Abstract
Gain-of-function mutations in isocitrate dehydrogenase (IDH) genes result in excessive production of (D)-2-hydroxyglutarate (D-2HG) which intrinsically modifies tumor cell epigenetics and impacts surrounding noncancerous cells through nonepigenetic pathways. However, whether D-2HG has a paracrine effect on endothelial cells in the tumor microenvironment needs further clarification. We quantified microvessel density by immunohistochemistry using tissue sections from 60 high-grade astrocytic gliomas with or without IDH mutation. Microvessel density was found to be reduced in tumors carrying an IDH mutation. Ex vivo experiments showed that D-2HG inhibited endothelial cell migration, wound healing, and tube formation by suppressing cell proliferation but not viability, possibly through reduced activation of the mTOR/STAT3 pathway. Further, D-2HG reduced fluorescent dextran permeability and decreased paracellular T-cell transendothelial migration by augmenting expression of junctional proteins thereby collectively increasing endothelial barrier function. These results indicate that D-2HG may influence the tumor vascular microenvironment by reducing the intratumoral vasculature density and by inhibiting the transport of metabolites and extravasation of circulating cells into the astrocytoma microenvironment. These observations provide a rationale for combining IDH inhibition with antitumor immunological/angiogenic approaches and suggest a molecular basis for resistance to antiangiogenic drugs in patients whose tumors express a mutant IDH allele.
Keywords: Astrocytic glioma, Brain vascular endothelial cell, (D)-2-hydroxyglutarate (D-2HG), Glioblastoma, Isocitrate dehydrogenase (IDH), Microvascular proliferation
INTRODUCTION
One of the most noteworthy discoveries in neuro-oncology in recent years is the identification of hotspot mutations in the isocitrate dehydrogenase (IDH) genes 1 and 2. These mutations exist in the vast majority of lower-grade diffuse astrocytomas and oligodendrogliomas as well as in a minor fraction of glioblastomas at the time of histology-based diagnosis (1–3). In the citric acid cycle, IDH catalyzes the oxidative decarboxylation of isocitrate to produce α-ketoglutarate (α-KG) and nicotinamide adenine dinucleotide phosphate hydrogenase (NADPH). IDH1 and IDH2 homologs are primarily cytoplasmic and mitochondrial enzymes, respectively, the physiological functions of which are not fully defined. It is known, however, that active site mutations in IDH result in a dominant gain-of-function catalyzing the NADPH-dependent reduction of 2-oxoglutarate to (D)-2-hydroxyglutarate (D-2HG or R-2HG) (4). This isomer is poorly metabolized relative to (L)-2-hydroxyglutarate and accumulates to high levels in cells expressing mutant IDH (4). D-2HG acts as an intrinsic antagonist of α-KG in tumor cells to competitively inhibit the activity of α-KG-dependent dioxygenases, including α-KG-regulated demethylases involved in epigenetic reprogramming as well as in metabolic and transcriptional alterations (5).
Thus, D-2HG impacts tumor cell biology but IDH-mutant glial cells also release a portion of the D-2HG (6) that accumulates in the surrounding milieu (7). Magnetic resonance imaging has quantified the total amount of D-2HG and L-2HG in IDH-mutant gliomas to nearly 10 mM (8). Using paired arterial-venous analysis, Xiong et al (9) showed that the amount of D-2HG released from patients with IDH-mutant gliomas is at least doubled compared to patients with IDH-wildtype gliomas. Up to 30 mM of D-2HG can accumulate within the tumor microenvironment (6), and modeling studies have shown that the concentration of D-2HG is up to 3 mM and may extend 2 centimeters beyond the tumor periphery (7). IDH mutation and its oncometabolite D-2HG modulate cells of the tumor microenvironment, for example by suppressing platelet activation and thereby reducing the risk of thrombosis (10), and by promoting neuronal activity, thereby increasing the risk of seizures (11, 12). Further, D-2HG reportedly impairs T-cell proliferation, chemotaxis, recruitment, and effector functions in IDH-mutant tumors (13–16). Most research shows that D-2HG promotes tumorigenesis in glioma cells carrying an IDH mutation (17, 18). However, some findings have contradicted this general concept suggesting that IDH mutation and D-2HG accumulation may be inherently tumor suppressive (5), thereby calling into question the potential antiglioma efficacy of mutant IDH inhibitors. Furthermore, the possible impact of IDH mutations and D-2HG on the intratumoral milieu poses a challenge to the treatment of cancers harboring an IDH mutation (5). Thus, the complex functions of D-2HG still need to be elucidated, in particular as a recent phase 3 trial reported that the mutant IDH inhibitor vorasidenib improved progression-free survival and delayed the time to the next intervention in patients with grade 2 IDH-mutant gliomas (19).
Glioma growth depends to a large extent on the formation of new blood vessels; infiltration of bone-marrow derived immune cells requires adequate vascular density (20). Angiogenesis is a complex process involving proliferation, migration, and differentiation of vascular endothelial cells in response to specific signals (21, 22). Some studies suggest that IDH mutation is associated with reduced vessel size in patients with a glioma (23, 24). In addition, the presence of the R132H mutation is a significant factor influencing the degree of tumor-associated vascular disruption and postsurgical residual vascular dysregulation (25). The clinical significance and a mechanistic understanding of these discoveries are unclear, however. In the present study, we investigated the possible influence of IDH mutation and D-2HG on the vascular microenvironment in gliomas. We examined microvascular density in astrocytic gliomas with and without IDH mutation and further determined whether D-2HG alters responses of cultured human brain microvascular endothelial (BME) cells.
