Abstract
In some cases, differentiating thrombotic thrombocytopenic purpura (TTP) from septic disseminated intravascular coagulation (DIC) without measuring ADAMTS13 activity is critical for urgent lifesaving plasma exchange. To investigate whether PLASMIC score without identifying the presence of schistocytes, D-dimer, fibrin/fibrinogen degradation products (FDP), FDP/D-dimer ratio, prothrombin time-international normalized ratio (PT-INR), lactate dehydrogenase (LD), hemoglobin (Hb), and LD/Hb ratio are useful in differentiating patients with TTP from those with septic DIC. Retrospective analysis was conducted on the medical records of the patients with septic DIC (32 patients) or TTP (16 patients). The PLASMIC score and other laboratory measurements all were helpful in differentiating TTP from septic DIC. When dichotomized between high risk (scores 6–7) and intermediate–low risk (scores 0–5), the PLASMIC score predicted TTP with a sensitivity of 75.0% and a specificity of 100%. However, 4 of 16 patients with TTP and 19 of 32 patients with septic DIC showed comparable PLASMIC scores of 4 or 5, making it difficult to distinguish between the two by PLASMIC score alone. Among the measurements examined, the LDH/Hb ratio was the most useful for differentiation. Receiver operating characteristic analysis of the LD/Hb ratio for predicting TTP revealed a cutoff of 53.7 (IU/10 g) (sensitivity 0.94, specificity 0.91). If the LD/Hb ratio was less than 53.7, it was unlikely that the patient had TTP. A combination of the LD/Hb ratio and the PLASMIC score may be useful for distinguishing between TTP and DIC and identifying patients who need rapid plasma exchange or caplacizumab administration.
Keywords: septic DIC, TTP, PLASMIC score, LD/Hb ratio, sepsis
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a thrombotic microangiopathy (TMA) resulting from severely diminished activity of the von Willebrand factor (VWF)-cleaving protease ADAMTS13. It is characterized by extensive intravascular thrombosis enriched with platelets, leading to thrombocytopenia, microangiopathic hemolytic anemia, and occasionally organ dysfunction. 1 Timely initiation of plasma exchange is crucial, as TTP is nearly always fatal without intervention, 2 and a deficiency in ADAMTS13 activity serves as a definitive diagnostic criterion. 3 However, due to lengthy turnaround times and limited accessibility of the ADAMTS13 assay, initial diagnosis of TTP primarily relies on clinical manifestations. Distinguishing TTP from other forms of TMA, such as hemolytic uremic syndrome (HUS) and atypical HUS, is challenging since these conditions exhibit similar clinical symptoms. To address this issue, Bendapudi et al 4 developed the PLASMIC score, which utilizes readily available laboratory parameters to identify TTP patients without measuring ADAMTS13 activity. The PLASMIC score has been validated5–8 and has demonstrated a remarkably high sensitivity (0.9–0.98) and specificity (0.46–0.92), making it a practical tool for selecting TTP patients from among TMA cases in clinical practice. Nonetheless, in real-world scenarios, clinicians unfamiliar with TTP diagnosis often face difficulties in differentiating TTP from sepsis-induced disseminated intravascular coagulation (DIC). This challenge arises due to the lack of specific laboratory tests for diagnosing DIC and the overlapping clinical manifestations, such as anemia, thrombocytopenia, bleeding, elevated D-dimer levels, multiple organ dysfunction, and occasionally the presence of fragmented red blood cells (schistocytes) seen in both TTP and septic DIC. Consequently, clinicians encounter delays in promptly and accurately identifying TTP patients, thus impeding the timely initiation of plasma exchange. Moreover, the PLASMIC score, employed to select TTP patients among those with TMA, defined by the presence of at least 1% schistocytes and platelet counts below 150 × 103/μL4, can pose challenges in emergency situations, particularly during nighttime, when determining the presence of at least 1% schistocytes in peripheral blood becomes arduous. Therefore, we decided to investigate the possibility of differentiating TTP from DIC using PLASMIC score and some laboratory measurements without measuring ADAMTS13 activity and confirming schistocytes.
Institutional Review Board approval was obtained for this study (No. 2146).
Study Design and Methods
Patients
This study included consecutive patients admitted to the Department of General Medicine in the Nara Medical University Hospital between December 2012 and February 2018, who were diagnosed with TTP or septic DIC by physicians in the Department of General Medicine. The diagnosis of patients was based on the Japanese Association for Acute Medicine (JAAM) criteria for DIC9,10 and the International Society on Thrombosis and Haemostasis (ISTH) consensus on the definition of TTP. 3 Retrospective analysis was conducted on the medical records of these patients.
ADAMTS13 Activity
The activity of ADAMTS13 and its inhibitor were measured in TTP patients using ADAMTS13-act-ELISA (Kainos, Tokyo, Japan).
