Abstract
Radiation oncology is an integral part of the multidisciplinary team caring for children with cancer. The primary goal of our committee is to enable the delivery of the safest dose of radiation therapy (RT) with the maximal potential for cure, and to minimize toxicity in children by delivering lower doses to normal tissues using advanced technologies like intensity-modulated RT (IMRT) and proton therapy. We provide mentorship for young investigators and are actively involved in educating the global radiation oncology community. We are leaders in the effort to discover novel radiosensitizers, radioprotectors, and advanced RT technologies that could help improve outcomes of children with cancer.
Radiation oncology is an integral part of the multidisciplinary team responsible for the management of children with cancer. The primary goals of the Children’s Oncology Group (COG) Radiation Oncology (RO) Committee include: 1). To deliver the safest dose of RT with the maximum potential for cure, based on the outcomes of prior COG studies; 2). To individualize the indications for, and total dose of radiation therapy (RT), based on molecular, pathologic and imaging biomarkers, in order to minimize toxicity for low-risk children by using less or no RT, and maximize tumor control rates for children with higher risk cancers by using higher doses of RT; 3) To limit the radiation exposure to normal tissues to minimize toxicity by using advanced technologies like IMRT and proton therapy; 4). To analyze tumor control and late toxicity outcomes from COG studies with the goal of refining RT guidelines for future protocols; 5). To survey RT practice patterns regarding advanced technologies like proton therapy and image-guided RT, in order to set national and international standards of care; 6). To promote education of the principles and practice of RT for our members and the global radiation oncology community, including low and middle income countries (SIOP and LMIC). 7). To provide mentorship and training for young investigators, in order to create a talented future generation of radiation oncologists; and 8). To conduct multidisciplinary research that includes biologists and industry, to discover novel radiosensitizers, radioprotectors and advanced novel RT techniques that could improve clinical outcomes in children with cancer. Currently in the RO Committee there are approximately 392 radiation oncologists and 45 physicists. The distribution of COG RT sites are as follows: USA 230, Puerto Rico 1, Canada 15, Australia 7 and New Zealand 2, including 21 proton therapy facilities (17 of which offer pencil beam scanning therapy). In this report we present a summary of the activities in the COG RO Committee during the last decade in pursuit of these goals.
1. Radiation Physics.
Medical Physicists and radiation oncologists work closely together to maximize the benefits of modern RT technologies in children. We have promoted utilization of IMRT, volumetric-modulated arc therapy (VMAT), and proton therapy in clinical trials to maximize the therapeutic ratio. We have reported practice guidelines for, cardiac-sparing whole lung IMRT,1 choose-wisely image guidance techniques,2 protection of the brainstem in proton and photon therapy,3 and pediatric total body irradiation (TBI).4 Particle therapy has seen a rapid growth in the last decade with transformative technologies such as pencil beam scanning that is poised to deliver highly conformal RT with the best normal tissue sparing in the history of RT, (Fig. 1). The RO Committee is leading efforts for the safe adoption and optimal use of particle therapy. We are also modernizing and standardizing TBI using VMAT and Tomotherapy technologies that may lead to lower late toxicities, (Fig. 2). Future goals include the introduction of artificial intelligence and automation in clinical trials and the development of predictive models using outcome data in the era of precision medicine.
Figure 1.
The mean RT doses to the supratentorial brain and left temporal lobe in 127 medulloblastoma patients on the ACNS0331 trial who received either involved field radiotherapy (IFRT, N=60 patients) or posterior fossa radiotherapy (PFRT, N=67 patients) after craniospinal irradiation. For both brain structures, doses paired comparisons with two-sample t test for equal means show statistically significant differences (p<0.05) for IFRT vs. PFRT and 3D CRT/IMRT vs. proton therapy, but not for IMRT vs. 3D CRT. (Data courtesy of Dr. Matthew Ladra, Children’s National Medical Center and Chia-Ho Hua Ph. D, St. Jude Children’s Hospital).
Figure 2.
A) 3D dose color wash distribution for an Acute Myeloid Leukemia (AML) patient treated with Volumetric Modulated Arc Therapy Total Body Irradiation (VMAT TBI). Five treatment isocenters were utilized for TBI: 3 VMAT isocenters treated in Head First Supine (HFS) position and 2 AP/PA isocenters treated in Feet First Supine (FFS) position. B). Dose Volume Histogram (DVH) comparisons between traditional 2D TBI (triangles) and VMAT TBI (squares) for the AML patient showing VMAT technique as superior in terms of lungs and kidneys sparing and TBI Planning Target Volume (PTV) dose coverage (Data courtesy of Dr. Susan M. Hiniker and Nataliya Kovalchuk Ph. D, Stanford University).
