovine ARC inhibited LH pulse frequency (33) and infusion of kisspeptin in sheep reinitiated LH pulses that had been blocked with an NK3R antagonist (40).
Thus, this study had 2 goals: (1) to assess the effects of lesioning ovine KNDy neurons on episodic LH secretion and (2) to test the role of ARC Kiss1R-containing cells in maintaining episodic LH secretion. KNDy neurons were lesioned with a cellular toxin (saporin [SAP]) conjugated to an agonist to NK3R (NK3-SAP), a reagent that was very effective in lesioning ovine NK3R-containing cells in the retrochiasmatic area (41) and has been used to lesion KNDy neurons in rats (42). Similarly, saporin conjugated to kisspeptin-54 (Kiss-SAP) was used to lesion ARC Kiss1R cells while leaving KNDy and GnRH neurons intact.
Materials and Methods
Animals
Adult blackface ewes were purchased from local farmers and maintained under ambient conditions in an open barn with access to water and food daily at West Virginia University. At least 6 days before surgery, ewes were moved to an indoor facility with controlled photoperiods simulating natural outdoor lighting. They had free access to water and a mineral lick and were fed a maintenance ration of Timothy Pellets (Standlee Hay Company Inc., Kimberly, ID) and Complete Pellets (Triple Crown, Wayzata, MN). Frequent blood samples (∼4 mL) were collected every 12 minutes for 4 hours by jugular venipuncture, dispensed into heparinized tubes, and the serum stored at −20 °C until assayed.
Surgical Procedures
Ewes were ovariectomized (OVX) via a mid-ventral approach using sterile techniques and gas anesthesia, as previously described (43), except that the ovarian blood supply was clamped and cauterized using an electrical ligature system (Liga-A-Sure, Medtronic, Minneapolis, MN), rather than being tied off with suture, before being cut. This improvement shortened the procedure considerably and minimized the amount of bleeding. Animals were treated with antibiotics (Excede, Zoetis, Parsippany-Troy Hills, NJ) and analgesic (Buprenorphine, Zoetis) before and after OVX. Saporin conjugates (Advanced Targeting Systems, San Diego, CA) were injected into the ARC. Methods were similar to those previously described in detail (41, 44). First, a small portion of the dorsal surface of the brain was exposed, the sagittal sinus ligated, and an 18-gauge needle was lowered into 1 lateral ventricle. Radiopaque dye was injected into the ventricle, and lateral and frontal radiography was used to lower bilateral stainless steel guide tubes (4 mm apart) to a position 1 to 1.5 mm posterior to the infundibular recess and 5 to 7 mm above the base of the third ventricle. A 1-µL Hamilton syringe with a fixed needle containing 1 μL of the saporin conjugates was inserted 3 mm beyond 1 guide tube and then lowered so the tip was 0.5 to 1.0 mm above the base of the ventricle. The 1-µL contents were then slowly injected over 2 minutes, and the needle was left in place for an additional 3 minutes. The procedure was then repeated on the other side of the hypothalamus, the syringe removed, and the guide tubes repositioned 2 to 3 mm rostrally. The saporin conjugate was again injected on both sides of the hypothalamus using the protocol described previously. Thus, because of the length of the ARC, 2 bilateral injections of saporin were made approximately equidistance from the infundibular recess. After the final injection, the syringe and guide tubes were removed, the surface of the brain covered with gel foam, and the skin sutured. In 1 case (to test the Kiss-SAP conjugate), unilateral injections were made in the preoptic area (POA), using this same procedure but with only a single injection. For neurosurgery, animals received dexamethasone (Patterson Veterinary, Bessemer, AL) and antibiotics (gentamycin twice daily, Patterson Veterinary) before and 4 days after surgery; for analgesia, they were given buprenorphine SR (Zoetis) the day before surgery and gabapentin (McCracken Pharmacy, WV) twice daily for 4 days postsurgery. All animal work was approved by the West Virginia University Animal Care and Use Committee and followed National Institutes of Health guidelines for the use of animals in research.
Saporin Conjugates
NK3-SAP ([MePhe7]-NKB conjugated to saporin) and Blank-SAP (a nonspecific peptide conjugated to saporin) were purchased from Advanced Targeting Systems (San Diego, CA) at concentrations of 700 ng/µL and 1.4 µg/µL, respectively. Blank-SAP (32 kDa molecular weight) was diluted to 700 ng/µL with sterile PBS (0.1 M phosphate buffer with 0.9% sodium chloride). Kiss-SAP (kisspeptin54-SAP) was custom prepared by Advanced Targeting Systems by conjugating human kisspeptin peptide (54 aa version) to saporin at a concentration of 1.4 µg/µL (36 kDa molecular weight) and diluted to 700 ng/µL in PBS immediately before use. Because we were able to inject 2 ewes each day, a sufficient volume for injections in 2 ewes was aliquoted into single sterile vials and stored at −20 °C (if used within 1 month) or −80 °C (if stored more than a month). Aliquots were thawed the morning of the injection; they were kept at 4 °C for that day, except when being used for injections, and disposed of after injections were completed in the 2 ewes.
In preliminary work to test the effectiveness of Kiss-SAP, a single unilateral injection (1 µL of 700 ng/µL) of this conjugate was made in the POA of 3 ewes, and paraformaldehyde-fixed tissue collected 3 weeks later as described later. The contralateral side was used as control and either received no injections (n = 2) or Blank-SAP (1 µL of 700 ng/µL, n = 1). Coronal sections (45-µm thick) were cut on a sliding microtome (Leica SM2010R, Leica, Deer Park, IL) through the POA and processed for GnRH using immunohistochemistry (IHC) as described later and in previous papers (45). Kiss-SAP eliminated all GnRH-immunoreactive neurons in the vicinity of the injection, but not those on the contralateral side.
Experimental Protocols
Experiment 1. Effect of NK3-SAP and Kiss-SAP injections in the ARC on episodic LH secretion
Ewes were OVX and 10 to 14 days later, frequent blood samples were collected for control (preinjection) LH pulse patterns. Then NK3-SAP (n = 7), Kiss-SAP (n = 6), or Blank-SAP (n = 7) were injected into the ARC and frequent blood samples collected 2 and 3 weeks later. Animals were euthanized 1 to 3 days later and tissue collected to determine the effectiveness of the lesions. Because of the intensive nature of the neurosurgical procedures, this experiment was done in 3 replicates over 2 years (2019-2020), with 2 in anestrus and 1 in the breeding season. No differences in any of the experimental outcomes among the replicates within experimental groups were detected, and thus the data from all 3 replicates were combined.
Experiment 2. Time course of SAP injections on episodic LH secretion
To determine if there was any recovery of episodic LH secretion after saporin-induced lesions, we repeated the protocol described in experiment 1 but extended the blood collections to 2 months after injections. As in experiment 1, a control blood collection was taken 2 weeks after OVX (preinjection) and Kiss-SAP (n = 4) or Blank-SAP (n = 3) injected into the ARC. Frequent blood samples were collected at 2, 3, 4, 6, and 8 weeks after injections and tissue were collected 1 to 3 days after the last blood collections. Initially, an NK3-SAP-injected group (n = 5) was also included in this study. However, it became evident after the first 3 blood collections that the NK3-SAP injections had not affected LH pulse patterns as in experiment 1 (see Results), so blood collections were discontinued, and tissue collected from these ewes. Subsequent histological analyses confirmed that these injections did not lesion most of the KNDy neurons and several of the animals only had partial loss in 1 hemisphere, probably because of COVID-related delays and thus prolonged shipping time. Therefore, data from this cohort of NK3-SAP ewes were excluded and only LH data from the Blank-SAP and Kiss-SAP injected ewes were analyzed.
Experiment 3. Characterization of Kiss1R-containing neurons in the ovine ARC
In an initial exploratory study, sections of paraformaldehyde-fixed tissue collected for earlier experiments from 3 ovary-intact luteal phase and 3 acutely (2 weeks) OVX ewes were used to determine if 11 different genes were found in Kiss1R-containing ARC neurons, using HiPlex RNAScope fluorescent in situ hybridization. These images were also used to examine the colocalization of these transcripts in TAC3R-containing cells. These ewes received no other experimental treatments before tissue collection.
Tissue Collection and Processing
Animals were treated with heparin (20 000 U IV) 10 minutes before and immediately before euthanasia via overdose (8-16 mL IV) of Euthasol (Patterson Veterinary). When breathing stopped and there was no eye reflex, the carotids were cut and the head removed and perfused with 6 L of 4% paraformaldehyde in 0.1 M phosphate buffer with 0.1% sodium nitrite. A block of tissue including the hypothalamus and POA was removed and stored in paraformaldehyde solution overnight at 4 °C. Tissue was then stored in RNAse free 30% sucrose in PBS at 4 °C for at least 1 week. Brains were blocked and embedded in Tissue OCT (Fisher, Tissue Plus OCT, Cat No. 4585) within 1 month after collection, stored at −80 °C, and sectioned coronally using a Cryostat (Leica CM3050S) at 12 μm. Sections were mounted on plus-charged microscope slides (Fisherbrand Colorfrost Plus, Cat. No. 12-550-17) and stored at −80 °C until processing for RNAScope or HiPlex ISH and IHC for GnRH.
Histological Analyses
Fluorescent in situ hybridization RNAscope for saporin injections experiments
Fluorescent in situ hybridization was conducted using RNAscope Fluorescent Multiplex Reagent Kit V2 (Advanced Cell Diagnostics, REF No: 323100, Lot No: 2003230003230; Newark, CA) following the accompanying instructions (Document #UM 323100) with minor modifications. Unless specified, all reagents used were obtained from Advanced Cell Diagnostics and all incubations were at room temperature (RT). All in situ and amplification steps took place in a humidified oven and slides were washed in RNAscope 50 × Wash Buffer (Ref No: 320058) for 2 minutes between all steps. Slides were removed from the −80 °C freezer and washed in PBS for 5 minutes; they were then baked at 60 °C for 30 minutes, postfixed in chilled 4% paraformaldehyde at 4 °C for 15 minutes, incubated in a hydrogen peroxide solution (Ref No: 322335) for 10 minutes, treated with Target Retrieval solution for 5 minutes at 98 °C (Ref No: 322001), washed in 100% ethanol, and air dried. Next, a hydrophobic barrier was created around the tissue using an Immedge hydrophobic barrier pen (Vector, Cat No. H-4000, Burlingame, CA) and sections were treated with RNAscope Protease III at 40 °C for 30 minutes (Ref No: 322337).
