Abstract
Endothelial caveolae are essential for a wide range of physiological processes and have emerged as key players in vascular biology. Our understanding of caveolar biology in endothelial cells has expanded dramatically since their discovery revealing critical roles in mechanosensation, signal transduction, eNOS regulation, lymphatic transport, and metabolic disease progression. Furthermore, caveolae are involved in the organization of membrane domains, regulation of membrane fluidity, and endocytosis which contribute to endothelial function and integrity. Additionally, recent advances highlight the impact of caveolae-mediated signaling pathways on vascular homeostasis and pathology. Together, the diverse roles of caveolae discussed here represent a breadth of cellular functions presenting caveolae as a defining feature of endothelial form and function. In light of these new insights, targeting caveolae may hold potential for the development of novel therapeutic strategies to treat a range of vascular diseases.
Introduction
In the early 1950’s, ultrastructure observations by Palade and Bruns using transmission electron microscopy identified 50–100 nm flask-shaped invaginations in apical and basal endothelial membranes1. These unique structures were later termed caveolae, meaning “little caves”, based on their cave-like appearance by Yamada in 19552 (Figure 1). Originally caveolae were thought to solely be used by the endothelium for endocytosis,3,4, 5 but since their discovery, caveolae have been implicated in several cell-physiology processes ranging from transcytosis to ion channel organization.6, 7, 8–11 This review will focus on recent discoveries in caveolae (~2013 to 2023) and their relationship to endothelial cell (EC) physiology.
Figure 1: Transmission electron micrograph image from cremaster arteriole showing caveola in a blood vessel.

Endothelial cells (EC) containing numerous caveolae (arrow), internal elastic laminae (IEL) and surrounding vascular smooth muscle cells (SMC). A red blood cell (RBC) can be seen in the lumen of the vessel. Caveolae shown here (arrows) display the classical flask shaped invagination protruding into the ECs. This image was made by Brian R. Duling.
Rothberg et al. first identified caveolins as the proteins responsible for the caveolae structure more than 30 years ago.12 There are three different isoforms of caveolins: caveolin-1 (cav1), cav2, and cav3.13–16 Cav1 and cav2 are most abundant in endothelium, adipocytes and fibrous tissue, while cav3 expression is limited to striated muscle cells.13–16 Caveolae assembly requires more than the cav1 protein alone. A group of filamentous membrane sculpting proteins are also necessary.17, 18 However, this review will focus on cav1 in its relation to the physiological function of caveolae in endothelium, although it is generally assumed other isoforms of caveolin and cavins facilitate caveolae formation.
Additionally, ECs are not the only vascular cell which contain caveolae. Caveolar structures can also be seen in vascular smooth muscle cells (SMCs).19 However, the prevalence and role of caveolae in ECs versus SMCs is different. ECs have a higher density of caveolae than SMCs as ECs utilized caveolae as their major plasmalemma vesicular structure.20, 21 Continuous ECs have a very high density of caveolae, while fenestrated caveolae have fewer caveolar structures.20 Caveolae in SMCs mainly serve as a signaling platform for angiotensin II through the angiotensin II receptor.22, 23 It has also been shown that caveolin-3 is present in SMCs and aids in caveolae based signaling.19 Clearly, caveolae in both vascular cells can serve as a signaling hubs however the scope of this review is focused on endothelial based caveolae.
Structure
Cav1 facilitates the unique shape of caveolae (Figure 1) making the structure of cav1 crucial for the physiological functions of caveolae in endothelium. Advances in cryoelectron microscopy and computational modeling have facilitated novel structural characterizations of cav1 (Figure 2).24, 25 Porta et al., found cav1 complexes to be composed of eleven protomers packed tightly together to form a disc. These discs are comprised of an outer “rim”, a central beta-barrel “hub”, and curved “spokes”. Computational modeling using these new structural components show the membraneassociate side of the cav1 complex can be deeply imbedded within the cytoplasmic leaflet of the plasma membrane and directly interact with the terminal carbons on the lipids of the outer leaflet. This differs from previous models in which cav1 was proposed to interact solely with the inner leaflet lipid of the plasma membrane. It is through this displacement of the cytoplasmic leaflet and connections with the outer leaflet that cav1 could create ordered membrane nanodomains containing proteins on one leaflet and lipid on the other acting as a sculpting protein to form the cave like structures of caveolae.25
Figure 2: Human caveolin-1 structure identifying novel protein domains.