2. Quality Assurance: Imaging and Radiation Oncology Core (IROC).5,6
RT quality assurance ensures uniform application of treatment for COG trials. All COG centers are credentialed for trial participation and, for most trials, must pass knowledge tests, both for contouring and treatment application. Specific technologies including IMRT, proton therapy and stereotactic therapy require additional credentialing. The management of imaging and radiotherapy data is crucial to the conduct of COG trials. Imaging and radiation therapy objects are acquired and collated by IROC and reviewed for compliance with protocol specifications. Data and image transfer tools can be applied in real time, so that imaging can define response, and be applied to clinical trials in an adaptive format (for example, to trigger additional pathways of randomization in a trial, as in Hodgkin lymphoma and Wilms tumors). As image-defined target definition becomes more complex with advanced imaging techniques, IROC is prepared to manage the challenges and improve care for the next generation of patients. The RO Committee is also committed to the analysis of the voluminous imaging and RT data at IROC, for correlation with tumor control and toxicity outcomes in COG protocols, (Table 1).
Table 1.
The COG Data Archive of the Imaging and Radiation Oncology Core (IROC) Rhode Island categorized by disease category, number of protocols, and broken down into number of subjects, number of diagnostic imaging studies and number of digital RT treatment plans.
| Disease category | #Protocols | #Patients | Accrual Years | DICOM Diagnostic Studies | DICOM RT Treatment Plans |
|---|---|---|---|---|---|
| AALL | 16 | 3,500 | 2001–2023 | 1,317 | 9 |
| ACNS/ACCL/ADVL | 35 | 3664 | 2001–2023 | 19454 | 1985 |
| AEWS | 7 | 1731 | 2001–2019 | 6,347 | 735 |
| AHOD | 11 | 3,174 | 2001–2023 | 33,587 | 1,161 |
| AHEP | 2 | 725 | 2009–2023 | 4,076 | N/A |
| ANBL | 17 | 3494 | 2001–2023 | 15,328 | 783 |
| ARAR/ARET | 6 | 322 | 2006–2017 | 1,236 | 94 |
| AREN | 6 | 9016 | 2006–2023 | 13,772 | 967 |
| ARST | 13 | 2,607 | 2002–2023 | 8,910 | 1334 |
3. Central Nervous System Tumors
COG ACNS0331 confirmed that reducing the radiation boost volume in newly diagnosed average-risk medulloblastoma (MB) is safe and does not compromise survival. In contradistinction, reducing the craniospinal irradiation (CSI) dose resulted in inferior survival but improved neurocognitive outcomes.7 ACNS0331 set the stage for the ACNS1422 that carved out WNT-driven average-risk MB as a population to test further reduction in CSI dose of 18 Gy down from 23.4 Gy. Similarly, the ACNS2031 trial will evaluate whether reduced therapy is warranted in the subset of Group 4 MB patients with chromosome 11 loss. The ACNS0332 study showed that radiosensitization with carboplatin improved event-free survival (EFS) by 19% at 5 years for children with high-risk group 3 MB, underscoring the importance of molecularly informed stratification and decision-making.8 We will conduct future studies using reduced-dose CSI of 23.4 Gy, for a subset of high-risk MB patients with relatively favorable molecular and/or histologic features, while continuing to seek effective radiosensitizers. For ependymomas, gross-total (GTR) or near-total resection (NTR) followed by RT are the cornerstones of therapy. Building upon ACNS0121, ACNS0831 adopted the same four-strata risk stratification while investigating the efficacy of post-radiation maintenance chemotherapy.9 The RT guidelines differed where the GTV-to-CTV expansion was reduced from 10 to 5 mm. Moreover, ACNS0831 mandated a cone-down treatment volume to exclude spinal cord, brainstem, or optic chiasm, after 54 Gy, when indicated. Additionally, more conservative brainstem dose constraints were assigned to patients receiving proton therapy, due to the higher proton biological effective dose (BED) and end-of-range uncertainties, as well as a few unexpected brainstem-related adverse events observed in these patients.3 For CNS germ cell tumors (GCT), ACNS1123 established the paradigm of response-based RT for patients with localized germinoma. This entails 18 Gy or 24 Gy whole ventricular irradiation (WVI) respectively, for patients with complete and partial responses to carboplatin and etoposide chemotherapy. A 12 Gy boost to the tumor bed ensues in either case. Reduced-dose WVI (18 Gy) was associated with no ventricular failures. Still, the study failed to statistically demonstrate the non-inferiority of 18 Gy WVI in terms of 3-year PFS compared to historical controls due to some confounding factors that are being addressed in the new dose-reduced WVI study.10 For localized non-germinomatous GCTs, ACNS0122 showed the best outcomes after chemotherapy, followed by CSI, with five-year EFS and OS of 84% and 93%, respectively ACNS1123 evaluated a reduced-dose 30.6 Gy WVI instead of 36 Gy CSI, with the tumor bed receiving a cumulative dose of 54 Gy. This stratum was prematurely closed after 10 recurrences, all confined to the spine, despite comparable 3-year PFS.12 These results influenced the design for the current ACNS2021 study, in which response-based 30.6 Gy whole ventricular and spinal canal irradiation is being evaluated. In children with atypical teratoid/rhabdoid tumor (ATRT) superior OS outcomes (43% at 4 years) were noted in the COG ACNS0333 compared to historical cohorts. These results have led to the management paradigm that includes surgery, two courses of chemotherapy, followed by three courses of high-dose chemotherapy with peripheral blood stem cell rescue and RT; involved field +/− CSI for localized and disseminated disease, respectively.13 We are committed to better quality survivorship after CNS RT in children. The COG phase 3 ACCL2031 study evaluates memantine for neurocognitive protection in children undergoing upfront cranial radiation, building upon the adult RTOG 0614 study results.
