Abstract
Venous leg ulcers (VLU) represent a major public health challenge. Little is known about the prevalence and incidence of VLU internationally. Published studies are usually reporting different estimates because of disparities in study designs and measurement methods. Therefore, we conducted a systematic literature review and meta‐analysis to identify the prevalence and incidence of VLU internationally and to characterise the population as reported in these studies. Studies were identified from searches in Medline (PubMed), CINAHL Complete (EBSCOhost), Embase, Scopus, Web of Science, LiSSa (Littérature Scientifique en Santé), Google Scholar and Cochrane Database of Systematic Reviews up to November 2022. Studies were included if their primary outcomes were reported as a period prevalence or point prevalence or cumulative incidence or incidence VLU rate. Fourteen studies met the inclusion criteria, 10 reporting estimates of prevalence, three reporting both prevalence and incidence estimates and one incidence. All were included in meta‐analyses. The results show a pooled prevalence of 0.32% and a pooled incidence of 0.17%. Our results highlighted an extreme heterogeneity across effect sizes for both prevalence and incidence, which prevent a meaningful interpretation of pooled indexes and argue for further studies with specific prevalence‐type reported and target population under study.
Keywords: incidence, meta‐analysis, prevalence, systematic review, venous leg ulcer
1. INTRODUCTION
Venous leg ulcers (VLU) are a major clinical challenge and the result of chronic venous insufficiency (CVI) and venous hypertension. 1 They manifest on the lower limb and represent between 60% and 80% of all leg ulcerations. 2 , 3 Their three‐months healing rate is estimated at 40% 4 and once healed up to 80% of patients develop a recurrence within 3 months. 5 The prevalence of VLUs is are reported around 1.08% 6 and the incidence being up to 1.33%. 7 The latter numbers are primarily based on estimates because of the lack of clinical registries for VLU. 9 The prevalence and incidence of VLU increase with age. 8 Age does negatively affect healing and recurrence 9 as well as treatment adherence. 10 People with VLUs often report having reduced health‐related quality of life because these wounds can be painful, malodorous, and exuding. 11 , 12
VLUs continue to be of international as well as local concern. Despite proper care, up to 20% of VLUs would not heal after 2 years. 13 , 14 , 15 They represent a considerable social and economic burden, with an estimated annual cost of £102 million sterling in the UK, 16 $32 billion in the United States, 17 and $3 billion in Australia AUD$ 3. 18 Despite this burden, there is no international systematic collation and review of existing prevalence and incidence studies. Such information on the epidemiology of VLU are necessary to inform decision‐making by health services to establish best strategies for prevention and management of VLU.
We therefore conducted a systematic literature review and meta‐analysis to identify the prevalence and incidence of VLU internationally and to characterise the population as reported in these studies. The following research questions were addressed:
What is the prevalence of VLUs for different settings according to internationally published studies?
What is the incidence of VLUs for different settings according to internationally published studies?
What are the determinants of VLUs in different settings as reported in these studies?
2. METHODS AND MATERIALS
2.1. Design
This systematic review was conducted following the Joanna Briggs Institute (JBI) methodology for prevalence and incidence systematic reviews. 19 , 20 the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement 21 were followed to report the research and structure of this manuscript. A review protocol was registered on PROSPERO international prospective register of systematic reviews (University of York, UK) on 15 October 2020 as (CRD42020205855) and published. 22
2.2. Eligibility criteria
Eligibility criteria were defined using the COCOPOP (Condition, Context, Population) approach of the JBI for incidence and prevalence systematic reviews. 23
2.2.1. Condition
The main variables of interest were the “prevalence” and/or the “incidence” of VLU's. VLU's had to be clinically diagnosed based on symptoms and/or examination such as by Doppler ultrasonography. We excluded studies reporting a prevalence or incidence of other chronic wounds such as arterial ulcer, diabetic foot ulcer, pressure ulcer, burns or surgical wounds.
2.2.2. Context
Studies conducted in any setting or context were included.
2.2.3. Participants
Studies were included if the VLU population was 18 years of age and older and the VLU diagnosis was reported within the study as well as if the primary outcomes were reported as a period prevalence or point prevalence or cumulative incidence or incidence rate of VLU.
2.3. Types of studies
Observational studies such as cohort studies, case control studies, cross sectional studies, intervention studies, regardless of language, sample size or year of publication, were eligible for inclusion. Excluded studies were editorials, letters, case studies, case series and animal studies.
2.4. Information sources and search strategy
A systematic search was performed up to June 16th 2021 in the following electronic bibliographic databases: Medline (via PubMed), Cumulative Index to Nursing and Allied Health Literature (CINAHL Complete, EBSCOhost), Embase, Scopus, Web of Science, LiSSa (Littérature Scientifique en Santé), Google Scholar, and Cochrane Database of Systematic Reviews. Search strategies were adapted in English or French according to the database. On November 9th 2022, an update was conducted to check for additional potential articles. No time limits or language restrictions were applied during the screening phase.
The search strategy was designed and conducted in collaboration with an experienced reference librarian of the HES‐SO University of Applied Sciences and Arts Western Switzerland, Geneva (MP) in consultation with the authors. The Peer Review of Electronic Search Strategies (PRESS) 2015 Guideline Statement 24 was used to guide the electronic literature search strategies. We applied controlled vocabulary (eg. Medical Subject Headings terms) with key words both in full and in various truncations to construct a comprehensive set of possible search terms for each concept. The defined terms were searched in the title, or title and abstract, or keywords of the publications. Boolean operators and proximity operators, including wildcards, AND, OR, parentheses, and quotations for each data base were used. The search terms, the complete search strategies and their results for each database are outlined in Table 1.
TABLE 1.
