Abstract
We have applied an under-explored backbone modification strategy to generate new analogues of peptides that activate two clinically important class B1 G protein-coupled receptors (GPCRs). Most peptide modification strategies involve changing side chains, or, less commonly, changing configuration at side chain-bearing carbons (i.e., L residues replaced by D residues). In contrast, backbone modifications alter the number of backbone atoms and/or the identities of backbone atoms relative to a poly-α-amino acid backbone. Starting from the peptide agonists PTH(1-34) (the first 34 residues of parathyroid hormone, used clinically as the drug teriparatide) and glucagon-like peptide-1 (7-36) (GLP-1(7-36)), we replaced native α-residue triads with a diad composed of a β-amino acid residue and a γ-amino acid residue. The β/γ diad retains the number of backbone atoms in the ααα triad. Because the β and γ residue each bear a single side chain, we implemented ααα→βγ replacements at sites that contained a Gly residue (i.e., at α residue triads that presented only two side chains). All seven of the α/β/γ-peptides derived from PTH(1-34) or GLP-1(7-36) bind to the cognate receptor (the PTHR1 or the GLP-1R), but they vary considerably in their activity profiles. Outcomes include functional mimicry of the all-α agonist, receptor-selective agonist activity, biased agonism or strong binding with weak activation, which could lead to antagonist development. Collectively, these findings demonstrate that ααα→βγ replacements, which are easily implemented via solid-phase synthesis, can generate peptide hormone analogues that display unique and potentially useful signaling behavior.
Graphical Abstract

INTRODUCTION
Long polypeptide hormones that activate class B1 G protein-coupled receptors (GPCRs) exert powerful effects on human physiology. Many human class B1 GPCRs are targeted by peptide drugs that treat diverse diseases. For example, agonists of the parathyroid hormone receptor-1 (PTHR1) are prescribed to treat osteoporosis or hypoparathyroidism,1 and agonists of the glucagon-like peptide-1 receptor (GLP-1R) are used to treat type 2 diabetes and obesity.2,3 Signaling by these and other GPCRs is complex for several reasons: (1) each receptor interacts with multiple intracellular partners (including G proteins, β-arrestins and GPCR kinases (GRKs));4,5 (2) signaling can occur over different time scales and in different cellular locations depending on activation mode;6,7 and (3) some receptors are engaged by more than one natural agonist,8-10 each with a distinct activity profile. This last feature is illustrated by the two natural agonists of the PTHR1, parathyroid hormone (PTH) and parathyroid hormone-related protein (PTHrP).1,8 PTH stimulates prolonged production of intracellular cAMP, presumably resulting from GS heterotrimer activation, and most cAMP is generated after the agonist-receptor complex has been internalized.6,7 In contrast, PTHrP causes transient cAMP production from agonist-receptor complexes at the cell surface; internalization of the PTHrP-PTHR1 complex terminates signaling.6,7 This system also provides an example of polypharmacology: PTH activates the PTHR2 in addition to the PTHR1, but PTHrP is selective for the PTHR1, and another peptide hormone, tuberoinfundibular peptide of 39 residues (TIP39), is selective for the PTHR2.8,11,12 The GLP-1R is activated by at least three endogenous polypeptides, GLP-1, glucagon and oxyntomodulin, which manifest different activation profiles.9,13,14
Unnatural GPCR agonists with distinctive signaling profiles relative to natural agonists are valuable tools for elucidating relationships between specific activation parameters and responses at the cell or organism level.2,3,15-17 Some non-natural signaling profiles may prove to be advantageous from a therapeutic perspective.18,19 Natural peptide agonists result from ribosomal synthesis; therefore, signaling profile differences among natural agonists arise from sequence differences, i.e., differences in side chain identity along a homochiral poly-α-amino acid backbone. Chemical synthesis provides access to distinct and complementary structural variations.20-23 For example, replacement of native α-amino acid residues with β-amino acid residues at one site or more can cause significant changes in an agonist’s relative propensities to stimulate cAMP production vs. β-arrestin recruitment; biased agonists of the PTHR124,25 or the GLP-1R26,27 have been generated in this way. In addition, α→β replacement in a dual agonist of the PTHR1 and PTHR2 can deliver highly selective agonists of either the PTHR1 or the PTHR2.28 Guichard and colleagues showed that α→ureido residue replacement near the N-terminus of exendin-4, a natural agonist of the GLP-1R, induced bias toward cAMP production relative to β-arrestin recruitment.29 Cai et al. have developed a novel family of δ-amino acid residues that can replace a pair of α-amino acid residues. A GLP-1 analogue with a fully unnatural backbone was >100-fold less active than GLP-1 itself,30 but activity was retained after one or two αα→δ modifications near the C-terminus of GLP-1 or glucagon.31,32
Modification of a natural peptide via α→β or α→ureido replacement extends the backbone by one or two atoms (Figure S1). It is therefore somewhat surprising that these types of modification can deliver potent analogues of peptide hormones. Many α→β or α→ureido replacements cause partial or complete loss of agonist activity;28,29,33-35 examples that retain the capacity for potent receptor activation must be discovered via systematic surveys. We wondered whether a backbone modification strategy that maintains the natural number of backbone atoms could provide potent hormone analogues with non-native signaling profiles. Here we describe outcomes of replacing three contiguous natural α-amino acid residues with a β residue + γ residue pair. Previous structural studies have shown that peptides containing α+β+γ combinations can adopt α-helix-like conformations,36-41 and that short α/β/γ-peptides can bind to proteins that naturally recognize α-helical peptides;42,43 however, the prior work does not reveal how ααα→βγ modification affects the stability of the helical conformation. These precedents encouraged us to explore the ααα→βγ replacement strategy in agonists of class B1 GPCRs, which are largely or entirely helical in the receptor-bound state.44-49
RESULTS AND DISCUSSION
Design Considerations
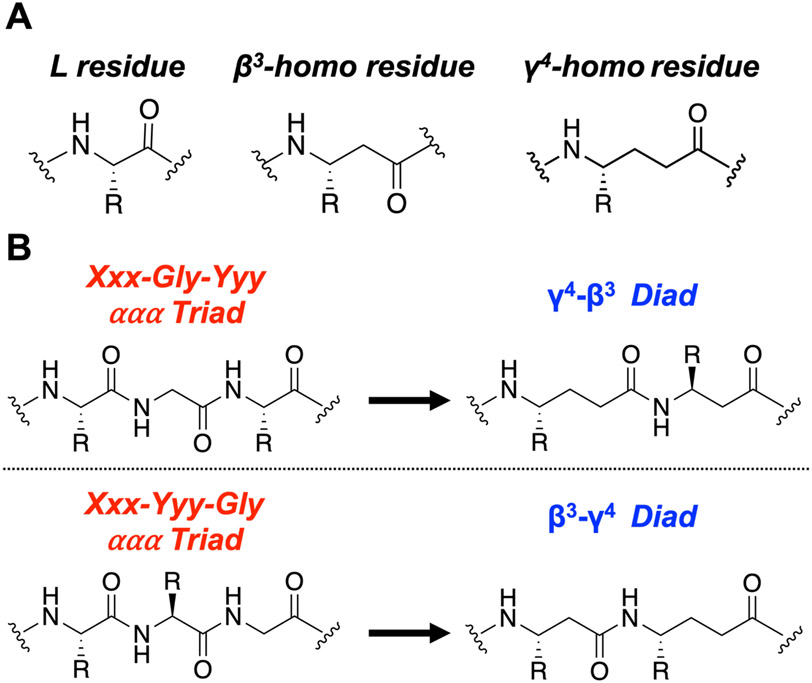
We sought to retain the natural complement of side chains as we evaluated the ααα→βγ replacement strategy. Most β- or γ-amino acids that are commercially available in protected forms suitable for solid-phase synthesis bear a single side chain, on the carbon adjacent to the amine group (β3 or γ4) (Figure 1A). These considerations directed our attention to ααα segments within natural hormones that contain a Gly residue, because such ααα segments bear only two side chains. We envisioned that an Xxx-Gly-Yyy triad could be replaced with a γ4-β3 diad, and an Xxx-Yyy-Gly triad could be replaced with a β3-γ4 diad (Figure 1B), which would allow retention of both side chains from the original ααα triad and approximate the natural positioning of those side chains along the new backbone. Gly-containing triads near the agonist N-terminus seemed most likely to produce changes in signaling profile because this portion of the peptide engages the transmembrane domain of the receptor, which, in turn, interacts with cytosolic partner proteins.50,51
Figure 1.
The ααα→βγ backbone replacement approach utilized in this work. (A) Generic structures of L-amino acid residues and β3- and γ4-homoamino acid residues. (B) Overview of two backbone replacements starting with either the Xxx-Gly-Yyy or Xxx-Yyy-Gly ααα triad; each of these replacements retains original side chains and the total number of backbone atoms found in the original ααα triad. Xxx or Yyy = any chiral residue; Gly = achiral Gly residue.
ααα→βγ Backbone Modification Generates PTHR1-selective and GS-biased Analogues of PTH(1-34)
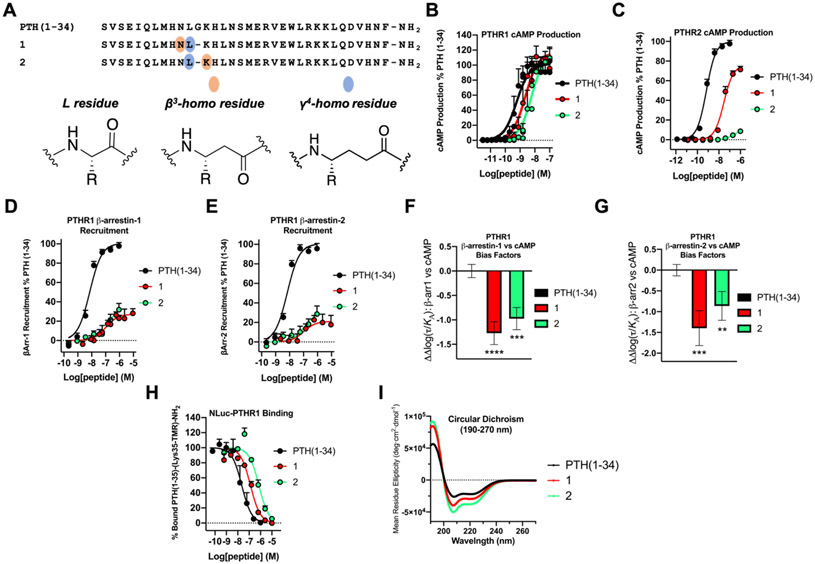
Full-length human PTH contains 84 residues, but the N-terminal fragment PTH(1-34) is fully active at the PTHR1; this fragment (teriparatide) is used to treat osteoporosis.52 Our initial studies focused on PTH(1-34) analogues 1 and 2, which contain ααα→βγ replacements that involve Gly12 (Figure 2A). These and all other peptides discussed here were synthesized as C-terminal amides, consistent with our previous studies.24-28,35 Luck et al. found that rat PTH(1-14) could activate the human PTHR1, and they reported an alanine-scan of this fragment.53 Rat PTH(1-14) differs from a human PTH(1-14) fragment by just one residue: Ala in place of Ser at position 1. This study showed that replacing Asn10, Leu11, Gly12 or Lys13 of rat PTH(1-14) with Ala had no significant impact on stimulation of PTHR1 cAMP production, but only one high agonist concentration was examined.
