SUMMARY
Invasive fungal infections (IFI) are life-threatening complications of intensive chemotherapy treatment, with the incidence in pediatric patients ranging from 2% to 21%. In this article, we describe our 5-year experience of IFI in pediatric oncology patients and its clinical manifestations with radiological findings, treatment and outcome. A retrospective and descriptive survey of IFI in children with hematologic neoplasms was conducted at the Department of Oncology and Hematology, Zagreb Children’s Hospital. Medical charts of children 0-17 years of age, of both sexes, treated for leukemias and lymphomas from January 2016 to December 2020 were reviewed. In a 5-year period, 60 patients were treated for hematologic malignancy, acute lymphoblastic leukemia (ALL) being the most prevalent diagnosis. IFI was verified in 9 (15%) children, predominantly in patients with ALL (75%). The specific causative agent was detected in one child, whereas other infections were classified as probable pulmonary aspergillosis. All the patients received standard prophylaxis with fluconazole and treatment with liposomal amphotericin B and voriconazole. The majority of our patients achieved recovery. IFI prevention, diagnosis and treatment remain a challenge. Uniform prophylaxis and therapy protocols, as well as environmental control are of vital importance for the development of better strategies in the prevention, early detection and treatment of IFI in pediatric hematology patients.
Key words: Antifungal agents, Hematologic neoplasms, Invasive fungal infection
Introduction
Invasive fungal infections (IFI) are life-threatening complications of treatment of hematologic malignancies, which remain a major cause of morbidity and mortality. In pediatric patients, the incidence of IFI ranges from 2% to 21% and depends on the intensity of treatment, underlying malignant condition, and presence of comorbidity factors (1-4). Early diagnosis and initiation of appropriate antifungal therapy are essential for a satisfactory clinical outcome. Unfortunately, the diagnosis of this opportunistic infection remains a challenge.
The issue of antifungal prophylaxis in pediatric patients is still a matter of debate. The use of most active agents is limited by the lack of approval in children. Information on optimal dosing and schedule of prophylactic administration in pediatric patients are scarce (5).
In this retrospective study, we assessed our 5-year experience with IFI in children treated for hematologic malignancies, as well as the time and type of clinical presentation, diagnostics, etiology, and specific antifungal treatment.
Patients and Methods
A retrospective and descriptive survey of IFI diagnosed in children with hematologic malignancies was conducted at the Department of Oncology and Hematology, Zagreb Children’s Hospital. Paper and computer medical charts of children aged 0-17 years, of both sexes, treated for leukemias and lymphomas from the beginning of January 2016 to the end of December 2020 were reviewed. Epidemiologic data (sex and age) and clinical information regarding malignant diagnosis (risk group and chemotherapy protocol), as well as IFI details (time and type of presentation, diagnostics, etiology, prophylaxis and specific antifungal treatment) were extracted and entered in the designated tables. Basic descriptive statistical analysis was performed. The study was carried out in accordance with the institutional ethical standards.
Results
Throughout the observed 5-year period, 60 patients were diagnosed with hematologic malignancy at our department. The majority of children (45%) were treated for acute lymphoblastic leukemia (ALL). Hodgkin lymphoma (HL) was the second most common diagnosis (23%), followed by non-Hodgkin lymphoma (18%) and acute myeloid leukemia (AML) (8%).
Invasive fungal infections were verified in 9 (15%) children, predominantly in patients with ALL (75%). The first patient diagnosed with IFI in 2016 was provided for at another institution, and was therefore excluded from further analysis. Almost two-thirds of cases were cohorted at the end of 2018 and the beginning of 2019. Three children were situated in fully equipped rooms (HEPA filter/laminar flow/positive pressure) when IFI diagnosis was made.
The mean age at diagnosis was 7.7 (range 1-17.5) years and the disease was equally distributed between the sexes. All children were diagnosed with IFI during first-line chemotherapy regimen. A greater number of IFI episodes (50%) was detected during the consolidation phase, most commonly presenting itself as prolonged fever with respiratory symptoms during hematologic aplasia. The mean duration of febrile neutropenia prior to the diagnosis of IFI was 37 days.
