Abstract
Background
The association between cigarette smoking and thyroid cancer has been reported in prospective cohort studies, but the relationship remains controversial. To investigate this potential correlation further, we employed Mendelian randomization methodology to evaluate the causative impact of smoking on thyroid cancer incidence.
Methods
From the genome‐wide association study and Sequencing Consortium of Alcohol and Nicotine use, we obtained genetic variants associated with smoking initiation and cigarettes per day (1.2 million individuals). We also extracted genetic variants associated with past tobacco smoking from the UK Biobank (424,960 individuals). Thyroid cancer outcomes were selected from the FinnGen GWAS (989 thyroid cancer cases and 217,803 control cases). Sensitivity analyses employing various approaches such as weighted median, MR‐Egger, and MR‐pleiotropy residual sum and outlier (MR‐PRESSO) have been executed, as well as leave‐one‐out analysis to identify pleiotropy.
Results
Using the IVW approach, we did not find evidence that any of the three smoking phenotypes were related to thyroid cancer (smoking initiation: odds ratio (OR) = 1.56, p = 0.61; cigarettes per day: OR = 0.85, p = 0.51; past tobacco smoking: OR = 0.80, p = 0.78). The heterogeneity (p > 0.05) and pleiotropy (p > 0.05) testing provided confirmatory evidence for the validity of our MR estimates.
Conclusions
The MR analysis revealed that there may not exist a causative link between smoking exposure and elevated incidence rates of thyroid malignancies.
Keywords: causality, Mendelian randomization, smoking, thyroid cancer
Short abstract
We used Mendelian randomization analysis to evaluate the causal relationship between smoking and thyroid cancer, and found that smoking was not associated with thyroid cancer.
1. INTRODUCTION
In most parts of the world, thyroid cancer incidence has significantly grown during the last few decades. 1 In the United States, thyroid carcinoma ranks 13th in terms of newly diagnosed tumors in the United States each year, with over 440,000 forecasted instances in 2022. 2 According to their pathogenesis, histopathological features, and clinical presentation, thyroid cancer are categorized into four main subtypes: differentiated (follicular or papillary) thyroid 999 cancers, poorly differentiated thyroid cancers, anaplastic thyroid cancers, and medullary thyroid cancers. 3 Differentiated cancers, which have a usually good prognosis, account for 80% of all thyroid cancers. 4 Thyroid cancer has been recognized as a cancer type with a significant related financial burden on patients. 5 The risk of thyroid cancer seems to be increased by some factors such as race, radiation exposure, and women, but no controllable risk factors have been identified for thyroid cancer. 6 To further reduce thyroid cancer burden and prevalence, we should pay more attention to other controllable risk factors, such as cigarette smoking. 7
Smoking is one of the leading causes of various types of cancer, including colorectal, prostate, lung, stomach, and cervix cancers. 8 However, the correlation between smoking and thyroid carcinoma remains elusive. Some studies show that smoking has a carcinogenic effect on thyroid cancer. 9 The negative relationship between smoking and thyroid cancer was reported in a cohort study that followed 96,855 adults for 5.9 years. 7 A meta‐analysis indicated that smokers have a lower risk of thyroid carcinoma (OR = 0.798; 95% CI = 0.681–0.935). 10 A Korean cohort study based on population data revealed that smoking exerts a protective effect against thyroid cancer. 11 Other studies showed that smoking does not significantly affect thyroid cancer. 12 Whether smoking is a causal factor in the emergence of thyroid cancer is still up for disagreement due to the contradictory findings from several independent studies.
To clarify the direction and strength of the association between smoking behavior and thyroid cancer, we implemented the MR analysis, a powerful tool from genetic epidemiology that uses genetic polymorphisms as instrumental variables (IVs) to minimize sources of bias stemming from confounding or reverse causation. 13 By applying this approach to disentangle the causal linkages between smoking exposure and thyroid malignant tumors, we aim to shed new light on the prevention of thyroid cancer.