Figure 2.
PTH(1-34) α/β/γ-analogues display PTHR1 selectivity and bias toward cAMP production and away from β-arrestin recruitment. (A) Sequences of PTH(1-34) and α/β/γ-analogues 1 and 2. (B) PTHR1 activation, via cAMP production, in HEK293 cells stably expressing the PTHR1 and the cAMP-sensing protein GloSensor. Data points represent the average of 4 independent experiments. (C) PTHR2 activation, via cAMP production, in HEK293 cells stably expressing PTHR2 and GloSensor. Data is derived from ≥4 independent experiments. (D,E) PTHR1 β-arrestin-1 and −2 recruitment dose-responses. Assays were performed in CHO-FlpIn cells stably transfected with the PTHR1-RLuc8 and β-arrestin-1-Venus or β-arrestin-2-Venus. Data represent the mean of ≥4 (β-arrestin-1) or ≥3 (β-arrestin-2) independent experiments. All cAMP, β-arrestin-1 and β-arrestin-2 data was normalized against PTH(1-34) and all uncertainties are expressed as SEM. (F,G) PTHR1 β-arrestin-1 or −2 vs. cAMP bias factors were generated from dose-response curves in Figures 2B, D, and E. Negative values indicate bias towards cAMP production. Bias factor uncertainties are expressed in SD. ** = p ≤ 0.01; *** = p ≤ 0.001; **** = p ≤ 0.0001; by one-way ANOVA followed by Dunnett test. (H) Competition binding measurements by bioluminescence resonance energy transfer (BRET) in HEK293 cells expressing the NLuc-PTHR1. The peptide tracer was a tetramethylrhodamine (TMR)-labeled derivative of PTH(1-34), PTH(1-35)-(Lys35-TMR)-NH2. Data (as IC50 values) represent the average of 3 independent experiments. (I) Circular dichroism data measured in 50 mM PBS buffer, pH 7.3, 20% 2,2,2-trifluoroethanol (TFE).
Each of the α/β/γ-analogues was a potent agonist of the PTHR1, as measured by stimulation of cAMP formation in HEK293 cells that stably express the receptor and the cAMP-sensing GloSensor protein. Peptides 1 and 2 were each only slightly less potent than PTH(1-34) itself at PTHR1 (Figure 2B and Table 1): EC50 for 1 was ~4-fold higher than for PTH(1-34), and EC50 for 2 was ~11-fold higher. The PTHR2 is also potently activated by PTH(1-34),28 but for this receptor the ααα→βγ substitutions caused substantial declines in agonist activity (Figure 2C and Table 1). EC50 for 1 was ~42-fold higher than for PTH(1-34), and activity of 2 was barely detectable at the highest concentration examined (1 μM). Thus, replacing the natural Leu-Gly-Lys triad with a γ4-homoleucine (γ4-hLeu)-β3-homolysine (β3-hLys) diad (peptide 2) converted a potent dual agonist to a highly selective PTHR1 agonist.
Table 1.
PTHR1 and PTHR2 Activation for PTH(1-34) and α/β/γ-Analogues 1 and 2.
| PTHR1 cAMP Production | PTHR2 cAMP Production | |||||||
|---|---|---|---|---|---|---|---|---|
| Peptide | pEC50 | EC50 (nM) |
EC50 relative |
% Max | pEC50 | EC50 (nM) |
EC50 relative |
% Max |
| PTH(1-34) | 9.2 ± 0.1 | 0.58 | 1 | 102 ± 4 | 9.2 ± 0.05 | 0.69 | 1 | 100 ± 2 |
| 1 | 8.6 ± 0.1 | 2.3 | 4 | 116 ± 6 | 7.5 ± 0.07 | 29 | 42 | 76 ± 3 |
| 2 | 8.2 ± 0.1 | 6.2 | 11 | 119 ± 7 | 6.6 ± 0.2 | 280 | 406 | 11 ± 2 |
For PTHR1 activation, measured via stimulation of intracellular cAMP, pEC50, EC50 and maximal response (% Max) values were derived from 4 independent experiments. For PTHR2 activation, pEC50, EC50 and % Max represent the average of ≥4 independent experiments. pEC50 indicates the negative logarithm of the half-maximal effective concentration (EC50). EC50 relative indicates cAMP potency normalized to PTH(1-34) by the quotient (α/β/γ-analogue/PTH(1-34)). pEC50 and % Max uncertainties are expressed as SEM.
α/β/γ-Peptides 1 and 2 differed considerably from PTH(1-34) in their ability to induce recruitment of β-arrestin-1 or −2 to the PTHR1 (Figure 2D,E and Table 2). Both backbone modifications caused a substantial decrease in potency (higher EC50) and resulted in a much lower β-arrestin recruitment maximum in these assays. Analysis with the Black-Leff operational model,54,55 using PTH(1-34) as the basis for comparison, indicated that both 1 and 2 display significant bias toward cAMP production relative to recruitment of either β-arrestin-1 or β-arrestin-2 (Figure 2F,G).
Table 2.
PTHR1 β-arrestin-1 and −2 Recruitment and NLuc-PTHR1 Affinity for PTH(1-34) and α/β/γ-Analogues 1 and 2.
| β-arrestin-1 Recruitment | β-arrestin-2 Recruitment | NLuc-PTHR1 Affinity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptide | pEC50 | EC50 (nM) |
EC50 relative |
% Max | pEC50 | EC50 (nM) |
EC50 relative |
% Max | pIC50 | IC50 (nM) |
IC50 relative |
| PTH(1-34) | 8.1 ± 0.1 | 7.2 | 1 | 101 ± 3 | 8.2 ± 0.1 | 6.8 | 1 | 101 ± 3 | 7.6 ± 0.1 | 22 | 1 |
| 1 | 7.0 ± 0.2 | 100 | 14 | 27 ± 3 | 7.0 ± 0.4 | 100 | 15 | 21 ± 4 | 6.9 ± 0.1 | 130 | 6 |
| 2 | 6.7 ± 0.3 | 190 | 26 | 39 ± 8 | 7.0 ± 0.4 | 110 | 16 | 30 ± 8 | 6.1 ± 0.1 | 720 | 33 |
For β-arrestin-1 recruitment, pEC50, EC50 and maximal response (% Max) values represent the average of ≥4 independent experiments. For β-arrestin-2 recruitment, pEC50, EC50 and % Max are the average of ≥3 independent experiments. pEC50 indicates the negative logarithm of the half-maximal effective concentration (EC50). NLuc-PTHR1 pIC50 and IC50 values are the average of 3 independent experiments. pIC50 indicates the negative logarithm of the half-maximal inhibitory concentration (IC50). EC50 and IC50 relative indicate β-arrestin-1 or −2 recruitment potency or NLuc-PTHR1 affinity normalized to PTH(1-34) by the quotient (α/β/γ-analogue/PTH(1-34)). pEC50, pIC50 and % Max uncertainties are expressed as SEM.
α/β/γ-Peptides 1 and 2 were evaluated for their ability to induce PTHR1 internalization in a β-arrestin-2-dependent manner. The experiments were conducted in U2OS cells that stably express the PTHR1, Enzyme Acceptor (EA)-tagged β-arrestin-2 and the ProLink tag localized to the endosomes. Consistent with the β-arrestin-2 recruitment data, both 1 and 2 were less effective than PTH(1-34) at inducing β-arrestin-2-mediated receptor internalization (Figure S3A and Table S3). Analysis using the Black-Leff operational model54,55 revealed that 1 and 2 both display bias toward β-arrestin-1 recruitment relative to internalization (Figure S3B). Peptide 2 shows bias also toward β-arrestin-2 recruitment relative to internalization (Figure S3C). Internalization of GPCRs is generally mediated by β-arrestins;56,57 however, some GPCRs can also internalize in a β-arrestin-independent manner.58 Results with α/β/γ-peptides 1 and 2 suggest that PTHR1 internalization and β-arrestin recruitment responses are not perfectly correlated with one another. Both GS-biased α/β/γ-peptides 1 and 2 displayed significant bias toward cAMP production relative to internalization (Figure S3D), an outcome that has been observed among previously reported GS-biased GLP-1R agonists.59,60
Affinities of α/β/γ-peptides 1 and 2 for the PTHR1 were evaluated via bioluminescence resonance energy transfer (BRET) measurements with an engineered version of the receptor that contains a nanoluciferase (NLuc) unit fused to the N-terminus at the extracellular side;61 this modification does not alter the PTHR1 activation, as measured by cAMP production. We compared the abilities of PTH(1-34), 1 and 2 to displace a tetramethylrhodamine(TMR)-labeled derivative of PTH(1-34), PTH(1-35)-(Lys35-TMR)-NH2. Both of the backbone-modified peptides bound somewhat less strongly than did PTH(1-34), with IC50 6-fold higher for 1 and 33-fold higher for 2 relative to PTH(1-34) (Figure 2H and Table 2).
The lower receptor internalization and affinities of agonists 1 and 2 relative to PTH(1-34) raise the possibility that the PTHR1 signaling they induce might originate largely from receptors at the cell surface. To investigate this possibility, we measured the duration of PTHR1 cAMP signaling induced by PTH(1-34), 1 and 2 in the absence or presence of the cell-impermeable PTHR1 antagonist PTH(7-34).62,63 Cells were stimulated with 10 nM agonist for 20 min, after which the agonist was washed away, and post-washout cAMP levels were monitored (Figure S4A). Under these conditions, PTH(1-34), 1 and 2 generate comparable cAMP production maxima (Figure 2B). Introduction of antagonist PTH(7-34) after the wash was expected to cause competitive displacement of agonist peptides bound to PTHR1 molecules on the cell surface, with concomitant termination of cAMP production stimulated by these receptors. However, the antagonist should not affect cAMP production stimulated by internalized agonist-PTHR1 complexes. In the absence of the antagonist, peptide 1 exhibited comparable duration of cAMP signaling relative to PTH(1-34), based on calculated area under curve (AUC) (Figure S4B). In contrast, when the PTHR1 antagonist was introduced, the AUC of 1 was ~40% lower compared to PTH(1-34) (Figure S4B), supporting the hypothesis that relative to PTH(1-34), a greater proportion of cAMP production stimulated by 1 originates from PTHR1 activation at the cell surface. For agonist 2, cAMP production was more transient relative to PTH(1-34) or 1 under each experimental condition, with the antagonist not exerting a significant effect on cAMP signaling between two conditions (Figure S4A,B).