4. Sarcomas
Successive COG rhabdomyosarcoma studies have yielded valuable results regarding RT and local control. From D9803, we learned that tumor size >5cm is the most significant clinical risk factor for local failure with definitive RT.14 As a result, the recent intermediate risk study, ARST1431, is studying dose escalation to 59.4 Gy (from 50.4 Gy) for tumors >5cm. We also saw local control decrease significantly in ARST0531, compared to the previous intermediate risk study, D9803.15 This result was unexpected as we had hoped for improved local control with earlier RT at week 4 (from week 13) and the use of a mild radiosensitizer, irinotecan, in the experimental arm. The reduction in local control was attributed to the reduction in cyclophosphamide dose between the two studies. EFS and overall survival (OS) were also lower in ARST0531, though the comparison to D9803 was only historical. Consequently, a future randomized comparison of cyclophosphamide dosing regimens is under consideration. Cyclophosphamide dose has also been found to correlate with local control in recent low risk studies, especially in girls with vaginal primary tumors when RT was also eliminated.16 Future protocols will strongly encourage vaginal brachytherapy for these patients. For Group III orbit primary tumors, it was shown that local control was inadequate when both cyclophosphamide dose was decreased and RT dose was reduced to 45 Gy.17 In future COG protocols, RT dose for orbit tumors will match other sites. COG is collecting molecular subclassification of RMS tumors to correlate with outcomes. We have demonstrated that Group I FOXO1+ tumors benefit from post-operative RT, while FOXO1- tumors do not. We also find that TP53 and MYOD1 mutations are associated with poorer local control and possible radioresistance. Future work will seek to confirm these findings and determine if alternate strategies are indicated for tumors with these mutations. We published results of the first COG risk-adapted study for non-rhabdomyosarcoma soft tissue sarcomas, ARST0032.18 The subsequent trial ARST1321 was a groundbreaking randomized study combining pediatric and adult patients, partnering with the NRG cooperative group. For all sarcomas, we have seen increasing use of advanced technologies including protons and stereotactic body radiotherapy (SBRT). We will have robust data within all our protocols to measure the impact of these technologies on patient outcomes.
5. Wilms Tumor
In AREN0533, children with favorable histology (FH) Wilms tumor and lung metastases showing complete response (CR) continued DD4A chemotherapy without whole lung irradiation (WLI). Patients with incomplete response (IR) or loss of heterozygosity at chromosomes 1p and 16q received WLI and 4 cycles of cyclophosphamide and etoposide in addition to DD4A chemotherapy (Regimen M). CR was achieved in 45.5% of the patients at 6 weeks after chemotherapy. For patients with CR, 4-year EFS and OS estimates were 79.5% and 96.1%, while for patients with IR, the corresponding 4-year estimates were 88.5% and 95.4%, respectively.18 In AREN0321, a higher flank dose (19.8 Gy) in addition to a carboplatin-based regimen resulted in fewer local relapses for stage III diffuse anaplastic Wilms tumor, compared to NWTS-5 when a dose of 10.8 Gy was utilized.19 The use of a cardiac-sparing IMRT for WLI is currently being explored in AREN1921 protocol for Stage II to IV diffuse anaplastic and relapsed Wilms tumor, and in the next favorable histology protocol, AREN 2231.1 A major change in AREN2231 protocol is the timing of flank/abdomen RT in Stage IV patients. To avoid overlap between sequential flank/abdomen and WLI fields that exposed the heart, liver and lungs to higher doses of RT in AREN0533, patients enrolled in AREN2231 will receive RT to these sites concurrently after the week 6 evaluation of CR/IR for lung metastasis. The presence of 1q gain has also been found to be an adverse prognostic factor and children with 1q gain will require WLI even if CR is achieved after 6 weeks of chemotherapy.21
6. Hodgkin Lymphoma
In the last decade, we have focused on advancing novel targeted systemic therapy through collaboration with industry, and to advance adolescent and young adult (AYA) approaches in collaboration with the adult NCTN groups. AHOD1331, AHOD1822, S1826 and AHOD2131 incorporated novel targeted chemotherapy and/or check point inhibitors in the front line setting, and AHOD1721 and EA4211 in the setting of retrieval therapy for relapsed or refractory disease. The positron emission tomography (PET) response adapted trial designs have significantly reduced the overall application and field extent of RT across these trials.22 In AHOD1331 bleomycin was substituted with antiCD30 antibody drug conjugate, brentuximab vedotin, resulting in an improvement in 3-year EFS of 92.1% (95%CI 88.4–94.7) versus 82.5% (95% CI 77.4 to 86.5) for children with advanced stage Hodgkin