Abstract
Introduction
There is variability in the information available for patients after carpal tunnel release (CTR). We aimed to establish (i) what advice should be provided regarding return to driving after CTR; (ii) how work activities should be categorised and defined in relation to CTR, and when patients should be recommended to return to these activities; (iii) what wound care and rehabilitation advice should be provided after CTR.
Methods
We developed consensus recommendations from an expert panel of hand surgeons, primary care surgeons and hand therapists using an electronic Delphi process. Participants were recruited from clinical organisations using pre-defined criteria. Delphi questionnaires included open text and tick-box responses. Consensus was defined as ≥75% agreement and summary feedback was provided after each round.
Results
There were 33 panellists (21 surgeons and 12 hand therapists), of which 27 (82%) completed all rounds. Expected return to driving was agreed as 5–14 days. Expected timescales were also agreed for return to seven selected occupational activities. Post-operative advice focused on using and moving the hand, rather than specific rehabilitation. While consensus was reached for most items, there were important areas of disagreement, including divergent views on driving with sutures in situ and the need to inform car insurers.
Conclusion
Recommendations from this study expand on existing advice by including functional descriptors for occupational activities and guidance timescales generated through a formal consensus process. Areas where consensus was not reached warrant further exploration to assess whether different practices impact clinical and functional outcomes for patients.
Keywords: Carpal tunnel release, patient information, work, driving, Delphi study
Introduction
Carpal tunnel release (CTR) is a common elective procedure.1,2 Many individuals undergoing CTR wish to return to work and/or driving after their surgery, and yet there is no evidence-based guidance advising when it might be appropriate for them to do so. The Royal College of Surgeons provides general advice for patients after CTR, including expected timescales for return to several broad job titles and a recommendation for patients to return to driving when they feel comfortable and safe. 3 However, it is unclear how this advice was generated, whether the advice is being used in practice, and whether it meets the needs of CTR patients.
Our previous programme of research found marked variation in reported and recommended return to work times after CTR. Firstly, our systematic review found that mean durations of work absence ranged from 4-168 days across 56 included studies; however, the types of work that participants were returning to was poorly reported. 4 Secondly, our survey of UK hand surgeons and hand therapists explored the earliest time points recommended for patients to safely return to three different occupational activities after CTR: desk-based activities (e.g. keyboard, mouse, writing, telephone); light manual activities (e.g. driving, delivery, stacking); and heavy manual activities (e.g. construction). Reported timescales ranged from 0–30 days; 1–56 days; and 1–90 days, respectively, suggesting notable variation in practice. 5
Thirdly, we recruited a cohort of ∼200 UK patients who underwent open CTR with a mini-incision and returned to work. 6 The median duration of work absence was 20 days (interquartile range [IQR] 12–33 days). Expected duration of work absence (as reported by patients at the time of listing for surgery) was strongly associated with actual duration of work absence, regardless of other occupational or clinical factors, such as the type of work, work contract, or health status. 6 Return to driving was not specifically assessed as part of this cohort study, but it was commonly mentioned in the open text comments, suggesting that this was a pertinent issue.
Finally, qualitative interviews with a sub-sample of this cohort identified a need for clearer recommendations from clinicians, including expected timescales for return to work and driving. 7 This was desired in advance of surgery to provide guidance for employers and to help individuals and workplaces plan for the post-operative period. Most interviewees recalled following the advice provided by their surgeon with respect to driving. The importance of appropriate guidance about driving after injury or surgery has been highlighted for other musculoskeletal injuries and conditions 8 ; however there are currently no clear recommendations for return to driving after CTR. Interestingly, interviewees were reluctant to return to work before the period stated on any sickness certification, as this was viewed as acting against medical advice. This further highlights the important role that clinicians have in informing and directing return to work outcomes.
A web search in a naïve browser for ‘When can I return to work after carpal tunnel surgery?’ returned >6 million results (Google 31/01/22). The first 10 hits included patient information material from five different international healthcare organisations, and reported the following contradictory suggestions:
“If you had open surgery on your dominant hand and you do repeated actions at work, you may be able to go back to work in 6–8 weeks… If the surgery was on the other hand and you don’t do repeated actions at work, you may be able to return to work in 7–1 days”. 9
“Some persons can return to work in 4–8 weeks with the right recovery plan… [for others] recovery can take several months, needing medication and therapy”. 10
“Follow your doctor’s specific instructions on when it’s okay to return to work and whether you will have any restrictions on your work activities”. 11
“Predicting who will be able to return to work (and how soon) after carpal tunnel surgery is not simple or straight forward”. 12
“Patients who had endoscopic surgery may be able to return to work after 2 weeks, but that’s extremely optimistic. Usually, it’s about a month later. However, those who had open release surgery will need more time”. 13
Pre- and post-operative information from clinicians has an important role in supporting recovery after injury and elective surgery. 14 For elective procedures, information about how long the individual might expect to be away from work, be unable to drive, or perform other activities of daily living will also contribute to shared decision-making around the suitability and timing of the proposed procedure. Variation in practice regarding work- and activity-related advice may have clinical and financial implications for patients, particularly if patients are advised to take extended periods of time off work without access to sick pay.
Wound care and rehabilitation strategies may also affect a patient’s ability to work or drive after CTR. Bulky dressings could impede hand function, or conversely, may be helpful in providing additional padding over the wound and facilitate function. A randomised controlled trial comparing the use of a bulky dressing for 2 weeks after CTR versus 24 h found no difference in patient outcomes; 15 the authors suggested that patients should be given the choice of when to remove their dressing. Post-operative rehabilitation strategies include movement exercises, targeted thenar strengthening and sensory relearning, however the latest Cochrane review found no high-quality evidence to support any specific rehabilitation interventions after CTR. 16
Considering the absence of definitive evidence informing when it might be safe and appropriate to return to driving and different types of work after CTR, we used a Delphi process to develop consensus recommendations from an expert panel of clinicians. We aimed to establish (i) what advice should be provided regarding return to driving after CTR; and (ii) how different work activities should be categorised and defined in relation to CTR, and when patients should be recommended to return to these different work activities. Following suggestions from our patient advisors, we also added an extra aspect to explore (iii) what wound care and rehabilitation advice should be provided after CTR.
