Abstract
Surgical Site Infection (SSI) is one of the common postoperative complications after gastric cancer surgery. Previous studies have explored the risk factors (such as age, diabetes, anaemia and ASA score) for SSI in patients with gastric cancer. However, there are large differences in the research results, and the correlation coefficients of different research results are quite different. We aim to investigate the risk factors of surgical site infection in patients with gastric cancer. We queried four English databases (PubMed, Embase, Web of Science and the Cochrane Library) and four Chinese databases (China National Knowledge Infrastructure, Chinese Biological Medicine Database, Wanfang Database and Chinese Scientific Journal Database (VIP Database)) to identify published literature related to risk factors for surgical site infection in patients with gastric cancer. Rev Man 5.4 and Stata 15.0 were used in this meta‐analysis. A total of 15 articles (n = 6206) were included in this analysis. The following risk factors were found to be significantly associated with surgical site infection in gastric cancer: male (OR = 1.28, 95% CI [1.06, 1.55]), age >60 (OR = 2.75, 95% CI [1.65, 4.57]), smoking (OR = 1.99, 95% CI [1.46, 2.73]), diabetes (OR = 2.03, 95% CI [1.59, 2.61]), anaemia (OR = 4.72, 95% CI [1.66, 13.40]), preoperative obstruction (OR = 3.07, 95% CI [1.80, 5.23]), TNM ≥ III (OR = 2.05, 95% CI [1.56, 2.70]), hypoproteinemia (OR = 3.05, 95% CI [2.08, 4.49]), operation time ≥3 h (OR = 8.33, 95% CI [3.81, 18.20]), laparotomy (OR = 2.18, 95% CI [1.61, 2.94]) and blood transfusion (OR = 1.44, 95% CI [1.01, 2.06]). This meta‐analysis showed that male, age >60, smoking, diabetes, anaemia, preoperative obstruction, TNM ≥ III, hypoproteinemia, operation time ≥3 h, open surgery and blood transfusion were the risk factors for SSI in patients with gastric cancer.
Keywords: gastric cancer, meta‐analysis, risk factors, surgical site infection
1. INTRODUCTION
Gastric cancer (GC) is one of the most common gastrointestinal malignancies in the world. According to data from the Global Cancer Epidemiology Database (GLOBOCAN) in 2020, there were about 1 089 000 cases and 769 000 deaths of gastric cancer in the world, with a global incidence of 5.6% and a mortality rate of 7.7%. 1 Currently, surgical operation is still the primary treatment for gastric cancer. 2 Surgical Site Infection (SSI) is one of the most common postoperative complications after gastric cancer operation, as well as one of the most common nosocomial infections with an incidence of about 30%. 3 , 4 The occurrence of SSI can result in a prolonged postoperative hospital stay and increased medical costs, affecting postoperative rehabilitation and the quality of life of patients. 5 , 6 Thus, it is very important to identify and treat the perioperative risk factors to reduce the occurrence of SSI in patients with gastric cancer.
Previous studies have explored the risk factors for SSI in patients with gastric cancer. However, there are large differences in the research results, and the correlation coefficients of different research results are quite different. For example, Kosuga et al. reported that male gender and chronic liver disease were independent risk factors for SSI in gastric cancer patients after surgery, whereas diabetes, anaemia, ASA score and hypoproteinemia were not associated with SSI. 7 In other previous studies, 8 , 9 ASA score, diabetes, smoking and duration of surgery were associated with SSI. The exact factors associated with SSI in patients with gastric cancer and the correlation coefficients between them remain unclear. Therefore, this study aimed to examine the risk factors of SSI among gastric cancer patients as valuable information for developing better interventions and management of gastric cancer.
2. METHODS
This study was registered to the international database of prospective registered systematic reviews (PROSPERO) with registration number CRD42022322277. We followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement. 10
2.1. Search strategy
We queried four English databases (PubMed, Embase, Web of Science and the Cochrane Library) and four Chinese databases (China National Knowledge Infrastructure, Chinese Biological Medicine Database, Wanfang Database and Chinese Scientific Journal Database (VIP Database)) to identify published literature related to risk factors for surgical site infection in patients with gastric cancer from the date of each database's inception up to December 2022. The main keywords were “stomach neoplasms” or “gastric cancer” or “gastric neoplasms” and “surgical wound infection” or “surgical site infections” and “risk factors” or “influence factors” or “dangerous factors.” The PubMed search strategy is provided in Data S1.
2.2. Eligibility criteria
We included studies that met the following conditions: (1) all gastric cancer patients with surgical treatment were included; (2) SSI diagnostic criteria are derived from Diagnostic Criteria for Nosocomial Infection or Centres for Disease Control and Prevention criteria; 11 , 12 (3) the case and control groups were defined according to the presence or absence of SSI after surgery of gastric cancer; (4) the risk factors related to SSI were reported; (5) the study design consisted of case–control study, cohort study, or other observational studies.
The following studies were excluded during screening: (1) with the risk factors for other types of infections, such as postoperative anastomotic fistula, pulmonary infection, abdominal infection and nosocomial infection; (2) published in a language other than English or Chinese; (3) studies on data errors, incomplete or unable to obtain full text; (4) duplicated studies and non‐primary studies (i.e., meetings, review articles and editorials) and (5) the level of the New Castle‐Ottawa Scale scores ≤4.
2.3. Quality assessment
We evaluated the overall quality of case–control studies and cohort studies using the New Castle‐Ottawa Scale (NOS). The evaluation contents include study population selection, inter‐group comparability and outcome/exposure factor measurement. The level of scores ≤4, 5–6 and ≥7 was graded as low quality, moderate quality and high quality. 13
2.4. Study selection and risk of bias assessment
To address the risk of bias, two researchers (M.X.C. and M.Y.C.) independently assessed all the titles and abstracts to remove articles that obviously did not meet the study criteria. The studies that met the eligibility criteria were included for further evaluation with a full‐text review. Data extraction from the accepted trials included: first author, year of publication, region of study, type of study, sample size and risk factors related to surgical site infection of gastric cancer. Differences of opinion were resolved by discussion between the two researchers. Two researchers (M.X.C. and M.Y.C) independently assessed the risk of bias of the New Castle‐Ottawa Scale (NOS). Any disagreements were adjudicated by consulting a third author (L.W.)