lymphoma.23 Involved site radiotherapy (ISRT) was used for mediastinal bulky disease and slow early responding lesions based on interim PET response, a huge evolutionary step from involved field radiotherapy (IFRT) to all sites of initial involvement. This strategy has currently resulted in a reduction of RT use from 76% on AHOD0831 to 55%.22, 24 While overall RT use is declining (S1826 <5% of pediatric patients and AHOD2131 expected <20%), the RT doses will increase from 21 Gy to 30 Gy with options for higher boost doses based on final PET response to 36–40 Gy. Despite the higher RT doses, increased toxicity is not expected due to the use of highly conformal RT technology (IMRT, proton therapy), smaller RT fields (e.g. residual site RT) and careful evaluation of normal tissue exposure. In fact, on AHOD1331, IMRT and proton therapy were used among 45% and 27% respectively, among patients receiving RT.25 In addition to investigating smaller fields and using advanced RT technology, future RT related strategies include exploring the use of RT consolidation in place of high dose chemotherapy and autologous stem cell transplant in the relapse refractory setting.26
7. Neuroblastoma
The benefit of RT in high-risk neuroblastoma (HR-NBL) was demonstrated in CCG3891, with 22 Gy to primary site (PS) providing superior local control compared to 10 Gy.27 Outcomes from ANBL0532 included superior 3 year EFS of 62% for patients receiving tandem autologous stem cell transplant (ASCT) compared to 48% after single transplant.28 The RT dose was 21.6 Gy to PS after complete resection, and for all persistent metastatic sites after induction chemotherapy. In patients with incomplete resection, the RT dose was escalated to 36 Gy. In ANBL1531, novel induction agents (131I-MIBG and ALK-inhibitors) were introduced. The RT dose guidelines were similar to ANBL0532 initially, however, after establishing that there was no improvement in tumor control with 36 Gy in ANBL0532 compared to historical controls receiving 21.6 Gy (5 year EFS 49% vs 51%, p = 0.5), 29 the RT guidelines were modified such that all patients would receive 21.6 Gy regardless of surgical resection status. Future goals include optimization of response to systemic therapy through use of chemoimmunotherapy and extended induction regimens for patients with poor initial response. The RT aims may include modest dose-de-escalation for patients with complete response to induction therapy.30 The role of metastatic site RT remains unclear, although RT to ≤5 sites is an established part of COG paradigm. Patterns of failure analysis from ANBL1531 is expected to shed light on the importance of metastatic site RT.
8. Bone Tumors
AEWS1031 showed the best survival outcomes to date for localized Ewing sarcoma, despite the negative results of the primary endpoint. Analysis of local control outcomes also revealed the lowest incidence of local failure (6% at 5 years) reported in a prospective trial of localized Ewing sarcoma, with the most significant improvement seen in patients receiving primary RT. Local failure rates for surgery, radiation and combined surgery and radiation were 5%, 7.7%, and 6.5%, respectively (p=0.33).31 Further analysis is ongoing, however preliminary results suggest that large tumor size is an important risk factor for increased risk of local failure and should be incorporated into local therapy risk stratification in future localized Ewing sarcoma studies. Future Ewing sarcoma studies will evaluate the feasibility, efficacy and toxicity of dose-escalation to 64.8 Gy for tumors at highest risk for local failure treated with definitive RT, including pelvic primary tumors and large tumors (≥200 mL at diagnosis). In addition, local control remained similar compared to prior studies after surgery and RT, despite using response-adapted RT to reduce RT volumes for favorable responders, in an effort to reduce the risk of musculoskeletal complications.32 A prospective study for metastatic Ewing sarcoma, AEWS1221 was also recently reported.33 Despite evidence supporting consolidation of metastatic sites with RT, compliance with recommendations was poor and no meaningful data were obtained to evaluate the efficacy of this approach.33 AEWS1221 also utilized SBRT to allow definitive RT administered at higher doses in rapid time frame (5 fractions) to improve compliance with RT recommendations for all metastatic bony sites. Although the trial was closed due to lack of efficacy of the experimental arm, 298 patients were enrolled including 74 with bony metastatic disease eligible for SBRT. Secondary analysis is ongoing and this will be the first COG trial to report detailed patterns of failure and local control of metastases-directed therapy in Ewing sarcoma. Similarly, the currently open trial in osteosarcoma, AOST2032, recommends comprehensive local therapy to metastatic sites including for the first time, the option of SBRT for bone and lung metastases.