While the lungs were affected in all IFI cases, systemic involvement (lungs, skin, spleen) was verified in one patient. A specific causative agent (Scedosporium) was detected in only one child, whereas other infections were classified as probable pulmonary aspergillosis. Fluconazole was standard prophylaxis in all eight patients, while voriconazole proved as an effective antifungal therapy leading to complete recovery in the majority of cases. Voriconazole was subsequently administered to 7 patients for a mean of 95 (range, 43-178) days, while one of the patients is still receiving the treatment.
Discussion
The incidence of IFI in children with ALL and lymphoma is reported to range from 0.01% to 22%, depending on the chemotherapy protocol, risk category and prophylaxis regimen (6-8). The 18.5% occurrence of IFI in our patients with ALL is, therefore, in concordance with literature data. On the other hand, episodes of IFI in children diagnosed with AML vary between 5% and 13% and are an indirect cause of death in 5%-18% of patients (9-12). In our study, only one patient diagnosed with AML developed IFI but with severe symptoms and involvement of multiple organs.
Patient-based analysis showed the major risk factors for IFI to be profound and long-lasting neutropenia, highly intensive chemotherapy, allogeneic stem cell transplantation, relapse, and AML (13-15). However, all of our patients experienced an IFI episode during the first-line treatment that did not include stem cell transplant, and ALL rather than AML proved as a risk factor. Nevertheless, neutropenia prior to IFI diagnosis was recorded in all eight patients that were treated according to the intermediate- or high-risk chemotherapy regimens. Furthermore, a greater number of IFI episodes (57%) were observed during the consolidation phase of treatment. Although this observation is not consistent with recent multicenter studies conducted in Europe and the United States, according to which IFI is most likely to occur during the induction phase, our patients still received high doses of chemotherapy based on the risk stratification throughout the consolidation phase that could increase the risk of developing opportunistic infection (16). Subsequently, we found another study of the adolescent and young adult population with results similar to ours, showing that children older than 10 years had higher rates of fungal infection following intensive consolidation or transplantation (17, 18).
Moreover, several studies have implicated that environmental factors have a direct influence on fungal spore concentration in the air in conventional patient rooms (contamination), as well as air filtration systems in air conditioners, thus increasing the risk of developing IFI (19). During March 2019, we quantitatively evaluated the presence of fungi and bacteria in the indoor air at our department. According to the Portuguese model, Diario da Republica n.°235/2013, 1° Suplemento, Serie I de 2013-12-04, the overall quantity of fungi and bacteria did not cross the reference value (the overall concentration of bacteria in the indoor air must be lower than the concentration outdoors +350 cfu/m3, while the overall concentration of molds in the indoor air must be lower than outdoors). The mandatory conditions to ensure adequate air quality include HEPA filters, positive air pressure (>15 Pa) in rooms with an air change of >20 volumes, as recommended by the consensus conference (20, 21).
Almost two-thirds of our cases were cohorted at the end of 2018 and the beginning of 2019. Our findings are consistent with the results reported by Bellanger et al., whose multivariate analysis showed that winter was especially associated with a higher risk of fungal aero-contamination, probably due to an active heating system (20). However, a significant number of IFI cases in this period were not affected solely by the winter season but by construction work in proximity.
According to the latest internationally accepted EORTC/MSG criteria, IFI are defined as proven (histopathological evidence for fungal elements in the affected tissue, with or without proven fungus by culture), probable (a combination of host factors, clinical and radiological features and mycologic evidence), and possible (clinical and imaging findings and host factors consistent with IFI but without mycologic support) (1). In our study, a specific causative agent (Scedosporium) was detected in one patient only through the skin furuncle biopsy on the left side of the thorax. Furthermore, small nodules surrounded by a halo (ground-glass opacification) were detected on computed tomography (CT) scan of the lungs (Fig. 1, patient 8 in Table 1). In addition, abdominal CT verified a hypodense inhomogeneous lesion in the spleen with post-contrast imbibition. Based on the above mentioned criteria, a proven systemic IFI with involvement of the lungs, skin and spleen was diagnosed. According to earlier studies, other non-Aspergillus molds are increasingly identified in cases of documented IFI, as observed in our patient. In the study by Mor et al., ten cases of Aspergillus infection and two Zygomycetes infections were detected, in addition to the infection with Fusarium spp. Their findings probably represent diverse etiologies of invasive mold infections and the current emergence of non-Aspergillus mold infections (22, 23).