Methods
Delphi design
The study was designed using an electronic Delphi format hosted with asynchronous data collection. This was chosen to provide flexibility for busy clinicians and to facilitate geographical diversity within the UK. This format also enabled anonymity, which was desirable to prevent the consensus process being driven by the loudest voice, or the most eminent individuals. 17
Questions were developed in response to the findings from our previous research5–7 and through discussion with our clinical and patient advisory groups. All questions were piloted with the clinical advisory group and amended based on their feedback. Further feedback was provided by the British Association of Hand Therapists (BAHT) Clinical Evidence Committee. We used a combination of multiple-choice and open questions, with space for any additional feedback after each topic. This process was repeated for each round of the study. The Delphi format involved three blocks of questions: the first asked about factors and timescales that were important for return to driving; the second asked about different occupational activities and timescales for return to these activities; and the final section asked about post-operative wound management and rehabilitation. This final section was not part of the initial study plan but was added following recommendations from the patient advisory group. Full text for each round of the Delphi is provided through the Open Science Framework repository. 18 The Delphi was hosted on Qualtrics (XM, Imperial College London) and could be completed on a phone, tablet or desktop.
Consensus was predefined as 75% agreement. 18 If ≥75% of respondents agreed with an item, it was included in the recommendations. If ≥75% of participants disagreed with an item, it was excluded. Where consensus was not reached, the item was progressed to the next round. We anticipated that up to three Delphi rounds would be required to reach consensus.
Eligibility criteria and recruitment
Eligible clinicians were those working as clinical hand surgeons, hand therapists or primary care surgeons in the UK. The study was limited to the UK because of contextual factors including access to NHS care, sickness certification and driving requirements. We wanted to include clinicians who routinely treated CTR patients. Surgeons were eligible if they had performed >50 CTR procedures in the previous 12 months, hand therapists were eligible if they had treated >20 CTR patients in the previous 12 months. This number was lower to reflect that not all CTR patients in the UK are routinely referred for hand therapy. 19 We used the number of patients seen, rather than years of experience to define study eligibility because we wanted panellists to reflect on current, rather than past practice.
The study was advertised through BAHT, the British Society for Surgery of the Hand (BSSH), the Reconstructive Surgical Trials Network (RSTN) and the Association of Surgeons in Primary Care (ASPC). Potential panellists were directed to an electronic expression of interest form which collected contact details and basic demographic information including clinical discipline, geographical location, gender, and ethnicity. In the case of oversubscription, we developed a purposive sampling strategy based on these characteristics. We aimed to enrol 10–12 participants from each professional group (surgeons and therapists). While there are no established criteria for sample size in Delphi studies, a response rate of ∼70% is commonly desired.20,21 For this reason, the target sample size was a total of 30 (∼15 for each profession).
Data management
Responses were analysed using descriptive statistics in Stata 15 (StataCorp). Open text responses were discussed with the clinical and patient advisory groups and categorised for analysis. After the first round, summary feedback was provided as part of the subsequent rounds. Feedback was not individualised for each participant.
Governance and approvals
The study was registered with Guy’s and St Thomas’ NHS Foundation Trust Occupational Therapy Clinical Audit Team (reference: 12,121) and approved for distribution by the four organisations that were approached: BAHT, BSSH, RSTN and ASPC. Study reporting followed the CREDES recommendations 22 (online supplementary File 1).
Patient and public involvement
Two advisory groups were set up specifically for this study. The clinical advisory group was recruited through an open call and personal recommendations. 10 individuals were chosen to represent different clinical and academic stakeholder groups: orthopaedic and plastic surgery; occupational health; primary care; and hand therapy. These individuals were not Delphi panellists. The patient advisory group comprised four individuals who had previously undergone CTR. These individuals were recruited via the research team and clinical advisory group members. They were reimbursed for their contribution to the study in accordance with the National Institute for Health Research guidance. 23
Results
Delphi panel
Forty-five clinicians registered an expression of interest. Of these, 12 were ineligible; six because they did not practise in the UK, and six because they did not treat the required number of CTR patients. The remaining 33 clinicians were invited to participate, of which all completed at least one round and 27 (82%) completed both rounds. Panel characteristics are described in Table 1. Round 1 was open for responses for 3 weeks from October to November 2021, and round 2 was open for responses for 4 weeks between December 2021 and January 2022. At this point, consensus had been reached or responses were approximately equally balanced, such that consensus was unlikely to be reached in a subsequent round. The decision to finish the study after round 2 was agreed with the advisory groups and through two online feedback meetings with Delphi panellists. Summary findings were also shared with all panellists by email and they were invited to comment.
Table 1.
Characteristics of the Delphi panel.
| Surgeons (n = 21) | Hand therapists (n = 12) | |
|---|---|---|
| Clinical background | ||
| Orthopaedic surgery | 18 | - |
| Plastic surgery | 2 | - |
| Primary care | 1 | - |
| Occupational therapy | - | 8 |
| Physiotherapy | - | 4 |
| Clinical grade | ||
| Consultant | 19 | - |
| Senior surgical trainee | 2 | - |
| Specialist (NHS band 7) | - | 6 |
| Senior specialist (NHS band 8a+) | - | 6 |
| CTR patients treated in last 12 months | ||
| 21–50 | - | 6 |
| >50 | 21 | 6 |
| Location | ||
| England | 17 | 8 |
| Northern Ireland | 1 | 2 |
| Scotland | 1 | 1 |
| Wales | 2 | 1 |
| Gender | ||
| Male | 15 | 2 |
| Female | 6 | 10 |
| Ethnicity | ||
| Asian/ Asian British | 4 | - |
| Black African/ Caribbean/ Black British | 1 | - |
| Other | 1 | - |
| White/ White British | 15 | 12 |
| Participation | ||
| Completed both rounds | 17 | 10 |
| Completed only round 1 | 1 | 2 |
| Completed only round 2 | 3 | - |
NHS: National Health Service; CTR: carpal tunnel release.