Sections were next incubated with RNAscope target and control probes at 40 °C for 2 hours. RNAscope probes specifically targeting sheep (Ovis aries-Oa) Kiss1 mRNA (Table 1) were used in analysis of sections from all 3 groups, whereas those targeting sheep Kiss1R and TAC3R mRNA (Table 1) were used with tissue from the Kiss-SAP- and NK3-SAP-injected ewes, respectively; all 3 probes were used to analyze tissue from Blank-SAP-injected ewes. Hence, sections of Kiss-SAP and Blank-SAP animals were processed for Kiss1R and Kiss1 mRNA, whereas sections of NK3R-SAP and Blank-SAP animals were processed for TAC3R and Kiss1 mRNA. Positive and negative control probes (Table 1) were included in each experiment to confirm RNA integrity (positive control) and image capture settings (negative control) for all animals in each RNAscope run and experiment. In preliminary work, we also confirmed the presence of Kiss1R in the vast majority of POA GnRH neurons as a positive control for this transcript.
Table 1.
RNAscope multiplex V2 and HiPlex probes
| Gene name | Probe name | Reference number | Lot number |
|---|---|---|---|
| Multiplex RNAscope | |||
| Kiss1 | RNAscope Probe—Oa-KISS1-C3 | 497471-C3 | 22117A |
| Kiss1R | RNAscope Probe—Oa-KISS1R | 543361 | 22101A |
| TACR3 | RNAscope Probe—Oa-TACR3 | 550441 | 22210B |
| Negative control | 3-Plex negative control | 320871 | 21106A |
| Positive control | RNAscope Probe—Oa-POLR2A Probe | 516171 | 21134B |
| HiPlex | |||
| TACR3 | RNAscope Probe—Oa-TACR3-T1 | 550441-T1 | 20300A |
| OPRK1 | RNAscope Probe—Oa-OPRK1-T2 | 550431-T2 | 20307A |
| Kiss1R | RNAscope Probe—Oa-KISS1R-T3 | 543361-T3 | 20301A |
| SLC17A6 | RNAscope Probe—SLC17A6-T4 | 834681-T4 | 20307A |
| GAD1 | RNAscope Probe—GAD1-T5 | 834691-T5 | 20301A |
| ESR1 | RNAscope Probe—ESR1-T6 | 834731-T6 | 20300A |
| TH | RNAscope Probe—Oa-TH-T7 | 593491-T7 | 20300A |
| POMC | RNAscope Probe—Oa-POMC-T11 | 593481-T11 | 20307A |
| AGRP | RNAscope Probe—Oa-AGRP-T9 | 593521-T9 | 20300A |
| TAC3 | RNAscope Probe—Oa-TAC3-01-T10 | 481411-T10 | 20301A |
| TAC1 | RNAscope Probe—Oa-TAC1-T8 | 1000161-T8 | 20309B |
| Kiss1 | RNAscope Probe—Oa-KISS1-T12 | 497471-T12 | 20301A |
| Negative control | RNAscope Hiplex12 control Probe Negative | 324341 | 19197A |
| Positive control | RNAscope Hiplex12 control Probe—Oa-Pooled Positive | N/A | 20056A |
RNAscope Multiplex V2 and HiPlex probes specifically targeting sheep (Ovis aries-Oa) mRNA used for NK3-SAP or Kiss-SAP lesion verifications in experiments 1 and 2.
AGRP, agouti-related neuropeptide; N/A, not available.
Next, sections were incubated with 4 drops of RNAscope Multiplex FL v2 Amp 1 at 40 °C for 30 minutes (Ref No: 323101), followed by FL v2 Amp 2 at 40 °C for 30 minutes (Ref No: 323102), then FL v2 Amp 3 at 40 °C for 15 minutes (Ref No: 323103), followed by FL v2 HRP-C1 at 40 °C for 15 minutes (Ref No: 323104). Sections were next incubated with 120 μL per slide of Cy5 + TSA at 40 °C for 30 minutes (Akoya Biosciences, Catalog No: NEL745001KT, Marlborough, MA; 1:1500 concentration in RNAscope TSA buffer [Ref No: 322809)], then in RNAscope Multiplex FL v2 HRP Blocker at 40 °C for 15 minutes (4 drops, Ref No: 323107), followed by RNAscope Multiplex FL v2 HRP-C3 at 40 °C for 15 minutes (Ref No: 323106). Sections were then incubated with 120 µL Cy3 + TSA at 40 °C for 30 minutes (Akoya Biosciences, Ref No: NEL744001KT, 1:1500 in RNAscope TSA buffer), followed by 4 drops of RNAscope Multiplex FL v2 HRP Blocker at 40 °C for 15 minutes. Finally, slides were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) for 30 seconds (Ref No: 323108), and coverslipped with ProLong Gold Antifade Mountant (Invitrogen, Ref No: P36930, Eugene, OR) and stored at 4 °C. Sixteen sections for each animal were included for RNAscope, which was conducted in separate runs that each included the NK3- or Kiss-SAP- and blank-SAP-treated animals for which tissues and LH samples were collected simultaneously.
Immunohistochemistry for GnRH for saporin injection experiments
For immunofluorescence detection of GnRH cells and fibers in the POA and medial basal hypothalamus (MBH), 6 sections through the organum vasculosum of the laminae terminalis and POA, and 16 sections paralleling the RNAscope sections in the ARC were processed for IHC. Slides were washed 4 times in PBS at RT for 5 minutes each on a shaker between all incubation and blocking steps, and all steps were performed at RT. Sections were treated with 1% H202 (Fisher, Cat No. H323-500) in PBS for 10 minutes. Sections were blocked with antibody dilution solution (PBS + 0.4% Triton-X [Fisher, Cat No. BP151-100] + 4% Normal Goat Serum [Jackson Immunoresearch Cat No. 005-000-121, West Grove, PA]). Next, a hydrophobic barrier was created around the sections using an Immedge hydrophobic barrier pen. Sections were incubated with the primary antibody Rabbit anti-GnRH (Immunostar, Cat No. 20075, Hudson, WI, 1:400; RRID: AB_572248) in a humidity chamber for 17 hours. Next, sections were incubated with secondary antibody Goat anti-Rabbit DyLight 550 (Thermo Fisher Cat No. 84541, 1:100 in PBS; RRID:AB_10942173) for 30 minutes. Finally, sections were counterstained with DAPI for 30 seconds (Invitrogen, Cat No. D1306), and coverslipped with ProLong Gold Antifade Mountant (Invitrogen Cat No. P36930) and stored at 4 °C.
MultiPlex RNAscope Hiplex for characterization of ARC Kiss1R or TAC3R cells
Fluorescent in situ hybridization was conducted using RNAscope HiPlex V2 assay (Advanced Cell Diagnostics) (46), which permits the detection of 12 different RNA targets on the same tissue. This procedure was used to assess colocalization of 8 neurotransmitters and 3 receptors with ARC Kiss1R or TAC3R cells and followed the accompanying instructions (UM #324419; RNAscope TM HiPlex12 Reagent Kit v2 [488, 550, 650] Assay) with minor modifications. Slides containing coronal sections of hypothalamic tissue prepared on the cryostat were used for the assay, which was performed according to the manufacturer's instructions. Briefly, sections were fixed in 4% paraformaldehyde for 5 minutes, dehydrated in 50%, 70%, and 100% ethanol for 5 minutes each, and then treated with protease reagent at 99 °C. After tissue pretreatment, all RNA targets were simultaneously hybridized by pooling the ACD Biotechne HiPlex probes (Table 1) targeting sheep neurotransmitters glutamate decarboxylate (GAD1, Cat no. 834691), vesicular glutamate transporter (solute carrier family 17 member 6 [SLC17A6], Cat no. 834681), substance P/neurokinin A (TAC1, Cat no. 100016) and tyrosine hydroxide (TH, Cat no. 593491), the neuropeptides pro-opiomelanocortin (POMC, Cat no. 593481), agouti-related neuropeptide (Cat no. 593521), kisspeptin (KISS1, Cat no 497471), and neurokinin B (TAC3, Cat no. 481411), and the receptors estrogen receptor alpha (ESR1, Cat no. 834731), neurokinin B-NK3 receptor (TAC3R, Cat no. 550441), kappa opioid receptor (OPRK1, Cat no. 550431), and kiss1 receptor (Kiss1R, Cat no. 543361). After hybridization, all probes were amplified simultaneously. Cleavable versions of fluorophores AF488, Atto550, and Atto647 were next applied to sections to target 3 probes simultaneously. Sections were counterstained with DAPI, and slides were coverslipped using ProLong Gold Antifade Mountant to prepare for confocal microscopy with an Olympus FluoView FV 3000 laser scanning microscope (Center Valley, PA). The complete ARC was then captured using the 20 × objective and the FV3000 multiarea imaging tool with mosaic stitching. After imaging, samples were treated with a sodium citrate solution to gently remove coverslips, and an RNAscope cleaving solution was used to remove bound fluorophores. Sections were then incubated with the next round of fluorophores targeted to the next set of amplified probes and reimaged with confocal microscopy in the same brain region of the same brain sections. This process of cleaving fluorophores, applying new fluorophores, and imaging using confocal microscopy was repeated until all 12 RNA targets had been imaged (in total 4 times). Negative control probes were included in all rounds and sections and images were inspected to confirm that cleavage of fluorophores from previous rounds was complete. Even though all probes are equally sensitive, probes for the 4 rounds (Table 1, T1-12) were designed such that the least abundant transcripts were visualized in the first round (T1-T3), and the most abundant transcripts were visualized in the fourth and final round (T10-T12).
LH Radioimmunoassay
LH concentrations in plasma (50-200 μL) were measured in duplicate with a RIA using anti-ovine LH (RRID: AB_2629449) and other reagents provided by Dr. Al Parlow (Harbor-UCLA Medical Center, CA), as previously described (45). Values are expressed in terms of the reference NIH-LH-S12. The sensitivity limit of the LH radioimmunoassay averaged 0.05 ng/tube (equivalent to 0.25 ng/mL) and the inter- and intra-assay coefficients of variation were 6.5% and 3.4%, respectively.