Fig. 1.
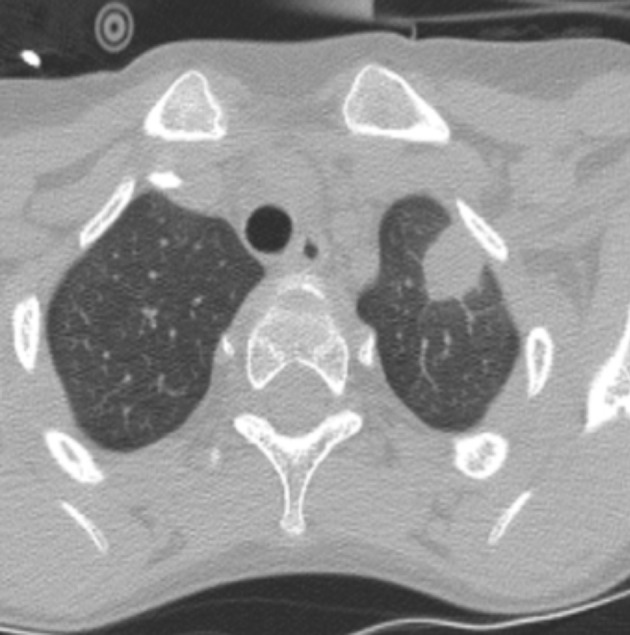
Spiculated subpleural lesion in the anterior part of the left upper lung lobe with ‘ground glass’ opacification (patient 8).
Table 1. Case description.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Age | 4 years and 1 month | 12 years and 9 months | 5 years and 6 months | 1 year and 4 months | 7 years and 4 months | 3 years and 1 month | 11 years and 2 months | 17 years and 17 months |
| Sex | Female | Male | Female | Female | Female | Male | Male | Male |
| Diagnosis | ALL | B-LL | ALL-Ph like | ALL | ALL | ALL | AML | B-LL |
| Risk group | IR | HR | HR | IR | HR | SR | IR | IR |
| Time of IFI | 9/2018 | 9/2018 | 1/2019 | 2/2019 | 3/2019 | 2/2020 | 8/2020 | 12/2020 |
| Protocol-phase | ALL IC- BMF 2009 Early intensification |
EURO LB-02 Consolidation |
ALL IC- BMF 2009 Early intensification |
ALL IC- BFM 2009 Consolidation |
ALL IC- BFM 2009 Consolidation |
ALL IC-BFM 2009 Reinduction |
Registry AML- BFM 2012 Consolidation |
B-NHL BFM 2004 Induction |
| Clinical presentation and laboratory findings |
Febrile neutropenia, increase in inflammatory parameters (CRP), cough, impaired respiratory function | Febrile neutropenia, normal inflammatory parameters, cough, deterioration of respiratory function (oxygen dependence) | Febrile neutropenia, persistence of high inflammatory parameters (CRP), cough, deterioration of respiratory function (oxygen dependence) | Febrile neutropenia, slightly elevated CRP, cough, deterioration of respiratory function (oxygen dependence) | Febrile neutropenia, increase in inflammatory parameters (CRP), cough, deterioration of respiratory function (oxygen dependence) | Prolonged fever, febrile neutropenia, increase in inflammatory parameters (CRP) | Prolonged fever, febrile neutropenia, increase in inflammatory parameters (CRP), furuncle on the left chest | Caugh, prolonged fever, febrile neutropenia, increase in inflammatory parameters (CRP) |
| Duration of FN in days prior to the diagnosis of IFI | 40 | 11 | 60 | 19 | 25 | 23 | 86 | 30 |
| Imaging | Thorax CT | Lung x-ray Thorax CT |
Lung x-ray Thorax CT |
Lung x-ray Thorax CT |
Lung x-ray Thorax CT |
Thorax CT | Thorax, abdomen and pelvis CT | Lung x-ray Thorax CT |
| Bronchoscopy | Yes | No | Yes | Yes | No | No | No | No |
| Etiology | - | - | - | - | - | - | Scedosporium | - |
| Prophylaxis (fluconazole) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Treatment | Voriconazole, Liposomal amphotericin B | Liposomal amphotericin B | Voriconazole, Liposomal amphotericin b | Voriconazole, Liposomal amphotericin B | Voriconazole, Liposomal amphotericin B | Voriconazole, Liposomal amphotericin B | Voriconazole, Liposomal amphotericin B | Voriconazole, Liposomal amphotericin B |
| Clinical outcome | Incomplete regression of changes | Complete regression of changes | Complete regression of changes after reactivation of fungal infection | Complete regression of changes | Complete regression of changes after reactivation of fungal infection | Complete regression of changes | Residual disease | Complete regression of changes |
ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; ALL IC-BFM 2009 = Randomized Trial of the International Berlin-Frankfurt-Münster Study Group for the Management of Childhood non-B Acute Lymphoblastic Leukemia; B-LL = B lymphoblastic lymphoma; B-NHL BFM 2004 = Randomized Trial of the International Berlin-Frankfurt-Münster Study Group for the Management of Childhood non-Hodgkin lymphoma; CRP = C-reactive protein; CT = computed tomography; EURO LB-02 = Treatment Protocol for Lymphoblastic Lymphoma; FN = febrile neutropenia; HR = high risk; IR = intermediate risk; Registry AML- BFM 2012 clinical registry of children and adolescents with acute myeloid leukemia; SR = standard risk.
Respiratory symptoms, predominantly cough, were the most prominent ones diagnosed in all patients alongside prolonged fever during hematologic aplasia (febrile neutropenia). Patient characteristics and IFI clinical course, diagnostics and treatment are shown in Table 1. The most remarkable imaging results are shown in Figures 1-4. All of the patients in our study had elevated C-reactive protein with normal or slightly elevated procalcitonin, and cough as one of the main symptoms. Thus, thorax CT was performed with findings of typical ground-glass opacification in some patients. CT of the lungs should be performed in high-risk patients with persistent fever for more than 96 hours who are unresponsive to broad-spectrum antibiotic therapy (24). Furthermore, endoscopically obtained bronchoalveolar lavage (BAL) for microbiological diagnosis can be performed in order to make more accurate and causative diagnosis of IFI (25). However, the diagnostic yield of BAL ranges between 25% and 50%, and depends on the causative organism, the risk profile of the patient analyzed, and the quality of the sample (26, 27). Moreover, the feasibility of performing bronchoscopy and obtaining large enough sample volumes is more challenging in neutropenic cancer children with lung infiltrates accompanied with deterioration of respiratory function. Bronchoscopy with BAL was performed in three of our patients and standard pathogen tests for identifying fungi in addition to common bacteria, mycoplasma, and tuberculosis were negative. The reasons for failure to identify the causative fungus in our patients could have been due to previous antifungal prophylaxis, the numbers of specimens obtained, the technique and time schedule for work-up of samples, and the interpretation of results (26). However, based on the clinical course, long-term febrile neutropenia unresponsive to standard treatment and radiological findings, all children were diagnosed as having IFI.
Fig. 2.
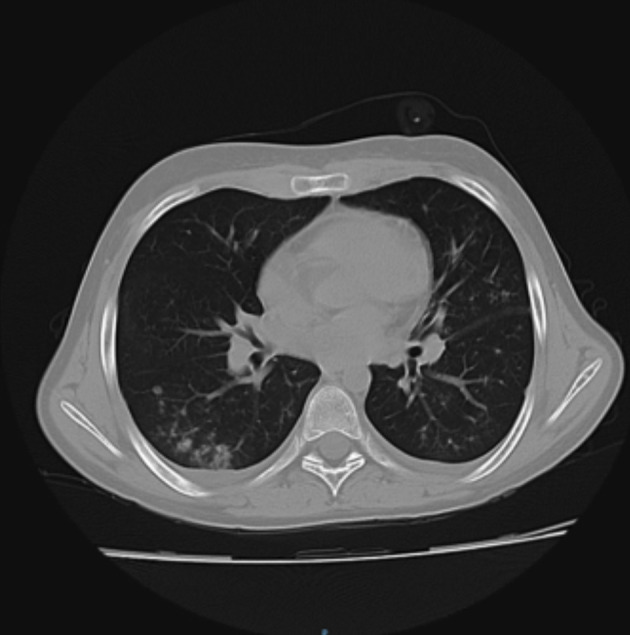
Small nodular infiltration of the lower lung bases, pronounced within the right lung base of partially confluent appearance (patient 2).