Factors to consider when returning to driving
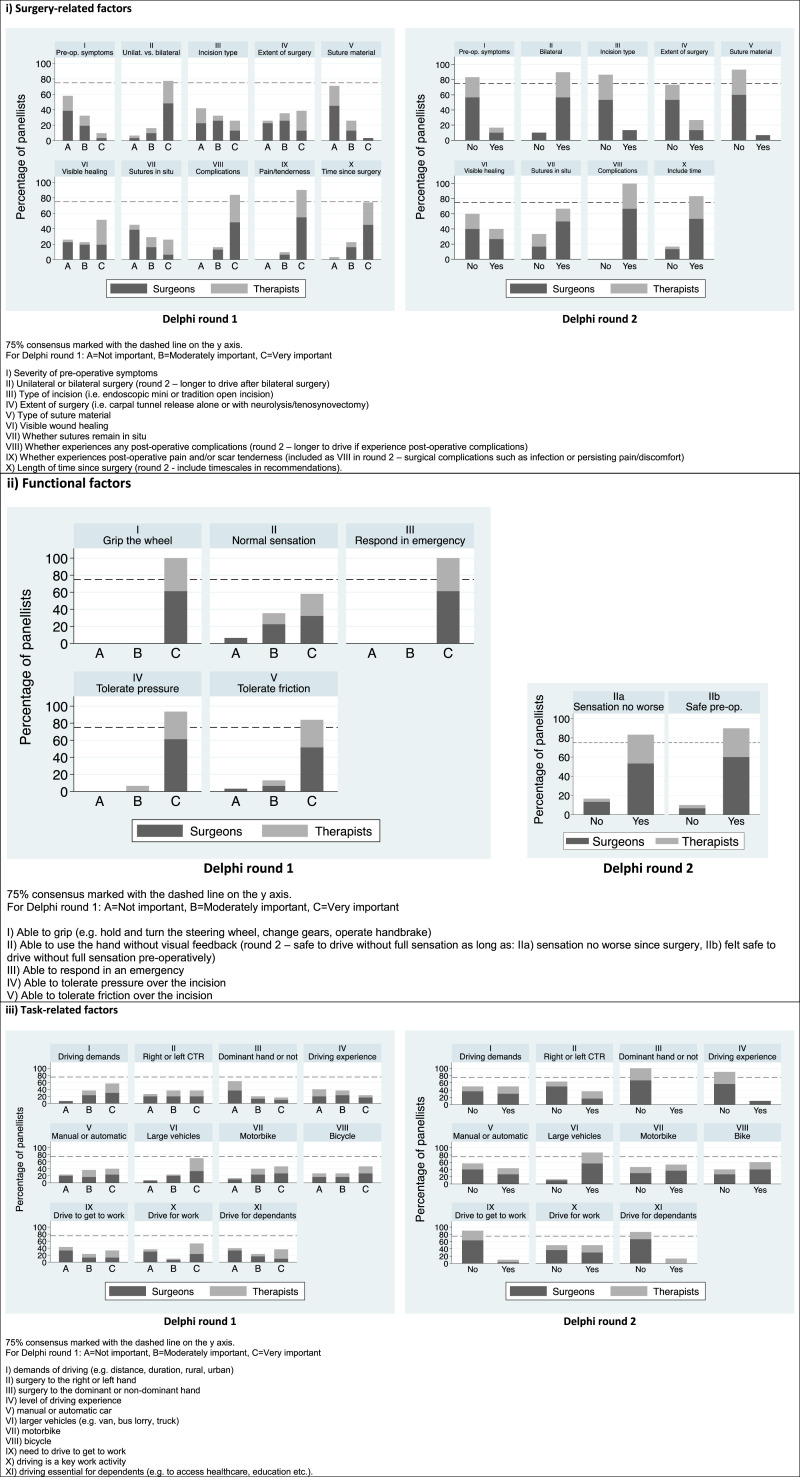
Panellists were asked to consider which surgical, functional, and task-related factors were important for return to driving and whether specific information for each factor should be included in general advice about return to driving after CTR. The breakdown of responses per item, clinical discipline and Delphi round are provided in Figure 1.
Figure 1.
Surgical, functional, and task-related factors to consider for return to driving after carpal tunnel release.
