Abstract
Costimulation serves as a critical checkpoint for T-cell activation, and several genetic variants affecting costimulatory pathways confer risk for autoimmune diseases. A single nucleotide polymorphism (rs763361) in the CD226 gene encoding a costimulatory receptor increases susceptibility to multiple autoimmune diseases, including type 1 diabetes. We previously found that Cd226 knockout protected NOD mice from disease, but the impact of CD226 on individual immune subsets remained unclear. Our prior reports implicate regulatory T cells (Tregs), as human CD226+ Tregs exhibit reduced suppressive function. Hence, we hypothesized that genomic Cd226 gene deletion would increase Treg stability and that Treg-specific Cd226 deletion would inhibit diabetes in NOD mice. Indeed, crossing NOD.Cd226−/− and a NOD Treg-lineage tracing strain resulted in decreased pancreatic Foxp3-deficient “ex-Tregs.” We generated a novel Treg-conditional knockout (TregΔCd226) strain that displayed decreased insulitis and diabetes incidence. CD226-deficient pancreatic Tregs had increased expression of the coinhibitory counter-receptor T-cell immunoreceptor with Ig and immunoreceptor tyrosine-based inhibitory motif domains (TIGIT). Moreover, NOD splenocytes treated with TIGIT-Fc fusion protein exhibited reduced T-cell proliferation and interferon-γ production following anti-CD3/CD28 stimulation. This study demonstrates that a CD226/TIGIT imbalance contributes to Treg instability in NOD mice and highlights the potential for therapeutic targeting this costimulatory pathway to halt autoimmunity.
Article Highlights
We previously found that Cd226 genomic knockout (gKO) in NOD mice reduced insulitis severity and diabetes incidence, but the impact on individual immune subsets remained unclear.
Human CD226+ regulatory T cells (Tregs) exhibit reduced suppressive function, suggesting Cd226 gKO would increase Treg stability, and Treg-specific Cd226 deletion would inhibit diabetes in NOD mice.
Treg-conditional Cd226 KO reduced insulitis and delayed diabetes onset in female NOD mice, while Cd226 gKO NOD mice displayed reduced Foxp3-deficient Tregs in pancreas and increased T-cell immunoreceptor with Ig and immunoreceptor tyrosine-based inhibitory motif domains (TIGIT) expression on Tregs.
CD226/TIGIT imbalance contributes to Treg instability in NOD mice and highlights the potential for therapeutic targeting this costimulatory pathway in type 1 diabetes.
Introduction
Costimulation is essential for the thymic development and peripheral activation of T lymphocytes, and gene variants converging on several costimulatory pathways confer risk for autoimmune diseases, including type 1 diabetes. The rs763361 (C>T) single nucleotide polymorphism within the CD226 gene, for example, increases susceptibility to type 1 diabetes, multiple sclerosis, and rheumatoid arthritis (1,2). CD226, also known as DNAX accessory molecule-1 (DNAM-1), is expressed on natural killer cells (3), CD8+ T cells (4), platelets (5), monocytes (6,7), and activated CD4+ T cells (8). CD226 competes with the coinhibitory receptors TIGIT (T-cell immunoreceptor with Ig and immunoreceptor tyrosine-based inhibitory motif domains) and CD96 to bind CD155 on antigen presenting cells (9). Upon ligation, CD226 initiates phosphorylation of its cytoplasmic tail, leading to downstream phosphatidylinositide 3-kinase/Akt and mitogen-activated protein kinase/Erk signaling, inducing T-cell activation and proliferation (3). The autoimmunity-associated Ser307 variant, encoded by the rs763361 (T) allele, potentially provides an additional cytoplasmic phosphorylation site, augmenting downstream signaling (10). Hence, there is a need to understand how CD226 activity contributes to the pathogenesis of type 1 diabetes and other autoimmune diseases.
CD4+ regulatory T cells (Tregs) play an important role in type 1 diabetes, with most type 1 diabetes immunotherapies seeking to augment Treg function or frequency relative to effector T-cell subsets (11,12). We previously demonstrated that interferon-γ (IFN-γ)–producing Tregs exhibit augmented expression of CD226 (13). However, the role of CD226 signaling in Treg stability and function during type 1 diabetes development remains unclear. To address this question, we reported that expanded human CD226+ Tregs had increased expression of IFN-γ as well as decreased T-cell suppression compared with CD226− Tregs (14). Building on this, we observed increased FOXP3+Helios+ purity and suppressive capacity in CD4+CD25+CD226− versus CD4+CD25+CD127lo/− sorted Tregs, supporting the potential utility of CD226 as a negative selection marker for improving Treg purity prior to expansion and adoptive cell therapy (15). Consistent with these observations, other groups have identified CD226 as a potential source of Treg instability, finding that blocking CD226 on human peripheral blood mononuclear cells diminished graft versus host disease development in a humanized mouse model by increasing CD4+FOXP3+ Treg frequencies and FOXP3 upregulation (16).
The causal role of CD226 signaling in autoimmune diabetes was previously studied by our group through the Cd226 genomic knockout (gKO) in the NOD mouse model of type 1 diabetes (17). Genetic deletion of Cd226 led to reduced diabetes incidence and insulitis severity. While Cd226 gKO appeared to primarily impact the pathogenicity of CD8+ T cells, adoptive transfer of mixed populations of Cd226+/+ and Cd226−/− NOD T cells into immunodeficient NOD.Prkdcscid recipients demonstrated a requirement for CD226 on CD4+ T cells to confer disease (17). In line with these findings, Wang et al. (18) demonstrated that CD226 deletion delayed disease onset in the experimental autoimmune encephalomyelitis (EAE) mouse model of multiple sclerosis. Interestingly, EAE-induced Cd226−/− mice had increased Tregs, and in vitro induced Tregs from Cd226−/− mice had increased proliferation (18). Other studies have found that blocking CD226 reduced T helper 1 (Th1)–mediated autoimmunity (8) and increased interleukin (IL)-10 production (19) in the EAE mouse model. While these observations collectively support the role of CD226 in promoting the loss of T-cell tolerance in two autoimmune mouse models, how CD226 signaling on Tregs specifically might modulate type 1 diabetes development in NOD mice remained unclear.
The current study investigates how CD226 signaling impacts Treg frequency and function in the context of the NOD mouse model. We hypothesized that Treg-selective Cd226 KO would improve Treg stability and subsequently reduce diabetes incidence in these mice. To test this, we generated two novel NOD strains, specifically, a Treg-fate reporter (20) Cd226 gKO and a Treg Cd226 conditional KO (cKO) strain, for detailed interrogation of Treg-specific contributions toward insulitis and autoimmune diabetes.
