Abstract
Patients with bronchopulmonary dysplasia (BPD) have shown clinical improvement after secundum atrial septal defect (ASD) closure. We sought to determine if this post‐ASD closure improvement is secondary to the expected course in BPD patients or related to the closure itself. A novel BPD‐ASD score was created to assess patients' clinical status (higher score = worse disease) and applied to 10 BPD‐ASD inpatients weighing ≤ 10 kg who underwent ASD closure. The score and its subcomponents were retrospectively calculated serially ranging from 8 weeks pre‐ to 8 weeks post‐intervention, and pre‐ and post‐intervention score slopes were created. These slopes were compared using mixed regression modeling with an interaction term. There was a significant difference in pre‐ versus post‐intervention slope with the most score drop the first week post‐intervention (−2.1 + /− 0.8, p = 0.014). The mean score also dropped through weeks 2 (slope −0.8 + /− 0.8, p = 0.013) and 4 (slope −1.0 + /− 0.5, p = 0.001) post‐intervention. There was a significant difference in pre‐ and post‐intervention slopes for diuretics (p = 0.018) and the combined score of respiratory support, FiO2 need, and respiratory symptoms (p = 0.018). This study demonstrated significant improvement in BPD‐ASD score, diuretic need, and respiratory status after ASD closure in BPD‐ASD patients ≤ 10 kg that was outside of the natural course of BPD. Our study was limited by its small, single‐center, retrospective nature. Future studies should be performed in a larger multicenter population to both validate the scoring system and compare to non‐intervention infants.
Keywords: neonatal lung disease & BPD, pediatrics, pulmonary hypertension
INTRODUCTION
Patients with bronchopulmonary dysplasia (BPD) have immature lung parenchyma and alveolar simplification. Embryologically, alveolar development and angiogenesis develop together, and therefore when there is immature alveolar development, there is abnormal development of pulmonary vascularity. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 The fragile lung beds of these patients have decreased alveolar‐capillary and vascular surface area for gas exchange, concerning for an increased susceptibility to and exaggerated respiratory effect from left to right shunts. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 Additionally, premature infants with BPD are at high risk of left ventricular diastolic dysfunction, making their pulmonary vasculature even more susceptible to untoward hemodynamic effects of intracardiac shunts. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 Due to the fragility of the alveolar‐capillary surface and increased left ventricle diastolic pressures, 1 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 these patients may be prone to pulmonary edema and vascularity changes when exposed to even the slightest excess pulmonary blood flow (Qp) from cardiac shunts.
In a retrospective review of 20,496 patients, 1314 (6%) were found to have an atrial septal defect (ASD) with an increased odds of developing BPD, making ASDs a potentially modifiable risk factor for BPD development. 21 Multiple single‐center studies have demonstrated improvement in symptoms and echocardiographic findings after infants with BPD undergo ASD closure. 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 However, the natural history of BPD is to improve over time, 31 so it is unclear if the improvement seen is secondary to the ASD closure or from the expected clinical course of BPD. Our study retrospectively applies a novel BPD–ASD score to evaluate if ASD closure is associated with an acute change in clinical course apart from the background clinical trajectory. This was evaluated by comparing the BPD‐ASD score slope over time before and after closure. It was our hypothesis that the slope of the BPD‐ASD score would acutely and significantly improve after ASD closure in infants with BPD and ASD, supporting the possibility that ASD closure results in clinical improvement. This score also allows for standardized measures of BPD‐ASD illness in inpatients that could be used in future studies.
METHODS
We performed a retrospective cohort study of all patients with BPD and ≤ 10 kg who underwent secundum ASD closure between 2020 and 2023 at Texas Children's Hospital. Inclusion criteria included (1) prematurity defined as gestational age at birth less than 37 weeks; (2) the presence of moderate to severe BPD, defined per Jensen et al. 32 ; and (3) a history of either surgical or catheterization‐based secundum ASD closure as an inpatient at a weight less than or equal to 10 kg. Exclusion criteria included other significant congenital heart disease. The institutional review board at Baylor College of Medicine approved this study.
A BPD‐ASD scoring system was developed by a multidisciplinary team consisting of a neonatologist, general pulmonologist, a specialist in pulmonary hypertension, and interventional cardiologist. This scoring system was presented to the BPD‐pulmonary hypertension subcommittee of the BPD Collaborative and modified based on recommendations. The scoring system consisted of assigning points based on the level of BPD severity, respiratory symptoms, diuretic use, growth, type of respiratory support, inspired fractional oxygen need (FiO2), and estimated right ventricular (RV) pressure using the degree of systolic ventricular septal flattening on echocardiogram (Table 1). The respiratory symptoms and support were determined by review of flowsheets and daily progress note for the 24 h before the time point being reviewed. The definition of growth < 10% was based on the Fenton 2013 growth chart calculator for patients up to 50 weeks corrected gestational age and the 2006 World Health Organization growth standard charts were used for patients over 50 weeks corrected gestational age to 2 years of age. 33 We chose to use a systolic septal configuration to estimate RV pressure by echocardiogram, as this parameter is the most consistently used method in our echocardiography lab to estimate RV pressure in this patient population. The maximum possible BPD‐ASD score using the scoring system was 15 points, with a higher score indicating a worse clinical status. This score was retrospectively applied to the patients at 1, 4, and 8 weeks pre‐intervention, at the time of ASD closure, and at 1, 2, 4, 6, 8 weeks post‐intervention (if data were available via chart review).
Table 1.
Bronchopulmonary dysplasia‐atrial septal defect scoring system consisting of points assigned for each variable.
|
1 |
|
|
|
1 |
|
1 |
|
3 |
|
|
|
1 |
|
2 |
|
3 |
|
|
|
0 |
|
1 |
|
|
|
1 |
|
2 |
|
3 |
|
|
|
0 |
|
1 |
|
2 |
|
|
|
0 |
|
1 |
|
2 |
Abbreviations: BID, twice a day; BPD, bronchopulmonary dysplasia; CGA, corrected gestational age; FiO2, inspired fractional oxygen; IV, intravenous; RR, respiratory rate; RV, right ventricle; TID, three times a day.