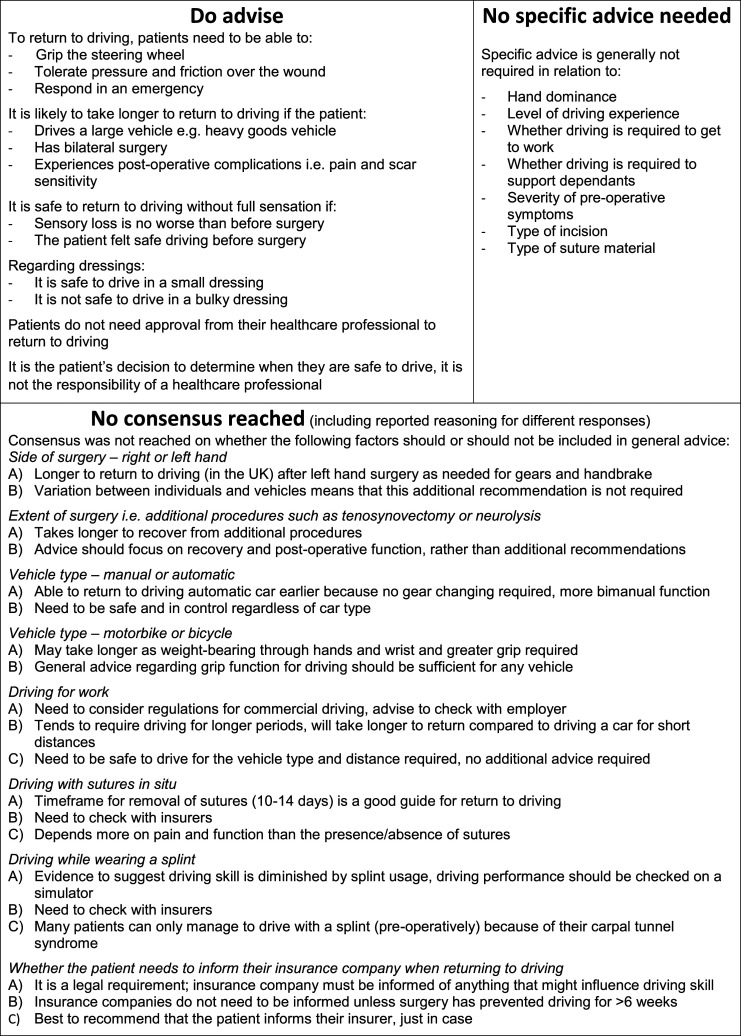
Panellists were also asked to consider three specific safety factors in relation to return to driving: dressings, sutures and splints. Consensus was only reached for two items: it is not safe to drive with a bulky dressing in situ (90%; surgeons 83%, therapists 100%); and it is safe to drive with a small dressing covering the wound (80%; surgeons 83%, therapists 75%). Consensus was not reached on whether patients should be advised to inform their insurance company when returning to driving (no = 65%; surgeons 60%, therapists 70%). In round two, it was unanimously agreed that it is the patient’s responsibility to determine when they are safe to return to driving (100%) and that approval from a healthcare professional is not required (93%; surgeons 95%, therapists 90%, Supplemental Material File 2 – Section A). Final consensus recommendations for driving after CTR are provided in Figure 2. For the items that did not reach consensus, the additional comments were explored to identify and categorise the key themes.
Figure 2.
Factors to consider when returning to driving after carpal tunnel release.
Timescales for return to driving
In round 1, panellists provided a suggested time point for return to driving that could be included in the general information for CTR patients. In round 2, panellists were asked whether they agreed with the summary data describing the reported time points. The median suggested time for return to driving was 14 days (IQR 5–14, range 1–30) and the median suggested time for return to driving for work was 21 days (IQR 14–28, range 1–42; Supplemental Material File 2 – Section B). In round 2, 83% of panellists (80% of surgeons and 90% of therapists) agreed that the summary data for these timescales should be included in general information for patients, either as median (50% agreement), interquartile range (33% agreement) or range (10% agreement).
Occupational activities
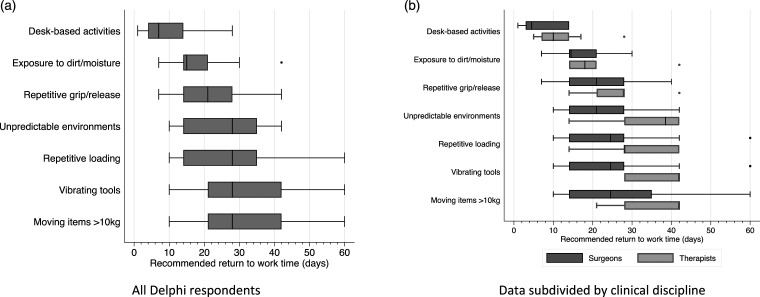
A list of eight different occupational activities was provided in round 1 and panellists were asked to select whether the activity should be included in general information for CTR patients, and to suggest a time point when it might be appropriate for patients to return to these activities. Seven activities reached consensus (Figure 3). Open text comments for the remaining category (lifting and stabilising >2 kg) indicated that there was perceived overlap between this category and repeated loading of the hand and wrist. This was confirmed with the responses in round 2 and therefore this activity was not included. In round 2, 90% of panellists (85% of surgeons and 100% of therapists) agreed that the timescales reported in round 1 should be included as general information for patients, reported either as median (50% agreement), interquartile range (47% agreement) or range (3% agreement).
Figure 3.
Suggested general return to work times for different occupational activities after carpal tunnel release. (a). All Delphi respondents and (b) Data subdivided by clinical discipline. Central markers show the median, grey boxes show the interquartile range, end markers show the range, dots indicate single outlier responses.
In round 1, panellists were asked to suggest general return to work advice. 14 individuals provided recommendations and these were classified into nine items. Panellists were asked whether to include each item as part of round 2. Four items reached consensus, these were: i) once the wound is healed, you can do tasks within pain/comfort limits (97%; surgeons 95%, therapists 100%); ii) aim to pace and modify tasks when returning to work (100%); iii) discuss with your manager or occupational health practitioner in advance and plan a phased return to work (83%; surgeons 80%, therapists 90%); and iv) the individual is best placed to decide whether they will be able to do their job (77%; surgeons 85%, therapists 60%, Supplemental Material File 2 – Section C).
Wound care and daily function
Consensus recommendations for post-operative dressings, when to wash the wound, and when to use the hand for daily function were reached in round 2. This included advice to remove any bulky dressing after 2–3 days (97%; surgeons 95%, therapists 100%); keep the wound dry for 10–14 days (90%; surgeons 85%, therapists 100%) and use the hand as soon as possible after surgery, ideally within the first 1–2 days (93%; surgeons 95%, therapists 90%, Supplemental Material File 2 – Section D).