Analyses
Lesion verification of TAC3R, Kiss1R and Kiss1 mRNA
Lesion verification was conducted in the rostral ARC (rARC), middle ARC (mARC), and caudal ARC (cARC) of NK3-SAP- (n = 4), Kiss-SAP- (n = 10), and Blank-SAP- (n = 10) treated ewes. In addition, verification of lesions was restricted to mARC in a subset of NK3-SAP animals (n = 3) in experiment 1. One Kiss-SAP animal in experiment 1 was excluded from analyses because of perfusion or sectioning artifacts that prohibited reliable analysis of cell counts. One Blank-SAP animal was accidentally not included in the RNAscope run for Kiss1R/Kiss1 analysis. For each animal, 16 sections through the ARC (4 sections rARC, 8 sections mARC, and 4 sections cARC), each separated by 144 µm, were included for RNAscope. Within 4 weeks after the RNAscope procedure was conducted, tissues were imaged using a Leica fluorescent microscope (Leica DMR) equipped with MicroBrightField Neurolucida (MicroBrightField Bioscience, version 2021.1.3, Williston, VT) to capture ARC images from each animal, using a 20 × objective. A 1-mm2 area of analysis in the ARC was delineated and cells were counted as positive for a transcript if 5 or more puncta of fluorescent labeling for a transcript were detected. Positive cell numbers in the right and left hemisphere for each section were counted and recorded separately. Right and left hemispheres throughout rARC, mARC, and cARC were compared within each animal using t-test to control for potential unilateral lesions. Once confirmed that significant differences between hemispheres were absent, cell averages/per hemisphere/per section were calculated for rARC (8 counts), mARC (16 counts), and cARC (8 counts) for each animal. In addition, cell counts for each animal were expressed as percentages of the average of Blank-SAP group for rARC, mARC, and cARC separately. Cell counts and percentages were used for statistical analyses by 2-way ANOVA to determine effects of saporin treatments (Blank-SAP vs NK3- or Kiss-SAP) in the 3 subareas within ARC (rARC, mARC, cARC). In addition, total ARC cell count averages per hemisphere were calculated for each animal based on all analyzed sections (32 counts), also expressed as percentages of the mean of the Blank-SAP control group and analyzed using t-test (Blank-SAP vs NK3-SAP or Kiss-SAP). All data sets passed tests of normality and equal variance and Holms-Sidak pairwise comparisons were conducted for ANOVAs. There were no differences between cohorts or experiments in any of the lesion analysis parameters.
DAPI cell counts
In each animal, 1 image in the middle of the mARC was selected for counting of all DAPI-labeled cells in a 0.2 × 0.2-mm area of analysis. Differences between Blank-SAP and NK3-SAP or Kiss-SAP groups were tested using t-test.
GnRH immunoreactivity
The numbers of cells immunoreactive for GnRH were counted on sections containing the organum vasculosum of the laminae terminalis (2-4 sections) and in sections containing the rostral and middle ARC (12 sections; no GnRH cells were detected in sections containing the cARC) in Blank-SAP and Kiss-SAP ewes and compared by t-test. Averages were calculated for the numbers of cells/section (containing both hemispheres) in POA and MBH. In addition, in the MBH, where few cells are detected, the total number of cells over all sections was also calculated (MBH: 12 sections). Group comparisons were conducted using t-tests. Qualitative analysis was conducted on fiber labeling throughout all sections and in the median eminence.
Pulsatile LH secretion
LH pulses were identified using the following criteria: (1) the peak was within 2 samples of the preceding nadir, (2) the peak was 2 SD greater than the preceding and subsequent nadirs, and (3) the amplitude was greater than the sensitivity of the assay. These criteria have been used successfully since 1980 (47) and reliably detect pulses in sheep, except at very fast frequencies (1.5-2 pulses/h) that only occur late in the follicular phase (48). Mean LH, LH pulse amplitude (peak minus preceding nadir), and interpulse interval (IPI) were calculated for each LH pulse pattern; LH pulse patterns with no or only 1 pulse occurred in some NK3-SAP-injected ewes and these were assigned IPI of 240 and 120 minutes, respectively. The coefficient of variation (CV) of IPIs (as a measure of the variability of episodic secretion) was calculated if at least 2 pulses occurred during the 4 hours of sampling; if the IPI before the first and after the last pulse were longer than other IPIs, they were included in the calculation of these CVs. No difference in LH pulse parameters were detected between the cohorts, so LH data from all ewes were used for determining the effects of these lesions in experiment 1. The effects of different saporin conjugates on time and treatment on the first 3 variables were statistically analyzed using 2-way ANOVA with repeated measure and post hoc comparisons carried out using the Holm-Sidak method. Because there were an insufficient number of LH pulses in the third week after injection of NK3-SAP to determine the CV of IPIs, 2 different data sets were analyzed, 1 that included all 3 treatments but only 2 time points (preinjection and 2 weeks after injection), and the other that included all 3 time points but excluded the data from the NK3-SAP-injected ewes. P < .05 was considered a significant difference.
RNAscope HiPlex analysis
Confocal files of ARC sections that underwent multiple rounds of in situ hybridization and imaging for 12 gene targets were converted into TIFF files and registered to each other using RNAscope HiPlex image registration software (Advanced Cell Diagnostics). CellProfiler software was used for unbiased quantification of targeted genes (49). To achieve this, we modified a previously published pipeline to enable automated quantification up to 12 gene targets per cell (50, 51). CellProfiler software identified cells via DAPI labeling, and RNA transcripts were identified when above background intensity, as determined by negative control sections, and a signal diameter equal or greater to 3 pixels and less than 30 pixels. Within the image, CellProfiler software quantified the number of overlying RNA transcripts for each gene within the border of each DAPI-labeled cell. A cell was deemed to express a target gene when 3 or more transcripts overlay DAPI. Using these parameters, the pipeline was configured to automatically quantify the number of cells in OVX and luteal ewes expressing each target gene, the percentage of Kiss1R-expressing or Tac3R-expressing cells that are colocalized with other gene targets, and the percentage of other gene targets that colocalize Kiss1R or TAC3R.
Results
Lesion Verification
Effects of saporin injections on TAC3R, Kiss1R, and Kiss1/KNDy neurons
Effects of NK3-SAP
Verification of effects of NK3-SAP was conducted in NK3-SAP (n = 4) and Blank-SAP (n = 10) animals throughout the entire ARC and NK3-SAP (n = 3) animals in mARC (Figs. 1 and 2, Tables 2 and 3). In Blank-SAP control animals, TAC3R-containing cells were located throughout the ARC without significant differences within the rostrocaudal continuum (Fig. 2D, Table 3). Moreover, TAC3R was expressed in 91 ± 3% of Kiss1/KNDy cells, replicating our previous findings using immunostaining (52). Coexpression of TAC3R in KNDy cells was evident throughout the ARC, averaging 93.7 ± 2.3% (rARC), 91.3 ± 2.8%, and 87.6 ± 4.3% (cARC) of Kiss1-expressing cells. Conversely, fewer TAC3R-expressing cells coexpressed Kiss1 (60.2 ± 4.2%). However, significant differences in numbers of Kiss1-expressing cells were detected when comparing rostrocaudal areas of ARC, with lowest numbers of Kiss1-expressing cells in rARC (Fig. 2E, Table 3), as has been previously described (7). Therefore, in rARC, only 27.8 ± 4.9% of TAC3R-cells coexpressed Kiss1.
Figure 1.
NK3-SAP injections lesion almost all KNDy cells. Images show RNAscope fluorescent ISH for TACR3R (red), Kiss1 (green), and DAPI (blue) in representative animals from NK3-SAP and Blank-SAP groups. A and E show low-magnification view of mARC in Blank-SAP (A) and NK3-SAP ewes (E), with squares showing locations of images in B-D and F-H. Scale bar in D depicts 50 µm (B-D, F-H) or 500 µm (A, E).
Figure 2.
Quantitative analysis of NK3-SAP lesions. NK3-SAP (gray bars) significantly reduced numbers (#; numbers of cells/per hemisection) of TAC3R (A) or Kiss1 cells (B) and expression of Kiss1 as percentages of the Blank-SAP average (C), compared with Blank-SAP control (white bars). These effects were noted throughout the rostrocaudal ARC (D, E; rostral: rARC, middle: mARC, caudal: cARC). Data are presented as group mean ± SEM with data for each animal shown as a circle (open: Blank-SAP; filled: NK3-SAP). *Statistically significant difference from Blank-SAP controls. #Statistically significant difference from rARC within the Blank-SAP group. For statistical information, see Table 3.
Table 2.
Percentages of Tac3R or Kiss1R, and Kiss1 cells after NK3-SAP or Kiss-SAP lesions
| Percentages (%) of Blank-SAP values | lesions | rARC | mARC | cARC | Total ARC |
|---|---|---|---|---|---|
| TAC3R cells | NK3-SAP | 17.1 ± 14.2 | 8.5 ± 6.3a | 15.4 ± 10.0 | 10.9 ± 7.8a |
| Blank-SAP | 100 ± 36.9 | 100 ± 21.3 | 100 ± 16.5 | 100 ± 18.1 | |
| Kiss1 cells | NK3-SAP | 6.6 ± 4.9a | 1.9 ± 1.0a | 5.3 ± 3.0a | 2.9 ± 1.5a |
| Blank-SAP | 100 ± 24.4 | 100 ± 13.7 | 100 ± 14.5 | 100 ± 9.3 | |
| Kiss1R cells | Kiss-SAP | 36 ± 6.5a | 27.5 ± 3.2a | 51.4 ± 6.6a | 35.4 ± 2.4a |
| Blank-SAP | 100 ± 15.4 | 100 ± 13.9 | 100 ± 10.5 | 100 ± 10.4 | |
| Kiss1 cells | Kiss-SAP | 51.6 ± 10.5 | 81.1 ± 13.4 | 113.5 ± 19.1 | 88.7 ± 13.0 |
| Blank-SAP | 100 ± 29.7 | 100 ± 17.2 | 100 ± 21.6 | 100 ± 14.8 |
NK3-SAP lesions significantly reduced TAC3R and Kiss1 cells, while Kiss-SAP lesions significantly reduced Kiss1R cells, but not Kiss1 cells. Data (mean ± SEM) are expressed as percentages of the average of Blank-SAP control groups.
a Statistically significant difference from Blank-SAP controls.
Table 3.
Statistical comparisons NK3-SAP lesion confirmation in ARC subregions
| Analysis | Factor 1: Blank- vs NK3-SAP | Factor2: r-m-cARC | Interaction | Pairwise comparisons Factor 1 |
Pairwise comparisons Factor 2 |
|---|---|---|---|---|---|
| # TAC3R cells | F(1,36) = 16.66; P = .0002a | F(2,36) = 2.86; P = .07 NS | F(2,36) = 2.15; P = .13 NS | rARC: P = .43 NS mARC: P = .007a cARC: P = .014a |
|
| TAC3R % of Blank-SAP | F(1,36) = 11.98; P = .0014a | F(2,36) = 0.011; P = .99 NS | F(2,36) = 0.011; P = .99 NS | rARC: P = .063 NS mARC: P = .041a cARC: P = .058 NS |
|
| # Kiss1 cells | F(1,36) = 38.5; P < .0001a | F(2,36) = 8.43; P = .001b | F(2,36) = 7.75; P = .0016a | rARC: 0.60 NS mARC: P < .001a cARC: P = .0002a |
In Blank-SAP: r-m: P < .001b r-c: P < .001b m-c: P = .0367b In NK3-SAP: NS |
| Kiss1% of Blank-SAP | F(1,36) = 31.24; P = < .0001a | F(2,36) = 0.007; P = .99 NS | F(2,36) = 0.007; P = .99 NS | rARC: P = .0032a mARC: P = .0021a cARC: P = .0028a |
Two-way ANOVA and Holm-Sidak pairwise comparisons for data presented in Fig. 2.
a Statistically significant difference between blank- and saporin-treated groups.
b Statistically significant difference between ARC regions within the blank or saporin groups.