Statistical analysis
Descriptive data are reported as median and interquartile range (IQR). First, a spaghetti plot was created with each line representing a unique patient demonstrating the BPD‐ASD score at each point to allow visual demonstration of changes in score over time. Second, to compare changes over time, mixed linear regression analysis accounting for repeated measures was performed with week as the independent variable, BPD‐ASD score as the dependent variable, and status pre‐ versus post‐intervention as the interaction term. A secondary mixed linear regression analysis was then performed adding interaction terms at the change from 1 to 2 weeks, 2 to 3 weeks, 3 to 4 weeks, and 4 to 8 weeks, to compare and assess the slopes in greater detail.
To assess the effects of each clinical variable on the total BPD‐ASD score, a sub‐analysis of the subcomponents of the scoring system was performed. For this analysis, the respiratory components of FiO2, respiratory support, and respiratory symptoms were combined into a respiratory score with a maximum total points of 8. The remaining subcomponents were diuretic use, growth, and estimated RV pressure by systolic septal flattening on echocardiogram. First, stacked area graphs were plotted for each of these subcomponents to visualize the pre‐ and post‐intervention trends. Mixed linear regression analysis of the pre‐ and post‐slopes for each subcomponent were then performed.
RESULTS
Ten patients met the inclusion criteria. Nine of these patients underwent cardiac catheterization device closure and one underwent surgical closure (patient #10) of the ASD due to the size of the defect and deficient ASD rims. There were no procedural or postprocedural complications. Table 2 demonstrates baseline data, length of stay, and respiratory support at the time of ASD closure and discharge for the patient cohort. The median pre‐procedure length of stay was 210 days (interquartile range [IQR]: 152–303) and post‐procedure median length of stay was 43 days (IQR: 25–67).
Table 2.
Baseline patient data, respiratory support, and length of stay.
| Patient | Sex | Birth GA (weeks) | Birthweight (g) | Total LOS (days) | Pre‐procedure LOS (days) | Procedure respiratory support | Postprocedure LOS (days) | Discharge respiratory support |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 30 | 765 | 17 | 13 | CPAP 8 | 4 | 1/8 LFNC |
| 2 | M | 28 | 720 | 56 | 46 | 1/2 LFNC | 10 | 1/4 LFNC |
| 3a | F | 24 | 730 | 483 | 438 | Hospital ventilator | 45 | Home ventilator |
| 4b | M | 24 | 815 | 257 | 217 | HFNC 4 L | 40 | 1/4 LFNC |
| 5b | F | 22 | 450 | 340 | 207 | HFNC 10 L | 133 | Trach/vent |
| 6 | F | 27 | 1090 | 393 | 331 | HFNC 4 L | 62 | RA |
| 7 a | F | 23 | 590 | 637 | 615 | Home ventilator | 22 | Home ventilator |
| 8 a | M | 24 | 500 | 246 | 213 | Hospital ventilator | 33 | Home ventilator |
| 9 b | F | 26 | 720 | 267 | 184 | Hospital ventilator | 83 | 1/2 LFNC |
| 10 b | F | 26 | 870 | 210 | 141 | Hospital ventilator | 69 | 1/8 LFNC |
| Median (IQR) | 25 (24–27) | 725 (626–803) | 262 (219–380) | 210 (152–303) | ‐ | 43 (25–67) | ‐ | |
Abbreviations: ASD, atrial septal defect; CPAP, continuous positive airway pressure; F, female; GA, gestational age; g, grams; HFNC, high flow nasal cannula; IQR, interquartile range; LOS, length of stay; LFNC, low flow nasal cannula; L, liters; M, male; RA, room airtrach; tracheostomy; vent, ventilator.
Patients with tracheostomy already in place at the time of ASD closure.
Patients being considered for tracheostomy at the time of ASD closure.
The decision to close the ASD was made by the bedside teams based on clinical criteria, including the inability to wean respiratory support, consideration for tracheostomy, high diuretic needs, and increased work of breathing including tachypnea and retractions. If a patient had pulmonary hypertension on catheterization but had reactive pulmonary vasculature, consideration was given for ASD closure to help limit the development of pulmonary edema with initiation of pharmacotherapy.
Indication for closure of the ASD in four patients was potential need for tracheostomy due to the inability to wean respiratory support from invasive ventilation (identified with “b” in Table 2), continuous positive pressure, or high‐flow nasal cannula. Of these four patients, one was weaned from invasive ventilation to room air within 4 weeks, one from high flow nasal cannula to low flow nasal cannula within 1 week, and one from invasive ventilation to 2 L low‐flow nasal cannula within 4 weeks after ASD closure. One of these four patients (#5) required invasive mechanical ventilation 6 weeks after the procedure and tracheostomy placement 12 weeks after the procedure. After ASD closure, all three patients with tracheostomy already in place at the time of ASD closure were able to have their ventilatory support weaned enough to be transitioned successfully from a hospital to home ventilator or reach low enough home ventilator settings to be safely discharged home within a maximum of 45 days (identified with “a” in Table 2).
Table 3 displays invasive hemodynamic data obtained at the time of cardiac catheterization, showing baseline data along with data during pulmonary vasoreactivity testing. Five patients were on pulmonary vasodilator therapy at the time of catheterization. At baseline, all patients had sub‐systemic RV pressure and all but one had an indexed pulmonary vascular resistance (PVRi) less than 6 Wood‐units.m2. Percent systemic right ventricle systolic pressure (RVSP) ranged from 43% to 68%. Three patients had elevated baseline left atrial or pulmonary artery wedge pressures, defined as a pressure ≥ 10 mmHg. 34
Table 3.