MATERIALS AND METHODS
Astrocytic glioma patient cohort
Formalin-fixed, paraffin-embedded (FFPE) tissue samples from 60 adult patients diagnosed with high-grade astrocytic gliomas of central nervous system (CNS) World Health Organization (WHO) grade 3 and 4, were used for the chromogenic immunohistochemistry part of this study. One FFPE tissue block was used per patient. All patients underwent initial surgery between 1997 and 2017 at the Department of Neurosurgery, Heinrich Heine University, Düsseldorf, Germany, or at the Department of Neurosurgery, Odense University Hospital, Odense, Denmark. The tumor samples were reclassified according to the WHO classification of CNS tumors 2021 (26), and in line with the European Association of Neuro-Oncology (EANO) guidelines (27). Of the 60 samples, 14 were IDH-mutant astrocytomas, CNS WHO grade 3; 14 were IDH-mutant astrocytomas, CNS WHO grade 4; and 32 were IDH-wildtype glioblastomas, CNS WHO grade 4. Immunohistochemistry and/or a next generation gene panel sequencing were used to determine the IDH and alpha-thalassemia/mental retardation, X-linked mutation status, as previously described (28–30). When needed for accurate classification, next generation gene panel sequencing or droplet digital PCR assays were employed to detect possible telomerase reverse transcriptase (TERT) promoter mutations, epidermal growth factor receptor amplification, and cyclin-dependent kinase inhibitor 2A loss as previously reported (28, 30, 31). In individual cases the Infinium Methylation 850K EPIC BeadChip array (Illumina, San Diego, CA), was used to assess the chromosome 7+/10− and the chromosome 1p-/19q- cytogenetic signatures as described elsewhere (28, 32). The use of patient data and tissue was approved by the Institutional Review Board of the Medical Faculty, Heinrich Heine University, Düsseldorf, Germany (Study No. 5848R), the Regional Scientific Ethical Committee of the Region of Southern Denmark (Project-ID: S-20150148), and the official Danish data registration authority (the Data Protection Authority, file number: 16/11065).
Immunohistochemistry and digital image analysis
Three-µm-thick sections from FFPE tissue blocks were mounted on FLEX IHC slides (Dako, Glostrup, Denmark) and loaded onto the Discovery Ultra autostainer (Ventana Medical Systems, Tucson, AZ). The tissue sections were subjected to a standard immunostaining protocol with deparaffinization, epitope retrieval in Cell Conditioning 1 buffer (CC1, No. 950-500, Ventana) for 32 minutes at 100°C, and blockade of endogenous peroxidase activity. Sections were then incubated with primary antibody against CD34 (dilution 1:50, clone EP88, Cell Marque, Rocklin, CA) for 16 minutes at 36°C. Detection was done using the OptiView DAB IHC Detection Kit (No. 760-700, Ventana). The tissue slides were then counterstained with Hematoxylin II (No. 790-2208, Ventana) and Bluing Reagent (No. 760-2037, Ventana), dehydrated, and cleared. Coverslips were mounted using Pertex Mounting Medium (No. 00811, HistoLab Products AB, Gothenburg, Sweden). A Hamamatsu Digital Slide Scanner (Hamamatsu, Japan) was used to digitize the slides. Digital image analysis was done in the Visiopharm software module Stereology (Visiopharm, Hoersholm, Denmark). Non-necrotic, vital tumor areas were manually outlined as regions of interest. Exclusion criteria included normal brain tissue, tumor-infiltrating regions, necrotic areas, and larger blood vessels (i.e. arteries, veins, large arterioles). Sample images were then collected in the outlined regions of interest using systematic uniform random sampling at 20× magnification, obtaining at least 5 images per tumor. The average area size of each sample image was 0.21 mm2. Automated digital image assessment and quantification were carried out in the Visiopharm APP author module using a pixel-based algorithm as previously described (28, 33). The output variable was the percentage of CD34-positive area, defined as the area of positive expression divided by the total area of interest multiplied by 100.
Antibodies and reagents
Fluorescein isothiocyanate (FITC) antihuman junctional adhesion molecule 1 (JAM-1), Allophycocyanin (APC) antihuman CD31, or FITC-conjugated isotypes were antihuman IgG1 monoclonal antibodies obtained from BioLegend (San Diego, CA). Propidium iodide (PI)- or APC-labeled isotypes and FITC Annexin V were purchased from BD Biosciences (Franklin Lakes, NJ). Antihuman CD3 (OKT3), and soluble 2 µg/mL antihuman CD28 were from BioLegend. Antirabbit IgG (7074 seconds), zona occludens 1 (ZO-1), ZO-2, CD2-associated protein (CD2AP), and primary antibodies for phosphorylated mammalian target of rapamycin (P-mTOR), total mTOR (T-mTOR), phosphorylated signal transducer and activator of transcription 3 (P-STAT3), and total STAT3 (T-STAT3) were purchased from Cell Signaling (Danvers, MA). Recombinant human interleukin-2, chemokine (C-C motif) ligand 19 (CCL19), and chemokine (C-X-C motif) ligand 21 (CXCL21) were obtained from PeproTech (Rocky Hill, NJ).
Primary human BME cells
Primary human BME cells were obtained from Angio-Proteomie (Boston, MA) and maintained at complete EBM-2 Endothelial growth medium (Lonza, No. 190860, Basel, Switzerland) at 37°C in 5% CO2. For BME cultures, flasks/petri dishes and inserts were precovered with quick coat solution (R&D Systems, Minneapolis, MN).
Wound healing assay
Wound healing assessments were performed in a µ-Dish cell culture system containing 2-well silicone inserts with a defined cell-free gap (ibidi GMBH, Gräfelfing, Germany) according to the manufacturer’s instructions. In brief, 70 µL of the BME cells (3×105 cells/mL, passages 6–8) were seeded into each chamber of the culture inserts and incubated for 16 hours to allow cell attachment and confluence. Next, 0, 10, or 20 mM D-2HG was added, and the cells were cultured for 8 hours before the inserts were carefully removed to expose cell-free gaps. Images of the gaps were then recorded after 4, 8, and 14 hours under an inverted microscope. Wound healing was assessed by analyzing gap areas from 3 randomly selected microscopic images using the ImageJ software (NIH) (34).
Tube formation assay
BME cells (passages 5–6) were seeded at 2 × 105 cells per 75-cm2 tissue culture flask using medium 200PRF supplemented with Low Serum Growth Supplement (LSGS) (Thermo Fisher Scientific, Waltham, MA). The medium was changed every other day until a cell confluency of approximately 80% was reached. Next, 50 µL of Gibco Geltrex (Thermo Fisher Scientific) was added to each well of a 96-well plate and incubated for 30 minutes at 37°C to allow the gel to solidify. Then 100 µL of unsupplemented medium 200PRF (Thermo Fisher Scientific) containing detached BME cells (3 × 105 cells/ml) were added to each well in the presence of different concentrations of D-2HG (0, 5, 10, or 20 mM) followed by incubation for 14–16 hours. Images were captured under an inverted microscope camera, and the average number of tube nodes, segments, meshes, and total tube lengths were counted or calculated in 3 random microscopic pictures using the ImageJ software “angiogenesis analyzer” as previously described (35).
Cell proliferation assay
BME cells (passages 4–8) were seeded in 96-well plates at 1 × 104 cells/well. After 4 hours, the original medium was replaced with medium containing 0, 5, 10, or 20 mM D-2HG and incubated for 24, 48, or 72 hours at 37°C. Cell proliferation was assessed by measuring the amount of incorporated bromodeoxyuridine (BrdU) using the Cell Proliferation ELISA-BrdU Kit (Millipore, Burlington, MA) according to the manufacturer’s instructions.