Available structural data suggest that peptide agonists are mostly or entirely α-helical when bound to the PTHR1;44,45,64,65 however, PTH(1-34) in aqueous solution is largely unfolded.66 Addition of a small proportion of 2,2,2-trifluoroethanol (TFE) enhances helicity.67 We used circular dichroism (CD) to compare the secondary structures of PTH(1-34), 1 and 2 in aqueous buffer containing 20 vol % TFE. PTH(1-34) manifested a strong α-helix signature, with minima at ~208 and ~222 nm (Figure 2I). α/β/γ-Peptides 1 and 2 displayed even stronger CD signatures at each minimum, in terms of mean residue ellipticity (MRE) (Figure 2I). In these cases, the minimum at lower wavelength was significantly more intense than the minimum at higher wavelength. This observation is consistent with previously reported CD data for peptides containing larger proportions of β and γ residues, which indicate that helical secondary structure is associated with a more intense minimum in the ~200-210 nm region.40,41 Overall, the CD data suggest that helical propensity is retained after ααα→βγ backbone modification in 1 and 2.
ααα→γ4β3 vs. ααα→γ4β2 Backbone Replacement in PTH(1-34)
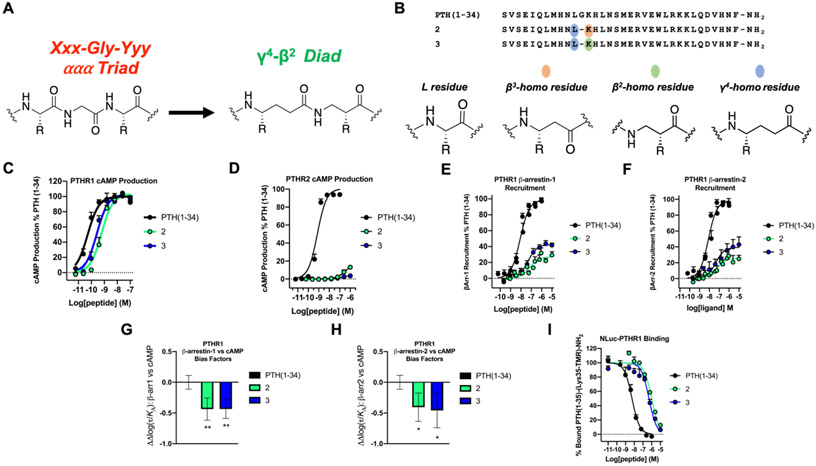
In PTH(1-34) α/β/γ-variant 2, a Xxx-Gly-Yyy triad was replaced with a γ4-β3 diad (Figures 1B and 2A). The γ4-β3 diad preserves both side chains found in the Xxx-Gly-Yyy triad; however, those side chains are separated by five backbone bonds in the γ4-β3 diad, while the corresponding side chains in the Xxx-Gly-Yyy ααα triad are separated by six backbone bonds. Use of a β2 residue in place of the β3 residue would generate a γ/β diad with side chains spaced by six backbone bonds (Figure 3A). However, in contrast to the broad commercial availability of Fmoc-protected β3-amino acids, very few Fmoc-protected β2-amino acids can be purchased, which makes a γ4-β3 design more accessible than a γ4-β2 design. To assess functional differences between γ4-β3 and γ4-β2 diads in isomeric α/β/γ-peptides, we prepared PTH(1-34) derivative 3 for comparison with 2 (Figure 3B). This effort required the synthesis of enantiopure Fmoc-β2-hLys(diBoc).68
Figure 3.
PTH(1-34) γ4-β3 (peptide 2) and γ4-β2 (peptide 3) analogues display comparable affinity and signaling outcomes at PTHR1. (A) Backbone replacement of Xxx-Gly-Yyy ααα triad with a γ4-β2 diad. For comparison, replacement of Xxx-Gly-Yyy ααα triad with a γ4-β3 diad is shown in Figure 1B. (B) Sequences of PTH(1-34) and α/β/γ-analogues 2 and 3. (C) PTHR1 activation, via cAMP production, in HEK293 cells stably expressing the PTHR1 and the cAMP-sensing protein GloSensor. Data points represent the average of 5 independent experiments. (D) PTHR2 activation, via cAMP production, in HEK293 cells stably expressing PTHR2 and GloSensor. Data is derived from 3 independent experiments. (E,F) PTHR1 β-arrestin-1 and −2 recruitment dose-responses. Assays were performed in CHO-FlpIn cells stably transfected with the PTHR1-RLuc8 and β-arrestin-1-Venus or β-arrestin-2-Venus. Data represent the mean of ≥5 (β-arrestin-1) or ≥4 (β-arrestin-2) independent experiments. All cAMP, β-arrestin-1 and β-arrestin-2 data was normalized against PTH(1-34) and all uncertainties are expressed as SEM. (G,H) PTHR1 β-arrestin-1 or −2 vs. cAMP bias factors were generated from dose-response curves in Figures 3C, E, and F. Negative values indicate bias towards cAMP production. Bias factor uncertainties are expressed in SD. * = p ≤ 0.05; ** = p ≤ 0.01; by one-way ANOVA followed by Dunnett test. (I) Competition binding measurements by bioluminescence resonance energy transfer (BRET) in HEK293 cells expressing the NLuc-PTHR1. The peptide tracer was a tetramethylrhodamine (TMR)-labeled derivative of PTH(1-34), PTH(1-35)-(Lys35-TMR)-NH2. Data (as IC50 values) represent the average of 3 independent experiments.
Comparisons between α/β/γ-peptides 2 and 3 revealed that the difference between β3-hLys and β2-hLys exerts very little impact on engagement with the PTH receptors. Thus, PTH(1-34) variant 3 was very similar to 2 in potency for stimulating cAMP production at the PTHR1 (Figure 3C and Table S4). As with 2, α/β/γ-analogue 3 did not activate the PTHR2 (Figure 3D and Table S4). These α/β/γ-peptide isomers displayed similar profiles in recruitment of β-arrestin-1 or β-arrestin-2 to the PTHR1 in terms of EC50, although peptide 3 displayed a slightly higher β-arrestin recruitment maximum in these assays relative to 2 (Figure 3E,F and Table S5). Each of these α/β/γ-peptides induced a modest bias toward cAMP production relative to β-arrestin recruitment at the PTHR1 (Figure 3G,H). In addition, peptide 3 bound to the NLuc-fused PTHR1 with a similar affinity relative to 2 (Figure 3I and Table S5). Overall, this comparison suggests that the synthetically demanding β2 subunit offers little or no benefit relative to the analogous β3 subunit in terms of affinity and activity, and our remaining efforts focused on ααα→βγ replacements containing β3 residues.
ααα→βγ Backbone Modification Impairs Signaling in Analogues of Abaloparatide
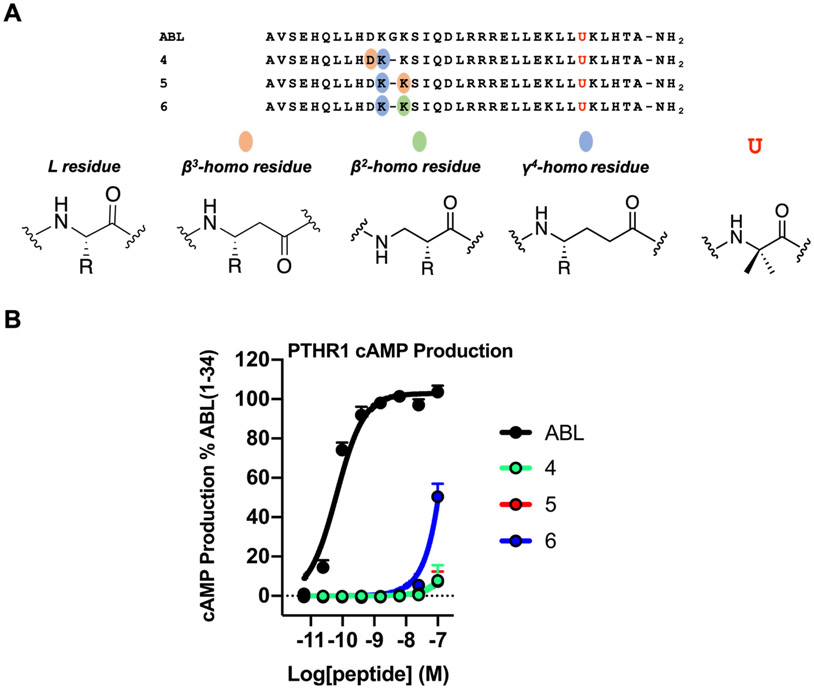
Abaloparatide (ABL), which is based on the first 34 residues of PTHrP, is used to treat osteoporosis.69 The sequence of ABL differs significantly from that of PTH(1-34); the two peptides share Gly12 and Lys13, but the Asn10-Leu11 diad of PTH(1-34) is replaced by Asp10-Lys11 diad in ABL (Figure S5). We examined three α/β/γ-peptide analogues of ABL, 4-6 (Figure 4A). In 4, the Asp-Lys-Gly triad is replaced by the β3-hAsp-γ4-hLys diad. In 5 and 6, the Lys-Gly-Lys triad is replaced by the γ4-hLys-β3-hLys or γ4-hLys-β2-hLys diad, respectively. In this system, ααα→βγ modification caused a profound loss of agonist activity at the PTHR1, as determined from cAMP production assays (Figure 4B). Thus, despite the common occurrence of Gly at position 12 of PTH(1-34) and ABL, these two peptides respond very differently to ααα→βγ backbone modification in terms of activating the PTHR1. We are unable to rationalize this difference. Recently reported cryo-EM structures of this receptor bound to PTH(1-34) or ABL are similar in overall geometry,45 and the biological effects of these two peptides are similar;70 however, their receptor-binding behaviors differ.61
Figure 4.
