Abstract
After skin injury, wound repair involves a complex process in which angiogenesis plays a crucial role. Previous research has indicated that fucoidan may aid in wound healing; we therefore hypothesised that fucoidan may speed up the process by promoting angiogenesis. In this study, we investigated the potential molecular mechanism underlying fucoidan's ability to accelerate wound healing by promoting angiogenesis. Using a full‐cut wound model, we observed that fucoidan significantly intensified wound closure and promoted granulation formation and collagen deposition. Immunofluorescence staining revealed that fucoidan also promoted wound angiogenesis, specifically by accelerating the migration of new blood vessels to the middle area of the wound. Furthermore, fucoidan demonstrated the ability to enhance the proliferation of human umbilical vein endothelial cells (HUVECs) damaged by hydrogen peroxide (H2O2) and to improve the formation of endothelial tubes. Mechanistic studies revealed that fucoidan upregulated the protein levels of the AKT/Nrf2/HIF‐1α signalling pathway, which plays a crucial role in angiogenesis. This was further confirmed using the inhibitor LY294002, which reversed the promotion of endothelial tube formation by fucoidan. Overall, our findings suggest that fucoidan can promote angiogenesis via the AKT/Nrf2/HIF‐1α signalling pathway and accelerate wound healing.
Keywords: AKT/Nrf2/HIF‐1α, angiogenesis, fucoidan, human umbilical vein endothelial cell, wound healing
1. INTRODUCTION
Skin is the largest organ of the human body, providing a crucial protective barrier against external threats. However, owing to the increasing incidence of refractory wounds, burns, and chronic diseases, skin wound repair remains a significant global health challenge. 1 The wound healing process is a complex series of cellular and biochemical reactions comprising the stages of inflammation, proliferation, and remodelling. 2 Angiogenesis, the formation of new blood vessels from the existing vascular system, occurs throughout the process of wound healing and is a hallmark of both normal growth and tissue regeneration and pathological states. 3 Angiogenesis is an essential repair process after injury and is an important internal self‐protection mechanism. The formation of new blood vessels is vital for wound healing, as it supplies the cells of the wound site with oxygen and nutrients. 4
Angiogenesis involves a complex interplay of molecular and cellular processes, with endothelial cells (ECs) playing a central role. 5 During angiogenesis, ECs form the tunica intima, which is then followed by the attachment of smooth muscle cells and pericytes to form the tunica media. Finally, the tunica adventitia is composed of connective tissue, collagen, and fibres. 6 , 7 Li et al. reported that copper‐containing composite hydrogels promoted EC proliferation and tube formation, accelerating wound healing, which was confirmed by CD31 and α‐SMA costaining in tissue on Day 7. 8 Yin et al. found that extracellular vesicles secreted by PCC7942 enhanced angiogenesis and wound healing, with visualisation of the wound on Day 12. 9 Moreover, Hou et al. found that deferoxamine (DFO) boosted neovascularisation and improved diabetic wound healing, with the effect of DFO being demonstrated through haematoxylin–eosin (H&E) staining, cell migration, and tube formation experiments. 10 Nonetheless, the intricacies of angiogenesis in wound repair, including the related signalling pathways and distribution of neovascularisation, have yet to be thoroughly investigated.
Fucoidan, a unique water‐soluble sulfated polysaccharide, exhibits diverse biological activities such as anti‐tumour, anti‐inflammatory, anti‐oxidant, and anti‐coagulant properties, which have been extensively investigated. 11 Chen et al. have demonstrated that fucoidan can regulate calcium levels in the body, thereby augmenting the sensitivity of keratinocytes to calcium ions, inducing keratinocyte differentiation under low calcium levels, and promoting epidermal barrier repair. 12 Moreover, studies have revealed that fucoidan mitigates lipid peroxidation, enhances antioxidant enzyme activity, suppresses the expression of inflammatory factors, and interacts with growth factors to promote dermal wound healing. 13 , 14 Recent research has suggested that fucoidan can regulate heparin‐binding growth factors, such as FGF‐2, to generate angiogenesis in vitro. 15 It can also expedite bone repair and promote angiogenesis by stimulating the release of vascular endothelial growth factors (VEGF), as well as by stimulating the expression of VEGF, nitric oxide synthesis, and eNOS activation, thereby increasing angiogenesis in ischemic regions. 16 , 17 However, the molecular mechanism by which fucoidan promotes angiogenesis during wound repair, particularly the distribution of neovascularisation in the wound area, remains poorly understood.
This study aimed to clarify the underlying function of fucoidan in wound healing. We evaluated the effect of fucoidan on wound healing rate, granulation formation, collagen deposition, angiogenesis‐related proteins, and the levels of AKT/Nrf2/HIF‐1α signalling molecules. Particularly, we investigated the distribution and status of neovascularisation in the wound area. Additionally, we examined how fucoidan impacted the proliferation and tube formation of ECs that were damaged by hydrogen peroxide.
2. MATERIALS AND METHODS
2.1. Experimental reagents and antibodies
Fucoidan was purchased from Macklin (Shanghai, China). HIF‐1α (Cat. #36169) and p‐Akt (Ser 473, Cat. #4060) antibodies were obtained from Cell Signalling (USA), and CD31 (Cat. ab281583) was purchased from Abcam (Cambridge, UK). The inhibitor LY294002 (Cat. 934 389–88‐5) was acquired from Sigma (USA), and Nrf2 (Cat. 16 396‐1‐AP), eNOS (Cat. 27 120‐1‐AP), VEGF (Cat. 19 003‐1‐AP), and GAPDH (Cat. 6004‐1‐lg) were obtained from Proteintech (Chicago, USA). The secondary antibodies (goat anti‐rabbit and goat anti‐mouse) were procured from Beyotime (Cat. A0208 and Cat. A0216, Shanghai, China), and the enhanced chemiluminescence (ECL) detection kit was obtained from Bio‐Rad (CA, USA).