Procedural and catheterization data.
| Patient data | Baseline catheterization data | Catheterization data with pulmonary vasodilator testing | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Procedure CGA | Procedure weight (g) | PH Medication (#) | RVSP/EDP (mmHg) | SBP/DBP/mean (mmHg) | RVSP (% SBP) | mPAp (mmHg) | LAP/Wedge (mmHg) | PVRi (Wu.m2) | PVR/SVR | Qp:Qs | RVSP/EDP (mmHg) | SBP/DBP/mean (mmHg) | RVSP (% SBP) | mPAp (mmHg) | LAP/wedge (mmHg) | PVRi (Wu.m2) | PVR/SVR | Qp:Qs |
| 1 | 51 | 3800 | 0 | 36/8 | 83/47/62 | 43 | 21 | 8 | 1.6 | 0.1 | 2.2 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| 2 | 52 | 4440 | 1 | 45/10 | 81/38/55 | 58 | 32 | 8 | 5.2 | 0.22 | 2.2 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| 3 | 86 | 9700 | 2 | 50/12 | 74/45/55 | 68 | 30 | 12 | 3.5 | 0.31 | 1.6 | 49/12 | 86/52/64 | 57 | 28 | 10 | 3.4 (O2 only) | 0.22 | 1.6 (O2 only) |
| 4 | 56 | 6510 | 0 | 45/13 | 76/44/53 | 59 | 26 | 9 | 4.7 | 0.33 | 1.1 | 35/11 | 67/44/52 | 52 | 20 | 7 | 2.7 | 0.21 | 1.3 |
| 5 | 52 | 5780 | 2 | 42/6 | 73/38/51 | 58 | 25 | 6 | 4.9 | 0.26 | 1.6 | 41/8 | 75/43/56 | 55 | 28 | 9 | 4.2 | 0.29 | 1.3 |
| 6 | 59 | 7060 | 0 | 49/10 | 74/45/56 | 66 | 32 | 7 | 5 | 0.26 | 1.7 | 46/8 | 73/42/53 | 63 | 28 | 8 | 3.7 | 0.17 | 2.6 |
| 7 | 111 | 9140 | 1 | 53/8 | 87/50/65 | 61 | 35 | 8 | 8 | 0.28 | 1.7 | 48/6 | 75/45/56 | 64 | 30 | 11 | 5.4 | 0.21 | 1.7 |
| 8 | 55 | 5660 | 0 | 48/17 | 71/45/55 | 68 | 33 | 13 | 4.1 | 0.19 | 2.4 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| 9 | 53 | 6630 | 1 | 52/11 | 76/46/58 | 68 | 37 | 14 | 4.3 | 0.28 | 1.7 | 38/8 | 84/53/64 | 45 | 26 | 10 | 2.1 | 0.15 | 2 |
| 10 (surgical) | 46 | 4100 | 0 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Median (IQR) | 54 (52–58) | 6145 (4745–6953) | ‐ | ‐ | ‐ | 60 (58–67) | 32 (26–33) | 8 (8–12) | 4.7 (4.1–5) | 0.26 (0.22–0.28) | 1.7 (1.6–2.2) | ‐ | ‐ | 56 (53–62) | 28 (26.5–28) | 9.5 (8.25–10) | 3.6 (2.9–4.1) | 0.21 (0.18–0.22) | 1.7 (1.4–1.9) |
Abbreviations: #, number; AP, left atrial pressure; CGA, corrected gestational age; DBP, diastolic blood pressure; EDP, end diastolic pressure; g, grams; IQR, interquartile range; O2, oxygen; PVR/SVR, pulmonary vascular resistance to systemic vascular resistance ratio; PVRi, indexed pulmonary vascular resistance; Qp:Qs, pulmonary blood flow to systemic blood flow ratio; RVSP, right ventricle systolic pressure; SBP, systolic blood pressure; Wu.m2, Wood.units.meters2.
Six patients underwent pulmonary vasodilator testing. With vasodilator testing, two patients (#3 and #9) had improvement in the percent systemic RVSP by more than 10%, while the other four had minimal change to the RVSP even with improvement in PVRi. All patients in which testing was performed had responsive PVRi to less than 6 Wood‐units.m2. Of the five patients on pulmonary vasodilator therapy at the time of catheterization, one was weaned off by 12 weeks. One patient with Trisomy 21 was not on pulmonary hypertension medication at the time of catheterization due to inability to tolerate initiation of therapy but was able to be maximized on full dose sildenafil therapy by 1 week after the ASD closure without development of pulmonary edema.
Table 4 demonstrates the number of patients for each variable at each time point that data were available. Since this was a retrospective study, protocols were not in place for follow‐up echocardiograms, so the majority of missing follow‐up data was secondary to the lack of echocardiogram at particular time points. Other variables were not obtainable in some patients due to those patients not being in our institution's hospital at those particular time points.
Table 4.
Number of patients with data for each BPD‐ASD scoring system variable at each time point.
| Weeks relative to intervention | BPD severity | Symptoms | Diuretics | Growth | Support | FiO2 | RV pressure | Total |
|---|---|---|---|---|---|---|---|---|
| −8 | 10 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| −4 | 10 | 9 | 9 | 9 | 9 | 9 | 9 | 9 |
| −1 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| 0 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| 1 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| 2 | 10 | 10 | 10 | 10 | 10 | 10 | 6 | 6 |
| 4 | 10 | 10 | 10 | 10 | 10 | 10 | 6 | 6 |
| 6 | 10 | 10 | 10 | 10 | 10 | 10 | 6 | 6 |
| 8 | 10 | 8 | 10 | 10 | 9 | 8 | 5 | 5 |
Abbreviations: ASD, atrial septal defect; BPD, bronchopulmonary dysplasia; FiO2, inspired fractional oxygen; RV, right ventricular.
