Abstract
Background
Surgical site infection (SSI) has a significant impact on patients’ morbidity and aesthetic results.
Objective
To identify risk factors for SSI in dermatologic surgery.
Patients and Methods
This prospective, single‐centre, observational study was performed between August 2020 and May 2021. Patients that presented for dermatologic surgery were included and monitored for the occurrence of SSI. For statistical analysis, we used a mixed effects logistic regression model.
Results
Overall, 767 patients with 1272 surgical wounds were included in the analysis. The incidence of SSI was 6.1%. Significant risk factors for wound infection were defect size over 10cm2 (OR 3.64, 95% confidence interval [CI] 1.80–7.35), surgery of cutaneous malignancy (OR 2.96, CI 1.41–6.24), postoperative bleeding (OR 4.63, CI 1.58–13.53), delayed defect closure by local skin flap (OR 2.67, CI 1.13–6.34) and localisation of surgery to the ear (OR 7.75, CI 2.07–28.99). Wound localisation in the lower extremities showed a trend towards significance (OR 3.16, CI 0.90–11.09). Patient‐related factors, such as gender, age, diabetes, or immunosuppression, did not show a statistically significant association with postoperative infection.
Conclusion
Large defects, surgery of cutaneous malignancy, postoperative bleeding, and delayed flap closure increase the risk for SSI. High‐risk locations are the ears and lower extremities.
Keywords: antibiotic prophylaxis, dermatologic surgical procedures, prospective studies, surgical wound infection, wound closure techniques
1. INTRODUCTION
Surgical site infection (SSI) is the most frequent complication in dermatologic surgery. 1 Previous studies reported an incidence between 0.7% and 8.7%. 2 , 3 , 4 , 5 , 6 It is associated with increased morbidity, treatment costs and impaired wound healing. 7 Given that a significant number of procedures involve the head and neck area, the latter is of highest importance, as it may also lead to poor aesthetic results.
In recent years, multiple studies assessed potential risk factors for SSI in skin surgery. 4 , 5 , 6 , 8 , 9 , 10 , 11 , 12 , 13 There is evidence that the lower extremities, lips, and the ear are at higher risk for postoperative infection. 14 In terms of surgery‐related risk factors, complex closure techniques including local flaps and skin grafts were significantly associated with SSI. In contrast, delayed wound closure or second‐intention healing did not show higher infection rates. 15 The latter was supported by a recent study that showed an incidence of less than 5% in lower‐extremity wounds that were allowed to heal by second intention. 13 Other identified risk factors were male gender and immunosuppression. Diabetes is commonly considered as a risk factor for postoperative wound infection. In dermatologic surgery, however, diabetes may not be associated with SSI. 16 , 17 The impact of other potentially relevant factors, such as defect size or delayed wound closure remains uncertain because of heterogeneous or insufficient data. 13 , 14 , 16 , 18 , 19
To prevent SSI, perioperative antibiotic prophylaxis (PAP) may be indicated in high‐risk individuals. Other indications for PAP include patients at high risk for infective endocarditis or hematogenous prosthetic joint infection, whereas the latter plays a minor role in dermatologic surgery. 20 , 21 Current recommendations for PAP in the prevention of wound infection associated with skin surgery mainly rely on an advisory statement by Wright and colleagues from 2008 or are extrapolated from guidelines on antibiotic prophylaxis from other surgical fields. 22 , 23 , 24 Considering the recent evidence on risk factors for SSI, updated recommendations that focus on dermatologic surgery are needed.
The aim of this study was to provide new high‐quality data that included potential risk factors, such as defect size or delayed wound closure, for which current evidence is heterogeneous or poor.
2. PATIENTS AND METHODS
2.1. Study design
This prospective observational study was conducted between August 2020 and May 2021 at the Department of Dermatology and Allergy, University Hospital, Ludwig Maximilian University (LMU) Munich, Germany. The study was approved by the local ethics committee (Nr. 20‐141). Prior to initiation, a study‐protocol was published on researchregistry.com (ID‐Nr. researchregistry5879). All adult in‐ and outpatients that presented for dermatologic surgery and could give oral and written consent were eligible. The study staff recorded baseline characteristics (body mass index [BMI], history of smoking, alcohol consumption, systemic antibiotics within weeks prior to surgery). Specific data concerning the hospital stay, such as on surgery, haemorrhagic complications, or histologic results, were collected retrospectively from medical charts. All data were recorded within Microsoft Excel 2010®.