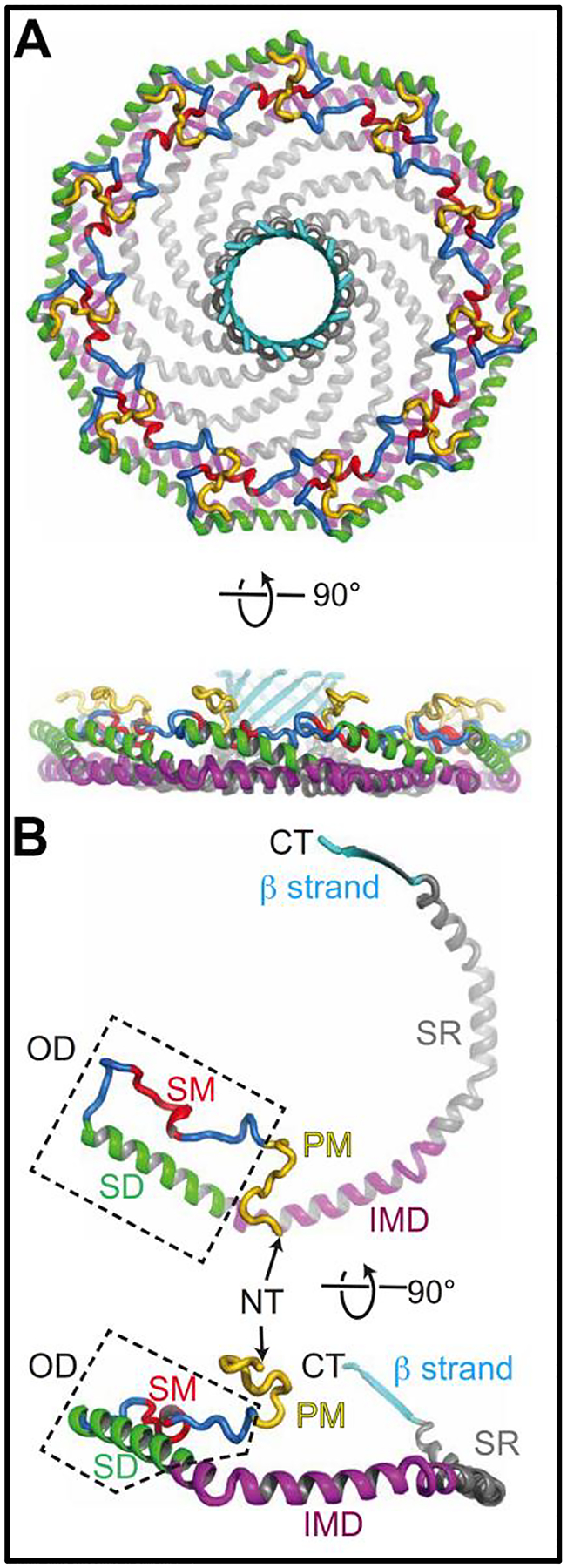
This figure has been taken from Porta et al. Science Advances 2022 (A) depicts a 3.5Å resolution of the Cav1 complex viewed from the cytoplasmic face. Below is the same structure but rotated 90°. (B) shows singular cav1 protomers with defined regions highlighted. Regions previously defined: Signature motif (SM), Scaffolding domain (SD) or in this manuscript referred to as CSD, intermembrane domain (IMD). Regions newly defined by Porta et al., Pin Motif (PM), Spoke region (SR), and the β strand.
The importance of cav1 structure and cellular membrane associations can be seen through the conservation of the cav1 structure across several species. Recent analysis using AlphaFold2 shows six common structural elements of cav1 are conserved from metazoan to human cells.26 The ability of cav1 protomers to combine and form discs is extensively preserved across species, providing evidence for a primordial association between caveolins and cellular membranes. Another conserved structural element of the cav1 protein which facilitates cellular signaling is the cav1 scaffolding domain (CSD).27 The function of various signaling molecules and enzymes can either be enhances or inhibited by binding the CSD.28 Cav1 interacts with a plethora of cellular components, these signaling networks form the foundation for the majority of caveolae related cellular processes.