Research Design and Methods
Mouse Strains
Animals were bred and housed in specific-pathogen-free facilities with ad libitum access to food and water. NOD.Foxp3-green fluorescent protein (GFP)-Cre.R26-loxP-STOP-loxP-yellow fluorescent protein (YFP) (20) and NOD.Cd226−/− (17) strains were crossed to generate NOD.Foxp3-GFP-Cre.R26-loxP-STOP-loxP-YFP.Cd226−/− mice. NOD Cd226 cKO (NOD.Cd226fl) mice were generated at The Jackson Laboratory (Bar Harbor, ME) and backcrossed with NOD mice for one generation before crossing with NOD.Foxp3-GFP-Cre mice (20). Cd226fl/+.Foxp3Cre/+ × Cd226fl/+.Foxp3+/+ breeder pairs were created, and subsequently, Cd226 Treg-specific cKO (i.e., Cd226fl/fl × Cd226fl/fl) and intact (Cd226+/+ × Cd226+/+) breeding schemes were used to obtain TregΔCd226 and wild-type (WT) TregWT mice for experiments. Studies were performed with protocols approved by the University of Florida Institutional Animal Care and Use Committee and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
PCR Confirmation of Cd226 gKO
Cd226 gKO allele genotyping was performed as previously described (17). The Foxp3-enhanced GFP (eGFP)-Cre transgene was genotyped using a SYBR Green PCR Master Mix (Thermo Fisher Scientific) to amplify the GFP allele (forward, GACCCTGAAGTTCATCTGCACC; reverse, CGGGTCTTGTAGTTGCCGTC) and internal control (forward, GGCAAAGGTGGAAATGAAGA; reverse, CTCAGACCACACAGGGAATG). A Roche LightCycler 480 was used to detect GFP and internal control amplicons at melting points of 86°C and 79°C, respectively. The Cd226fl allele was genotyped by amplifying with SYBR Green PCR Master Mix (forward, CTGGCACAGAGGACACACTC; reverse, GCACAGGAAAGAAGTTTCAGC) on a Roche LightCycler 480. Samples were assessed based on melting profiles for Cd226fl (∼200 base pairs) versus WT alleles (∼180 base pairs).
DNA Confirmation of Treg Cd226 cKO
Splenocytes from mice were stained for CD4 and CD8α, then CD8+ T cells, CD4+GFP− conventional T cells (Tconv), and CD4+GFP+ Tregs were sorted on a FACSAria III (BD Biosciences). Sorted cells were processed with a DNeasy kit (QIAGEN), and 2 ng of DNA was amplified with primers complementary to Cd226 gene sequences flanking exon 2 (forward, TTGTTGCACAGAGCTAAGTCTG; reverse, CCACCTGGGTTAAGTTATGCG). Samples were then run on a 1.5% agarose (w/v) gel with a 100-base pair ladder (Thermo Fisher Scientific).
Flow Cytometry
Euglycemic Cd226 WT, gKO, TregΔCd226, and TregWT mice (12 weeks old) were humanely euthanized, and the thymus, spleen, pancreatic draining lymph nodes (PLN), and pancreas were harvested and processed, as previously described (17). Cells were stained with a flow cytometry panel (Supplementary Table 1) (17), collected on a Cytek Aurora spectral flow cytometer, and analyzed with FlowJo 10 software (BD Biosciences). Intracellular IL-10 cytokine staining was performed by stimulating splenocytes with phorbol 12-myristate 13-acetate (PMA; 20 ng/mL), ionomycin (1 μg/mL), and GolgiStop (BD Biosciences) for 3 h prior to staining.
In Vitro Treg Polarization
CD4+ T cells from NOD or NOD.Cd226−/− mouse splenocytes were isolated using an EasySep magnetic bead isolation kit (STEMCELL Technologies), then polarized into Tregs by stimulating with plate-bound α-CD3ε (1 μg/mL) and α-CD28 (1 μg/mL) in the presence of recombinant human IL-2 (300 IU/mL; National Institutes of Health) and mouse transforming growth factor-β (5 ng/mL; BioLegend). Media were replenished on day 3, and cultures were harvested on day 5. Cells were stained for Live/Dead Near-Infrared (L/D NIR), CD4, CD8α, CD226, and TIGIT, then fixed, permeabilized, and stained for Foxp3 and Helios (Supplementary Table 1). Data were collected on a Cytek Aurora flow cytometer.
Insulitis and Dacryoadenitis Scoring
Pancreata and lacrimal glands were fixed overnight in 10% formalin in PBS, washed with PBS, and stored in 70% ethanol. Pancreata were paraffin-embedded, sectioned at 250-μm intervals, stained with hematoxylin and eosin (H&E), and imaged with an Aperio Scancope CS slide scanner. A blinded observer scored three sections of pancreas per mouse (mean number of islets per mouse: 59.7; SD 12.4) for insulitis severity according to previously published criteria (21). Fixed lacrimal glands from male mice were paraffin-embedded, sectioned, stained with H&E, and scored as previously described (22).
Diabetes Incidence Study
Blood glucose measurements from TregΔCd226, TregWT, and NOD.Cd226fl/fl.Foxp3+/+ mice were taken weekly beginning at 7 weeks of age by collecting a small drop of blood (<5 µL) onto an AlphaTRAK 2 glucose monitor via tail vein prick. Animals registering blood glucose >250 mg/dL had a confirmatory screening 24 h later, and two consecutive readings >250 mg/dL indicated the onset of diabetes (17). Mice were monitored until diabetes onset or 30 weeks of age, at which time they were humanely euthanized.
TIGIT-Fc Proliferation Assay and ELISA
Splenocytes from NOD (WT) mice were treated with 5 μg/mL TIGIT-Fc or human IgG isotype control for 30 min at 37°C and washed with PBS. Cells were treated with CellTrace Violet (CTV; Invitrogen) according to the manufacturer’s instructions and subsequently stimulated with plate-bound α-CD3ε (2 μg/mL) and α-CD28 (1 μg/mL) or plate-bound α-CD3ε (2 μg/mL) and plate-bound CD155-Fc (1 μg/mL; BioLegend) at 37°C. Supernatants were collected at day 2, and IL-10 and IFN-γ concentrations were measured with ELISA kits according to the manufacturer’s recommendations (BioLegend). On day 4, cells were collected, stained for L/D NIR, CD4, and CD8α, acquired on a Cytek Aurora Flow Cytometer, and analyzed using FlowJo 10 software.
Phosphoflow Cytometry
Splenocytes from TregΔCd226 and TregWT mice were plated in a 96-well plate at a concentration of 250,000 cells in 250 μL of well volume with plate-bound α-CD3ε (2 μg/mL) and plate-bound CD155-Fc (1 μg/mL; BioLegend) for 15 or 30 min at 37°C. Stimulations were halted with Cytofix Fixation Buffer (BD Biosciences), followed by a 10-min incubation at 37°C and wash with PBS. Cells were stained with L/D NIR for 15 min and quenched with stain buffer. Fixed cells were permeabilized with 90% methanol for 1 h and washed with stain buffer twice. Permeabilized cells were stained for CD4, CD8α, phosphorylated ribosomal protein S6 (pS6; pS235/pS236), and phosphorylated Akt (pAkt; pS473) overnight at 4°C (Supplementary Table 1). Data were collected with a Cytek Aurora flow cytometer and analyzed using FlowJo software.
In Vitro Proliferation Assay
Splenocytes from TregΔCd226 and TregWT mice were stained with CTV according to the manufacturer’s instructions. Cells were incubated in a 24-well plate at a concentration of 500,000 cells/mL on plate-bound α-CD3ε (2 μg/mL) and soluble α-CD28 (1 μg/mL) or plate-bound α-CD3ε (2 μg/mL) and plate-bound CD155-Fc (1 μg/mL) for 96 h at 37°C. Cells were then harvested and stained with L/D NIR, α-CD4, and α-CD8α, as described above, then acquired on a Cytek Aurora flow cytometer. Data were analyzed using FlowJo 10 software.
Statistical Analyses
Statistical analyses were performed with GraphPad Prism 9 software (GraphPad Software). Flow cytometry data were analyzed using multiple t tests with Šidák-Bonferroni multiple comparisons correction, one-way ANOVA with the Bonferroni multiple comparisons test, and two-way ANOVA with Šidák multiple comparisons correction. Insulitis scoring was compared by χ2 test. Diabetes incidence curves were compared by log-rank (Mantel-Cox) test. ELISA data were analyzed by two-way ANOVA. P values <0.05 were considered significant.