Our investigation utilized an MR approach to evaluate the putatively causal connection between cigarette smoking and the likelihood of developing thyroid carcinomas by leveraging published large‐scale genome‐wide association studies (GWAS) data sets relating to varying smoking characteristics (smoking initiation, cigarettes per day, and past tobacco use).
2. METHODS
2.1. Genetic variants associated with smoking
The GWAS summary statistics of tobacco use were extracted from the UK Biobank and the GWAS and Sequencing Consortium of Alcohol and Nicotine use (GSCAN) for three smoking phenotypes. Genetic IVs for the exposure were selected at the genome‐wide significance level (p < 5 × 10−8, linkage disequilibrium [LD] r 2 < 0.01) across a 1 Mb region. 14 The study design of MR analysis is presented in Figure 1.
FIGURE 1.
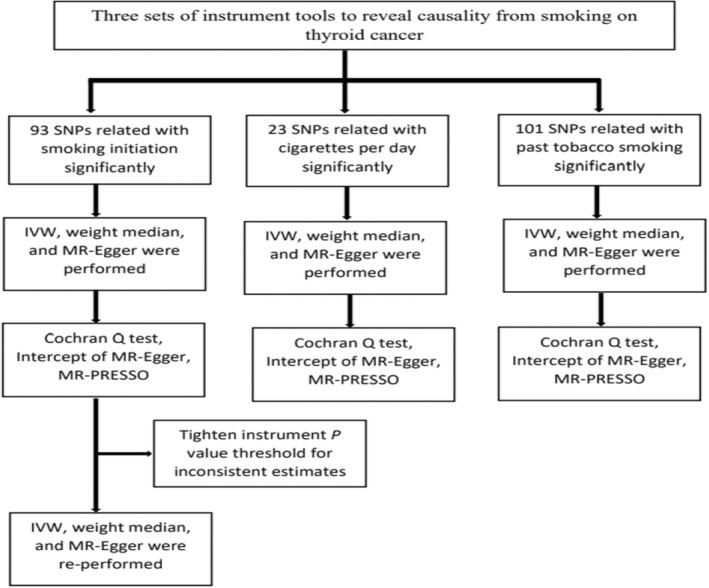
Workflow of the MR study demonstrating the link between thyroid cancer and smoking. IVW, inverse variance weighted; MR, Mendelian randomization; MR‐PRESSO, MR Pleiotropy Residual Sum and Outlier; SNP, single‐nucleotide polymorphisms.
We extracted IVs of smoking initiation and cigarettes per day from the GSCAN. For smoking initiation GWAS, they included 607,291 European individuals. Smoking initiation was defined as having ever been a regular smoker in life (current or former). A total of 93 SNPs related to smoking initiation were identified (Supplementary Material, Table S1). For cigarettes per day GWAS, they included 337,334 European individuals and identified 23 significant SNPs (Supplementary Material, Table S2). For past tobacco smoking GWAS, they included 424,960 Europeans and a total of 101 SNPs were significant (Supplementary Material, Table S3). In the Supplementary Material, more information on these smoking phenotypes is available.
To assess the presence of weak instrument bias, we computed estimates of the strength of the relationship between the genetic instruments and smoking phenotypes utilizing the F statistic (F = beta2/se2). By doing so, we aimed to determine whether the instruments possess sufficient explanatory power to influence the outcome variable. The F statistical range of the instrument SNPs used in MR analysis is 29.8–961, exceeding the suggested F > 10 threshold. 15
2.2. GWAS summary data for thyroid cancer
The GWAS summary data for thyroid cancer were extracted from FinnGen (https://r5.finngen.fi/), including 989 patients and 217,803 controls of European ancestry. The Cancer Registry data were used to identify cases of thyroid cancer, according to the code C73 of the International Classification of Diseases‐10.