A spaghetti plot with BPD‐ASD score for each patient over time from 8 weeks pre‐ to 8 weeks post‐intervention is shown in Figure 1a. In all but one patient, the scores appear to drop rapidly after intervention. Notably, patient #4, who had a low pulmonary blood flow to systemic blood flow ratio (Qp:Qs) of 1.1 at baseline and 1.3 with pulmonary vasodilator testing underwent ASD closure due to excessive diuretic need, respiratory symptoms, and right heart dilation. This patient demonstrated a similar benefit with ASD closure, as demonstrated by a decline in BPD‐ASD score from 8 at intervention to 5 at 1 week and to 3 by 8 weeks. Figure 1b demonstrates pre‐ and post‐intervention regression lines split by intervention only. There was a significant difference in pre‐ versus post‐intervention slope when split only by time of intervention (p = 0.048) and not by weeks post‐intervention. Figure 1c demonstrates a regression line and 95% interval lines fit to the measures scores and comparisons of the pre‐ and post‐intervention rates of change by grouped weeks. There was a significant difference when pre‐intervention slope (0.0 + /− 0.1) was compared with week 1 post‐intervention slope (−2.1 + /− 0.8, p = 0.014), week 2 to week 3 post‐intervention slope (−0.8 + /− 0.8, p = 0.013), and week 3 to week 4 (−1.0 + /− 0.5) post‐intervention slope (−1.0 + /− 0.5, p = 0.001). After week 4, there was no longer a significant negative slope in the score.
Figure 1.

(a) Spaghetti plot showing each individual bronchopulmonary dysplasia (BPD)‐atrial septal defect (ASD) score over time with time “0” marking the intervention, and scores 8 weeks before and 8 weeks after the intervention. (b) A simplified graph, with the only breakpoint at intervention, to allow the analysis of aggregate slopes with 95% confidence limits (regression lines) before and after intervention. This shows that while the pre‐intervention slope was no different than 0, the score post‐intervention was significantly decreasing, and the pre‐ and post‐intervention slopes were significantly different than each other. (c) Graph with slopes and 95% confidence limits before and after intervention, with additional division of time periods post‐intervention to evaluate the speed of change in relationship to intervention timing. Before intervention, the BPD‐ASD score was stable with no change over time. The week after the intervention the score drops an average of 2.2 ± 0.8 points, which is a significant slope change (p = 0.014). The mean score continues to significantly drop through weeks 2 and 4 post‐intervention. After week 4, there is no longer a significant drop in score. *p < 0.05.
A sub‐analysis of each subcomponent of the BPD‐ASD score was performed. Each patient in the cohort was grouped by point assignment for each variable. The percentage of patients with higher diuretic use, higher respiratory scores, and higher estimated RV pressure appeared to decrease after intervention (Figure 2). The percentage of patients with systemic RV pressure before ASD closure did not appear to change after closure. Growth did not appear to have a difference in slope from pre‐ to post‐intervention either, as it was improving throughout the entire time course. When the pre‐ and post‐intervention slopes of the diuretics, respiratory, growth, and estimated RV pressure were compared, there was a significant difference between pre‐ and post‐intervention slopes for diuretics (p = 0.018) and respiratory score (p = 0.018). While the pre‐ and post‐intervention slope of the estimated RV pressure score did not demonstrate significance, the post‐intervention slope demonstrated a significant difference compared with a slope of 0 while the pre‐intervention slope did not demonstrate a significant difference compared with a slope of 0 (Figure 3). One of two patients (#7) with post‐intervention systemic RV pressure readings by echocardiogram underwent the ASD closure at the corrected gestational age of 111 weeks and had already undergone tracheostomy, making accurate septal assessment in the standard parasternal short axis view difficult due to artifactual interference with sonography from the severity of the lung disease. The second of the two patients was patient #5 who required escalation of respiratory support and intubation 6 weeks after the procedure. Once intubated and provided with adequate respiratory support, the septal flattening resolved immediately.
Figure 2.
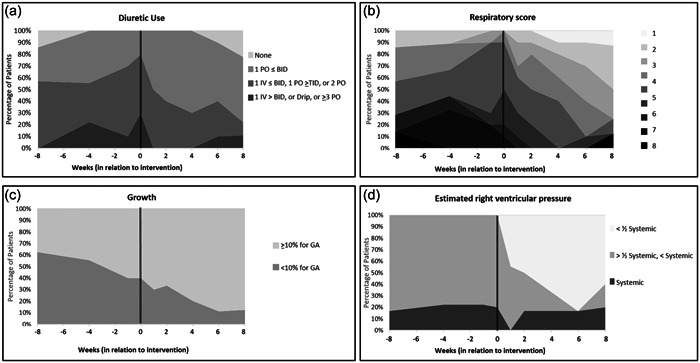
Stacked area graphs for each subcomponent of the bronchopulmonary dysplasia (BPD)‐atrial septal defect (ASD) score, showing by shade the proportion of subjects with each subcomponent score. (a) The proportion of patients with high diuretic scores decreases after the intervention. (b) Respiratory score, which is a combination score consisting of the FiO2 need, respiratory support, and respiratory symptoms scores. After the intervention, the majority of the patients had a lower score than before the intervention. (c) Growth score, showing no difference in improvement of growth pre‐ and post‐intervention. (d) Estimated right ventricular (RV) pressure, which shows that after the intervention the majority of patients ultimately had a normal RV pressure.
Figure 3.