The PLASMIC Score, PT-INR, D-Dimer, FDP, FDP/D-Dimer Ratio, LD, and LD/Hb Ratio
The PLASMIC score was calculated using admission data. The study aimed to determine the usefulness of the PLASMIC score in distinguishing TTP from septic DIC, without identifying the presence of schistocytes, and to investigate whether D-dimer, fibrin/fibrinogen degradation products (FDP), FDP/D-dimer ratio, prothrombin time-international normalized ratio (PT-INR), lactate dehydrogenase (LD), and LD/hemoglobin (Hb) ratio levels could aid in differentiating between TTP and DIC. Differences between the two groups were assessed using a t-test. Because the receiver operating characteristic (ROC) analysis can define the optimal cut-point value as the value whose sensitivity and specificity are the closest to the value of the area under the ROC curve and the absolute value of the difference between the sensitivity and specificity values is minimum, ROC analysis was conducted to determine the optimal cutoff points for each measurement in predicting TTP. Statistical analyses were performed using Easy R (EZR) version 1.3.6. 11
Results
Patients
The study included patients with septic DIC (n = 32) and TTP (n = 16). All TTP patients had ADAMTS13 activity levels below 0.5% and positive ADAMTS13 inhibitor titers ranging from 0.6 to 28.7 BU/mL (Table 1).
Table 1.
Characteristics of Patients with TTP and Those with Septic DIC.
| Disease | Age | Sex | ADAMTS13 activity (%) or underlying disease | Inhibitor titer (BU/mL) | PT-INR | FDP (μg/mL) | D-dimer (μg/mL) | FDP/D-dimer ratio | LD (IU/mL) | Hb (g/dL) | LD/Hb (IU/10g) | CK (IU/mL) | PLASMIC score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TTP | 72 | M | <0.5 | 2.4 | 1.4 | 44.9 | 22 | 2.0 | 2271 | 4.2 | 540.7 | 292 | 6 |
| TTP | 47 | F | <0.5 | 11.4 | 1.0 | 17.6 | 7.2 | 2.4 | 1555 | 7 | 222.1 | 90 | 7 |
| TTP | 55 | M | <0.5 | 12.9 | 1.1 | 16.3 | 7.2 | 2.3 | 2037 | 5.7 | 357.4 | 216 | 6 |
| TTP | 67 | F | <0.5 | 17.8 | 1.0 | 3.2 | 1.6 | 2.0 | 703 | 8.8 | 79.9 | 53 | 6 |
| TTP | 61 | M | <0.5 | 10 | 1.1 | 3 | 1.5 | 2.0 | 835 | 6.8 | 122.8 | 60 | 6 |
| TTP | 36 | M | <0.5 | 17.6 | 1.0 | 2.7 | 1.4 | 1.9 | 478 | 8 | 59.8 | 35 | 6 |
| TTP | 38 | M | <0.5 | 4.6 | 1.0 | 4.8 | 1.6 | 3.0 | 671 | 12.5 | 53.7 | 976 | 5 |
| TTP | 66 | M | <0.5 | 2.9 | 1.1 | 32.7 | 12.6 | 2.6 | 2737 | 14.6 | 187.5 | 299 | 4 |
| TTP | 76 | F | <0.5 | 1.2 | 1.0 | 3.9 | 2 | 2.0 | 554 | 7.8 | 71.0 | 196 | 6 |
| TTP | 65 | F | <0.5 | 28.7 | 1.0 | 4.9 | 2.4 | 2.0 | 429 | 9.9 | 43.3 | 73 | 5 |
| TTP | 49 | F | <0.5 | 4.9 | 1.1 | 12.5 | 4.7 | 2.7 | 1554 | 7.6 | 204.5 | 179 | 6 |
| TTP | 26 | M | <0.5 | 0.6 | 1.0 | 51.6 | 20.9 | 2.5 | 1910 | 5.7 | 335.1 | 131 | 6 |
| TTP | 74 | M | <0.5 | 6.5 | 1.1 | 21.9 | 9.2 | 2.4 | 1451 | 11.9 | 121.9 | 323 | 5 |
| TTP | 85 | M | <0.5 | 1 | 1.0 | 12.4 | 4.8 | 2.6 | 901 | 12.3 | 73.3 | 113 | 6 |
| TTP | 65 | M | <0.5 | 2.9 | 1.1 | 22.8 | 9.8 | 2.3 | 1498 | 7.3 | 205.2 | 390 | 6 |
| TTP | 88 | M | <0.5 | 2.3 | 1.1 | 12.3 | 4.8 | 2.6 | 809 | 9.6 | 84.3 | 58 | 6 |
| DIC | 79 | M | GI perforation | NA | 1.08 | 15.9 | 6.4 | 2.5 | 436 | 8.9 | 49.0 | 573 | 5 |
| DIC | 70 | M | Iliopsoas abscess | NA | 1.17 | 39.4 | 17.6 | 2.2 | 279 | 12.2 | 22.9 | 187 | 3 |