As expected based on previous work with NK3-SAP in sheep (41), injection of NK3-SAP eliminated approximately 90% of ARC TAC3R/NK3R-containing cells (10.9 ± 7.8% of Blank-SAP values; P = .011; Figs. 1 and 2, Table 2). TAC3R cells were lesioned throughout the ARC (Fig. 2D) and averaged 17.1 ± 14.2% (rARC), 8.5 ± 6.3% (mARC; P = .022), and 15.4 ± 10.0% (cARC; P = .011) of Blank-SAP values (Table 2). Statistical significance was not reached in rARC because of lower numbers of TAC3R-positive cells in this part of the ARC, leading to high variability in the data (Fig. 2). Moreover, the NK3-SAP injections lesioned virtually all KNDy cells throughout ARC (2.9 ± 1.5% of Blank-SAP values; P < .0001), based on Kiss1 mRNA expression (Figs. 1 and 2B, 2C, 2E; Table 2). Kiss1 cells were lesioned throughout the ARC and averaged 6.6 ± 4.9% (rARC; P = .036), 1.9 ± 1.0% (mARC; P = .0008), and 5.3 ± 2.9% (cARC; P < .0001) of Blank-SAP values (Table 2). There was little variability in lesion effectiveness across ewes, with the number of Kiss1 neurons in any region ranging from 0 to 4 cells per section, per hemisphere (0.0%-11.5% of Blank-SAP values). In the additional cohort of 3 ewes in experiment 1, lesions were similar, resulting in 0.2% mARC Kiss1 cells and 0.13% mARC TAC3R of Blank-SAP control values. As noted previously, the NK3-SAP injections in experiment 2 were ineffective and thus analyzed separately. Indeed, lesion confirmation showed that in 2 ewes, there was little or no decrease in KNDy cell number (87% and 64% of Blank-SAP controls), whereas the other 3 had partial or mostly unilateral decreases (25%, 30%, and 45% of Blank-SAP controls). Finally, NK3-SAP injections did not cause overall cell loss because there were no effects on DAPI-labeled cells (73 ± 15.1 and 72 ± 8.3 cells/area of analysis in NK3-SAP and Blank-SAP animals, respectively; P = .94), indicative of the cell specificity of the NK3-SAP lesions.
Effects of Kiss-SAP
Verification of effects of Kiss-SAP was conducted in Kiss-SAP (n = 9) and Blank-SAP (n = 9) animals throughout the entire ARC (Figs. 3 and 4, Tables 2 and 4). In Blank-SAP control ewes, cells expressing Kiss1R were observed scattered throughout the ARC with lower numbers in rARC. Kiss1R mRNA was not expressed in KNDy cells because less than 1% of ARC Kiss1 cells expressed Kiss1R (0.64 ± 0.19%) and only 2% of ARC Kiss1R cells expressed Kiss1 (1.93 ± 0.43%). Injections of Kiss-SAP significantly decreased the numbers of Kiss1R cells throughout the ARC by approximately two-thirds to 35.4 ± 2.4% of Blank-SAP controls (Figs. 3 and 4A, 4D; Table 4) and averaged 35.9 ± 6.5% (rARC; P = .0015), 27.5 ± 3.2% (mARC; P = .0012), and 51.4 ± 6.6% (cARC; P = .0012) of Blank-SAP values (Table 2). In contrast, injection of Kiss-SAP had no significant effect on the number of KNDy neurons in any region of the ARC (Figs. 3 and 4B, 4C, 4E; Table 4) and Kiss-SAP-treated ewes had 85.7 ± 12.7% Kiss1-expressing neurons compared to Blank-SAP controls (P = .47).
Figure 3.
Kiss-SAP injections lesion majority of Kiss1R-expressing cells, but do not lesion KNDy cells. Images show RNAscope fluorescent ISH for Kiss1R (red), Kiss1 (green), and DAPI (blue) in representative animals from Kiss-SAP and Blank-SAP groups. A and E show low-magnification view of mARC in Blank-SAP (A) and Kiss-SAP ewes (E), with squares showing locations of images in B-D and F-H. Scale bar in D depicts 50 µm (B-D, F-H) or 500 µm (A, E).
Figure 4.
Quantitative analysis of Kiss-SAP lesions. Kiss-SAP (gray bars) significantly reduced numbers (#; numbers of cells/per hemisection) of Kiss1R (A) but not Kiss1 cells (B) or expression of Kiss1 as percentages of the Blank-SAP average (C), compared with Blank-SAP control (white bars). Reduced Kiss1R was noted throughout the rostrocaudal ARC (D; rostral: rARC, middle: mARC, caudal: cARC), whereas Kiss cells were not different from Blank-SAP control in all areas of the ARC (E) and significantly higher in mARC and cARC in both treatment groups compared with rARC. Data are presented as group mean ± SEM with data for each animal shown as a circle (open: Blank-SAP; filled: Kiss-SAP). *Statistically significant difference from Blank-SAP controls. #Statistically significant difference from rARC within the Blank-SAP group. For statistical information, see Table 4.
Table 4.
Statistical comparisons Kiss-SAP lesion confirmation in ARC subregions
| Analysis | Factor 1: Blank- vs Kiss-SAP | Factor 2: r-m-cARC | Interaction | Pairwise comparisons Factor 1 |
Pairwise comparisons Factor 2 |
|---|---|---|---|---|---|
| # Kiss1R cells | F(1,48) = 54.98; P < .0001a | F(2,48) = 14.16; P < .0001b | F(2,48) = 3.04; P = .057 NS | rARC: P = .012a mARC: P < .0001a cARC: P = .0001a |
In Blank-SAP: r-m: P = .0002b r-c: P = .0002b m-c: P = ..91 NS In Kiss-SAP: r-m: P = .41 NS r-c: P = .017b m-c: P = .087 NS |
| Kiss1R % of Blank-SAP | F(1,48) = 54.02; P < .0001a | F(2,48) = 0.69; P = .51 NS | F(2,48) = 0.69; P = .51 NS | rARC: P < .0001a mARC: P < .0001a cARC: P = .0016a |
|
| # Kiss1 cells | F(1,48) = 0.37; P = .55 NS | F(2,48) = 25.1; P < .0001b | F(2,48) = 0.69; P = .51 NS | In Blank-SAP: r-m: P < .0001b r-c: P = .011b m-c: P = .022b In Kiss-SAP: r-m: P = .0001b r-c: P = .0008b m-c: P = .48 NS |
|
| Kiss1% of Blank-SAP | F(1,48) = 1.25; P = .30 NS | F(2,48) = 1.25; P = .30 NS | F(2,48) = 1.26; P = .27 NS |
Two-way ANOVA and Holm-Sidak pairwise comparisons for data presented in Fig. 4.
a Statistically significant difference between saporin-treated groups.
b Statistically significant difference between ARC regions.
Kiss-SAP lesions did not reduce overall numbers of DAPI-stained cells in mARC (75.5 ± 8.8 and 79.2 ± 10.2 cells/area of analysis in Kiss-SAP and Blank-SAP animals, respectively; P = .84). Moreover, Kiss-SAP injections had no significant effects on numbers of GnRH cells in either the POA or MBH (Table 5, Fig. 5B and 5E). As expected, there were few GnRH cell bodies in the MBH, with many sections having none. To ensure that the absence of any effect of the Kiss-SAP injections was not due to so few GnRH cells/section, we also compared the total number of GnRH cells/ewe; again, Kiss-SAP had no significant effect (Table 5). Consistent with these data on GnRH cell bodies, there were no obvious differences in immunoreactivity, localization, or density of GnRH fibers in the MBH or median eminence between these 2 treatments (Fig. 5).
Table 5.
Lack of effects of Kiss-SAP injections on GnRH cell number in the POA and MBH
| Treatment | POA (AV cells/section) | MBH (AV cells/section) | MBH (sum of cells) |
|---|---|---|---|
| Blank-SAP | 18.0 ± 1.6 | 0.36 ± 0.05 | 4.0 ± 0.5 |
| Kiss-SAP | 16.1 ± 1.9 | 0.44 ± 0.06 | 5.1 ± 0.8 |
Analysis of GnRH-immunoreactive cells in POA and MBH of Kiss-SAP and Blank-SAP-injected ewes. Data (mean ± SEM) are expressed as average cells per section (AV cells/section) or sum of all cells in all sections included in analysis (sum of cells). No significant differences between groups were detected.
Figure 5.
Effect of Kiss-SAP injections on GnRH immunoreactive cells and axons in MBH. A to C show a GnRH cell in ARC of Kiss-SAP-injected ewe and representative GnRH axons in the ME immediately below the ARC. Squares show areas of higher magnification in B and C of the GnRH neuron (arrow) and axon terminals. D shows GnRH axons at a similar anatomical level in the MBH of a Blank-SAP-injected ewe with a higher magnification of the area in square shown in F. In the same Blank-SAP ewe, E illustrates a representative cell. Scale bars depict 200 µm (A, D) or 50 µm (B, C, E, F).
Effects of Lesions
Experiment 1. Effects of saporin injections on LH pulse patterns
Injection of Blank-SAP had no obvious effect on LH pulse patterns, but both NK3-SAP and Kiss-SAP injections dramatically inhibited LH concentrations and LH pulse amplitude (Figs. 6 and 7). Pulses were seen in almost all ewes injected with Kiss-SAP but were only occasionally evident following NK3-SAP injections, particularly in week 3 (Fig. 6). Statistical analyses indicated a significant effect of time, treatment, and an interaction of the 2 on mean LH concentrations and LH pulse amplitude (Table 6), with values on weeks 2 and 3 after NK3-SAP and Kiss-SAP injections below preinjection values. The effect of NK3-SAP on LH concentrations was significantly greater than Kiss-SAP at 3 weeks, but not at 2 weeks, whereas there was no significant difference between the inhibitory effects of NK3-SAP and Kiss-SAP on LH pulse amplitude. In contrast, these 2 saporin conjugates had differential effects on IPI and the variability of pulse occurrence. There were again significant effects of time and treatment (Table 6), with NK3-SAP only increasing IPI and Kiss-SAP only increasing the CV of IPI (Fig. 7C and 7D). Note that we could not run a single complete analysis on the latter variable because CVs could not be determined for the effects of NK3-SAP in most ewes on week 3. However, a comparison of all 3 treatments for preinjection and 2 weeks postinjection indicated that Kiss-SAP produced a significant increase in CV, whereas NK3-SAP did not. Similarly, a comparison of Kiss-SAP and control injections at all 3 time points indicated that Kiss-SAP increased the variability of IPI at both posttreatment time points.