Possible complications and scar care
Panellists were asked about the provision of advice for scar massage and the risk of possible post-operative complications. Consensus was reached in the first round and included recommendations to massage the scar once the wound is closed and the sutures removed (93%; surgeons 94%, therapists 92%), and advice about the possibility of pillar pain (93%; surgeons 94%, therapists 92%), scar sensitivity (100%) and ongoing symptoms (100%, Supplemental Material File 2 – Section E).
Hand and wrist exercises
Only one item reached consensus in relation to hand and wrist exercises: patients should be advised to regularly move their fingers, thumb, and wrist after surgery (97%; surgeons 95%, therapists 100%). There was no agreement over the utility of median nerve glides, sensory retraining, thumb motor control activities or the timing of any home exercises programme (Supplemental Material File 2 – Section F).
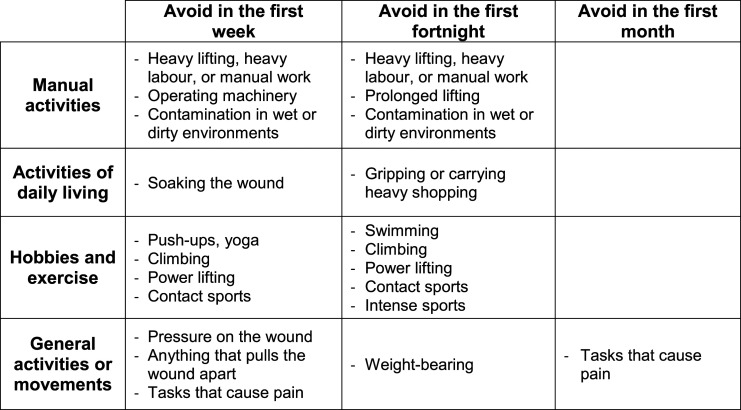
Activities during the first month after surgery
Following a recommendation from our patient advisory group, we asked panellists to list any activities that patients should be advised to avoid during the first week, fortnight and month after surgery. This was an open text question in round 1, with panellists selecting whether they agreed or disagreed with the advice in round 2. Consensus was reached for 11 activities to be avoided during the first week, 10 to be avoided in the first fortnight and one to be avoided in the first month (Figure 4). Full data are provided in Supplemental Material File 2 – Section G.
Figure 4.
Activities to avoid during the first month after carpal tunnel release.
Discussion
The aims of this Delphi study were to establish (i) what advice should be provided regarding return to driving after CTR; (ii) how different work activities should be categorised and defined in relation to CTR, and when patients should be recommended to return to these different work activities; and (iii) what wound care and rehabilitation advice should be provided after CTR.
The a priori level of consensus (75%) was met over two rounds to achieve agreement on the key considerations for returning to driving or specific work activities, and for post-operative wound care and rehabilitation. There were four key instances where consensus was reached within one clinical group, but not maintained when all participants were combined. Two related to driving and two related to post-operative exercises: 80% of surgeons agreed that the extent of surgery (whether there were additional procedures, such as neurolysis or tenosynovectomy) was not important for return to driving. This is compared with 60% of hand therapists. Comments from panellists were divided into those suggesting that general advice should include a recommendation that it would take longer to return to driving after more extensive surgery; and those suggesting that the advice should be kept simple and based on pain and functional ability. The patient advisory group agreed with the latter and suggested that this item could be confusing for patients as they might not know whether their surgery involved additional procedures.
Driving with sutures in situ was reported as safe by 75% of surgeons compared with 50% of hand therapists. We acknowledge that the timing of suture removal may not fully reflect the degree of wound healing, but whether it was safe to drive with the ‘stitches in’ was an important point for our patient advisory group and was also highlighted in previous qualitative research. 7 There is no specific UK DVLA (Driver and Vehicle Licensing Agency) guidance regarding driving with volar hand or wrist sutures 24 and we were unable to find any evidence to support or refute the safety of driving with sutures in situ. However, the DVLA information for driving after surgery does indicate that “Drivers do not need to notify DVLA of surgical recovery unless it is likely to affect driving and persist for more than 3 months. License holders wishing to drive after surgery should establish with their own doctors when it would be safe to do so.” 24
More than 75% of panellists agreed that it was safe to drive with a small dressing in situ, 90% disagreed that it was safe to drive in a bulky dressing, and there was no consensus on whether it was safe to drive in a splint (50% disagreed in round 1, 60% disagreed in round 2). We did not define the term ‘bulky dressing’, however, this is a commonly used term within hand surgery and hand therapy and there were no comments from panellists in either round requesting a definition.
In our experience, patients commonly ask whether it is appropriate for them to drive in their dressing, sutures, or splint, but there is little evidence to inform a response. A review of driving with upper limb immobilisation found only two studies assessing the use of wrist splints. 25 One found that driving in a splint with the fingers and thumb free had no effect on driving ability, 26 while the other found no effect during normal driving conditions, but impaired driving performance during hazardous conditions. 27 Importantly, these studies assessed healthy volunteers and therefore do not incorporate other contextual factors after surgery, such as pain or oedema.
In the absence of robust evidence, the consensus reached in this Delphi study offers clinicians and patients key guidance to support a return to driving. Patients may expect to be able to return to driving between 5–14 days after CTR, but this will be person-specific. Essentially, it is the patient’s responsibility to determine when they are safe to drive and this should incorporate being able to grip the steering wheel, tolerate pressure and friction over the wound and being able to respond to hazards.