Fig. 3.
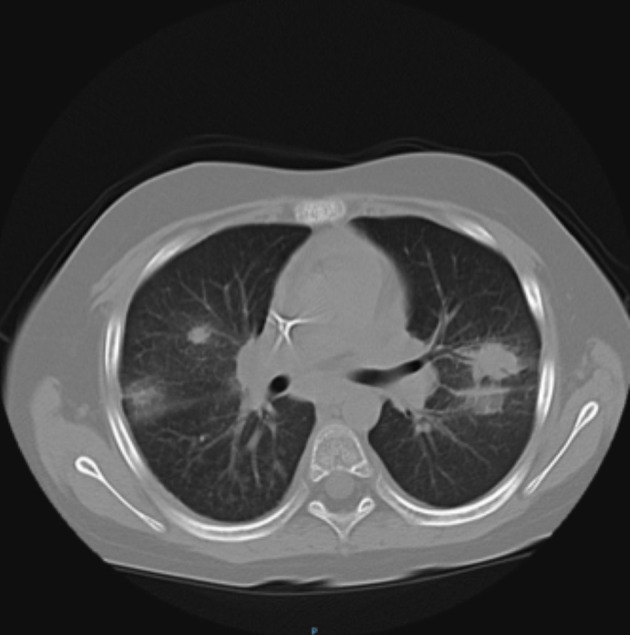
Multiple, different-sized round infiltrates bilaterally in the pulmonary parenchyma with ‘ground glass’ interstitial pattern (patient 3).
Fig. 4.
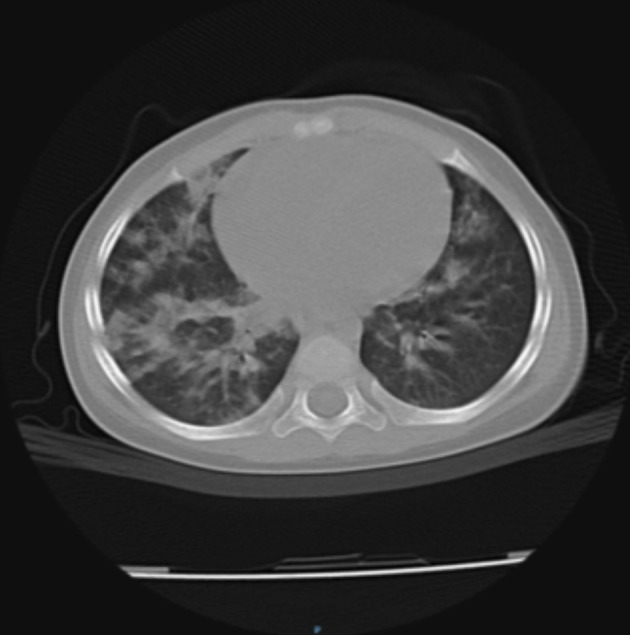
Multiple confluent lesions bilaterally in the pulmonary parenchyma and pulmonary hila (patient 4).
The most commonly recommended agent for antifungal prophylaxis is fluconazole, although it is not sufficiently active against Aspergillus spp., the second most common cause of IFI (28). Extended-spectrum azoles (voriconazole, posaconazole) possess anti-mold activity, whereas echinocandins (caspofungin) exhibit fungicidal activity against Candida spp. However, in our research, we did not detect invasive candidiasis; a shift towards mold infection might be the effect of fluconazole prophylaxis which lacks the activity against molds (29-32). According to the new guidelines from May 2020, the recommended antifungal prophylaxis in patients diagnosed with AML, relapsed ALL at a high risk of IFI, patients undergoing allogeneic hematopoietic stem cell transplant (HSCT) and those receiving systemic immunosuppression for the treatment of graft-versus-host disease includes echinocandins or mold-active azoles (33). Our retrospective research included only one patient diagnosed with ALL during 2020, but stratified as standard risk lymphoblastic leukemia. Therefore, in our 5-year study, all of the patients received standard prophylaxis (fluconazole). All of them were treated with liposomal amphotericin B as the first-line treatment according to the guidelines for febrile neutropenia (34-36). In the majority of patients, following a thorax CT scan, antifungal therapy was changed to voriconazole due to persistent and prolonged fever and a high suspicion of Aspergillus infection. Therapeutic drug monitoring of voriconazole was performed every two weeks (37). Furthermore, voriconazole was the first choice of treatment for the patient with verified hyalohyphomycosis that constitutes a heterogeneous group of fungi including Scedosporium. According to the literature, the mortality associated with IFI has been decreasing in recent years with early detection but remains quite significant. A recent French study in HSCT centers showed a 12-week mortality of 18% in patients with probable/proven aspergillosis, while in an Italian study the mortality was 27% in patients with AML and aspergillosis, and 1-year mortality of approximately 70% was recorded in US patients undergoing HSCT and developing IFI (38-41).