9. Late Effects Committee
Despite the remarkable success in improving survival, both RT and chemotherapy can cause debilitating or even fatal late effects in children that are critical to understand, mitigate, or prevent.35 The function of the Late Effects subcommittee includes: (1) review of existing normal tissue RT dose constraints in COG protocols; (2) generate new evidence-based dose constraints for future protocols; (3) interface with the COG Late Effects Survivorship Guidelines committee for refinement of surveillance recommendations (http://www.survivorshipguidelines.org/); (4) identify knowledge gaps relating to RT and combined modality normal tissue toxicities; (5) support academic endeavors of young investigators to address these gaps and training them in radiation late effects. These efforts led to the integration of COG radiation oncologists and medical physicists into the Pediatric Normal Tissue Effects in the Clinic (PENTEC) consortium to form 19 organ-specific task forces.36 These task forces have investigated radiation-associated normal tissue damage as a function of patient age, RT dose and fraction size, RT volume, functional organization of the organ, and the impact of ancillary cytotoxic therapy. Several of these reports have been published, and all 19 will appear in a dedicated issue of the International Journal of Radiation Oncology, Biology, and Physics in 2023. These reports will provide the best available data on which to make decisions in treating children for cancer with RT, and inform scientists on necessary future directions for research. The COG Dose Constraints task force successfully completed compilation of a comprehensive set of evidence-based normal tissue dose-constraints guidelines to harmonize these recommendations across COG protocols. Further, our discipline is also actively involved in late effects outcome analysis conducted by the Childhood Cancer Survivor Study and National Wilms Tumor Late Effects Study
10. Education and Young Investigator (YI) Mentorship
The COG RO discipline prioritizes education, mentorship, training and academic productivity especially among YIs. We published a special edition of Pediatric Blood and Cancer journal on the use of radiotherapy for pediatric cancers, that included 21 articles with over 100 authors. The majority of papers were led by YIs.37 Further, all RT protocols in COG have at least one YI paired with a senior discipline member in order to foster a culture of opportunity, mentorship and learning. In an effort to promote education among COG members and others globally, we have comprehensive subject presentations on all pediatric cancers that are available freely on the non-password protected IROC website (www.qarc.org).
Conclusions and Future Plans
While the cure rates of children with cancer have improved significantly in modern times, there is still a need for improved tumor control in advanced cancers. The discovery of novel radiosensitizers could improve current rates of tumor control. Based on preclinical data, our discipline is working to advance clinical trials with novel radiosensitizers like ATM inhibitor AZD 139037 and tyrosine kinase inhibitor catequentinib38 (anlotinib) in children and young adults. RT is also an important cause for the induction of late treatment-related toxicities including secondary malignancies that significantly impairs the quality of life of childhood cancer survivors. Novel RT techniques such as IMRT and proton therapy will provide opportunities for improved quality of survival. Our members are also in the forefront of laboratory and clinical research using a highly novel RT technique called FLASH RT, that involves the delivery of ultra-high dose rate radiation to tumors with the potential to reduce RT-related complications, (Fig. 3).39 The discovery of novel genetic biomarkers linked to the development of late RT induced toxicities will improve our understanding of risks of RT and refine treatment recommendations and surveillance guidelines for high-risk survivors.40 The COG RO Committee continues to work in several novel areas of research sponsored by the NCI including: 1). Study of the mechanistic interactions and biologic consequences of RT, prioritizing a comprehensive study of patient (genomic and epigenomics), tumor and treatment (chemotherapy, RT, dosimetry) factors, together with longitudinal multiomics (pre and post-therapy) to improve our understanding of the effects of RT on normal tissues (RFA-CA-22–046); 2). Discovery of novel radiosensitizers (PAR-22–198, PAR-22–199); and 3). Discovery of novel targeted radionuclide therapy (PAR-22–139, PAR-22–140). We hope that these innovations in radiation oncology and biology can be harnessed for the benefit of children and childhood survivors of cancer.