BME monolayer permeability assay
Cell culture inserts with polycarbonate microporous membranes of 6.5-mm diameter and 1-µm pore size (Costar, Cambridge, MA) were coated with Quick Coat Solution at 37°C for 5 minutes. BME cells (passages 3–8, concentration 3.5 × 104 cells/mL) in 100 μL EBM-2 were added to each insert, and 600 μL EBM-2 was added to the lower transwell chamber. The BME cells were exposed to 0 or 20 mM D-2HG from day 4 to day 7. After 7 days in culture, the formation of confluent BME monolayers was confirmed by microscopy. To measure the permeability of the monolayers, each insert was washed 3 times with medium and transferred to a fresh 24-well plate. Then, 100 and 600 μL of phenol red-free RPMI 1640 medium with 10% fetal calf serum (FCS) containing 1 μg/mL FITC-dextran (molecular weight 40 KD, Sigma-Aldrich, St. Louis, MO) was added to the upper chamber and lower chamber, respectively. The 24-well plate with transwell inserts was incubated for another 4 hours at 37°C. The amount of FITC-dextran passing through the monolayer into the lower chambers were determined utilizing a Fluorescent Microplate Reader (Biotek, Winooski, VT) with excitation and emission wavelengths of 492 and 520 nm, respectively.
Human T-cell isolation and activation
Human primary T cells were isolated from peripheral blood mononuclear cells of healthy donors by negative selection using magnetic beads (BioLegend) according to the manufacturer’s protocol. The purity was >97% as determined by CD3 staining followed by flow cytometric analysis. Purified T cells were resuspended at 1 × 106 cells/mL and then activated with plate-bound 5 µg/mL antihuman CD3 (OKT3) and soluble 2 µg/mL antihuman CD28 plus 30 U/mL recombinant interleukin-2 in RPMI 1640 medium containing 10% FCS at 37°C, 5% CO2 for 24 hours.
T-cell transwell migration assay
Primary BME cells (passages 3–8) were seeded onto cell culture inserts with polycarbonate microporous membranes of 6.5 mm diameter and 8 µm pore size (Costar, Cambridge, MA) and cultured for 7 days to allow monolayers to form. On day 4, either 0 or 20 mM D-2HG was added into the wells containing the culture inserts, and on day 7 the formation of BME monolayers in all inserts was confirmed by microscopy. The inserts were then gently rinsed at least 3 times with RPMI 1640 medium to remove residual D-2HG and quickly transferred to a new 24-well plate containing 600 µL of fresh RPMI 1640 plus 10% FCS, with 50 ng/mL human CCL19 and 50 ng/mL human CXCL21 in the lower chambers. Next, 100 µL of activated purified human T cells (1–2 × 105 cells/mL) were added to the BME monolayers in the upper chamber and incubated at 37°C for 4 hours. The number of migrated T cells was assessed by taking 100 µL of cell-containing medium from the lower chamber into an Eppendorf tube and adding 400 µL 0.4% trypan blue (final concentration 0.32%). The number of migrated T cells was counted microscopically using hemocytometry. The percentage of migrated cells was calculated as the number of cells in the lower chamber divided by the total number of T cells added to the upper chamber multiplied by 100. Transwells with unactivated T cells in the upper chamber and without chemokine in the lower chamber were used as the negative controls. The percentage of spontaneous T-cell migration was <5%.
Flow cytometry
Single cell suspensions were incubated for 20 minutes on ice with various combinations of the following antibodies: APC antihuman CD31, FITC antihuman JAM-1, FITC Annexin V as well as appropriate PI-, APC-, or FITC-conjugated isotype controls. Staining was performed with 1 µg of each antibody per 1 × 106 cells. All the flow cytometry experiments were performed by gating 10,000–30,000 cells with a BD FACSCalibur flow cytometry and FlowJo version 10 software.
Western blotting
For Western blotting of the mTOR and STAT3 pathways, confluent BME cells (passages 6–8) were detached by exposure to 0.025% trypsin for 4 minutes, starved with serum-free medium 199 (Thermo Fisher Scientific) for 4 hours in 15 mL Eppendorf tubes, and then exposed to 0 or 20 mM D-2HG for 20 minutes in serum-free medium 199. Endothelial cell growth culture medium (EBM-2) was then added to each tube. The cells were incubated for defined times and washed twice with cold phosphate-buffered saline (PBS) before lysis and analysis. For ZO-1, ZO-2, and CD2AP Western blotting, BME cells were seeded into 6-well plates in culture medium (EBM-2) for 4 hours and washed before exposure to 0 or 20 mM D-2HG for 72 hours. BME cells were lysed in 200 μL RIPA buffer (10 mM Tris-Cl [pH 8.0], 1 mM EDTA, 0.5 mM EGTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 140 mM NaCl) (Abcam, Cambridge, United Kingdom) per well on ice and then scraped into 1.5 mL Eppendorf tubes, and lysed on ice for 30 minutes. Cells were cleared by centrifugation at 130,000g for 30 minutes at 4°C, and these supernatants were mixed with 4 × loading buffer supplemented with 10% β-mercaptoethanol (Thermo Fisher Scientific) and heated at 95°C for 15 minutes. Protein concentration was determined using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Equivalent amounts of protein (15–20 μg) for each sample were resolved by electrophoresis in SDS-polyacrylamide gels. Following electrophoresis, methanol-activated polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA) were placed over the gels, and proteins were eluted overnight at a constant voltage of 30 V. PVDF membranes were then blocked with 5% milk for 1 hour followed by incubation with the primary antibody (1:1000) overnight at 4°C. Detection was done using HRP-conjugated secondary antirabbit IgG (7074 seconds, Cell Signaling) diluted 1:4000 in 2.5% milk. Unbound proteins were removed by washing with 0.05% (V/V) Tween 20 in PBS (No. 0283C285, Bioexpress, Kaysville, UT). For exposure, the protein expression levels in the PVDF membranes were assessed using enhanced chemifluorescence (Thermo Fisher Scientific) and the laser scanner LAS-3000 (FujiFilm, Tokyo, Japan). If necessary, bound antibodies were removed using stripping buffer (No. 21059, Thermo Fisher Scientific) for 5–10 minutes at room temperature, and samples were reblotted in the same manner as described above. ImageJ was used to quantify the relative intensity of the Western-blot bands.