2.2. Experimental animals and models
Male ICR mice of 8–10 weeks of age were supplied. The mice were anaesthetised, and their back hair was shaved. Then, 5–0 sutures were used to attach 0.5‐mm‐thick silicone ring splints (outer diameter: 16 mm, inner diameter: 8 mm) to both the sides of their backs. A 6‐mm round skin biopsy punch (Acuderm Inc., FL, USA) was used to create two full‐thickness cutaneous wounds. To prevent the mice from gnawing at the splint, the wound site was covered with a transparent dressing (3 M Health Care, Germany) and bandaged. The ICR mice were divided into a control group and a fucoidan group to evaluate the impact of fucoidan on wound healing. In situ administration (20 μl) was done to the wound area of mice every 3 days until wound closure. The control group was treated with sterile water as the solvent, and the fucoidan group was treated with fucoidan of 1.2% concentration. The wound area was measured and tracked using ImageJ on Days 0, 3, 6, 9, 12, and 14 after dosing, to determine wound closure.
2.3. Histological analysis
The mice were euthanised on Days 7 and 14 after surgery, and skin tissue from the wound area was collected for histological analysis. The collected tissue samples were fixed in 4% paraformaldehyde for 24 h, rinsed with running water for 5 h, dehydrated in a gradient of ethanol (70%, 80%, 95%, and 100%), embedded in paraffin, and cut into 5‐μm sections, which were then affixed onto slides for subsequent staining. H&E and Masson's trichrome staining were performed following the manufacturer's instructions.
2.4. Western blot analysis
Tissue or cell proteins were extracted using the RIPA lysis buffer containing a protease inhibitor mixture, phosphatase inhibitor mixture, and PMSF (phenylmethylsulfonyl fluoride; Beyotime, Shanghai, China). The extracted proteins were then centrifuged at 12,000 rpm at 4°C for 15 min, and the supernatant was collected. Bradford assay (Bio‐Rad, Richmond, USA) was employed to determine the protein concentration, and SDS‐PAGE (sodium dodecyl sulfate‐polyacrylamide electrophoresis) was performed to separate the protein extracts. The separated extracts were then transferred onto polyvinylidene difluoride membranes (Bio‐Rad). Subsequently, the membranes were blocked with 5% skimmed milk prepared with PBST (phosphate buffered saline with Tween) at room temperature for 2 h, followed by incubation with the primary antibody overnight at 4°C. After washing with PBST, the membranes were cultured with horseradish peroxidase‐conjugated secondary antibodies (Beyotime) for 2 h. Images were obtained using a Chemi DocXRS+ imaging system (Bio‐Rad), and protein bands were quantified using Quantity‐One software.
2.5. Immunofluorescence staining
The skin tissue was sectioned into 5‐μm slices following fixation, dehydration, and embedding in paraffin. The sections were dried for 30 min at 60°C, then dewaxed with xylene, hydrated through an ethanol gradient, and immersed in a solution of 3% H2O2 produced with 80% methanol at room temperature for 15 min to quench endogenous peroxidase activity. To retrieve the antigen, the slices were subjected to heat treatment in a buffer containing 10 mM sodium citrate (pH 6.0). Next, the samples were rinsed with PBS and blocked for 30 min with 5% bovine serum albumin (BSA) before incubation with the primary antibody overnight at 4°C. After re‐warming and washing with PBST, the sections were incubated with the fluorescently labelled secondary antibody at 37°C. The tablets were then sealed with an anti‐fluorescence quencher containing DAPI (diamidino phenylindole). Images were acquired using a Leica microscope.
Secondary antibodies labelled with Alexa Fluor 488 or Alexa Fluor 647 (Cat. ab150077 or Cat. ab150075, Abcam) were used for immunofluorescence staining. DAPI (Cat. P0131, Beyotime) served as an anti‐fluorescence quencher.
2.6. Cell culture and treatment
Human umbilical vein endothelial cells (HUVECs) were obtained from Zhongqiao Xinzhou (Shanghai) and cultured in endothelial cell medium (ECM) (Sciencell, California, USA) supplemented with endothelial cell growth supplement (ECGS), 1% penicillin/streptomycin (P/S), and 5% foetal bovine serum (FBS) at 37°C with 5% CO2.
HUVECs were treated with H2O2 to create an in vitro injury model. 18 Next, HUVECs were treated with 300 μg/ml fucoidan for 24 h, followed by the addition of 150 μmol/L H2O2 for 1 h. To further investigate the signalling pathway, cells were pre‐treated with the inhibitor LY294002 for 30 min before treatment with H2O2 and fucoidan.
2.7. CCK‐8 assay
Cell proliferation was determined using a CCK‐8 kit. 19 HUVECs were seeded in 96‐well plates after trypsin digestion. After 12 h, cells were exposed to varying concentrations of fucoidan (0, 1, 10, 100, 300, and 500 μg/ml) for 24 h in serum‐free media. Subsequently, 10 μl of CCK‐8 solution (Beyotime) was added per well, and a blank control group was set. The plate was then incubated for 2 h in the dark, and absorbance values at 450 nm were measured using a microplate reader.
2.8. In vitro tube formation assay
The matrigel tube formation assay is widely used to evaluate angiogenesis in in vitro experiments. 20 , 21 Matrigel solution (ABW, China, Cat. 0827045) was thawed overnight at 4°C and placed in pre‐refrigerated 48‐well plates on ice. Then, 100 μl of matrigel solution was added per well, and the well plate was placed in incubation for 1 h at 37°C to allow them to solidify. HUVECs were pre‐treated with fucoidan and H2O2 as described above, and the treated cells were then seeded in a well plate pre‐coated with matrigel. Tube formation was observed using an inverted fluorescence microscope (DM IL LED Fluo, Leica, Mannheim, Germany). The length of tubes and the number of branch points in endothelial tubes were quantified using ImageJ.
2.9. Statistical analysis
Data were presented as mean ± standard error of the mean (SEM). Analysis was conducted using SPSS software. Student's t‐test was used to compare two groups, while one‐way analysis of variance (ANOVA) and Tukey's post hoc test were implemented to compare three or more groups. P < .05 was considered a statistically significant difference.
3. RESULTS
3.1. Fucoidan accelerated wound healing in mice
To assess the efficacy of fucoidan in promoting skin wound healing, a full‐thickness skin wound model was employed in this study. The ICR mice were treated with sterile water or 1.2% fucoidan topically. As seen in Figure 1A, the fucoidan group showed significantly accelerated wound healing as compared with the control group, with a notable difference observed from day 6 after the operation (Figure 1C). To illustrate the wound healing process more comprehensively, a schematic representation is used (Figure 1B). The results indicate that fucoidan can effectively expedite the wound healing process by promoting wound closure and enhancing repair mechanisms.
FIGURE 1.