Data and Resource Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request. The novel mouse strains generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Results
CD226 Is Upregulated on Foxp3-Deficient Treg and Effector/Memory Treg Subsets
Previous studies have shown that expression of CD226 on murine CD4+ Tconv is upregulated during initial T-cell receptor (TCR) activation (8). We hypothesized that CD226 would be similarly upregulated in activated Tregs. Indeed, our analysis of previously published human RNA sequencing data sets (23) revealed that CD226 transcription was increased in activated Tregs compared with resting Tregs (24). To confirm that this observation extended to mice, we stimulated NOD splenocytes with α-CD3ε and α-CD28 monoclonal antibodies and found that CD226 surface expression was increased on CD4+Foxp3− Tconv and CD4+Foxp3+ Tregs, but not CD8+ T cells compared with the unstimulated condition (Supplementary Fig. 1). Therefore, CD226 expression patterns are generally maintained between human and NOD mouse CD4+ T cells.
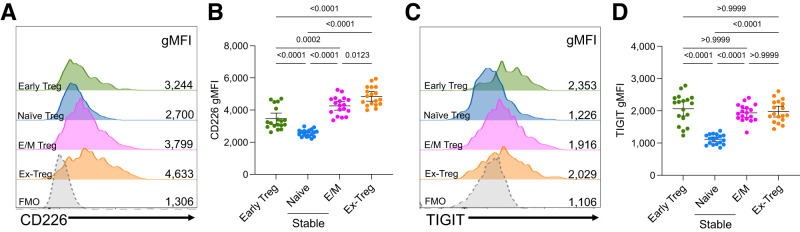
To determine whether this observation extended to activated murine Tregs in vivo, we assessed CD226 levels in pancreatic Tregs of NOD.Foxp3-GFP-Cre.R26-loxP-STOP-loxP-YFP Treg-lineage tracing mice (20). This strain contains a Foxp3 promoter-driven transgene expressing an eGFP-Cre recombinase, facilitating the excision of a stop codon, and allowing the translation of a Rosa26 promoter-driven YFP transcript. This provides the ability to discern GFP+YFP− early Tregs that recently initiated Foxp3 expression, GFP+YFP+ stable Tregs, and GFP−YFP+ Foxp3-deficient (ex-Tregs) that have lost Foxp3 expression. Ex-Tregs were previously found to be enriched in a CD44+CD62L− effector/memory (E/M) phenotype and transferred disease into NOD.Rag2−/− recipients (20); hence, we predicted that ex-Tregs would have upregulated CD226 levels compared with early Tregs and stable Tregs. As expected, CD226 surface expression was increased on ex-Tregs compared with early Tregs (1.4-fold; P < 0.0001), CD44−CD62L+ naive stable Tregs (1.9-fold; P < 0.0001), and E/M stable Tregs (1.1-fold; P = 0.0123), with the lowest expression levels observed on naive stable Tregs (Fig.1A and B and Supplementary Fig. 2). Similarly, surface expression of TIGIT was increased on ex-Tregs compared with naive stable Tregs (1.8-fold; P < 0.0001) but was comparable to early Tregs and E/M stable Tregs (Fig.1C and D). In line with prior studies demonstrating that CD226 disrupts FOXP3 expression (16) while TIGIT restores Treg suppression (25), our data suggest an imbalance of CD226:TIGIT signaling contributes to ex-Treg development within the NOD pancreas.
Figure 1.
CD226 and TIGIT expression levels in Tregs and ex-Tregs. Islet-infiltrating cells from prediabetic 12-week-old female Treg-fate tracking NOD mice were assessed through spectral flow cytometry. Half-offset histograms (A) and quantification (B) of CD226 gMFI on GFP+YFP− early Treg, GFP+YFP+CD44−CD62L+ naive stable Treg, GFP+YFP+CD44+CD62L− E/M stable Treg, and GFP−YFP+ ex-Treg subsets. Fluorescence minus one (FMO) negative-staining controls display CD4+ T cells. Half-offset histograms (C) and quantification (D) of TIGIT gMFI on subsets as defined in B. Lines indicate the mean and 95% CI. P values are displayed and were determined from one-way ANOVA with the Bonferroni multiple comparisons test (n = 18).
CD226-Deficient CD4+ T Cells Produce Fewer Ex-Tregs After In Vitro Treg Polarization
The increased expression of CD226 in pancreatic early and ex-Tregs suggested that CD226 may affect peripheral Treg development and stability. To address this, we polarized bulk CD4+ T cells from spleens of Treg-lineage tracing NOD mice under naive T cell (Th0)- and Treg-skewing conditions to determine the expression pattern of CD226 on in vitro-induced Tregs (Supplementary Fig. 3A) (26). Interestingly, there were substantial numbers of ex-Tregs in addition to early and stable Tregs after 5 days of Treg polarization (Supplementary Fig. 3B). We found that all subsets examined (i.e., CD4+ Tconv, early, stable, and ex-Tregs) had increased CD226 and decreased TIGIT expression in the Treg-polarized compared with the Th0-polarized cultures (Supplementary Fig. 3C and D), suggesting that newly in vitro polarized Tregs have skewed CD226 and TIGIT expression.
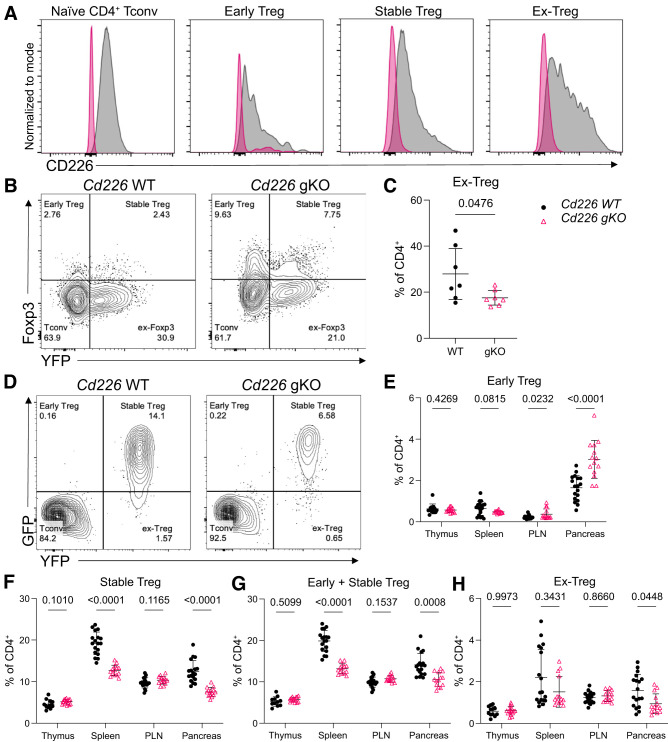
To determine the impact of CD226 on induced Treg stability and ex-Treg generation, we crossed NOD Treg-lineage tracing (20) and NOD.Cd226 gKO strains (17). We first confirmed that CD226 was diminished on naive CD4+ Tconv as well as early, stable, and ex-Treg subsets from NOD.Cd226 gKO Treg-lineage tracing mice (Fig.2A). We then performed Treg polarization on CD4+ T cells from spleens of NOD (WT) and NOD.Cd226−/− (gKO) Treg-lineage tracing mice and found that there were significantly decreased frequencies of ex-Tregs induced from Cd226 gKO versus Cd226 WT CD4+ T cells (Fig.2B and C). Overall, this suggests that CD226 signaling contributes to ex-Treg generation in vitro.
Figure 2.