Because of the restrictions related to the COVID‐19 pandemic, only punch biopsies and shave excisions were performed at the outpatient surgery unit. During these procedures, patients presented and were operated whilst wearing their own clothes, while the medical staff wore clean non‐sterile gowns, facemasks, surgical caps, and sterile gloves. The surgical site was disinfected with octenidine hydrochloride and covered with a surgical drape. Inpatients wore a specific theatre dress. In line with recommendations of the German Working Group ‘Hygiene in Hospital & Practice’, 25 surgeons and assistants of the inpatient operation theatre wore surgical scrubs and sterile gowns, surgical caps and sterile gloves. According to COVID‐19 infection control measures, the surgical team used filtering face piece 2 (FFP‐2) masks, instead of standard surgical masks. Prior to incision, the surgical site was disinfected by using disinfectant containing either providone‐iodine and propanol or octenidine hydrochloride and propanol. Surgery was performed in mostly local, but also general anaesthesia. During multistep procedures, such as microscopically controlled surgery, open wounds were covered with sterile dressings. Wound closure techniques included simple sutures, local flaps (e.g., rotation flap, transposition flap), skin grafting, or second intention healing.
Perioperative antibiotic prophylaxis to prevent SSI was administered according to local guidelines, which were implemented according to an antibiotic stewardship program. These included a single‐shot of intravenous (i.v.) cephazolin 2 g 30 min prior to the incision in patients with sentinel lymph node biopsies in the axilla or a fixed‐combination of Ampicillin 2 g and Clavulanic acid 1 g i.v. for interventions involving the groins. Prophylaxis for infective endocarditis when indicated was given according to the guidelines of the European Society of Cardiology. 20
The endpoint of interest was the occurrence of SSI. It was defined as the presence of typical clinical signs of infection at the surgical site (pain, tenderness, warmth, erythema and/or heat, and the appearance of purulent drainage) as diagnosed by the treating physician (e.g., surgeon, dermatologist, or primary care physician).
2.2. Follow‐up
Whenever possible, patients were followed‐up in our outpatient clinic. Sutures were removed after 5 to 7 days on the face, and 10 to 12 days on the scalp, neck, trunk, and extremities. Fourteen days after surgery, all patients were followed‐up by phone call and asked for any surgery‐related adverse event or systemic antibiotic treatment after discharge.
2.3. Exclusion criteria
Exclusion criteria were signs of infection at the surgical site prior to surgery, if the patient had to be transferred to an external department for further surgery, or if the intervention was cancelled.
2.4. Statistical analysis
One patient could have several wound lesions, and thus, all wound lesions were nested within the patient. Descriptive statistics were conducted either on the patient or lesion level. Mean values for lesion characteristics were thus calculated using intercept‐only linear mixed effects models with a random intercept per patient.
The SSI incidence was modelled using a mixed effects logistic regression model with patient and lesion characteristics as fixed effects. To account for the multilevel data structure, a random intercept was included per patient. At first, each of the patient and lesion characteristics was included separately in the model. All factors which reached statistical significance (P ≤ 0.05) or a statistical trend (P ≤ 0.1) were subsequently included in combination. Loss of statistical significance or a trend in this step leads to exclusion of the respective factor from the final model. This approach did not apply to age and gender, which were included in the final model irrespectively of the corresponding P‐values, as it is common in epidemiological research.
All statistics were performed in R, version 4.0.3. Functions of the lme4 and sjPlot packages were applied to calculate mixed effects models and extract reader‐friendly results.
3. RESULTS
From 804 patients that initially met inclusion criteria, 767 individuals with 1272 surgical sites were finally included in the per‐protocol analysis (Figure 1). Baseline patient and lesion characteristics can be found in Tables 1 and 2.
FIGURE 1.
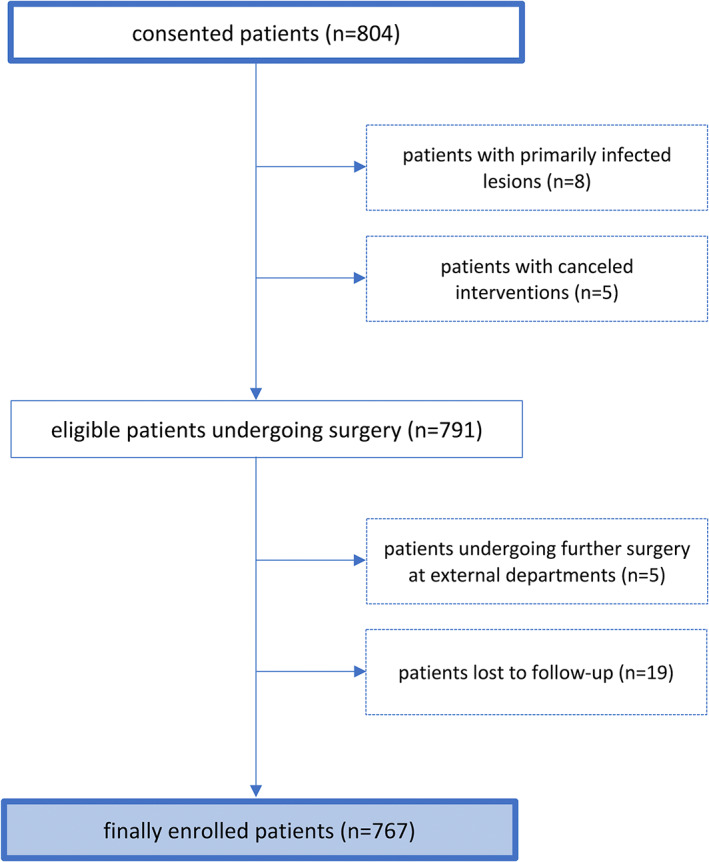
Flowchart of the study sample.