Despite all of the above mentioned antifungal agents and environmental control, IFI prevention, diagnosis and treatment still remain a challenge. Larger prospective, multicenter studies using uniform prophylaxis and treatment protocols are of vital importance for the development of better strategies for the prevention, early detection and treatment of IFI in pediatric hematology patients.
References
- 1.Cesaro S, Tridello G, Castagnola E, et al. Retrospective study on the incidence and outcome of proven and probable invasive fungal infections in high-risk pediatric onco-hematological patients. Eur J Haematol. 2017;99(3):240–8. 10.1111/ejh.12910 [DOI] [PubMed] [Google Scholar]
- 2.Groll AH, Kurz M, Schneider W, et al. Five-year survey of invasive aspergillosis in pediatric cancer centres: epidemiology, management, and long-term survival. Mycoses. 1999;42(7-8):431–42. 10.1046/j.1439-0507.1999.00496.x [DOI] [PubMed] [Google Scholar]
- 3.Hovi L, Saarinen-Pihkal UM, Vettenrant K, et al. Invasive fungal infections in pediatric bone marrow transplantation recipients: single center experience of 10 years. Bone Marrow Transplant. 2000;26(9):999–1004. 10.1038/sj.bmt.1702654 [DOI] [PubMed] [Google Scholar]
- 4.Castagnola E, Rossi MR, Cesaro S, et al. Incidence of bacteremia and invasive mycoses in children with acute non-lymphoblastic leukemia: results from a multi-centre Italian study. Pediatr Blood Cancer. 2010;55(6):1103–7. 10.1002/pbc.22750 [DOI] [PubMed] [Google Scholar]
- 5.Ramos JT, Romero CA, Belda S. Clinical practice update of antifungal prophylaxis in immunocompromised children. Rev Esp Quimioter. 2019;32(5):410–25. [PMC free article] [PubMed] [Google Scholar]
- 6.O’Reilly MA, Govender D, Kirkwood AA, et al. The incidence of invasive fungal infections in children, adolescents and young adults with acute lymphoblastic leukaemia/lymphoma treated with the UKALL2011 protocol: a multicentre retrospective study. Br J Haematol. 2019;186(2):327–9. 10.1111/bjh.15798 [DOI] [PubMed] [Google Scholar]
- 7.Afzal S, Ethier MC, Dupuis LL, et al. Risk factors for infection related outcomes during induction therapy for childhood acute lymphoblastic leukemia. Pediatr Infect Dis J. 2009;28(12):1064–8. 10.1097/INF.0b013e3181aa6eae [DOI] [PubMed] [Google Scholar]
- 8.Hale KA, Shaw PJ, Dalla-Pozza L, et al. Epidemiology of paediatric invasive fungal infections and a case-control study of risk factors in acute leukaemia or post stem cell transplant. Br J Haematol. 2010;149(2):263–72. 10.1111/j.1365-2141.2009.08072.x [DOI] [PubMed] [Google Scholar]
- 9.Johnston DL, Lewis V, Yanofsky R, et al. Invasive fungal infections in paediatric acute myeloid leukaemia. Mycoses. 2013;56(4):482–7. 10.1111/myc.12063 [DOI] [PubMed] [Google Scholar]
- 10.Kaya Z, Gursel T, Kocak U, et al. Invasive fungal infections in pediatric leukemia patients receiving fluconazole prophylaxis. Pediatr Blood Cancer. 2009;52(4):470–5. 10.1002/pbc.21868 [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi R, Kaneda M, Sato T, et al. The clinical feature of invasive fungal infection in pediatric patients with hematologic and malignant diseases: a 10-year analysis at a single institution in Japan. J Pediatr Hematol Oncol. 2008;30(12):886–90. 10.1097/MPH.0b013e3181864a80 [DOI] [PubMed] [Google Scholar]
- 12.Lehrnbecher T, Varwig D, Kaiser J, et al. Infectious complications in pediatric acute myeloid leukemia: analysis of the prospective multi-institutional clinical trial AML-BFM 93. Leukemia. 2004;18(1):72–7. 10.1038/sj.leu.2403188 [DOI] [PubMed] [Google Scholar]