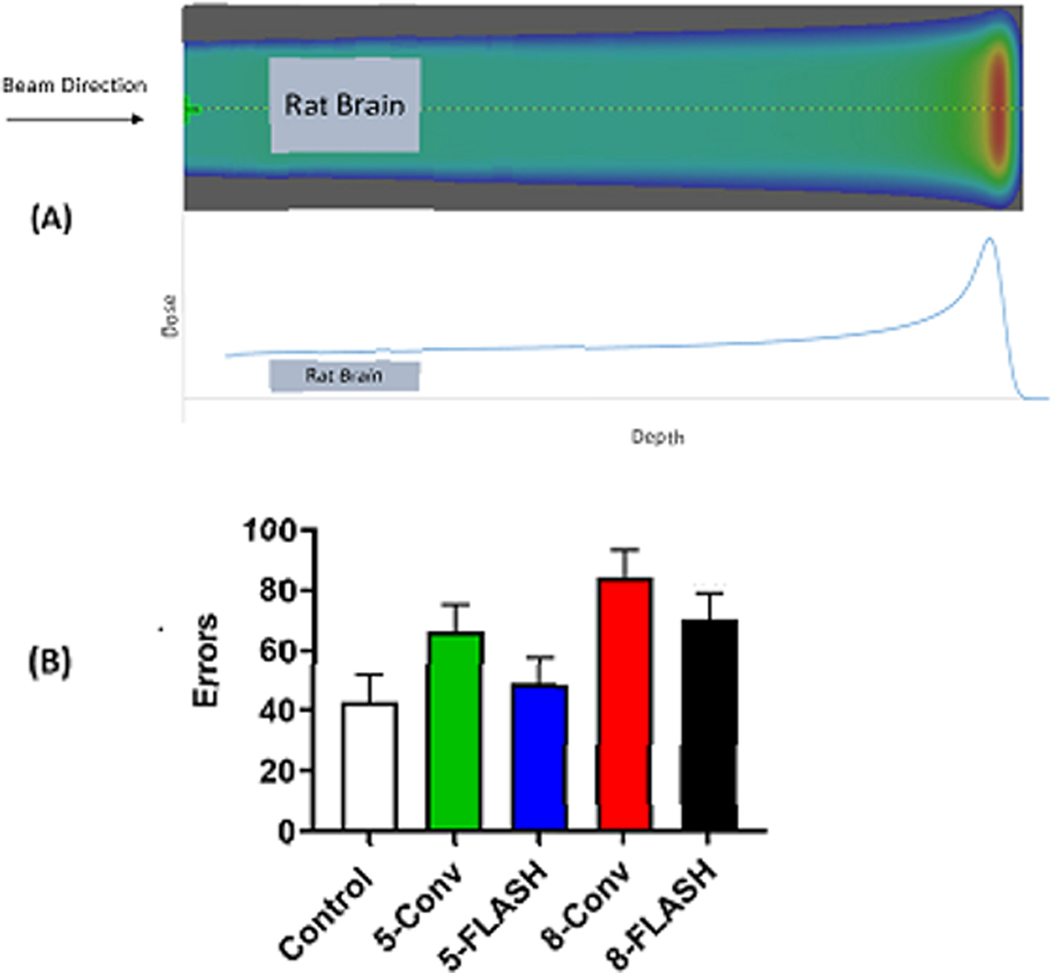
Figure 3.
Pre-clinical results of the impact of ultra-high dose rate (FLASH) protons (100 Gy/s) versus conventional dose rate (1 Gy/s) protons on cognition in 11 day-old rats (n=19). The whole brain was irradiated with a single transmission radiation field, with the Bragg peak itself stopping outside of the rat. (A) shows the proton treatment beam with the rat brain target superimposed within the plateau region of the beam. Rats received total doses of 0 (Control), 5, or 8 Gy proton radiation in a single fraction. They were then raised to adulthood and tested for learning using the Cincinnati Water Maze. (B) shows the effect of radiation on errors, 2 trials/day for 10 days. The 5 Gy FLASH group made significantly fewer errors than the 5 Gy conventional dose rate. (p < 0.05). No benefit to FLASH was seen at 8 Gy. (Data courtesy of Charles Vorhees, PhD, Cincinnati Children’s Hospital).
Funding Acknowledgement:
1U24CA180803, U10CA180886, U10CA180899, U10CA098543, U10CA098413.
Abbreviations
- RT
Radiation therapy
- Gy
Gray
- IMRT
Intensity Modulated Radiation Therapy
- COG
Children’s Oncology Group
- SIOP
International Society of Paediatric Oncology
- LMIC
Low-and middle-income countries
- VMAT
volumetric-modulated arc therapy
- TBI
Total Body Irradiation
- IROC
Imaging and Radiation Oncology Core
- CSI
Craniospinal Irradiation
- MB
Medulloblastoma
- GTR
Gross total resection
- NTR
Near total resection
- CNS
Central Nervous System
- GCT
Germ Cell tumor
- WVI
Whole ventricular Irradiation
- BED
Biological Effective Dose
- ATRT
Atypical teratoid/rhabdoid tumor
- SBRT
Stereotactic body radiotherapy
- RMS
Rhabdomyosarcoma
- FH
Favorable Histology
- WLI
Whole lung Irradiation
- ISRT
Involved Site Radiotherapy
- IFRT
Involved Field Radiotherapy
- PET
Positron Emission Tomography
- NBL
Neuroblastoma
- ASCT
Autologous Stem Cell Transplant
- MIBG
Meta Iodo Benzyl Guanidine
- PENTEC
Pediatric Normal Tissue Effects in the Clinic
- YI
Young Investigator
Footnotes
Conflicts of Interest: None
References
- 1.Kalapurakal JA, Gopalakrishnan M, Walterhouse DO et al. Cardiac-Sparing Whole Lung IMRT in Patients With Pediatric Tumors and Lung Metastasis: Final Report of a Prospective Multicenter Clinical Trial. Int J Radiat Oncol Biol Phys. 2019. Jan 1;103(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hua CH, Vern-Gross TZ, Hess CB et al. Practice patterns and recommendations for pediatric image-guided radiotherapy: A Children’s Oncology Group report. Pediatr Blood Cancer. 2020. Oct;67(10):e28629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas-Kogan D, Indelicato D, Paganetti H et al. National Cancer Institute Workshop on Proton Therapy for Children: Considerations Regarding Brainstem Injury. Int J Radiat Oncol Biol Phys. 2018. May 1;101(1):152–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rassiah P, Esiashvili N, Olch AJ et al. Practice Patterns of Pediatric Total Body Irradiation Techniques: A Children’s Oncology Group Survey. Int J Radiat Oncol Biol Phys. 2021. Dec 1;111(5):1155–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding L, Bradford C, Kuo IL et al. Radiation Oncology: Future vision for quality assurance and data management in clinical Trials and Translational Science. Front Oncol. 2022; 12:931294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FitzGerald TJ, Followill D, Laurie F et al. Quality assurance in radiation oncology. Pediatr Blood Cancer. 