2.3. MR analyses
For each instrumental variable, the Wald ratio was used to evaluate the influence of exposure on the result. Then, we used the inverse variance weighted (IVW) method to combine each instrumental variable's effect size. This method is based on the premise that instruments can only affect a result through exposure and not through any other channel. 16 The weighted median and MR‐Egger methods were employed to supplement IVW estimates. A stricter instrument p value threshold was set if the estimations from the methods used in our investigation were inconsistent. 17 All MR analysis approaches have consistent MR results, which could provide more reliable predictions in a wider range of scenarios. 18 The MR analysis was combined using a random‐effect model.
2.4. Sensitivity analyses
The heterogeneity was evaluated using the Cochrane's Q value from the IVW approach, with a p value <0.05 indicating statistical significance. 19 , 20 The MR‐Egger intercept and MR‐PRESSO methods were used to detect horizontal pleiotropy (directional pleiotropy was assumed to exist if p < 0.05), 21 MR‐PRESSO includes three components: (i) identification of horizontal pleiotropy; (ii) horizontal pleiotropy correction by the elimination of outliers; (iii) analyzing the causal estimates before and after the outlier correction to determine whether there are any discrepancies. 22 A leave‐one‐out approach was employed to evaluate the effect of a particular SNP on the outcome of the MR analysis.
The genetic relationships between smoking and the risk of thyroid cancer were displayed using scatter plots and funnel plots to visually evaluate the consistency of MR estimations and any potential bias. 23
MR analyses were performed by the R package “TwoSampleMR” (version 0.5.6) and “MRPRESSO” (version 1.0) in R (version 4.2.0).
3. RESULTS
3.1. MR Analysis of smoking with risk of thyroid cancer
To investigate the causal association between smoking‐related SNPs and thyroid cancer, we conducted a two‐sample MR study using genetic data from participants of European descent. Our analysis involved utilizing three different methods for estimating instrumental variables (IVs), including the IVW, WM, and MR‐Egger. By evaluating interactions between these inputs and outcomes, our goal was to assess the validity of inferring a causal relationship. In the standard IVW method, we found smoking initiation increased the risk for thyroid cancer significantly (OR = 1.54, 95% CI = 1.01–2.35, p = 0.047), while opposing results were observed using the MR‐Egger (OR = 1.24, 95% CI = 0.15–10.66, p = 0.842) and WM approaches (OR = 1.48, 95% CI = 0.82–2.71, p = 0.195). Since the MR estimates for IVW and MR‐Egger were incompatible, we implemented stringent criteria by setting the instrument p value threshold at 1 × 10−8. This adjustment resulted in the inclusion of 59 SNPs as instrumental variables. 14 The MR estimations became nonsignificant, the IVW (OR = 1.56, 95% CI: 0.96–2.53, p = 0.070) did not detect evidence of the impact of smoking on thyroid cancer, while the MR‐Egger (OR = 1.97, 95% CI: 0.17–23.51, p = 0.592) also provided inconclusive findings regarding direct effects of smoking on disease risk. Finally, the WM estimate did not identify a statistically significant relationship either (OR = 1.48, 95% CI: 0.72–3.08, p = 0.282). No evident heterogeneity was found, as indicated by the Cochran Q‐test derived p value (IVW: Q = 54.29, df = 58, p = 0.61; ME‐Egger: Q = 54.26, df = 57, p = 0.58) (Table 1). We found that cigarettes per day (OR = 0.85, 95% CI = 0.61–1.20, p = 0.369); past tobacco smoking (OR = 0.80 95% CI = 0.47–1.38, p = 0.429) were not causally associated with thyroid cancer risk using IVW method. Furthermore, the results of MR‐Egger regression and WM approaches also support this finding. The MR estimates of smoking in thyroid cancer using conventional MR analysis (MR‐Egger, WM, and IVW) are presented in Figure 2.
TABLE 1.