Figure 6.
Effect of lesioning ARC NK3R- or Kiss1R- containing cells on LH pulse patterns. Panels depict episodic LH secretion before (left), and 2 and 3 weeks after (middle and right, respectively) injections of Blank-SAP (top), NK3-SAP (middle), or Kiss-SAP (bottom). ^Peak of LH pulse.
Figure 7.
Effect of lesioning ARC NK3R, or Kiss1R-containing, cells on LH pulse parameters. Panels present mean (± SEM) LH concentration (A), LH pulse amplitude (B), interpulse interval (IPI) (C), coefficient of variation of IPI (D), before (open bars), 2 weeks (light gray bars), and 3 weeks (dark gray bars) after injection of Blank-SAP, NK3-SAP, or Kiss-SAP. Circles depict data from individual ewes. *Significantly different from corresponding Blank-SAP value. #Significantly different from preinjection value. ≠Significantly different from corresponding NK3-SAP value.
Table 6.
Statistical analyses of LH pulse parameters
| Variable | Time | Treatment | Interaction |
|---|---|---|---|
| Experiment 1 | |||
| LH Levels | F(2) = 36.3, P < .001 | F(2) = 15.3, P < .001 | F(4) = 10.1, P < .001 |
| Amplitude | F(2) = 36.4, P < .001 | F(2) = 24.1, P < .001 | F(4) = 7.1, P < .001 |
| IPI | F(2) = 5.27, P = .010 | F(2) = 6.57, P < .007 | F(4) = 5.52, P < .001 |
| CV of IPI pre- vs week 2 | F(1) = 11.9, P = .003 | F(2) = 4.31, P = .032 | F(2) = 2.36, P = .129 |
| CV of IPI Blank- vs Kiss-SAP | F(2) = 7.42, P = .003 | F(1) = 15.9, P = .002 | F(2) = 2.87, P = .078 |
| Experiment 2 | |||
| LH Levels | F(5) = 7.39, P = .001 | F(1) = 64.7, P = .001 | F(5) = 9.73, P < .001 |
| Amplitude | F(5) = 8.74, P < .001 | F(1) = 62.3, P < .001 | F(5) = 5.45, P = .002 |
| IPI | F(5) = 1.62, P = .2 | F(1) = 4.33, P = .11 | F(5) = .32, P = .89 |
| CV of IPI | F(5) = 2.45, P = .069 | F(1) = 9.88, P = .035 | F(5) = 1.23, P = .33 |
Experiment 2. Time course of SAP injections of episodic LH secretion
As illustrated in Fig. 8, the effects of Kiss-SAP injections in weeks 2 and 3 after injection were very similar to those in experiment 1: LH concentration and pulse amplitude were markedly reduced, IPI was not affected, and the CV of IPI increased significantly (Table 6). These effects largely lasted for the duration of the experiment, with LH concentrations and pulse amplitude being significantly lower than in controls in weeks 2, 4, 6, and 8 after injection, and the CV of IPI being significantly higher (Fig. 8). Interestingly, the inhibition of LH pulse amplitude by the Kiss-SAP lesions appeared to diminish over time and amplitude was significantly higher (P = .004, critical level = .005 using pairwise multiple comparisons) at 8 weeks, than at 2 weeks, postinjection. Based on comparison of Kiss1R expression in these ewes with its expression in experiment 1, which used tissue collected 3 weeks after injection, the injection-induced decrease in Kiss1R-containing cells was similar between these 2 time points (3 weeks: 3.2 ± 0.5 cells/section vs 8 weeks: 3.9 ± 0.5 cells/section).
Figure 8.
Time course of the effects of lesioning Kiss1R neurons on LH pulse parameters. Panels present mean (± SEM) LH concentration (A), LH pulse amplitude (B), interpulse interval (IPI) (C), coefficient of variation of IPI (D), before and 2, 3, 4, 6, and 8 weeks after injection of Blank-SAP (open circles) or Kiss-SAP (solid circles). *Significantly different from corresponding Blank-SAP value. #Significantly different from preinjection value. ǂSignificantly different from Kiss-SAP value at 2 weeks. There was an overall significant effect of treatment on the CVs of IPI, but no pairwise comparisons reached significance.
Because there was no difference in Kiss1R expression in Kiss-SAP-injected ewes between experiments 1 and 2, we combined these data and the data on LH pulse parameters at week 3 postinjection from both experiments to determine if the number of Kiss1R-containing cells had a significant effect on either LH pulse amplitude or the variability of intervals between pulses. There was a significant (P = .005) negative effect of Kiss1R cell number on the CV of IPI and a significant (P = .002) positive effect on LH pulse amplitude (Fig. 9).
Figure 9.
Correlation of pulse parameters with Kiss1R expression. Panels present the linear regression of the variability of interpulse intervals (A) and LH pulse amplitude (B) at 3 weeks after injection with the mean number of Kiss1R-containing cells/section in the middle and caudal regions of the ARC.
Phenotype of Kiss1R Cells
Experiment 3. Characterization of Kiss1R-containing neurons in the ovine ARC
HiPlex analysis of 12 transcripts known to be expressed in sheep ARC was conducted in intact luteal phase and OVX ewes to take a first step toward identification of the neurochemical phenotype of ARC Kiss1R cells (Fig. 10). Table 7 lists the average numbers of neurons expressing 1 of the 12 transcripts included in this analysis. There were no significant differences in cells with Kiss1R mRNA between OVX and luteal-phase ewes, and coexpression of 11 different mRNAs with Kiss1R mRNA in the ovine ARC was based on 36 ± 6.4 (luteal) and 62 ± 23 (OVX) Kiss1R cells. Numbers of Kiss1R cells did not differ from several other transcripts in mARC, except were significantly lower than numbers of cells expressing OPRK1 (P < .0001), ESR1 (P < .0001), and GAD1 (P < .001), which were most abundant in the mARC. In replication of findings described previously, a small percentage of Kiss1R cells coexpressed Kiss1 (coexpression was noted in 3.1%-3.9% of Kiss1R cells and 3.9%-1.3% of Kiss1 cells in OVX and luteal ewes, respectively (Table 7)). The highest colocalization was seen with GABAergic (70%-66% of Kiss1R cells), and glutamatergic (24%-33% of Kiss1R cells) markers (Fig. 10B). Kiss1R cells coexpressed estrogen receptors (ESR1 47%-50%), KOR (OPRK1 29%-48%), and NK3R (TAC3R 14%-22%). Finally, smaller portions of Kiss1R cells coexpressed tachykinin-mRNA transcripts for NKB (TAC3 15-12%) and substance P/neurokinin A (TAC1 11%-9%), and for transcripts encoding TH (21%-15%), agouti-related neuropeptide (9%-10%), or POMC (4-2%) (Fig. 10B, Table 7). Conversely, within each of the cells expressing 1 of these 11 mRNAs, coexpression with Kiss1R mRNA was restricted to a fairly small subpopulation (Table 7). Finally, 9.5% (OVX)–17.7% (luteal) of Kiss1R cells did not coexpress the 11 transcripts included in this analysis. The same analysis was also conducted for TAC3R-expressing cells, confirming high coexpression of TAC3R with Kiss1, TAC3, and ESR1 (Table 7). A sizable portion of TAC3R cells also coexpressed GAD1 (25-40%), SLC17AC (29-48%), and OPRK1 (65-66%). TAC3R cells were almost fully characterized for coexpression in this analysis and only 1.2% (OVX)–1.1% (luteal) did not coexpress any of the 11 transcripts.
Figure 10.
RNAscope HiPlex analysis of coexpression of neurotransmitters and receptors with Kiss1R in mARC neurons. (A) Representative confocal composite image of the ARC showing 12 mRNA transcripts labeled with Hiplex RNAscope. (B) The mean (± SEM) percentage of Kiss1R-positive neurons that also contain mRNA for 1 of 11 other markers for neurotransmitters or receptors in OVX (gray bars) and luteal (black bars) groups (n = 3 each). (C) Higher power confocal image from the same section showing localization of Kiss1R mRNA (blue) with cells containing either GAD1 (orange) or SLC17A6 (salmon) mRNA. DAPI-labeled nuclei are gray. Note that because this was an exploratory study, with only 3 animals/treatment, a more complete experiment is needed to determine if there is any effect of steroids on the colocalization of these transcripts in ovine Kiss1R cells.
Table 7.