2021; 68 Suppl 2:e28609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michalski JM, Janss AJ, Vezina LG et al. Children’s Oncology Group Phase III Trial of Reduced-Dose and Reduced-Volume Radiotherapy With Chemotherapy for Newly Diagnosed Average-Risk Medulloblastoma. J Clin Oncol. Aug 20 2021;39(24):2685–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leary SES, Packer RJ, Li Y, et al. Efficacy of Carboplatin and Isotretinoin in Children With High-risk Medulloblastoma: A Randomized Clinical Trial From the Children’s Oncology Group. JAMA Oncol. Sep 1 2021;7(9):1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merchant TE, Bendel AE, Sabin ND, et al. Conformal Radiation Therapy for Pediatric Ependymoma, Chemotherapy for Incompletely Resected Ependymoma, and Observation for Completely Resected, Supratentorial Ependymoma. J Clin Oncol. Apr 20 2019;37(12):974–983. doi: 10.1200/jco.18.01765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy ES, Dhall G, Fangusaro J, et al. A Phase 2 Trial of Response-Based Radiation Therapy for Localized Central Nervous System Germ Cell Tumors: Patterns of Failure and Radiation Dosimetry for Nongerminomatous Germ Cell Tumors. Int J Radiat Oncol Biol Phys. May 1 2022;113(1):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman S, Bouffet E, Fisher PG, et al. Phase II Trial Assessing the Ability of Neoadjuvant Chemotherapy With or Without Second-Look Surgery to Eliminate Measurable Disease for Nongerminomatous Germ Cell Tumors: A Children’s Oncology Group Study. J Clin Oncol. Aug 1 2015;33(22):2464–71. doi: 10.1200/jco.2014.59.5132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fangusaro J, Wu S, MacDonald S, et al. Phase II Trial of Response-Based Radiation Therapy for Patients With Localized CNS Nongerminomatous Germ Cell Tumors: A Children’s Oncology Group Study. J Clin Oncol. Dec 1 2019;37(34):3283–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddy AT, Strother DR, Judkins AR, et al. Efficacy of High-Dose Chemotherapy and Three-Dimensional Conformal Radiation for Atypical Teratoid/Rhabdoid Tumor: A Report From the Children’s Oncology Group Trial ACNS0333. J Clin Oncol. Apr 10 2020;38(11):1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolden SL, Lyden ER, Arndt CA et al. Local control for Intermediate-Risk Rhabdomyosarcoma: Results from D9803 according to Histology, Group, Site and Size: A Report from the Children’s Oncology Group. Int J Radiat Oncol Biol Phys, 93(5):1071–1076, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casey DL, Chi YY, Donaldson SS et al. Increased local failure for patients with intermediate-risk rhabdomyosarcoma on ARST0531: A report from the Children’s Oncology Group. Cancer 125(18):3242–3248, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walterhouse DO, Pappo AS, Meza JL et al. Reduction of cyclophosphamide dose for patients with subset 2 low-risk rhabdomyosarcoma is associated with an increased risk of recurrence: A report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group Cancer 123(12):2368–2375, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ermoian RP, Breneman J, Walterhouse DO et al. 45Gy is Not Sufficient Radiotherapy Dose for Group III Orbital Embryonal Rhabdomyosarcoma after less than Complete Response to 12 weeks of ARST0331 Chemotherapy. A Report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. Pediatr Blood Cancer 64(9), 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spunt SL, Million L, Chi YY et al. A risk-based treatment strategy for non-rhabdomyosarcoma soft-tissue sarcomas in patients younger than 30 years (ARST0332): a Children’s Oncology Group prospective study Lancet Oncol 21(1):145–161, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dix DB, Seibel NL, Chi YY, et al. Treatment of Stage IV Favorable histology Wilms tumor with lung metastases: A report from the Children’s Oncology Group AREN0533 study. J Clin Oncol 2018; 36: 1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daw NC, Chi YY, Kalapurakal JA, et al. Activity of vincristine and irinotecan in diffuse anaplastic Wilms tumor and therapy outcomes of Stage II to IV disease: results of the Children’s Oncology Group AREN0321 study. J Clin Oncol 2020; 38:1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gratias EJ, Dome JS, Jennings LJ, et al. Association of 1q gain with inferior survival in favorable-histology Wilms tumor: a report from the Children’s Oncology Group. J Clin Oncol 2016; 34: 3189–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parikh RR, Kelly KM, Hodgson DC et al. Patterns of Initial Relapse from a Phase 3 Study of Response-Based Therapy for High-Risk Hodgkin Lymphoma (AHOD0831): A Report from the Children’s Oncology Group. Int J Radiat Oncol Biol Phys. 2022. Mar 15;112(4):890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castellino SM, Pei Q, Parsons SK et al. Brentuximab Vedotin with Chemotherapy in Pediatric High-Risk Hodgkin’s Lymphoma. N Engl J Med. 2022. Nov 3;387(18):1649–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoppe Bradford S, McCarten Kathleen M