| DIC | 66 | F | Cellulitis | NA | 1.29 | 16.7 | 7.6 | 2.2 | 670 | 11.8 | 56.8 | 1623 | 4 |
| DIC | 57 | M | TTS | NA | 1.04 | 32.1 | 12.4 | 2.6 | 1882 | 17.1 | 110.1 | 45510 | 3 |
| DIC | 66 | M | UTI | NA | 1.5 | 68.9 | 35.7 | 1.9 | 339 | 9.2 | 36.8 | 103 | 3 |
| DIC | 68 | F | TSW infection | NA | 1.11 | 32.2 | 20 | 1.6 | 248 | 9.8 | 25.3 | 60 | 5 |
| DIC | 67 | F | Pneumonia | NA | 1.37 | 33 | 17.9 | 1.8 | 488 | 9.8 | 49.8 | 1482 | 5 |
| DIC | 58 | F | Pneumonia | NA | 2.37 | 23.5 | 9 | 2.6 | 444 | 9.5 | 46.7 | 122 | 5 |
| DIC | 74 | M | Pneumonia | NA | 2.18 | 130.2 | 56.6 | 2.3 | 1040 | 9.2 | 113.0 | 1333 | 4 |
| DIC | 84 | M | SPIPF | NA | 2.61 | 56.2 | 28.5 | 2.0 | 695 | 13.9 | 50.0 | 856 | 2 |
| DIC | 82 | M | Meningitis | NA | 1.06 | 87.1 | 38.9 | 2.2 | 315 | 11.9 | 26.5 | 99 | 4 |
| DIC | 66 | F | Enterocolitis | NA | 1.08 | 191 | 100 | 1.9 | 193 | 5 | 38.6 | 26 | 5 |
| DIC | 65 | M | IE | NA | 1 | 17.2 | 7.1 | 2.4 | 408 | 15.4 | 26.5 | 21 | 5 |
| DIC | 74 | F | Pneumonia | NA | 1.19 | 35.9 | 21.4 | 1.7 | 318 | 8.4 | 37.9 | 1372 | 3 |
| DIC | 77 | F | Acute pancreatitis | NA | 1.22 | 113.7 | 66.6 | 1.7 | 489 | 14.2 | 34.4 | 419 | 5 |
| DIC | 77 | M | Ileus | NA | 1.32 | 43.2 | 25.8 | 1.7 | 595 | 13.4 | 44.4 | 3484 | 3 |
| DIC | 68 | M | GI perforation | NA | 1.09 | 7.5 | 3 | 2.5 | 344 | 11.5 | 29.9 | 236 | 3 |
| DIC | 87 | F | GI perforation | NA | 0.97 | 8.9 | 4.8 | 1.9 | 213 | 15.4 | 13.8 | 62 | 4 |
| DIC | 82 | M | Perineal abscess | NA | 1.27 | 621 | 331 | 1.9 | 234 | 8.8 | 26.6 | 764 | 4 |
| DIC | 86 | F | SMA thrombosis | NA | 1.55 | 31.5 | 18.8 | 1.7 | 465 | 13.5 | 34.4 | 122 | 2 |
| DIC | 74 | M | Deep mycosis | NA | 3.01 | 2.9 | 1.6 | 1.8 | 255 | 9.6 | 26.6 | 34 | 4 |
| DIC | 88 | F | NOMI | NA | 1.92 | 35.2 | 13.5 | 2.6 | 419 | 12 | 34.9 | 6758 | 2 |
| DIC | 61 | M | Deep mycosis | NA | 1.17 | 16.9 | 11.2 | 1.5 | 256 | 10.5 | 24.4 | 524 | 3 |
| DIC | 81 | M | Pneumonia | NA | 1.21 | 16.1 | 19.7 | 0.8 | 300 | 9 | 33.3 | 158 | 3 |
| DIC | 44 | F | UTI | NA | 2.09 | 130.2 | 126.9 | 1.0 | 330 | 8.5 | 38.8 | 172 | 4 |
| DIC | 56 | F | Meningitis | NA | 1.11 | 5.7 | 2.7 | 2.1 | 346 | 12.7 | 27.2 | 468 | 4 |
| DIC | 67 | F | Prosthetic joint infection | NA | 1.79 | 38.8 | 49.2 | 0.8 | 385 | 9.1 | 42.3 | 92 | 3 |
| DIC | 61 | F | UTI | NA | 1.35 | 15.8 | 6.8 | 2.3 | 321 | 10.2 | 31.5 | 164 | 5 |
| DIC | 70 | F | UTI | NA | 1.72 | 12.2 | 11.2 | 1.1 | 313 | 8.7 | 36.0 | 150 | 3 |
| DIC | 79 | F | GI perforation | NA | 1.01 | 11.7 | 6.7 | 1.7 | 127 | 9.6 | 13.2 | 135 | 4 |
| DIC | 80 | M | Pneumonia | NA | 1.16 | 75 | 39.83 | 1.9 | 442 | 13.9 | 31.8 | 38 | 4 |
| DIC | 72 | M | Hepatic abscess | NA | 1.1 | 73 | 45.3 | 1.6 | 491 | 14.6 | 33.6 | 1376 | 4 |
TTP, thrombotic thrombocytopenic purpura; DIC, disseminated intravascular coagulation; M, male; F, female; PT-INR, prothrombin time-international normalized ratio; FDP, fibrin/fibrinogen degradation product; LD, lactate dehydrogenase; Hb, hemoglobin; CK, creatine kinase; GI, gastrointestinal; TSS, toxic shock syndrome; UTI, urinary tract infection; TSW, thoracoabdominal stab wound; SPIPF, streptococcus pneumonia-induced purpura fulminans; SMA, superior mesenteric artery; NOMI, nonocclusive mesenteric ischemia.