ABL α/β/γ-analogues 4-6 are weak activators of PTHR1. (A) Sequences of ABL and α/β/γ-analogues 4, 5 and 6. Letter (U) denotes 2-aminoisobutyric acid. (B) PTHR1 activation, via cAMP production, in HEK293 cells stably expressing the PTHR1 and the cAMP-sensing protein GloSensor. Data points represent the average of ≥3 independent experiments. Data was normalized against ABL and all error bars uncertainties are expressed as SEM. Note: data points for ABL α/β/γ-analogues 4 and 5 overlap.
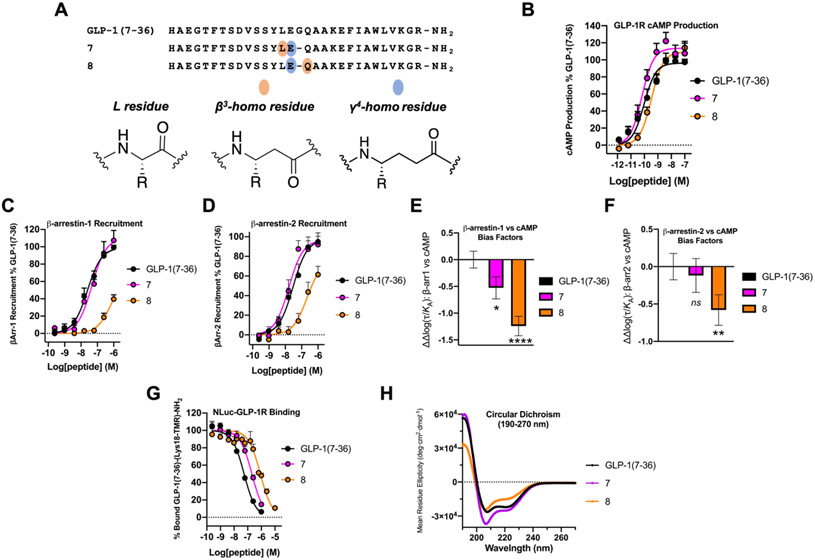
ααα→βγ Backbone Modification Involving Gly22 Generates Potent Analogues of GLP-1(7-36)
To broaden our assessment of the ααα→βγ backbone modification strategy, we examined two replacements involving Gly22 of GLP-1(7-36) (Figure 5A; the mature form of this hormone has His7 at the N-terminus). Adelhorst et al. surveyed the impact of replacing each native residue in GLP-1(7-36) with Ala.71 This study showed that substituting Ala for Leu20, Gly22 or Gln23 had relatively little impact on potency for stimulation of cAMP production in plasma membranes purified from cells that endogenously expressed rat GLP-1R. However, replacing Glu21 with Ala caused a substantial potency decline. These findings suggest that retaining a side chain corresponding to that of Glu21 would be important for retaining activity in GLP-1 analogues.
Figure 5.
Evaluation of ααα→βγ replacements at Gly22 of GLP-1R agonist, GLP-1(7-36). (A) Sequences of GLP-1(7-36) and α/β/γ-analogues 7 and 8. (B) GLP-1R activation, measured via stimulation of intracellular cAMP, in HEK293 cells transiently expressing the GLP-1R and stably expressing the GloSensor protein. Data points represent the average of ≥3 independent experiments. (C,D) β-arrestin-1 and −2 recruitment dose-response curves for GLP-1(7-36), 7 and 8. Assays were performed in HEK293 cells transiently transfected with GLP-1R-RLuc8 and GFP2-tagged β-arrestin-1 or β-arrestin-2. Data represent the mean of ≥2 (β-arrestin-1) and ≥3 (β-arrestin-2) independent experiments. All cAMP, β-arrestin-1 and β-arrestin-2 data was normalized against GLP-1(7-36) and all uncertainties are expressed as SEM. (E,F) GLP-1R β-arrestin-1 or −2 vs. cAMP bias factors generated from curves in Figures 5B, C, and D. Negative values indicate bias towards cAMP production. Bias factor uncertainties are expressed in SD. ns= non-significant; * = p ≤ 0.05; ** = p ≤ 0.01; **** = p ≤ 0.0001; by one-way ANOVA followed by Dunnett test. (G) Competition NLuc-GLP-1R affinity measurements in HEK293 cells. GLP-1(7-36)-(Lys18-TMR)-NH2 was used as a labeled peptide tracer. IC50 values represent the average of ≥3 independent experiments. (H) Circular dichroism data for GLP-1(7-36), 7 and 8 measured in 50 mM PBS buffer, pH 7.3, 20% 2,2,2-trifluoroethanol (TFE).
In α/β/γ-peptide 7, the Leu-Glu-Gly triad is replaced with the β3-hLeu-γ4-hGlu diad, while in 8 the Glu-Gly-Gln triad is replaced with the γ4-hGlu-β3-hGln diad. Both of these α/β/γ-analogues of GLP-1(7-36) were very potent in terms of stimulating cAMP production via the GLP-1R (Figure 5B and Table 3). EC50 for 7 was indistinguishable from that of GLP-1(7-36), while EC50 of 8 was ~4-fold higher relative to GLP-1(7-36). Thus, as observed for PTH(1-34) analogues 1 and 2 and PTHR1, ααα→βγ modification involving the native Gly22 in GLP-1(7-36) provides analogues that retain a native-like ability to activate GS via the GLP-1R. For both 7 and 8, the duration of GLP-1R cAMP signaling with or without antagonist, (Exendin(9-39)), was more transient relative to GLP-1(7-36) (Figure S6).
Table 3.
GLP-1R cAMP Production and β-arrestin-1 and −2 Recruitment for GLP-1(7-36) and α/β/γ-Analogues 7 and 8.
| cAMP Production | β-arrestin-1 Recruitment | β-arrestin-2 Recruitment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptide | pEC50 | EC50 (nM) |
EC50 relative |
% Max | pEC50 | EC50 (nM) |
EC50 relative |
% Max | pEC50 | EC50 (nM) |
EC50 relative |
% Max |
| GLP-1(7-36) | 10.0 ± 0.1 | 0.096 | 1 | 96 ± 4 | 7.6 ± 0.1 | 25 | 1 | 100 ± 2 | 7.5 ± 0.1 | 29 | 1 | 97 ± 6 |
| 7 | 10.2 ± 0.1 | 0.065 | 0.7 | 114 ± 5 | 7.3 ± 0.1 | 46 | 2 | 110 ± 7 | 7.8 ± 0.1 | 16 | 0.6 | 97 ± 6 |
| 8 | 9.5 ± 0.1 | 0.34 | 4 | 109 ± 3 | 6.2 ± 0.2 | 710 | 28 | 40[#] | 6.7 ± 0.2 | 220 | 8 | 61[#] |
For GLP-1R cAMP production, pEC50, EC50 and maximal response (% Max) values are derived from ≥3 independent experiments. For β-arrestin-1 and −2 recruitment to the GLP-1R, pEC50, EC50 and % Max represent ≥2 and ≥3 independent experiments, respectively. pEC50 indicates the negative logarithm of the half-maximal effective concentration (EC50). EC50 relative indicates cAMP or β-arrestin recruitment potency normalized to GLP-1(7-36) by the quotient (α/β/γ-analogue/GLP-1(7-36)). pEC50 and % Max uncertainties are expressed as SEM. Symbol [#] describes % Max measured at the highest concentration (1 μM) for 8 because the dose-response curve failed to reach saturation.
The GLP-1(7-36)-derived α/β/γ-peptides 7 and 8 differed significantly from one another in their ability to induce recruitment of β-arrestin-1 or β-arrestin-2 to the GLP-1R (Figure 5C,D and Table 3). In both assays, 7 was indistinguishable from GLP-1(7-36), while 8 was less potent. α/β/γ-Peptide 8 displayed a significant bias toward cAMP production relative to recruitment of either β-arrestin, with GLP-1(7-36) as the basis of comparison (Figure 5E,F). α/β/γ-Peptide 7 may display a small bias toward cAMP production and away from β-arrestin-1 (Figure 5E).
The ability of α/β/γ-peptides 7 and 8 to induce GLP-1R internalization mirrored their ability to recruit β-arrestins when compared to GLP-1(7-36) (Figure S7A and Table S6). Both backbone modifications caused a significant bias toward cAMP production relative to receptor internalization (Figure S7D), consistent with observations made for PTH(1-34) α/β/γ-peptides 1 and 2 (Figure S3D).
Binding of GLP-1(7-36) analogues 7 and 8 to the receptor was evaluated via BRET with cells expressing an NLuc-GLP-1R fusion protein;35 the NLuc-GLP-1R is comparable to the native GLP-1R in terms of signal transduction, as monitored by stimulation of cAMP production. α/β/γ-Peptides 7 and 8 were compared with GLP-1(7-36) for the ability to displace a TMR-labeled derivative of GLP-1(7-36), GLP-1(7-36)-(Lys18-TMR)-NH2, from the receptor.72 Each ααα→βγ modification caused a modest decrease in affinity relative to GLP-1(7-36), with IC50 increased by ~4-fold for 7 and by ~16-fold for 8 (Figure 5G and Table 4).
Table 4.
NLuc-GLP-1R Affinity for GLP-1(7-36) and α/β/γ-Analogues 7 and 8.
| NLuc-GLP-1R Affinity | |||
|---|---|---|---|
| Peptide | pIC50 | IC50 (nM) | IC50 relative |
| GLP-1(7-36) | 7.3 ± 0.04 | 54 | 1 |
| 7 | 6.7 ± 0.05 | 200 | 4 |
| 8 | 6.1 ± 0.05 | 850 | 16 |
Competition NLuc-GLP-1R pIC50 and IC50 values are the average of ≥3 independent experiments. pIC50 indicates the negative logarithm of the half-maximal inhibitory concentration (IC50). IC50 relative indicates binding affinity normalized to GLP-1(7-36) by the quotient (α/β/γ-analogue/GLP-1(7-36)).
Multiple cryo-EM structures of the GLP-1R bound to agonists indicate that these peptides are largely or entirely helical when bound.35,73-75 GLP-1 is unfolded in aqueous solution,76 but addition of TFE induces helicity.67 We compared the folding propensities of GLP-1(7-36), 7 and 8 in aqueous buffer containing 20 vol % TFE. GLP-1(7-36) itself displays a strong α-helical signature under these conditions (Figure 5H). The CD signatures of 7 and 8 suggest helicity as well, although as noted for PTH(1-34) α/β/γ-peptides 1 and 2 (Figure 2I), the minimum at 208 nm is significantly more intense than the minimum at 222 nm in these cases (Figures 5H). This trend is consistent with the hypothesis that the relative intensities of these minima are influenced by the ααα→βγ backbone modification.