2.5. Statistical analysis
This meta‐analysis was performed using RevMan 5.4 (Cochrane Collaboration) and STATA 15.0 (Stata Corp). Outcomes were presented as the odds ratios (ORs) with 95% confidence intervals (CIs). Heterogeneity across the studies was tested with the Q‐test (test level α = 0.1). The I 2 statistics were used as a quantitative measure of heterogeneity and the I 2 value of 25% to 50%, 50% to 75% and ≥75% was considered to indicate low heterogeneity, moderate heterogeneity and high heterogeneity, respectively. 14 A fixed‐effects model was used if there was no significant heterogeneity (P ≥ .10 and I 2 ≤ 50%); otherwise, a random‐effects model was used. We performed a sensitivity analysis to assess the stability of the Meta‐analysis results. A funnel plot and Egger's test were used to further determine the publication bias if there were a sufficient number of included trials (10 trials). P < .05 was considered significant.
3. RESULTS
3.1. Selection of studies
A total of 3273 articles were identified through our database search, of which 390 were duplicate studies. After reading the titles and abstracts, we excluded 2853 studies because the articles did not meet our eligibility criteria. After reading the full texts, 14 case–control studies and 1 cohort study were eligible for inclusion in this meta‐analysis. All studies were published in English or Chinese between 2012 and 2021. In total, 6206 patients with surgical treatment were included in this analysis, including 668 patients with surgical site infections, and 13 contributing factors were extracted. The prevalence of SSI in all included studies was reported to vary between 4.7% and 50%. The outcome of quality assessment (NOS score) for these studies was as follows: one study scored 8 15 ; nine studies scored 7 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 ; five studies scored 6. 25 , 26 , 27 , 28 , 29 The selection process is presented in Figure 1. Detailed information about the included studies is shown in Tables 1 and 2.
FIGURE 1.
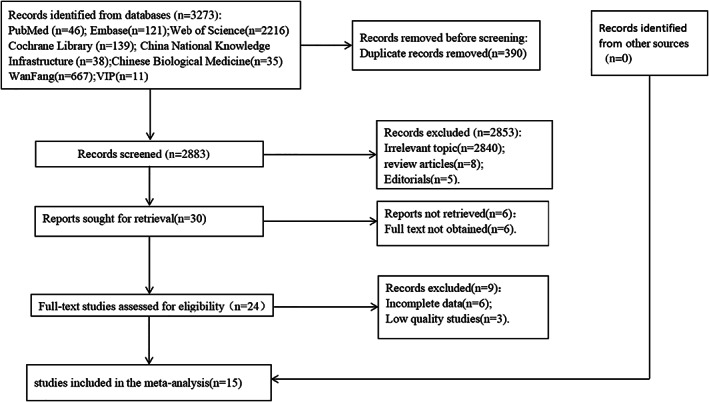
Flow chart of literature selection.
TABLE 1.
Basic characteristics of the 15 included studies.
| First author | Year | Country | Study design | Total (n) | SSI group (n) | SSI prevalence | Study population characteristics | Significant factors | |
|---|---|---|---|---|---|---|---|---|---|
| Liu 16 | 2012 | China | Case–control | 280 | 30 | 10.7% |
Mean age: NA Range age: NA Gender: NA |
③ | |
| Shi 25 | 2013 | China | Case–control | 96 | 13 | 13.54% |
Mean age: NA Range age: NA Gender: NA |
⑪ | |
| Hirao 17 | 2013 | Japan | Case–control | 355 | 24 | 7% |
Mean age: NA Range age: 35 to 84 Gender Male: 240 (67.61%) Female: 115 (32.39%) |
① | |
| Hu 18 | 2014 | China | Case–control | 412 | 39 | 9.47% |
Mean age: 45.28 Range age: 27 to 76 Gender Male: 282 (68.45%) Female: 130 (31.55%) |
①③⑧⑨ | |
| Dai 19 | 2014 | China | Case–control | 492 | 97 | 19.72% |
Mean age: 59.13 Range age: 26 to 79 Gender Male: 302 (61.38%) Female: 190 (38.62%) |
①④⑤⑧⑬ | |
| Wang 20 | 2015 | China | Case–control | 287 | 26 | 9.06% |
Mean age: 51.7 Range age: 30 to 91 Gender Male:191 (66.55%) Female:96 (33.45%) |
①②⑤⑧⑬ | |
| Endo 26 | 2015 | Japan | Case–control | 685 | 42 | 6.1% |
Mean age: NA Range age: 60 to 74 Gender Male: 484 (70.66%) Female: 201 (29.34%) |
① | |
| Yuji 21 | 2017 | Japan | Case–control | 384 | 18 | 4.7% |
Mean age: 67 Range age: 32 to 88 Gender Male: 264 (68.75%) Female: 120 (31.25%) |
①⑩⑫ | |
| Xu 22 | 2018 | China | Case–control | 410 | 50 | 12.0% |
Mean age: 73 Range age: 65 to 92 Gender Male: 330 (80.49%) Female: 80 (19.51%) |
①⑤⑥⑦⑨ | |
| Chen 27 | 2018 | China | Case–control | 223 | 39 | 17.5% |
Mean age: 61.5 Range age: NA Gender Male: 137 (61.43%) Female: 86 (38.57%) |
①③④⑤⑨⑩⑫ | |
| Kim 23 | 2019 | Korea | Case–control | 1038 | 58 | 5.6% |
Mean age: 59 Range age: 50 to 67 Gender Male: 654 (63.00%) Female: 384 (37.00%) |
①③④⑤⑧⑫ | |
| Kim 15 | 2019 | Korea | Cohort | 353 | 25 | 7.1% |
Mean age: 71 Range age: 67 to 75 Gender Male: 232 (65.72%) Female: 121 (34.28%) |
①③④⑤⑥⑧⑨⑫ | |
| Ye 24 | 2020 | China | Case–control | 160 | 38 | 23.75% |
Mean age: NA Range age: >18 Gender Male: 110 (68.75%) Female: 50 (31,25%) |
①④⑤⑩⑪⑫⑬ | |
| Gong 28 | 2021 | China | Case–control | 180 | 90 | 50% |
Mean age: 63.32 Range age: 45 to –79 Gender Male: 48 (26.67%) Female: 132 (73.33%) |
①②⑤⑦⑩ | |
| Zhang 29 | 2021 | China | Case–control | 851 | 79 | 9.28% |
Mean age: 58.35 Range age: NA Gender Male: 548 (64.39%) Female: 303 (35.61%) |
①④⑤⑥⑨⑩⑫ | |
Abbreviations: ① sex; ② age>60; ③ smoking; ④ hypertension; ⑤ diabetes; ⑥ anaemia; ⑦Preoperative obstruction; ⑧ tumour lymph node metastasis (TNM) ≥ III; ⑨ hypoproteinemia; ⑩ ASA ≥ 3; ⑪ operation time ≥ 3 h; ⑫ surgery ways; ⑬ blood transfusion; NA, not available.