PLASMIC Score
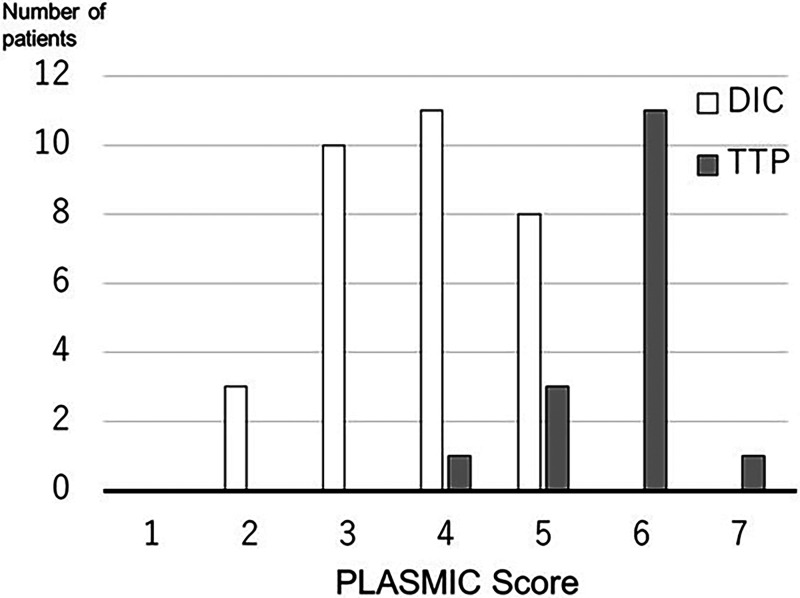
The PLASMIC scores ranged from 2 to 5 points for septic DIC patients and from 4 to 7 points for TTP patients (Figure 1). Among the total of 48 patients, 11 had PLASMIC scores of 6 or 7, indicating a high-risk group, and all of them were TTP patients. When dichotomized into high risk (scores 6–7) and intermediate-low risk (scores 0–5), the PLASMIC score predicted TTP with a sensitivity of 0.75 [95% confidence interval: 0.48 to 0.93], specificity of 1.00 [95% CI: 0.84 to 1.00], positive predictive value (PPV) of 100% [95% CI: 64.0 to 100.0], and negative predictive value (NPV) of 88.9% [95% CI: 73.9 to 96.9]. This implies that patients with a PLASMIC score of 6 or 7 can be diagnosed as having TTP rather than septic DIC, without confirming the presence of schistocytes. When dichotomized into intermediate–high risk (scores 5–7) and low risk (scores 0–4), the PLASMIC score demonstrated a sensitivity of 0.94 [95% CI: 0.70 to 1.00], specificity of 0.75 [95% CI: 0.57 to 0.89], PPV of 65.2% [95% CI: 42.7 to 83. 6], and an NPV of 96.0% [95% CI: 79.6 to 99.9]. However, among the cohort of 16 patients diagnosed with TTP, a total of 3 individuals demonstrated a PLASMIC score of 4 or 5 points. In contrast, within the group of 32 patients diagnosed with septic DIC, a substantial 19 patients exhibited an identical PLASMIC score. Consequently, relying solely on the PLASMIC score becomes challenging in practical settings to differentiate TTP patients from those with septic DIC, especially when the PLASMIC score indicates a value of 4 or 5.
Figure 1.
PLASMIC scores of patients with septic DIC or TTP. The open bar represents the distribution of the PLASMIC score in septic DIC patients, and the closed bar represents the distribution of the PLASMIC score in TTP patient. Abbreviations: DIC, disseminated intravascular coagulation; TTP, thrombotic thrombocytopenic purpura.
D-Dimer, FDP, and FDP/D-Dimer Ratio
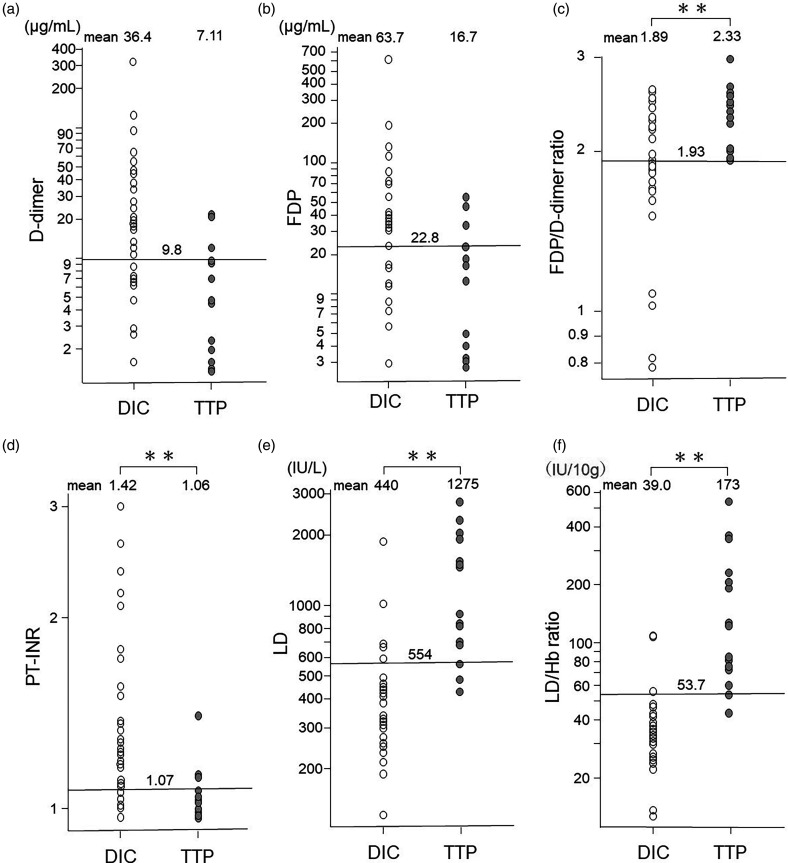
The mean value of D-dimer in patients with TTP was 7.11 μg/mL, which was not significantly lower than that observed in patients with septic DIC (36.4 μg/mL, t = 0.06) as shown in Figure 2a. ROC analysis demonstrated an AUC of 0.80 [95% CI: 0.67 to 0.93] for the D-dimer in diagnosing TTP, with a cutoff point of 9.8 (sensitivity 0.81 [95% CI: 0.54 to 0.96], specificity 0.69 [95% CI: 0.50 to 0.84]). Similarly, the mean value of FDP in TTP patients was 16.7 μg/mL, which did not significantly differ from the value in septic DIC patients (63.7 μg/mL, t = 0.10) as shown in Figure 2b. The AUC of the FDP in ROC analysis for TTP diagnosis was 0.75 [95% CI: 0.65 to 0.90], with a cutoff point of 22.8 (sensitivity 0.81 [95% CI: 0.54 to 0.96], specificity 0.63 [95% CI: 0.44 to 0.79]). Additionally, we calculated the FDP/D-dimer ratio and compared it between TTP and septic DIC patients. The average FDP/D-dimer ratio for TTP patients was 2.33, significantly higher than that observed in patients with septic DIC (1.89, t = 0.003) as shown in Figure 2c. The AUC of the FDP/D-dimer ratio in ROC analysis for TTP diagnosis was 0.78 [95% CI: 0.65 to 0.91], with a cutoff point of 1.93 (sensitivity 1.00 [95% CI: 0.71 to 1.00], specificity 0.56 [95% CI: 0.38 to 0.74]).