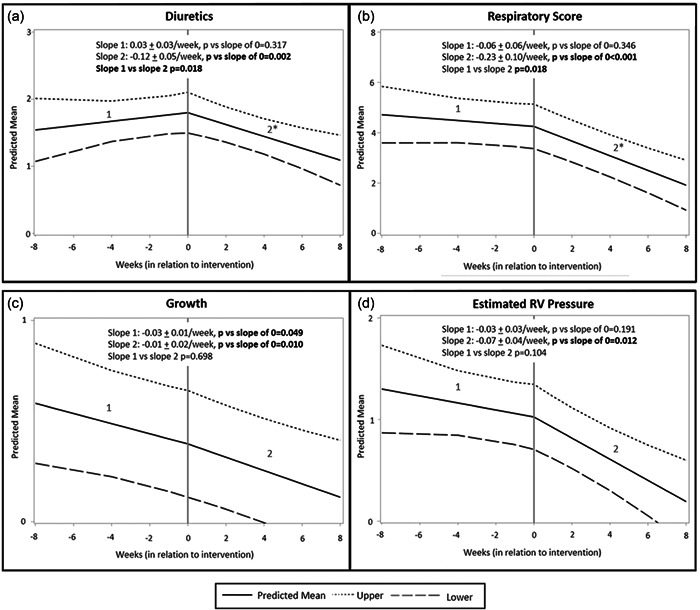
Regression analysis with 95% interval lines fit for each subcomponent of the bronchopulmonary dysplasia (BPD)‐atrial septal defect (ASD) scoring system was performed with the only breakpoint at the time of intervention. (a) The pre‐intervention diuretic score was not significantly different than a slope of 0. The post‐intervention diuretic score slope was significantly different than a slope of 0 (decreasing) and the post‐intervention score slope was significantly different than the pre‐intervention diuretic score slope (p = 0.018). (b) Respiratory score consisted of the total points assigned for FiO2 need, respiratory symptoms, and respiratory support. The pre‐intervention respiratory score slope was not significantly different than 0. The post‐intervention respiratory score slope was significantly different than 0 (decreasing) and the post‐intervention score slope was significantly different than the pre‐intervention respiratory score slope. (c) The growth score slope was significantly different than 0 (decreasing) for both pre‐ and post‐intervention and there was no significant difference between slope 1 and slope 2. (d) The pre‐intervention estimated RV pressure score slope was no different than 0, but the post‐intervention estimated RV pressure score was significantly different than 0, indicating a significant decrease in estimated RV pressure score. However, there was no difference between pre‐ and post‐intervention slopes. *p ≤ 0.05.
DISCUSSION
Our study demonstrates improvement in patient clinical status after ASD closure in patients ≤ 10 kg with BPD. We also demonstrate the utility of a novel BPD‐ASD scoring system that allows for more objective assessment of this patient cohort over time. By demonstrating a significant improvement in the slope of the BPD‐ASD score after ASD closure, early ASD closure appears to have significant benefit in this particular population. This improvement was most prominent in the first‐week post‐intervention, and continued up to 4 weeks post‐intervention. When a subanalysis of the components used in the BPD‐ASD score was performed, significant improvement in diuretic use and a respiratory status was demonstrated. The slope of estimated RV pressure by echocardiogram for pre‐ and post‐intervention was not significantly different, though the post‐intervention slope was significantly different when compared with no change at all. This suggests that perhaps with more patients, the pre‐ and post‐intervention estimated RV pressure slope might result in a significant difference. In addition, many patients had a very prolonged length of stay and ASD closure might have expedited their ability to discharge home by being transitioned from intravenous to oral diuretics and to respiratory support compatible with discharge home. Three of four patients being considered for tracheostomy were able to avoid a tracheostomy after ASD closure.
Several single‐center descriptive studies have demonstrated improvement in the respiratory status of BPD patients with ASD who undergo secundum ASD closure both surgically and by catheterization. 23 , 24 , 25 , 27 , 28 , 29 , 30 In a recent retrospective case–control study, six patients who underwent transcatheter occlusion of their ASD demonstrated a faster rate of improvement in their BPD severity compared with those patients who did not undergo ASD closure. 22 Nevertheless, all studies to date have been low‐powered and mostly descriptive in nature. Our study adds to the literature by demonstrating that the improvement in clinical status is expedited after ASD closure is performed rather than just secondary to the natural improvement that can occur in BPD patients and that this improvement seems to be primarily secondary to improved respiratory and diuretic status. In addition, this benefit seems to be seen immediately and up to 4 weeks post‐intervention.
Remarkably, the median pre‐procedure hospital length of stay was 210 days and median post‐procedure length of stay was 42.5 days. While this may imply that closure of these patients' ASDs was performed late in the patients' hospitalization and that they would be discharged in the same timeframe despite the ASD closure, given that 9 out of 10 of the patients were on non‐dischargeable respiratory support at the time of catheterization, one could possibly infer that the ASD closure expedited discharge home by allowing transition to respiratory support suitable for discharge. It is unclear if the total length of stay would have been shorter if secundum ASD closure was performed sooner than the median corrected gestational age and weight of 54 weeks and 6145 g.
Another finding was the significant difference in the slope of estimated RV pressure after ASD closure compared with a slope that would be reflective of no improvement at all. This is consistent with previous data described in which pulmonary hypertension improved after ASD closure. 23 , 30 , 35 Vyas Read demonstrated a 2.44‐fold higher prevalence of pulmonary hypertension 36 in 57 patients and Choi demonstrated an odds ratio of 3.8 for the presence of pulmonary hypertension in an atrial shunt group compared with non‐atrial shunt group. 35 This does not answer the question as to whether the ASD is an aggravator for pulmonary hypertension or just associated with pulmonary hypertension in these patients, but given that multiple studies, including ours, have demonstrated improvement in pulmonary hypertension after ASD closure, it could possibly be inferred that ASDs in these patients contribute to, rather than relieve, pulmonary hypertension. In addition, of the six patients who underwent vasodilator testing during catheterization, only two had improvement in percent RV pressure more than 10% while the other four had minimal or no change to the right ventricle pressure despite an improvement in PVRi. Given the improvement in PVRi and minimal change in RVSP, this indicates that the extra volume to the right heart from an ASD in a patient with BPD may be a contributor to elevated pulmonary artery pressures in some of these patients. Answering this question is important, as pulmonary hypertension in the presence of BPD is associated with higher rates of mortality 37 , 38 , 39 , 40 with as high of an odds ratio as 3.15 compared with those patients without pulmonary hypertension, 37 in addition to a 3.8‐fold increased risk for tracheostomy. 41 Notably, all patients in our study had between 43% and 68% systemic RVSP at the time of cardiac catheterization with Qp:Qs ranging from 1.1 to 2.4 at baseline. Even the patients with relatively lower Qp:Qs demonstrated improvement in their BPD‐ASD score and were able to be transitioned to respiratory support appropriate for home. This implies that even with a relatively low Qp:Qs by cardiac catheterization, due to the reduced vascular surface area and the overall fragile nature of their lungs, 10 patients may still benefit from ASD closure. This finding is consistent with other studies that have had similar findings. 23 , 24 Lastly, no patient suffered mortality or had a pulmonary hypertension event requiring the ASD as a “pop‐off” after the ASD closure.