Consensus was not reached on whether CTR patients need to inform their insurance company on return to driving. We searched for guidance from the five largest UK car insurance companies Admiral Group, AXA, Direct Line Group, AVIVA and Liverpool Victoria (as reported by NimbleFins 28 ), using the search terms ‘driving after surgery’ (search date, 31/03/22). Only AVIVA provided general customer advice, recommending “as long as the doctor has given you the all clear and you feel OK to drive, then it is absolutely fine. You don’t need to provide proof and we don’t need to note anything on your policy” 29 As with the DVLA advice discussed above, this places the emphasis on the doctor to give approval to drive, highlighting the importance of patients receiving clear information from the hand surgery team which includes permission to make the personal decision about whether they feel safe to drive. Clearer and standardised advice from insurance companies would clarify the situation for both clinicians and patients.
Several of the comments from Delphi panellists expressed concern about providing general return to driving advice, emphasising that the patient is responsible for making the decision about when they are safe to drive. Feedback from our patient advisory group and previous qualitative interviews indicate that patients are looking for advice about driving from their healthcare professionals, including expected timescales. 7 As clinicians, we need to be familiar with the location-specific regulations relating to driving after surgery. We also need to ensure that we equip our patients with the information they need to make their own decision about when to drive. Currently, there appears to be a disconnect between the information that patients would like, the information that clinicians are prepared to provide, and the requirements for insurers.
The Delphi panel identified seven different occupational activities that they believed were relevant for patients returning to work after CTR and provided consensus timescales for expected return to these activities. Both the activities and the timescales were endorsed by our patient advisory group with the caveat that the additional descriptors used in the Delphi questionnaire 18 should also be included to ensure that the information was meaningful. Previous qualitative interviews with CTR patients found a perceived lack of clarity over the work-related advice, including difficulty interpreting terms, such as ‘light duties’. 7 The recommendations generated in the current study provide guidance related to upper limb function, rather than a job title or general descriptors of ‘light’ or ‘modified’ activities. These timescales were developed to help CTR patients plan their return to work, however we are aware that other factors will influence return to work in practice, such as the availability of graded return to usual hours and/or activities.
Consensus was reached on advice to keep the wound dry for 10–14 days (unless otherwise advised), yet existing guidance suggests that there is no benefit of keeping sutured wounds dry when compared to washing with sterile saline or tap water, even within the first 48 hours after surgery. 30 Comments about wound care highlighted concerns about infection when washing the wound before 10 days, particularly if patients soaked the wound or did not allow it to dry fully. However, these concerns do not appear to be fully substantiated by the available evidence, with current NICE Guidelines advising that patients may safely shower 48 h after surgery. 31
More than 75% of hand therapists recommended routine median nerve glides and thumb motor control exercises after CTR, but consensus was not reached across both clinical groups. Interestingly, 75% of surgeons recommended that thumb motor control exercises should not be routinely recommended. This was the only response where the two clinical groups held strongly opposing views. There is a lack of evidence supporting any postoperative rehabilitation interventions after CTR, including those described above. Sensory relearning exercises were not found to improve sensory or functional outcomes after CTR 32 however 30% of therapists and 40% of surgeons suggested that sensory relearning should be routinely offered. The latest Cochrane review suggested that rehabilitation interventions should be a decision between the clinician and patient with an open discussion about the lack of high-quality evidence. 16
Future research is needed to explore whether specific post-operative rehabilitation is required after CTR, and if so, which patients would be the most likely to benefit from targeted interventions.
Compared with surgeons, hand therapists recommended longer time periods for return to all occupational activities and driving. A similar pattern was found in our previous survey. 5 This was discussed as part of the panellist feedback sessions, with participants suggesting that hand therapists’ perceptions may be influenced by their experience of primarily treating patients with post-operative complications or functional difficulties after CTR. This feedback aligns with our previous survey which found that only 14% of surgeons routinely referred CTR patients for hand therapy input within the NHS. 5 Alternatively, it was suggested that therapists may have a better understanding of their patients’ occupational requirements and are therefore able to provide more realistic timescales.
Limitations
Delphi panellists were a self-selected group of individuals who responded to a research invitation. The decisions made by the panel may therefore not be fully reflective of the views of the broader population of clinicians. We used a purposive sampling strategy to maximise diversity, however several clinical and demographic characteristics were underrepresented, including plastics, primary care and non-consultant level surgeons. Furthermore, all hand therapy panellists were White or White British. We took steps to maximise the opportunity for clinicians to be involved, including advertising the study using email bulletins, Website links and social media posts from relevant clinical organisations, and employing asynchronous data collection to increase the convenience of participation. We also chose to use an anonymous survey-based process to avoid dominant voices from influencing the group and to encourage participation from individuals who might not otherwise feel comfortable sharing their views.
The panel comprised more hand surgeons than therapists. As patients are not routinely referred to hand therapy after CTR, 5 the pool of potentially eligible hand therapists may have been quite small. The findings were not weighted according to clinical discipline and will therefore have a greater contribution from the surgeons as the majority group. However, the final consensus recommendations were agreed by all Delphi panellists, plus the patient and clinical advisory groups.
Conclusions
The guidance and timescales provided by this study were generated through a formal consensus process and included clinicians from different disciplines involved in the care of CTR patients.
In summary, after CTR, patients might expect to return to driving in 5–14 days and should be advised to plan for this timescale. However, it will likely take longer if the patient experiences any post-operative complications, has bilateral surgery, or needs to drive a larger vehicle.