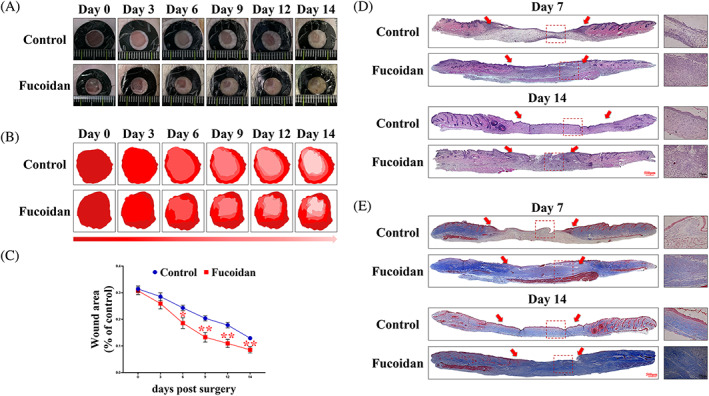
Effect of fucoidan on wound healing. (A) Representative images of wounds in the fucoidan and control groups at predetermined time points after surgery. (B) Dynamic wound healing process of the control and fucoidan groups. (C) Statistical analysis of wound area percentage in each group at different time points. *P < .05 and **P < .01 versus control, n = 6. (D, E) H&E staining and Masson staining were performed on 7 and 14 days after surgery to observe granulation and collagen deposition at the wound edge. The scale bars are 500 and 75 μm, respectively.
Histological examination of the wound tissue sections stained with H&E showed that the fucoidan group had a greater granulation thickness on postoperative Days 7 and 14 compared with the control group (Figure 1D), signifying that fucoidan enhances the speed of granulation formation. Furthermore, Masson's trichrome staining showed a significant increase in collagen deposition in the fucoidan‐treated group compared with the control group (Figure 1E). These findings suggest that fucoidan can accelerate wound healing by augmenting the production of collagen and granulation tissue.
3.2. Fucoidan induced angiogenesis in vivo
To investigate the effect of fucoidan on angiogenesis during wound repair, CD31 expression was evaluated through western blotting on Day 7. The results showed a significant increase in CD31 levels in the fucoidan group compared with the control group, with a triple expression level (Figure 2A). Direct observation of subcutaneous blood vessels in the wound skin on Day 7 showed that the fucoidan group had blood vessels twice the size of those in the control group (Figure 2B). To further confirm the angiogenesis state, the EC marker CD31 was costained with the vascular smooth muscle cell marker α‐SMA. Compared with the control group, the fucoidan group displayed more complete vascular structures, indicating that fucoidan could promote complete blood vessels (Figure 2C).
FIGURE 2.
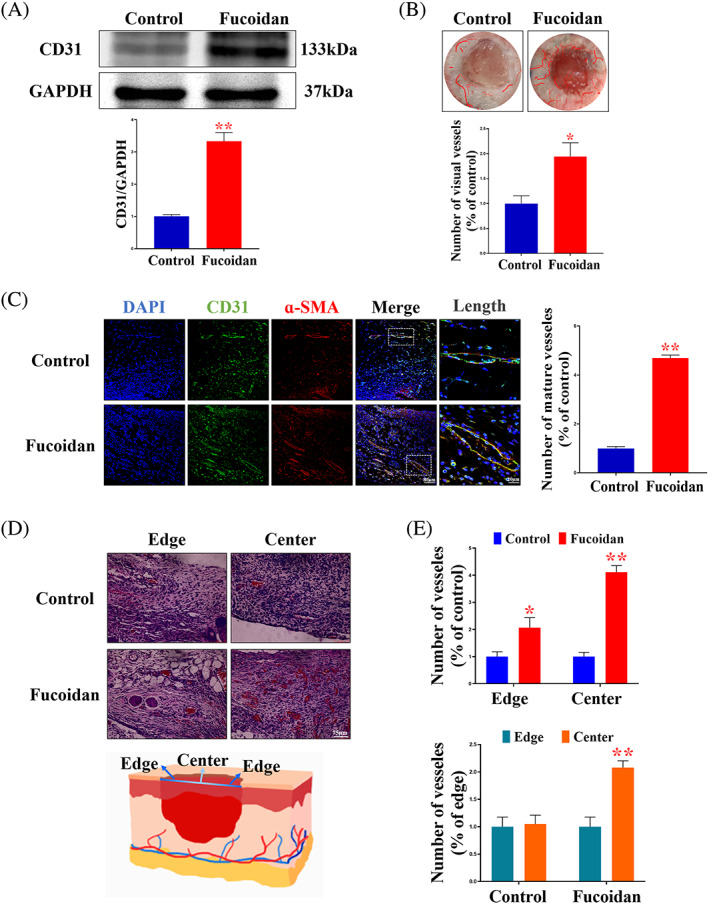
Fucoidan promotes angiogenesis in vivo. (A) Expression and quantification of CD31 protein in each group on Day 7 after surgery *P < .05 and **P < .01 versus the control, n = 3. (B) Quantification of the number of visible blood vessels and degree of vascularisation in the wound on Day 7 following surgery. *P < .05 and **P < .01 versus the control, n = 6. (C) Immunofluorescence staining and quantification of CD31 and α‐SMA in each group on the 7th day after surgery at the wound edge. Scale bars are 80 and 20 μm, respectively. *P < .05 and **P < .01 versus the control, n = 6. (D) Distribution of visual vessels observed using H&E staining on Day 7 following surgery. Scale bar = 75 μm. (E) Quantification of the number of blood vessels at the edge and middle of the wound in each group and distribution of blood vessels in the same tissue. *P < .05 and **P < .01 versus the control, n = 6.
On Day 7, the formation and distribution of neovascularisation in the wound area were characterised by H&E staining. The fucoidan group was seen to have a larger number of blood vessels in the whole wound area compared with the control group (Figure 2D). In the edge area, the number of blood vessels in the fucoidan group was twice that of the control group, while in the central area, the number of blood vessels in the fucoidan group was even higher. Furthermore, a comparison of the number of blood vessels in the central and edge regions of the wound within the same group showed a significant difference in the number of neovascularisations in the fucoidan group. The number of mature blood vessels in the central region was twice that in the edge region (Figure 2E).