Search terms and – strategy.
| Full search strategies MEDLINE via PubMed | |
| (“venous leg ulcer*”[Title/Abstract] OR (“varicose ulcer”[MeSH Terms] AND “leg ulcer”[MeSH Terms])) AND (“prevalence”[Title] OR “incidence”[Title] OR “occurrence”[Title] OR “epidemiolog*”[Title]) | |
|
Filters: none Remarks: none |
Number of articles: 57 |
| Date: 10.06.2021 | |
| Full search strategies CINAHL | |
| (TI “venous leg ulcer*” OR AB “venous leg ulcer*” OR ((MH “Venous Ulcer”) AND (MH “Leg ulcer”))) AND (TI prevalence OR TI incidence OR TI occurrence OR TI epidemiolog*) | |
|
Filters: none Remarks: none |
Number of articles: 22 |
| Date: 10.06.2021 | |
| Full search strategies Embase | |
| (‘varicosis’/de AND ‘leg ulcer’/de) OR (‘venous leg ulcer*’:ti,ab,kw) AND (prevalence:ti OR incidence:ti OR occurrence:ti OR epidemiolog*:ti OR ‘prevalence’/exp/mj OR ‘ulcer incidence’/exp) | |
|
Filters: none Remarks: ‘venous leg ulcer*’:ti,ab,kw: search Title, Abstract, et author keywords Prevalence/exp/mj: search with exploded and major topic Incidence/exp: search with exploded term |
Number of articles: 72 |
| Date: 10.06.2021 | |
| Full search strategies Web of Science | |
| (TS = [“venous leg ulcer*”]) AND TI = (prevalence or incidence or occurrence or epidemiolog*): | |
|
Filters: none Remarks: TS corresponds to the search in Title, Abstract, AND other keywords |
Number of articles: 37 |
| Date: 14.06.2021 | |
| Full search strategies Cochrane Database of systematic reviews | |
| (Venous leg ulcer* OR [varicose ulcer AND venous leg]):ti,ab,kw AND (prevalence or incidence or occurrence or epidemiolog*):ti | |
|
Filters: none Remarks: same results ans same references if using only ‘venous leg ulcer’ |
Number of articles: 3 |
| Date: 16.06.2021 | |
| Full search strategies LiSSa | |
| ((ulcère variqueux.tl) OU (ulcère variqueux.mc) OU (ulcère veineux.tl) OU (ulcère veineux.mc)) ET ((ulcère de la jambe.tl) OU (ulcère de la jambe.mc)) ET ((prévalence.ti) OU (incidence.ti) OU (occurrence.ti) OU (epidemiolog*.ti)) | |
|
Filters: none Remarks: ((ulcère variqueux.tl) OU (ulcère variqueux.mc) OU (ulcère veineux.tl) OU (ulcère veineux.mc)) ET ((ulcère de la jambe.tl) OU (ulcère de la jambe.mc)): recherche dans Titre, mots‐clés et résumé |
Number of articles: 4 |
| Date: 14.06.2021 | |
| Full search strategies Scopus | |
| (TITLE‐ABS‐KEY [“venous leg ulcer*” OR “varicose ulcer” AND “leg ulcer”]) AND (TITLE [“prevalence” OR “incidence” OR “occurrence” OR “epidemiolog*”]) | |
|
Filters: none Remarks: none |
Number of articles: 54 |
| Date: 16.06.2021 | |
| Full search strategies Google Scholar | |
| “venous leg ulcer” intitle:prevalence|incidence|occurrence|epidemiology | |
|
Filters: none Remarks: | corresponds to the operator OR No *, as it does not have quite the same function as in other tools and google scholar recognises plurals of nouns and some derived terms automatically |
Number of articles: 103 |
| Date: 14.06.2021 | |
2.5. Selection process
All references were imported into a single EndNote library version X9 file and deduplicated. To facilitate the screening process, the remaining references were then exported from the EndNote Library into the Rayyan platform. 25 Two authors (CS and GG) independently screened the titles and abstracts for those matching the eligibility criteria. We then retrieved the full‐texts of the relevant eligible studies. The same authors independently assessed the full texts for study characteristics. The excluded studies were listed in a table including the reason for exclusion. We resolved any discrepancies between the reviewers involving a third reviewer (SP).
2.6. Data extraction & risk of bias assessment
Two reviewers (CS, GG) extracted and managed independently the study details by using an electronic data collection form developed by SP, PB and MP. The following information was extracted: Study details (e.g., study title, authors, year of publication and journal), study method (e.g., aim, setting, study design, outcomes and method of data analysis), and results (eg. prevalence n/N (%) or (‰), proportion and 95% confidence intervals (CI), incidence n/N (‰), proportion and 95% CI, duration of the recruitment or the study, and population characteristics). If studies reported prevalence's and/or incidences of various wound aetiologies, we only extracted the data specific to VLUs. We contacted the authors for data that were unclear or missing. Any disagreements between the reviewers were resolved through discussion by involving a third reviewer (PB).
Risk of bias assessment was conducted by two independent reviewers (CS, GG). A third reviewer (SP) was consulted for any disagreements. We used the JBI quality appraisal tools depending on the study design 26 to assess the methodological quality. For each study, the percentage of “Yes” was calculated on applicable questions. The average percentage of “Yes” was calculated for all included studies as well. In addition, the percentage of “Yes” was calculated for each question of the check list when applicable.
2.7. Statistical methods
An initial random effect meta‐analysis of the prevalence and incidences effect sizes were conducted with the R package meta. Random effect meta‐analysis is the gold standard in the field when several true effects are suspected to exist in the population. Pooled effects were estimated, together with 95% Confidence Intervals and 95% Prediction Intervals. Heterogeneity between effects sizes was assessed using the Cochran Q test and the I 2 statistic. Cochran Q test allows us to test the null hypothesis of no heterogeneity. I 2 statistic accounts for the percentage of variability in the effect sizes not due to sampling error. An I 2 value above 75% accounts for a substantial heterogeneity. 27
The meta‐analyses were performed with double arcsine transformations to stabilise the variance. 28 The results were then back transformed for interpretation. Moderator's analyses were performed for prevalence to assess the explanatory role of several predictors in the observed heterogeneity between effect sizes. Those predictors were: the nature of the population (VLU people in a regional sample, VLU patients among the total number of patients receiving care or VLU patients among the residents of a region), the type of prevalence reported (point or period prevalence), the way VLU case was ascertained (clinical assessment or health records [no wound inspection]), the origin of the sample (classified in two categories, Europe and non‐Europe), the year of publication, the mean age of the sample and the percentage of women present in the sample. No moderators' analyses were performed for the incidences due to the very low number of papers included in the analysis.
2.8. Changes to the protocol
Some minor changes were made to the published protocol. An updated version of EndNote library X9 and not X8 as declared in the protocol was used. We had also a change in the persons who conducted the selection process (CS and GG) and we adapted the statistical test according to the publication of Barendregt et al. 28 Additional information not mentioned in the protocol, i.e. “country” and “population characteristics” were extracted from the studies. Quality assessment of the studies was performed using the JBI critical appraisal tools for studies reporting prevalence data instead of the GRADE assessment tool.
3. RESULTS
3.1. Study selection
The systematic literature search yielded a total of 354 publications, leading to 188 articles for assessment for full‐text review. One hundred and forty‐three were excluded after full‐text review. Of these, seven were excluded because of being a wrong population, not an original article (n = 11) or the general leg ulcer prevalence and/or incidence numbers (n = 6) (see flow diagram Figure 1). Therefore, we included in this systematic review, 14 studies that reported prevalence estimates and/or incidence estimates (Tables 2 and 3). Nearly all included studies were in English (12/14). Two studies were in German, but as the first author is a native German speaker, there was no needed for any language support.