All of our patients met the published American Heart Association pediatric pulmonary hypertension guidelines that ASD closure should be considered if PVRi is < 6 Wood‐units.m2 and pulmonary vascular resistance to systemic vascular resistance ratio is < 0.3 at baseline or if there is PVRi reversibility to < 6 Wood‐units.m2 with a pulmonary vascular resistance to systemic vascular resistance ratio of < 0.3. However, this recommendation is for patients with normal lung development and does not account for the unique myocardium, vascularity, and alveolar development of premature infants with BPD 10 who may benefit from a more aggressive approach to ASD closure. While “small” ASDs are typically not considered for closure, this is only in patients “with no other risk factors” per the AHA Scientific Statement, 42 which would not apply to the BPD patient population. Risk factors in BPD are myriad in a disease that is hallmarked by decreased angiogenesis and alveolarization and abnormal vascular function and structure, leading to decreased alveolar‐capillary surface area and exaggerated effects of left to right shunts. The decreased vessel number in addition to the abnormal vessel function and structure can lead to leaky capillary vessels and pulmonary hypertension with even the slightest extra pulmonary blood flow. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 Additionally, the vessels are hyper‐sensitive to hemodynamic stress, which can cause long‐term endothelial injury, smooth muscle cell proliferation, precocious maturation of immature mesenchymal cells into mature smooth muscle cells, and the incorporation of fibroblasts/myofibroblasts into the vessel wall, all contributing to pulmonary hypertension development. 1 Outside of the studies already mentioned, studies have also been published demonstrating abnormal left ventricle structure and diastology 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 in premature infants with BPD. Certainly elevated left ventricular filling pressures could also affect the development of pulmonary hypertension and the development of increased capillary pressure leading to a “leaky” pulmonary bed and therefore lower tolerance of excess pulmonary blood flow. The pathophysiology of pulmonary hypertension and respiratory compromise is different than in an idiopathic pulmonary hypertension patient and should be taken into consideration when considering treatment plans for BPD patients.
The catheterization risks such as vascular injury, device embolization, device erosion, and arrhythmia, 42 coupled with the surgical risks such as pericardial effusion, arrhythmia, and infection 43 must be considered when determining patient suitability for ASD closure. Fortunately, we had no complications with any of the catheterizations or the surgical procedure. This is consistent with a total of 46 combined patients with a weight under 10 kg from previously published studies having no complications with catheterization‐based closure 23 , 24 , 28 and 111 combined patients under 10 kg from previously published studies having minimal complications with surgical ASD closure. 27 , 29 , 30
Our study is limited by the single‐center nature of this work. Additionally, this institution has the robust support of a pulmonary hypertension association accredited center of comprehensive care that may not be generalizable to all centers. The study was retrospective with associated limitations, including limited follow‐up data on some patients and varying approaches to the use of pulmonary vasodilators, feeds, and diuretics by care teams. The number of patients included in the study is few due to the rarity of the procedure, making extensive statistical analysis difficult. The developed BPD‐ASD scoring system is limited in its ability to assess ASD closure in patients with tracheostomy, as many markers of improvement, such as rate, positive end‐expiratory pressure, and peek inspiratory pressure, are not included in the scoring system. Modifications to account for this limitation are something to consider moving forward with the scoring system.
In conclusion, our study demonstrated improvement in BPD‐ASD clinical score status primarily through improved respiratory score and diuretic use that is attributable to closure of an ASD in BPD patients less than 10 kg. There is also some improvement in estimated RV pressure by echocardiogram in BPD patients who underwent closure of a secundum ASD. While the risks/benefits of ASD closure must be weighed in this population, when selected appropriately, these patients appear to have improvement in their clinical course. Future studies to validate the scoring system should be performed in a larger multicenter population and also with a comparison cohort.
AUTHOR CONTRIBUTIONS
Melissa K. Webb: Conceptualization; methodology; investigation; formal analysis; data curation; writing—original draft; writing—review & editing; supervision; project administration. Milenka Cuevas Guaman: Methodology; investigation; data curation; writing—original draft; writing—review & editing. S. Kristen Sexson Tejtel: Conceptualization; methodology; investigation; formal analysis; writing—original draft; writing—reviewing & editing. Neil Cambronero: Conceptualization; investigation; data curation; formal analysis; writing—review & editing. Ryan D. Coleman: Conceptualization; investigation; data curation; formal analysis; writing— review & editing. Corey A. Chartan: Conceptualization; investigation; data curation; formal analysis; writing—review & editing. Betul Yilmaz Furtun: Conceptualization; investigation; data curation; writing—review & editing. Shaine A. Morris: Conceptualization; methodology; investigation; formal analysis; writing—review & editing. Nidhy P. Varghese: Conceptualization; methodology; investigation; data curation; writing—original draft; writing—review & editing. Natalie M. Villafranco: Conceptualization; methodology; investigation; supervision/oversight; data curation; formal analysis; writing—original draft; writing—reviewing & editing.
CONFLICTS OF INTEREST STATEMENT
Dr. Varghese receives royalties as an UptoDate reviewer; receives honoraria from Practice Point Communications as a speaker; receives research support from Janssen Pharmaceutical company; and receives an honoraria from the Pulmonary Hypertension Association for Professional development. Dr. Morris served on the scientific advisory board for Aytu Biopharma for a clinical trial for Vascular Ehlers‐Danlos syndrome until November 2022. Dr. Sexson Tejtel served on the Sobi scientific advisory board in 2022 and was provided a paid trip through the company regarding inflammatory heart conditions. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the Baylor College of Medicine (H‐50051). Informed consent was not required for inclusion of patient data in the study due to the retrospective nature of the study. All efforts were made to limit risk of data disclosure, including information being deidentified and stored on a secure workstation in a password‐protected system only accessible to the investigators. No contact with the patient or intervention in their healthcare was performed.