Statistics
Statistical analyses were performed using GraphPad Prism software version 6. One-way ANOVA was used to compare multiple groups, and Student unpaired t-test or Mann-Whitney U-test was used to compare 2 groups. The results were presented as mean±standard error of the mean (SEM). The significance level was set at p < 0.05.
RESULTS
Astrocytic gliomas with IDH mutation have lower CD34-positive blood vessel density
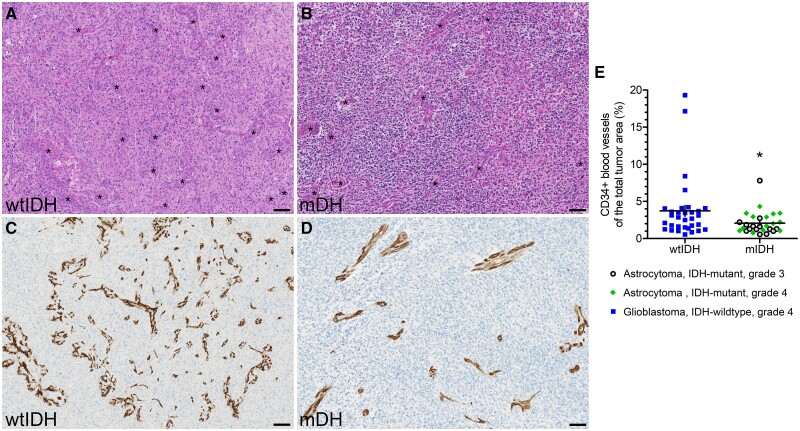
To investigate whether the locally accumulated high levels of D-2HG as a result of the IDH mutations might affect local endothelial cells, we stained IDH-wildtype and IDH-mutant tumors with an antibody against the endothelial marker CD34. Using hematoxylin and eosin staining, we had previously observed that microvessel density appeared to be higher in IDH-wildtype tumors (Fig. 1A, B). CD34 expression was both more abundant and more widely dispersed in IDH-wildtype glioblastomas (Fig. 1C) compared to IDH-mutant astrocytomas (Fig. 1D). IDH-mutant astrocytomas appeared to be particularly devoid of the smallest vascular structures. The quantified presence of CD34-positive microvessels was significantly lower in IDH-mutant than IDH-wildtype tumors (mean±SEM: 2.1 ± 0.3% vs 3.7 ± 0.7%; p = 0.030) (Fig. 1E), suggesting that D-2HG may negatively regulate the proliferation or survival of brain vascular endothelial cells and thereby reduce angiogenesis.
Figure 1.
Endothelial cell vascular density is reduced in IDH-mutant astrocytic gliomas. (A, B) Representative hematoxylin and eosin stained images of an IDH-wildtype (wtIDH) glioblastoma and IDH-mutant (mIDH) astrocytoma, CNS WHO grade 4. Asterisks indicate blood vessels. (C, D) Representative images of CD34 expression levels in a wtIDH glioblastoma and mIDH astrocytoma, CNS WHO grade 4. (E) The expression level of CD34 was used as a marker of endothelial cell vasculature and quantified using automated digital image analysis. The microvascular density was significantly lower in mIDH astrocytic gliomas, CNS WHO grade 3 or 4, compared to wtIDH glioblastomas, CNS WHO grade 4. Horizontal and vertical lines in panel E indicate mean±SEM, and data were analyzed by Mann-Whitney U-test. Each dot in panel E represents a single patient, and asterisk (*) signifies p < 0.05. Scale bar: 100 µm.
D-2HG inhibits proliferation of primary human BME cells without affecting their survival
To explain the possible reason for the reduced level of endothelial cell in situ, the growth rate of primary BME cells was assessed at different concentrations of D-2HG using a BrdU proliferation assay. These experiments showed that D-2HG significantly reduced the incorporation of BrdU into the newly synthesized DNA of BME cells in a concentration-dependent manner after 1 day (Fig. 2A) and 3 days (Fig. 2B) of exposure. We then used flow cytometry to determine whether the reduced cell number reflected a loss of membrane integrity or an induction of apoptosis and found that the BME populations remained viable without loss of plasma membrane phospholipid asymmetry even after exposure of up to 20 mM D-2HG for 24 hours (Fig. 2C, D). Collectively, these results suggest that D-2HG impairs proliferation of BME cells, but not their survival.
Figure 2.
D-2HG suppress BME cells proliferation but not viability. (A, B) BME cells were cultured in media with 0, 5, 10, and 20 mM of D-2HG together with BrdU. Levels of incorporated BrdU in the cell lysates was measured by ELISA and compared on day 1 (A) and day 3 (B). (C, D) Apoptosis was analyzed by Annexin V and PI staining. BME cells were cultured in media containing 0 (C) or 20 mM (D) D-2HG for 24 hours. Cell death and apoptosis were analyzed after staining with PI and Annexin V followed by flow cytometry analyses. Results are provided as mean+SEM from 3 experiments. Significance levels: ***p < 0.001 and ****p < 0.0001.
D-2HG inhibits migration of human BME cells
As vasculogenesis requires endothelial cell migration, we assessed the effect of D-2HG on the rate of wound closure in BME monolayers. BME cells were grown in normal medium to reach confluency after which the medium was replaced with one containing 0, 10, or 20 mM D-2HG. Following an 8-hour incubation period the culture inserts were removed to form uniform gaps in the monolayer. The recovery from this structural wound was then monitored by quantifying the rate of gap closure over the subsequent 4, 8, and 14 hours. These experiments showed that D-2HG inhibited BME wound healing at all 3 time points in a concentration-dependent manner (Fig. 3A, B), indicating that D-2HG directly affects endothelial cell growth and migration.
Figure 3.
D-2HG reduces migration of BME cells. (A) In a wound healing assay, BME cells were grown to confluence, and then structural gaps were made by removal of inserts. The cells were further cultured in media containing 0, 10, or 20 mM D-2HG and imaged using an Axio observer microscope at 5× magnification at 4, 8, and 14 hours. (B) Quantification of the wound healing area ratio compared to the control group (0 mM D-2HG) at 4, 8, and 14 hours. Results are provided as mean+SEM from ≥4 experiments, and data were analyzed by 1-way ANOVA. Significance levels: **p < 0.01, ***p < 0.001, and ****p < 0.0001.