FIGURE 1.
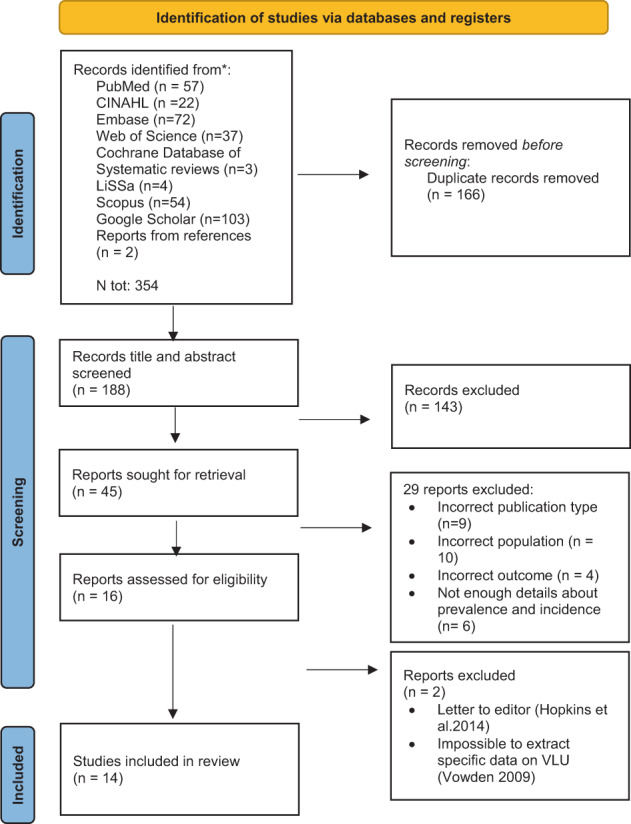
Prisma flow chart.
TABLE 2.
Venous leg ulcer prevalence: characteristics of included studies.
| Author, year, Journal | Country | Aim | Data collection | Age (mean[SD]) | Sex (% women) | Outcome ascertainment | Prevalence type (duration of data collection) | N population | n VLU | VLU prevalence in % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| VLU patients among the residents of a region | ||||||||||
| Vowden et al., 2009, Journal of Tissue Viability | United Kingdom | Prevalence, management and characteristics of leg ulcers in Bradford population | Survey to care providers | 75.3 (14.9) | 57 | Patients records, no wound inspection | Point prevalence (6 d) | 487 975 | 195 | 0.04 (0.035‐0.046) |
| Moffatt et al., 2004, QJM An International Journal of Medicine | United Kingdom | Prevalence and cause of leg ulcers in a London population | Questionnaire to health professionals | 75 (na), 31‐94 a | 64 | Clinical assessment by trained research nurses (Doppler [ABPI>0.8], Duplex, photoplethysmography, open ≥4 wks) | Point prevalence (4 wks) | 252 463 | 49 b | 0.02 b (0.014‐0.026) |
| Srinivasaiah et al., 2007, Journal of Wound Care | United Kingdom | Prevalence, management and characteristics of wounds in north‐east England | Data collection in health establishments by a study team | na | na | Patients records and verbal feedbacks, no wound inspection | Point prevalence (2 d) | 590 000 | 260 | 0.04 b (0.039‐0.05) |
| VLU patients and self‐treating people in a regional sample | ||||||||||
| Baker et al., 1991, British Journal of Surgery | Australia | Prevalence and characteristics of venous leg ulcers in a Perth metropolitan population | Recruitment by referral from care providers and by self‐referral (via local newspapers) | 75 (na) c , 20‐99 a | 64.5 | Clinical assessment by study team (photoplethysmography, Doppler [ABPI>0.9], open ≥4 wks) | Point prevalence (3 mos) | 238 000 | 148 b | 0.06 (0.053‐0.073) |
| Rabe et al., 2003, Phlebologie | Germany | Prevalence and severity of CVD in urban and rural Bonn residential population | Questionnaire to random a sample of adults | 48 (16) | 56 | Clinical assessment by physicians and study team (CEAP, Doppler, Duplex) | Point prevalence (16 mos) | 3072 | 3 | 0.10 (0.02‐0.285) |
| Zolotukhin et al., 2017, European Journal of Vascular and Endovascular Surgery | Russia | Prevalence and risk factors of CVD in a rural community of central Russia | Invitation to participate to all community residents | 53.5 (17.8) | 63 | Clinical assessment by vascular surgeons and study team (CEAP, Duplex) | Point prevalence (2 mos) | 703 | 1 | 0.14 (0.004‐0.79) |
| VLU patients among patients receiving care | ||||||||||
| Khan et al., 2013, Phlebology | Pakistan | Prevalence and clinical pattern of CVD in primary care settings in Pakistan | Physicians requested to invite patients to participate | 39 (13.3) | 47.4 | Clinical examination by participating doctors according to CEAP | Point prevalence (4 mos) | 3000 | 18 | 0.60 b (0.36‐0.95) |
| Laible et al., 2002, Pflege | Germany | Prevalence of leg ulcers in home‐care setting in north‐Rhine Westphalia | Questionnaire to a random sample of home nursing services | 77.5 (10) | 68.7 | Patients records, no wound inspection | Point prevalence (1 wks) | 12 156 | 123 b | 1.01 b (0.84‐1.21) |
| Vuylsteke et al., 2018, Angiology | 23 countries | Prevalence of CVD and CVI in general practitioners practice in 23 countries | General practitioners requested to invite consecutive patients | 51.8 (na) | 70.7 | Clinical examination by participating general practitioners (trained for the study) according to CEAP | Point prevalence (47 mos) | 99 359 | 672 | 0.68 b (0.63‐0.73) |
| Kreft et al., 2020, Angiology | Germany | Prevalence and mortality of CVD and CVI in the German population | Random sample of insurants from databse of largest German health insurer | >50 | 57 | Health insurance database records, ICD‐10‐GM codes, no wound inspection | Period prevalence (1 y) | 269 670 b | 2454 | 0.91 (0.88‐0.95) |
| Margolis et al., 2002, Journal of the American Academy of Dermatology | United Kingdom | Prevalence and incidence of venous leg ulcers in the elderly | Random sample of patients from the General Practice Research Database | 65‐95 a | na | General practice research database records, OXMIS codes, validation of case definition | Period prevalence (1 year) | 50 000 | 845 b | 1.69 (1.58‐1.81) |
| Berenguer et al., 2019, International Wound Journal | Spain | Trends of venous leg ulcers'prevalence and incidence in a primary care centre in Barcelona | Idenfication of records of patients with VLU in health history database from 2010 to 2014 | 79.3 (13.7) | 54.8 | Primary care research database records, ICD codes, no wound inspection | Period prevalence (1 year) | 30332.4 d | 47.2 d | 0.16 d (0.11‐0.21) |
| Homs‐Romero et al., 2021, Journal of Nursing Scholarship | Spain | Validity of CVD diagnoses in primary care research database to estimate CVD, CVI and venous leg ulcers prevalence and incidence | Idenfication of records of patients with VLU in health history database | 50.7 (18.4) | 52.8 | Primary care research databae records, ICD codes, validation of case definition | Point prevalence (na) | 4 074 308 | 13 595 | 0.33 (0.328‐0.339) |
Abbreviations: ABPI, ankle brachial pressure index; CEAP, clinical etiological anatomical and pathophysiological; CVD, chronic venous disease; CVI, chronic venous insufficiency; ICD, international classification of diseases; na, not available; OXMIS, Oxford medical information system.