TABLE 1.
Numbers and percentages of patients showing lesions with or without surgical site infection (SSI) or both according to the presence or absence of patient‐related risk factors (n = 767).
| Patient characteristics | SSI n (%) | SSI and no SSI n (%) | No SSI n (%) |
|---|---|---|---|
| Sex | |||
| Female, n (%) | 17 (2.2) | 11 (1.4) | 332 (43.3) |
| Male, n (%) | 14 (1.8) | 30 (3.9) | 363 (47.3) |
| Diabetes | |||
| Yes, n (%) | 5 (0.7) | 5 (0.7) | 64 (8.3) |
| No, n (%) | 26 (3.4) | 36 (4.7) | 631 (82.3) |
| Smoking | |||
| Active, n (%) | 4 (0.5) | 4 (0.5) | 94 (12.3) |
| Previous, n (%) | 8 (1.0) | 15 (2.0) | 139 (18.1) |
| No, n (%) | 19 (2.5) | 22 (2.9) | 462 (60.2) |
| Coronary artery disease | |||
| Yes, n (%) | 2 (0.3) | 4 (0.5) | 70 (9.1) |
| No, n (%) | 29 (3.8) | 37 (4.8) | 625 (81.5) |
| History of hematologic cancer | |||
| Yes, n (%) | 1 (0.1) | 0 (0.0) | 13 (1.7) |
| No, n (%) | 30 (3.9) | 41 (5.3) | 682 (88.9) |
| HIV | |||
| Yes, n (%) | 0 (0.0) | 1 (0.1) | 11 (1.4) |
| No, n (%) | 31 (4.0) | 40 (5.2) | 684 (89.2) |
| Glucocorticoids | |||
| Yes, n (%) | 4 (0.5) | 4 (0.5) | 46 (6.0) |
| No, n (%) | 27 (3.5) | 37 (4.8) | 649 (84.6) |
| Biologicals | |||
| Yes, n (%) | 1 (0.1) | 2 (0.3) | 24 (3.1) |
| No, n (%) | 30 (3.9) | 39 (5.1) | 671 (87.5) |
| Anticoagulation | |||
| Yes, n (%) | 1 (0.1) | 10 (1.3) | 80 (10.4) |
| No, n (%) | 30 (3.9) | 31 (4.0) | 615 (80.2) |
| Antiplatelet | |||
| Yes, n (%) | 3 (0.4) | 11 (1.4) | 110 (14.3) |
| No, n (%) | 28 (3.7) | 30 (3.9) | 585 (76.3) |
| Total | 31 (4.0) | 41 (5.3) | 695 (90.6) |
TABLE 2.
Numbers and percentages of lesions with or without surgical site infection (SSI) according to the presence or absence of lesion‐related risk factors (n = 1272).