TABLE 2.
Quality evaluation results of included studies in Meta‐analysis of SSI risk factors in gastric cancer patients.
| First author | Study population selection | Inter‐group comparability | Outcome/exposure factor measurement | Total |
|---|---|---|---|---|
| Liu 16 | 3 | 2 | 2 | 7 |
| Shi 25 | 3 | 1 | 2 | 6 |
| Hirao 17 | 3 | 2 | 2 | 7 |
| Hu 18 | 3 | 2 | 2 | 7 |
| Dai 19 | 3 | 2 | 2 | 7 |
| Wang 20 | 3 | 2 | 2 | 7 |
| Endo 26 | 3 | 1 | 2 | 6 |
| Yuji 21 | 3 | 2 | 2 | 7 |
| Xu 22 | 3 | 2 | 2 | 7 |
| Chen 27 | 3 | 1 | 2 | 6 |
| Kim 23 | 3 | 2 | 2 | 7 |
| Kim 15 | 4 | 2 | 2 | 8 |
| Ye 24 | 3 | 2 | 2 | 7 |
| Gong 28 | 3 | 1 | 2 | 6 |
| Zhang 29 | 3 | 1 | 2 | 6 |
3.2. Effect of general factors on SSI
In this study, General factors included gender, age >60 and smoking. Low heterogeneity was observed between studies in terms of sex, age >60 and smoking (I 2 ≤ 50%, P ≥ .10), so we used a fixed‐effects model for meta‐analysis. The meta‐analysis results showed that the male (OR = 1.28, 95% CI [1.06, 1.55], P = .01), age >60 years old (OR = 2.75, 95% CI [1.65, 4.57], P = .0001), smoking (OR = 1.99, 95% CI [1.46, 2.73], P < .0001) were significant risk factors for SSI in gastric cancer. The forest plots of the meta‐analysis are presented in Figure 2. The main outcomes of the meta‐analysis are shown in Table 3.
FIGURE 2.
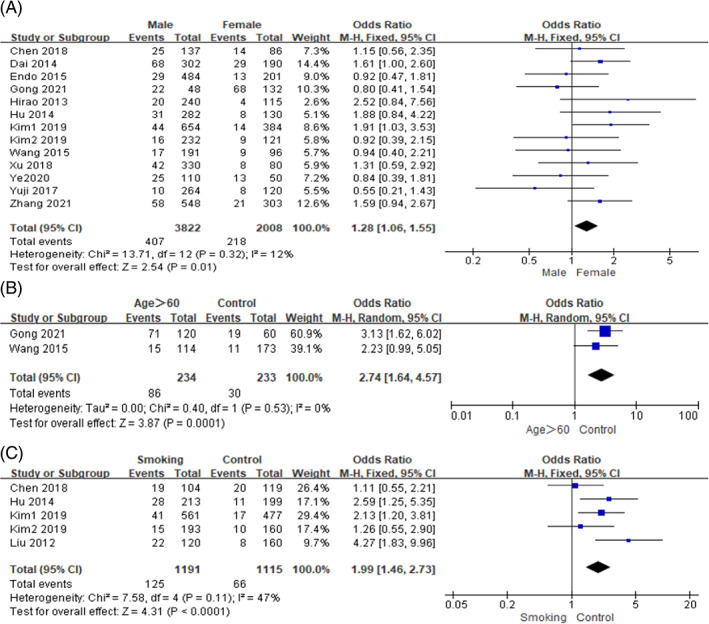
Forest plots of the meta‐analysis of (A) sex, (B) age >60, (C) smoking.
TABLE 3.
The main outcomes of the meta‐analysis.
| Significant factors | No. of studies | I 2 (%) | Q test (P) | OR (95% CI) | P value | |
|---|---|---|---|---|---|---|
| General factors | Sex | 13 15 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 26 , 27 , 28 , 29 | 12 | 0.32 | 1.28 (1.06, 1.55) | .01 a |
| Age > 60 | 2 20 , 28 | 0 | 0.53 | 2.75 (1.65, 4.57) | .0001 a | |
| Smoking | 5 15 , 16 , 18 , 23 , 27 | 47 | 0.11 | 1.99 (1.46, 2.73) | <.0001 a | |
| Diseases factors | Hypertension | 6 15 , 19 , 23 , 24 , 27 , 29 | 0 | 0.71 | 1.24 (0.94, 1.64) | .12 a |
| Diabetes | 9 15 , 19 , 20 , 22 , 23 , 24 , 27 , 28 , 29 | 12 | 0.33 | 2.03 (1.59, 2.61) | <.00001 a | |
| Anaemia | 3 15 , 22 , 29 | 58 | 0.09 | 4.72 (1.66, 13.40) | .004 b | |
| Preoperative obstruction | 2 22 , 28 | 0 | 0.99 | 3.07 (1.80, 5.23) | <.0001 a | |
| TNM ≥ III | 5 15 , 18 , 19 , 20 , 23 | 24 | 0.26 | 2.05 (1.56, 2.70) | <.00001 a | |
| Hypoproteinemia | 5 15 , 18 , 19 , 22 , 27 | 0 | 0.52 | 3.05 (2.08, 4.49) | <.00001 a | |
| Surgical operation factors | ASA ≥ 3 | 5 21 , 24 , 27 , 28 , 29 | 0 | 0.78 | 1.22 (0.87, 1.72) | .25 a |
| Operation time ≥3 h | 2 24 , 25 | 0 | 0.39 | 8.33 (3.81, 18.20) | <.00001 a | |
| Surgery ways | 6 15 , 21 , 23 , 24 , 27 , 29 | 0 | 0.74 | 2.18 (1.61, 2.94) | <.00001 a | |
| Blood transfusion | 3 19 , 20 , 24 | 38 | 0.20 | 1.44 (1.01, 2.06) | .04 a | |
Note: I 2 statistic was defined as the proportion of heterogeneity not due to chance or random error. The significance of statistics is shown in bold.