Work-related recommendations from this study expand on existing advice by including functional descriptors for occupational activities, rather than using potentially ambiguous terms, such as ‘light’ or ‘modified’ duties. Patients and clinicians can use the following timescales to guide return to specific work activities:
1. Desk-based activities (e.g. writing or typing using a keyboard and mouse, filing or handling documents) 4–14 days
2. Exposure to dirt, dust, chemicals or fluids (e.g. cleaning, kitchen work, construction, industrial roles, gardening, healthcare, animal care) 14–21 days
3. Repetitive gripping and releasing (e.g. hand-sorting or picking, assembly, hairdressing, cleaning, gardening, driving for work, kitchen or hospitality work) 14–28 days
4. Unpredictable hand or wrist contact (e.g. child care, primary teaching, custodial or emergency services, healthcare, animal care) 14–35 days
5. Repeated loading of the hand and wrist (e.g. hammering, sawing, child care, massage, sports training, delivering cardiopulmonary resuscitation, assembly) 14–35 days
6. Use of hand-held vibrating tools (e.g. dental or surgical tools, drilling, construction and building work, use of pneumatic hammers or drills) 21–42 days
7. Lifting or stabilising items over 10 kg (e.g. delivery, stacking shelves, stockroom management, construction, maintenance, gardening, custodial or rescue services, some healthcare roles) 21–42 days
Wound care recommendations are to remove any bulky dressings 2–3 days after surgery and to keep the wound clean and dry for 10–14 days. Rehabilitation recommendations are to move the fingers, wrist and thumb regularly and to use the hand as soon as possible after surgery, ideally within the first 1–2 days.
We aim to further disseminate this guidance in easy-to-read formats to a wide range of stakeholders including hand surgeons, hand therapists, occupational health professionals, primary care clinicians and patients. Areas where consensus was not reached warrant further exploration to assess whether different post-operative practices or advice provision influence clinical and functional outcomes for patients.
Supplemental Material
Supplemental Material for Driving, work, wound care and rehabilitation after carpal tunnel release: Consensus recommendations from a UK Delphi study by Lisa Newington, Ira Madan and Fiona Sandford in Hand Therapy
Supplemental Material for Driving, work, wound care and rehabilitation after carpal tunnel release: Consensus recommendations from a UK Delphi study by Lisa Newington, Ira Madan and Fiona Sandford in Hand Therapy
Acknowledgements
Thank you to the Dephi Panellists for giving up their time to take part in this study: Sue Fullilove, Ryan Trickett, Rob Poulter, Shamim Umarji, Tim Halsey, Katherine Browne, Will Mason, Chris Little, Shahrier Sarker, Darren Roberts, Ali Phillips, Anthea Davy, Irshad Patel, Andrew Smith, Michael David, Phil Grieve, Russel Walker, Claire Simpson, Meg Birks, Philip Sauve, Anna Simpson, Lisa Small, Jan Beaumont, Elise van der Veen, Daniel Ceh, Donna Kennedy, Christy Fowler, Lynne Nicholls, Janey Milligan, Holly Newton, Sally van Liefland, Daniel Harte and Lesslie Ponraja. Thank you also to the clinical advisory group for their support with study design and content: Laura Ingham, Karen Walker-Bone, David Warwick, Peter Sharpe, Nandor Pillai, Megha Soni, Matt Gardiner and Emma Reay. Finally, thank you to the patient advisors for sharing their experiences, contributing to the study design, and providing helpful feedback throughout: Lyn Bailey, Carol McCarthy, Helen Wilson and Cassie Thomas.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: LN and FS were supported by the British Association of Hand Therapists 2020 Research Grant.
Ethical approval: Approval was provided by the Guy’s and St Thomas’ NHS Foundation Trust Occupational Therapy Clinical Audit Team (reference: 12,121).
Informed consent: The purpose of the Delphi study and links to the protocol were provided at the start of each round. Panellists were asked to opt in to participate.
Guarantor: LN.
Contributorship: LN and FS conceived the study and developed the protocol. All authors contributed to the Delphi survey questions. LN analysed the data with review by FS. LN wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Lisa Newington https://orcid.org/0000-0001-6954-2981
Ira Madan https://orcid.org/0000-0003-2200-7329
Fiona Sandford https://orcid.org/0000-0002-1778-891X
References
- 1.Bebbington E, Furniss D. Linear regression analysis of Hospital Episode Statistics predicts a large increase in demand for elective hand surgery in England. J Plast Reconstr Aesthet Surg 2015; 68: 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burton CL, Chen Y, Chesterton LS, et al. Trends in the prevalence, incidence and surgical management of carpal tunnel syndrome between 1993 and 2013: An observational analysis of UK primary care records. BMJ Open 2018; 8: e020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Royal College of Surgeons . Get well soon: helping you make a speedy recovery after carpal tunnel release. London, UK: Royal College of Surgeons, https://www.rcseng.ac.uk/patient-care/recovering-from-surgery/carpal-tunnel-release/download-full-pdf-version/ (accessed 27 January 2022). [Google Scholar]
- 4.Newington L, Stevens M, Warwick D, et al. Sickness absence after carpal tunnel release: a systematic review of the literature. Scand J Work Environ Health 2018; 44: 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newington L, Francis K, Ntani G, et al. Return to work recommendations after carpal tunnel release: a survey of UK hand surgeons and hand therapists. J Hand Surg Eur 2018; 43: 875–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newington L, Ntani G, Warwick D, et al. Sickness absence after carpal tunnel release: a multicentre prospective cohort study. BMJ Open 2021; 11: e041656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newington L, Brooks C, Warwick D, et al. Return to work after carpal tunnel release surgery: a qualitative interview study. BMC Musculoskelet Disord 2019; 20: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts C, Protzer L. ‘“Doctor, can I drive ?”’: The need for a rational approach to return to driving after musculoskeletal injury. Injury 2016; 47: 513–515. [DOI] [PubMed] [Google Scholar]
- 9.My Health Alberta Canada . Carpal tunnel release: what to expect at home, https://myhealth.alberta.ca/Health/aftercareinformation/pages/conditions.aspx?hwid=ug3907 (2020, accessed 31 January 2022).