3.3. Fucoidan facilitated angiogenesis by modulating the AKT/Nrf2/HIF‐1α signalling pathway
The expression of proteins implicated in angiogenesis (eNOS and VEGF) and the AKT/Nrf2/HIF‐1α signalling pathway was examined via western blot to verify whether fucoidan's influence on wound healing was linked with angiogenesis regulation (Figure 3A). The results showed that fucoidan significantly increased the expression levels of eNOS, VEGF, AKT, Nrf2, and HIF‐1α proteins (Figure 3B). Immunofluorescence costaining of CD31 and signalling pathway proteins was carried out to confirm the presence of the AKT/Nrf2/HIF‐1α signalling pathway in ECs because these cells are essential for angiogenesis development. Our findings confirmed that Nrf2 and HIF‐1α were present in ECs (Figure 3C, D).
FIGURE 3.

Fucoidan enhances angiogenesis through the AKT/Nrf2/HIF‐1α signalling pathway. (A) Western blot analysis was performed to determine the expression levels of angiogenesis‐related and signalling pathway‐related proteins in the fucoidan and control groups on Day 7 after surgery. (B) Quantitative analysis of the expression levels of angiogenesis‐related and signalling pathway‐related proteins in each group. *P < .05 and **P < .01 versus the control, n = 3. (C) Immunofluorescence costaining was used to detect the expression and localisation of Nrf2 and CD31 at the wound edge of mice on Day 7 after surgery. Scale bar = 20 μm. (D) Immunofluorescence costaining was used to investigate the expression and distribution of HIF‐1α and CD31 at the wound edge on Day 7 following surgery. Scale bar = 20 μm.
3.4. Fucoidan restored H2O2 ‐damaged tube formation in HUVEC in vitro
HUVEC proliferation is critical for angiogenesis development. 22 To explore the impact of fucoidan on HUVEC proliferation, the viability of HUVECs exposed to fucoidan at various concentrations was assessed by CCK‐8 assay (Figure 4A). Fucoidan concentrations ranging from 1 to 100 μg/mL did not affect HUVEC proliferation, but concentrations of 300 and 500 μg/mL significantly increased HUVEC viability. HUVECs damaged with 150 μmol/L H2O2 were treated with fucoidan at various concentrations to identify the appropriate treatment concentration. Our results showed that fucoidan at 100, 300, and 500 μg/mL improved HUVEC vitality and decreased H2O2‐induced damage. Based on these findings, we selected 300 μg/mL of fucoidan to protect HUVECs from H2O2‐induced damage.
FIGURE 4.

Fucoidan promotes HUVEC proliferation, while H2O2 damages tube formation. (A) The impact of various concentrations of fucoidan in serum‐free ECM medium on cell proliferation and the effect of different fucoidan concentrations on HUVEC proliferation under hydrogen peroxide treatment, were investigated. *P < .05 and **P < .01 versus the control or the H2O2, n = 6. (B) Representative images of endothelial tube formation in each group are presented. Scale bar = 50 μm. (C) Quantification of endothelial tube length and branch point numbers. *P < .05 and **P < .01 versus the H2O2, n = 3. (D) The protein expression levels of p‐Akt, Nrf2, HIF‐1α, eNOS, and VEGF in HUVECs treated with hydrogen peroxide and fucoidan were measured using western blot. (E) Quantitative analysis of the expression levels of angiogenesis‐related proteins in each group. *P < .05 and **P < .01 versus H2O2, n = 3.
Furthermore, an in vitro tube formation assay was conducted to investigate how fucoidan affected HUVEC tube formation. The formation of endothelial tubes was significantly reduced in cells treated with 150 μmol/L H2O2 compared with untreated cells when the treated cells were seeded in matrigel, and fucoidan was found to be capable of reversing the tube formation (Figure 4B). Additionally, the total length of the accumulated tube structures and their branch points were significantly increased when treated with 300 μg/mL fucoidan (Figure 4C). These results suggest that fucoidan can promote tube formation in HUVECs.
To examine how fucoidan affected the expression levels of angiogenesis‐related proteins in cells damaged by H2O2, we evaluated the expression levels of eNOS, VEGF, HIF‐1α, Nrf2, and p‐Akt proteins (Figure 4D). The results showed that the protein expression levels of eNOS, VEGF, HIF‐1α, Nrf2, and p‐Akt were reduced after H2O2 incubation. The addition of fucoidan caused the upregulation of angiogenesis‐related protein expression levels in the injured cells (Figure 4E). These findings suggest that fucoidan can rescue EC injury induced by H2O2.
3.5. The inhibitor LY294002 abolished the effect of fucoidan on endothelial tube formation
To investigate the function of AKT/Nrf2/HIF‐1α signalling in in vitro tube formation assays, we used the inhibitor LY294002 to assess how fucoidan affected tube formation in ECs. The results in Figure 5A show that the promoting effect of fucoidan on H2O2‐damaged HUVEC tube formation was inhibited by LY294002 (20 μmol/L), leading to a significant reduction in the overall length of the tube structure and the number of branch points. Compared with the fucoidan group, the LY294002 + fucoidan group showed a 50% decrease in the total length of the tube structure and the number of branching points (Figure 5B). Furthermore, the protein expression levels of p‐Akt, Nrf2, and HIF‐1α were markedly reduced after adding the inhibitor LY294002, in comparison with the fucoidan treatment group (Figure 5C). Additionally, the upregulation of eNOS and VEGF protein levels induced by fucoidan were negated by the addition of LY294002 (Figure 5D).
FIGURE 5.

The inhibitor LY294002 abolished the effect of fucoidan on tube formation in H2O2‐damaged cells. HUVECs were seeded on matrigel and pre‐treated with the inhibitor LY294002 (20 μmol/L) for 30 min, followed by cotreatment with fucoidan (300 μg/ml) and H2O2 (150 μmol/L). (A) Representative image of endothelial tube formation after pre‐treatment with LY294002 inhibitor. Scale bars = 50 μm. (B) Quantification of total tube length and branch point numbers in endothelial tubes. *P < .05 versus H2O2, # P < .05 and ## P < 0.01 versus fucoidan, n = 3. (C, D) The protein expression levels of p‐Akt, Nrf2, HIF‐1α, eNOS, and VEGF were determined and quantified by western blotting. *P < .05 versus H2O2, # P < .05 and ## P < .01 versus fucoidan, n = 3.
