Abstract
Spinal cord compression caused by cancer metastasis is a medical emergency that should be managed positively. Both multiple myeloma and lung cancer can lead to metastatic deposits in the spinal column to induce compression of the spinal cord. However, co-occurring multiple myeloma and lung cancer in a single patient causing spinal cord compression are rarely reported in the literature. We describe a case of a 61-year-old female with multiple myeloma and lung cancer whose radiologic characteristics of spinal cord compression mimicked those of metastatic lung cancer. Finally, the diagnosis was multiple myeloma. We showed the systematic imaging manifestations of metastatic multiple myeloma and discussed their therapeutic management.
Keywords: Multiple myeloma, Metastatic lung cancer, Spinal cord compression, Diagnostic challenge
Introduction
Metastatic spinal cord compression can be a devastating complication of advanced cancer and has the potential to cause immobilizing pain and impairment of neurological function [1]. Among the various cancers with metastatic potential, lung cancer (LC), the most commonly diagnosed cancer, is frequently associated with bone metastases, especially in the spine bones, which may induce metastatic spinal cord compression and threaten the quality of life of patients [2]. Multiple myeloma (MM), the second most common hematologic malignancy, is another tumor that can present with spinal cord compression [3]. MM and LC have been shown to co-occur in a single case in the literature; however, MM mimicking metastatic LC to cause spinal cord compression is rarely reported [4]. In this report, we present a case of simultaneous diagnosis with MM and LC. However, the spinal cord compression caused by MM shares similar imaging findings with metastatic LC. The diagnosis was finally revealed as MM, which was confirmed by pathological examination. We showed a positive therapeutic outcome by partial tumor resection combined with chemotherapy.
Case Report
Investigations
A 61-year-old female presented with increasing back pain for 2 months. This pain was accompanied by numbness located below her umbilicus involving both of her legs. Over the previous month, her symptoms had become intrusive, resulting in difficulty walking. The patient reported no history of smoking or alcohol consumption. No weight loss or bowel or bladder dysfunction was reported.
On examination, she had reduced sensation from the umbilicus to the foot involving both of her legs. Her tendon reflexes were brisk in both of the lower limbs. Weakness (three out of five) in her right lower extremity was noted. The rest of the neurologic examination was unremarkable. Laboratory analysis revealed iron deficiency anemia and hypoalbuminemia. The erythrocyte sedimentation rate was 33 mm/h. The coagulation factors only showed a slight increase in activated partial thromboplastin time (32.3 s). Her serum tumor markers showed increased ferroprotein of 272.68 ng/mL (normal range is 4.63 to 204 ng/mL) and a mildly elevated cytokeratin 19 fragment at 4.01 ng/mL (normal range is 0.1 to 3.3 ng/mL). Table 1 shows the main lab results of the patient.
Table 1. Lab Results.
| Test | Patient results | Reference range |
|---|---|---|
| WBC | 6.37 | 3.5 - 9.5 × 109/L |
| RBC | 2.34a | 3.8 - 5.1 × 1012/L |
| Hemoglobin | 74a | 115 - 150 g/L |
| Platelets | 116a | 125 - 350 × 109/L |
| Albumin | 35.8a | 40 - 55 g/L |
| Total protein | 78.6 | 65 - 85 g/L |
| CRP | 11.4a | 0 - 3.48 mg/L |
| ESR | 34a | 0 - 20 mm/h |
| Aspartate aminotransferase | 15 | < 35 U/L |
| Alanine aminotransferase | 25.6 | < 40 U/L |
| Total bilirubin | 5.4 | 5 - 24 µmol/L |
| Creatinine | 55 | 45 - 84 µmol/L |
| β2-microglobulin | 3.48 | 1 - 3 mg/L |
| Carcinoembryonic antigen | 1.39 | 0 - 5 ng/mL |
| Alpha fetal protein | 1.56 | 0 - 8.78 ng/mL |
| Carbohydrate antigen125 | 14.7 | 0 - 35 U/mL |
| Ferroprotein | 272.68a | 4.63 - 204 ng/mL |
| Cytokeratin 19 fragment | 4.01* | 0.1 - 3.3 ng/mL |
| Squamous cell carcinoma antigen | 0.5 | 0 - 1.5 ng/mL |
| Carbohydrate antigen 19-9 | 8.88 | 0 - 27 U/mL |
| Calcium | 2.10* | 2.19 - 2.54 mmol/L |
aAbnormal result. WBC: white blood cell; RBC: red blood cell; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate.