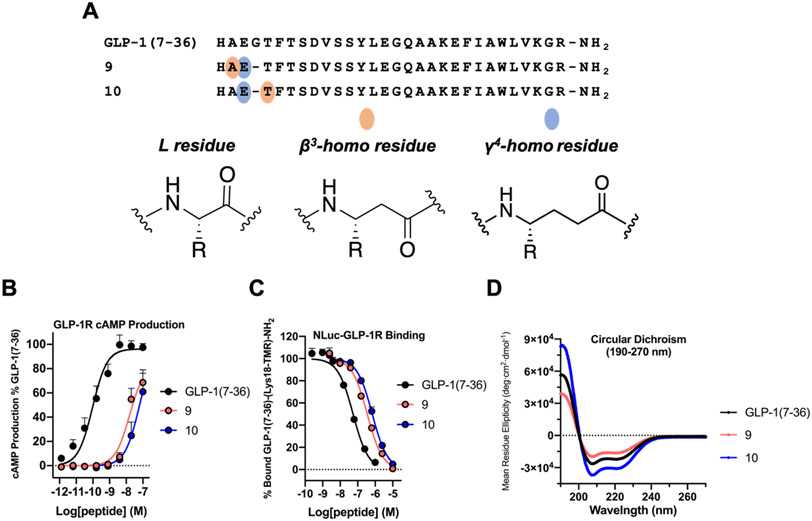
ααα→βγ Backbone Modification Involving Gly10 Generates Analogues of GLP-1(7-36) that Bind Tightly but are Weak GLP-1R Agonists
A second evaluation of the ααα→βγ modification strategy in GLP-1(7-36) was conducted via modifications involving Gly10 (Figure 6A). Adelhorst et al. found that replacing Glu9 or Thr11 with Ala had no significant effect on agonist potency, as manifested by stimulation of cAMP production via rat GLP-1R; replacing Gly10 with Ala, however, led to a substantial potency decline.71
Figure 6.
ααα→βγ replacements at the N-terminus (Gly10) of GLP-1(7-36) are not well tolerated. (A) Sequences of GLP-1(7-36) and α/β/γ-analogues 9 and 10. (B) GLP-1R activation, measured via cAMP production, in HEK293 cells transiently expressing the GLP-1R and stably expressing the GloSensor protein. Data points represent the average of ≥3 independent experiments. The cAMP data was normalized against GLP-1(7-36) and all uncertainties are expressed as SEM. (C) Competition affinity measurements in HEK293 cells transiently transfected with the NLuc-GLP-1R. GLP-1(7-36)-(Lys18-TMR)-NH2 was used as a labeled peptide tracer. IC50 values represent the average of ≥3 independent experiments. (D) Circular dichroism data for GLP-1(7-36), 9 and 10 measured in 50 mM PBS buffer, pH 7.3, 20% 2,2,2-trifluoroethanol (TFE).
In α/β/γ-peptide 9, the Ala-Glu-Gly triad is replaced with the β3-hAla-γ4-hGlu diad, while in 10 the Glu-Gly-Thr triad is replaced with the γ4-hGlu-β3-hThr diad. In contrast to modifications involving Gly22, the modifications involving Gly10 caused profound decreases in GLP-1R activation (Figure 6B and Table 5). EC50 values for stimulating cAMP production were >100-fold higher for 9 and 10 relative to GLP-1(7-36). Neither of these α/β/γ-peptides caused detectable recruitment of β-arrestin-1 or β-arrestin-2 to the GLP-1R (Figure S8), which prevented calculations of cAMP vs. β-arrestin bias. Neither 9 or 10 induced measurable GLP-1R internalization (Figure S9).
Table 5.
GLP-1R Activation and NLuc-GLP-1R Affinity for GLP-1(7-36) and α/β/γ-Analogues 9 and 10.
| GLP-1R cAMP Production | NLuc-GLP-1R Affinity | ||||||
|---|---|---|---|---|---|---|---|
| Peptide | pEC50 | EC50 (nM) |
EC50 relative |
% Max | pIC50 | IC50 (nM) |
IC50 relative |
| GLP-1(7-36) | 10.0 ± 0.1 | 0.096 | 1 | 96 ± 4 | 7.3 ± 0.04 | 54 | 1 |
| 9 | 7.8 ± 0.1 | 16 | 167 | 69[#] | 6.5 ± 0.04 | 350 | 6 |
| 10 | 7.2 ± 0.3 | 62 | 646 | 61[#] | 6.2 ± 0.03 | 680 | 13 |
For GLP-1R activation, pEC50, EC50 and maximal response (% Max) represent the mean of ≥3 independent experiments. For NLuc-GLP-1R competition binding experiments, pIC50 and IC50 values are derived from ≥3 independent experiments. pEC50 and pIC50 indicate the negative logarithm of the half-maximal effective or inhibitory concentration (EC50, or IC50, respectively). EC50 or IC50 relative indicates cAMP potency or NLuc-GLP-1R affinity normalized to GLP-1(7-36) by the quotient (α/β/γ-analogue/GLP-1(7-36)). pEC50, pIC50 and % Max uncertainties are expressed as SEM. Symbol [#] describes the maximal response measured at the highest concentration (100 nM) for 9 or 10 when their corresponding dose-response curves failed to reach saturation.
Despite the weak agonist behavior of α/β/γ-peptides 9 and 10, both displayed high affinity for the receptor (Figure 6C and Table 5). The competition BRET assay involving the NLuc-GLP-1R revealed that the IC50 values for GLP-1(7-36) analogues 9 and 10 were similar to those of GLP-1(7-36) analogues 7 and 8 (Figure 5G and Table 4), each of which displayed a native-like ability to activate GS via the GLP-1R. CD analysis of 9 and 10 suggested that these α/β/γ-peptides have significant propensities to adopt helical secondary structure (Figure 6D).
The binding data suggest that the inability of 9 and 10 to induce a robust cAMP response or strongly recruit β-arrestins does not arise from lack of engagement with the orthosteric site of the receptor. Instead, the weak activities of 9 and 10 seem to reflect deficiencies in their abilities to stabilize GLP-1R conformations required for coupling with intracellular partners. This impact of ααα→βγ modification near the N-terminus of GLP-1 on agonist activity is consistent with other evidence that the GLP-1R71,77 and other class B1 GPCRs53,78-80 are very sensitive to modifications in the N-terminal regions of agonists. Guichard et al. identified a ureido substitution for Ala8 of GLP-1(7-37) that retained native-like activity, but this group also found that homologous urethane or γ residue substitutions for Ala8 caused dramatic declines in potency for stimulation of cAMP production.29 Bai et al. examined homologous β residue substitutions for Ala8, Glu9 and Gly10 of GLP-1(7-36) and observed a decline in potency in each case.81 We examined α- and β-amino acid substitutions at Gly10 and concluded that deviations from helical secondary structure near the agonist N-terminus are important for activation of the GLP-1R.35 It is possible that the ααα→βγ modifications in this region of GLP-1 disfavor that local conformation required for signal transduction.
CONCLUSIONS
We have shown that potent agonists of two biomedically important class B1 GPCRs can be generated by a backbone modification strategy that has not previously been evaluated in this context, replacement of a contiguous α residue triad with a β/γ diad. ααα→βγ replacements were evaluated in one region of PTH(1-34) and in two regions of GLP-1(7-36). The resulting α/β/γ-peptides displayed substantial affinity for the PTHR1 or the GLP-1R, respectively. These observations are consistent with reports that a β/γ diad can replace an α residue triad in an α-helix like conformation,36-41 since both PTH(1-34) and GLP-1(7-36) appear to be fully or largely α-helical when bound to the receptor.44,45,73 Most of the α/β/γ-peptides we examined manifested activity profiles that differed significantly from that of the corresponding all-α peptide. GLP-1(7-36) analogue 7 most closely approached the prototype activity profile, while GLP-1(7-36) analogue 8 and PTH(1-34) analogues 1-3 displayed biased agonism, favoring cAMP production over β-arrestin recruitment. Biased agonists are potentially useful as tools for probing receptor function at the cell or organism level, and new strategies to achieve bias could have implications for future therapeutic strategies.18,19
PTH(1-34)-derived α/β/γ-peptides 2 and 3 selectively activated the PTHR1 over the PTHR2 (Figure 3C,D and Table S4). The similar functional profiles of these regioisomers suggests that there is little difference between β/γ diads containing a β3 or β2 residue in terms of receptor binding and activation.
A substantial loss of signaling potency was observed for GLP-1(7-36)-derived α/β/γ-peptides 9 and 10 relative to GLP-1(7-36) itself. These high-affinity α/β/γ-peptides could be forerunners of new GLP-1R antagonists, which are of interest for treating post-bariatric hypoglycemia.82 Similar declines in potency were observed among ABL-derived α/β/γ-peptides 4-6.
Backbone modification via replacement of α-amino acid residues with β-amino acid residues protects nearby amide bonds from the action of proteolytic enzymes.83 Similar effects can be anticipated for ααα→βγ replacements. Because the new α/β/γ-peptides described above contain ≥ 29 residues, it is unlikely that the modification of a single ααα triad would provide meaningful protection from proteolysis in vivo. However, our results suggest that ααα→βγ modification could be combined with backbone modifications at other sites to generate biologically active peptides that display substantial resistance to degradation.33
Since most of the α/β/γ-peptides described above were easily prepared from commercial starting materials, including protected β3- or γ4-amino acids, via conventional solid-phase synthesis, and since these α/β/γ-peptides manifested diverse and potentially useful behaviors, this work should encourage broader exploration of hormone analogues containing ααα→βγ replacements. Our initial efforts were limited to α triads containing a Gly residue, so that we could preserve all native side chains. Alanine-scan studies, however, have revealed multiple sites in GLP-1, PTH(1-34) and other Class B1 GPCR agonists at which side chain identity is not important in terms of receptor engagement.53,71,77-79,84 Future studies will reveal whether these sites are promising sites for ααα→βγ replacement.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported, in part, by National Institutes of Health grant R01 GM056414. L.M.T. was supported in part by a Chemistry-Biology Interface Training Grant (T32 GM008505). We are grateful for additional support provided by the William F. Vilas Trust Estate.