4. DISCUSSION
Previous research has suggested that fucoidan can enhance wound healing by promoting granulation formation, growth factor expression, and skin reconstruction, among other mechanisms. 13 , 23 However, the mechanism underlying the effect of fucoidan on wound healing remains elusive. Angiogenesis is a critical process that occurs throughout the wound healing process, and the development of a functional vascular network is essential for wound repair. Despite previous reports indicating that fucoidan can promote angiogenesis in vitro, its impact on angiogenesis and its distribution during wound healing are not yet fully understood. Moreover, previous studies have suggested that the AKT/Nrf2, Nrf2/HIF‐1α, and AKT/HIF‐1α signalling pathways are involved in angiogenesis, but the effect of the AKT/Nrf2/HIF‐1α pathway on regulating angiogenesis in wound healing is still not clear. Our study found that fucoidan can accelerate wound healing by promoting angiogenesis via the AKT/Nrf2/HIF‐1α signalling pathway (Figure 6).
FIGURE 6.
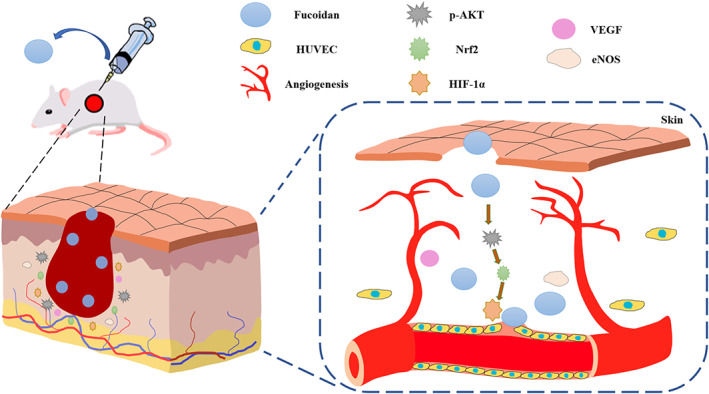
Schematic diagram showing that fucoidan enhances angiogenesis through activation of the AKT/Nrf2/HIF‐1α pathway, leading to accelerated wound healing.
The primary stage of wound healing involves the formation of granulation tissue, which consists of numerous capillaries and fibroblasts. Capillaries provide essential nutrients for granulation tissue growth, while fibroblasts play a crucial role in wound contraction. 24 , 25 The extracellular matrix's fibronectin serves as a scaffold for collagen fibres and cell migration, contributing to granulation tissue formation. 26 Ronan et al. have previously reported that fucoidan can enhance fibroblast proliferation in an in vitro wound model. 14 Additionally, Park et al. found that fucoidan accelerates angiogenesis and collagen deposition in wound tissues, promoting epithelial re‐formation. 13 In our study, we also observed that fucoidan can promote granulation formation and collagen deposition, thereby enhancing wound healing. However, the underlying molecular mechanism of fucoidan in wound healing requires further investigation.
Vascular reconstruction is of paramount importance for wound healing. 27 Angiogenesis, as a key step in wound repair, is a promising therapeutic target. In the inflammatory and oxidative stress environment, blood vessels act as transportation channels to deliver oxygen to the wounded tissue while also continuously delivering anti‐inflammatory, anti‐oxidant, and growth factors to promote wound healing. 28 , 29 , 30 During the wound healing process, macrophages migrate to the wound area, disrupt adhesion between ECs by releasing inflammatory factors, and release vascular growth factors to drive vascular germination. 31 EC migration occurs at the top of the new blood vessels, which then migrate to the wound area and form capillary structures through proliferation and differentiation. This neovascularisation provides metabolic support for tissue repair and hastens wound healing. 32 Adherence of ECs to smooth muscle cells is critical for maintaining vascular stability and integrity during angiogenesis. 33 The state of blood vessels is typically assessed by costaining the EC marker CD31 with the α‐SMA marker, which represents mature smooth muscle cells, using immunofluorescence. 34 Wound neovascularisation provides nutrients for cell migration and promotes wound healing. 35 Giraux et al. reported that fucoidan enhances HUVEC proliferation and migration in vitro. 36 Chen et al. evaluated the degree of wound angiogenesis using fluorescent staining of CD31 with α‐SMA. 37 Liu et al. examined angiogenesis in vivo by costaining CD31 with α‐SMA and used matrigel to identify tube formation in vitro. 38 Feng et al. employed H&E staining to assess neovascularisation in the wound. 4 Consistent with the results, immunofluorescence staining showed that fucoidan enhanced the stability and integrity of blood vessels, as confirmed by in vitro tube formation experiments. Furthermore, H&E staining analysis indicated that fucoidan promoted wound neovascularisation. Analysis of the distribution of neovascularisation in the wound showed that fucoidan significantly improved angiogenesis in the middle area of the wound. This suggests that fucoidan accelerates new blood vessel migration to the wound centre and assists in the re‐establishment of the vascular network. Rapid vascularisation also enhances granulation tissue formation and speeds up wound healing. Despite investigations on the distribution of neovascularisation in wounds, further research is warranted to evaluate the dynamic changes of blood vessels during the entire healing process.
The process of angiogenesis involves cellular proliferation, migration, and differentiation, with EC proliferation serving as a crucial initiating step. 39 In vitro studies have indicated that exposure to 200 μmol/L H2O2 can significantly reduce the viability of ECs. 18 , 40 In accordance with prior laboratory pre‐experiments, our investigation established that treatment with 150 μmol/L H2O2 notably reduced the survival rate of ECs. Fucoidan has been shown to promote EC proliferation in a dose‐dependent manner, 41 and we discovered that treatment with 300 μg/mL fucoidan significantly increased cell proliferation and protected the cells from H2O2‐induced damage. The formation of tubes by HUVECs is regarded as an essential indicator of angiogenesis. Wang et al. found that NO can promote the endothelial differentiation process of stem/promoter cells (SPCs) and evaluated the EC function of SPC extension through tube formation assay. 42 Consistent with these findings, our study found that fucoidan could promote HUVEC proliferation and effectively restore damaged cells to form vascular structures.