Diagnosis
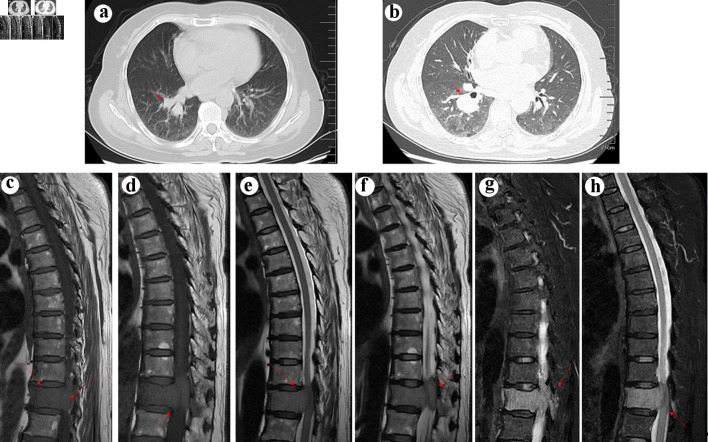
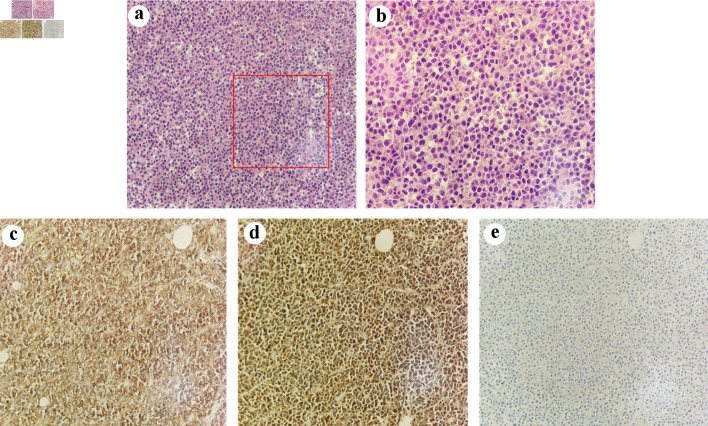
Pulmonary nodules with enlarged hilar and mediastinal lymph nodes were found in the right lung on chest computed tomography (CT) (Fig. 1). Subsequent magnetic resonance imaging (MRI) of the spine revealed a homogeneous mass lesion arising from the T11 vertebral body. The lesion involved the vertebral body and its appendix of T11 with extension into the thoracic canal and compression of the spinal cord, which highly supported the diagnosis of metastatic malignancy (Fig. 1). The patient was preliminarily diagnosed with metastatic epidural spinal cord compression from LC based on the results of imaging and laboratory examination. Considering that radical resection of metastatic tumors may not affect overall survival, we planned to treat the patient with direct surgical resection and decompression followed by spinal stabilization to improve her quality of life [5]. However, pathologic examination revealed that the tissue obtained from the spine during the operation was CD38- and CD138-positive, whereas kappa and lambda were negative, supporting the diagnosis of MM (Fig. 2). Finally, the diagnosis of LC and MM derived from the spine were determined. As for LC, we consulted a respiratory physician who staged them as T2N2M0 stage III-A. However, the lung lesion was undetermined by biopsy.
Figure 1.
(a) Computed tomography (CT) scan showing a neoplasm in the right lung (arrow). (b) Enhanced CT scan confirmed the existence of neoplasm in the right lung (arrow). Arrows indicate the location of the tumor. T1 (c and d), T2 (e and f), and fat-suppressed T2WI weighted spin echo magnetic resonance image of the thoracic spine in the sagittal planes (g, h). The tumor (arrow) showed hyperintensity on T1WI, mild hypointensity on T2WI, and hyperintensity on fat-suppressed T2WI, mimicking metastatic lung cancer.