Range.
Calculated (not reported by author).
Median (IQR).
Average 5 years.
TABLE 3.
Venous leg ulcer incidence: characteristics of included studies.
| Author, year | Country | Aim | Data collection | Age (mean[SD]) | Sex (% women) | Outcome ascertainment | N population | n VLU | VLU incidence in ‰ (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| VLU patients among patients receiving care | |||||||||
| Margolis et al., 2002, Journal of the American Academy of Dermatology | United Kingdom | Prevalence and incidence of venous leg ulcers in the elderly | Random sample of patients from the General Practice Research Database from 1988 to 1996 | 65‐95 a | na | 6 mos ulcer‐free period after registration, patients with a new VLU during follow‐up time, OXMIS codes, validation of case definition | 207 062 | 2394 | 11.56 (11.11‐12.03) |
| Berenguer et al., 2019, International Wound Journal | Spain | Trends of venous leg ulcers'prevalence and incidence in a primary care centre in Barcelona | Idenfication of records of patients with VLU in primary care health history research database from 2010 to 2014 | 79.3 (13.7) | 54.8 | Patients with a new VLU in the year of study, ICD codes, no wound inspection | 30 332.4 b | 25.6 b | 0.86 c (0.56‐1.26) |
| Homs‐Romero et al., 2021, Journal of Nursing Scholarship | Spain | Validity of CVD diagnoses in primary care research database to estimate CVD, CVI and venous leg ulcers prevalence and incidence | Idenfication of records of patients with VLU in primary care health history research database of 2016 | 48.4 (18.2) | 50.1 | Patients newly diagnosed with VLU in 2016, ICD codes, validation of case definition | 3 934 171 | 865 | 0.22 (0.21‐0.24) |
| Goh et al., 2020, BMJ open | Singapore | Estimate the chronic wounds incidence of a multi‐ethnic Asian population | Identification of patients with chronic wounds in Singapore's nationwide acute care claims database from 2010 to 2017 | 74 (63‐84) d | 49 | Admissions for a new VLU in the year of study, IDC‐9‐AM and ICD‐10‐AM codes | 3 965 796 | 598 | 0.15 c (0.14‐0.16) |
Abbreviations: CVD, chronic venous disease; CVI, chronic venous insufficiency; ICD, international classification of diseases; na, not available; OXMIS, Oxford medical information system.
Range.
Average 5 years.
Calculated (not reported by author).
Median (IQR).
3.2. Quality assessment
According to JBI assessment tool for prevalence studies, most included studies showed adequate quality (average percentage of “Yes” 64.9%, SD 19.7%) (Table 4). Higher results were obtained for items associated with sample frame, methods for sampling and sample size. Most studies used appropriate sampling strategies for the targeted populations and described the study settings in details. Lower results were obtained for items regarding the reliability of condition measurement, the comprehensibility of response rate and the appropriateness of the statistical analysis used. All studies were included in the analysis, including the ones with relatively low percentage of “yes” (33.3%), as their prevalence estimates were in the same range than other estimates. Very high heterogeneity was shown between the studies as reflected by their distribution in the funnel plot (Figure 2). However there does not seem to be publication bias regarding study‐size or their representativeness.
TABLE 4.
JBI critical appraisal for studies reporting prevalence and incidence data.
| Citation | Was the sample frame appropriate to address the target population? | Were study participants sampled in an appropriate way? | Was the sample size adequate? | Were the study subjects and the setting described in detail? | Was the data analysis conducted with sufficient coverage of the identified sample? | Were valid methods used for the identification of the condition? | Was the condition measured in a standard, reliable way for all participants? | Was there appropriate statistical analysis? | Was the response rate adequate, and if not, was the low response rate managed appropriately? | %Y |
|---|---|---|---|---|---|---|---|---|---|---|
| Berenguer Pérez M et al. 2019. | Y | Y | Y | N | Y | Y | N | N | N/A | 62.50 |
| Goh OQ et al, 2020. | N | Y | Y | Y | Y | Y | N | Y | N/A | 75.00 |
| Homs‐Romero E et al. 2021. | Y | Y | Y | Y | Y | Y | N | Y | N/A | 87.50 |
| Khan AF et al. 2013. | Y | U | U | Y | U | U | Y | N | U | 33.33 |
| Kreft D et al. 2020. | Y | Y | Y | Y | Y | Y | N | Y | N/A | 87.50 |
| Margolis DJ et al. 2002. | Y | Y | Y | N | Y | Y | N | Y | N/A | 75.00 |
| Moffatt CJ et al. 2004. | Y | Y | Y | Y | U | Y | Y | U | U | 66.67 |
| Rabe E et al. 2003. | Y | Y | N | Y | Y | Y | Y | N | Y | 77.78 |
| Srinivasaiah N et al. 2007. | Y | Y | Y | N | U | N | N | U | U | 33.33 |
| Vowden KR et al. 2009. | Y | Y | Y | Y | Y | U | N | U | Y | 66.67 |
| Zolotukhin IA et al. 2017. | Y | Y | Y | Y | Y | Y | Y | N | Y | 88.89 |
| Vuylsteke ME et al. 2018. | Y | Y | Y | Y | U | Y | Y | N | U | 66.67 |
| Baker SR et al. 1991. | Y | U | U | N | U | Y | Y | N | U | 33.33 |
| Laible J et al. 2002. | Y | U | Y | U | U | Y | Y | Y | U | 55.56 |
| Total | 92.85714286 | 78.57142857 | 78.57142857 | 64.28571429 | 57.14285714 | 78.57142857 | 50 | 28.57142857 | 33.33333333 | 64.98 |
Abbreviations: N, No; N/A, not applicable; U, Unclear; Y, Yes.