Mendelian randomization estimates for the association between thyroid cancer and smoking.
| Smoking phenotypes | IVW | Weighted median | ME‐Egger | MR‐PRESSO | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Cochran Q Statistics (df) | p | OR (95% CI) | OR (95% CI) | Cochran Q Statistics (df) | p | Intercept (se) | p | p | |
| Smoking initiation | 1.54 (1.01–2.35) | 69.99 (84) | 0.86 | 1.48 (0.82–2.71) | 1.24 (0.15–10.66) | 69.95 (83) | 0.85 | 0.0056 (0.028) | 0.84 | 0.86 |
| Smoking initiation after tightening threshold | 1.56 (0.96–2.53) | 54.29 (58) | 0.61 | 1.48 (0.72–3.08) | 1.97 (0.17–23.51) | 54.26 (57) | 0.58 | −0.0065 (0.034) | 0.85 | 0.62 |
| Cigarettes per day | 0.85 (0.61–1.20) | 20.09 (21) | 0.51 | 0.75 (0.47–1.17) | 0.82 (0.45–1.49) | 20.07 (20) | 0.45 | 0.0035 (0.02) | 0.86 | 0.56 |
| Past tobacco smoking | 0.80 (0.47–1.38) | 82.04 (93) | 0.78 | 0.65 (0.29–1.44) | 0.64 (0.07–6.17) | 81.99 (92) | 0.76 | 0.0046 (0.02) | 0.83 | 0.79 |
FIGURE 2.
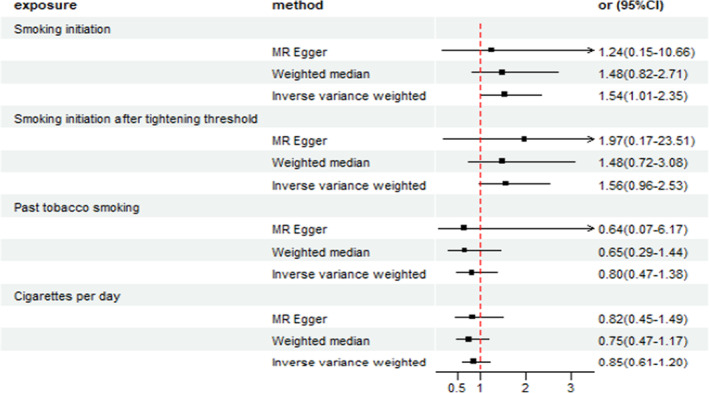
Odds ratio plot for smoking initiation, cigarettes per day, and past tobacco smoking. CI: confidence interval; OR: odds ratio.
MR‐PRESSO identified no outlier variants in all analyses (for smoking initiation, p = 0.86; for smoking initiation after tightening threshold, p = 0.62; for cigarettes per day, p = 0.56; for past tobacco smoking, p = 0.79) (Table 1). The MR‐Egger method's intercepts showed that there is no evidence for the potential pleiotropy of a single SNP (for smoking initiation, intercept = 0.0056, p = 0.84; for smoking initiation after tightening threshold, intercept = −0.0065, p = 0.85; for cigarettes per day, intercept = 0.0035, p = 0.86; for past tobacco smoking, intercept = 0.0046, p = 0.83) (Table 1) (Supplementary Material, Figures S1, S4, and S7). Simultaneously checked the stability of our observations using the “leave one out method” (Supplementary Material, Figures S2, S5, and S8). Additionally, the funnel plot was symmetry, showing no pleiotropy (Supplementary Material, Figures S3, S6, and S9). These evaluations confirm the reliability of our research results (Table 1).
4. DISCUSSION
To comprehensively assess whether smoking has a causal impact on the incidence of thyroid cancer, we used a two‐sample MR approach, and we found no conclusive evidence to support the causative role of genetically predicted cigarette smoking on the risk of thyroid cancer.