- 13.Sano H, Kobayashi R, Suzuki D, Kishimoto K. Bacteremia during neutropenia is a predictive factor for invasive fungal infection in children. Pediatr Int. 2013;55(2):145–50. 10.1111/ped.12031 [DOI] [PubMed] [Google Scholar]
- 14.Abbasi S, Shenep JL, Hughes WT, Flynn PM. Aspergillosis in children with cancer: a 34-year experience. Clin Infect Dis. 1999;29(5):1210–9. 10.1086/313445 [DOI] [PubMed] [Google Scholar]
- 15.Dornbusch HJ, Manzoni P, Roilides E, et al. Invasive fungal infections in children. Pediatr Infect Dis J. 2009;28(8):734–7. 10.1097/INF.0b013e3181b076b1 [DOI] [PubMed] [Google Scholar]
- 16.Wang SS, Kotecha RS, Blyth CC, et al. Invasive fungal infections in children with acute lymphoblastic leukaemia: results from four Australian centres, 2003‐2013. Pediatr Blood Cancer. 2019;66(10):e27915. 10.1002/pbc.27915 [DOI] [PubMed] [Google Scholar]
- 17.Gramatges MM, Rabin KR. The adolescent and young adult with cancer: state of the art – acute leukemias. Curr Oncol Rep. 2013;15(4):317–24. 10.1007/s11912-013-0325-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sung L, Gamis A, Alonzo TA, et al. Infections and association with different intensity of chemotherapy in children with acute myeloid leukemia. Cancer. 2009;115(5):1100–8. 10.1002/cncr.24107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boff C, Zoppas B, Aquino VR, et al. The indoor air as a potential determinant of the frequency of invasive aspergillosis in the intensive care. Mycoses. 2013;56(5):527–31. 10.1111/myc.12070 [DOI] [PubMed] [Google Scholar]
- 20.Bellanger AP, Reboux G, Demonmerot F, et al. Fungal aerocontamination exposure risk for patients in 3 successive locations of a pediatric hematology unit department: influence of air equipment and building structure on air quality. Am J Infect Control. 2017;45(10):e109–13. 10.1016/j.ajic.2017.04.283 [DOI] [PubMed] [Google Scholar]
- 21.Consensus conference . Preventing the risk of Aspergillus infection in immunocompromised patients. Bull Cancer. 2001;88(6):589–600. [PubMed] [Google Scholar]
- 22.Mor M, Gilad G, Kornreich L. Invasive fungal infections in pediatric oncology. Pediatr Blood Cancer. 2011;56(7):1092–7. 10.1002/pbc.23005 [DOI] [PubMed] [Google Scholar]
- 23.Pongas GN, Lewis RE, Samonis G, et al. Voriconazole-associated zygomycosis: a significant consequence of evolving antifungal prophylaxis and immunosuppression practices. Clin Microbiol Infect. 2009;15:93–7. 10.1111/j.1469-0691.2009.02988.x [DOI] [PubMed] [Google Scholar]
- 24.Lehrnbecher T, Robinson P, Ruhnke M, et al. Guideline for the management of fever and neutropenia in children with cancer and hematopoietic stem-cell transplantation recipients: 2017 update. J Clin Oncol. 2017;35(18):2082–94. 10.1200/JCO.2016.71.7017 [DOI] [PubMed] [Google Scholar]
- 25.Warris A, Lehrnbecher T. Progress in the diagnosis of invasive fungal disease in children. Curr Fungal Infect Rep. 2017;11(2):35–44. 10.1007/s12281-017-0274-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maschmeyer G, Beinert T, Buchheidt D, Cornely OA, Einsele H, Heinz W, et al. Diagnosis and antimicrobial therapy of lung infiltrates in febrile neutropenic patients: Guidelines of the infectious diseases working party of the German Society of Haematology and Oncology. Eur J Cancer. 2009;45(14):2462–72. 10.1016/j.ejca.