FIGURE 2.
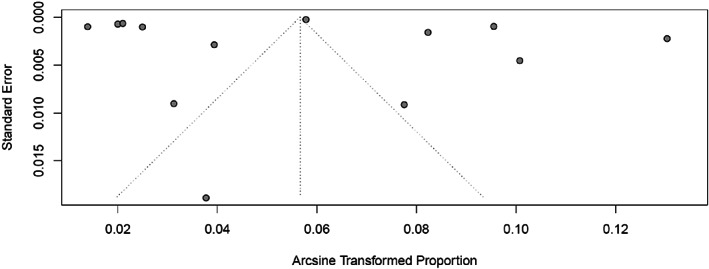
Study distribution (funnel plot).
3.3. Study characteristics
The characteristics of the 14 studies included are described in Tables 2 and 3. The studies were published from 1991 to 2021 and were conducted in 26 different countries, among which most of them were European countries (64%). Among them, 10 studies reported prevalence only, three reported prevalence and incidence, and one reported incidence only. Most of the studies focused on chronic venous disorders (n = 6), few of them only on VLU (n = 3) or on leg ulcerations (n = 3), one on chronic wounds, and one more broadly on wounds. VLU‐specific prevalence or incidence were mostly not reported, but data specific to VLU could be extracted from all studies. These data represented a total number of 6 111 038 observations with a total number of 18 410 VLU events for prevalence and 8 137 361 observations with 3883 VLU events for incidence. The studies were all observational studies with either a transversal 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 or a longitudinal 8 , 38 , 39 , 40 , 41 design. A mix of retrospective and prospective data collection were undertaken in different settings including primary care facilities, 29 , 30 , 32 , 34 general practitioners' medical practice, 36 home‐care services, 35 health insurance databases (acute or primary care private and/or public facilities), 38 , 40 primary care research database, 8 , 39 , 41 and population of a region. 31 , 33 , 37 When reported, patients' mean age ranged from 39 to 79.3 years old and percentage of women ranged from 47.4% to 70.7%.
3.4. Outcome measurement
The way VLU cases were attributed is presented in Tables 2 and 3. Six out of 14 of the included studies stated having performed a clinical assessment reporting ABPI (Ankle Brachial Pressure Index) with a cut off of 0.8 or 0.9, Doppler ultrasound, Duplex ultrasound, photoplethysmography, the CEAP classification (Clinical‐Aetiology‐Anatomy‐Pathophysiology), or a combination of these diagnostic tools. 30 , 31 , 33 , 34 , 36 , 37 In two of them the duration of the wound was reported. 30 , 31 In the eight remaining studies, VLU estimates were based on patients' health records without any wound inspection. 8 , 29 , 32 , 35 , 38 , 39 , 40 , 41 The records came either from care facilities registers, from health insurance databases or from research databases. In the databases, the diagnosis of VLU was reported according to ICD‐9 or ICD‐10 diagnostic codes (International Classification of Diseases), or OXMIS coding algorithms (Oxford Medical Information System). Two studies performed a validation of case definition. 39 , 41
3.5. VLU prevalence
Ten studies (77%) reported a point prevalence and three studies 8 , 38 , 41 a period prevalence (1 year). Due to disparities in study designs and measurement methods the prevalence varied between studies (see Table 2). In three studies 29 , 30 , 32 VLU point prevalence was expressed as the number of patients with VLU identified in health care facilities, on the total population of a region. It ranged from 0.02% to 0.04%. Other studies (n = 3), 31 , 33 , 37 reported their point prevalence as VLU events in a sample population including both patients receiving treatment from health care professionals and people that self‐treat, ranging from 0.06% to 0.14%. In the remaining studies (n = 7), 8 , 34 , 35 , 36 , 38 , 39 , 41 prevalence estimates consisted in VLU events identified in health care systems and reported on the corresponding population of patients receiving treatment from health care professionals. These estimates ranged from 0.33% to 1.01% for point prevalence and from 0.16% to 1.69% for period prevalence.
3.6. VLU incidence
One study 40 estimated VLU incidence in an acute care health insurance claims database (excluding outpatient services and people that self‐treat) to 0.15‰ person‐years. In the three remaining studies 8 , 39 , 41 VLU incidence was estimated in primary care research databases and ranged from 0.22‰ to 11.56‰ person‐years (see Table 3).
3.7. Meta‐analysis
3.7.1. Prevalence: pooled effect
Random effect meta‐analysis of prevalence on 13 studies revealed a pooled effect size of 0.0032 with 95% confidence interval between [0.0013; 0.0060] and 95% prediction interval ranging between [0.0000; 0.0201] (Figure 3). The Cochran Q test was significant (Q = 10 727.92, df = 12, P < .001) and (I 2 statistic = 99.9%).
FIGURE 3.
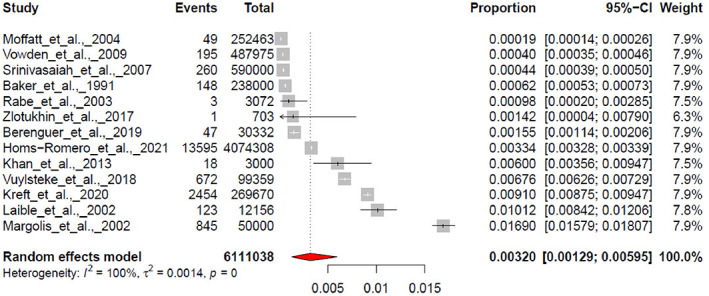
Pooled effect estimated for the prevalence.
3.7.2. Prevalence: moderator analyses
Moderator analysis revealed that the nature of the population explained a substantial amount 42 of heterogeneity in the effect's sizes with a pseudo R 2 of 60.49% (k = 13) (Table 5). The type of prevalence reported (point/period prevalence) exhibited a moderate contribution with a R 2 of 17.03% (k = 13). The contribution of other investigated moderators was negligible.
TABLE 5.
Moderators analysis.
| Moderator | k | R 2 (%) | QM | df | P‐value |
| Population | 13 | 60.49 | 19.87 | 2 | <.0001 |
| Type of prevalence reported | 13 | 17.03 | 3.41 | 1 | .06 |
| Case ascertainment | 13 | 0 | 1.05 | 1 | .31 |
| Origin | 13 | 0 | 0.23 | 1 | .63 |
| Quality of study | 13 | 0 | 0.12 | 1 | .73 |
| Year of publication | 13 | 0 | 0.28 | 1 | .60 |
| Mean age | 10 | 0.31 | 0.94 | 1 | .33 |
| Percentage of women | 11 | 0 | 0.08 | 1 | .78 |
Abbreviations: df, degree of freedom; k, number of studies; QM, omnibus test; R 2 (%), coefficient of determination en %.