D-2HG inhibits tube formation by cultured BME cells
To establish an in vitro model of the phenotype observed in the patient tissue and to explore the underlying mechanism, we determined whether D-2HG had a direct effect on endothelial cell function using culture conditions that enabled cultured BME cells to organize into tube-like structures. We exposed BME cells to pathophysiological concentrations of D-2HG, and after 2 weeks we analyzed the number, length, and complexity of the tubes formed over this period. D-2HG had a strong, concentration-dependent effect on endothelial cell tube formation (Fig. 4A). Estimation of the changes in the number of tubes (Fig. 4B) and their length (Fig. 4C) revealed a significant reduction at each D-2HG concentration. Further, the number of tube segments (Fig. 4D) and intersecting nodes (Fig. 4E) were also suppressed in a concentration-dependent manner, suggesting that the complexity of the tube formation was significantly altered by chronic exposure to D-2HG.
Figure 4.
D-2HG reduces BME cell tube formation and complexity. (A) In the tube formation assays, BME cells were cultured in media containing 0, 5, 10, or 20 mM D-2HG. Tube formation was imaged using an Axio observer microscope at 5× magnification. The bar charts illustrate the quantification of the fold change in tube number (B), tube length (C), segment number (D), and node number (E) relative to the control group (0 mM D-2HG). Results are provided as mean+SEM from ≥4 experiments, and data were analyzed by 1-way ANOVA. Significance levels: *p < 0.05, **p < 0.01, and ****p < 0.0001.
D-2HG improves endothelial cell barrier function
To determine whether D-2HG modulates BME permeability, BME cells were cultured on transwell insert in the absence or presence of 20 mM D-2HG. FITC-dextran was then added to the monolayer in the upper chambers, and the level of fluorescence in the bottom chambers was quantified at different time points allowing assessment of the BME monolayer permeability. These experiments showed that 20 mM D-2HG exposure significantly reduced the leakage of FITC-dextran across the monolayer (Fig. 5A, B). We also examined the rate of T-cell transendothelial migration by adding activated human T cells to the BME monolayers which had been pretreated with 0 or 20 mM D-2HG using the chemokines CCL19 and CXCL21 as chemoattractants. These experiments showed that D-2HG significantly impaired the number of T cells migrating across the BME monolayer (Fig. 5C, D).
Figure 5.
D-2HG impairs endothelial cell barrier permeability and T-cell transmigration. BME cells were cultured as monolayers in transwell inserts in the absence or the presence of 20 mM D-2HG. (A) Dextran permeability assay: BME paracellular permeability was assessed by measuring FITC-dextran leakage. FITC-dextran levels in the lower chambers were calculated using a FITC-dextran fluorescence concentration standard curve (20 minutes: p = 0.028, n = 10; 60 minutes: p = 0.016, n = 8; 120 minutes: p = 0.0028, n = 9; 180 minutes: p = 0.017, n = 8; 240 minutes: p = 0.016, n = 8). (B) Scatter plot of leaked dextran levels in the lower chamber after 240 minutes (p = 0.016). (C, D) T-cell transendothelial cell assays: T cells were purified from human peripheral blood monocytes by negative selection and then activated overnight with anti-CD3 and anti-CD28. After 3 gentle washes of endothelial monolayer with media to get rid of the D-2HG in the culture media, the activated T cells were added to the upper chambers of transwell inserts with 50 ng/mL human CCL19 and CXCL21 in the lower chamber. The percentage of T cells migrating through the BME cells monolayer in the lower chamber at (C) 1.5 hours (p = 0.039, n = 6) or (D) 4 hours (p = 0.0009, n = 16) was calculated. Results are provided as mean+SEM from ≥3 experiments, and data were analyzed by unpaired t-test. Significance levels: *p < 0.05, **p < 0.01, and ***p < 0.001.
D-2HG upregulates junctional protein expression and reduces mTOR/STAT3 phosphorylation in BME cells
To examine whether D-2HG impacts the expression of the junctional scaffold proteins that regulate cell-cell interaction and transcellular permeability, we cultured BME cells for 3 days in the absence or presence of D-2HG and performed Western blotting on the whole-cell lysates. The abundance of the tight junction proteins ZO-1 and ZO-2 were significantly increased in D-2HG-treated cell lysates (Fig. 6A–C). Similar results were obtained for the expression of the adaptor molecule CD2AP (Fig. 6D, E), which notably plays a vital role in maintaining the integrity of the blood-brain barrier (36) and in modulating inflammatory cell adhesion and paracellular migration (37). Using flow cytometry we found that D-2HG-exposed cells had an increased surface expression of the tight junction scaffolding protein JAM-1 (Fig. 6F, G), which is known to regulate intercellular contact (38). Furthermore, 20 mM D-2HG reduced rapid phosphorylation of mTOR phosphorylation in response to serum stimulation (Fig. 7A, B). As a serine/threonine kinase, the mTOR protein is triggered by signals from upstream stimuli. These signals increase mTOR activity, which then stimulates downstream targets, including the transcriptional component STAT3. When activated, STAT3 binds to DNA and controls the production of genes that are essential for cell growth, metabolism, and other cellular functions, including endothelial cell proliferation and tube formation (39). We found that D-2HG exposure inhibited STAT3 phosphorylation (Fig. 7C, D). Together, these results indicate that D-2HG may enhance endothelial cell barrier function and block endothelial cell proliferation and tube formation by inhibiting the mTOR/STAT3 signaling pathway.
Figure 6.
D-2HG increases endothelial cell junctional protein expression. (A–E) BME cells were plated in a 6-well plate and cultured with or without 20 mM D-2HG for 72 hours. The cell lysate was subjected to Western blotting using antibodies specific for ZO-1 and ZO-2 (A), and CD2AP (D) using β-actin as loading control (A, D). Bar charts of the quantified and adjusted band densities of ZO-1 (B), ZO-2 (C), and CD2AP (E) after adjustment to comparing the D-2HG exposed group to the control group (W/O). (F) Flow chart illustrating the cell surface expression levels of JAM-1 after exposure to 0 or 20 mM D-2HG. (G) Scatter plot showing the JAM-1 expression levels represented as MFI (mean fluorescence intensity) fold compared to the control group (W/O). Results are provided as mean+SEM from ≥3 experiments, and data were analyzed by unpaired t-test. Significance levels: *p < 0.05, **p < 0.01, ****p < 0.0001. See Supplementary Data for original Western blots.
Figure 7.
D-2HG inhibits mTOR and STAT3 phosphorylation in BME cells. (A–D) BME cells were starved for 4 hours, treated with 0 or 20 mM D-2HG for 20 minutes, and cultured in complete media. Cells were harvested at 0, 5, 15, or 60 minutes and analyzed by Western blot. β-actin was used as a loading control. The expression patterns of P-mTOR (Tyr397) (A, C), P-STAT3 (Thr202/Tyr204) (B, D), and β-actin expression patterns were analyzed using ImageJ software. The bar charts represent the ratio of phosphorylation/total band density of mTOR (C) and STAT3 (D) normalized to the control group (W/O). Results are provided as mean+SEM from ≥3 experiments, and data were analyzed by unpaired t-test. Significance levels: ***p < 0.001, and ****p < 0.0001. See Supplementary Data for original Western blots.