Figure 2.
Distribution of the values of D-dimer (a), FDP (b), FDP/D-dimer ratio (c), PT-INR (d), LD (e), and LD/Hb ratio (f) in patients with septic DIC and those with TTP. The number written at the top of the figure for each measurement is the mean value for each group. The long horizontal lines in each figure are the cutoff values for identifying TTP patients for each measurement. D-dimer and FDP were not significantly different between septic DIC and TTP, but FDP/D-dimer ratio, PT-INR, LD, and LD/Hb ratio were significantly different between the two groups. The LD/Hb ratio values showed the least overlap between the two groups. Abbreviations: FDP, fibrin/fibrinogen degradation products; LD/Hb, lactate dehydrogenase/hemoglobin; TTP, thrombotic thrombocytopenic purpura; DIC, disseminated intravascular coagulation; AUC, area under the curve; ROC, receiver operating characteristic.
PT-INR
The mean value of PT-INR in patients with TTP was 1.06, significantly lower than that observed in patients with septic DIC (1.42, t = 0.008) as shown in Figure 2d. ROC analysis indicated an AUC of 0.84 [95% CI: 0.71 to 0.96] for PT-INR in diagnosing TTP, with a cutoff point of 1.07 (sensitivity 0.81 [95% CI: 0.54 to 0.95], specificity 0.84 [95% CI: 0.67 to 0.95]).
LD and LD/Hb Ratio
The mean value of LD in patients with TTP was 1275 U/L, which was significantly higher than that observed in patients with septic DIC (440 U/L, t < 0.001) as shown in Figure 2e. ROC analysis unveiled an impressive AUC of 0.92 [95% CI: 0.85 to 1.0] for the LD in facilitating TTP diagnosis, with a discerning cutoff point of 554 (sensitivity 0.88 [95% CI: 0.62 to 0.98], specificity 0.84 [95% CI: 0.67 to 0.95], PPV 73.7% [95% CI: 48.8 to 90.9], and an NPV 93.1% [95% CI: 77.2 to 99.2]).
Moreover, we calculated and compared the LD/Hb ratio between TTP patients and those with septic DIC. The average of the LD/Hb ratio for TTP patients stood remarkably higher at 173, a significant contrast to that of DIC (39.0, p < 0.001), as illustrated in Figure 2f. ROC analysis indicated an AUC of 0.96 [95% CI: 0.91 to 1.0] for the LD/Hb ratio in TTP diagnosis (Table 2), with an impactful cutoff point of 53.7 (IU/10 g) (sensitivity 0.94 [95% CI: 0.70 to 1.00], specificity 0.91 [95% CI: 0.75 to 0.98], PPV 83.3 [95% CI: 58.6 to 96.4], NPV 96.7% [95% CI: 82.8 to 99.9]). Thus, the LD/Hb ratio demonstrated superiority in differentiating TTP from septic DIC. Remarkably, among the 16 patients diagnosed with TTP, all but one displayed an LD/Hb ratio equal to or exceeding 53.7 (IU/10 g). The exception, a truly exceptional case, exhibited an LD/Hb ratio of 43.3 (IU/10 g), alongside a PLASMIC score of 5, ADAMTS13 activity below 0.5%, an inhibitor titer of 2 BU/mL, and a platelet count of 3.0 × 104/µL, indicative of a severe manifestation of TTP. Nonetheless, this particular patient presented no signs of renal dysfunction, showed low levels of hemolysis throughout the disease course, and even had only slight positivity in urine occult blood during the diagnostic phase before treatment, which might elucidate the relatively modest values of LD and LD/Hb ratio. Hence, we confidently deemed an LD/Hb ratio of 53.7 (IU/10 g) or higher as the decisive threshold for TTP diagnosis.