Further investigations regarding the nature of the populations revealed that the three subgroups were significantly different (Q = 35.15, df = 2, P < .0001) with a pooled effect size of 0.00063 [0.00053; 0.00073] for VLU people identified in a regional sample (k = 3), a pooled effect of 0.00034 [0.00020; 0.00051] for VLU patients among the residents of a region (k = 3) and 0.00694 [0.00374; 0.01112] for VLU patients among the total number of patients receiving care (k = 7) (Figure 4).
FIGURE 4.
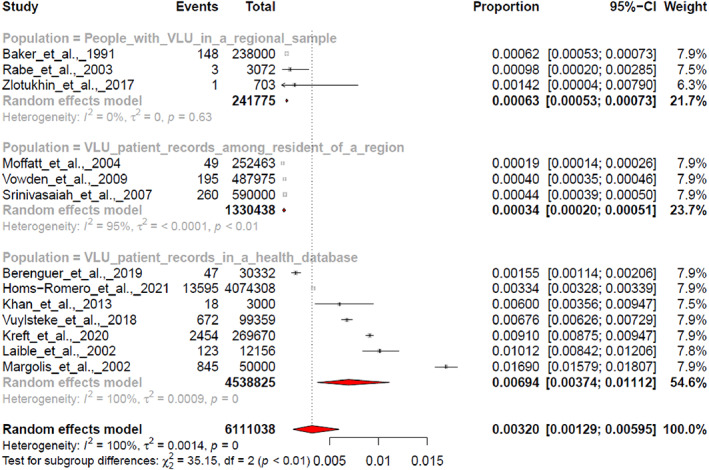
Pooled effect estimated for the prevalence in three identified population subgroups.
3.7.3. Incidence: pooled effect
Incidence random effect meta‐analysis on four papers revealed a pooled effect of 0.00168 with 95% confidence interval between [0.0000; 0.00725], a 95% Prediction interval of [0.0000; 0.0651], significant Q test (Q = 7232.32, df = 3, P < .001) and the heterogeneity of I 2 = 100% (Figure 5).
FIGURE 5.
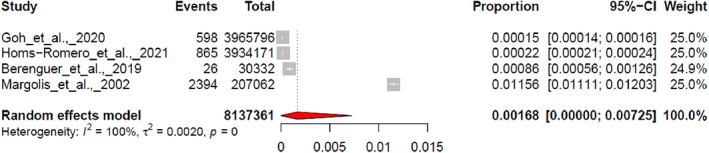
Pooled effect estimated for the incidence.
4. DISCUSSION
This study provides conservative estimates of VLU prevalence and incidence in different settings. Ten of the 14 included studies report only prevalence rates, three a mix of prevalence and incidence, and one report incidence rate only. All included studies were observational studies. Our meta‐analysis of prevalence included 13 studies and resulted in a pooled VLU prevalence of 0.32% (95% CI 0.129%‐0.595%). However, because of the small amount of included studies, the variety of methodological approaches, the different settings and the use of different data collection methods, this result should be considered with caution. Nevertheless, the results are in alignment with other systematic reviews and meta‐analysis reporting prevalence of chronic wounds. 43 , 44
Our analyses show that the pooled prevalence of VLU patients among the residents of a region was estimated at 0.034% (95% CI 0.020%‐0.051%), at 0.063% (95% CI 0.053%‐0.073%) for VLU people (includes VLU patients and people that self‐treats) in a regional sample, and at 0.694% (95% CI 0.374%‐1.112%) for VLU patients among patients receiving care. These results are in the same range than previous results reporting a combined VLU prevalence estimates of three epidemiological studies conducted in Sweden, which ranged between 0.22% and 0.4%. 45 , 46 , 47
Our systematic review included four studies reporting rates of incidences. All were incorporated in the meta‐analysis with a pooled VLU incidence of 0.168% (95% CI 0.000%‐0.725%). This proportion represents about a 1/4th of the reported pooled prevalence of 0.694% in the corresponding population group (VLU patients among patients receiving care). This would indicate that one out of four VLUs is newly developed. This observation is slightly higher, but in a close range than a previous report (unpublished results) that mentions a ratio of one out of 10. 46 Because of the small amount of studies included in this meta‐analysis and the extremely high heterogeneity of 99.9% observed between studies these results should also be considered with caution.
This study is not without limitations. Even though our eligibility criteria were fairly inclusive, only a very small number of studies could be retained. This low number shows that prevalence and incidence studies within the population of VLU's are rarely carried out. This may be because of a variety of the definition of a VLU, 48 or a lack of systematic measure and data collection methods. In addition, it cannot be excluded that some studies were not identified by our search strategy i.e. the term prevalence/incidence Is not mentioned neither in the title or abstract. Even though our objective was to describe the prevalence and incidence of VLU internationally, the articles included in our study consisted mainly of publications from Europe and other developed countries (86%), with only two reports from emerging countries (Pakistan and Russia). This makes it difficult to estimate an international prevalence of VLU's. We therefore need more methodologically robust studies from different countries around the globe to tailor strategies for the management and prevention of VLU in different settings. In addition, our meta‐analysis of VLU prevalence reveals a very high heterogeneity between studies (I 2 = 99.9%) and a very broad 95% prediction interval ranging between 0.00% to 2.01%. This latter indicates an extremely large band of predicted estimated prevalence for future studies on VLU, that could lie between 0.00% and 2.01% with a probability of 95%. Therefore, the comparison of results between studies is challenging and the reliability of the results as well as the methodologies used must be carefully analysed. One crucial point that accounts for the high heterogeneity in this review is the nature of the VLU population, as indicated by a moderator analysis with a pseudo R 2 of 60.49%.
Our analysis identified three different approaches to reporting prevalence that help to explain a significant amount of the heterogeneity. A statistically significant (P < .0001) difference of VLU prevalence estimates does exist between the population subgroups. This is shown in the proportion of VLU events in residents of a region, where VLU events are identified only in people receiving care (pooled prevalence 0.034%). It has to be noted that in these studies, prevalence data of self‐treated cases were not included. These data are, as highlighted by previous studies, 45 underestimated and are not representative for the general population. This can lead to challenging access of this population in regards to the implementation of the best VLU practice. The proportion of VLU people in a regional sample was 0.063% (pooled prevalence). This pooled estimate is higher than in previous subgroup as it includes both patients that receive care and people that self‐treats and is thus more representative of the prevalence in the general population. However, sample size in these studies is small and therefore the number of patients with VLU identified is too low so that reliable prevalence can be made. When calculating the proportion of VLU patients in the total number of patients receiving care (pooled prevalence 0.694%), patients that self‐treat their VLU are again not included. This value must be higher than those of the two previous groups as the reference population is restricted to the healthcare system and cannot been taken as representative for the general population. These observations reveal the variability of how prevalence can be reported and highlights the need to set up international consensus for systematic collation and review of prevalence and incidence to establish the epidemiological profile of VLU at an international level.