RNAscope HiPlex analysis of ARC neurons
| Cell no. | % KISS1R with | % with KISS1R | % TAC3R with | % with TAC3R | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Target | OVX | Luteal | OVX | Lutealal | OVX | Lutealal | OVX | Lutealal | OVX | Lutealal |
| AGRP | 102 ± 40 | 65.7 ± 32.6 | 9.9 ± 4.4 | 46.6 ± 5.7 | 4.7 ± 1.0 | 4.3 ± 2.3 | 3.7 ± 1.1 | 5.2 ± 3.6 | 7.5 ± 1.5 | 6.4 ± 0.4 |
| ESR1 | 627 ± 76.9 | 527.3 ± 67.2 | 49. ± 0.8 | 46.6 ± 6.6 | 5 ± 1.9 | 3.5 ± 1.2 | 63.4 ± 9.7 | 88.4 ± 5.6 | 18.5 ± 3 | 18.7 ± 4.7 |
| GAD1 | 923.8 ± 73.8 | 665 ± 44.2a | 70.4 ± 3.9 | 66.1 ± 10.6 | 4.7 ± 1.9 | 3.7 ± 1.0 | 40.4 ± 8.9 | 24.7 ± 9.0 | 7.9 ± 1.6 | 3.4 ± 0.6 |
| KISS1 | 82.8 ± 5.6 | 48.5 ± 19 | 3.9 ± 2.2 | 3.1 ± 2.2 | 3.9 ± 2.6 | 1.3 ± 0.9 | 37.6 ± 6.7 | 33.1 ± 11.2 | 79.7 ± 7.4 | 80.2 ± 2.9 |
| KISS1R | 61.5 ± 22.5 | 36 ± 6.4 | N/A | N/A | N/A | N/A | 7.4 ± 3.3 | 7.3 ± 5.0 | 21.5 ± 4.5 | 14.4 ± 4 |
| OPRK1 | 571.8 ± 36.5 | 371.3 ± 12.6a | 48 ± 4.5 | 28.9 ± 10.1 | 5 ± 1.7 | 3.1 ± 1.4 | 64.7 ± 8.4 | 65.7 ± 10.4 | 20.3 ± 2.7 | 18.8 ± 4.7 |
| POMC | 43.5 ± 3.1 | 51.8 ± 10.4 | 1.8 ± 0.1 | 3.6 ± 1.4 | 3.1 ± 0.6 | 2 ± 0.4 | 0.9 ± 0.3 | 0.7 ± 0.4 | 3.7 ± 1.4 | 1.6 ± 1 |
| SLC17A6 | 313.7 ± 40.6 | 174.3 ± 6.9a | 32.6 ± 2.7 | 23.6 ± 8.3 | 7.3 ± 3.6 | 5.4 ± 2.6 | 48 ± 5.0 | 29.7 ± 8.3 | 29.2 ± 3.4 | 16.1 ± 0.3 |
| TAC1 | 60.8 ± 18.1 | 27.8 ± 4.1 | 8.5 ± 1.7 | 11.1 ± 5.0 | 9.3 ± 5.0 | 15.1 ± 6.9 | 7.3 ± 3.6 | 8.1 ± 6.0 | 20.6 ± 5.9 | 18.1 ± 8.2 |
| TAC3 | 111.3 ± 11.6 | 91.3 ± 111.3 | 12.6 ± 4.1 | 15.1 ± 2.8 | 6.4 ± 2.5 | 10.1 ± 7.3 | 48.9 ± 6.6 | 63.8 ± 7.8 | 78.5 ± 5.9 | 20.6 ± 5.4 |
| TAC3R | 180.5 ± 11 | 108.7 ± 26.9 | 21.5 ± 4.5 | 14.4 ± 4.0 | 7.4 ± 3.3 | 7.3 ± 5.0 | N/A | N/A | N/A | N/A |
| TH | 53.2 ± 31.9 | 39.2 ± 17.8 | 15.4 ± 7.3 | 20.8 ± 6.6 | 19.3 ± 1.6 | 20.7 ± 2.6 | 7.5 ± 4.8 | 9.9 ± 7.6 | 42.3 ± 22.9 | 14.7 ± 3.7 |
Average numbers (cell no.) and percentages (%) of cells expressing transcripts included in RNAscope HiPlex analysis (mean ± SEM). Two-way ANOVA analyses on data for cell no. revealed that there was an overall effect of OVX (OVX vs luteal; F(1,52) = 26.18; P < .0001), transcripts (between the 12 transcripts; F(12,52) = 566.2; P < .0001), and an interaction (F(12,52) = 1.99; P = .0447). Pairwise comparisons were only conducted for OVX vs luteal within each transcript. Two-way ANOVA analyses on data for percentages (%) failed to show significant effect of OVX, and only an overall effect of transcripts was detected. Therefore, pairwise comparisons between OVX and luteal within each transcript were not performed on any of the percentage data.
a Statistically significant difference from OVX.
Discussion
This study demonstrated that lesioning KNDy and other ARC NK3R-containing neurons produces a different effect on episodic LH secretion than lesions of ARC Kiss1R neurons. Although both types of lesions decreased LH pulse amplitude, disrupting KNDy neurons also inhibited pulse frequency without affecting how regular the remaining pulses occurred. In contrast, lesions of Kiss1R-containing cells did not alter LH pulse frequency but did produce a more irregular pattern of these LH pulses.
The effects of the NK3-saporin conjugate confirm and extend a report that similar injections inhibited mean LH concentrations in OVX rats (42) by demonstrating an inhibition of both LH pulse amplitude and pulse frequency. These results add further support to the growing body of evidence that KNDy neurons comprise an integral component of the GnRH pulse generator (11, 13, 18, 53). The most compelling functional evidence for this comes from cell-specific transgenic studies in mice (18) and rats (53), but studies in larger animals (ie, sheep and goats) have necessarily relied on less direct anatomical and pharmacological approaches (44). Thus, this is the first report in sheep that lesions of KNDy neurons disrupt episodic LH secretion. One caveat to interpreting these functional data is that NK3-SAP also lesioned non-KNDy NK3R-containing neurons in the ARC. However, based on the other data implicating KNDy neurons in generation of GnRH and LH pulses in sheep and goats, the simplest explanation for these results is that ablation of the KNDy network was the key subpopulation responsible for suppression of LH pulses in these ewes. Injections of NK3-SAP in experiment 2 failed to alter LH pulse patterns because they produced limited destruction of KNDy neurons, ranging from 25% to 87% of Kiss1-containing neurons remaining. These data thus support the conclusion that 30%, and possibly less, of the KNDy network is sufficient for normal episodic LH secretion, a number similar to that in rats (53).
Injections of Kiss-SAP that lesioned most Kiss1R-containing cells in the ARC, without affecting KNDy cell number, had 2 significant effects on episodic LH secretion, inhibiting LH pulse amplitude and producing an irregular pulse pattern. The simplest explanation for the decrease in amplitude, that these injections also lesioned GnRH cells, can be ruled out because there were no differences in GnRH cell bodies or fibers between Kiss-SAP- and Blank-SAP-injected ewes. The lack of effect of the Kiss-SAP injections on GnRH expression is likely because of (1) Kiss-SAP not being taken up by GnRH axons or terminals and transported back to cell bodies in the MBH and POA and (2) Kiss-SAP not diffusing far enough laterally where most GnRH cell bodies are located in the ovine MBH (54). It is possible that these conjugates did produce small changes in GnRH neurons that were not detected with IHC, but it is unlikely that a small decrease in GnRH would produce the dramatic inhibition of LH pulse amplitude observed (55). It is somewhat surprising that the decrease in LH pulse amplitude produced by the Kiss-SAP injections was similar to that produced by the NK3-SAP injections (Figs. 6 and 7) because approximately one-third of ARC Kiss1R-positive neurons remained, a level of loss that if it occurred for KNDy neurons would be compatible with normal LH pulse amplitude. This might reflect that KNDy neurons function as a network, and as such may require more extensive destruction to affect its output to GnRH neurons. Alternatively, the number, rather than the percentage, of neurons may be the critical variable because these values were similar for the remaining KNDy (1.3 neurons/section) and Kiss1R (4 neurons/section) cells following the lesions.
It is also interesting that there was some recovery of LH pulse amplitude 8 weeks after injection of Kiss-SAP (experiment 2). Because, as expected saporin produced permanent lesions, this was not caused by a parallel increase in the number of Kiss1R-expressing cells. Thus, it most likely reflects some compensation by other systems that can stimulate LH pulse amplitude. This is not surprising given the extensive redundancies in the neuroendocrine systems controlling GnRH secretion but does indicate the need for caution in interpreting the lack of effect of embryonic genetic knockouts of ARC Kiss1R neurons or their contents. Identifying where this compensation occurs requires further work, but it is unlikely to be at the pituitary because the decrease in GnRH release caused by the lesions would be expected to decrease, not increase, pituitary GnRH receptors (56). Of the other 2 possibilities, GnRH or KNDy neurons, the latter seems more likely because neural plasticity often involves compensatory changes for the loss of synaptic inputs by increasing synapses from other afferents.
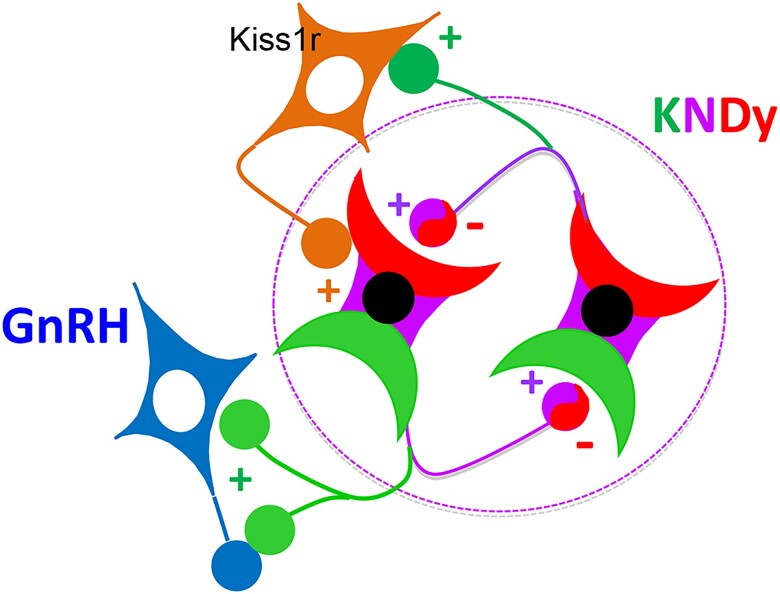
The other dramatic effect of the Kiss-SAP injections was a significant increase in the variability of IPIs in each ewe. Interestingly, this increase, which reflects a loss of the regular, hourly, release of LH normally seen in these OVX ewes, was only seen with the Kiss-SAP, and not the NK3-SAP, lesions. One simple explanation for this loss of regular pulses is that the amplitude had decreased to such an extent that small pulses were not detected with the statistical method used to identify each pulse. Two observations, however, argue strongly against this possibility. First, the inability to detect small LH pulses that are present should increase IPI following Kiss-SAP lesions, but this was not seen. Second, no significant increase in variability was observed 2 weeks after NK3-SAP injection, although this conjugate produced slightly lower LH pulse amplitudes than did Kiss-SAP (Fig. 7). Thus, we conclude that ARC Kiss1R neurons normally contribute to both the amplitude and regularity of episodic LH secretion in ewes. Moreover, these effects appear to be proportional to the number of Kiss1R-neurons in the ARC (Fig. 9). In contrast, disruption of these Kiss1R-containing neurons had no effect on LH pulse frequency. Thus, it appears that they are not a critical component of the GnRH pulse generator. Based on these results, we propose that ARC Kiss1R neurons are part of a positive feedback circuit that receives input from kisspeptin released from KNDy neurons during a pulse and feeds back to reinforce the amplitude and regular release of GnRH/LH pulses (Fig. 11).
Figure 11.