ACKNOWLEDGMENTS
The authors have no funding to report.
Webb MK, Cuevas Guaman M, Sexson Tejtel SK, Cambronero N, Coleman RD, Chartan CA, Yilmaz Furtun B, Morris SA, Varghese NP, Villafranco NM. Atrial septal defect closure is associated with improved clinical status in patients ≤ 10 kg with bronchopulmonary dysplasia. Pulm Circ. 2023;13:e12299. 10.1002/pul2.12299
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in FigShare at https://doi.org/10.6084/m9.figshare.23800728.
REFERENCES
- 1. Mourani PM, Abman SH. Pulmonary hypertension and vascular abnormalities in bronchopulmonary dysplasia. Clin Perinatol. 2015;42:839–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lassus P, Turanlahti M, Heikkilä P, Andersson LC, Nupponen I, Sarnesto A, Andersson S. Pulmonary vascular endothelial growth factor and Flt‐1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med. 2001;164(10Pt1):1981–1987. [DOI] [PubMed] [Google Scholar]
- 3. Kumar H. Growth factors in the fetal and neonatal lung. Front Biosci. 2004;9:464–480. [DOI] [PubMed] [Google Scholar]
- 4. D'Angio T. The role of vascular growth factors in hyperoxia‐induced injury to the developing lung. Front Biosci. 2002;7:d1609–d1623. [DOI] [PubMed] [Google Scholar]
- 5. Thébaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med. 2007;175:978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H. Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol. 2002;282:L811–L823. [DOI] [PubMed] [Google Scholar]
- 7. Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L600–L607. [DOI] [PubMed] [Google Scholar]
- 8. Thébaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, Archer SL. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in Hyperoxia‐Induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation. 2005;112:2477–2486. [DOI] [PubMed] [Google Scholar]
- 9. Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, FLT‐1, and TIE‐2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;164(10Pt1):1971–1980. [DOI] [PubMed] [Google Scholar]
- 10. Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, Hanna BD, Rosenzweig EB, Raj JU, Cornfield D, Stenmark KR, Steinhorn R, Thébaud B, Fineman JR, Kuehne T, Feinstein JA, Friedberg MK, Earing M, Barst RJ, Keller RL, Kinsella JP, Mullen M, Deterding R, Kulik T, Mallory G, Humpl T, Wessel DL. Pediatric pulmonary hypertension guidelines from the American Heart Association and American thoracic society. Circulation. 2015;132:2037–2099. [DOI] [PubMed] [Google Scholar]
- 11. Mourani PM, Ivy DD, Rosenberg AA, Fagan TE, Abman SH. Left ventricular diastolic dysfunction in bronchopulmonary dysplasia. J Pediatr. 2008;152(2):291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bokiniec R, Własienko P, Borszewska‐Kornacka M, Szymkiewicz‐Dangel J. Evaluation of left ventricular function in preterm infants with bronchopulmonary dysplasia using various echocardiographic techniques. Echocardiography. 2017;34:567–576. [DOI] [PubMed] [Google Scholar]
- 13. Di Maria MV, Younoszai AK, Sontag MK, Miller JI, Poindexter BB, Ingram DA, Abman SH, Mourani PM. Maturational changes in diastolic longitudinal myocardial velocity in preterm infants. J Am Soc Echocardiogr. 2015;28(9):1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aldana‐Aguirre JC, Eckersley L, Hyderi A, Hirose A, Boom J, Kumaran K, Hornberger LK. Influence of extreme prematurity and bronchopulmonary dysplasia on cardiac function. Echocardiography. 2021;38:1596–1603. [DOI] [PubMed] [Google Scholar]
- 15. Helfer S, Schmitz L, Bührer C, Czernik C. Tissue doppler‐derived strain and strain rate during the first 28 days of life in very low birth weight infants. Echocardiography. 2014;31:765–772. [DOI] [PubMed] [Google Scholar]
- 16. Desa DJ. Myocardial changes in immature infants requiring prolonged ventilation. Arch Dis Child. 1977;52:138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Melnick G, Pickoff AS, Ferrer PL, Peyser J, Bancalari E, Gelband H. Normal pulmonary vascular resistance and left ventricular hypertrophy in young infants with bronchopulmonary dysplasia: an echocardiographic and pathologic study. Pediatrics. 1980;66(4):589–596. [PubMed] [Google Scholar]
- 18. Sullivan RT, Tandel MD, Bombal S, et al. Role of left atrial hypertension associated with bronchopulmonary dysplasia. Front Pediatr. 2022. 10.3389/f[ed/2-22/1012136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xie L, Chee Y, Wong K, Cheung Y. Cardiac mechanics in children with bronchopulmonary dysplasia. Neonatology. 2016;109(1):44–51. [DOI] [PubMed] [Google Scholar]
- 20. Yates AR, Welty SE, Gest AL, Cua CL. Myocardial tissue doppler changes in patients with bronchopulmonary dysplasia. J Pediatr. 2008;152:766–770. [DOI] [PubMed] [Google Scholar]
- 21. Kumar KR, Clark DA, Kim EM, Perry JD, Wright K, Thomas SA, Thompson EJ, Greenberg RG, Smith PB, Benjamin DK, Laughon MM, Clark RH, Hornik CP. Association of atrial septal defects and bronchopulmonary dysplasia in premature infants. J Pediatr. 2018;202(11):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haregu F, McCulloch M, Vergales B, Garrod A, Conaway M, Hainstock M. Transcatheter occlusion of left‐To‐right shunts in premature infants with bronchopulmonary dysplasia. Neonatology. 2023;120(1):57–62. [DOI] [PubMed] [Google Scholar]