Up to now, the relationship between smoking and thyroid cancer has remained unclarified. 7 , 10 , 24 , 25 The majority of prior epidemiological research used case–control methodologies, which failed to clarify the causal relationship between thyroid cancer and smoking. Evidence on the relationship between smoking and thyroid cancer is inconsistent. A large cohort study reported that smoking was significantly associated with a lower risk of thyroid cancer, 7 a finding confirmed by another meta‐analysis. 20 At the same time, a population‐based Korean cohort study also indicated that smoking reduces the risk of thyroid cancer, both in men and women. 11 However, not all research supports this conclusion. In prospective cohort studies carried out in North American populations, researchers found no statistically significant association between cigarette smoking and the incidence of thyroid cancer over a median follow‐up period spanning approximately 15–20 years. This null finding may be indicative of the limited or non‐existent influence exerted by smoking on thyroid carcinogenesis. 12 , 26 In light of the substantial gender disparity in thyroid cancer incidence, whereby men experience a much lower burden compared to females. 27 Specifically, in most studies, statistical significance has been observed between current smoking habits and thyroid tumors exclusively among women rather than men. 28 , 29 In addition, different types of thyroid cancer vary greatly. However, previous observational studies have not examined the effects of smoking on different subtypes of thyroid cancer. Therefore, further investigation is needed to test the relationship between smoking and different subtypes of thyroid cancer.
The variability of experimental design and analysis methods adopted by different researchers may be the main reason for the differences in research conclusions on the relationship between tobacco use and thyroid malignant tumors. Additional potential sources of inconsistency encompass differences in study participant characteristics such as age, demographics, and other comorbidities; and distinctions in classification systems used to define cases of thyroid cancer. Moreover, the differences in environmental exposure, lifestyle, diagnostic techniques, medical practice, and monitoring strategies in different research environments must be considered. In summary, while past empirical works have attempted to account for possible confounding factors. However, certain unmeasured variables remain possible sources of bias. 7 , 30 , 31 , 32 Our study addressed these concerns by employing MR analysis of multiple instrumental variables, subsequently demonstrating no evidence of a causative association between cigarette smoking and incident occurrences of thyroid carcinoma. Collectively, these null findings suggest that factors other than smoking contribute substantially to thyroid cancer pathogenesis. Thyroid cancer cells could evade immune surveillance by establishing an immunosuppressive microenvironment. 33 Furthermore, BRAF (rapidly accelerating fibrosarcoma homolog B) and RAS (rat sarcoma) gene mutations associated with impaired immunity activate the PI3K signaling pathway, enhancing cell growth, survival, and angiogenesis in thyroid cancer cells. 34 , 35 Future investigations are warranted to better understand the etiologic determinants of this malignancy.
Though our research indicated that there was no causality between smoking and thyroid cancer, it was possible that smoking might have a potential impact on the development of thyroid cancer. First, cigarette smoke contains thiocyanate, which impacts the thyroid by competitively inhibiting the uptake and organification of iodine in the gland. 36 Previous research findings demonstrate that, among individuals who smoke, there exists a trend toward decreased circulatory concentrations of thyroid stimulating hormone (TSH), as well as the bioactive metabolites of thyroxine (T4) and triiodothyronine (T3). 37 , 38 Furthermore, it has previously been postulated that altered regulation of TSH may be pivotal in promoting thyroid cancer. 39 Smoking may also affect the risk of thyroid cancer by changing the sex steroid hormone levels. 40 , 41 Thyroid cancer was also associated with autoimmune thyroiditis (mainly Graves' disease and Hashimoto's disease). Previous investigations have suggested that nicotine present in tobacco can skew dysfunctional Th1 and Th17 immune responses toward a protective Th2 response, thereby potentially reducing the likelihood of autoimmune thyroiditis. 36 Additionally, the alkaloid compound anatabine derived from tobacco plants has been demonstrated to attenuate both the prevalence and severity of autoimmune thyroiditis. 42
This study possesses several noteworthy advantages due to its use of extensive datasets and meticulous methodology. We leveraged the potential of MR to reduce issues related to reverse causality and confounding. By adopting this strategy, we were able to minimize these concerns compared to traditional observational approaches. Furthermore, our MR design yielded an F statistics value greater than 10, suggesting a minimal possibility of weak instrument variable bias. Additional support for the validity of our findings comes from the absence of horizontal pleiotropy among instrumental variables observed via the application of MR‐Egger intercepts and MR‐PRESSO estimations. Finally, the successful implementation of the “leave one out” technique underscores the dependable nature of our conclusions. These outcomes highlight the rigor of our methodological approach and substantiate our research findings.