Proposed model for role of ARC Kiss1R neurons in the generation of GnRH pulses in ewes. Kisspeptin (green) released from the KNDy neurons during a pulse stimulate ARC Kiss1R cells, which in turn release a stimulatory neurotransmitter on to KNDy neurons. This neurotransmitter increases release of NKB and/or kisspeptin from the KNDy neural network (within circle), increasing GnRH pulse amplitude. It also stimulates dynorphin release, strengthening the signal that terminates each pulse, which might be important for the regular occurrence of pulses. Dynorphin would also inhibit Kiss1R cells that also contain KOR at pulse termination, ending this stimulatory input.
One key question that arises from this model is the neurotransmitters and receptors (besides Kiss1R) found in these ARC neurons. Based on the screen of colocalization of gene markers for 8 different neurotransmitters/neuropeptides, the most likely candidates are GABA and glutamate (Fig. 10). Only a low percentage of Kiss1R neurons, ranging from 2.7% for POMC to 18% for TH, also contained mRNA for one of the other 6 neurotransmitters/neuropeptides. Because the model proposes that these Kiss1R neurons stimulate the KNDy neural network, glutamate could reasonably be considered the key neurotransmitter. The report that local administration of MK801, an antagonist to the NMDA glutamate receptor, had no effect on LH pulse patterns in ewes (33) does not rule out a role for glutamate because the AMPA receptor may be involved, as it appears to be in mice (57). Alternatively, GABA could be the important neurotransmitter because GABA can have stimulatory or inhibitory effects via the GABA-A receptor, depending on which Cl− transporter is present within a neuron (58). NKCC1 pumps Cl− into the cell so Cl− flows out of cells containing NKCC1 when the GABA-A channel opens and thus depolarizes them. In contrast, KCC2 pumps Cl− out so that opening the GABA-A channel in neurons containing KCC2 hyperpolarizes the cell. Interestingly, expression of these transporters in KNDy neurons is heterogeneous, with 28% containing only NKCC1, 16% containing only KCC2, and 45% expressing both (59). Thus, GABA would likely be stimulatory to a significant portion of KNDy neurons, which might be sufficient to reinforce ongoing network activity. Although there is less information available on the neurotransmitters found in rodent ARC Kiss1R cells, the available data strongly indicate some significant species differences, which raises the possibility that these ARC Kiss1R neurons perform different functions in these species. Specifically, more than 60% of ARC Kiss1R neurons in rats coexpress POMC (22), which contrasts with the low expression of POMC in ovine ARC Kiss1R neurons. In contrast, a similar percentage of them contain TH in ewes (18%) and rats (11%).
The other genes found to highly colocalize with Kiss1R in the ARC are the genes for KOR and ERα. The relatively high expression of ERS1 in this subpopulation of Kiss1R-containing cells is in marked contrast with the other major subpopulation of hypothalamic cells that express Kiss1R (ie, GnRH neurons (60)). This expression raises the possibility that these ARC Kiss1R neurons may help mediate the feedback actions of estradiol. In light of the dramatic effect of Kiss-SAP injections on LH pulse amplitude, estradiol inhibition of this population could be a mechanism by which this steroid inhibits GnRH and LH pulse amplitude in sheep (61). The report that estradiol inhibits Kiss1R expression in the rat ARC (62) is consistent with this possibility. Although these experiments were not designed to test the role of dynorphin-KOR signaling in pulse generation, the relatively high coexpression of the gene for KOR (OPRK1) in Kiss1R neurons raises the possibility that KOR inhibits these Kiss1R neurons. If so, this would complement the inhibitory actions of dynorphin on both KNDy and GnRH cells at pulse termination in sheep (38). In contrast, based on the relatively low expression TAC3R in Kiss1R containing cells, it seems unlikely that NKB release from KNDy neurons plays an important role in this potential positive feedback circuit.
The distribution of these genes in TAC3R cells is consistent with previous IHC data on the expression of NK3R (52): almost all ovine Kiss1 neurons contained TAC3R, but only 35% to 40% of TAC3R cells contained Kiss1. It is also interesting to note that, in contrast to the limited colocalization of these genes in Kiss1R cells, 5 neurotransmitter gene markers (and 2 receptor genes) were found in at least 30% of TAC3R cells (Table 7). Some of this high expression represents overlap of these genes in the same cells (eg, KISS1, TAC3, SLC17A6, and OPRK1 in KNDy neurons), but most of these genes are also found in non-KNDy cells. This likely reflects the more extensive effects of NKB on physiological systems controlled by hypothalamic neurons than those of kisspeptin. However, it raises the possibility that lesions of non-KNDy neurons that contain NK3R may contribute to the inhibition of LH pulse frequency and amplitude in this study. Given the wealth of data pointing to KNDy neurons as the GnRH pulse generator (see Introduction), it seems most likely that other NK3R-containing neurons would play only supportive roles but the resolution of this issue awaits further work.
Two lines of evidence from transgenic mice are not consistent with the proposed role for ARC Kiss1R cells. First, selective reinsertion of Kiss1R into only GnRH neurons is sufficient to restore fertility in mice with a global Kiss1R knockout (63). However, this report does not completely rule out a role for other Kiss1R-containing neurons in maintaining normal GnRH/LH levels, because episodic LH secretion was not monitored. Specifically, lower amplitude GnRH pulses could be occurring in these mice because a small percentage of GnRH neurons is sufficient to maintain tonic LH secretion in this species (55). Second, selective knock out of the kisspeptin gene in KNDy neurons did not affect the frequent bursts in intracellular calcium levels in the KNDy network that correlate with LH pulses and are thought to be a manifestation of the GnRH pulse generator (64). Thus, it is likely that this positive feedback circuit does not play an important role in maintaining normal episodic GnRH secretion in mice.
In summary, these results provide the first functional data that KNDy neurons are a key component of the GnRH pulse generator in sheep. They also identify a population of ovine ARC Kiss1R neurons as important modulators of episodic GnRH/LH secretion that form the basis of a possible positive feedback circuit within the ARC that strengthens the activity of the GnRH pulse generator. The hypothetical existence of this circuit in sheep provides a simple explanation for the report that infusion of kisspeptin restored LH pulse patterns in OVX ewes in which pulses had been blocked with an NK3R antagonist (40). The kisspeptin infusion would activate this external circuit, which, in turn, would stimulate KNDy neurons, replacing the need for their activation by NKB. This proposed positive feedback circuit may also occur in humans because an IV injection of kisspeptin reset the GnRH pulse generator in normal men (29) and kisspeptin infusions induce pulsatile LH secretion in patients without functional NKB receptors (28). In the absence of NK3R in these patients, the KNDy network would be present but there would be no signal to initiate a pulse and thus no stimulatory neurotransmitter release from nearby Kiss1R-containing neurons. Under these circumstances, exogenous kisspeptin infusion would stimulate the ARC Kiss1R cells so they would release their neurotransmitter, activating KNDy neurons. This would initiate kisspeptin release from KNDy cells onto Kiss1R cells, further increasing their activity, so the circuit between KNDy and Kiss1R neurons would function as a positive feedback loop, replacing the role of NKB in KNDy network, and thus inducing episodic GnRH secretion. Thus, action of kisspeptin on non-GnRH neurons may contribute to the clinically beneficial effects of this peptide (30).
Acknowledgments
The authors thank Miroslav Valent, Gail Sager, and Dr. John Connors for technical assistance with radioimmunoassay and animal surgeries. They also thank the veterinarians and veterinary technicians of the WVU Office of Laboratory Animal Research for veterinary care and assistance with surgeries, Heather Bungard for care of the sheep, and Dr. Al Parlow for reagents used to measure LH.
Abbreviations
- ARC
arcuate nucleus
- cARC
caudal arcuate nucleus
- CV
coefficient of variation
- DAPI
4′,6-diamidino-2-phenylindole
- IHC
immunohistochemistry
- IPI
interpulse interval
- KOR
kappa opioid receptor
- mARC
middle arcuate nucleus
- MBH
medial basal hypothalamus
- NKB
neurokinin B
- OVX
ovariectomized
- POA
preoptic area
- POMC
pro-opiomelanocortin
- rARC
rostral arcuate nucleus
- RT
room temperature
- SAP
saporin
- TH
tyrosine hydroxide
Contributor Information
Robert L Goodman, Department of Physiology and Pharmacology, West Virginia University, Morgantown, WV 26506, USA.
Aleisha M Moore, Brain Health Research Institute, Kent State University, Kent, OH 44242, USA; Department of Biological Sciences, Kent State University, Kent, OH 44242, USA.
Kayla Onslow, Brain Health Research Institute, Kent State University, Kent, OH 44242, USA; Department of Biological Sciences, Kent State University, Kent, OH 44242, USA.
Stanley M Hileman, Department of Physiology and Pharmacology, West Virginia University, Morgantown, WV 26506, USA.
Steve L Hardy, Department of Physiology and Pharmacology, West Virginia University, Morgantown, WV 26506, USA.
Elizabeth C Bowdridge, Department of Physiology and Pharmacology, West Virginia University, Morgantown, WV 26506, USA.
Burgundy A Walters, Brain Health Research Institute, Kent State University, Kent, OH 44242, USA; Department of Biological Sciences, Kent State University, Kent, OH 44242, USA.
Sami Agus, Brain Health Research Institute, Kent State University, Kent, OH 44242, USA; Department of Biological Sciences, Kent State University, Kent, OH 44242, USA.
Max J Griesgraber, Department of Physiology and Pharmacology, West Virginia University, Morgantown, WV 26506, USA.
Eliana G Aerts, Department of Physiology and Pharmacology, West Virginia University, Morgantown, WV 26506, USA.
Michael N Lehman, Brain Health Research Institute, Kent State University, Kent, OH 44242, USA; Department of Biological Sciences, Kent State University, Kent, OH 44242, USA.
Lique M Coolen, Brain Health Research Institute, Kent State University, Kent, OH 44242, USA; Department of Biological Sciences, Kent State University, Kent, OH 44242, USA.
Funding
This work was supported by National Institutes of Health Grant R01 HD082135 (to R.L.G. and M.N.L.).