Abbreviations: ASA, American Society of Anesthesiologists; CI, confidence interval; OR, odds ratio; TNM, distant staging of local lymph node metastasis of primary tumour.
Fixed‐effects model was performed.
Random‐effects model was performed.
3.3. Effect of disease factors on SSI
In this study, the disease factors included hypertension, diabetes, anaemia, preoperative obstruction, TNM and hypoproteinemia. Among them, low heterogeneity was observed between studies in terms of hypertension, diabetes, preoperative obstruction, TNM and hypoproteinemia (I 2 ≤ 50%, P ≥ .10), so we used a fixed‐effects model for meta‐analysis. Whereas moderate heterogeneity was observed between studies in terms of anaemia (I 2 > 50%, P < .10), and the random‐effects model was used for meta‐analysis. On the basis of the pooled ORs and corresponding 95% CIs, the following risk factors were found to be significantly associated with SSI in gastric cancer:diabetes (OR = 2.03, 95% CI [1.59, 2.61], P < .0001), anaemia (OR = 4.72, 95% CI [1.66, 13.40], P = .004), preoperative obstruction (OR = 3.07, 95% CI [1.80, 5.23], P < .0001), TNM ≥ III (OR = 2.05, 95% CI [1.56, 2.70], P < .0001) and hypoproteinemia (OR = 3.05, 95% CI [2.08, 4.49], P < .0001). But no statistically significant difference was observed in terms of hypertension (OR = 1.24, 95% CI [0.94, 1.64], P = .12). The forest plots of the meta‐analysis are presented in Figures 3 and 4, and the main outcomes of the meta‐analysis are shown in Table 3.
FIGURE 3.
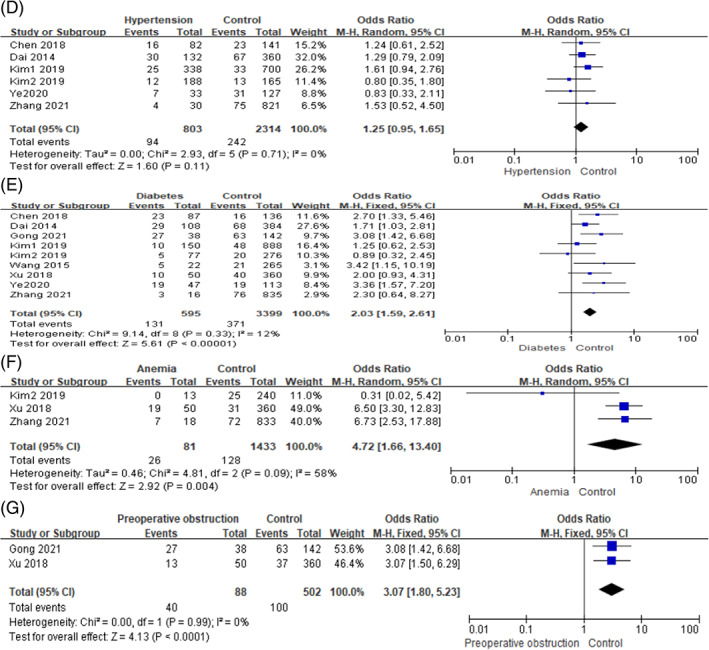
Forest plots of the meta‐analysis of (D) hypertension, (E) diabetes, (F) anaemia, (G) preoperative obstruction.
FIGURE 4.
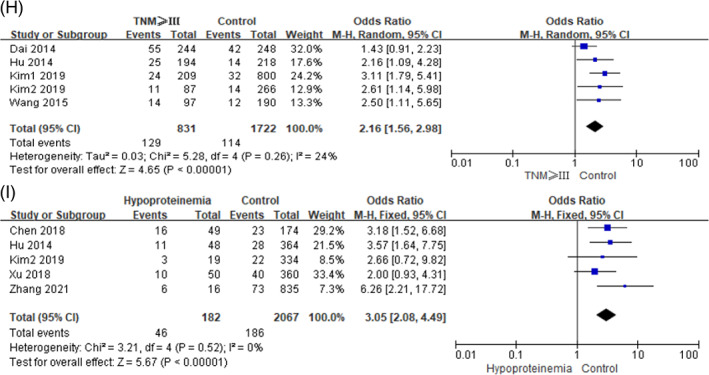
Forest plots of the meta‐analysis of (H) tumour lymph node metastasis (TNM) ≥ III, (I) hypoproteinemia.
3.4. Effect of surgical operation factors on SSI
In this study, the surgical operation factors included ASA score, operation time, surgery ways and blood transfusion. Because low heterogeneity was observed between studies in terms of these factors (I 2 ≤ 50%, P ≥ .10), we used a fixed‐effects model for meta‐analysis. The following risk factors were found to be significantly associated with SSI in gastric cancer: Operation time ≥ 3 h (OR = 8.33, 95% CI [3.81, 18.20], P < .0001), laparotomy (OR = 2.18, 95% CI [1.61, 2.94], P < .0001) and blood transfusion (OR = 1.44, 95% CI [1.01, 2.06], P = .04). ASA score was not significantly associated with SSI in gastric cancer (OR = 1.22, 95% CI [0.87, 1.72], P = .25). The forest plots of the meta‐analysis are presented in Figure 5. The main outcomes of the meta‐analysis are shown in Table 3.
FIGURE 5.
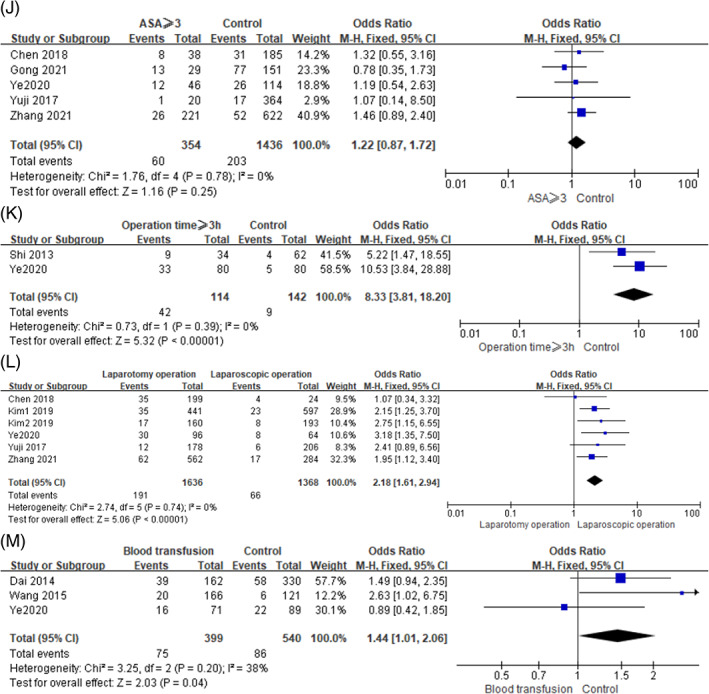
Forest plots of the meta‐analysis of (J) ASA ≥ 3, (K) operation time ≥3 h, (L) surgery ways, (M) blood transfusion.