Decreased ex-Tregs following in vitro Treg polarization and in pancreata of female Cd226 gKO NOD mice. A: Overlaid histograms plotting CD226 expression in naive CD4+ Tconv, early, stable, and ex-Treg subsets in spleens from Cd226 WT and gKO. Representative contour plots (B) and quantification (C) of Foxp3−YFP+ ex-Tregs as the percentage of CD4+ T cells from Cd226 WT and gKO Treg-lineage tracing from NOD mice after 5 days of Treg-polarizing culture conditions (see Supplementary Fig. 3A). The P value is displayed for an unpaired t test (n = 7). Representative contour plots of splenic CD4+ T cells (D) and quantification of early (E), stable (F), total Tregs (G) and ex-Tregs (H) as the percentage of CD4+ T cells in thymus, spleen, PLN, and pancreas from female Cd226 WT (n = 18) and Cd226 gKO (n = 14) Treg fate-reporter NOD mice. Lines indicate the mean, and error bars display the SD. P values are displayed and were determined using multiple t tests with Šidák-Bonferroni multiple comparisons correction.
Pancreata of Cd226 gKO NOD Mice Have Reduced Ex-Tregs
To determine the impact of Cd226 gKO on in vivo Treg stability in the NOD mouse, we assessed T-cell subset frequencies in various tissues from 12-week-old Cd226 WT and gKO Treg-lineage tracing strains (Fig.2D). Interestingly, thymic frequencies of early, stable, total, and ex-Tregs were not significantly altered between Cd226 WT and gKO mice (Fig.2E–H), demonstrating that germ line loss of Cd226 did not impair thymic Treg development. However, we found that early Tregs were increased in the PLN (P = 0.0232) and pancreas (P < 0.0001) (Fig.2E), while stable Tregs were decreased in frequency in the spleen (P < 0.0001) and the pancreas (P < 0.0001) of Cd226 gKO NOD mice (Fig.2F). Total Tregs (i.e., the sum of early and stable Tregs) followed trends similar to stable Tregs alone (Fig.2G), altogether suggesting that CD226 deficiency altered peripheral Treg maintenance and/or recruitment in NOD mice. Interestingly, ex-Treg frequencies were also reduced in the pancreata of female Cd226 gKO mice (P = 0.0448) (Fig.2H). This suggests that CD226 signaling promotes Foxp3 instability in the context of an inflammatory microenvironment, such as the pancreata in NOD mice.
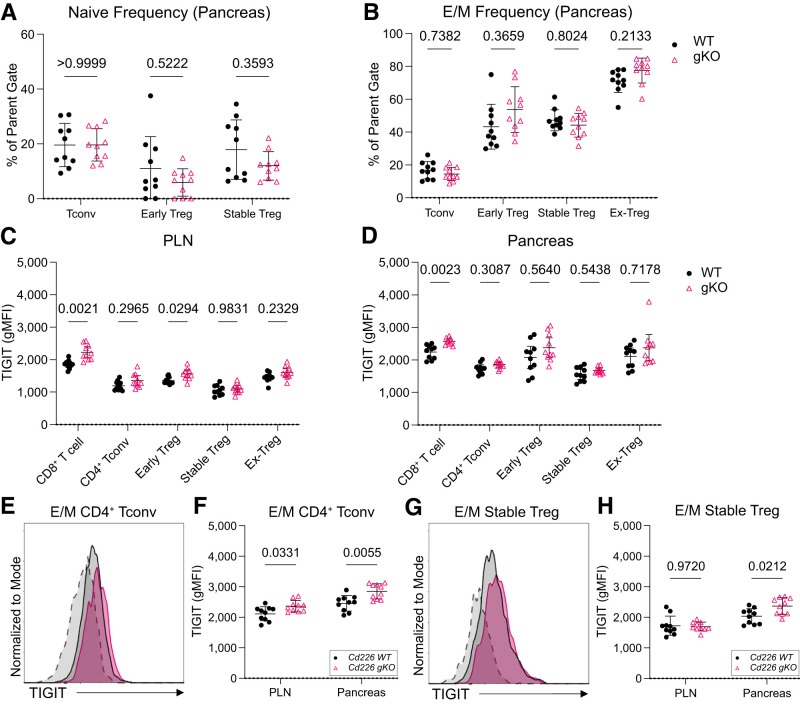
Pancreatic E/M CD4+ Tconv and E/M Stable Tregs in Cd226 gKO Mice Have Higher TIGIT Expression
Next, we quantified naive and E/M CD4+ frequencies to determine whether Cd226 deficiency altered CD4+ activation and differentiation. We found that pancreatic naive and E/M T-cell subset frequencies were maintained between Cd226 WT and gKO mice (Fig.3A and B), suggesting that Cd226 ablation did not impair Tconv or Treg memory differentiation. Based on the competing actions of CD226 and TIGIT (27), we assessed whether TIGIT expression was altered following CD226 deficiency. We found that TIGIT was significantly increased on CD8+ T cells in the PLN (1.2-fold; P = 0.0021) and pancreas (1.1-fold; P = 0.0023) of Cd226 gKO mice (Fig.3C and D). And, while TIGIT was unaltered in bulk CD4+ Tconv, stable Tregs, and ex-Tregs (Fig.3C and D), there was increased TIGIT expression on pancreatic E/M CD4+ Tconv (1.2-fold; P = 0.0055) and E/M-stable Tregs (1.2-fold; P = 0.0212) from Cd226 gKO versus WT mice (Fig.3E–H). This suggests that loss of CD226 coupled with increased TIGIT may contribute to the diabetes protection previously observed in the NOD.Cd226−/− strain (17).
Figure 3.
Pancreatic E/M CD4+ Tconv and stable Tregs have increased TIGIT expression in Cd226 gKO mice. Frequency of naive (CD44−CD62L+) (A) and E/M (CD44+CD62L−) (B) Tconv and Treg subsets from the pancreata of 12-week-old female Cd226 WT and gKO Treg fate-tracking NOD mice. Quantification of TIGIT gMFI in CD8+ T cells, GFP−YFP− CD4+ Tconv, GFP+YFP− early Treg, GFP+YFP+ stable Treg, GFP−YFP+ ex-Treg in the PLN (C) and pancreas (D) of 12-week-old female mice. Overlaid histograms showing TIGIT expression on pancreatic E/M CD4+ Tconv cells (E) and quantification of TIGIT expression on E/M CD4+ Tconv (F) in the PLN and pancreas of 12-week-old female Cd226 WT and gKO Treg fate-tracking NOD mice. FMO negative staining controls are displayed in the dashed histogram. G and H: Same as E and F but for pancreatic E/M stable Tregs. Lines indicate the mean, and error bars display the SD. P values are shown for multiple t tests with the Šidák-Bonferroni multiple test correction (n = 10).