Disclosures
The authors have nothing to disclose.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111(5):1737‐1739. [DOI] [PubMed] [Google Scholar]
- 2. Levine JE, Ramirez VD. In vivo release of luteinizing hormone-releasing hormone estimated with push-pull cannulae from the mediobasal hypothalami of ovariectomized, steroid-primed rats. Endocrinology. 1980;107(6):1782‐1790. [DOI] [PubMed] [Google Scholar]
- 3. Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202(4368):631‐633. [DOI] [PubMed] [Google Scholar]
- 4. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614‐1627. [DOI] [PubMed] [Google Scholar]
- 5. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100(19):10972‐10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goodman RL, Lehman MN, Smith JT, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752‐5760. [DOI] [PubMed] [Google Scholar]
- 8. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859‐11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479‐3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124‐3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moore AM, Coolen LM, Porter DT, Goodman RL, Lehman MN. KNDy cells revisited. Endocrinology. 2018;159(9):3219‐3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herbison AE. The gonadotropin-releasing hormone pulse generator. Endocrinology. 2018;159(11):3723‐3736. [DOI] [PubMed] [Google Scholar]
- 13. Goodman RL, Herbison AE, Lehman MN, Navarro VM. Neuroendocrine control of gonadotropin-releasing hormone: pulsatile and surge modes of secretion. J Neuroendocrinol. 2022;34(5):e13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goodman RL, Coolen LM, Lehman MN. Unraveling the neural mechanisms underlying the GnRH pulse generator: an update. In: Ulloa-Aguirre A, ed. Cellular Endocrinology in Health and Disease. 2nd ed. Elsevier; 2021:123‐148. [Google Scholar]
- 15. Hrabovszky E, Sipos MT, Molnar CS, et al. Low degree of overlap between kisspeptin, neurokinin B, and dynorphin immunoreactivities in the infundibular nucleus of young male human subjects challenges the KNDy neuron concept. Endocrinology. 2012;153(10):4978‐4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lehman MN, He W, Coolen LM, Levine JE, Goodman RL. Does the KNDy model for the control of gonadotropin-releasing hormone pulses apply to monkeys and humans? Semin Reprod Med. 2019;37(2):71‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498(5):712‐726. [DOI] [PubMed] [Google Scholar]
- 18. Clarkson J, Han SY, Piet R, et al. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci U S A. 2017;114(47):E10216‐E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han SY, McLennan T, Czieselsky K, Herbison AE. Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc Natl Acad Sci U S A. 2015;112(42):13109‐13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qiu J, Nestor CC, Zhang C, et al. High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife. 2016;5:e16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Voliotis M, Li XF, De Burgh R, et al. The origin of GnRH pulse generation: an integrative mathematical-experimental approach. J Neurosci. 2019;39(49):9738‐9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higo S, Iijima N, Ozawa H. Characterisation of Kiss1r (Gpr54)-expressing neurones in the arcuate nucleus of the female rat hypothalamus. J Neuroendocrinol. 2017;29(2):1‐8. [DOI] [PubMed] [Google Scholar]
- 23. Smith JT, Li Q, Yap KS, et al. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152(3):1001‐1012. [DOI] [PubMed] [Google Scholar]
- 24. de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154(8):2750‐2760. [DOI] [PubMed] [Google Scholar]
- 25. George JT, Veldhuis JD, Roseweir AK, et al. Kisspeptin-10 is a potent stimulator of LH and increases pulse frequency in men. J Clin Endocrinol Metab. 2011;96(8):E1228‐E1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. George JT, Veldhuis JD, Tena-Sempere M, Millar RP, Anderson RA. Exploring the pathophysiology of hypogonadism in men with type 2 diabetes: kisspeptin-10 stimulates serum testosterone and LH secretion in men with type 2 diabetes and mild biochemical hypogonadism. Clin Endocrinol. 2013;79(1):100‐104. [DOI] [PubMed] [Google Scholar]
- 27. Skorupskaite K, George JT, Veldhuis JD, Millar RP, Anderson RA. Interactions between neurokinin B and kisspeptin in mediating estrogen feedback in healthy women. J Clin Endocrinol Metab. 2016;101(12):4628‐4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Young J, George JT, Tello JA, et al. Kisspeptin restores pulsatile LH secretion in patients with neurokinin B signaling deficiencies: physiological, pathophysiological and therapeutic implications. Neuroendocrinology. 2013;97(2):193‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chan Y-M, Butler JP, Pinnell NE, et al. Kisspeptin resets the hypothalamic GnRH clock in men. J Clin Endocrinol Metab. 2011;96(6):E908‐E915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anderson RA, Millar RP. The roles of kisspeptin and neurokinin B in GnRH pulse generation in humans, and their potential clinical application. J Neuroendocrinol. 2022;34(5):e13081. [DOI] [PubMed] [Google Scholar]
- 31. Li X-F, Kinsey-Jones J-S, Cheng Y, et al. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS One. 2009;4(12):e8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ohkura S, Takase K, Matsuyama S, et al. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol. 2009;21(10):813‐821. [DOI] [PubMed] [Google Scholar]
- 33. Goodman RL, Hileman SM, Nestor CC, et al. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154(11):4259‐4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Noritake K, Matsuoka T, Ohsawa T, et al. Involvement of neurokinin receptors in the control of pulsatile luteinizing hormone secretion in rats. J Reprod Dev. 2011;57(3):409‐415. [DOI] [PubMed] [Google Scholar]
- 35. Fergani C, Mazzella L, Coolen LM, et al. Do substance P and neurokinin A play important roles in the control of LH secretion in ewes? Endocrinology. 2016;157(12):4829‐4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fergani C, Navarro V. Expanding the role of tachykinins in the neuroendocrine control of reproduction. Reproduction. 2016;153(1):R1‐R14. [DOI] [PubMed] [Google Scholar]
- 37. Grachev P, Li XF, Kinsey-Jones JS, et al. Suppression of the GnRH pulse generator by neurokinin B involves a kappa-opioid receptor-dependent mechanism. Endocrinology. 2012;153(10):4894‐4904. [DOI] [PubMed] [Google Scholar]
- 38. Weems PW, Coolen LM, Hileman SM, et al. Evidence that dynorphin acts upon KNDy and GnRH neurons during GnRH pulse termination in the ewe. Endocrinology. 2018;159(9):3187‐3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fraser GL, Hoveyda HR, Clarke IJ, et al. The NK3 receptor antagonist ESN364 interrupts pulsatile LH secretion and moderates levels of ovarian hormones throughout the menstrual cycle. Endocrinology. 2015;156(11):4214‐4225. [DOI] [PubMed] [Google Scholar]
- 40. Clarke IJ, Li Q, Henry BA, Millar RP. Continuous kisspeptin restores luteinizing hormone pulsatility following cessation by a neurokinin B antagonist in female sheep. Endocrinology. 2018;;159(2):639‐646. [DOI] [PubMed] [Google Scholar]
- 41. Goodman RL, He W, Lopez JA, et al. Evidence that the LH surge in ewes involves both neurokinin B-dependent and -independent actions of kisspeptin. Endocrinology. 2019;160(12):2990‐3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153(6):2800‐2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McCosh RB, Szeligo BM, Bedenbaugh MN, et al. Evidence that endogenous somatostatin inhibits episodic, but not surge, secretion of LH in female sheep. Endocrinology. 2017;158(6):1827‐1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goodman RL, Ohkura S, Okamura H, Coolen LM, Lehman MN. KNDy hypothesis for generation of GnRH pulses: evidence from sheep and goats. In: Herbison AE, Plant TM, eds. The GnRH Neuron and its Control. John Wiley and Sons; 2018:289‐324. [Google Scholar]
- 45. Goodman RL, Coolen LM, Anderson GM, et al. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959‐2967. [DOI] [PubMed] [Google Scholar]
- 46. Wang F, Flanagan J, Su N, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14(1):22‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goodman RL, Karsch FJ. Pulsatile secretion of luteinizing hormone: differential suppression by ovarian steroids. Endocrinology. 1980;107(5):1286‐1290. [DOI] [PubMed] [Google Scholar]
- 48. Karsch FJ, Foster DL, Bittman EL, Goodman RL. A role for estradiol in enhancing luteinizing hormone pulse frequency during the follicular phase of the estrous cycle of sheep. Endocrinology. 1983;113(4):1333‐1339. [DOI] [PubMed] [Google Scholar]
- 49. Carpenter AE, Jones TR, Lamprecht MR, et al. Cellprofiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7(10):R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moore AM, Lohr DB, Coolen LM, Lehman MN. Prenatal androgen exposure alters KNDy neurons and their afferent network in a model of polycystic ovarian syndrome. Endocrinology. 2021;162(11):bqab158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Erben L, He M-X, Laeremans A, Park E, Buonanno A. A novel ultrasensitive in situ hybridization approach to detect short sequences and splice variants with cellular resolution. Mol Neurobiol. 2018;55(7):6169‐6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Amstalden M, Coolen LM, Hemmerle AM, et al. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2010;22(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nagae M, Uenoyama Y, Okamoto S, et al. Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator. Proc Natl Acad Sci U S A. 2021;118(5):e2009156118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lehman MN, Robinson JE, Karsch FJ, Silverman A-J. Immunocytochemical localization of luteinizing hormone-releasing hormone (LHRH) pathways in the sheep brain during anestrus and the mid-luteal phase of the estrous cycle. J Comp Neurol. 1986;244(1):19‐35. [DOI] [PubMed] [Google Scholar]
- 55. Herbison AE, Porteous R, Pape J-R, Mora JM, Hurst PR. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology. 2008;149(2):597‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Turzillo AM, Nett TM. Regulation of GnRH receptor gene expression in sheep and cattle. J Reprod Fertil Suppl. 1999;54:75‐86. [PubMed] [Google Scholar]
- 57. Han SY, Morris PG, Kim JC, et al. Mechanism of kisspeptin neuron synchronization for pulsatile hormone secretion in male mice. Cell Rep. 2023;42(1):111914. [DOI] [PubMed] [Google Scholar]
- 58. Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J Neuroendocrinol. 2011;23(7):557‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Porter DT, Moore AM, Cobern JA, et al. Prenatal testosterone exposure alters GABAergic synaptic inputs to GnRH and KNDy neurons in a sheep model of polycystic ovarian syndrome. Endocrinology. 2019;160(11):2529‐2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lehman MN, Karsch FJ. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and beta-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology. 1993;133(2):887‐895. [DOI] [PubMed] [Google Scholar]
- 61. Evans NP, Dahl GE, Mauger D, Karsch FJ. Estradiol induces both qualitative and quantitative changes in the pattern of gonadotropin-releasing hormone secretion during the presurge period in the ewe. Endocrinology. 1995;136(4):1603‐1609. [DOI] [PubMed] [Google Scholar]
- 62. Ozaki S, Higo S, Iwata K, Saeki H, Ozawa H. Region-specific changes in brain kisspeptin receptor expression during estrogen depletion and the estrous cycle. Histochem Cell Biol. 2019;152(1):25‐34. [DOI] [PubMed] [Google Scholar]
- 63. Kirilov M, Clarkson J, Liu X, et al. Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nat Commun. 2013;4(1):2492. [DOI] [PubMed] [Google Scholar]
- 64. Liu X, Yeo S-H, McQuillan HJ, et al. Highly redundant neuropeptide volume co-transmission underlying episodic activation of the GnRH neuron dendron. eLife. 2021;10:e62455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.