- 23. Yung D, Jackson EO, Blumenfeld A, Redding G, DiGeronimo R, McGuire JK, Riker M, Tressel W, Berkelhamer S, Eldredge LC. A multidisciplinary approach to severe bronchopulmonary dysplasia is associated with resolution of pulmonary hypertension. Front Pediatr. 2023;11:1077422. 10.3389/fped.2023.1077422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thomas VC, Vincent R, Raviele A, Diehl H, Qian H, Kim D. Transcatheter closure of secundum atrial septal defect in infants less than 12 months of age improves symptoms of chronic lung disease. Congenit Heart Dis. 2012;7:204–211. [DOI] [PubMed] [Google Scholar]
- 25. Lim DS, Matherne GP. Percutaneous device closure of atrial septal defect in a premature infant with rapid improvement in pulmonary status. Pediatrics. 2007;119:398–400. [DOI] [PubMed] [Google Scholar]
- 26. Zussman ME, Freire G, Cupp SD, Stapleton GE. Closure of a secundum atrial septal defect in two infants with chronic lung disease using the Gore HELEX septal occluder. Cardiol Young. 2016;26:79–83. [DOI] [PubMed] [Google Scholar]
- 27. Lammers A, Hager A, Eicken A, Lange R, Hauser M, Hess J. Need for closure of secundum atrial septal defect in infancy. J Thorac Cardiovasc Surg. 2005;129(6):1353–1357. [DOI] [PubMed] [Google Scholar]
- 28. Wyss Y, Quandt D, Weber R, Stiasny B, Weber B, Knirsch W, Kretschmar O. Interventional closure of secundum type atrial septal defects in infants less than 10 kilograms: indications and procedural outcome. J Interv Cardiol. 2016;29:646–653. [DOI] [PubMed] [Google Scholar]
- 29. Charisopoulou D, Bini RM, Riley G, Janagarajan K, Moledina S, Marek J. Repair of isolated atrial septal defect in infants less than 12 months improves symptoms of chronic lung disease or shunt‐related pulmonary hypertension. Cardiol Young. 2020;30:511–520. 10.1017/S1047951120000463 [DOI] [PubMed] [Google Scholar]
- 30. Tsuda T, Davies RR, Radtke W, Pizarro C, Bhat AM. Early surgical closure of atrial septal defect improves clinical status of symptomatic young children with underlying pulmonary abnormalities. Pediatr Cardiol. 2020;41:1115–1124. 10.1007/s00246-020-02361-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Choi YJ, Stoecklin B, Hemy NR, Hall GL, Doherty DA, Simpson SJ, Pillow JJ. Pulmonary gas exchange improves over the first year in preterm infants with and without bronchopulmonary dysplasia. Neonatology. 2021;118(1):98–105. [DOI] [PubMed] [Google Scholar]
- 32. Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, Kirpalani H, Laughon MM, Poindexter BB, Duncan AF, Yoder BA, Eichenwald EC, DeMauro SB. The diagnosis of bronchopulmonary dysplasia in very preterm infants. an Evidence‐Based approach. Am J Respir Crit Care Med. 2019;200(6):751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chou JH, Roumiantsev S, Singh R. PediTools electronic growth chart calculators: applications in clinical care, research, and quality improvement. J Med Internet Res. 2020;22(1):e16204. 10.2196/16204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Allen HD, Driscoll DJ, Shaddy RE, et al. Moss and Adams' heart disease: in infants, children, and adolescents, including the fetus and young adult. 8th ed. Philadelphia: Lippincott Willis & Wilkins; 2013. [Google Scholar]
- 35. Choi EK, Jung YH, Kim HS, Shin SH, Choi CW, Kim EK, Kim BI, Choi JH. The impact of atrial left‐to‐right shunt on pulmonary hypertension in preterm infants with moderate or severe bronchopulmonary dysplasia. Pediatr Neonatol. 2015;56:317–323. [DOI] [PubMed] [Google Scholar]
- 36. Vyas‐Read S, Guglani L, Shankar P, Travers C, Kanaan U. Atrial septal defects accelerate pulmonary hypertension diagnoses in premature infants. Front Pediatr. 2018;6:342. 10.3389/fped.2018.00342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lagatta JM, Hysinger EB, Zaniletti I, Wymore EM, Vyas‐Read S, Yallapragada S, Nelin LD, Truog WE, Padula MA, Porta NFM, Savani RC, Potoka KP, Kawut SM, DiGeronimo R, Natarajan G, Zhang H, Grover TR, Engle WA, Murthy K. The impact of pulmonary hypertension in preterm infants with severe bronchopulmonary dysplasia through 1 year. J Pediatr. 2018;203:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. DeVries LB, Heyne RJ, Ramaciotti C, Brown LS, Jaleel MA, Kapadia VS, Burchfield PJ, Brion LP. Mortality among infants with evolving bronchopulmonary dysplasia increases with major surgery and with pulmonary hypertension. J Perinatol. 2017;37:1043–1046. [DOI] [PubMed] [Google Scholar]
- 39. Bhat R, Salas AA, Foster C, Carlo WA, Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics. 2012;129:e682–e689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, Mullen MP. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120(6):1260–1269. [DOI] [PubMed] [Google Scholar]
- 41. Murthy K, Savani RC, Lagatta JM, Zaniletti I, Wadhawan R, Truog W, Grover TR, Zhang H, Asselin JM, Durand DJ, Short BL, Pallotto EK, Padula MA, Dykes FD, Reber KM, Evans JR. Predicting death or tracheostomy placement in infants with severe bronchopulmonary dysplasia. J Perinatol. 2014;34:543–548. [DOI] [PubMed] [Google Scholar]
- 42. Feltes TF, Bacha E, Beekman RH, Cheatham JP, Feinstein JA, Gomes AS, Hijazi ZM, Ing FF, de Moor M, Morrow WR, Mullins CE, Taubert KA, Zahn EM. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American heart association. Circulation. 2011;123:2607–2652. [DOI] [PubMed] [Google Scholar]
- 43. Galal MO, Wobst A, Halees Z, Hatle L, Schmaltz AA, Khougeer F, De Vol E, Fawzy ME, Abbag F, Fadley F, Duran CMG. Peri‐operative complications following surgical closure of atrial septal defect type II in 232 patients—a baseline study. Eur Heart J. 1994;15:1381–1384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in FigShare at https://doi.org/10.6084/m9.figshare.23800728.


