Obesity is one of the most prevalent metabolic diseases worldwide, and it increases the risk of developing type 2 diabetes, heart failure, cancer, and other diseases (1). White adipocytes are specialized cells that store enormous amounts of lipids, predominantly in the form of triglycerides, and they release free fatty acids into the circulation in response to lipolytic stimuli. In addition, adipocytes communicate with other cells by secreting a complex set of hormones, cytokines, and metabolites that maintain white adipose tissue (WAT) homeostasis and regulate the function of distant organs, such as the heart, liver, pancreas, and brain (2). In obesity, shifts in adipocyte-derived signals contribute to the development of local and systemic inflammation, pathologic tissue remodeling in WAT, and adverse metabolic sequelae such as insulin resistance (3,4). Increasing our understanding of how adipocytes communicate with neighboring and distant cells may reveal new therapeutic targets to treat obesity or associated metabolic diseases.
An emerging body of literature indicates that adipocytes release extracellular vesicles (EVs) that contain a wide variety of cargo, including lipids, proteins, nucleic acids, and even organelles such as mitochondria (5–8). These EVs mediate cross talk between cells within WAT but can also exert systemic effects on distant organs. For example, adipocyte-derived extracellular vesicles (AdEVs) can either impair or enhance insulin sensitivity in hepatocytes and muscle cells (9,10), and they can also increase pancreatic β-cell production of insulin (11). However, the mechanisms that regulate the release of EVs by adipocytes are not fully understood.
In this issue of Diabetes, Huang et al. (12) report that lipolysis leads to activation of the DNA repair enzyme p53 to stimulate release of AdEVs. They demonstrate that the lipolysis-inducing compounds forskolin and isoproterenol lead to the release of EVs by 3T3-L1 adipocytes. Interestingly, this process was not EV specific, as inducing lipolysis also stimulated the release of many free proteins, such as fatty acid binding protein 4 (FABP4). Secretion of free proteins and AdEVs is suppressed by the p53 inhibitor pifithrin-α or when expression of p53 is reduced by shRNA-mediated knockdown. Consistent with this result, serum from p53-deficient mice had reduced EV particle counts and decreased levels of FABP4. Gain-of-function studies showed that activation of p53 led to the release of more AdEVs and FABP4 from adipocytes. Nutlin, a compound that indirectly activates p53, also led to the release of more AdEVs. Since p53 is classically involved in DNA damage repair, the authors used doxorubicin to induce DNA damage and found that this treatment led to the release of more AdEVs in a p53-dependent manner. Interestingly, in mice with ERCC1 haploinsufficiency, which causes increased DNA damage that goes unrepaired, p53 expression is upregulated, and this is associated with increased FABP4 in serum in male but not female mice, a sex dependency that warrants further investigation. Overall, these studies indicate that lipolysis activates p53 to induce release of AdEVs in vitro and in vivo (Fig. 1), and the findings are consistent with those from prior studies showing that diet-induced obesity is associated with increased DNA damage, p53 activation, and release of proinflammatory factors that promote WAT inflammation and insulin resistance (13).
Figure 1.