Pei Qinglin et al. Importance of Central Imaging Review in a Pediatric Hodgkin Lymphoma Trial Using Positron Emission Tomography Response Adapted Radiation Therapy. Int J Radiat Oncol Biol Phys. 2023. Mar 2;S0360–3016(23)00164–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mailhot Vega RB, Castellino SM, Pei Q et al. Evaluating Disparities in Proton Radiation Therapy Use in AHOD1331, a Contemporary Children’s Oncology Group Trial for Advanced-Stage Hodgkin Lymphoma. Int J Part Ther. 2021. Oct 28;8(3):55–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mailhot Vega RB, Harker-Murray PD et al. Radiotherapy Use in Refractory and Relapsed Adolescent and Young Adult Hodgkin Lymphoma: A Report from the Children’s Oncology Group. Int J Radiat Oncol Biol Phys. 2023. Apri 26:S0360–3016(23)00381–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haas-Kogan DA, Swift PS, Selch M, et al. Impact of radiotherapy for high-risk neuroblastoma: a Children’s Cancer Group study. Int J Radiat Oncol Biol Phys. 2003. May 1;56(1):28–39. [DOI] [PubMed] [Google Scholar]
- 28.Park JR, Kreissman SG, London WB, et al. Effect of Tandem Autologous Stem Cell Transplant vs Single Transplant on Event-Free Survival in Patients With High-Risk Neuroblastoma A Randomized Clinical Trial. JAMA. 2019;322(8):746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu KX, Naranjo A, Zhang FF, et al. Prospective Evaluation of Radiation Dose Escalation in Patients With High-Risk Neuroblastoma and Gross Residual Disease After Surgery: A Report From the Children’s Oncology Group ANBL0532 Study. J Clin Oncol. 2020. Aug 20;38(24):2741–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casey DL, Kushner BH, Cheung NV, et al. Reduced-Dose Radiation Therapy to the Primary Site is Effective for High-Risk Neuroblastoma: Results From a Prospective Trial. Int J Radiat Oncol Biol Phys. 2019. Jun 1;104(2):409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leavey PJ, Laack NN, Krailo MD et al. Phase III Trial Adding Vincristine-Topotecan-Cyclophosphamide to the Initial Treatment of Patients With Nonmetastatic Ewing Sarcoma: A Children’s Oncology Group Report. J Clin Oncol. 2021. Dec 20;39(36):4029–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmed S, Laack NN, Binitie O et al. Factors associated with Local Control and Survival in Localized Ewing Sarcoma Patients Treated on AEWS1031; A report from the Children’s Oncology Group. Annual Meeting of Connective Tissue Oncology Society, November 2022 [Google Scholar]
- 33.DuBois SG, Krailo MD, Glade-Bender J et al. Randomized Phase III Trial of Ganitumab With Interval-Compressed Chemotherapy for Patients With Newly Diagnosed Metastatic Ewing Sarcoma: A Report From the Children’s Oncology Group. J Clin Oncol. 2023. Apr 10;41(11):2098–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. N Engl J Med 2016;374:833–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Constine LS; Olch AJ; Jackson A et al. “Pediatric Normal Tissue Effects in the Clinic (PENTEC): an international collaboration to assess normal tissue radiation dose-volume-response relationships for children with cancer”. Int J Radiat Oncol Biol Phys. 2021. doi. 10.1016/j.ijrobp.2020.10.040 [DOI] [PubMed] [Google Scholar]
- 36.Kalapurakal JA, John C, Breneman JC, Geert O, Janssens GO et al. Introduction to the COG/SIOP/PROS supplement on radiation oncology. Pediatr Blood Cancer 2021; 68 Suppl 2: e28768. [DOI] [PubMed] [Google Scholar]
- 37.Durant ST, Zheng L, Wang Y et al. The brain-penetrant clinical ATM inhibitor AZD390 radiosensitizes and improves survival of preclinical brain tumor models. Sci Adv. 2018; 4(6): eaat1719. doi: 10.1126/sciadv.aat1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chi Y, Fang Z, Hong X et al. Safety and efficacy of Anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft-tissue sarcoma. Clin Cancer Res. 2018. Nov 1;24(21):5233–5238. [DOI] [PubMed] [Google Scholar]
- 39.Anthony E. Mascia AD, Daugherty EC, Yongbin Zhang Y et al. Proton FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases The FAST-01 Nonrandomized Trial. JAMA Oncol. 2023;9(1):62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morton LM, Sampson JN, Armstrong GT et al. Genome-Wide Association Study to Identify Susceptibility Loci That Modify Radiation-Related Risk for Breast Cancer After Childhood Cancer. J Natl Cancer Inst. 2017. Nov; 109(11): djx058. [DOI] [PMC free article] [PubMed] [Google Scholar]