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 27.Mukkada S, Kirby J, Aoiwattanakul N, Hayden RT, Caniza MA. Use of fungal diagnostics and therapy in pediatric cancer patients in resource-limited settings. Curr Clin Microbiol Rep. 2016;3(3):120–31. 10.1007/s40588-016-0038-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hicheri Y, Cook G, Cordonnier C. Antifungal prophylaxis in haematology patients: the role of voriconazole. Clin Microbiol Infect. 2012;18(2):1–15. 10.1111/j.1469-0691.2012.03772.x [DOI] [PubMed] [Google Scholar]
- 29.Dvorak CC, Fisher BT, Sung L, et al. Antifungal prophylaxis in pediatric hematology/oncology: new choices & new data. Pediatr Blood Cancer. 2012;59(1):21–6. 10.1002/pbc.23415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashley ESD, Lewis R, Lewis JS, et al. Pharmacology of systemic antifungal agents. Clin Infect Dis. 2006;43:28–39. 10.1086/504492 [DOI] [Google Scholar]
- 31.Reboli AC, Rotstein C, Pappas P, et al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007;356(24):2472–82. 10.1056/NEJMoa066906 [DOI] [PubMed] [Google Scholar]
- 32.Lehrnbecher T, Fisher BT, Phillips B. Clinical practice guideline for systemic antifungal prophylaxis in pediatric patients with. cancer and hematopoietic stem-cell transplantation recipients. J Clin Oncol. 2020;38(27):3205–16. 10.1200/JCO.20.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mora-Duarte J, Betts R, Rotstein C, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med. 2002;347(25):2020–9. 10.1056/NEJMoa021585 [DOI] [PubMed] [Google Scholar]
- 34.Wingard JR. Antifungal prophylaxis in pediatric hematology/oncology: new choices & new data. Pediatr Blood Cancer. 2012;59(1):21–6. 10.1002/pbc.23415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vardakas KZ, Michalopoulos A, Falagas M. Fluconazole versus itraconazole for antifungal prophylaxis in neutropenic patients with haematological malignancies: a meta-analysis of randomised-controlled trials. Br J Haematol. 2005;131(1):22–8. 10.1111/j.1365-2141.2005.05727.x [DOI] [PubMed] [Google Scholar]
- 36.Penack O, Schwartz S, Martus P, et al. Low-dose liposomal amphotericin B in the prevention of invasive fungal infections in patients with prolonged neutropenia: results from a randomized, single center trial. Ann Oncol. 2006;17(8):1306–12. 10.1093/annonc/mdl128 [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Chan C, Colantonio D, Seto W. Therapeutic drug monitoring of voriconazole in children. Ther Drug Monit. 2012;34(1):77–84. 10.1097/FTD.0b013e31823f3516 [DOI] [PubMed] [Google Scholar]
- 38.Pagono L, Mayor S. Invasive fungal infections in high-risk patients: report from TIMM-8 2017. Future Sci OA. 2018;4(6):FSO307. 10.4155/fsoa-2018-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pagano L, Dragonetti G, Cattaneo C, et al. Changes in the incidence of candidemia and related mortality in patients with hematologic malignancies in the last ten years. A SEIFEM 2015-B report. Haematologica. 2017;102(10):e407–10. 10.3324/haematol.2017.172536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pagano L, Caira M, Candoni A, et al. Invasive aspergillosis in patients with acute myeloid leukemia: a SEIFEM-2008 registry study. Haematologica. 2010;95(4):644–50. 10.3324/haematol.2009.012054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) database. Clin Infect Dis. 2010;50(8):1091–100. 10.1086/651263 [DOI] [PubMed] [Google Scholar]