| Lesion characteristics | SSI n (%) | No SSI n |
|---|---|---|
| Malignancy | ||
| Yes, n (%) | 14 (1.1) | 632 (49.7) |
| No, n (%) | 64 (5.0) | 562 (44.2) |
| Ulceration | ||
| Yes, n (%) | 14 (1.1) | 98 (7.7) |
| No, n (%) | 64 (5.0) | 1096 (86.2) |
| Histologic diagnosis | ||
| BCC, n (%) | 31 (2.4) | 298 (23.4) |
| SCC, n (%) | 17 (1.3) | 173 (13.6) |
| Melanoma, n (%) | 4 (0.3) | 52 (4.1) |
| Acne inversa, n (%) | 2 (0.2) | 14 (1.1) |
| Condyloma, n (%) | 1 (0.1) | 14 (1.1) |
| Others, n (%) | 23 (1.8) | 642 (50.5) |
| Localization | ||
| Lips, n (%) | 1 (0.1) | 36 (2.8) |
| Nose, n (%) | 7 (0.6) | 129 (10.1) |
| Ear, n (%) | 10 (0.8) | 40 (3.1) |
| Scalp, n (%) | 12 (0.9) | 123 (9.7) |
| Hand, n (%) | 2 (0.2) | 34 (2.7) |
| Lower extremities, n (%) | 7 (0.6) | 49 (3.9) |
| Upper extremities, n (%) | 3 (0.2) | 41 (3.2) |
| Trunk, n (%) | 7 (0.6) | 188 (14.8) |
| Others, n (%) | 29 (2.3) | 553 (43.5) |
| Planned wound closure | ||
| Simple, immediate, n (%) | 27 (2.1) | 619 (48.7) |
| Simple, delayed, n (%) | 3 (0.2) | 119 (9.4) |
| Flap, immediate, n (%) | 2 (0.2) | 10 (0.8) |
| Flap, delayed, n (%) | 22 (1.7) | 79 (6.2) |
| Graft, immediate, n (%) | 3 (0.2) | 14 (1.1) |
| Graft, delayed, n (%) | 15 (1.2) | 102 (8.0) |
| Secondary intention, n (%) | 6 (0.5) | 251 (19.7) |
| Secondary haemorrhage | ||
| Yes, n (%) | 9 (0.7) | 20 (1.6) |
| No, n (%) | 69 (5.4) | 1174 (92.3) |
| Perioperative antibiotic prophylaxis | ||
| Yes, n (%) | 1 (0.1) | 20 (1.6) |
| No, n (%) | 77 (6.1) | 1174 (92.2) |
| Endocarditis prophylaxis | ||
| Yes, n (%) | 0 (0.0) | 24 (1.9) |
| No, n (%) | 78 (6.1) | 1170 (91.2) |
| Total | 78 (6.1) | 1194 (93.9) |
Surgical site infections occurred in 78 of the 1272 surgical wounds (6.1%). The final mixed effects model including patient and lesion characteristics that showed a significant impact on the SSI rate and the factors of gender and age are provided in Table 3. The corresponding odds ratios are illustrated in Figure 2.
TABLE 3.
Odd ratios of individual risk factors for surgical site infection according to the final mixed effects logistical regression model.
| Fixed effects on wound infection | |||
|---|---|---|---|
| Predictors | Odds ratios | CI | P |
| (Intercept) | 0.01 | 0.00–0.07 | <0.001 |
| Age (continuous variable, one unit represents one decade) | 0.92 | 0.77–1.09 | 0.341 |
| Sex (reference category: male) | 0.73 | 0.42–1.28 | 0.271 |
| Malignancy (reference category: benignancy) | 2.96 | 1.41–6.24 | 0.004 |
| Defect size >10 cm2 (reference category: <10 cm2) | 3.64 | 1.80–7.35 | <0.001 |
| Haemorrhage (reference category: no haemorrhage) | 4.63 | 1.58–13.53 | 0.005 |
| Delayed simple closure (reference category: immediate simple closure) | 0.38 | 0.10–1.41 | 0.146 |
| Immediate flap closure (reference category: immediate simple closure) | 2.01 | 0.35–11.48 | 0.433 |
| Delayed flap closure (reference category: immediate simple closure) | 2.67 | 1.13–6.34 | 0.026 |
| Immediate graft closure (reference category: immediate simple closure) | 2.91 | 0.63–13.37 | 0.169 |
| Delayed graft closure (reference category: immediate simple closure) | 1.15 | 0.46–2.85 | 0.765 |
| Secondary intention (reference category: immediate simple closure) | 0.58 | 0.21–1.63 | 0.302 |
| Nose (reference category: trunk) | 0.97 | 0.26–3.55 | 0.964 |
| Ears (reference category: trunk) | 7.75 | 2.07–28.99 | 0.002 |
| Lips (reference category: trunk) | 0.56 | 0.05–6.38 | 0.640 |
| Upper extremities (reference category: trunk) | 2.11 | 0.47–9.54 | 0.330 |
| Lower extremities (reference category: trunk) | 3.16 | 0.90–11.09 | 0.072 |
| Hands (reference category: trunk) | 2.27 | 0.37–14.09 | 0.378 |
| Scalp (reference category: trunk) | 1.70 | 0.53–5.50 | 0.376 |
| Other areas (reference category: trunk) | 1.17 | 0.45–3.07 | 0.742 |
| Random effects | |||
| σ2 | 3.29 | ||
| τ00 Patient | 0.44 | ||
| ICC | 0.12 | ||
| NPatient | 758 | ||
| Observations | 1238 | ||
| Marginal R2/Conditional R2 | 0.313/0.394 | ||
Note: P < 0.05 are in bold.
FIGURE 2.

Forest plot illustrating the odds ratios derived by the final mixed effects model.