However, several limitations were also present in our study. First, we limited the research to people of European ancestry, which decreased the possibility of population structure‐related bias but constrained the applicability of our findings to other populations. Second, despite the large sample size, the proportion of cases in the results is relatively low and may have an impact on MR estimation results. Finally, performing subgroup analysis is difficult when employing GWAS summary‐level data. A further study including thyroid cancer subgroups can be considered in the future.
5. CONCLUSION
This is the inaugural MR study to examine the correlation between smoking and thyroid cancer. Our comprehensive outcomes do not corroborate the existence of a direct association between smoking and thyroid cancer occurrence. In the future, a large GWAS dataset is necessary to scrutinize the causal connection between cigarette smoking and thyroid cancer.
AUTHOR CONTRIBUTIONS
Hongzhan Jiang: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); resources (equal); software (equal); visualization (equal); writing – original draft (equal). Yi Li: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); resources (equal); software (equal); visualization (equal); writing – review and editing (equal). Jiali Shen: Conceptualization (equal); visualization (equal); writing – review and editing (equal). Huihui Lin: Conceptualization (equal); writing – review and editing (equal). Siyue Fan: Writing – review and editing (equal). Rongliang Qiu: Writing – review and editing (equal). Jiaxi He: Writing – review and editing (equal). Ende Lin: Writing – review and editing (equal). Lijuan Chen: Conceptualization (equal); funding acquisition (equal); writing – review and editing (equal).
FUNDING INFORMATION
This research was supported by the Xiamen Municipal Science & Technology Project (No. 3502Z20209025).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
We used publicly available summary‐level data. No additional patient consent and ethical approval are required.
Supporting information
Data S1.
ACKNOWLEDGMENTS
The present investigation acknowledges the contributions of various investigators whose valuable datasets were essential for conducting this analysis. Additionally, the authors appreciate the open sharing of summary statistics from multiple GWASs by the wider research community.
Jiang H, Li Yi, Shen J, et al. Cigarette smoking and thyroid cancer risk: A Mendelian randomization study. Cancer Med. 2023;12:19866‐19873. doi: 10.1002/cam4.6570
Hongzhan Jiang and Yi Li contributed equally to this work.
DATA AVAILABILITY STATEMENT
All the data used in the current research are publicly available GWAS summary data.
REFERENCES
- 1. Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:965212. doi: 10.1155/2013/965212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7‐33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 3. Chmielik E, Rusinek D, Oczko‐Wojciechowska M, et al. Heterogeneity of thyroid cancer. Pathobiol: J Immunopathol Mol Cell Biol. 2018;85:117‐129. doi: 10.1159/000486422 [DOI] [PubMed] [Google Scholar]
- 4. Chen DW, Lang BHH, McLeod DSA, Newbold K, Haymart MR. Thyroid cancer. Lancet (London, England). 2023;401:1531‐1544. doi: 10.1016/S0140-6736(23)00020-X [DOI] [PubMed] [Google Scholar]
- 5. Uppal N, Cunningham Nee Lubitz C, James B. The cost and financial burden of thyroid cancer on patients in the US: a review and directions for future research. JAMA Otolaryngol Head Neck Surg. 2022;148:568‐575. doi: 10.1001/jamaoto.2022.0660 [DOI] [PubMed] [Google Scholar]
- 6. Vigneri R, Malandrino P, Vigneri P. The changing epidemiology of thyroid cancer: why is incidence increasing? Curr Opin Oncol. 2015;27:1‐7. doi: 10.1097/CCO.0000000000000148 [DOI] [PubMed] [Google Scholar]