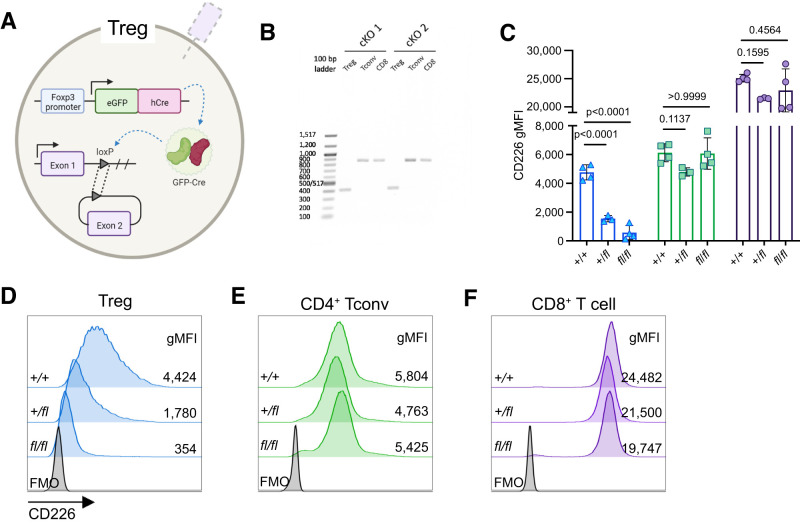
Generation of Cd226fl/fl Treg-Specific cKO NOD Mice
While the Cd226 ablation resulted in increased early Tregs along with reduced stable Tregs and ex-Tregs within the pancreas, this phenotype could also be due to Treg-extrinsic factors related to the diabetes protection observed in NOD.Cd226−/− mice (17). To investigate the intrinsic role of CD226 on Tregs and how it contributes to diabetes pathogenesis, we generated a NOD.Foxp3-GFP-Cre.Cd226fl cKO strain to selectively reduce CD226 expression in Foxp3-expressing Tregs (Fig.4A). Gel electrophoresis of the Cd226 gene in sorted CD4+GFP+ Treg, CD4+GFP− Tconv, and CD8+ T cells from Cd226fl/fl.Foxp3-GFP-Cre+/− (TregΔCd226) splenocytes showed a truncated Cd226 amplicon (381 base pairs) from Tregs compared with CD4+ Tconv and CD8+ T cells (868 base pairs), confirming successful cKO (Fig.4B). CD226 expression was reduced on Tregs in a Cd226fl allele-dose dependent manner but was maintained in CD4+ Tconv and CD8+ T cells (Fig.4C–F). Overall, these data validate the selectiveness of the TregΔCd226 NOD strain, leading us to study the impacts of this genetic modification on diabetes pathogenesis.
Figure 4.
CD226 gMFI is selectively reduced in Treg cKO NOD mice. A: Schematic of Cd226 exon 2 excision in Tregs of TregΔCd226 mice. B: CD4+GFP+ Tregs, CD4+GFP− Tconv, and CD8+ T cells were sorted from splenocytes from two TregΔCd226 (cKO) mice on a BD FACSAria III. Genomic DNA was isolated from sorted T-cell populations, and PCR was performed using primers amplifying the region flanking the flox insertions surrounding exon 2 of the Cd226 gene. PCR amplicons were run on a 1.5% agarose gel with a 100-base pair DNA ladder. Scatter plot showing CD226 gMFI (C) and representative histograms of splenic CD4+GFP+ Tregs (blue triangles) (D), CD4+GFP− Tconv (green squares) (E), and CD8+ T cells (purple circles) (F) of 12-week-old prediabetic female Cd226+/+ (n = 4), +/fl (n = 3), and fl/fl (n = 3) NOD mice vs. fluorescence minus one (FMO) control. Lines represent the mean, and error bars display the SD. P values are shown for analysis by one-way ANOVA with the Bonferroni multiple comparisons test.
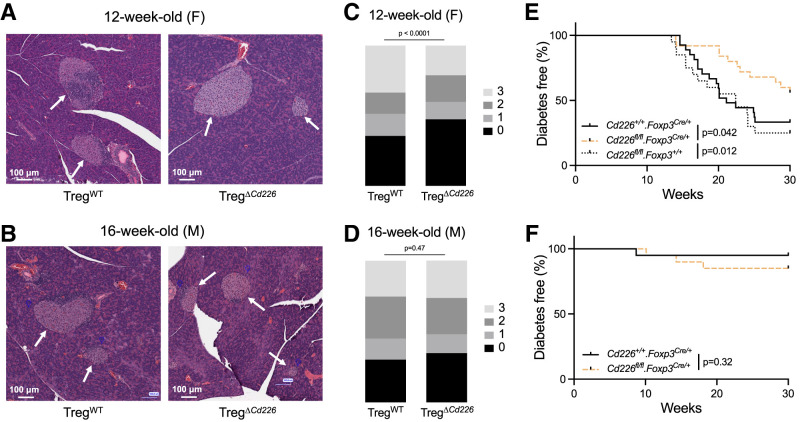
Treg Cd226 cKO Reduced Insulitis and Diabetes Incidence in Female NOD Mice
To determine the effect of the Treg-selective Cd226 deletion on diabetes pathogenesis in NOD mice, we performed insulitis scoring (21) on H&E-stained pancreas sections from 12-week-old female and 16-week-old male TregΔCd226 and TregWT mice (Fig.5A and B). These ages were selected based on when mice in our colony were closest to potential disease onset (17). Insulitis scores were reduced in 12-week-old female TregΔCd226 compared with TregWT mice (P < 0.0001), while insulitis severity was comparable in 16-week-old male TregΔCd226 and TregWT NOD mice (P = 0.47) (Fig.5C and D). We next monitored TregΔCd226, TregWT, and Cd226fl/fl.Foxp3+/+ mice to assess diabetes incidence. As expected, diabetes incidence was not significantly different between female TregWT and Cd226fl/fl.Foxp3+/+ control groups (P = 0.5985). In line with our insulitis findings, female TregΔCd226 mice had reduced diabetes incidence (44.0% [n = 11 of 25]) compared with TregWT (66.7% [n = 18 of 27], P = 0.042) and Cd226fl/fl.Foxp3+/+ controls (75.0% [n = 15 of 20], P = 0.012) (Fig.5E), whereas diabetes incidence in male mice was unchanged between TregΔCd226 (15.0% [n = 3 of 20]) and TregWT genotypes (5.0% [n = 1 of 20], P = 0.3367) (Fig.5F). Dacryoadenitis was previously found to be decreased in male NOD Cd226 gKO mice (17). However, there was no significant difference in dacryoadenitis scores in TregΔCd226 mice compared with TregWT mice (P = 0.1613) (Supplementary Fig. 4A and B). Thus, efforts to further characterize the TregΔCd226 NOD strain were focused on female mice.
Figure 5.
Treg Cd226 cKO reduces insulitis and diabetes incidence in female NOD mice. A and B: Representative H&E-stained pancreas sections with white arrows denoting islets of Langerhans. C and D: Stacked bar graphs depicting insulitis scores from 12-week-old female (F) and 16-week-old male (M) Cd226+/+.Foxp3Cre/+ (female, n = 8 mice and 512 islets; male, n = 5 mice and 252 islets) and Cd226fl/fl.Foxp3Cre/+ (female, n = 8 mice and 443 islets; male, n = 3 mice and 262 islets) NOD mice. P values shown were determined by χ2 test. Diabetes incidence curves from female (Cd226+/+.Foxp3Cre/+, n = 27; Cd226fl/fl.Foxp3Cre/+, n = 20; Cd226fl/fl.Foxp3+/+, n = 20) (E) and male (Cd226+/+.Foxp3Cre/+, n = 20; Cd226fl/fl.Foxp3Cre/+, n = 20) NOD strains (F). P values shown for log-rank (Mantel-Cox) test.