As shown much of the heterogeneity between studies might originate from the nature of the population, however considerable variability persists within each identified subgroup of population. The way prevalence is assessed, namely point prevalence versus period prevalence estimates represent an important methodological variation that must obviously be considered. In the included studies, the variation in how prevalence is measured appears to explain only a moderate part of the heterogeneity in this meta‐analysis (R 2 = 17.03%). This is likely because only a small number of studies (3/13) report period prevalence, the majority of them (10/13) measure point prevalence. Again, a consensus on the type of prevalence to report would facilitate comparisons.
How a VLU case is ascertained may also have a significant impact on the accuracy of the prevalence or incidence estimate. A differential diagnosis should be made especially as in most studies leg ulcer events of venous origin must be sorted out from other causes of leg ulceration or from other CVD categories. Evidence demonstrates that 41% of VLU patients never got diagnosed 6 and if they got diagnosed there is a delay from wound appearance to the first evaluation by a physician of 8 days (IQR 1‐32 days, range 0‐314 days). 49 A lack of clear diagnostic reduce confidence in the outcome and lead to a risk of over‐ or under‐estimating the prevalence or incidence.
Our results show that in more than half of the included studies (54%), case ascertainment was based on patient records and the authors did not undergo de novo clinical assessment. Among them, two groups did achieve case ascertainment validation using questionnaire addressed to practitioners. In this questionnaire practitioners had to review a sample of medical records, but no patient was examined. The other half (46%) of the studies performed clinical assessments according to clinical practice guidelines. Our moderator analysis indicates that whether de novo VLU clinical assessment is performed or not appears to explain a negligible part (R 2 = 0.35%) of the heterogeneity observed in our prevalence pooled effect. This indicates that other sources of variability between the included studies are responsible for the high heterogeneity. These sources of variation could be found in the use of divergent diagnostic tools or protocols between studies, or more broadly at other methodological levels, in the use of different approaches. This finding further highlights the importance of using tools and protocols that are as standardised as possible, to obtain results that can be compared.
5. CONCLUSION
To guaranty the quality of this systematic review and meta‐analysis, we followed the PRISMA statement. The results show highly variable estimates of VLU prevalence and incidence. Comparison between studies is difficult because of the varying nature of the study population and the type of prevalence/incidence reported. We believe that diagnostic tools used as well as protocols may also have influenced estimates. Prevalence and incidence studies with clear definitions of population characteristics and comparable estimates are now needed. We therefore recommend a standardisation of the methodology to conduct future internationally representative and report them as prevalence and incidence studies to understand the magnitude of VLUs and retrieve them for further systematic reviews.
ACKNOWLEDGMENTS
This article is part of a larger project funded by the Swiss National Science Foundation number 10531C_185332.
Probst S, Saini C, Gschwind G, et al. Prevalence and incidence of venous leg ulcers—A systematic review and meta‐analysis. Int Wound J. 2023;20(9):3906‐3921. doi: 10.1111/iwj.14272
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Santler B, Goerge T. Chronic venous insufficiency – a review of pathophysiology, diagnosis, and treatment. J Dtsch Dermatol Ges. 2017;15(5):538‐556. [DOI] [PubMed] [Google Scholar]
- 2. Körber A, Jockenhöfer F, Sondermann W, Stoffels‐Weindorf M, Dissemond J. First manifestation of leg ulcers: analysis of data from 1000 patients. Der Hautarzt. 2017;68(6):483‐491. [DOI] [PubMed] [Google Scholar]
- 3. Körber A, Klode J, Al‐Benna S, et al. Etiology of chronic leg ulcers in 31,619 patients in Germany analyzed by an expert survey. J Dtsch Dermatol Ges. 2011;9(2):116‐121. [DOI] [PubMed] [Google Scholar]
- 4. Rajhathy EM, Murray HD, Roberge VA, Woo KY. Healing rates of venous leg ulcers managed with compression therapy: a secondary analysis of data. J Wound Ostomy Continence Nurs. 2020;47(5):477‐483. [DOI] [PubMed] [Google Scholar]
- 5. Abbade LPF, Lastoria S. Venous ulcer: epidemiology, physiopathology, diagnosis and treatment. Int J Dermatol. 2005;44(6):449‐456. [DOI] [PubMed] [Google Scholar]
- 6. Guest JF, Fuller GW, Vowden P. Cohort study evaluating the burden of wounds to the UK's National Health Service in 2017/2018: update from 2012/2013. BMJ Open. 2020;10(12):e045253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Margolis DJ. The incidence and prevalence of venous leg ulcers:different data from old and new epidemiologic studies? J Wound Care. 2020;29(Suppl 7B):81. [Google Scholar]
- 8. Berenguer Pérez M, López‐Casanova P, Sarabia Lavín R, González de la Torre H, Verdú‐Soriano J. Epidemiology of venous leg ulcers in primary health care: incidence and prevalence in a health Centre‐a time series study (2010‐2014). Int Wound J. 2019;16(1):256‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raffetto JD, Ligi D, Maniscalco R, Khalil RA, Mannello F. Why venous leg ulcers have difficulty healing: overview on pathophysiology, clinical consequences, and treatment. J Clin Med. 2020;10(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weller CD, Richards C, Turnour L, Team V. Patient explanation of adherence and non‐adherence to venous leg ulcer treatment: a qualitative study. Front Pharmacol. 2021;12:663570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Probst S, Séchaud L, Bobbink P, Skinner MB, Weller CD. The lived experience of recurrence prevention in patients with venous leg ulcers: an interpretative phenomenological study. J Tissue Viability. 2020;29(3):176‐179. [DOI] [PubMed] [Google Scholar]
- 12. González‐Consuegra RV, Verdú J. Quality of life in people with venous leg ulcers: an integrative review. J Adv Nurs. 2011;67(5):926‐944. [DOI] [PubMed] [Google Scholar]