Footnotes
SUPPORTING INFORMATION
Table of reagents and resources, Instrumentation acknowledgments, Supporting Figures S1-S11, Supporting Tables S1-S8, Experimental procedures for peptide synthesis, cell culture, PTHR1, PTHR2 or GLP-1R cAMP production, β-arrestin-1 or −2 recruitment, NLuc-PTHR1 or NLuc-GLP-1R competition binding, PTHR1 or GLP-1R cAMP washout assay, circular dichroism, synthesis of Fmoc-β2-hLys(diBoc), MALDI and UPLC characterization of peptides
The authors declare the following competing financial interest(s): S.H.G. is a cofounder of Longevity Biotech, Inc., which is pursuing biomedical applications of α/β-peptides.
REFERENCES
- (1).Cheloha RW; Gellman SH; Vilardaga JP; Gardella TJ PTH Receptor-1 Signalling—Mechanistic Insights and Therapeutic Prospects. Nature Reviews Endocrinology 2015, 11 (12), 712–724. 10.1038/nrendo.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Davenport AP; Scully CCG; de Graaf C; Brown AJH; Maguire JJ Advances in Therapeutic Peptides Targeting G Protein-Coupled Receptors. Nat Rev Drug Discov 2020, 19 (6), 389–413. 10.1038/s41573-020-0062-z. [DOI] [PubMed] [Google Scholar]
- (3).Suzuki R; Brown GA; Christopher JA; Scully CCG; Congreve M Recent Developments in Therapeutic Peptides for the Glucagon-like Peptide 1 and 2 Receptors. J Med Chem 2020, 63 (3), 905–927. 10.1021/ACS.JMEDCHEM.9B00835. [DOI] [PubMed] [Google Scholar]
- (4).Wootten D; Christopoulos A; Marti-Solano M; Babu MM; Sexton PM Mechanisms of Signalling and Biased Agonism in G Protein-Coupled Receptors. Nature Reviews Molecular Cell Biology 2018, 19 (10), 638–653. 10.1038/s41580-018-0049-3. [DOI] [PubMed] [Google Scholar]
- (5).Gurevich VV; Gurevich EV GPCR Signaling Regulation: The Role of GRKs and Arrestins. Front Pharmacol 2019, 10 (125). 10.3389/FPHAR.2019.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Vilardaga JP; Jean-Alphonse FG; Gardella TJ Endosomal Generation of CAMP in GPCR Signaling. Nat Chem Biol 2014, 10 (9), 700–706. 10.1038/nchembio.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Ferrandon S; Feinstein TN; Castro M; Wang B; Bouley R; Potts JT; Gardella TJ; Vilardaga JP Sustained Cyclic AMP Production by Parathyroid Hormone Receptor Endocytosis. Nat Chem Biol 2009, 5 (10), 734–742. 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Gardella TJ; Vilardaga JP International Union of Basic and Clinical Pharmacology. XCIII. The Parathyroid Hormone Receptors—Family B G Protein–Coupled Receptors. Pharmacol Rev 2015, 67 (2), 310–337. 10.1124/PR.114.009464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).de Graaf C; Donnelly D; Wootten D; Lau J; Sexton PM; Miller LJ; Ahn JM; Liao J; Fletcher MM; Yang D; Brown AJH; Zhou C; Deng J; Wang MW Glucagon-Like Peptide-1 and Its Class B G Protein–Coupled Receptors: A Long March to Therapeutic Successes. Pharmacol Rev 2016, 68 (4), 954–1013. 10.1124/PR.115.011395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Sexton PM; Christopoulos A To Bind or Not to Bind: Unravelling GPCR Polypharmacology. Cell 2018, 172 (4), 636–638. 10.1016/J.CELL.2018.01.018. [DOI] [PubMed] [Google Scholar]
- (11).Usdin TB The PTH2 Receptor and TIP39: A New Peptide–Receptor System. Trends Pharmacol Sci 2000, 21 (4), 128–130. 10.1016/S0165-6147(00)01455-3. [DOI] [PubMed] [Google Scholar]
- (12).Usdin TB; Hoare SRJ; Wang T; Mezey É; Kowalak JA TIP39: A New Neuropeptide and PTH2-Receptor Agonist from Hypothalamus. Nature Neuroscience 1999, 2 (11), 941–943. 10.1038/14724. [DOI] [PubMed] [Google Scholar]
- (13).Pocai A. Action and Therapeutic Potential of Oxyntomodulin. Mol Metab 2014, 3 (3), 241–251. 10.1016/J.MOLMET.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Darbalaei S; Yuliantie E; Dai A; Chang R; Zhao P; Yang D; Wang MW; Sexton PM; Wootten D Evaluation of Biased Agonism Mediated by Dual Agonists of the GLP-1 and Glucagon Receptors. Biochem Pharmacol 2020, 180, 114150. 10.1016/J.BCP.2020.114150. [DOI] [PubMed] [Google Scholar]
- (15).White AD; Peña KA; Clark LJ; Maria CS; Liu S; Jean-Alphonse FG; Lee JY; Lei S; Cheng Z; Tu CL; Fang F; Szeto N; Gardella TJ; Xiao K; Gellman SH; Bahar I; Sutkeviciute I; Chang W; Vilardaga JP Spatial Bias in CAMP Generation Determines Biological Responses to PTH Type 1 Receptor Activation. Sci Signal 2021, 14 (703), 5944. 10.1126/SCISIGNAL.ABC5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Hargrove DM; Alagarsamy S; Croston G; Laporte R; Qi S; Srinivasan K; Sueiras-Diaz J; Wisniewski K; Hartwig J; Lu M; Posch AP; Wisniewska H; Schteingart CD; Rivière PJM; Dimitriadou V Pharmacological Characterization of Apraglutide, a Novel Long-Acting Peptidic Glucagon-like Peptide-2 Agonist, for the Treatment of Short Bowel Syndrome. J. Pharmacol. Exp. Ther 2020, 373 (2), 193–203. 10.1124/JPET.119.262238. [DOI] [PubMed] [Google Scholar]
- (17).Naimi RM; Hvistendahl M; Nerup N; Ambrus R; Achiam MP; Svendsen LB; Grønbæk H; Møller HJ; Vilstrup H; Steensberg A; Jeppesen PB Effects of Glepaglutide, a Novel Long-Acting Glucagon-like Peptide-2 Analogue, on Markers of Liver Status in Patients with Short Bowel Syndrome: Findings from a Randomised Phase 2 Trial. EBioMedicine 2019, 46, 444–451. 10.1016/J.EBIOM.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Chen XT; Pitis P; Liu G; Yuan C; Gotchev D; Cowan CL; Rominger DH; Koblish M; Dewire SM; Crombie AL; Violin JD; Yamashita DS Structure-Activity Relationships and Discovery of a g Protein Biased μ Opioid Receptor Ligand, [(3-Methoxythiophen-2-Yl)Methyl]({2-[(9 r)-9-(Pyridin-2-Yl)-6-Oxaspiro-[4.5]Decan-9-Yl]Ethyl})Amine (TRV130), for the Treatment of Acute Severe Pain. J Med Chem 2013, 56 (20), 8019–8031. 10.1021/JM4010829. [DOI] [PubMed] [Google Scholar]
- (19).Willard FS; Douros JD; Gabe MBN; Showalter AD; Wainscott DB; Suter TM; Capozzi ME; van der Velden WJC; Stutsman C; Cardona GR; Urva S; Emmerson PJ; Holst JJ; D’Alessio DA; Coghlan MP; Rosenkilde MM; Campbell JE; Sloop KW Tirzepatide Is an Imbalanced and Biased Dual GIP and GLP-1 Receptor Agonist. JCI Insight 2020, 5 (17), e140532. 10.1172/JCI.INSIGHT.140532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Zhang Y; Herling M; Chenoweth DM General Solution for Stabilizing Triple Helical Collagen. J Am Chem Soc 2016, 138 (31), 9751–9754. 10.1021/JACS.6B03823. [DOI] [PubMed] [Google Scholar]
- (21).Chingle R; Proulx C; Lubell WD Azapeptide Synthesis Methods for Expanding Side-Chain Diversity for Biomedical Applications. Acc Chem Res 2017, 50 (7), 1541–1556. 10.1021/ACS.ACCOUNTS.7B00114. [DOI] [PubMed] [Google Scholar]
- (22).George KL; Horne WS Foldamer Tertiary Structure through Sequence-Guided Protein Backbone Alteration. Acc Chem Res 2018, 51 (5), 1220–1228. 10.1021/ACS.ACCOUNTS.8B00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Horne WS; Grossmann TN Proteomimetics as Protein-Inspired Scaffolds with Defined Tertiary Folding Patterns. Nature Chemistry 2020, 12, 331–337. 10.1038/s41557-020-0420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Liu S; Jean-Alphonse FG; White AD; Wootten D; Sexton PM; Gardella TJ; Vilardaga JP; Gellman SH Use of Backbone Modification to Enlarge the Spatiotemporal Diversity of Parathyroid Hormone Receptor-1 Signaling via Biased Agonism. J Am Chem Soc 2019, 141 (37), 14486–14490. 10.1021/JACS.9B04179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Liu S; Yu Z; Daley EJ; Bingman CA; Bruchs AT; Gardella TJ; Gellman SH Altered Signaling at the PTH Receptor via Modified Agonist Contacts with the Extracellular Domain Provides a Path to Prolonged Agonism in Vivo. Proc Natl Acad Sci U S A 2022, 119 (48), e2212736119. 10.1073/PNAS.2212736119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Hager MV; Johnson LM; Wootten D; Sexton PM; Gellman SH β-Arrestin-Biased Agonists of the GLP-1 Receptor from β-Amino Acid Residue Incorporation into GLP-1 Analogues. J Am Chem Soc 2016, 138 (45), 14970–14979. 10.1021/JACS.6B08323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Hager MV; Clydesdale L; Gellman SH; Sexton PM; Wootten D Characterization of Signal Bias at the GLP-1 Receptor Induced by Backbone Modification of GLP-1. Biochem Pharmacol 2017, 136, 99–108. 10.1016/J.BCP.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Liu S; Cheloha RW; Watanabe T; Gardella TJ; Gellman SH Receptor Selectivity from Minimal Backbone Modification of a Polypeptide Agonist. Proc Natl Acad Sci U S A 2018, 115 (49), 12383–12388. 