Table 2.
Performance Metrics of Various Measurements for Identifying TTP.
| Cut off point | AUC | 95%CI | sensitivity | 95%CI | specificity | 95%CI | |
|---|---|---|---|---|---|---|---|
| D-dimer | 9.8 | 0.80 | 0.67–0.93 | 0.81 | 0.54–0.96 | 0.69 | 0.50–0.84 |
| FDP | 22.8 | 0.75 | 0.61–0.90 | 0.81 | 0.54–0.96 | 0.63 | 0.44–0.79 |
| FDP/D-dimer | 1.93 | 0.78 | 0.65–0.91 | 1.00 | 0.71–1.00 | 0.56 | 0.38–0.74 |
| PT-INR | 1.07 | 0.84 | 0.71–0.96 | 0.81 | 0.54–0.95 | 0.84 | 0.67–0.95 |
| LD | 554 | 0.92 | 0.85–1.00 | 0.88 | 0.62–0.98 | 0.84 | 0.67–0.95 |
| LD/Hb | 53.7 | 0.96 | 0.91–1.00 | 0.94 | 0.70–1.00 | 0.91 | 0.75–0.98 |
Intriguingly, all septic DIC patients with a PLASMIC score of 5 exhibited an LD/Hb ratio lower than 53.7, strongly suggesting the potential use of the LD/Hb ratio alongside the PLASMIC score to discriminate between TTP and septic DIC patients. Furthermore, within the septic DIC cohort, the three patients displaying LD/Hb ratios surpassing 53.7 (IU/10 g) manifested serum creatine kinase (CK) levels exceeding 1000 IU/L, while none of the TTP patients demonstrated CK levels surpassing 1000 IU/L (Table 1).
Discussion
In the realm of practical clinical scenarios, differentiating between patients with TTP and those afflicted with septic DIC often poses a challenging task. This difficulty arises due to the unavailability of immediate results for ADAMTS13 activity and the presence or absence of schistocytes in peripheral blood, particularly during emergency situations and nighttime hours. However, a rapid differential diagnosis is necessary to enable prompt plasma exchange to save the lives of patients with TTP. The primary distinction between septic DIC and TTP lies in the fact that septic DIC patients have serious infections and fibrin thrombi throughout the body, whereas TTP patients have no serious underlying disease and platelet thrombi throughout the body. However, identifying the presence of a serious infection is sometimes surprisingly difficult in actual clinical practice as it may be overshadowed by other potential causes such as connective tissue diseases or malignancies. Moreover, the early stages of a severe infection can manifest with TTP-like pathological features. Intriguingly, an experimental porcine model of septic DIC induced by intraperitoneal injection of lipopolysaccharide revealed that as early as 12 h after injection, platelet thrombi, rather than fibrin clots, were frequently observed in the kidneys, effectively simulating a TTP-like condition. 12 After that sustained stimulation to vascular endothelium and macrophages by lipopolysaccharide may consequently trigger the coagulation system and lead to DIC. These experimental findings highlight the occasional difficulty, even at the pathological level, in distinguishing between septic TTP and septic DIC. Moreover, surprisingly, the D-dimer values, indicating fibrin thrombus formation, in patients with TTP in actual clinical practice usually are all elevated than normal as shown in Table 1. Considering the above, we decided to investigate whether the PLASMIC score, which was developed to select TTP patients among TMAs that have more than 1% of schistocytes and platelets less than 150,000/μL, would be useful in differentiating TTP patients from septic DIC patients.
Several diagnostic criteria exist for septic DIC, including those formulated by the International Society on Thrombosis and Haemostasis (ISTH), 13 the Japanese Society of Thrombosis and Hemostasis (JSTH), and the Japanese Association for Acute Medicine (JAAM).9,10 In our study, we employed the diagnostic criteria established by JAAM, as it is specifically designed to detect DIC at an earlier or milder stage compared to the criteria of ISTH and JSTH. Consequently, it may have the potential to identify abnormal coagulation patterns resembling TTP in the early phase of septic DIC, which may present a condition that can make us hesitate to distinguish between the two in clinical practice.
Among the cohort of 16 patients diagnosed with TTP, a total of 13 individuals were classified within the high PLASMIC score category, exhibiting a score of 6 or higher. Strikingly, none of the patients affected by septic DIC were found to belong to this specific group. This observation suggested that the positive predictive value is 100%, indicating the possibility that the patient has TTP when a patient shows a PLASMIC score of 6 or higher. Importantly, these findings highlight that employing a PLASMIC score of 6 or 7, without necessitating the evaluation of schistocytes, not only enables the identification of TTP patients within the spectrum of TMAs but also allows for the reliable differentiation between TTP and septic DIC cases. However, it is worth noting that a notable proportion of septic DIC patients (19 out of 32 cases) and TTP patients (3 out of 16 cases) presented with a PLASMIC score of 4 or 5. This poses a considerable challenge in accurately distinguishing between these two groups when solely based on the PLASMIC score alone.