3.5. Sensitivity analysis results of risk factors
A sensitivity analysis was performed for risk factors (male, age >60 years, smoking, diabetes, anaemia, preoperative obstruction, TNM, hypoproteinemia, operation time ≥3 h, laparotomy and blood transfusion) by switching random and fixed effects models. The sensitivity analysis results showed that there was no statistical significance when the random‐effects model was used for the blood transfusion factor (OR = 1.44, 95% CI [0.87, 2.37], P = .16), indicating the result was unstable. However, the meta‐analysis results for other factors did not change the significance, indicating that the results were robust. The sensitivity results of meta‐analyses are shown in Table 4.
TABLE 4.
Sensitivity analysis results of risk factors.
| Risk factors | Random‐effects model | Fixed‐effects model |
|---|---|---|
| OR (95% CI) P | OR (95% CI) P | |
| Male | 1.24 (1.01, 1.54) .04 | 1.28 (1.06, 1.55) .01 |
| Age >60 | 2.74 (1.64, 4.57) .0001 | 2.75 (1.65, 4.57) .0001 |
| Smoking | 1.98 (1.27, 3.10) .003 | 1.99 (1.46, 2.73) <.0001 |
| Diabetes | 2.06 (1.56, 2.72) <.00001 | 2.03 (1.59, 2.61) <.00001 |
| Anaemia | 4.72 (1.66, 13.40) .004 | 4.74 (2.83, 7.94) <.00001 |
| Preoperative obstruction | 3.07 (1.81, 5.20) <.0001 | 3.07 (1.80, 5.23) <.0001 |
| TNM ≥ III | 2.16 (1.56, 2.98) <.00001 | 2.05 (1.56, 2.70) <.00001 |
| Hypoproteinemia | 3.15 (2.14, 4.63) <.00001 | 3.05 (2.08, 4.49) <.00001 |
| Operation time ≥3 h | 8.02 (3.64, 17.67) <.00001 | 8.33 (3.81, 18.20) <.00001 |
| Laparotomy operation | 2.17 (1.61, 2.93) <.00001 | 2.18 (1.61, 2.94) <.00001 |
| Blood transfusion | 1.44 (0.87, 2.37) .16 | 1.44 (1.01, 2.06) .04 |
3.6. Evaluation of the publication bias
A total of 10 studies included data on sex factor, so we used the funnel plot and Egger's test to further determine the publication bias. The results showed that the P value was .634, indicating no publication bias on sex factor (Figures 6 and 7).
FIGURE 6.
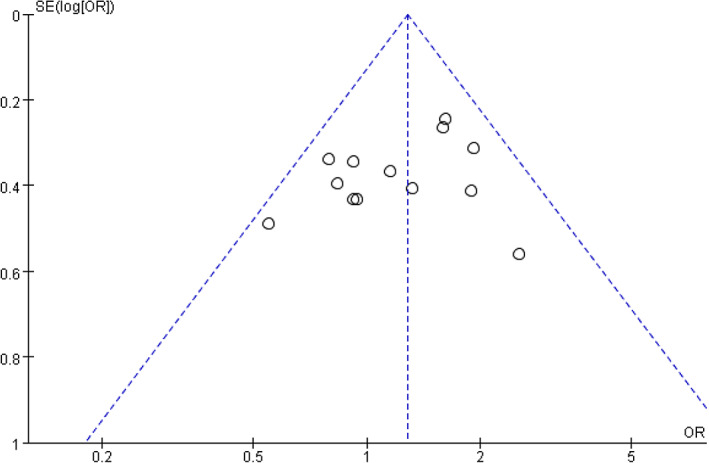
Funnel plot of sex as a risk factor.
FIGURE 7.
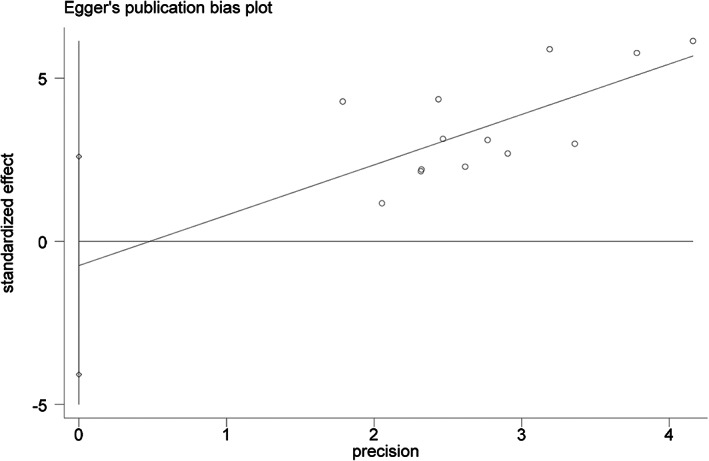
Egger plot of sex as a risk factor.
4. DISCUSSION
This meta‐analysis, including 15 articles with 6206 participants, showed that male, age >60, smoking, diabetes, anaemia, preoperative obstruction, TNM ≥ III, hypoproteinemia, operation time ≥3 h, laparotomy operation and blood transfusion were identified as risk factors for the development of SSI after surgical operation of gastric cancer patients, with low heterogeneity between results. Our findings provided much stronger and more sufficient evidence to identify and evaluate the risk factors for SSI after gastric cancer surgery.