DISCUSSION
Mutations at the catalytic site of IDH1 or IDH2 are common in diffuse gliomas. However, the biological significance of these mutations and the resulting oncometabolite D-2HG, which is excessively produced by IDH-mutant cells, remains somewhat enigmatic (6, 17, 40). In this study, we quantified microvascular density in tumor tissue samples of IDH-wildtype versus IDH-mutant high-grade astrocytic gliomas. We found that patients with IDH-mutant astrocytic gliomas had significantly reduced number of blood vessels within their tumors, suggesting that IDH mutation may negatively affect the proliferation and/or survival of endothelial cells. This finding is supported by a transcriptome study which found an altered transcriptomic signature correlating with decreased vasculogenesis in gliomas expressing mutant IDH (23). Similarly, Sun et al (41) reported that the presence of IDH mutation correlated with poorer pericyte coverage of microvessels in gliomas.
Functional exploration of the “oncometabolite” D-2HG showed that D-2HG had a direct effect on endothelial cell biology, affecting several complex functions of BME cells, but also that the direction of these changes was not uniform. We found that D-2HG reduced endothelial cell growth and migration but did not impact their viability. While D-2HG reduced the organization of endothelial cells into tubular scaffolds, the intercellular interactions were enhanced by stimulating junctional protein expression impairing transcellular flow across the endothelial cell monolayer. Some studies have shown similar results. Gene expression analyses in histologically low-grade diffuse gliomas demonstrated that angiogenic genes, e.g. angiopoietin 2 and serpin family H, are upregulated in IDH-wildtype tumors leading to increased endothelial cell migration and matrix remodeling (23). In addition, genotype/imaging phenotype correlation analysis reported that IDH-mutant gliomas have a more balanced level of pro- and antiangiogenic factors compared to IDH-wildtype tumors. In contrast, pathways involved in hypoxia, vasculogenesis, and angiogenesis are upregulated in IDH-wildtype gliomas resulting in elevated levels of proangiogenic molecules (23). Furthermore, research has shown that the metabolite S-2-hydroxyglutarate (S-2HG) plays a crucial role in promoting endothelial quiescence and restraining angiogenic activity (42). The D-2HG and S-2HG enantiomers are 2 conformations of 2-hydroxyglutarate that may have similar functions.
Interestingly, our data suggest that D-2HG inhibited endothelial cell proliferation without compromising cell survival. At the same time, D-2HG increased expression of tight junction proteins in endothelial cells, thereby reducing the transport of metabolites and circulating cells including T cells between the vascular endothelial space. Reportedly, IHD-mutant gliomas contain fewer tumor-infiltrating T cells within the tumor microenvironment compared to their IDH-wildtype counterparts (16), and this may thus partly be explained by stronger barrier function and lower microvessel density in gliomas harboring an IDH mutation. Andrade et al (42) also reported that S-2HG inhibited cell proliferation and caused cell cycle arrest at the G0/G1 phase in endothelial cells without inducing metabolic distress, senescence, or apoptotic cell death. It is well-established that tumor vascularization is characterized by a large vascular endothelial space, high vascular permeability, and structural disorder (43). Studies have shown that vascular leakage raises intratumoral fluid pressure and reduces tumor blood flow, which can hinder the delivery of anticancer drugs, including immunotherapies (44). Abnormal leakiness of blood vessels may also promote tumor cell dissemination and metastasis (44). These findings imply that IDH mutation and D-2HG may hold some tumor suppressive functions. We therefore hypothesize that IDH-mutant tumorigenesis is at least partly self-limiting. Several clinical and experimental studies support this speculation. First, IDH-mutant diffuse gliomas, regardless of malignancy grade, are less aggressive, easier to remove, and correlate with a better prognosis than IDH-wildtype diffuse gliomas (45). Second, IDH-wildtype high-grade gliomas exhibit larger areas of blood oxygen level-dependent (BOLD) signal aberration beyond the tumor margin, suggesting the existence of widespread tumor-induced vascular disorder within the brain (25). Specifically, antiangiogenic drug therapy can ameliorate the extent of BOLD signal abnormalities in the surroundings of the tumor, indicating that changes in BOLD signals may serve as a biomarker for the efficacy of antiangiogenic therapy (46). High intratumoral levels of D-2HG may impair the efficacy of antiangiogenic drugs, which could explain why patients with an IDH-mutant glioma respond poorly to antiangiogenic agents (47).
We found that D-2HG suppressed mTOR/STAT3 phosphorylation in endothelial cells. Reportedly, inhibition of the mTOR/STAT3 pathway impairs tumor angiogenesis (39), and blocking mTOR signaling was found to hinder endothelial cell proliferation and migration as well as reduce angiogenesis in several animal models (48, 49). In addition, mTOR inhibition can affect endothelial cell morphology and impair endothelial cell elongation as well as tube formation by overstabilizing microtubules to disrupt cytoskeletal organization (50). Previous work also reported that D-2HG suppresses ATP synthase function leading to reduced mTOR signaling and cell growth (51). Furthermore, STAT3 is known to play a key role in angiogenesis, endothelial cell proliferation, and the development of vascular barrier function (21, 22). Interestingly, D-2HG was shown to suppress the protein level expression of STAT1, leading to reduced chemokines levels and CD8-positive cytotoxic T-cell migration (15). We also found that the endothelial cell adhesion molecule JAM-1 was increased when D-2HG was present. In addition, D-2HG upregulated the expression of the tight junction proteins ZO-1, ZO-2, and CD2AP. ZO-1, ZO-2, and CD2AP are critical for the maintenance of endothelial barrier function and the regulation of vascular permeability and paracellular transport (52, 53). CD2AP is believed to serve as a connection between membrane proteins and the cytoskeleton (54), and JAM-1 has been reported to enhance the junctional localization of ZO-1 and ZO-2 (55, 56). Interestingly, blocking the mTOR pathway can promote endothelial cell JAM-1 expression to protect the vascular barrier function in models of Alzheimer disease (53). Taken together, these results indicate that blockade of the mTOR pathway can inhibit endothelial cell proliferation, migration, and angiogenesis as well as upregulate the expression of intercellular junctional proteins to protect the barrier function of the endothelial cell monolayer.