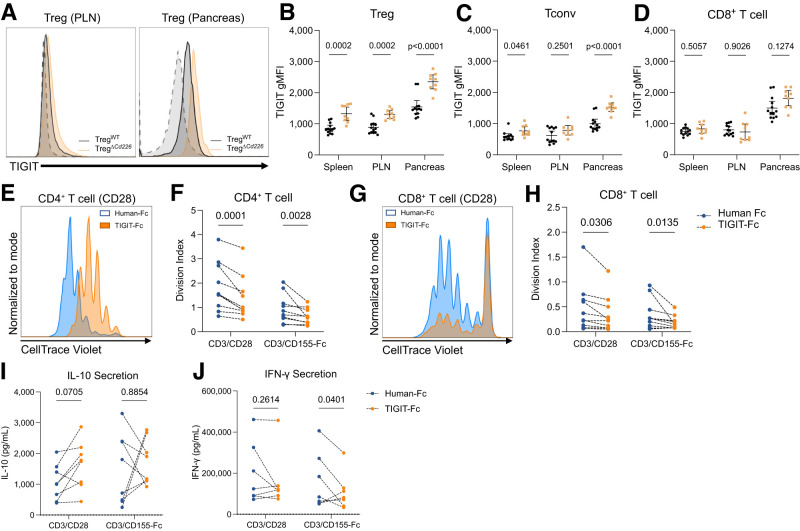
TIGIT Surface Expression Is Increased on Tregs and Tconv in TregΔCd226 Mice
To determine the basis of the diabetes protection in female TregΔCd226 NOD mice, we assessed whether Treg-specific Cd226 cKO altered IL-10 cytokine expression. We found that there was no change in the frequency of IL-10+ cells within Tregs, Tconv, and CD8+ T cells in splenocytes stimulated with PMA/ionomycin (Supplementary Fig. 4C–E). We then used flow cytometry to phenotype T cells from spleen, PLN, and pancreas (Supplementary Fig. 5A). We observed that bulk CD8+ and CD4+, Tconv, and Treg frequencies were unchanged in these tissues from TregΔCd226 versus TregWT mice (Supplementary Fig. 5B–E). We also found that there were no significant differences of naive and E/M T-cell frequencies within CD8+ T cells, CD4+ Tconv, and Tregs (Supplementary Fig. 5F–K). Furthermore, TregΔCd226 and TregWT mice showed no significant differences in thymic or peripheral Treg frequencies in the thymus, spleen, PLN, and pancreas (Supplementary Fig. 6) (28–31). Overall, these data suggest that Treg Cd226 cKO did not alter T-cell frequencies or memory Treg formation.
Due to the opposing roles of CD226 and TIGIT (9) and the increased TIGIT observed on pancreatic E/M CD4+ T cells in Cd226 gKO mice (Fig. 3), we examined TIGIT geometric mean fluorescence intensity (gMFI) in female TregΔCd226 mice. Interestingly, Treg TIGIT expression was increased in the spleen (P = 0.0002), PLN (P = 0.0002), and pancreas (P < 0.0001) of TregΔCd226 mice (Fig.6A and B). There was also increased TIGIT expression on CD4+ Tconv in the spleen (P = 0.0461) and pancreas (P < 0.0001), but not PLN (P = 0.2501), of TregΔCd226 mice (Fig.6C). Furthermore, there was no change in TIGIT expression on CD8+ T cells in the tissues examined (Fig.6D). This suggests that increased TIGIT signaling in Tregs and Tconv, secondary to the Treg-specific loss of CD226, contributes to the delayed disease progression in female TregΔCd226 NOD mice.
Figure 6.
Tregs and Tconv from TregΔCd226 mice show increased TIGIT expression compared with TregWT mice. A: Overlaid histograms of TIGIT surface expression on PLN and pancreatic Tregs from TregWT and TregΔCd226 mice with unstained controls (dashed line). Quantification of TIGIT gMFI on CD4+GFP+ Tregs (B), CD4+GFP− Tconv (C), and CD8+ T cells (D) from 12-week-old female TregWT (black circles, n = 13) and TregΔCd226 (gold squares, n = 9) mice. Lines indicate the mean, and error bars indicate the SD. P values shown for multiple t tests with Šidák-Bonferroni multiple comparisons correction. Representative histograms showing CTV dye dilution and division indices of CD4+ (E and F) and CD8+ (G and H) T cells in NOD splenocytes (n = 9) treated with Fc isotype control (blue) or mouse TIGIT-Fc (orange) and stimulated with plate-bound α-CD3ε/α-CD28 or α-CD3ε/CD155-Fc. Concentrations of IL-10 (I) and IFN-γ (J) in culture supernatants (n = 8) after 2 days of stimulation as described for E–H. P values are shown for two-way ANOVA with the Bonferroni correction.
Previous studies have shown that TIGIT binding to CD155 on dendritic cells (DCs) can promote a tolerogenic phenotype (32). Therefore, we investigated whether recombinant TIGIT-Fc could block CD155-CD226 signaling to recapitulate the immunomodulation observed in CD226-deficient NOD mice. We treated WT NOD splenocytes with Fc control or TIGIT-Fc and stimulated with α-CD3ε/α-CD28 or α-CD3ε/CD155-Fc to engage CD226 and TIGIT. We found that TIGIT-Fc reduced CD4+ and CD8+ T-cell proliferation in response to both stimulation conditions (Fig.6E–H). Supernatant cytokine measurements revealed that while IL-10 concentrations were similar between treatment conditions, TIGIT-Fc resulted in decreased IFN-γ after α-CD3ε/CD155-Fc stimulation compared with Fc control-treated cultures (P = 0.0401) (Fig.6I and J). Overall, these experiments suggest that TIGIT-Fc could promote immune tolerance in NOD mice by reducing T-cell proliferation and IFN-γ secretion.
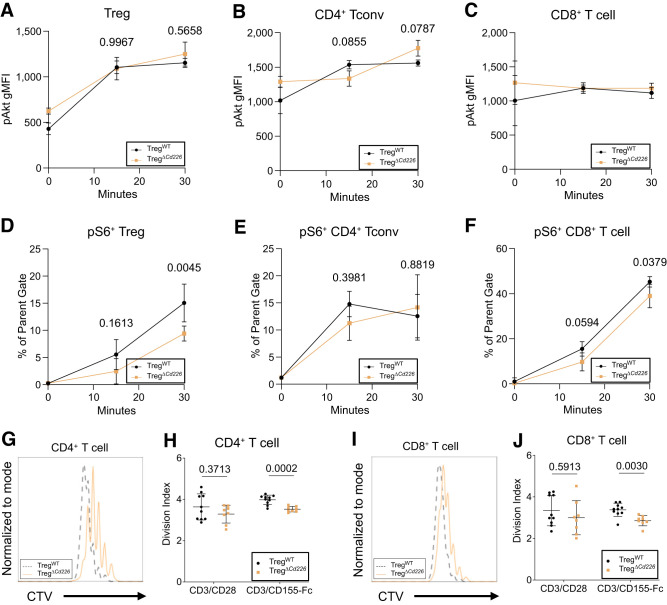
Reduced Proliferation of TregΔCd226 Effector T Cells Following In Vitro Stimulation
Next, we investigated whether increased TIGIT on CD4+ T cells in TregΔCd226 mice (Fig.6A–C) altered their response to CD155 ligand. We performed short time-course stimulation studies with plate-bound α-CD3ε/CD155-Fc to determine the magnitude of TCR and costimulatory signaling via measurement of pAkt (pS473) and pS6 (pS235/pS236) (16). While there were no significant differences in pAkt in Treg, Tconv, or CD8+ T cells from TregΔCd226 and TregWT splenocytes (Fig.7A–C), there were decreased frequencies of pS6+ Tregs (P = 0.0045) and CD8+ T cells (P = 0.0379) from TregΔCd226 mice 30 min after stimulation (Fig.7D–F). Additionally, we observed reduced proliferation of CD8+ (P = 0.0001) and CD4+ T cells (P = 0.0001) from TregΔCd226 splenocytes after α-CD3ε/CD155-Fc stimulation (Fig.7G–J). Altogether, these findings suggest that reduced CD4+ and CD8+ T-cell proliferation in the context of TIGIT-CD155 costimulation, with the latter being secondary to reduced S6 phosphorylation downstream of TCR signaling, could be a key mechanism by which insulitis and diabetes incidence are inhibited in TregΔCd226 NOD mice.