10.1073/PNAS.1815294115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Fremaux J; Venin C; Mauran L; Zimmer R; Koensgen F; Rognan D; Bitsi S; Lucey MA; Jones B; Tomas A; Guichard G; Goudreau SR Ureidopeptide GLP-1 Analogues with Prolonged Activity in Vivo via Signal Bias and Altered Receptor Trafficking. Chem Sci 2019, 10 (42), 9872–9879. 10.1039/C9SC02079A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Sang P; Zhou Z; Shi Y; Lee C; Amso Z; Huang D; Odom T; Nguyen-Tran VTB; Shen W; Cai J The Activity of Sulfono-γ-AApeptide Helical Foldamers That Mimic GLP-1. Sci Adv 2020, 6 (20), eaaz4988. 10.1126/SCIADV.AAZ4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Shi Y; Lee C; Sang P; Amso Z; Huang D; Zhong W; Gu M; Wei L; Nguyen-Tran VTB; Zhang J; Shen W; Cai J α/Sulfono-γ-AA Peptide Hybrids Agonist of GLP-1R with Prolonged Action Both in Vitro and in Vivo. Acta Pharm Sin B 2023, 13 (4), 1648–1659. 10.1016/J.APSB.2022.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Sang P; Zeng H; Lee C; Shi Y; Wang M; Pan C; Wei L; Huang C; Wu M; Shen W; Li X; Cai J α/Sulfono-γ-AApeptide Hybrid Analogues of Glucagon with Enhanced Stability and Prolonged in Vivo Activity. J Med Chem 2021, 64 (18), 13893–13901. 10.1021/ACS.JMEDCHEM.1C01289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Cheloha RW; Chen B; Kumar NN; Watanabe T; Thorne RG; Li L; Gardella TJ; Gellman SH Development of Potent, Protease-Resistant Agonists of the Parathyroid Hormone Receptor with Broad β Residue Distribution. J Med Chem 2017, 60 (21), 8816–8833. 10.1021/ACS.JMEDCHEM.7B00876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Fremaux J; Venin C; Mauran L; Zimmer RH; Guichard G; Goudreau SR Peptide-Oligourea Hybrids Analogue of GLP-1 with Improved Action in Vivo. Nature Communications 2019, 10 (1), 1–9. 10.1038/s41467-019-08793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Cary BP; Deganutti G; Zhao P; Truong TT; Piper SJ; Liu X; Belousoff MJ; Danev R; Sexton PM; Wootten D; Gellman SH Structural and Functional Diversity among Agonist-Bound States of the GLP-1 Receptor. Nat Chem Biol 2022, 18 (3), 256–263. 10.1038/s41589-021-00945-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Sawada T; Gellman SH Structural Mimicry of the β-Helix in Aqueous Solution with an Isoatomic α/β/γ-Peptide Backbone. J Am Chem Soc 2011, 133 (19), 7336–7339. 10.1021/JA202175A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Dinesh B; Basuroy K; Shamala N; Balaram P Structural Characterization of Folded Pentapeptides Containing Centrally Positioned β(R)Val, γ(R)Val and γ(S)Val Residues. Tetrahedron 2012, 68 (23), 4374–4380. 10.1016/J.TET.2012.02.034. [DOI] [Google Scholar]
- (38).Basuroy K; Dinesh B; Shamala N; Balaram P Promotion of Folding in Hybrid Peptides through Unconstrained γ Residues: Structural Characterization of Helices in (Aγγ)n and (Aγα)n Sequences. Angewandte Chemie International Edition 2013, 52 (11), 3136–3139. 10.1002/ANIE.201209324. [DOI] [PubMed] [Google Scholar]
- (39).Karle IL; Pramanik A; Banerjee A; Bhattacharjya S; Balaram P ω-Amino Acids in Peptide Design. Crystal Structures and Solution Conformations of Peptide Helices Containing a β-Alanyl-γ-Aminobutyryl Segment. J Am Chem Soc 1997, 119 (39), 9087–9095. 10.1021/JA970566W. [DOI] [Google Scholar]
- (40).Shin YH; Mortenson DE; Satyshur KA; Forest KT; Gellman SH Differential Impact of β and γ Residue Preorganization on α/β/γ-Peptide Helix Stability in Water. J Am Chem Soc 2013, 135 (22), 8149–8152. 10.1021/JA403319Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Shin YH; Gellman SH Impact of Backbone Pattern and Residue Substitution on Helicity in α/β/γ-Peptides. J Am Chem Soc 2018, 140 (4), 1394–1400. 10.1021/JACS.7B10868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Shin YH; Yang H Exploration of α/β/γ-Peptidomimetics Design for BH3 Helical Domains. Chemical Communications 2022, 58 (7), 945–948. 10.1039/D1CC05758H. [DOI] [PubMed] [Google Scholar]
- (43).Grison CM; Miles JA; Robin S; Wilson AJ; Aitken DJ An α-Helix-Mimicking 12,13-Helix: Designed α/β/γ-Foldamers as Selective Inhibitors of Protein–Protein Interactions. Angewandte Chemie - International Edition 2016, 55 (37), 11096–11100. 10.1002/anie.201604517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Kobayashi K; Kawakami K; Kusakizako T; Miyauchi H; Tomita A; Kobayashi K; Shihoya W; Yamashita K; Nishizawa T; Kato HE; Inoue A; Nureki O Endogenous Ligand Recognition and Structural Transition of a Human PTH Receptor. Mol Cell 2022. 10.1016/J.MOLCEL.2022.07.003. [DOI] [PubMed] [Google Scholar]
- (45).Cary BP; Gerrard EJ; Belousoff MJ; Fletcher MM; Jiang Y; Russell IC; Piper SJ; Wootten D; Sexton PM Molecular Insights into Peptide Agonist Engagement with the PTH Receptor. Structure 2023, 31 (6), 668–676. 10.1016/J.STR.2023.04.002. [DOI] [PubMed] [Google Scholar]
- (46).Chang R; Zhang X; Qiao A; Dai A; Belousoff MJ; Tan Q; Shao L; Zhong L; Lin G; Liang YL; Ma L; Han S; Yang D; Danev R; Wang MW; Wootten D; Wu B; Sexton PM Cryo-Electron Microscopy Structure of the Glucagon Receptor with a Dual-Agonist Peptide. Journal of Biological Chemistry 2020, 295 (28), 9313–9325. 10.1074/JBC.RA120.013793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Qiao A; Han S; Li X; Li Z; Zhao P; Dai A; Chang R; Tai L; Tan Q; Chu X; Ma L; Thorsen TS; Reedtz-Runge S; Yang D; Wang MW; Sexton PM; Wootten D; Sun F; Zhao Q; Wu B Structural Basis of Gs and Gi Recognition by the Human Glucagon Receptor. Science (1979) 2020, 367 (6484), 1346–1352. 10.1126/SCIENCE.AAZ5346. [DOI] [PubMed] [Google Scholar]
- (48).Sun W; Chen LN; Zhou Q; Zhao LH; Yang D; Zhang H; Cong Z; Shen DD; Zhao F; Zhou F; Cai X; Chen Y; Zhou Y; Gadgaard S; van der Velden WJC; Zhao S; Jiang Y; Rosenkilde MM; Xu HE; Zhang Y; Wang MW A Unique Hormonal Recognition Feature of the Human Glucagon-like Peptide-2 Receptor. Cell Res 2020, 30 (12), 1098–1108. 10.1038/s41422-020-00442-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Liang YL; Khoshouei M; Glukhova A; Furness SGB; Zhao P; Clydesdale L; Koole C; Truong TT; Thal DM; Lei S; Radjainia M; Danev R; Baumeister W; Wang MW; Miller LJ; Christopoulos A; Sexton PM; Wootten D Phase-Plate Cryo-EM Structure of a Biased Agonist-Bound Human GLP-1 Receptor–Gs Complex. Nature 2018, 555 (7694), 121–125. 10.1038/nature25773. [DOI] [PubMed] [Google Scholar]
- (50).Pal K; Melcher K; Xu HE Structure and Mechanism for Recognition of Peptide Hormones by Class B G-Protein-Coupled Receptors. Acta Pharmacologica Sinica 2012, 33 (3), 300–311. 10.1038/aps.2011.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Cong Z; Liang YL; Zhou Q; Darbalaei S; Zhao F; Feng W; Zhao L; Xu HE; Yang D; Wang MW Structural Perspective of Class B1 GPCR Signaling. Trends Pharmacol Sci 2022, 43 (4), 321–334. 10.1016/j.tips.2022.01.002. [DOI] [PubMed] [Google Scholar]
- (52).Neer RM; Arnaud CD; Zanchetta JR; Prince R; Gaich GA; Reginster J-Y; Hodsman AB; Eriksen EF; Ish-Shalom S; Genant HK; Wang O; Mellström D; Oefjord ES; Marcinowska-Suchowierska E; Salmi J; Mulder H; Halse J; Sawicki AZ; Mitlak BH Effect of Parathyroid Hormone (1-34) on Fractures and Bone Mineral Density in Postmenopausal Women with Osteoporosis. N Engl J Med 2001, 344 (19), 1434–1441. 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- (53).Luck MD; Carter PH; Gardella TJ The (1–14) Fragment of Parathyroid Hormone (PTH) Activates Intact and Amino-Terminally Truncated PTH-1 Receptors. Molecular Endocrinology 1999, 13 (5), 670–680. 10.1210/MEND.13.5.0277. [DOI] [PubMed] [Google Scholar]
- (54).Black JW; Leff P Operational Models of Pharmacological Agonism. Proceedings of the Royal Society of B: Biological Sciences 1983, 220 (1219), 141–162. 10.1098/RSPB.1983.0093. [DOI] [PubMed] [Google Scholar]
- (55).Kenakin T; Watson C; Muniz-Medina V; Christopoulos A; Novick S A Simple Method for Quantifying Functional Selectivity and Agonist Bias. ACS Chem Neurosci 2012, 3 (3), 193–203. 10.1021/CN200111M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Ahn S; Nelson CD; Garrison TR; Miller WE; Lefkowitz RJ Desensitization, Internalization, and Signaling Functions of β-Arrestins Demonstrated by RNA Interference. Proc Natl Acad Sci U S A 2003, 100 (4), 1740–1744. 10.1073/PNAS.262789099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Luttrell LM “Location, Location, Location”: Activation and Targeting of MAP Kinases by G Protein-Coupled Receptors. J Mol Endocrinol 2003, 30 (2), 117–126. 10.1677/JME.0.0300117. [DOI] [PubMed] [Google Scholar]