3.1. Patient‐related risk factors
Most participants with postoperative infection were male, but the SSI risk was not significantly different compared with females (OR 0.73, 95% confidence interval [CI] 0.42–1.28, P = 0.271). Age was not statistically associated with wound infection (OR 0.92, CI 0.77–1.09, P = 0.341). In terms of comorbidities, neither diabetes, smoking, abnormal BMI, coronary artery disease, history of hematologic cancer, HIV, or immunosuppressive medication had a statistically relevant impact on the occurrence of postoperative infection, and these were thus not included in the final mixed effects model.
3.2. Malignancy and defect size
Surgical wounds were associated with a significantly higher risk for SSI after surgery of malignant tumours (OR 2.96, CI 1.41–6.24, P = 0.004). Ulcerated skin tumours did not show statistically higher infection rates. Wounds with a defect size exceeding 10cm2 were significantly more likely to develop infection (OR 3.64, CI 1.80–7.35, P < 0.001).
3.3. Surgery‐related risk factors
Postoperative bleeding led to a significantly higher SSI risk (OR 4.63, CI 1.58–13.53, P = 0.005). However, patients with anticoagulant or anti‐platelet medication did not show a statistically higher SSI rate. The same applied for patients that did not receive any perioperative antibiotic prophylaxis. Compared with immediate simple wound closure, only delayed closure with local flaps showed significantly higher SSI rates (OR 2.67, CI 1.13–6.34, P = 0.026). In contrast, immediate defect closure with local flaps did not cause more wound infections (OR 2.01, CI 0.35–11.48, P = 0.433). The same accounted for defects that were closed by skin grafting immediately (OR 2.91, CI 0.63–13.37, P = 0.169) or delayed (OR 1.15, CI 0.46–2.85, P = 0.765), as well as for surgical wounds, which were left for second intention healing (OR 0.58, CI 0.21–1.63, P = 0.302).
3.4. Body site
Compared with surgery on the trunk, interventions involving the ears showed a significantly higher SSI rate (OR 7.75, CI 2.07–28.99, P = 0.02). The lower extremity had a strong tendency towards a higher infection risk, without statistical significance (OR 3.16, CI 0.90–11.09, P = 0.072). Other body sites did not show significantly increased SSI risks.
4. DISCUSSION
This prospective observational study provides new and additional evidence on risk factors for SSI in dermatologic surgery. Overall, 6.1% of all surgical wounds showed signs of infection. Patient‐related risk factors, such as gender or age and comorbidities, particularly immunosuppression or diabetes, were not shown to have a significant impact on the occurrence of SSI. Large defects (>10 cm2) and surgery of cutaneous malignancy were significantly associated with SSI. In terms of surgery‐related risk factors, postoperative bleeding and delayed defect closure with a local flap were associated with significantly higher infection rates. High‐risk locations for infection were the ears and lower extremities.
These risk factors were identified using a mixed effects logistic regression model, which is an extension to the commonly used multivariable logistic regression model and accounts for the dependency of lesions within one patient.
Although some authors reported an incidence of up to 8.7%, most studies had lower infection rates. 3 , 5 , 6 , 8 , 9 , 10 , 26 , 27 Because of SARS‐CoV‐2‐related restrictions, procedures in our outpatient surgery unit were strongly limited. Most patients that needed simple excision of benign lesions were referred to another dermatologic clinic or private practice. Therefore, the study sample consisted of proportionally more patients that had complex skin surgery in an inpatient setting, which may have accounted for the higher occurrence of SSI. For instance, the large prospective study by Liu and colleagues reported an incidence of SSI of only 4.0%. 5 In contrast to our study, however, the authors performed most interventions in an outpatient clinic, including significantly more simple excisions (80.1% vs. 50.7%), less delayed defect closures (4.1% vs. 26.8%) and less local flaps (6.2% vs. 8.8%) or skin grafts (1.7% vs. 10.6%).
However, 97,5% of the study participants were followed up 14 days after surgery in our outpatient clinic or by phone call. Thus, the results are based on robust data, indicating that the true risk for SSI in complex dermatologic surgery may be higher as generally considered.
In contrast to previous findings, our data did not show any association between patient‐related factors and wound infection. For instance, SSI did not occur significantly more often in men. Although immunosuppression and diabetes are commonly considered as risk factors for SSI, our data did not show a significant impact of these comorbidities on the infection rate. 16 , 17 In line with recent publications, however, our findings confirmed that higher age was not associated with SSI. 6 , 16
There is evidence that complex closure technique such as local flaps or skin grafts cause higher infection rates. 15 Interestingly, in this sample, significantly more wound infections were only seen after delayed closure with local skin flaps. This may be because of better aseptic conditions if defects are closed directly after excision. However, delayed skin grafting was not associated with wound infection.