- 13. Callam MJ, Ruckley CV, Dale JJ, Harper DR. Hazards of compression treatment of the leg: an estimate from Scottish surgeons. Br Med J. 1987;295(6610):1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chaby G, Senet P, Ganry O, et al. Prognostic factors associated with healing of venous leg ulcers: a multicentre, prospective, cohort study. Br J Dermatol. 2013;169(5):1106‐1113. [DOI] [PubMed] [Google Scholar]
- 15. Raju S, Fredericks R. Evaluation of methods for detecting venous reflux: perspectives in venous insufficiency. Arch Surg. 1990;125(11):1463‐1467. [DOI] [PubMed] [Google Scholar]
- 16. Urwin S, Dumville JC, Sutton M, Cullum N. Health service costs of treating venous leg ulcers in the UK: evidence from a cross‐sectional survey based in the north west of England. BMJ Open. 2022;12(1):e056790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and Medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21(1):27‐32. [DOI] [PubMed] [Google Scholar]
- 18. Weller C, Evans S. Venous leg ulcer management in general practice – practice nurses and evidence based guidelines. Aust Fam Physician. 2012;41(5):331‐337. [PubMed] [Google Scholar]
- 19. Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3(3):123‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147‐153. [DOI] [PubMed] [Google Scholar]
- 21. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Probst S, Weller CD, Bobbink P, et al. Prevalence and incidence of venous leg ulcers‐a protocol for a systematic review. Syst Rev. 2021;10(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Chapter 5: systematic reviews of prevalence and incidence. In: Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis. JBI; 2020:175‐182. [Google Scholar]
- 24. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40‐46. [DOI] [PubMed] [Google Scholar]
- 25. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan‐a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. JBI . Critical appraisal tools. 2022. https://jbi.global/critical-appraisal-tools
- 27. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta‐analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974‐978. [DOI] [PubMed] [Google Scholar]
- 29. Vowden KR, Vowden P. The prevalence, management and outcome for patients with lower limb ulceration identified in a wound care survey within one English health care district. J Tissue Viability. 2009;18(1):13‐19. [DOI] [PubMed] [Google Scholar]
- 30. Moffatt CJ, Franks PJ, Doherty DC, Martin R, Blewett R, Ross F. Prevalence of leg ulceration in a London population. QJM. 2004;97(7):431‐437. [DOI] [PubMed] [Google Scholar]
- 31. Baker SR, Stacey MC, Jopp‐McKay AG, Hoskin SE, Thompson PJ. Epidemiology of chronic venous ulcers. Br J Surg. 1991;78(7):864‐867. [DOI] [PubMed] [Google Scholar]
- 32. Srinivasaiah N, Dugdall H, Barrett S, Drew PJ. A point prevalence survey of wounds in north‐East England. J Wound Care. 2007;16(10):413‐419. [DOI] [PubMed] [Google Scholar]
- 33. Zolotukhin IA, Seliverstov EI, Shevtsov YN, et al. Prevalence and risk factors for chronic venous disease in the general Russian population. Eur J Vasc Endovasc Surg. 2017;54(6):752‐758. [DOI] [PubMed] [Google Scholar]
- 34. Khan AF, Chaudhri R, Ashraf MA, Mazaffar MS, Zawar‐ul‐Imam S, Tanveer M. Prevalence and presentation of chronic venous disease in Pakistan: a multicentre study. Phlebology. 2013;28(2):74‐79. [DOI] [PubMed] [Google Scholar]
- 35. Laible J, Mayer H, Evers GC. Prevalence of ulcus cruris in home care nursing. An epidemiological study in North Rhine‐Westphalia. Pflege. 2002;15(1):16‐23. [DOI] [PubMed] [Google Scholar]
- 36. Vuylsteke ME, Colman R, Thomis S, Guillaume G, Van Quickenborne D, Staelens I. An epidemiological survey of venous disease among general practitioner attendees in different geographical regions on the globe: the final results of the vein consult program. Angiology. 2018;69(9):779‐785. [DOI] [PubMed] [Google Scholar]
- 37. Rabe E, Pannier‐Fischer F, Bromen K, et al. Bonn vein study by the German Society of Phlebology: epidemiological study to investigate the prevalence and severity of chronic venous disorders in the urban and rural residential populations. Phlebologie. 2003;32(1):1‐14. [Google Scholar]
- 38. Kreft D, Keiler J, Grambow E, Kischkel S, Wree A, Doblhammer G. Prevalence and mortality of venous leg diseases of the deep veins: an observational cohort study based on German health claims data. Angiology. 2020;71(5):452‐464. [DOI] [PubMed] [Google Scholar]
- 39. Homs‐Romero E, Romero‐Collado A, Verdu J, Blanch J, Rascon‐Hernan C, Marti‐Lluch R. Validity of chronic venous disease diagnoses and epidemiology using validated electronic health records from primary care: a real‐world data analysis. J Nurs Scholarsh. 2021;53(3):296‐305. [DOI] [PubMed] [Google Scholar]
- 40. Goh OQ, Ganesan G, Graves N, Ng YZ, Harding K, Tan KB. Incidence of chronic wounds in Singapore, a multiethnic Asian country, between 2000 and 2017: a retrospective cohort study using a nationwide claims database. BMJ Open. 2020;10(9):e039411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol. 2002;46(3):381‐386. [DOI] [PubMed] [Google Scholar]
- 42. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Publishers; 1988. [Google Scholar]
- 43. Martinengo L, Olsson M, Bajpai R, et al. Prevalence of chronic wounds in the general population: systematic review and meta‐analysis of observational studies. Ann Epidemiol. 2019;29:8‐15. [DOI] [PubMed] [Google Scholar]
- 44. McCosker L, Tulleners R, Cheng QL, et al. Chronic wounds in Australia: a systematic review of key epidemiological and clinical parameters. Int Wound J. 2019;16(1):84‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Briggs M, Closs SJ. The prevalence of leg ulceration: a review of the literature. EWMA J. 2003;3(2):14‐20. [Google Scholar]
- 46. Nelzén O. Patients with Chronic Leg Ulcer: Aspects on Epidemiology, Aetiology, Clinical History, Prognosis and Choice of Treatment [Doctoral Thesis, Comprehensive Summary]. Uppsala: Acta Universitatis Upsaliensis; 1997. [Google Scholar]
- 47. Nelzen O. Prevalence of venous leg ulcer: the importance of the data collection method. Phlebolymphology. 2008;15(4):143‐150. [Google Scholar]
- 48. Kyaw BM, Järbrink K, Martinengo L, Car J, Harding K, Schmidtchen A. Need for improved definition of "chronic wounds" in clinical studies. Acta Derm Venereol. 2018;98(1):157‐158. [DOI] [PubMed] [Google Scholar]
- 49. Ahmajärvi K, Isoherranen K, Venermo M. Cohort study of diagnostic delay in the clinical pathway of patients with chronic wounds in the primary care setting. BMJ Open. 2022;12(11):e062673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