In addition to the PLASMIC score, which measurements are useful in differentiating TTP from septic DIC was examined. All measurements except D-dimer and FDP were significantly different between the two groups. Although Vincent et al 14 reported that abnormal coagulation profile including the D-dimer level can differentiate DIC from TMAs; surprisingly, none of the TTP patients had D-dimer within normal limits. Therefore, it was challenging to differentiate between TTP and DIC based solely on the presence or absence of elevated D-dimer or FDP levels. However, it was considered that when the D-dimer level was above 9.8 μg/mL or the FDP level was above 22.8 μg/mL, the likelihood of TTP was low. Additionally, the FDP/D-dimer ratio was calculated and compared between the two groups. This approach was motivated by the understanding that during hyperfibrinolysis, the FDP/D-dimer ratio tends to increase. 15 Consequently, it was hypothesized that plasminogen activator inhibitor 1 secreted from an injured vascular endothelium caused by sepsis in patients with septic DIC would suppress fibrinolysis 16 and exhibit a lower FDP/D-dimer ratio, providing a means for discrimination between the two groups. Indeed, the FDP/D-dimer ratio was significantly higher in the TTP group, and no TTP cases were observed with an FDP/D-dimer ratio below 1.93. Therefore, an FDP/D-dimer ratio below 1.93 was considered indicative of the absence of TTP. The PT-INR showed a greater AUC and a higher negative predictive value than this FDP/D-dimer ratio indicated that a PT-INR exceeding 1.07 rendered it improbable for a patient to have TTP. This observation suggests that despite the presence of elevated D-dimer levels in individuals with TTP, signifying the occurrence of fibrin thrombus formation, the extent of fibrin thrombus formation in TTP patients may be insufficient to precipitate a consumptive reduction in coagulation factors which prolong PT-INR.
There are various reports on LD levels in patients with TTP, and LD levels are considered very important in TTP. One is that the mortality rate increases when LD levels are 10 times higher than normal. 17 Another is that substituting LD for the hemolysis indicator in the PLASMIC score has been reported as diagnostically useful, albeit with lower specificity. 18 Moreover, Zhao et al 19 considered that LD might be a potent element for the early diagnosis of TTP; however, they did not ascertain the usefulness of LD values in differentiating TTP from septic DIC. The present study demonstrated that the LD value showed greater AUC than D-dimer, FDP, FDP/D-dimer ratio, or PT-INR to identify TTP patients. Furthermore, the LD value has higher sensitivity and specificity, so that patients with LD values below 554 IU/L did not seem to be TTP patients. LD is present in various tissues, including the heart, red blood cells, liver, kidneys, brain, lungs, and skeletal muscles, and is elevated in many diseases. But elevated LD in patients with TTP may be due to hemolysis. Hence, in order to obtain a more representative measure of hemolysis severity in TTP, we employed the ratio of LD to Hb levels. As can be seen in Figure 1f, the LD/Hb ratio values showed the least overlap between TTP and septic DIC patients, and the ROC analysis showed a surprisingly high AUC of 0.96 in Table 2, which was considered excellent for selecting TTP patients. At a cutoff point of 53.7 IU/10 g, the sensitivity and specificity were 0.94 and 0.91, respectively. Furthermore, the NPV of the LD/Hb ratio for TTP was remarkably high at 96.7%, suggesting that an LD/Hb ratio below 53.7 IU/10 g effectively rules out TTP. The PLASMIC score incorporates the presence or absence of hemolysis, but as a measure of the degree of hemolysis, the LD/Hb ratio may provide additional information for a TTP diagnosis. Notably, the LD/Hb ratio was 53.7 IU/10 g or greater in 15 out of 16 TTP patients (all but 1 exceptional case mentioned in the results), whereas only 3 septic DIC patients with PLASMIC scores of 3 or 4 showed LD/Hb ratios above 53.7 IU/10 g. The cause of the elevated LD/Hb ratio in these septic DIC patients is likely due to rhabdomyolysis because they had an increased level of CK, which is common in sepsis, 20 rather than hemolysis. Furthermore, the CK levels in these patients were over 1000 IU/ml, suggesting that if the cause of the elevated LD level is due to rhabdomyolysis, the condition may require an elevated CK above 1000 for the LD/Hb ratio to be above 53.7 IU/10 g.
From the above, we believe that the PLASMIC score and LD/Hb ratio can be used together to differentiate patients with TTP from those with septic DIC. In essence, irrespective of the presence or absence of schistocyte, when a patient exhibits a PLASMIC score ranging from 6 to 7, they can be classified as suffering from TTP. Moreover, even with a score falling between 4 and 5, if the LD/Hb ratio surpasses 53.7 IU/10 g and CK levels remain within the normal range, there is a strong likelihood of TTP manifestation. Naturally, by consulting other PT-INR values and FDP/D-dimer ratios as well, we can confidently ascertain the presence of TTP in patients as shown in this study.
There are several limitations to this study. The sample size is small and it is a retrospective study. Future prospective studies with larger sample sizes are needed. Although the septic DIC patients in this study had a variety of underlying diseases, LD and Hb may vary with an underlying diseases and should be investigated in septic DIC patients with more underlying diseases. However, in contrast to assessing ADAMTS13 levels or identifying the presence of schistocytes, the PLASMIC score and certain aforementioned laboratory test outcomes, notably the LD/Hb ratio, can be readily obtained from any clinical laboratory. These measurements hold the potential to distinguish TTP patients from those with septic DIC and encourage for prompt plasma exchange or caplacizumab administration. 21
Conclusion
The combination of the LD/Hb ratio with the PLASMIC score may be useful to distinguish between TTP and septic DIC and to identify patients with TTP who need rapid plasma exchange.