Caveolae as mechano-sensors
Caveolae have been hypothesized to be dynamic mechano-sensors that provide a buffer for cellular membrane tension.29 Cells can respond to acute pressure and mechanical stimulation by disassembly and reassembly of caveolae.30 Early research into how caveolae respond to and regulate membrane tension found mouse carotid arteries lacking the cav1 protein failed to respond to shear stress.31 Flow mediated dilation was restored with the re-expression of cav1 into global cav1 knockout mice.31
Current research into caveolae mechanosensation has focused on identifying molecular mechanisms for pressure induced caveolae disassembly. It is demonstrated that EH domain containing 2 (EHD2), an ATPase, is rapidly released from caveolae under mechanical stress and translocated to the nucleus to serve as a transcription factor for caveolae associated proteins.32 The data presented in Torrino et al. also showed EHD2 is required for proper maintenance of caveolae reserves and cycling. Caveolae’s mechanical sensing abilities have also been investigated in the context of pressure induced vascular inflammation.33 Rat carotid arteries exposed to high intraluminal pressures ex vivo displayed decreased number of caveolae, suggesting caveolar disassembly.33 The link between caveolar disassembly and inflammation was mechanistically probed using ECs in culture transfected with shRNA to cav1 and exposed to high pressures. Upon high pressure exposure, cells without cav1 had decreased inflammatory markers when compared to control cells. This data suggests pressure induced inflammation in the vascular wall occurs through a caveolae dependent mechanism. Additionally, recent work by Liu et al, implicate cav1 in EC shear stress sensation resulting in oxidative stress from mitochondrial dysfunction.34 The authors implicate the role of a cav1-miR-7–5-p-mitophagy pathway in the production of reactive oxygen species and decreased nitric oxide availability.
Current evidence suggests endothelial caveolae can be dynamically regulated in response to physiological stimuli such as blood flow and increased pressure. How these processes differ amongst the endothelial subtypes (i.e. arterial, venous, lymphatic) has yet to be demonstrated. However, the existing data build a strong role for caveolae as endothelial pressure sensors, giving weight to the central role caveolae have in maintaining homeostasis in endothelium.
Caveolae as a signaling hub
Perhaps the most well characterized function of caveolae in the endothelium is to serve as a scaffold for signaling complexes and cytoskeletal components creating signaling microdomains at the plasma membrane.35–42 This body of literature continues to grow as more signaling pathways are identified to be cav1-dependent.
DeLalio et al. first demonstrated cav1 modulates purine release through pannexin 1 (Panx1) channels in vascular smooth muscle cells (SMC).43 In these experiments, Cav1fl/fl mice with an SMMHC CreERT2 (SMC specific) on a C57Bl6 background were used for the first time.43 These same Cav1fl/fl mice were then crossed with a Cdh5CreERT2 (EC specific) mouse to examine their role in endothelial cell purinergic signaling with Panx1. EC cav1 has been shown to be an essential scaffolding protein for the cross talk between Panx1 and other purinergic signaling channels like P2Y2R.44 EC caveolae support and maintain spatial proximity between individual proteins in the Panx1-P2Y2R signaling pathway. This was demonstrated both by loss of ATP induced vasodilation in pulmonary arteries from mice lacking EC cav1, as well as proximity ligation assays between Panx1, P2Y2R, and cav1. These studies provided evidence cav1 is required for the spatial organization of Panx1 and other purinergic components.
Daneva et al. also demonstrated a unique coupling between endothelial cav1 and the channel function of transient receptor potential cation channel member 4 (TRPV4). During homeostatic conditions, in murine lung endothelium, cav1 and TRPV4 cooperate to keep pulmonary arterial pressure low by continuing a steady flow of calcium ions through TRPV4 via anchoring the second messenger PKC.45 Genetic deletion of endothelial cav1 increased right ventricular systolic pressure as well as pulmonary arterial pressure.45 These physiological changes in pressure were due to the impairment of endothelial TRPV4 channel activity at caveola.
The highlighted examples above and the existing body of literature surrounding endothelial caveolae and cellular signaling process highlight a common theme with caveolin—their essential role in facilitating a signaling microdomain in endothelium. This clustering of proteins in close proximity facilities membrane channel crosstalk and the rapid and specific propagation of signaling cascades. Caveolae spatially regulate signal transduction by sequestering proteins into a caveolae microdomain ensuring the proximity of signaling partners. The specific co-enrichment of proteins within a pathway to a caveola facilitates the proper propagation of protein signaling and cellular function.