Figure 2.
Pathological images of the tumor. (a) (H&E, × 200) and (b) (H&E, × 400) show the morphology of tumor cells. Immunohistochemical findings. The tumor cells expressed CD38 (c, original magnification × 200) and kappa (d, original magnification × 200) without expression of lambda (e, original magnification × 200). H&E: hematoxylin and eosin.
Treatment
She received partial tumor resection combined with decompression and spinal stabilization for spinal cord compression. Then, the patient was transferred to the local oncology department for further management of MM with chemotherapy. However, the lung lesion was still untreated in her local hospital.
Follow-up and outcomes
After surgery, the back pain and numbness of her legs were significantly reduced. She is 4 months postdiagnosis and remains disease free.
Discussion
Bone is a common site for metastasis of advanced tumors, whose clinical features include pain, hypercalcemia, pathologic fracture, and spinal cord or nerve root compression [6]. For malignancies with bone spread, the risks of developing spinal cord compression or collapse are increased [7]. Spinal cord compression can be the presenting symptom of metastatic malignancy, which may cause worsening pain and neurologic impairment [1]. The known origins of primary tumors with metastatic spinal cord compression include breast cancer, epithelioid mesothelioma, thyroid carcinoma, and sarcoma [8]. Among them, LC has also been shown to have a high rate of bone metastasis, especially spinal metastasis. The lesions of bone metastases from LC are usually osteolytic with poor prognosis, and the prognosis of metastatic spinal cord compression of LC is even worse [2, 9]. The imaging tests of metastatic LC include masses in the lobe with enlarged hilar or mediastinal lymph nodes combined with a collapsed vertebral body with spinal cord compression [10]. Here, we report a rare example of spinal cord compression resulting from soft tissue epidural MM of the thoracic spine. Our case was unique in that malignant LC co-occurred with MM, and the clinical characteristics of spinal cord compression caused by MM were identical to those of metastatic LC, which is a diagnostic challenge.
MM is a common hematological malignant tumor of proliferative plasma cells originating from the postgerminal lymphoid B-cell lineage [11]. One of the most common presentations of MM is bone pain caused by osteolysis [12]. Other manifestations of lytic lesions or osteopenia include hypercalcemia, pathological fracture, vertebral collapse, and spinal cord compression [13]. Although vertebral involvement is common in MM, spinal cord compression usually occurs from pathological fractures of the affected vertebra [14, 15]. Furthermore, extraosseous soft tissue epidural MM causing cord compression has rarely been described as yet, accounting for only 5% of the reported cases [15]. In our patient, the tumor mass originated from the T11 vertebrae and extended into the thoracic canal and compression of the spinal cord, which is a high index of suspicion for metastatic LC. Furthermore, a serum cytokeratin 19 fragment was identified as a biomarker for LC and human hepatocellular carcinoma, which is related to the metastatic behavior of the tumors [16, 17]. Both the increased serum cytokeratin 19 fragment and imaging findings favor the diagnosis of metastatic LC. However, the pathological diagnosis of the compression was MM, which is far from our initial diagnosis. Thus, our case highlights that imaging may provide local assessment of malignancies; however, histopathology is still vital for a final diagnosis. In view of this, clinicians should remain alert for the co-occurrence of two simultaneous tumors. Adequate tissue biopsy is important for prompt diagnosis and treatment.