In this meta‐analysis, smoking, male and age >60 were found to significantly increase the risk of SSI. Smoking is currently recognised as one of the risk factors for SSI. Our results indicated that the risk of SSI was 1.99 times higher in smokers with gastric cancer than in non‐smokers. A meta‐analysis study of 500 000 patients 30 reported that smoking increased the incidence of SSI by 79% after surgery. A previous study reported that smoking could cause tissue hypoxia and hypoperfusion that led to the obstruction of nutrient transport and changes in the immune response, which would result in attenuated inflammatory response mechanisms and bactericidal mechanisms. 31 These reasons can cause poor wound healing and increase infection. Our results indicated that the risk of SSI was 1.28 times higher in men than in women, which may be associated with bad habits of daily life (such as smoking, drinking, etc.) in male gastric cancer patients, being consistent with Kosuga 7 , 21 but not with Hirao. 17 In addition, this study showed that age >60 was 2.75 times as likely to increase the risk of SSI in gastric cancer patients. In elderly patients, physiological functions of organs decline with age, and other chronic diseases are common, resulting in decreased immunity and anti‐infection ability of the body, which may lead to an increased risk of SSI. 32 , 33 , 34 In perioperative management, it is notable that although gender and age are uncontrollable factors, more attention should be paid to elderly patients and male patients, and corresponding preventive measures should be given. Meanwhile, the patients should be advised to quit smoking early before surgery to reduce the occurrence of SSI.
Among the disease factors, our review showed that diabetes, anaemia, preoperative obstruction, TNM ≥ III and hypoproteinemia significantly increased the risk of SSI in patients with gastric cancer. In this study, the risk of SSI in gastric cancer patients with diabetes was 2.03 times higher than in non‐diabetes patients, which was consistent with the previous study. 8 Martin et al. reported that diabetes was 1.53 times as likely to develop surgical site infections, and it also was an independent risk factor for SSI for multiple surgical procedure types. 35 For gastric cancer patients with diabetes, the phagocytosis and bactericidal ability of WBC were reduced due to a decrease in their own immunity and hyperglycemia, which resulted in the decreased anti‐infection ability of patients and increased the opportunity of postoperative SSI. 20 In addition, abnormal blood glucose metabolism resulted in increased protein decomposition and decreased collagen synthesis, and the high blood glucose environment made it easy to breed bacteria, which prolonged postoperative wound healing time, led to an increased probability of SSI. 36 Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection recommended that it was necessary to implement perioperative glucose control and maintain blood glucose target levels less than 200 mg/dL in patients with and without diabetes. 37 A consensus report of the American College of Surgeons and Surgical Infection Society indicated that better short‐term perioperative glycemic control (110–150 mg/dL) was important to lower the SSI risk. 38 Meanwhile, postoperative or intraoperative regulation combined with postoperative regulation of blood glucose could also significantly reduce the incidence of SSI.
Our results also indicated that anaemia, preoperative obstruction, TNM ≥ III and hypoproteinemia increased the risk of SSI in gastric cancer patients by 4.74 times, 3.07 times, 2.05 times and 3.05 times, respectively. Digestive and absorption disorders and long‐term consumption of the disease may cause anaemia and hypoproteinemia and consequently raise malnutrition and reduce immune protein synthesis in patients with gastric cancer, resulting in inadequate surgical tolerance. 39 Weber et al. showed that anaemia (crude OR = 1.32, 95% CI [1.0, 1.7]) was significantly associated with an increased odds of SSI. 40 Patients with preoperative obstruction may have insufficient energy intake and malnutrition, which affect postoperative wound healing. In addition, the higher TNM stage was found to be associated with an increased risk of SSI. The higher the TNM stage, the greater the energy consumption of patients, which could raise the risk of malnutrition and decrease resistance of disease, resulting in increased difficulty of operation and prolonged operation time. 41 Chinese expert consensus on perioperative nutritional therapy for gastric cancer (2019 edition) 42 recommended that nutritional risk screening and nutritional assessment should be performed for all gastric cancer patients, and nutritional therapy should be performed 7 to 14 days before surgery for patients with moderate to severe malnutrition and undergoing major surgery, which was beneficial to reduce SSI. Meanwhile, for malnourished patients with gastric cancer, both the American Society for Parenteral and Enteral Nutrition (ASPEN) and the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines recommend oral/enteral feeding whenever possible. 43 , 44 Offering patients drink and food at will from day 1 after total gastrectomy was recommended by the ERAS consensus guidelines. 45 Therefore, in the perioperative period, the high‐risk population for SSI should be identified as early as possible, and individualised management should be taken to reduce the impact of disease factors on SSI, which can help promote postoperative recovery of patients.
Among the surgical operation factors, our review showed that operation time ≥3 h, laparotomy operation and blood transfusion significantly increased the risk of SSI in patients with gastric cancer. In our study, we found that operation time ≥3 h and laparotomy could increase the risk of SSI in gastric cancer patients by 8.33 times and 2.18 times, respectively, which was consistent with previous studies. 46 , 47 , 48 Operation time was considered to reflect complexity of the surgery 49 and it could increase the risk of SSI in gastric cancer patients by 1.52 times. 48 Inokuchi et al. also found that laparotomy surgery was associated with a significantly higher incidence of SSI than laparoscopic surgery, the former could increase the risk of SSI in gastric cancer patients by 0.5 times. 47 The prolonged operation time and laparotomy operation may lead to a prolonged period of contact between the abdominal cavity and external pathogenic microorganisms, increased intraoperative blood loss and physical trauma. 50 At the same time, blood vessels were in a state of contraction that aggravated the conditions of ischemia and hypoxia, which contributed to the increased risk of postoperative infection. 28 In our study, blood transfusion was also identified as a risk factor for the development of SSI. It had been reported that blood transfusion could cause immunosuppression and reduce the ability of anti‐infection, and the risk of infection would increase by 5% with every unit of concentrated red blood cells injected. 51 , 52
The limitations of this meta‐analysis ought to be taken into account: (1) we included only observational studies (case–control study and cohort study) published in English or Chinese, which may lead to publication bias; (2) some risk factors were included in less literature, which may be the cause of affecting the reliability of the review results; (3) because the complete details of some exposure factors were not available, related analyses based on NRS 2002 score, body mass index, length of hospital stay, or surgical season could not be performed in this meta‐analysis, which led to the loss of some information, resulting in the occurrence of reporting bias. Due to limitations in the quality and quantity of included studies, larger sample studies would be required to identify the precise indications for the above conclusions.