According to the literature, lips, lower extremities, and ears are considered as high‐risk body sites for SSI. 14 In this study, only ears showed a significant association with SSI. In accordance with the observational study by Amici and colleagues, our data yielded a link between postoperative haemorrhage and wound infection. 28 Anti‐coagulation or anti‐aggregation therapy, however, were not statistically associated with SSI, which again is in accordance with the literature. 4 , 12 , 29 There is conflicting data whether defect size may influence the infection rate. 5 , 13 , 18 , 19 , 28 , 30 This study compared defects of over 10cm2 with smaller wounds and found larger defect size to be an independent risk factor. In a multivariable logistic regression model Liu et al. showed that wounds exceeding 3 cm2 were associated with a higher infection risk. 5 Similar results were seen in studies that focused on wounds of the lower limbs, although the specific closure type significantly influenced the infection rate. 18 , 19 In contrast, Molina et al. did not find that defect size had an impact in defects of the lower extremity that were allowed to heal by second intention. 13 Wounds of the lower limbs, however, may not be comparable in terms of vascularization and their microbiome as compared with those in other body parts, such as the face. 31 Thus, the role of defect size may depend on the anatomic location and the technique of wound closure.
This study has certain limitations that are primarily because of its single‐centre design. Because of the SARS‐Cov‐2 pandemic, there was a significant decrease in the number of cases, because many patients postponed their intervention or had elective simple surgery of benign lesions in an external department or private practice. This had a significant impact on our sample size. In addition, it may have introduced bias, as proportionally more interventions with higher infection risk were included. Given these limitations, study results may be generalizable to patients that undergo complex dermatologic surgery. Hence, the risk of SSI may be lower in minor skin surgery in an outpatient setting, where certain risk factors are less frequent or do not apply. 32
Recent studies indicate that many dermatologic surgeons overprescribe and heterogeneously make use of PAP. 33 , 34 Given the risk of microbial resistance and potential adverse events, antibiotic prophylaxis should be restricted to high‐risk individuals. Clinical trials that have assessed antibiotic prophylaxis in dermatologic surgery were mostly underpowered and yielded conflicting data. 35 , 36 , 37 , 38 , 39 In addition, risk factors for SSI among the study populations were heterogeneous. Thus, there is an unmet need for well‐conducted controlled clinical trials that analyse the efficacy of PAP in patients with high infection risk. The findings of this study are an important contribution to future trials, as they may help to define high‐risk individuals that qualify for inclusion.
5. CONCLUSION
In dermatologic surgery, large defects, surgery of cutaneous malignancy, postoperative bleeding, and delayed defect closure by local flaps were significantly associated with SSI. High‐risk locations for infection were the ears and lower extremities. Neither diabetes nor immunosuppression had a relevant impact on the occurrence of postoperative wound infection.
FUNDING INFORMATION
This study was funded by a grant from the Medical Faculty of the Ludwig Maximilian University Munich (Verein zur Förderung von Wissenschaft und Forschung an der Medizinischen Fakultät der LMU München e.V. [WiFoMed]).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGEMENT
Open Access funding enabled and organized by Projekt DEAL.
Schlager JG, Patzer K, Wallmichrath J, et al. Surgical site infection in skin surgery—An observational study. Int Wound J. 2023;20(9):3514‐3522. doi: 10.1111/iwj.14224
Justin Gabriel Schlager, Kathrin Patzer, Benjamin Kendziora, and Daniela Hartmann contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. O'Neill JL, Lee YS, Solomon JA, et al. Quantifying and characterizing adverse events in dermatologic surgery. Dermatol Surg. 2013;39(6):872‐878. [DOI] [PubMed] [Google Scholar]
- 2. Maragh SL, Brown MD. Prospective evaluation of surgical site infection rate among patients with Mohs micrographic surgery without the use of prophylactic antibiotics. J Am Acad Dermatol. 2008;59(2):275‐278. [DOI] [PubMed] [Google Scholar]