Decompression and spinal stabilization are important to reduce pain, preserve motor and sensory function and improve quality of life for patients with metastatic spinal cord compression [15]. For patients with spinal cord compression due to MM, studies have shown that partial tumor resection combined with chemotherapy can be an effective treatment owing to minor surgical trauma, fewer complications, and favorable pain control [3]. However, the treatment methods for MM with spinal cord compression and LC are inexperienced and inconclusive. Some attempts have been proposed, such as surgical decompression of the spine, local surgery for LC, or targeted lung tumor treatment, but the efficiency of treatment is unreported or unsatisfactory [4, 14]. Recently, the use of zoledronic acid every 12 weeks has been shown to reduce pain in patients with bone metastases due to breast cancer, prostate cancer, or MM, whose risk of skeletal events is comparable to the standard dosing interval of every 4 weeks [18]. Furthermore, daratumumab plus lenalidomide and dexamethasone increased the prognosis in patients with newly diagnosed MM [19]. In addition, there are currently other treatment options for younger patients, including bortezomib, lenalidomide, dexamethasone (+/-), daratumumab with/without consolidating high-dose chemotherapy, and autologous stem cell transplantation [20]. Moreover, daratumumab has been reported to be safe and effective for MM with LC, displayed benefits in survival and can induce complete remission to proceed to LC surgery [21, 22]. In our case, the patient underwent partial surgical resection of the tumor followed by spinal stabilization, which achieved pain relief and the protection of her sensory function. Then, the patient was transferred to the oncology department in her local hospital for further management. Oncologic treatments were arranged with chemotherapy for MM rather than LC, which includes bortezomib, lenalidomide, and dexamethasone. She is still alive in the 4-month follow-up, whose LC had no progression. And she continues her chemotherapy treatment now. Therefore, systematic therapeutic approaches, including pain management, immunotherapy and chemotherapy with or without surgery, for patients with MM and LC are needed to achieve better clinical results [22]. In our opinion, we can treat two diseases according to their threats to life, or we can choose a proper oncologic therapeutic regimen to treat the two diseases at the same time. However, this is inconclusive, and studies with a high level of evidence are needed to investigate the optimal therapy for this condition.
Acknowledgments
None to declare.
Funding Statement
This work was supported by the Natural Science Foundation of Shandong Province (grant no. ZR2023QH517; ZR2022QH252).
Conflict of Interest
The authors declared no conflict of interest.
Informed Consent
Written informed consent was obtained from the patient.
Author Contributions
CJC and TBY participated in the drafting, writing, and revising of the manuscript. YDL, YW, XZ, YBQ, YGW, and KNZ participated in the data selection and analysis. CJC, YJR, and YY contributed to the study concept and acquired and analyzed the data.
Data Availability
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- 1.Lawton AJ, Lee KA, Cheville AL, Ferrone ML, Rades D, Balboni TA, Abrahm JL. Assessment and management of patients with metastatic spinal cord compression: a multidisciplinary review. J Clin Oncol. 2019;37(1):61–71. doi: 10.1200/JCO.2018.78.1211. [DOI] [PubMed] [Google Scholar]
- 2.da Silva GT, Bergmann A, Santos Thuler LC. Prognostic factors in patients with metastatic spinal cord compression secondary to lung cancer: a systematic review of the literature. Eur Spine J. 2015;24(10):2107–2113. doi: 10.1007/s00586-015-4157-x. [DOI] [PubMed] [Google Scholar]
- 3.Qian J, Jing J, Tian D, Yang H. Partial tumor resection combined with chemotherapy for multiple myeloma spinal cord compression. Ann Surg Oncol. 2014;21(11):3661–3667. doi: 10.1245/s10434-014-3754-y. [DOI] [PubMed] [Google Scholar]