5. CONCLUSION
In conclusion, this meta‐analysis showed that male gender, age >60, smoking, diabetes, anaemia, preoperative obstruction, TNM ≥ III, hypoproteinemia, operation time ≥3 h, open surgery and blood transfusion were the risk factors for SSI in patients with gastric cancer. Identification of these risk factors in patients with gastric cancer would contribute to formulating relevant prevention and intervention measures to reduce the development of SSI after gastric cancer surgery.
FUNDING INFORMATION
This research received specific grant from Institute of Science and Technology of National Health Commission of the People's Republic of China (No.2021KYSHX016010201).
Supporting information
Data S1. Supporting information.
Chen M, Liang H, Chen M, et al. Risk factors for surgical site infection in patients with gastric cancer: A meta‐analysis. Int Wound J. 2023;20(9):3884‐3897. doi: 10.1111/iwj.14264
Muxin Chen and Hao Liang contributed equally to this work.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Ma YW, Li YM. Research progress in surgical treatment of gastric cancer. Med Rev. 2021;27(18):3609‐3615. [Google Scholar]
- 3. Wataru M et al. Comparisons of postoperative complications and nutritional status after proximal laparoscopic gastrectomy with Esophagogastrostomy and double‐tract reconstruction. Yonago Acta Med. 2020;63(4):335‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of nosocomial surgical site infections,1992: a modification of CDC definitions of surgical wound infections. Infect Control HospEpidemiol. 1992;13(10):606‐608. [PubMed] [Google Scholar]
- 5. Surgical Infection and Critical Care Medicine Group , Surgery Branch of Chinese Medical Association , Professional Committee of Surgeons of Surgeons Branch of Chinese Medical Doctor Association . Chinese guideline for the prevention of surgical site infection. Chinese J Gastrointest Surg. 2019;22(4):301‐314. [Google Scholar]
- 6. Liu J, Wang Y, Fu Y. Comparative analysis of effect of chlorhexidine and povidone‐iodine on preventing surgical site infection: a systematic evaluation. Nurs Res. 2021;35(14):2497‐2503. [Google Scholar]
- 7. Kosuga T, Ichikawa D, Komatsu S, et al. Clinical and surgical factors associated with organ/space surgical site infection after laparoscopic gastrectomy for gastric cancer. Surg Endosc. 2017;31(4):1667‐1674. doi: 10.1007/s00464-016-5156-7 [DOI] [PubMed] [Google Scholar]
- 8. Han WH, Oh YJ, Eom BW, Yoon HM, Kim YW, Ryu KW. Prognostic impact of infectious complications after curative gastric cancer surgery. Eur J Surg Oncol. 2020;46(7):1233‐1238. doi: 10.1016/j.ejso.2020.04.032 [DOI] [PubMed] [Google Scholar]
- 9. Liu X, Duan X, Xu J, et al. Impact of intra‐operative intraperitoneal chemotherapy on organ/space surgical site infection in patients with gastric cancer. J Hosp Infect. 2015;91(3):237‐243. doi: 10.1016/j.jhin.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ministry of Health, PRC . Diagnostic criteria for nosocomial infections(proposed). Chin Med J (Engl). 2001;05:61‐67. [Google Scholar]
- 12. National Healthcare Safety Network, Centers for Disease Control and Prevention . Surgical Site Infection (SSI) Event. http://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf. Published January 2017. Accessed January 25, 2017
- 13. Wells G, Shea B, O'Connell J. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of non‐randomised studies in meta‐analyses. Appl Eng Agric. 2014;18(6):727‐734. [Google Scholar]
- 14. The Cochrane Collaboration, Sterne JAC, Egger M, Moher D. Higgins JPT, Green S. Addressing reporting biases. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 2011. http://www.cochrane-handbook.org. Accessed May 17, 2023.
- 15. Kim JH, Kim J, Lee WJ, et al. The incidence and risk factors for surgical site infection in older adults after gastric cancer surgery: a STROBE‐compliant retrospective study. Medicine. 2019;98(32):e16739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu XF. Related factors for incision infections after gastric cancer surgery. Chinese J Hosp Infecol. 2012;22(16):3529‐3531. [Google Scholar]
- 17. Hirao M, Tsujinaka T, Imamura H, et al. Overweight is a risk factor for surgical site infection following distal gastrectomy for gastric cancer. Gastric Cancer. 2013;16(2):239‐244. [DOI] [PubMed] [Google Scholar]
- 18. Hu JG, Wang BG, Wang B. Risk factors for surgical incision infection in gastric cancer patients after surgery. Chinese J Hosp Infec. 2014;24(1):151‐153. [Google Scholar]
- 19. Dai XQ, Li J, Zhang YY. Risk factors and nursing countermeasures for incision infections after operation of gastric cancer. Chinese J Microecol. 2014;26(12):1434‐1436. [Google Scholar]
- 20. Wang RT, Wu HG, Wu ZQ, et al. Analysis of surgical site infections of gastric cancer patients. Chinese J Hosp Infec. 2015;25(3):632‐634. [Google Scholar]
- 21. Yuji T, Tadanobu S, Hiromi Y, et al. Identification of predictors of surgical site infection in patients with gastric cancer undergoing surgery with curative intent. Int Surg. 2017;102(3–4):157‐164. [Google Scholar]
- 22. Xu HB, Cai WL, Wang WM, et al. Risk factors for surgical site infectious in postoperative elderly gastric cancer patients. Chinese J Gen Surg. 2018;33(4):276‐279. [Google Scholar]
- 23. Kim JH, Kim J, Lee WJ, et al. A high visceral‐to‐subcutaneous fat ratio is an independent predictor of surgical site infection after gastrectomy. J Clin Med. 2019;8(4):494. doi: 10.3390/jcm8040494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ye X, Jin CC, Gao C, et al. Etiological characteristics and related factors for postoperative surgical site infection in radical gastrectomy patients. Chinese J Hosp Infec. 2020;30(9):1369‐1372. [Google Scholar]
- 25. Shi ZG, Chen WS, Zhong DM, et al. Analysis of surgical incision infection factors in elderly patients with gastric cancer. Chinese Med Innov. 2013;10(12):118‐119. [Google Scholar]