Figure 7.
TregΔCd226 splenocytes have reduced proliferation in response to α-CD3ε/CD155-Fc stimulus. pAkt gMFI in CD4+GFP+ Treg (A), CD4+GFP− Tconv (B), and CD8+ (C) T cells in TregWT or TregΔCd226 splenocytes after 15 and 30 min of plate-bound α-CD3ε/CD155-Fc stimulation. Quantification of pS6+ in CD4+GFP+ Treg (D), CD4+GFP− T cells (E), and CD8+ T cells (F) from TregWT and TregΔCd226 splenocytes (n = 4) after 15 and 30 min of stimulation with plate-bound α-CD3ε/CD155-Fc. P values are shown for two-way ANOVA with Šidák multiple comparisons correction. Overlaid histograms showing CTV dye dilution and division indices of CD4+ (G and H) and CD8+ (I and J) T cells from TregWT (n = 10) and TregΔCd226 (n = 7) splenocytes after 4 days of stimulation with plate-bound α-CD3ε/CD155-Fc. Lines indicate the mean, and error bars indicate the SD. P values called with t tests.
Discussion
In this study, we determined that CD226 signaling in Tregs contributes to diabetes pathogenesis in female NOD mice. In agreement with previous reports (23,24), we found that E/M stable Tregs highly expressed CD226. Additionally, early Tregs and ex-Tregs had significantly higher CD226 expression compared with naive Tregs, which has not been previously reported. Thymic Treg development was not impaired in NOD.Cd226−/− mice, contrasting with the central and peripheral Treg defects observed in NOD.Cd28−/− mice (33–35). Interestingly, Cd226 ablation led to decreased pancreatic ex-Treg frequency, highlighting that the excess activation from CD226 signaling contributes to Treg destabilization in an inflammatory microenvironment (14,36). In line with this notion, others have shown that CD226-deficient murine Tregs have greater TIGIT signaling and, in turn, improved maintenance of Foxp3 expression (16). The diabetes protection (17) and reduced pancreatic ex-Tregs in female Cd226 gKO mice prompted us to interrogate the role of CD226 specifically on Treg function in NOD disease, apart from the risk conferred through CD226 activity within other immune cell subsets.
Our study of the TregΔCd226 strain revealed that our KO was Treg selective and delayed disease onset in female NOD mice. Interestingly, pancreatic Tregs and Tconv from TregΔCd226 mice had increased TIGIT expression compared with TregWT. A similar phenotype was observed in the Cd226−/− EAE mouse model in which central nervous system Tregs had increased CTLA-4 and TIGIT expression compared with Cd226+/+ EAE mice (37). We observed decreased pS6+ in Treg and CD8+ T cells and reduced T-cell proliferation in TregΔCd226 versus TregWT splenocytes stimulated with α-CD3ε/CD155-Fc. However, discerning the contributions of CD226-deficiency and increased TIGIT in response to CD155 stimuli is difficult. We surmise that the inhibition of effector T-cell proliferation in TregΔCd226 mice may be due to enhanced Treg suppression mediated through increased TIGIT-CD155 interactions, which others have shown to increase IL-10 production by CD4+ T cells and Tregs (38,39) as well as DCs (32). We would speculate that CD226-deficient Tregs may prevent diabetes pathogenesis by reducing effector T-cell activation and proliferation, mediated by increased TIGIT-CD155 signaling.
The beneficial role of increased TIGIT in the TregΔCd226 strain is supported by the suppressive effects of TIGIT-Fc on NOD splenocytes. TIGIT-Fc has been shown to promote the generation of immunoregulatory DCs in multiple autoimmune mouse models (32,40,41) by inducing Erk phosphorylation and Il10 transcription in DCs (40). While we did not observe significantly increased IL-10 production from TIGIT-Fc–treated NOD splenocytes, this may be due to our use of anti-CD3 rather than an antigen-presenting cell–directed stimuli such as lipopolysaccharide (32,40). We did observe decreased IFN-γ after TIGIT-Fc treatment, which was reported by other groups and attributed to improved inhibition of CD8+ T cells by DCs derived from TIGIT-Fc–treated bone marrow (40). The immune modulatory effects of TIGIT-Fc warrant further investigation as a therapeutic agent in NOD mice.
The delayed diabetes incidence in genomic and Treg-conditional Cd226 KO NOD mice demonstrates the utility of the NOD model in studying interventions modulating CD226/TIGIT signaling and how they affect type 1 diabetes pathogenesis. Future investigation may include crossing NOD Treg-lineage tracing and Cd226 cKO strains to determine how intrinsic CD226 activity in Tregs affects their development into ex-Tregs. Additionally, the NOD.Cd226fl/fl mice could readily be crossed with additional strains, such as NOD.Gzmb-Cre and NOD.Cd8-Cre, to study effects of natural killer cells and CD8+ T cell-specific Cd226 cKO. The mechanistic studies reported herein support strategies to block CD226 signaling, particularly on Tregs, to interrupt type 1 diabetes progression. Future studies are needed to evaluate therapeutics modulating this costimulatory pathway for the ability to delay or prevent autoimmune diabetes in the NOD preclinical model in support of future translation to clinical trials.
This article contains supplementary material online at https://doi.org/10.2337/figshare.24019158.
Article Information
Acknowledgments. The authors would like to acknowledge Thinzar Myint (University of Florida) for project coordination and support and also thank Kayla Nguyen and Jin-Ju Lee (University of Florida) for technical assistance with blood glucose monitoring. Treg-fate tracking NOD mice were a kind gift from Dr. Jeffrey Bluestone (University of California, San Francisco).
Funding. Project funding was provided by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK106191 to T.M.B. and F30 DK128945 to P.T.), The Leona M. and Harry B. Helmsley Charitable Trust (2004-03813 to T.M.B.), JDRF (3-PDF-2022-1137-A-N to M.R.S.), and Diabetes Research Connection (Project #45 to M.R.S.).
The funders played no role in the design and conduct of the study, including the collection, analysis, or interpretation of the data, nor the manuscript’s preparation, review, or approval.
Duality of Interest. T.M.B. discloses a provisional patent (UF#-14989) pertaining to the use of CD226 as a method for Treg isolation. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. P.T. contributed to data curation, formal analysis, funding acquisition, investigation, methodology, project administration, validation, and visualization, and wrote, reviewed, and edited the manuscript. M.E.B. contributed to investigation, methodology, formal analysis, and visualization, and reviewed and edited the manuscript. L.K.S., J.M.A., W.-I.Y., and Y.-G.C. contributed to the investigation and reviewed and edited the manuscript. A.L.P. reviewed and edited the manuscript. M.R.S. contributed to funding acquisition, investigation, and methodology, and reviewed and edited the manuscript. T.M.B. contributed to study conceptualization, funding acquisition, methodology, resources, and supervision, and reviewed and edited the manuscript. T.M.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented as a poster at the Federation of Clinical Immunology Societies (FOCIS) Annual Meeting, San Francisco, CA, 21–24 June 2022.
Funding Statement
Project funding was provided by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK106191 to T.M.B. and F30 DK128945 to P.T.), The Leona M. and Harry B. Helmsley Charitable Trust (2004-03813 to T.M.B.), JDRF (3-PDF-2022-1137-A-N to M.R.S.), and Diabetes Research Connection (Project #45 to M.R.S.).