Nrf2 and HIF‐1α are recognised as having regulatory effects on angiogenesis, 43 , 44 with recent research indicating that Nrf2 is activated during angiogenesis and its absence results in a reduction in vessel density. 45 Additionally, Li et al. demonstrated that loss of Nrf2 significantly inhibits VEGF expression, migration of brain microvascular endothelial cells (BMECs), and tube formation. 46 Earlier studies pointed out that HIF‐1α could regulate the expression of growth factors such as VEGF and PDGF‐β during wound healing. 47 , 48 Liu et al. showed that HIF‐1α knockdown impaired tube formation in endothelial progenitor cells. 49
Several research studies have established the role of the AKT signalling pathway in regulating angiogenesis and wound healing. 50 , 51 Previous studies have suggested that AKT regulates Nrf2 and HIF‐1α. Qing et al. reported that tetramethylpyrazine increases flap survival by encouraging angiogenesis via the AKT/Nrf2 pathway. 52 Chen et al. demonstrated that theaflavin could enhance the formation of EC tubes damaged by H2O2 through the PI3K/Akt/Nrf2 pathway, inhibit cell apoptosis, and promote wound healing. 53 Similarly, VAP‐PLGA microspheres have been found to promote the healing of chronic skin ulcer wounds by encouraging the growth, migration, and tube formation of adipose‐derived stem cells through the PI3K/AKT/HIF‐1α pathway. 54 In this study, we have shown that the Nrf2/HIF‐1α pathway also plays a regulatory role in angiogenesis during wound healing. Hydrogen sulfide donors have been found to upregulate vascular endothelial growth factor via the Nrf2/HIF‐1α pathway and stimulate EC migration and tube formation. 55 Nrf2 knockdown has been shown to decrease HIF‐1α expression, indicating that Nrf2 can promote angiogenesis of diabetic foot ulcers by regulating the MALAT1/HIF‐1α loop. 56 However, the regulatory effect of the AKT/Nrf2/HIF‐1α pathway on angiogenesis during wound healing has not been fully established. In our study, we analysed the expression of relevant signalling molecules via western blot in both in vivo and in vitro models and found that fucoidan activates the AKT/Nrf2/HIF‐1α signalling pathway, enhancing eNOS and VEGF expression.
As a PI3K inhibitor, LY294002 markedly suppresses the downstream phosphorylation of AKT and hence is commonly employed as an AKT inhibitor. 57 , 58 The incorporation of LY294002 results in AKT phosphorylation inhibition and subsequent suppression of both HIF‐1α and Nrf2 expressions. 59 , 60 Furthermore, the expressions of eNOS and VEGF were significantly inhibited. 61 , 62 Consistent with the previous reports, we also observed a significant reduction in the expression levels of p‐AKT, Nrf2, HIF‐1α, eNOS, and VEGF upon LY294002 addition in vitro. Additionally, LY294002 effectively counteracted the promoting effect of fucoidan in endothelial tube formation.
5. CONCLUSION
This study demonstrated that fucoidan accelerated wound healing by augmenting granulation formation and collagen deposition. Additionally, fucoidan promoted the formation and stabilisation of blood vessels, accelerated the migration of blood vessels to the central area of the wound, and simultaneously upregulated the AKT/Nrf2/HIF‐1α signalling pathway to encourage wound repair. Furthermore, in vitro experiments revealed that fucoidan facilitated HUVEC proliferation. In the model of H2O2‐induced injury, fucoidan significantly increased angiogenesis‐related protein expression and promoted endothelial tube formation in damaged cells. The addition of LY294002 inhibited the regulation of the AKT/Nrf2/HIF‐1α signalling pathway and reversed the promoting effect of fucoidan on the tube formation of HUVEC damaged by H2O2. These findings show that fucoidan promotes angiogenesis and accelerates wound healing through the AKT/Nrf2/HIF‐1α signalling pathway.
AUTHOR CONTRIBUTIONS
Wenting Wen contributed to data curation, methodology, and writing the original draft. Liangliang Yang, Xin Wang performed investigation and reviewed and edited the article. Hongyu Zhang and Fangfang Wu contributed to methodology and formal analysis. Ke Xu contributed to conceptualisation, data curation, and methodology. Shaodong Chen and Zhiyong Liao contributed to conceptualisation and data curation.
FUNDING INFORMATION
This study was jointly supported by the Zhejiang Provincial Natural Science Foundation of China (LQ22H150004), the Lishui City Science and Technology Project (2022GYX30), the Wenzhou Science and Technology Innovation Project (ZY2020026), and the Ningbo Key Research and Development Program (No. 2022Z146).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no potential conflicts of interest.
ETHICS STATEMENT
All animals were kept in a pathogen‐free environment and fed ad lib. The procedures for care and use of animals were approved by the Experimental Animal Ethics Committee of Zhejiang Wenzhou Medical University (WYDW2020‐073) and all applicable institutional and governmental regulations concerning the ethical use of animals were followed.
Wen W, Yang L, Wang X, et al. Fucoidan promotes angiogenesis and accelerates wound healing through AKT/Nrf2/HIF‐1α signalling pathway. Int Wound J. 2023;20(9):3606‐3618. doi: 10.1111/iwj.14239
Wenting Wen, Liangliang Yang, and Xin Wang contributed equally to this work.
Contributor Information
Hongyu Zhang, Email: hyzhang@wmu.edu.cn.
Ke Xu, Email: godxu1987@163.com.
Zhiyong Liao, Email: zyliao@wzu.edu.cn.
DATA AVAILABILITY STATEMENT
The labeled dataset used to support the findings of this study is available from the corresponding author upon request.