- 4.Xiao PP, Luo BQ, Fan W, Chen XY, Dong ZG, Huang JM, Zhang Y. et al. Simultaneous presentation of multiple myeloma and lung cancer: case report and gene bioinformatics analysis. Front Oncol. 2022;12:859735. doi: 10.3389/fonc.2022.859735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slimack NP, Liu JC, Koski T, McClendon J Jr, O'Shaughnessy BA. Metastatic gastrointestinal stromal tumor to the thoracic and lumbar spine: first reported case and surgical treatment. Spine J. 2012;12(1):e7–12. doi: 10.1016/j.spinee.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 6.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 7.Sutcliffe P, Connock M, Shyangdan D, Court R, Kandala NB, Clarke A. A systematic review of evidence on malignant spinal metastases: natural history and technologies for identifying patients at high risk of vertebral fracture and spinal cord compression. Health Technol Assess. 2013;17(42):1–274. doi: 10.3310/hta17420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wanman J, Grabowski P, Nystrom H, Gustafsson P, Bergh A, Widmark A, Crnalic S. Metastatic spinal cord compression as the first sign of malignancy. Acta Orthop. 2017;88(4):457–462. doi: 10.1080/17453674.2017.1319179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Confavreux CB, Pialat JB, Belliere A, Brevet M, Decroisette C, Tescaru A, Wegrzyn J. et al. Bone metastases from lung cancer: A paradigm for multidisciplinary onco-rheumatology management. Joint Bone Spine. 2019;86(2):185–194. doi: 10.1016/j.jbspin.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Fichte S, Brodhun M, Gottinger S, Rosahl S, Klisch J, Gerlach R. Vertebral and pulmonary actinomycosis mimicking metastatic lung cancer. J Neurol Surg A Cent Eur Neurosurg. 2013;74(Suppl 1):e188–192. doi: 10.1055/s-0033-1342930. [DOI] [PubMed] [Google Scholar]
- 11.Chen CJ, Ci LF, Wang Y, Zhang GH, Ren YJ, Qi YB, Wu YG. et al. Multiple myeloma: an unexpected cause of dysphagia after cervical spine surgery. World J Oncol. 2023;14:321–323. doi: 10.14740/wjon1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukkamalla SKR, Malipeddi D. Myeloma bone disease: a comprehensive review. Int J Mol Sci. 2021;22(12):6208. doi: 10.3390/ijms22126208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panaroni C, Yee AJ, Raje NS. Myeloma and bone disease. Curr Osteoporos Rep. 2017;15(5):483–498. doi: 10.1007/s11914-017-0397-5. [DOI] [PubMed] [Google Scholar]
- 14.Ha KY, Kim YH, Kim HW. Multiple myeloma and epidural spinal cord compression: case presentation and a spine surgeon's perspective. J Korean Neurosurg Soc. 2013;54(2):151–154. doi: 10.3340/jkns.2013.54.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trivedi RJ. Spinal cord compression as a consequence of spinal plasmacytoma in a patient with multiple myeloma: a case report. Clin Pract. 2021;11(1):124–130. doi: 10.3390/clinpract11010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao J, Lv F, Li J, Wu Z, Qi J. Serum cytokeratin 19 fragment, CK19-2G2, as a newly identified biomarker for lung cancer. PLoS One. 2014;9(7):e101979. doi: 10.1371/journal.pone.0101979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Tang ZY, Tian B, Ye SL, Qin LX, Xue Q, Sun RX. Serum CYFRA 21-1 level reflects hepatocellular carcinoma metastasis: study in nude mice model and clinical patients. J Cancer Res Clin Oncol. 2006;132(8):515–520. doi: 10.1007/s00432-006-0098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Himelstein AL, Foster JC, Khatcheressian JL, Roberts JD, Seisler DK, Novotny PJ, Qin R. et al. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48–58. doi: 10.1001/jama.2016.19425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Facon T, Kumar SK, Plesner T, Orlowski RZ, Moreau P, Bahlis N, Basu S. et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(11):1582–1596. doi: 10.1016/S1470-2045(21)00466-6. [DOI] [PubMed] [Google Scholar]
- 20.Leleu X, Gorsh B, Bessou A, Paka P, De Nascimento J, Colin X, Landi S. et al. Survival outcomes for patients with multiple myeloma in France: A retrospective cohort study using the Systeme National des Donnees de Sante national healthcare database. Eur J Haematol. 2023;111(1):125–134. doi: 10.1111/ejh.13976. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Deng H, Zhang L, Dai Y, Li X, Lin X, Wei J. et al. Daratumumab for refractory IgD multiple myeloma with lung cancer and persistent thrombocytopenia: a case report. Clin Lab. 2021;67(11) doi: 10.7754/Clin.Lab.2021.210405. [DOI] [PubMed] [Google Scholar]
- 22.Morse W, Nawaz H, Choudhry AA. Combination of chemotherapeutic agents and biological response modifiers (immunotherapy) in triple-negative/Her2( +) breast cancer, multiple myeloma, and non-small-cell lung cancer. J Egypt Natl Canc Inst. 2023;34(1):58. doi: 10.1186/s43046-022-00159-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.