There are some limitations to our study. First, although we observed reduced microvessel levels in IDH-mutant glioma specimens, our results were mainly derived from ex vivo experiments manipulating angiogenesis and vascular barrier function. Second, we used relatively high concentrations of D-2HG that are found within centimeters of the intracranial gliomas. Seeing that the D-2HG levels at these locations are only modeled, we do not know the actual concentration of D-2HG experienced by tumor microvasculature. Therefore, our results need to be further explored in an in vivo setting.
In conclusion, we discovered that IDH-mutant high-grade astrocytic tumors had reduced CD34-positive microvessel density compared to IDH-wildtype glioblastomas. The oncometabolite D-2HG significantly inhibited angiogenic responses ex vivo without destroying pre-existing blood vessels. Instead, the remaining vessels demonstrated enhanced paracellular barrier function with augmented expression of tight junction proteins. Our data indicate that the role of D-2HG on the glioma microvasculature and microenvironment is complex, implying that it cannot simply be delimited as a tumor promoting metabolite. In addition, our results may provide a molecular basis for resistance to antiangiogenic drugs in glioma patients whose tumors carry an IDH mutation.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the staff of the Department of Immunology, Lerner Research Institute, Cleveland Clinic, for excellent technical support.
Contributor Information
Chun Cao, Department of Hematology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China; Department of Inflammation and Immunity, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio, USA.
Lingjun Zhang, Department of Inflammation and Immunity, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio, USA.
Mia D Sorensen, Department of Pathology, Odense University Hospital, Odense, Denmark; Department of Clinical Research, University of Southern Denmark, Odense, Denmark.
Guido Reifenberger, Institute of Neuropathology, Medical Faculty, Heinrich Heine University, and University Hospital Düsseldorf, Düsseldorf, Germany; German Cancer Consortium (DKTK), Partner Site Essen/Düsseldorf, Düsseldorf, Germany.
Bjarne W Kristensen, Department of Pathology, Odense University Hospital, Odense, Denmark; Department of Clinical Research, University of Southern Denmark, Odense, Denmark; Department of Pathology, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark; Department of Clinical Medicine and Biotech Research and Innovation Center (BRIC), University of Copenhagen, Copenhagen, Denmark.
Thomas M McIntyre, Department of Cardiovascular & Metabolic Sciences, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio, USA.
Feng Lin, Department of Inflammation and Immunity, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio, USA.
FUNDING
This work is supported in part by grants NIH R01 DK 10358 (FL), Cleveland Clinic Center of Excellence in Cancer-Associated Thrombosis Award (FL and TMM), and VeloSano Pilot Project Award (TMM and FL).
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
SUPPLEMENTARY DATA
Supplementary Data can be found at academic.oup.com/jnen.
REFERENCES
- 1. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med 2009;360:765–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008;321:1807–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu S, Cadoux-Hudson T, Schofield CJ.. Isocitrate dehydrogenase variants in cancer—Cellular consequences and therapeutic opportunities. Curr Opin Chem Biol 2020;57:122–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009;462:739–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang LE. Friend or foe-IDH1 mutations in glioma 10 years on. Carcinogenesis 2019;40:1299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Horbinski C. What do we know about IDH1/2 mutations so far, and how do we use it? Acta Neuropathol 2013;125:621–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Linninger A, Hartung GA, Liu BP, et al. Modeling the diffusion of D-2-hydroxyglutarate from IDH1 mutant gliomas in the central nervous system. Neuro Oncol 2018;20:1197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi C, Ganji SK, DeBerardinis RJ, et al. 2-Hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med 2012;18:624–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xiong N, Gao X, Zhao H, et al. Using arterial-venous analysis to characterize cancer metabolic consumption in patients. Nat Commun 2020;11:3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Unruh D, Schwarze SR, Khoury L, et al. Mutant IDH1 and thrombosis in gliomas. Acta Neuropathol 2016;132:917–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feyissa AM, Worrell GA, Tatum WO, et al. Potential influence of IDH1 mutation and MGMT gene promoter methylation on glioma-related preoperative seizures and postoperative seizure control. Seizure 2019;69:283–9 [DOI] [PubMed] [Google Scholar]
- 12. Chen H, Judkins J, Thomas C, et al. Mutant IDH1 and seizures in patients with glioma. Neurology 2017;88:1805–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bunse L, Pusch S, Bunse T, et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med 2018;24:1192–203 [DOI] [PubMed] [Google Scholar]
- 14. Richardson LG, Nieman LT, Stemmer-Rachamimov AO, et al. IDH-mutant gliomas harbor fewer regulatory T cells in humans and mice. OncoImmunology 2020;9:1806662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kohanbash G, Carrera DA, Shrivastav S, et al. Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas. J Clin Invest 2017;127:1425–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang L, Sorensen MD, Kristensen BW, et al. D-2-Hydroxyglutarate is an intercellular mediator in IDH-mutant gliomas inhibiting complement and T cells. Clin Cancer Res 2018;24:5381–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ježek P. 2-Hydroxyglutarate in cancer cells. Antioxid Redox Signal 2020;33:903–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Du X, Hu H.. The roles of 2-hydroxyglutarate. Front Cell Dev Biol 2021;9:651317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mellinghoff IK, van den Bent MJ, Blumenthal DT, et al. ; INDIGO Trial Investigators. Vorasidenib in IDH1- or IDH2-mutant low-grade glioma. N Engl J Med 2023;389:589–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Popov S, Jury A, Laxton R, et al. IDH1-associated primary glioblastoma in young adults displays differential patterns of tumour and vascular morphology. PLoS One 2013;8:e56328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kang SH, Yu MO, Park KJ, et al. Activated STAT3 regulates hypoxia-induced angiogenesis and cell migration in human glioblastoma. Neurosurgery 2010;67:1386–95; discussion 1395. [DOI] [PubMed] [Google Scholar]
- 22. Chen Z, Han ZC.. STAT3: A critical transcription activator in angiogenesis. Med Res Rev 2008;28:185–200 [DOI] [PubMed] [Google Scholar]