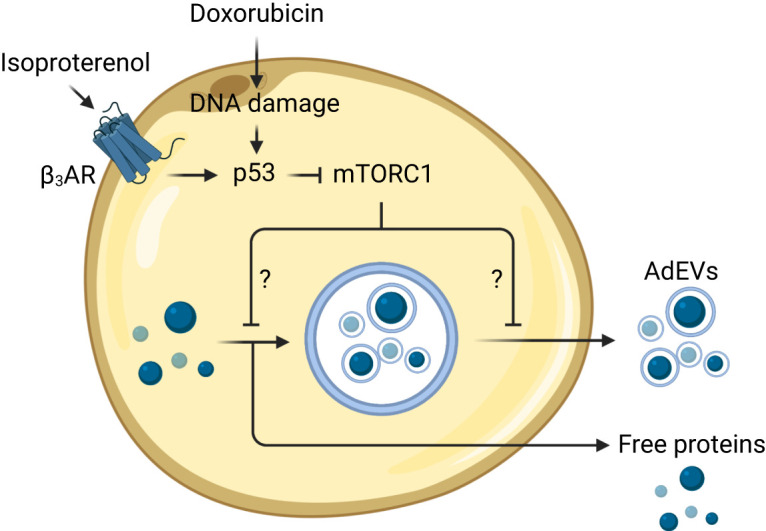
Lipolysis and DNA damage promote the release of EVs by adipocytes in a p53-dependent manner. Lipolytic stimuli activate p53 and promote the release of lipid-rich AdEVs. DNA damage and pharmacologic activation of p53 suppress the activity of mTORC1, which relieves the inhibition on free protein release and the packaging and/or release of AdEVs. The resultant AdEVs contain various types of cargo, including cytosolic, nuclear, and mitochondrial proteins. Conversely, genetic deletion or inhibition of p53 leads to impaired release of AdEVs and other secreted free proteins. The mechanisms by which mTORC1 regulates p53-dependent release of adipocyte-derived proteins and EVs are not yet well defined. β3AR, β-3 adrenergic receptor. Created with BioRender.com.
The mechanisms by which p53 regulates the release of AdEVs are not yet clear. As was shown previously in HeLa cells (14), the authors identified a potential role for mammalian target of rapamycin (mTOR). They showed 1) that p53 activation with nutlin or doxorubicin inhibits mTOR activity and S6 phosphorylation at serines 240/244 and 235/236 and 2) that inhibition of mTOR complex 1 (mTORC1) with torin or rapamycin leads to increased release of AdEVs. While numerous studies have identified how p53 and genotoxic stress regulate the activity of mTORC1 (15), it is not clear how mTORC1 regulates the production or release of AdEVs. It has been shown that mTORC1 physically interacts with Rab27a, a small GTPase that is required for exosome secretion (14); however, Rab27a function is only one part of the highly complex and coordinated process of EV biogenesis. It was demonstrated that the AdEVs released after lipolysis tend to be 130–400 nm in diameter, with predominant EV subsets that are 130, 180, 331, and 380 nm, on average. These subsets contain a diverse set of proteins that lack secretion signals and are typically localized to the cytoplasm and organelles such as the nucleus and mitochondria. However, AdEVs released after p53 activation tend to be smaller. This result suggests the p53-mTORC1 pathway may regulate the release of a subset of smaller AdEVs and that there are likely other pathways that regulate the release of larger AdEVs.
There are many questions that remain unanswered about the functional relevance of AdEVs released in response to lipolytic stimuli. One open area for investigation is whether lipolysis and p53 activation lead to selective packaging of contents into AdEVs. Huang et al. (12) use only a small number of markers, such as TSG101 and CD63, to characterize AdEVs, but they provide proteomic data on conditioned media, indicating that AdEVs can contain numerous proteins, including those localized to mitochondria. Whether p53 is involved in packaging certain types of cargo, such as mitochondria, within AdEVs prior to release is a particularly interesting question in light of recent studies that report that AdEVs containing oxidatively damaged mitochondria are delivered to tissue-resident macrophages for degradation (16) but can also be released into the circulation for delivery to distant organs, such as the heart, to protect against ischemia-reperfusion injury (5,17). While these observations suggest that AdEVs, including those that contain mitochondrial components, can have beneficial effects locally and systemically, in some circumstances AdEVs can contribute to pathology in obesity (18). It remains unknown whether the lipolysis-p53-mTORC1 pathway identified by Huang et al. (12) regulates the release of specific subsets of AdEVs, whether this pathway regulates AdEV production in the endolysosomal system or at the plasma membrane, or whether these AdEVs confer beneficial and/or deleterious effects on WAT or on distant organs. Further research into these topics may reveal previously unknown biological pathways that can be targeted therapeutically to treat metabolic diseases such as obesity.
Article Information
Funding. C.C. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R00-DK122019) and the American Heart Association (23IPA1054013). J.R.B. is supported by the National Institutes of Health Office of the Director (DP5 OD028125) and the Burroughs Wellcome Fund (CAMS #1019648).