- 7. Cho A, Chang Y, Ahn J, Shin H, Ryu S. Cigarette smoking and thyroid cancer risk: a cohort study. Br J Cancer. 2018;119:638‐645. doi: 10.1038/s41416-018-0224-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1‐1438. [PMC free article] [PubMed] [Google Scholar]
- 9. Sokić SI, Adanja BJ, Vlajinac HD, Janković RR, Marinković JP, Zivaljević VR. Risk factors for thyroid cancer. Neoplasma. 1994;41:371‐374. [PubMed] [Google Scholar]
- 10. Lee J‐H, Chai YJ, Yi KH. Effect of cigarette smoking on thyroid cancer: meta‐analysis. Endocrinol Metab (Seoul). 2021;36:590‐598. doi: 10.3803/EnM.2021.954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yeo Y, Han K, Shin D‐W, et al. Changes in smoking, alcohol consumption, and the risk of thyroid cancer: a population‐based Korean cohort study. Cancer. 2021;13:2343. doi: 10.3390/cancers13102343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iribarren C, Haselkorn T, Tekawa IS, Friedman GD. Cohort study of thyroid cancer in a San Francisco Bay area population. Int J Cancer. 2001;93:745‐750. doi: 10.1002/ijc.1377 [DOI] [PubMed] [Google Scholar]
- 13. Smith GD, Ebrahim S. “Mendelian randomization”: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1‐22. doi: 10.1093/ije/dyg070 [DOI] [PubMed] [Google Scholar]
- 14. Liu M, Jiang Y, Wedow R, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51:237‐244. doi: 10.1038/s41588-018-0307-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burgess S, Thompson SG, CRP CHD Genetics Collaboration . Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40:755‐764. doi: 10.1093/ije/dyr036 [DOI] [PubMed] [Google Scholar]
- 16. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. 2015;44:512‐525. doi: 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ong J‐S, MacGregor S. Implementing MR‐PRESSO and GCTA‐GSMR for pleiotropy assessment in Mendelian randomization studies from a practitioner's perspective. Genet Epidemiol. 2019;43:609‐616. doi: 10.1002/gepi.22207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lawlor DA, Tilling K, Davey SG. Triangulation in aetiological epidemiology. Int J Epidemiol. 2016;45:1866‐1886. doi: 10.1093/ije/dyw314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanderson E, Davey Smith G, Windmeijer F, Bowden J. An examination of multivariable Mendelian randomization in the single‐sample and two‐sample summary data settings. Int J Epidemiol. 2019;48:713‐727. doi: 10.1093/ije/dyy262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR‐egger method. Eur J Epidemiol. 2017;32:377‐389. doi: 10.1007/s10654-017-0255-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verbanck M, Chen C‐Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693‐698. doi: 10.1038/s41588-018-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology. 2017;28:30‐42. doi: 10.1097/EDE.0000000000000559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kitahara CM, Linet MS, Beane Freeman LE, et al. Cigarette smoking, alcohol intake, and thyroid cancer risk: a pooled analysis of five prospective studies in the United States. Cancer Causes Control. 2012;23:1615‐1624. doi: 10.1007/s10552-012-0039-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guignard R, Truong T, Rougier Y, Baron‐Dubourdieu D, Guénel P. Alcohol drinking, tobacco smoking, and anthropometric characteristics as risk factors for thyroid cancer: a countrywide case‐control study in New Caledonia. Am J Epidemiol. 2007;166:1140‐1149. doi: 10.1093/aje/kwm204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Navarro Silvera SA, Miller AB, Rohan TE. Risk factors for thyroid cancer: a prospective cohort study. Int J Cancer. 2005;116:433‐438. doi: 10.1002/ijc.21079 [DOI] [PubMed] [Google Scholar]
- 27. Jee SH, Samet JM, Ohrr H, Kim JH, Kim IS. Smoking and cancer risk in Korean men and women. Cancer Causes Control. 2004;15:341‐348. doi: 10.1023/B:CACO.0000027481.48153.97 [DOI] [PubMed] [Google Scholar]