- 23. Kickingereder P, Sahm F, Radbruch A, et al. IDH mutation status is associated with a distinct hypoxia/angiogenesis transcriptome signature which is non-invasively predictable with rCBV imaging in human glioma. Sci Rep 2015;5:16238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guo H, Kang H, Tong H, et al. Microvascular characteristics of lower-grade diffuse gliomas: Investigating vessel size imaging for differentiating grades and subtypes. Eur Radiol 2019;29:1893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Englander ZK, Horenstein CI, Bowden SG, et al. Extent of BOLD vascular dysregulation is greater in diffuse gliomas without isocitrate dehydrogenase 1 R132H mutation. Radiology 2018;287:965–72 [DOI] [PubMed] [Google Scholar]
- 26. WHO Classification of Tumours Editorial Board. Central Nervous System Tumours. 5th edition. Lyon, France: International Agency for Research on Cancer (IARC; ); 2021. [Google Scholar]
- 27. Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 2021;18:170–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sørensen MD, Dahlrot RH, Boldt HB, et al. Tumour-associated microglia/macrophages predict poor prognosis in high-grade gliomas and correlate with an aggressive tumour subtype. Neuropathol Appl Neurobiol 2018;44:185–206 [DOI] [PubMed] [Google Scholar]
- 29. Dahlrot RH, Kristensen BW, Hjelmborg J, et al. A population-based study of low-grade gliomas and mutated isocitrate dehydrogenase 1 (IDH1). J Neurooncol 2013;114:309–17 [DOI] [PubMed] [Google Scholar]
- 30. Petersen JK, Boldt HB, Sørensen MD, et al. Targeted next-generation sequencing of adult gliomas for retrospective prognostic evaluation and up-front diagnostics. Neuropathol Appl Neurobiol 2021;47:108–26 [DOI] [PubMed] [Google Scholar]
- 31. Wolter M, Felsberg J, Malzkorn B, et al. Droplet digital PCR-based analyses for robust, rapid, and sensitive molecular diagnostics of gliomas. Acta Neuropathol Commun 2022;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feber A, Guilhamon P, Lechner M, et al. Using high-density DNA methylation arrays to profile copy number alterations. Genome Biol 2014;15:R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petterson SA, Sørensen MD, Kristensen BW.. Expression profiling of primary and recurrent glioblastomas reveals a reduced level of Pentraxin 3 in recurrent glioblastomas. J Neuropathol Exp Neurol 2020;79:975–85 [DOI] [PubMed] [Google Scholar]
- 34. Schneider CA, Rasband WS, Eliceiri KW.. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ouchani F, Jeanne A, Thevenard J, et al. Ethoxyfagaronine, a synthetic analogue of fagaronine that inhibits vascular endothelial growth factor-1, as a new anti-angiogeneic agent. Invest New Drugs 2015;33:75–85 [DOI] [PubMed] [Google Scholar]
- 36. Cochran JN, Rush T, Buckingham SC, et al. The Alzheimer’s disease risk factor CD2AP maintains blood-brain barrier integrity. Hum Mol Genet 2015;24:6667–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schaefer A, van Duijn TJ, Majolee J, et al. Endothelial CD2AP binds the receptor ICAM-1 to control mechanosignaling, leukocyte adhesion, and the route of leukocyte diapedesis in vitro. J Immunol 2017;198:4823–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hartmann C, Schwietzer YA, Otani T, et al. Physiological functions of junctional adhesion molecules (JAMs) in tight junctions. Biochim Biophys Acta Biomembr 2020;1862:183299. [DOI] [PubMed] [Google Scholar]
- 39. Yang F, Zhang W, Li D, et al. Gadd45a suppresses tumor angiogenesis via inhibition of the mTOR/STAT3 protein pathway. J Biol Chem 2013;288:6552–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang J, Yu J, Tu L, et al. Isocitrate dehydrogenase mutations in glioma: From basic discovery to therapeutics development. Front Oncol 2019;9:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sun C, Zhao Y, Shi J, et al. Isocitrate dehydrogenase1 mutation reduces the pericyte coverage of microvessels in astrocytic tumours. J Neurooncol 2019;143:187–96 [DOI] [PubMed] [Google Scholar]
- 42. Andrade J, Shi C, Costa ASH, et al. Control of endothelial quiescence by FOXO-regulated metabolites. Nat Cell Biol 2021;23:413–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ahir BK, Engelhard HH, Lakka SS.. Tumor development and angiogenesis in adult brain tumor: Glioblastoma. Mol Neurobiol 2020;57:2461–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martin JD, Seano G, Jain RK.. Normalizing function of tumor vessels: Progress, opportunities, and challenges. Annu Rev Physiol 2019;81:505–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mirchia K, Richardson TE.. Beyond IDH-mutation: Emerging molecular diagnostic and prognostic features in adult diffuse gliomas. Cancers (Basel) 2020;12:1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Laufer S, Mazuz A, Nachmansson N, et al. Monitoring brain tumor vascular heamodynamic following anti-angiogenic therapy with advanced magnetic resonance imaging in mice. PLoS One 2014;9:e115093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schiff D, de Groot JF.. Lower-grade gliomas: The wrong target for bevacizumab. Neuro Oncol 2018;20:1559–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Falcon BL, Barr S, Gokhale PC, et al. Reduced VEGF production, angiogenesis, and vascular regrowth contribute to the antitumor properties of dual mTORC1/mTORC2 inhibitors. Cancer Res 2011;71:1573–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang S, Amato KR, Song W, et al. Regulation of endothelial cell proliferation and vascular assembly through distinct mTORC2 signaling pathways. Mol Cell Biol 2015;35:1299–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tsuji-Tamura K, Ogawa M.. Dual inhibition of mTORC1 and mTORC2 perturbs cytoskeletal organization and impairs endothelial cell elongation. Biochem Biophys Res Commun 2018;497:326–31 [DOI] [PubMed] [Google Scholar]
- 51. Fu X, Chin RM, Vergnes L, et al. 2-Hydroxyglutarate inhibits ATP synthase and mTOR signaling. Cell Metab 2015;22:508–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yeung D, Manias JL, Stewart DJ, et al. Decreased junctional adhesion molecule – A expression during blood-brain barrier breakdown. Acta Neuropathol 2008;115:635–42 [DOI] [PubMed] [Google Scholar]
- 53. Van Skike CE, Jahrling JB, Olson AB, et al. Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer’s disease and vascular cognitive impairment. Am J Physiol Heart Circ Physiol 2018;314:H693–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. González-Mariscal L, Tapia R, Chamorro D.. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta 2008;1778:729–56 [DOI] [PubMed] [Google Scholar]
- 55. Haas AJ, Zihni C, Ruppel A, et al. Interplay between extracellular matrix stiffness and JAM-A regulates mechanical load on ZO-1 and tight junction assembly. Cell Rep 2020;32:107924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Monteiro AC, Sumagin R, Rankin CR, et al. JAM-A associates with ZO-2, afadin, and PDZ-GEF1 to activate Rap2c and regulate epithelial barrier function. Mol Biol Cell 2013;24:2849–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.