- 3. Heal CF, Buettner PG, Drobetz H. Risk factors for surgical site infection after dermatological surgery. Int J Dermatol. 2012;51(7):796‐803. [DOI] [PubMed] [Google Scholar]
- 4. Nakamura Y, Sasaki K, Ishizuki S, et al. Invasive and in situ lesions of squamous cell carcinoma are independent factors for postoperative surgical‐site infection after outpatient skin tumors surgery: a retrospective study of 512 patients. J Dermatol. 2021;48(4):497‐501. [DOI] [PubMed] [Google Scholar]
- 5. Liu X, Sprengers M, Nelemans PJ, Mosterd K, Kelleners‐Smeets NWJ. Risk factors for surgical site infections in dermatological surgery. Acta Derm Venereol. 2018;98(2):246‐250. [DOI] [PubMed] [Google Scholar]
- 6. Nemer KM, Ko JJ, Hurst EA. Complications after Mohs micrographic surgery in patients aged 85 and older. Dermatol Surg. 2021;47(2):189‐193. [DOI] [PubMed] [Google Scholar]
- 7. Badia JM, Casey AL, Petrosillo N, Hudson PM, Mitchell SA, Crosby C. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect. 2017;96(1):1‐15. [DOI] [PubMed] [Google Scholar]
- 8. Schimmel J, Belcher M, Vieira C, Lawrence N, Decker A. Incidence of surgical site infections in second intention healing after dermatologic surgery. Dermatol Surg. 2020;46(12):1492‐1497. [DOI] [PubMed] [Google Scholar]
- 9. Balakirski G, Kotliar K, Pauly KJ, et al. Surgical site infections after dermatologic surgery in immunocompromised patients: a single‐center experience. Dermatol Surg. 2018;44(12):1525‐1536. [DOI] [PubMed] [Google Scholar]
- 10. Balakirski G, Löser CR, Dippel E, et al. Surgical site infections after microscopically controlled skin surgery in immunocompromised patients: a retrospective two‐center cohort study. Arch Dermatol Res. 2020;312(7):491‐499. [DOI] [PubMed] [Google Scholar]
- 11. Basu P, Goldenberg A, Cowan N, Eilers R, Hau J, Jiang SIB. A 4‐year retrospective assessment of postoperative complications in immunosuppressed patients following Mohs micrographic surgery. J Am Acad Dermatol. 2019;80(6):1594‐1601. [DOI] [PubMed] [Google Scholar]
- 12. Schmitt A, DePry J, Tsai S, Bordeaux J. Retrospective evaluation of the safety of large skin flap, large skin graft, and interpolation flap surgery in the outpatient setting. Dermatol Surg. 2018;44(12):1537‐1546. [DOI] [PubMed] [Google Scholar]
- 13. Molina GE, Yu SH, Neel VA. Observations regarding infection risk in lower‐extremity wound healing by second intention. Dermatol Surg. 2020;46(10):1342‐1344. [DOI] [PubMed] [Google Scholar]
- 14. Schlager JG, Ruiz San Jose V, Patzer K, French LE, Kendziora B, Hartmann D. Are specific body sites prone for wound infection after skin surgery? A systematic review and meta‐analysis. Dermatol Surg. 2022;48(4):406‐410. [DOI] [PubMed] [Google Scholar]
- 15. Schlager JG, Hartmann D, Ruiz San Jose V, Patzer K, French LE, Kendziora B. Procedure‐related risk factors for surgical site infection in dermatologic surgery. Dermatol Surg. 2022;48:1046‐1050. [DOI] [PubMed] [Google Scholar]
- 16. Schlager JG, Hartmann D, Wallmichrath J, et al. Patient‐dependent risk factors for wound infection after skin surgery: a systematic review and meta‐analysis. Int Wound J. 2022;19(7):1748‐1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwartzman G, Khachemoune A. Surgical site infection after dermatologic procedures: critical reassessment of risk factors and reappraisal of rates and causes. Am J Clin Dermatol. 2021;22(4):503‐510. [DOI] [PubMed] [Google Scholar]
- 18. Niklinska EB, Hicks A, Wheless L, Hanlon A. Characteristics of lower extremity infection rates following Mohs micrographic surgery. Dermatol Surg. 2021;47(12):1547‐1550. [DOI] [PubMed] [Google Scholar]
- 19. Nathan NR, O'Connor DM, Tiger JB, Sowerby LM, Olbricht SM, Luo S. Factors associated with surgical site infection of the lower extremity: a retrospective cohort study. J Am Acad Dermatol. 2020;83(1):274‐276. [DOI] [PubMed] [Google Scholar]
- 20. Habib G, Lancellotti P, Antunes MJ, et al. ESC guidelines for the management of infective endocarditis: The task force for the management of infective endocarditis of the european society of cardiology (ESC). Endorsed by: European Association for Cardio‐Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075‐3128. [DOI] [PubMed] [Google Scholar]
- 21. Sollecito TP, Abt E, Lockhart PB, et al. The use of prophylactic antibiotics prior to dental procedures in patients with prosthetic joints: evidence‐based clinical practice guideline for dental practitioners–a report of the American dental association council on scientific affairs. J Am Dent Assoc. 2015;146(1):11‐16.e8. [DOI] [PubMed] [Google Scholar]