- 28. Kabat GC, Kim MY, Wactawski‐Wende J, Rohan TE. Smoking and alcohol consumption in relation to risk of thyroid cancer in postmenopausal women. Cancer Epidemiol. 2012;36:335‐340. doi: 10.1016/j.canep.2012.03.013 [DOI] [PubMed] [Google Scholar]
- 29. Meinhold CL, Ron E, Schonfeld SJ, et al. Nonradiation risk factors for thyroid cancer in the US radiologic technologists study. Am J Epidemiol. 2010;171:242‐252. doi: 10.1093/aje/kwp354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. An S‐Y, Kim SY, Oh DJ, Min C, Sim S, Choi HG. Obesity is positively related and tobacco smoking and alcohol consumption are negatively related to an increased risk of thyroid cancer. Sci Rep. 2020;10:19279. doi: 10.1038/s41598-020-76357-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yeo Y, Shin DW, Han K, et al. Smoking, alcohol consumption, and the risk of thyroid cancer: a population‐based Korean cohort study of 10 million people. Thyroid. 2022;32:440‐448. doi: 10.1089/thy.2021.0675 [DOI] [PubMed] [Google Scholar]
- 32. Kitahara CM, McCullough ML, Franceschi S, et al. Anthropometric factors and thyroid cancer risk by histological subtype: pooled analysis of 22 prospective studies. Thyroid. 2016;26:306‐318. doi: 10.1089/thy.2015.0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jin Y, Liu M, Sa R, Fu H, Cheng L, Chen L. Mouse models of thyroid cancer: bridging pathogenesis and novel therapeutics. Cancer Lett. 2020;469:35‐53. doi: 10.1016/j.canlet.2019.09.017 [DOI] [PubMed] [Google Scholar]
- 34. de Biase D, Cesari V, Visani M, et al. High‐sensitivity BRAF mutation analysis: BRAF V600E is acquired early during tumor development but is heterogeneously distributed in a subset of papillary thyroid carcinomas. J Clin Endocrinol Metab. 2014;99:E1530‐E1538. doi: 10.1210/jc.2013-4389 [DOI] [PubMed] [Google Scholar]
- 35. Landa I, Ibrahimpasic T, Boucai L, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016;126:1052‐1066. doi: 10.1172/JCI85271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wiersinga WM. Smoking and thyroid. Clin Endocrinol (Oxf). 2013;79:145‐151. doi: 10.1111/cen.12222 [DOI] [PubMed] [Google Scholar]
- 37. Soldin OP, Goughenour BE, Gilbert SZ, Landy HJ, Soldin SJ. Thyroid hormone levels associated with active and passive cigarette smoking. Thyroid. 2009;19:817‐823. doi: 10.1089/thy.2009.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Belin RM, Astor BC, Powe NR, Ladenson PW. Smoke exposure is associated with a lower prevalence of serum thyroid autoantibodies and thyrotropin concentration elevation and a higher prevalence of mild thyrotropin concentration suppression in the third National Health and nutrition examination survey (NHANES III). J Clin Endocrinol Metab. 2004;89:6077‐6086. doi: 10.1210/jc.2004-0431 [DOI] [PubMed] [Google Scholar]
- 39. Boelaert K, Horacek J, Holder RL, Watkinson JC, Sheppard MC, Franklyn JA. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine‐needle aspiration. J Clin Endocrinol Metab. 2006;91:4295‐4301. doi: 10.1210/jc.2006-0527 [DOI] [PubMed] [Google Scholar]
- 40. Brand JS, Chan M‐F, Dowsett M, et al. Cigarette smoking and endogenous sex hormones in postmenopausal women. J Clin Endocrinol Metab. 2011;96:3184‐3192. doi: 10.1210/jc.2011-1165 [DOI] [PubMed] [Google Scholar]
- 41. Kumar A, Klinge CM, Goldstein RE. Estradiol‐induced proliferation of papillary and follicular thyroid cancer cells is mediated by estrogen receptors alpha and beta. Int J Oncol. 2010;36:1067‐1080. doi: 10.3892/ijo_00000588 [DOI] [PubMed] [Google Scholar]
- 42. Caturegli P, De Remigis A, Ferlito M, et al. Anatabine ameliorates experimental autoimmune thyroiditis. Endocrinology. 2012;153:4580‐4587. doi: 10.1210/en.2012-1452 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
All the data used in the current research are publicly available GWAS summary data.