Duality of Interest. J.R.B. has pending and issued patents related to mitochondrial transfer and obesity, has been a consultant for DeciBio and Flagship Pioneering within the past 12 months, receives royalties from Springer Nature, and is on the scientific advisory board for LUCA Science, Inc. No other potential conflicts of interest relevant to this article were reported.
Funding Statement
C.C. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R00-DK122019) and the American Heart Association (23IPA1054013). J.R.B. is supported by the National Institutes of Health Office of the Director (DP5 OD028125) and the Burroughs Wellcome Fund (CAMS #1019648).
Footnotes
See accompanying article, p. 1560.
References
- 1. Hales C, Carroll M, Fryar C, Ogden C.. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. National Center for Health Statistics Data Brief no. 360. Hyattsville, MD, National Center for Health Statistics, 2020 [Google Scholar]
- 2. Scheja L, Heeren J.. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat Rev Endocrinol 2019;15:507–524 [DOI] [PubMed] [Google Scholar]
- 3. Brestoff JR, Artis D.. Immune regulation of metabolic homeostasis in health and disease. Cell 2015;161:146–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Auger C, Kajimura S.. Adipose tissue remodeling in pathophysiology. Annu Rev Pathol 2023;18:71–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crewe C, Funcke J-B, Li S, et al. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell Metabolism 2021;33:1853–1868.e1811 [DOI] [PMC free article] [PubMed]
- 6. Flaherty SE 3rd, Grijalva A, Xu X, Ables E, Nomani A, Ferrante AW Jr. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science 2019;363:989–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thomou T, Mori MA, Dreyfuss JM, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017;542:450–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crewe C, Joffin N, Rutkowski JM, et al. An endothelial-to-adipocyte extracellular vesicle axis governed by metabolic state. Cell 2018;175:695–708.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kranendonk ME, Visseren FL, van Herwaarden JA, et al. Effect of extracellular vesicles of human adipose tissue on insulin signaling in liver and muscle cells. Obesity (Silver Spring) 2014;22:2216–2223 [DOI] [PubMed] [Google Scholar]
- 10. Fuchs A, Samovski D, Smith GI, et al. Associations among adipose tissue immunology, inflammation, exosomes and insulin sensitivity in people with obesity and nonalcoholic fatty liver disease. Gastroenterology 2021;161:968–981.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kulaj K, Harger A, Bauer M, et al. Adipocyte-derived extracellular vesicles increase insulin secretion through transport of insulinotropic protein cargo. Nat Commun 2023;14:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang Y, Hertzel AV, Fish SR, et al. TP53/p53 facilitates stress-induced exosome and protein secretion by adipocytes. Diabetes 2023;72:1560–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vergoni B, Cornejo PJ, Gilleron J, et al. DNA damage and the activation of the p53 pathway mediate alterations in metabolic and secretory functions of adipocytes. Diabetes 2016;65:3062–3074 [DOI] [PubMed] [Google Scholar]
- 14. Zou W, Lai M, Zhang Y, et al. Exosome release is regulated by mTORC1. Adv Sci (Weinh) 2018;6:1801313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cui D, Qu R, Liu D, Xiong X, Liang T, Zhao Y.. The cross talk between p53 and mTOR pathways in response to physiological and genotoxic stresses. Front Cell Dev Biol 2021;9:775507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosina M, Ceci V, Turchi R, et al. Ejection of damaged mitochondria and their removal by macrophages ensure efficient thermogenesis in brown adipose tissue. Cell Metab 2022;34:533–548.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borcherding N, Jia W, Giwa R, et al. Dietary lipids inhibit mitochondria transfer to macrophages to divert adipocyte-derived mitochondria into the blood. Cell Metab 2022;34:1499–513.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rome S, Blandin A, Le Lay S.. Adipocyte-derived extracellular vesicles: state of the art. Int J Mol Sci 2021;22:1788. [DOI] [PMC free article] [PubMed] [Google Scholar]