Footnotes
P.T. and M.E.B. are co-first authors.
References
- 1. Hafler JP, Maier LM, Cooper JD, et al. ; International Multiple Sclerosis Genetics Consortium (IMSGC) . CD226 Gly307Ser association with multiple autoimmune diseases. Genes Immun 2009;10:5–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qiu Z-X, Zhang K, Qiu X-S, Zhou M, Li W-M.. CD226 Gly307Ser association with multiple autoimmune diseases: a meta-analysis. Hum Immunol 2013;74:249–255 [DOI] [PubMed] [Google Scholar]
- 3. Zhang Z, Wu N, Lu Y, Davidson D, Colonna M, Veillette A.. DNAM-1 controls NK cell activation via an ITT-like motif. J Exp Med 2015;212:2165–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gilfillan S, Chan CJ, Cella M, et al. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J Exp Med 2008;205:2965–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kojima H, Kanada H, Shimizu S, et al. CD226 mediates platelet and megakaryocytic cell adhesion to vascular endothelial cells. J Biol Chem 2003;278:36748–36753 [DOI] [PubMed] [Google Scholar]
- 6. Reymond N, Imbert A-M, Devilard E, et al. DNAM-1 and PVR regulate monocyte migration through endothelial junctions. J Exp Med 2004;199:1331–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vo AV, Takenaka E, Shibuya A, Shibuya K.. Expression of DNAM-1 (CD226) on inflammatory monocytes. Mol Immunol 2016;69:70–76 [DOI] [PubMed] [Google Scholar]
- 8. Dardalhon V, Schubart AS, Reddy J, et al. CD226 is specifically expressed on the surface of Th1 cells and regulates their expansion and effector functions. J Immunol 2005;175:1558–1565 [DOI] [PubMed] [Google Scholar]
- 9. Fourcade J, Sun Z, Chauvin J-M, et al. CD226 opposes TIGIT to disrupt Tregs in melanoma. JCI Insight 2018;3:121157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaud G, Roncagalli R, Chaoui K, et al. The costimulatory molecule CD226 signals through VAV1 to amplify TCR signals and promote IL-17 production by CD4+ T cells. Sci Signal 2018;11:eaar3083. [DOI] [PubMed] [Google Scholar]
- 11. Jacobsen LM, Newby BN, Perry DJ, Posgai AL, Haller MJ, Brusko TM.. Immune mechanisms and pathways targeted in type 1 diabetes. Curr Diab Rep 2018;18:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Atkinson MA, Roep BO, Posgai A, Wheeler DCS, Peakman M.. The challenge of modulating β-cell autoimmunity in type 1 diabetes. Lancet Diabetes Endocrinol 2019;7:52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McClymont SA, Putnam AL, Lee MR, et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol 2011;186:3918–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fuhrman CA, Yeh W-I, Seay HR, et al. Divergent phenotypes of human regulatory T cells expressing the receptors TIGIT and CD226. J Immunol 2015;195:145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown ME, Peters LD, Hanbali SR, et al. Human CD4+CD25+CD226- Tregs demonstrate increased purity, lineage stability, and suppressive capacity versus CD4+CD25+CD127lo/- Tregs for adoptive cell therapy. Front Immunol 2022;13:873560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sato K, Yamashita-Kanemaru Y, Abe F, et al. DNAM-1 regulates Foxp3 expression in regulatory T cells by interfering with TIGIT under inflammatory conditions. Proc Natl Acad Sci USA 2021;118:e2021309118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shapiro MR, Yeh W-I, Longfield JR, et al. CD226 deletion reduces type 1 diabetes in the NOD mouse by impairing thymocyte development and peripheral T cell activation. Front Immunol 2020;11:2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang N, Yi H, Fang L, et al. CD226 attenuates Treg proliferation via Akt and Erk signaling in an EAE model. Front Immunol 2020;11:1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang R, Zeng H, Zhang Y, et al. CD226 ligation protects against EAE by promoting IL-10 expression via regulation of CD4+ T cell differentiation. Oncotarget 2016;7:19251–19264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou X, Bailey-Bucktrout SL, Jeker LT, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol 2009;10:1000–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xue S, Posgai A, Wasserfall C, et al. Combination therapy reverses hyperglycemia in NOD mice with established type 1 diabetes. Diabetes 2015;64:3873–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barr JY, Wang X, Meyerholz DK, Lieberman SM.. CD8 T cells contribute to lacrimal gland pathology in the nonobese diabetic mouse model of Sjögren syndrome. Immunol Cell Biol 2017;95:684–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Monaco G, Lee B, Xu W, et al. RNA-seq signatures normalized by mRNA abundance allow absolute deconvolution of human immune cell types. Cell Rep 2019;26:1627–1640.e1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shapiro MR, Thirawatananond P, Peters L, et al. De-coding genetic risk variants in type 1 diabetes. Immunol Cell Biol 2021;99:496–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lucca LE, Axisa P-P, Singer ER, Nolan NM, Dominguez-Villar M, Hafler DA. TIGIT signaling restores suppressor function of Th1 Tregs. JCI Insight 2019;4:e124427 [DOI] [PMC free article] [PubMed]
- 26. Flaherty S, Reynolds JM.. Mouse naive CD4+ T cell isolation and in vitro differentiation into T cell subsets. J Vis Exp 2015. (98):52739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA.. The TIGIT/CD226 axis regulates human T cell function. J Immunol 2012;188:3869–3875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thornton AM, Korty PE, Tran DQ, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol 2010;184:3433–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yadav M, Louvet C, Davini D, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med 2012;209:1713–1722, S1–S19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weiss JM, Bilate AM, Gobert M, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med 2012;209:1723–1742, S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singh K, Hjort M, Thorvaldson L, Sandler S.. Concomitant analysis of Helios and Neuropilin-1 as a marker to detect thymic derived regulatory T cells in naïve mice. Sci Rep 2015;5:7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu X, Harden K, Gonzalez LC, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol 2009;10:48–57 [DOI] [PubMed] [Google Scholar]
- 33. Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 2000;12:431–440 [DOI] [PubMed] [Google Scholar]
- 34. Tang Q, Henriksen KJ, Boden EK, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol 2003;171:3348–3352 [DOI] [PubMed] [Google Scholar]
- 35. Tai X, Cowan M, Feigenbaum L, Singer A.. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol 2005;6:152–162 [DOI] [PubMed] [Google Scholar]
- 36. Dean JW, Peters LD, Fuhrman CA, et al. Innate inflammation drives NK cell activation to impair Treg activity. J Autoimmun 2020;108:102417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang N, Liang S, Jin J, et al. CD226 attenuates Treg suppressive capacity via CTLA-4 and TIGIT during EAE. Immunol Res 2019;67:486–496 [DOI] [PubMed] [Google Scholar]
- 38. Joller N, Hafler JP, Brynedal B, et al. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol 2011;186:1338–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Joller N, Lozano E, Burkett PR, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity 2014;40:569–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang D, Hu W, Xie J, et al. TIGIT-Fc alleviates acute graft-versus-host disease by suppressing CTL activation via promoting the generation of immunoregulatory dendritic cells. Biochim Biophys Acta Mol Basis Dis 2018;1864:3085–3098 [DOI] [PubMed] [Google Scholar]
- 41. Fu W, Cai R, Ma Z, et al. TIGIT-Fc as a Potential Therapeutic Agent for Fetomaternal Tolerance. Front Immunol 2021;12:649135. [DOI] [PMC free article] [PubMed] [Google Scholar]