- (58).Moo E. von; van Senten JR; Bräuner-Osborne H; Møller TC Arrestin-Dependent and -Independent Internalization of G Protein–Coupled Receptors: Methods, Mechanisms, and Implications on Cell Signaling. Mol Pharmacol 2021, 99 (4), 242–255. 10.1124/MOLPHARM.120.000192. [DOI] [PubMed] [Google Scholar]
- (59).van der Velden WJC; Smit FX; Christiansen CB; Møller TC; Hjortø GM; Larsen O; Schiellerup SP; Bräuner-Osborne H; Holst JJ; Hartmann B; Frimurer TM; Rosenkilde MM GLP-1 Val8: A Biased GLP-1R Agonist with Altered Binding Kinetics and Impaired Release of Pancreatic Hormones in Rats. ACS Pharmacol Transl Sci 2021, 4 (1), 296–313. 10.1021/ACSPTSCI.0C00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Jones B; Buenaventura T; Kanda N; Chabosseau P; Owen BM; Scott R; Goldin R; Angkathunyakul N; Corrêa IR; Bosco D; Johnson PR; Piemonti L; Marchetti P; Shapiro AMJ; Cochran BJ; Hanyaloglu AC; Inoue A; Tan T; Rutter GA; Tomas A; Bloom SR Targeting GLP-1 Receptor Trafficking to Improve Agonist Efficacy. Nat Commun 2018, 9 (1), 1–17. 10.1038/s41467-018-03941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Yu Z; Cary BP; Kim TW; Nguyen KD; Gardella TJ; Gellman SH Kinetic and Thermodynamic Insights into Agonist Interactions with the Parathyroid Hormone Receptor-1 from a New NanoBRET Assay. ACS Chem Biol 2022. 10.1021/ACSCHEMBIO.2C00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).McKee RL; Goldman ME; Caulfield MP; Dehaven PA; Levy JJ; Nutt RF; Rosenblatt M THE 7–34-FRAGMENT OF HUMAN HYPERCALCEMIA FACTOR IS A PARTIAL AGONIST/ANTAGONIST FOR PARATHYROID HORMONESTIMULATED CAMP PRODUCTION. Endocrinology 1988, 122 (6), 3008–3010. 10.1210/ENDO-122-6-3008. [DOI] [PubMed] [Google Scholar]
- (63).Horiuchi N; Holick MF; Potts JT; Rosenblatt M A Parathyroid Hormone Inhibitor in Vivo: Design and Biological Evaluation of a Hormone Analog. Science (1979) 1983, 220 (4601), 1053–1055. 10.1126/SCIENCE.6302844. [DOI] [PubMed] [Google Scholar]
- (64).Zhao LH; Ma S; Sutkeviciute I; Shen DD; Edward Zhou X; de Waal PW; Li CY; Kang Y; Clark LJ; Jean-Alphonse FG; White AD; Yang D; Dai A; Cai X; Chen J; Li C; Jiang Y; Watanabe T; Gardella TJ; Melcher K; Wang MW; Vilardaga JP; Eric Xu H; Zhang Y Structure and Dynamics of the Active Human Parathyroid Hormone Receptor-1. Science (1979) 2019, 364 (6436), 148–153. 10.1126/SCIENCE.AAV7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Ehrenmann J; Schöppe J; Klenk C; Rappas M; Kummer L; Doré AS; Plückthun A High-Resolution Crystal Structure of Parathyroid Hormone 1 Receptor in Complex with a Peptide Agonist. Nature Structural & Molecular Biology 2018, 25 (12), 1086–1092. 10.1038/S41594-018-0151-4. [DOI] [PubMed] [Google Scholar]
- (66).Marx UC; Adermann K; Bayer P; Forssmann WG; Rösch P Solution Structures of Human Parathyroid Hormone Fragments HPTH(1–34) and HPTH(1–39) and Bovine Parathyroid Hormone Fragment BPTH(1–37). Biochem Biophys Res Commun 2000, 267 (1), 213–220. 10.1006/BBRC.1999.1958. [DOI] [PubMed] [Google Scholar]
- (67).Russ Lehrman S; Tuls JL; Lund M Peptide α-Helicity in Aqueous Trifluoroethanol: Correlations with Predicted α-Helicity and the Secondary Structure of the Corresponding Regions of Bovine Growth Hormone. Biochemistry 1990, 29 (23), 5590–5596. 10.1021/BI00475A025. [DOI] [PubMed] [Google Scholar]
- (68).Chi Y; English EP; Pomerantz WC; Horne WS; Joyce LA; Alexander LR; Fleming WS; Hopkins EA; Gellman SH Practical Synthesis of Enantiomerically Pure B2-Amino Acids via Proline-Catalyzed Diastereoselective Aminomethylation of Aldehydes. J Am Chem Soc 2007, 129 (18), 6050–6055. 10.1021/JA070063I. [DOI] [PubMed] [Google Scholar]
- (69).Shirley M. Abaloparatide: First Global Approval. Drugs 2017, 77 (12), 1363–1368. 10.1007/S40265-017-0780-7. [DOI] [PubMed] [Google Scholar]
- (70).Sato T; Verma S; Khatri A; Dean T; Goransson O; Gardella TJ; Wein MN Comparable Initial Engagement of Intracellular Signaling Pathways by Parathyroid Hormone Receptor Ligands Teriparatide, Abaloparatide, and Long-Acting PTH. JBMR Plus 2021, 5 (5), e10441. 10.1002/JBM4.10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Adelhorst K; Hedegaard BB; Knudsen LB; Kirk O Structure-Activity Studies of Glucagon-like Peptide-1. Journal of Biological Chemistry 1994, 269 (9), 6275–6278. 10.1016/S0021-9258(17)37366-0. [DOI] [PubMed] [Google Scholar]
- (72).Gibadullin R; Cary BP; Gellman SH Differential Responses of the GLP-1 and GLP-2 Receptors to N-Terminal Modification of a Dual Agonist. J Am Chem Soc 2023, 145 (22), 12105–12114. 10.1021/JACS.3C01628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Zhang X; Belousoff MJ; Zhao P; Kooistra AJ; Truong TT; Ang SY; Underwood CR; Egebjerg T; Šenel P; Stewart GD; Liang YL; Glukhova A; Venugopal H; Christopoulos A; Furness SGB; Miller LJ; Reedtz-Runge S; Langmead CJ; Gloriam DE; Danev R; Sexton PM; Wootten D Differential GLP-1R Binding and Activation by Peptide and Non-Peptide Agonists. Mol Cell 2020, 80 (3), 485–500.e7. 10.1016/J.MOLCEL.2020.09.020. [DOI] [PubMed] [Google Scholar]
- (74).zhang Y; Sun B; Feng D; Hu H; Chu M; Qu Q; Tarrasch JT; Li S; Sun Kobilka T; Kobilka BK; Skiniotis G Cryo-EM Structure of the Activated GLP-1 Receptor in Complex with a G Protein. Nature 2017, 546 (7657), 248–253. 10.1038/NATURE22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Deganutti G; Liang YL; Zhang X; Khoshouei M; Clydesdale L; Belousoff MJ; Venugopal H; Truong TT; Glukhova A; Keller AN; Gregory KJ; Leach K; Christopoulos A; Danev R; Reynolds CA; Zhao P; Sexton PM; Wootten D Dynamics of GLP-1R Peptide Agonist Engagement Are Correlated with Kinetics of G Protein Activation. Nature Communications 2022, 13 (1), 1–18. 10.1038/s41467-021-27760-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Neidigh JW; Fesinmeyer RM; Prickett KS; Andersen NH Exendin-4 and Glucagon-like-Peptide-1: NMR Structural Comparisons in the Solution and Micelle-Associated States. Biochemistry 2001, 40 (44), 13188–13200. 10.1021/BI010902S. [DOI] [PubMed] [Google Scholar]
- (77).Gallwitz B; Witt M; Paetzold G; Morys-Wortmann C; Zimmermann B; Eckart K; Fölsch UR; Schmidt WE Structure/Activity Characterization of Glucagon-Like Peptide-1. Eur J Biochem 1994, 225 (3), 1151–1156. 10.1111/J.1432-1033.1994.1151B.X. [DOI] [PubMed] [Google Scholar]
- (78).Chabenne J; Chabenne MDM; Zhao Y; Levy J; Smiley D; Gelfanov V; DiMarchi R A Glucagon Analog Chemically Stabilized for Immediate Treatment of Life-Threatening Hypoglycemia. Mol Metab 2014, 3 (3), 293–300. 10.1016/J.MOLMET.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).DaCambra MP; Yusta B; Sumner-Smith M; Crivici A; Drucker DJ; Brubaker PL Structural Determinants for Activity of Glucagon-like Peptide-2. Biochemistry 2000, 39 (30), 8888–8894. 10.1021/BI000497P. [DOI] [PubMed] [Google Scholar]
- (80).Wiśniewski K; Sueiras-Diaz J; Jiang G; Galyean R; Lu M; Thompson D; Wang YC; Croston G; Posch A; Hargrove DM; Wiśniewska H; Laporte R; Dwyer JJ; Qi S; Srinivasan K; Hartwig J; Ferdyan N; Mares M; Kraus J; Alagarsamy S; Rivière PJM; Schteingart CD Synthesis and Pharmacological Characterization of Novel Glucagon-like Peptide-2 (GLP-2) Analogues with Low Systemic Clearance. J Med Chem 2016, 59 (7), 3129–3139. 10.1021/ACS.JMEDCHEM.5B01909. [DOI] [PubMed] [Google Scholar]
- (81).Bai X; Niu Y; Zhu J; Yang AQ; Wu YF; Ye XS A New GLP-1 Analogue with Prolonged Glucose-Lowering Activity in Vivo via Backbone-Based Modification at the N-Terminus. Bioorg Med Chem 2016, 24 (6), 1163–1170. 10.1016/J.BMC.2016.01.036. [DOI] [PubMed] [Google Scholar]
- (82).Tan M; Lamendola C; Luong R; McLaughlin T; Craig C Safety, Efficacy and Pharmacokinetics of Repeat Subcutaneous Dosing of Avexitide (Exendin 9-39) for Treatment of Post-Bariatric Hypoglycaemia. Diabetes Obes Metab 2020, 22 (8), 1406–1416. 10.1111/DOM.14048. [DOI] [PubMed] [Google Scholar]
- (83).Werner HM; Cabalteja CC; Horne WS Peptide Backbone Composition and Protease Susceptibility: Impact of Modification Type, Position, and Tandem Substitution. ChemBioChem 2016, 17 (8), 712–718. 10.1002/CBIC.201500312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Alaña I; Parker JC; Gault VA; Flatt PR; O’Harte FPM; Malthouse JPG; Hewage CM NMR and Alanine Scan Studies of Glucose-Dependent Insulinotropic Polypeptide in Water. Journal of Biological Chemistry 2006, 281 (24), 16370–16376. 10.1074/JBC.M510414200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.