- 10.The Centre for Minimally Invasive Surgery . How soon can I return to work after minimally invasive carpal tunnel surgery? https://cmisurgery.net/orthopedic/how-soon-can-i-return-to-work-after-minimally-invasive-carpal-tunnel-surgery/ (2022, accessed 31 January 2022).
- 11.The Center Orthopedic and Neurosurgical Care and Research . What to Expect After Carpal Tunnel Release. Bend, OR: The Center Orthopedic and Neurosurgical Care and Research, https://www.thecenteroregon.com/medical-blog/carpal-tunnel-surgery-recovery/ (2021, accessed 31 January 2022). [Google Scholar]
- 12.EOrthopod.com . Will you return to work after carpal tunnel surgery? https://eorthopod.com/news/will-you-return-to-work-after-carpal-tunnel-surgery/ (2021, accessed 31 January 2022).
- 13.Carpal Rx . Guide: Recovery Time for Carpal Tunnel Surgery. Fort Pierce, FL: Carpal Rx, https://www.carpalrx.com/recovery-time-for-carpal-tunnel-surgery (2020, accessed 31 January 2022). [Google Scholar]
- 14.Jaensson M, Dahlberg K, Nilsson U, et al. Factors influencing day surgery patients’ quality of postoperative recovery and satisfaction with recovery: a narrative review. Perioper Med 2019; 8: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams AM, Baker PA, Platt AJ, et al. The impact of dressings on recovery from carpal tunnel decompression. J Plast Reconstr Aesthet Surg 2008; 61: 1493–1495. DOI: 10.1016/j.bjps.2007.10.006 [DOI] [PubMed] [Google Scholar]
- 16.Peters S, Page M, Coppieters M, et al. Rehabilitation following carpal tunnel release. Cochranhe Database Syst Rev 2013; 6: CD004158. [DOI] [PubMed] [Google Scholar]
- 17.Holloway K. Doing the E-Delphi: Using online survey tools. Comput informatics. Nurs 2012; 30: 347–350. [DOI] [PubMed] [Google Scholar]
- 18.Newington L. Return to driving and work after carpal tunnel release: what should we advise our patients? A UK Delphi study. Open Science Framework, https://osf.io/qhsxk/ (2021, accessed 24 March 2022).
- 19.Newington L. Return to Work after Carpal Tunnel Release: What Should We Advise Our Patients? Southampton, UK: University of Southampton, https://eprints.soton.ac.uk/438720/ (2019, accessed 27 January 2022). [Google Scholar]
- 20.Akins RB, Tolson H, Cole BR, et al. Stability of response characteristics of a Delphi panel: application of bootstrap data expansion. BMC Med Res Methodol 2005; 5: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasson F, Keeney S, Mckenna HP, et al. Research guidelines for the Delphi Survey Technique Research guidelines for the Delphi survey technique. J Adv Nurs 2000; 32: 1008–1015. [PubMed] [Google Scholar]
- 22.Jünger S, Payne S, Brine J, et al. Guidance on conducting and REporting DElphi Studies (CREDES) in palliative care: recommendations based on a methodological systematic review. Palliat Med 2017; 31: 684–706. [DOI] [PubMed] [Google Scholar]
- 23.National Institute for Health Research . Payment Guidance for Researchers and Professionals. England, UK: National Institute for Health Research, https://www.nihr.ac.uk/documents/payment-guidance-for-researchers-and-professionals/27392 (2020, accessed 27 January 2022). [Google Scholar]
- 24.Driver and Vehicle Licensing Agency . Driving After Surgery. Swansea, UK: Driver and Vehicle Licensing Agency, https://www.gov.uk/guidance/miscellaneous-conditions-assessing-fitness-to-drive#driving-after-surgery (2021, accessed 24 March 2022). [Google Scholar]
- 25.Sandvall BK, Friedrich JB. Driving with upper extremity immobilization: a comprehensive review. J Hand Surg Am 2015; 40: 1042–1047. [DOI] [PubMed] [Google Scholar]
- 26.Blair S, Chaudhri O, Gregori A, et al. Doctor, can I drive with this plaster? An evidence based response. Injury 2002; 33: 55–56. [DOI] [PubMed] [Google Scholar]
- 27.Gregory JJ, Stephens AN, Steele NA, et al. Effects of upper-limb immobilisation on driving safety. Injury 2009; 40: 253–256. [DOI] [PubMed] [Google Scholar]
- 28.NimbleFins . Top 10 Largest UK Car Insurance Companies 2022. London, UK: NimbleFins, https://www.nimblefins.co.uk/largest-car-insurance-companies (2022, accessed 31 March 2022). [Google Scholar]
- 29.AVIVA . Can I Drive After an Operation? London, UK: AVIVA, https://www.aviva.co.uk/faq/answer/motor/4759/ (2022, accessed 31 March 2022). [Google Scholar]
- 30.Harrison C, Wade C, Gore S, et al. Postoperative washing of sutured wounds. Ann Med Surg 2016; 11: 36–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Institute for Health and Care Excellence . Surgical site infections: prevention and treatment, https://www.nice.org.uk/guidance/ng125/chapter/recommendations (2020, accessed 15 June 2022). [PubMed]
- 32.Jerosch-Herold C, Houghton J, Miller L, et al. Does sensory relearning improve tactile function after carpal tunnel decompression? A pragmatic, assessor-blinded, randomized clinical trial. J Hand Surg Eur 2016; 41: 948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Driving, work, wound care and rehabilitation after carpal tunnel release: Consensus recommendations from a UK Delphi study by Lisa Newington, Ira Madan and Fiona Sandford in Hand Therapy
Supplemental Material for Driving, work, wound care and rehabilitation after carpal tunnel release: Consensus recommendations from a UK Delphi study by Lisa Newington, Ira Madan and Fiona Sandford in Hand Therapy