- 26. Endo S, Tsujinaka T, Fujitani K, et al. Risk factors for superficial incisional surgical site infection after gastrectomy: analysis of patients enrolled in a prospective randomized trial comparing skin closure methods. Gastric Cancer. 2016;19(2):639‐644. [DOI] [PubMed] [Google Scholar]
- 27. Chen J, Wei CQ, Wu WD. Preoperative prognostic nutritional index predicts postoperative surgical site infections in patients with gastric cancer. Chinese Med Innov. 2018;15(28):10‐14. [Google Scholar]
- 28. Gong YL, Niu XK, Guo Y. Analysis of influencing factors of operation site infection after radical surgery for gastric cancer. Cancer Progress. 2021;19(21):2218‐2220. [Google Scholar]
- 29. Zhang G, Cao WC, Lin F, et al. Study on risk factors for surgical site infection in radical gastric surgery. Chinese J Disinfect. 2021;38(8):638‐640. [Google Scholar]
- 30. Sørensen LT. Wound healing and infection in surgery: the clinical impact of smoking and smoking cessation: a systematic review and meta‐analysis. Arch Surg. 2012;147(4):373‐383. [DOI] [PubMed] [Google Scholar]
- 31. Ban KA, Minei JP, Laronga C, et al. Executive summary of the American College of Surgeons/surgical infection society surgical site infection guidelines—2016 update. Surg Infect (Larchmt). 2017;18(4):379‐382. [DOI] [PubMed] [Google Scholar]
- 32. Xu YJ, Jia ZZ, Li J, et al. Research progress of infective factors and preventive measures of surgical site infection in general surgery. Med Rev. 2020;26(8):1578‐1582. +1587. [Google Scholar]
- 33. Li GL, Li YN, Pan ZJ, et al. Study on related influencing factors for incision infection and drug sensitivity in patients undergoing gastric surgery. Chinese J Hosp Infecol. 2021;31(15):2323‐2326. [Google Scholar]
- 34. Wang DD, Zhang JH, Yan S, et al. Enhanced recovery after surgery in elderly patients received splenectomy combined with pericardial devascularization. Chinese Clinic Res. 2022;35(2):198‐201. [Google Scholar]
- 35. Martin ET, Kaye KS, Knott C, et al. Diabetes and risk of surgical site infection: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2016;37(1):88‐99. doi: 10.1017/ice.2015.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. He LQ. Operation room nursing measures to prevent postoperative incision infection in patients with diabetes mellitus. Chinese Med Guide. 2021;19(24):109‐110. [Google Scholar]
- 37. Berríos‐Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017 [published correction appears in JAMA Surg. 2017 Aug 1;152(8):803]. JAMA Surg. 2017;152(8):784‐791. doi: 10.1001/jamasurg.2017.0904 [DOI] [PubMed] [Google Scholar]
- 38. Ban KA, Minei JP, Laronga C, et al. American College of Surgeons and surgical infection society: surgical site infection guidelines, 2016 update. J Am Coll Surg. 2017;224(1):59‐74. doi: 10.1016/j.jamcollsurg.2016.10.029 [DOI] [PubMed] [Google Scholar]
- 39. Zhao JH, Gu ST, Tian L, et al. Indicators and risk factors of postoperative pulmonary infection in patients after laparoscopic radical resection of gastric cancer. Chinese J Hosp Infec. 2019;29(3):403‐406. [Google Scholar]
- 40. Weber WP, Zwahlen M, Reck S, et al. The association of preoperative anemia and perioperative allogeneic blood transfusion with the risk of surgical site infection. Transfusion. 2009;49(9):1964‐1970. doi: 10.1111/j.1537-2995.2009.02204.x [DOI] [PubMed] [Google Scholar]
- 41. Hu ZZ, Liao YY, Zhou YF, et al. Risk factors for surgical incision infection in patients with colorectal cancer:a meta‐analysis. J Nurs. 2021;28(8):23‐27. [Google Scholar]
- 42. Li ZY, Yan C, Li S. Consensus of Chinese expert panel on perioperative nutrition therapy of gastric cancer (2019 edition). Chin J Pract Surg. 2020;40(2):145‐151. [Google Scholar]
- 43. ASPEN Board of Directors and the Clinical Guidelines Task Force . Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients [published correction appears in JPEN J Parenter Enteral Nutr 2002 Mar‐Apr;26(2):144]. JPEN J Parenter Enteral Nutr. 2002;26(1 Suppl):1SA‐138SA. [PubMed] [Google Scholar]
- 44. Weimann A, Braga M, Carli F, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. 2017;36(3):623‐650. doi: 10.1016/j.clnu.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 45. Mortensen K, Nilsson M, Slim K, et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) society recommendations. Br J Surg. 2014;101(10):1209‐1229. doi: 10.1002/bjs.9582 [DOI] [PubMed] [Google Scholar]
- 46. Hennessey DB, Burke JP, Ni‐Dhonochu T, et al. Risk factors for surgical site infection following colorectal resection: a multi‐institutional study. Int J Colorectal Dis. 2016;31(2):267‐271. [DOI] [PubMed] [Google Scholar]
- 47. Inokuchi M, Sugita H, Otsuki S, Sato Y, Nakagawa M, Kojima K. Laparoscopic distal gastrectomy reduced surgical site infection as compared with open distal gastrectomy for gastric cancer in a meta‐analysis of both randomized controlled and case‐controlled studies. Int J Surg. 2015;15:61‐67. doi: 10.1016/j.ijsu.2015.01.030 [DOI] [PubMed] [Google Scholar]
- 48. Utsumi M, Yamada T, Yamabe K, et al. Differences in risk factors for surgical site infection between laparotomy and laparoscopy in gastrointestinal surgery. PLoS One. 2022;17(9):e0274887. doi: 10.1371/journal.pone.0274887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ushiku H, Hosoda K, Yamashita K, et al. A risk model for surgical site infection in the gastric cancer surgery using data of 790 patients. Dig Surg. 2015;32(6):472‐479. doi: 10.1159/000440703 [DOI] [PubMed] [Google Scholar]
- 50. Zhan Y, Wang W, Li ZH, et al. Comparison of the effect of laparoscopic radical gastric cancer surgery and traditional open surgery in the treatment of early gastric cancer. Cancer Progress. 2021;19(2):178‐182. [Google Scholar]
- 51. Li L, Dong L, Zhang Y, et al. Clinical study of surgical perioperative blood transfusion and postoperative infection. Chinese J Hosp Infec. 2016;26(12):2805‐2807. [Google Scholar]
- 52. Chen H. New progress in prevention and control of surgical site infection. Chinese J Clinic. 2016;44(4):12‐17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