REFERENCES
- 1. Russo J, Fiegel J, Brogden NK. Rheological and drug delivery characteristics of poloxamer‐based diclofenac sodium formulations for chronic wound site analgesia. Pharmaceutics. 2020;12(12):1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25(1):9‐18. [DOI] [PubMed] [Google Scholar]
- 3. Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873‐887. [DOI] [PubMed] [Google Scholar]
- 4. Feng ZH, Chen J, Yuan PT, et al. Urolithin A promotes angiogenesis and tissue regeneration in a full‐thickness cutaneous wound model. Front Pharmacol. 2022;13:806284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249‐257. [DOI] [PubMed] [Google Scholar]
- 6. Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010;22(5):617‐625. [DOI] [PubMed] [Google Scholar]
- 7. Betz C, Lenard A, Belting HG, Affolter M. Cell behaviors and dynamics during angiogenesis. Development. 2016;143(13):2249‐2260. [DOI] [PubMed] [Google Scholar]
- 8. Li Y, Xu T, Tu Z, et al. Bioactive antibacterial silica‐based nanocomposites hydrogel scaffolds with high angiogenesis for promoting diabetic wound healing and skin repair. Theranostics. 2020;10(11):4929‐4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yin H, Chen CY, Liu YW, et al. PCC7942 secretes extracellular vesicles to accelerate cutaneous wound healing by promoting angiogenesis. Theranostics. 2019;9(9):2678‐2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hou Z, Nie C, Si Z, Ma Y. Deferoxamine enhances neovascularization and accelerates wound healing in diabetic rats via the accumulation of hypoxia‐inducible factor‐1α. Diabetes Res Clin Pract. 2013;101(1):62‐71. [DOI] [PubMed] [Google Scholar]
- 11. Sanjeewa KKA, Jeon YJ. Fucoidans as scientifically and commercially important algal polysaccharides. Mar Drugs. 2021;19(6):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen Y, Li X, Gan X, et al. Fucoidan from Undaria pinnatifida ameliorates epidermal barrier disruption via keratinocyte differentiation and CaSR level regulation. Mar Drugs. 2019;17(12):660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park JH, Choi SH, Park SJ, et al. Promoting wound healing using low molecular weight fucoidan in a full‐thickness dermal excision rat model. Mar Drugs. 2017;15(4):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O'Leary R, Rerek M, Wood EJ. Fucoidan modulates the effect of transforming growth factor (TGF)‐beta1 on fibroblast proliferation and wound repopulation in in vitro models of dermal wound repair. Biol Pharm Bull. 2004;27(2):266‐270. [DOI] [PubMed] [Google Scholar]
- 15. Kim BS, Park JY, Kang HJ, Kim HJ, Lee J. Fucoidan/FGF‐2 induces angiogenesis through JNK‐ and p38‐mediated activation of AKT/MMP‐2 signalling. Biochem Biophys Res Commun. 2014;450(4):1333‐1338. [DOI] [PubMed] [Google Scholar]
- 16. Kim BS, Yang SS, You HK, Shin HI, Lee J. Fucoidan‐induced osteogenic differentiation promotes angiogenesis by inducing vascular endothelial growth factor secretion and accelerates bone repair. J Tissue Eng Regen Med. 2018;12(3):e1311‐e1324. [DOI] [PubMed] [Google Scholar]
- 17. Liu T, Wang Z, Chen X, et al. Low molecular‐weight fucoidan protects against hindlimb ischemic injury in type 2 diabetic mice through enhancing endothelial nitric oxide synthase phosphorylation. J Diabetes. 2018;10(11):820‐834. [DOI] [PubMed] [Google Scholar]
- 18. Liao L, Gong LH, Zhou MT, Xue X, Li Y, Peng C. Leonurine ameliorates oxidative stress and insufficient angiogenesis by regulating the PI3K/Akt‐eNOS signaling pathway in H2O2‐induced HUVECs. Oxidative Med Cell Longev. 2021;2021:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao HL, Wu BQ, Luo Y, et al. Exogenous hydrogen sulfide ameliorates high glucose‐induced myocardial injury & inflammation via the CIRP‐MAPK signaling pathway in H9c2 cardiac cells. Life Sci. 2018;208:315‐324. [DOI] [PubMed] [Google Scholar]
- 20. Liu S, Yao L, Wang Y, et al. Immunomodulatory hybrid micro‐nanofiber scaffolds enhance vascular regeneration. Bioact Mater. 2023;21:464‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Akgol S, Kalkan BM, Yucel D, Kocabas F. SC1 limits tube formation, branching, migration, expansion and induce apoptosis of endothelial cells. Vasc Pharmacol. 2021;141:106903. [DOI] [PubMed] [Google Scholar]
- 22. Naito H, Iba T, Takakura N. Mechanisms of new blood‐vessel formation and proliferative heterogeneity of endothelial cells. Int Immunol. 2020;32(5):295‐305. [DOI] [PubMed] [Google Scholar]
- 23. Song YS, Li H, Balcos MC, et al. Fucoidan promotes the reconstruction of skin equivalents. Korean J Physiol Pharmacol. 2014;18(4):327‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu K, Chai B, Zhang K, et al. Topical application of fibroblast growth factor 10‐PLGA microsphere accelerates wound healing via inhibition of ER stress. Oxidative Med Cell Longev. 2020;2020:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kiya K, Kubo T. Neurovascular interactions in skin wound healing. Neurochem Int. 2019;125:144‐150. [DOI] [PubMed] [Google Scholar]
- 26. Pearlstein E. Plasma membrane glycoprotein which mediates adhesion of fibroblasts to collagen. Nature. 1976;262(5568):497‐500. [DOI] [PubMed] [Google Scholar]
- 27. Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res. 2017;58(1–2):81‐94. [DOI] [PubMed] [Google Scholar]
- 28. Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49(1):35‐43. [DOI] [PubMed] [Google Scholar]
- 29. Guerra A, Belinha J, Jorge RN. Modelling skin wound healing angiogenesis: a review. J Theor Biol. 2018;459:1‐17. [DOI] [PubMed] [Google Scholar]
- 30. Okonkwo UA, DiPietro LA. Diabetes and wound angiogenesis. Int J Mol Sci. 2017;18(7):1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martin P, Gurevich DB. Macrophage regulation of angiogenesis in health and disease. Semin Cell Dev Biol. 2021;119:101‐110. [DOI] [PubMed] [Google Scholar]
- 32. Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60(1):107‐114. [DOI] [PubMed] [Google Scholar]
- 33. Breikaa RM, Denman K, Ueyama Y, et al. Loss of Jagged1 in mature endothelial cells causes vascular dysfunction with alterations in smooth muscle phenotypes. Vasc Pharmacol. 2022;145:107087. [DOI] [PubMed] [Google Scholar]
- 34. Pang L, Tian P, Cui X, et al. In situ photo‐cross‐linking hydrogel accelerates diabetic wound healing through restored hypoxia‐inducible factor 1‐alpha pathway and regulated inflammation. ACS Appl Mater Interfaces. 2021;13(25):29363‐29379. [DOI] [PubMed] [Google Scholar]