- 22. National Institute for Health and Care Excellence: Clinical Guidelines . Surgical site infections: prevention and treatment. Health and Care Excellence (NICE) Copyright © NICE 2020. London: National Institute for; 2020. [Google Scholar]
- 23. World Health Organization . Global Guidelines for the Prevention of Surgical Site Infection. [Internet]. 2nd ed. Geneva: World Health Organization; 2018. [cited 2022 Feb 11]:184 Available from: https://apps.who.int/iris/handle/10665/277399 [Google Scholar]
- 24. Wright TI, Baddour LM, Berbari EF, et al. Antibiotic prophylaxis in dermatologic surgery: advisory statement 2008. J Am Acad Dermatol. 2008;59(3):464‐473. [DOI] [PubMed] [Google Scholar]
- 25. AWMF Working Group “Hospital & Practice Hygiene” of AWMF. Hygieneanforderungen beim ambulanten Operieren. [Internet]. 2018[cited 2022 Feb 11] Available from: https://www.awmf.org/uploads/tx_szleitlinien/029-014l_S1_Hygieneanforderungen-beim-ambulanten-Operieren_2019-07.pdf
- 26. Bordeaux JS, Martires KJ, Goldberg D, Pattee SF, Fu P, Maloney ME. Prospective evaluation of dermatologic surgery complications including patients on multiple antiplatelet and anticoagulant medications. J Am Acad Dermatol. 2011;65(3):576‐583. [DOI] [PubMed] [Google Scholar]
- 27. Kulichová D, Geimer T, Mühlstädt M, Ruzicka T, Kunte C. Surgical site infections in skin surgery: a single center experience. J Dermatol. 2013;40(10):779‐785. [DOI] [PubMed] [Google Scholar]
- 28. Amici JM, Rogues AM, Lasheras A, et al. A prospective study of the incidence of complications associated with dermatological surgery. Br J Dermatol. 2005;153(5):967‐971. [DOI] [PubMed] [Google Scholar]
- 29. Dixon AJ, Dixon MP, Askew DA, Wilkinson D. Prospective study of wound infections in dermatologic surgery in the absence of prophylactic antibiotics. Dermatol Surg. 2006;32(6):819‐826. discussion 26–7. [DOI] [PubMed] [Google Scholar]
- 30. Heal C, Buettner P, Browning S. Risk factors for wound infection after minor surgery in general practice. Med J Aust. 2006;185(5):255‐258. [DOI] [PubMed] [Google Scholar]
- 31. Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. 2018;16(3):143‐155. [DOI] [PubMed] [Google Scholar]
- 32. Matos S, Sturm B, Buhnerkempe M, Larson R, Wilson M. Risk factors for infection after minor dermatologic procedures: a case‐control study. Dermatol Surg. 2021;47(12):1562‐1565. [DOI] [PubMed] [Google Scholar]
- 33. Balakirski G, Felcht M, Bayer H, Schmitt L. Analysis of the status quo of perioperative antibiotic prophylaxis in dermatosurgery in Germany: results of the DESSI‐study. J Dtsch Dermatol Ges. 2019;17(7):703‐713. [DOI] [PubMed] [Google Scholar]
- 34. Barbieri JS, Fix WC, Miller CJ, et al. Variation in prescribing and factors associated with the use of prophylactic antibiotics for Mohs surgery: a single‐institution retrospective study. Dermatol Surg. 2020;46(7):868‐875. [DOI] [PubMed] [Google Scholar]
- 35. Mourad A, Gniadecki R, Taher M. Oral and Intraincisional antibiotic prophylaxis in Mohs surgery: a systematic review and meta‐analysis. Dermatol Surg. 2020;46(4):558‐560. [DOI] [PubMed] [Google Scholar]
- 36. Rosengren H, Heal CF, Buttner PG. Effect of a single prophylactic preoperative oral antibiotic dose on surgical site infection following complex dermatological procedures on the nose and ear: a prospective, randomised, controlled, double‐blinded trial. BMJ Open. 2018;8(4):e020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosengren H, Heal CF, Buettner PG. Effect of a single preoperative dose of Oral antibiotic to reduce the incidence of surgical site infection following below‐knee dermatological flap and graft repair. Dermatol Pract Concept. 2019;9(1):28‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cherian P, Gunson T, Borchard K, Tai Y, Smith H, Vinciullo C. Oral antibiotics versus topical decolonization to prevent surgical site infection after Mohs micrographic surgery: a randomized, controlled trial. Dermatol Surg. 2013;39(10):1486‐1493. [DOI] [PubMed] [Google Scholar]
- 39. Smith SC, Heal CF, Buttner PG. Prevention of surgical site infection in lower limb skin lesion excisions with single dose oral antibiotic prophylaxis: a prospective randomised placebo‐controlled double‐blind trial. BMJ Open. 2014;4(7):e005270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