Footnotes
Author Contributions: NN and KY performed data and sample collection, statistical analysis, interpretation of the results, and writing of the manuscript. NY, AK, AS, SS, HK, RY, SO, KS, MM, and HF performed data and sample collection. KY and KN designed the research, interpreted the results, and wrote the paper.
MM, the inventor of the ELISA used to assess ADAMTS13 activity, received funding from Chugai Pharmaceutical. The remaining authors declare no conflicts of interest..
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Nobushiro Nishimura https://orcid.org/0000-0001-5163-0481
Kiyomi Yoshimoto https://orcid.org/0000-0001-5823-8239
Noritaka Yada https://orcid.org/0000-0002-8434-7399
Kenji Nishio https://orcid.org/0000-0003-0851-9643
References
- 1.Sadler JE. Pathophysiology of thrombotic thrombocytopenic purpura. Blood. 2017;130(10):1181-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korkmaz S, Keklik M, Sivgin S, et al. Therapeutic plasma exchange in patients with thrombotic thrombocytopenic purpura: a retrospective multicenter study. Transfus Apher Sci. 2013;48(3):353-358. [DOI] [PubMed] [Google Scholar]
- 3.Scully M, Cataland S, Coppo P, et al. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost. 2017;15(2):312-322. [DOI] [PubMed] [Google Scholar]
- 4.Bendapudi PK, Hurwitz S, Fry A, et al. Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: a cohort study. Lancet Haematol. 2017;4(4):e157-e164. [DOI] [PubMed] [Google Scholar]
- 5.Li A, Khalighi PR, Wu Q, et al. External validation of the PLASMIC score: a clinical prediction tool for thrombotic thrombocytopenic purpura diagnosis and treatment. J Thromb Haemost. 2018;16(1):164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schonermarck U, Ries W, Schroppel B, et al. Relative incidence of thrombotic thrombocytopenic purpura and haemolytic uraemic syndrome in clinically suspected cases of thrombotic microangiopathy. Clin Kidney J. 2020;13(2):208-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiscia GL, Ostuni A, Cascavilla N, et al. Validation of PLASMIC score and follow-up data in a cohort of patients with suspected microangiopathies from southern Italy. J Thromb Thrombolysis. 2018;46(2):174-179. [DOI] [PubMed] [Google Scholar]
- 8.Wynick C, Britto J, Sawler D, et al. Validation of the PLASMIC score for predicting ADAMTS13 activity <10% in patients with suspected thrombotic thrombocytopenic purpura in Alberta, Canada. Thromb Res. 2020;196:335-339. [DOI] [PubMed] [Google Scholar]
- 9.Gando S, Iba T, Eguchi Y, et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med. 2006;34(3):625-631. [DOI] [PubMed] [Google Scholar]
- 10.Gando S, Saitoh D, Ogura H, et al. A multicenter, prospective validation study of the Japanese association for acute medicine disseminated intravascular coagulation scoring system in patients with severe sepsis. Crit Care. 2013;17(3):R111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bockmeyer CL, Reuken PA, Simon TP, et al. ADAMTS13 Activity is decreased in a septic porcine model. Significance for glomerular thrombus deposition. Thromb Haemost. 2011;105(1):145-153. doi: 10.1160/TH10-03-0153 [DOI] [PubMed] [Google Scholar]
- 13.Taylor FB, Jr., Toh CH, Hoots WK, et al. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327-1330. [PubMed] [Google Scholar]
- 14.Vincent JL, Castro P, Hunt BJ, et al. Thrombocytopenia in the ICU: disseminated intravascular coagulation and thrombotic microangiopathies-what intensivists need to know. Crit Care. 2018;22(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oshiro A, Yanagida Y, Gando S, et al. Hemostasis during the early stages of trauma: comparison with disseminated intravascular coagulation. Crit Care. 2014;18(2):R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe R, Wada H, Miura Y, et al. Plasma levels of total plasminogen activator inhibitor-I (PAI-I) and tPA/PAI-1 complex in patients with disseminated intravascular coagulation and thrombotic thrombocytopenic purpura. Clin Appl Thromb Hemost. 2001;7(3):229-233. [DOI] [PubMed] [Google Scholar]
- 17.Benhamou Y, Assie C, Boelle PY, et al. Development and validation of a predictive model for death in acquired severe ADAMTS13 deficiency-associated idiopathic thrombotic thrombocytopenic purpura: the French TMA reference center experience. Haematologica. 2012;97(8):1181-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liam CCKTJ-, Yap YY, Sathar Jet al. Validating lactate dehydrogenase (LDH) as a component of the PLASMIC predictive tool (PLASMIC-LDH). Blood Res. 2023;58(1):36-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao N, Zhou L, Hu X, et al. A modified PLASMIC score including the lactate dehydrogenase/the upper limit of normal ratio more accurately identifies Chinese thrombotic thrombocytopenic purpura patients than the original PLASMIC score. J Clin Apher. 2020;35(2):79-85. [DOI] [PubMed] [Google Scholar]
- 20.Kumar AA, Bhaskar E, Palamaner Subash Shantha G, et al. Rhabdomyolysis in community acquired bacterial sepsis–a retrospective cohort study. PLoS One. 2009;4(9):e7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng XL, Vesely SK, Cataland SR, et al. ISTH guidelines for treatment of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2020;18(10):2496-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]