- 35. Wang CG, Lou YT, Tong MJ, et al. Asperosaponin VI promotes angiogenesis and accelerates wound healing in rats via up‐regulating HIF‐1α/VEGF signaling. Acta Pharmacol Sin. 2018;39(3):393‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Giraux JL, Matou S, Bros A, Tapon‐Bretaudière J, Letourneur D, Fischer AM. Modulation of human endothelial cell proliferation and migration by fucoidan and heparin. Eur J Cell Biol. 1998;77(4):352‐359. [DOI] [PubMed] [Google Scholar]
- 37. Chen A, He H, Ma G, et al. Biodegradable copolypeptide hydrogel prodrug accelerates dermal wound regeneration by enhanced angiogenesis and epithelialization. RSC Adv. 2018;8(19):10620‐10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu L, Wang W, Hong W, et al. Photothermal 2D nanosheets combined With Astragaloside IV for antibacterial properties and promoting angiogenesis to treat infected wounds. Front Bioeng Biotechnol. 2021;9:826011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100(6):782‐794. [DOI] [PubMed] [Google Scholar]
- 40. Sheng Y, Qiu YT, Wang YM, Chi CF, Wang B. Novel antioxidant collagen peptides of siberian sturgeon (Acipenserbaerii) Cartilages: the preparation, characterization, and cytoprotection of H2O2‐damaged human umbilical vein endothelial cells (HUVECs). Mar Drugs. 2022;20(5):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zemani F, Benisvy D, Galy‐Fauroux I, et al. Low‐molecular‐weight fucoidan enhances the proangiogenic phenotype of endothelial progenitor cells. Biochem Pharmacol. 2005;70(8):1167‐1175. [DOI] [PubMed] [Google Scholar]
- 42. Wang F, Qin K, Wang K, et al. Nitric oxide improves regeneration and prevents calcification in bio‐hybrid vascular grafts via regulation of vascular stem/progenitor cells. Cell Rep. 2022;39(12):110981. [DOI] [PubMed] [Google Scholar]
- 43. Florczyk U, Jazwa A, Maleszewska M, et al. Nrf2 regulates angiogenesis: effect on endothelial cells, bone marrow‐derived proangiogenic cells and hind limb ischemia. Antioxid Redox Signal. 2014;20(11):1693‐1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dewhirst MW, Cao Y, Li CY, Moeller B. Exploring the role of HIF‐1 in early angiogenesis and response to radiotherapy. Radiother Oncol. 2007;83(3):249‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wei Y, Gong J, Thimmulappa RK, Kosmider B, Biswal S, Duh EJ. Nrf2 acts cell‐autonomously in endothelium to regulate tip cell formation and vascular branching. Proc Natl Acad Sci U S A. 2013;110(41):E3910‐E3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li L, Pan H, Wang H, et al. Interplay between VEGF and Nrf2 regulates angiogenesis due to intracranial venous hypertension. Sci Rep. 2016;6:37338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Botusan IR, Sunkari VG, Savu O, et al. Stabilization of HIF‐1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci U S A. 2008;105(49):19426‐19431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guo J, Hu Z, Yan F, et al. Angelica dahurica promoted angiogenesis and accelerated wound healing in db/db mice via the HIF‐1α/PDGF‐β signaling pathway. Free Radic Biol Med. 2020;160:447‐457. [DOI] [PubMed] [Google Scholar]
- 49. Liu Y, Ran H, Xiao Y, et al. Knockdown of HIF‐1α impairs post‐ischemic vascular reconstruction in the brain via deficient homing and sprouting bmEPCs. Brain Pathol. 2018;28(6):860‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liang P, Jiang B, Li Y, et al. Autophagy promotes angiogenesis via AMPK/Akt/mTOR signaling during the recovery of heat‐denatured endothelial cells. Cell Death Dis. 2018;9(12):1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wei P, Zhong C, Yang X, et al. Exosomes derived from human amniotic epithelial cells accelerate diabetic wound healing via PI3K‐AKT‐mTOR‐mediated promotion in angiogenesis and fibroblast function. Burns Trauma. 2020;8:tkaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Qing L, Wu P, Zhou Z, Yu F, Tang JY. Tetramethylpyrazine improved the survival of multiterritory perforator flaps by inducing angiogenesis and suppressing apoptosis via the Akt/Nrf2 pathway. Drug Des Devel Ther. 2019;13:1437‐1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen D, Wu Z, Wu LN, Jiang J, Hu GN. Theaflavin Attenuates TBHP‐induced endothelial cells oxidative stress by activating PI3K/AKT/Nrf2 and accelerates wound healing in rats. Front Bioeng Biotechnol. 2022;10:830574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jiang W, Zhang J, Zhang X, Fan C, Huang J. VAP‐PLGA microspheres (VAP‐PLGA) promote adipose‐derived stem cells (ADSCs)‐induced wound healing in chronic skin ulcers in mice via PI3K/Akt/HIF‐1α pathway. Bioengineered. 2021;12(2):10264‐10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ling K, Xu A, Chen Y, Chen X, Li Y, Wang W. Protective effect of a hydrogen sulfide donor on balloon injury‐induced restenosis via the Nrf2/HIF‐1α signaling pathway. Int J Mol Med. 2019;43(3):1299‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jayasuriya R, Dhamodharan U, Karan AN, Anandharaj A, Rajesh K, Ramkumar KM. Role of Nrf2 in MALAT1/ HIF‐1α loop on the regulation of angiogenesis in diabetic foot ulcer. Free Radic Biol Med. 2020;156:168‐175. [DOI] [PubMed] [Google Scholar]
- 57. Bian H, Yang Q, Ma T, et al. Beneficial effects of extracts from Lucilia sericata maggots on burn wounds in rats. Mol Med Rep. 2017;16(5):7213‐7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Di Y, Chen XL. Inhibition of LY294002 in retinal neovascularization. Int J Ophthalmol. 2018;11(8):1284‐1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mu Q, Lv K, Yu J, et al. Hydrogen repairs LPS‐induced endothelial progenitor cells injury. Front Pharmacol. 2022;13:894812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen J, Gu Z, Wu M, et al. C‐reactive protein can upregulate VEGF expression to promote ADSC‐induced angiogenesis by activating HIF‐1α via CD64/PI3k/Akt and MAPK/ERK signaling pathways. Stem Cell Res Ther. 2016;7(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shen CC, Chen B, Gu JT, et al. The angiogenic related functions of bone marrow mesenchymal stem cells are promoted by CBDL rat serum via the Akt/Nrf2 pathway. Exp Cell Res. 2016;344(1):86‐94. [DOI] [PubMed] [Google Scholar]
- 62. Zhang Y, Han F, Gu L, et al. Adipose mesenchymal stem cell exosomes promote wound healing through accelerated keratinocyte migration and proliferation by activating the AKT/HIF‐1α axis. J Mol Histol. 2020;51(4):375‐383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The labeled dataset used to support the findings of this study is available from the corresponding author upon request.