Caveolae as regulators of endothelial nitric oxide synthase (eNOS)
Among their wide-reaching functions in ECs, the ability of caveolae and more specifically cav1 to regulate endothelial nitric oxide synthase (eNOS) activity has been studied extensively.46, 47 Functioning as the primary source of nitric oxide (NO) in ECs, eNOS is a constitutively expressed enzyme synthesizing NO from L-arginine and oxygen.48 Integral to the regulation of eNOS activity are its physical interactions with cav1.49, 50 Under basal conditions in vascular ECs, eNOS is colocalized with caveolae in membrane lipid rafts. Within the caveolae, cav1 stabilizes eNOS in an inactive conformation through a direct interaction between the eNOS calmodulin (CaM) binding domain and the cav1 scaffolding domain.51, 52 In this state, NO production is largely diminished, until an influx of Ca2+ (and subsequently Ca2+-bound CaM) promotes the dissociation of eNOS from cav1 and the binding of eNOS to CaM and other activators.53 This interaction is critical to the eNOS feedback inhibition loop, as evidenced by increased cav1-eNOS co-immunoprecipitation in response to VEGF-/Ca2+-/thrombininduced activation of eNOS. Moreover, eNOS-derived NO itself is sufficient to promote phosphorylation of caveolin-1 by Src kinases, increasing cav1’s affinity for eNOS and subsequent inhibition of eNOS activity.54
In the absence of cav1, dysregulation of eNOS presents in a number of ways–the most common of which is eNOS uncoupling in which eNOS produces superoxide rather than NO. This triggers the subsequent overproduction of reactive oxygen species (ROS), as well as a disruption of physiologic NO-dependent vasodilation.55–57 Notably, this increase in oxidative stress is also sufficient to drive the phosphorylation of cav1 and a subsequent reduction in caveolae density, as shown in early studies performed on human umbilical vein endothelial cells.58 In the aortas and mesenteric arteries of spontaneously hypertensive rats, it was observed there was a marked decrease of caveolae, consistent with observed caveolae disassembly in rat carotids exposed to high intraluminal pressure.33 This observation correlated with both a significant decrease in NO production and a reduction in endothelium-dependent vasodilation. After confirming that alterations in NO production were a result of increased eNOS uncoupling, it was hypothesized that this observed dysregulation of vasodilation was likely a result of ROS-mediated inactivation of NO.59 Inflammation-induced depletion of cav1 in the murine pulmonary vasculature demonstrates a similar phenotype, with a marked increase in eNOS uncoupling and subsequent activation/dysfunction of the endothelium.60 On the other hand, global cav1-knockout mice demonstrate both increased basal eNOS activity and enhancement of NO-dependent vasodilation upon treatment with acetylcholine. This occurred at a magnitude consistent with that observed in endothelium-specific eNOS transgenic mice of the same background, and was rescued by endothelium-specific reconstitution of cav161. Taken together, these studies illustrate the role of cav1 in maintaining homeostasis within the endothelium, but also in mediating physiologic NO-dependent processes, by stabilizing and mediating eNOS structure and function.
Caveolae in lymphatics
The role of caveolae in the lymphatic system has been understudied until recent years where literature in the field has only recently begun to expand.
Traditionally, lymphatic vessels have been implicated in the removal of interstitial fluid mainly composed of water, electrolytes, proteins, glucose, fatty acids and cellular products.62 Lymphatic endothelial cells (LECs) which line the lumen of the lymphatic vasculature, perform many of these transport functions. For example, Triacca et al. demonstrated caveolae in lymphatic endothelial cells (LECs) are essential cross-cellular transporters. Using whole mount staining, it was found that endogenous and exogenous albumin accumulation occurred in the LECs located in skin of BALB/cByJ mice following subcutaneous administration of fluorescently labelled albumin.63 Moreover, flow cytometry showed that the levels of albumin uptake by LECs are similar to albumin uptake by dermal ECs. Fluorescence imaging showed that in LECs cav1 as well as clathrin colocalize with albumin, confirming the albumin-dependent uptake. However, to confirm this, LECs were treated with the dynamin inhibitor, which is crucial for cav1 and clathrin function, and it was shown that use of the dynasore inhibitor blocked intracellular albumin accumulation. In addition to this, an in vitro method was developed to differentiate quantitative intracellular solvent uptake versus trans-endothelial transport by LECs, where LECs subjected to 1μm/s flow have higher expression of cav1 and clathrin, as well as increased albumin and dextran uptake.63
Additionally, other studies using mouse models with a global cav1 knock-out and an LEC specific cav1 knock-out showed an increase in macromolecule tissue uptake by lymphatic system with loss of cav1.64 In conjunction with these results it was also observed that phasic contractility of the lymphatic vessels decreased resulting in reduced lymphatic transport function. Together, these studies provide the initial basis for further study of caveolae mediated transport in the lymphatic system.
Caveolae in metabolic disease
The role of EC caveolae in the development and progression of cardiovascular and metabolic disease has been of interest for several years.65–71 Historically, caveolae have been implicated in cardio-metabolic disease for their role in LDL transcytosis promoting atherogenesis. Using a cav1 null mouse, Ramirez et al. found the absence of caveolae inhibited LDL transport across the endothelium and prevented both proatherogenic fibronectin deposition and disturbed flow-mediated EC inflammation.72 Additionally, unique cav1 expression and caveolae distribution were observed in atheroprone (highly enriched for cav1) and athero-resistant areas (decreased cav1 abundance) of aortic arches in C57BL/6 mice.
Recent work has demonstrated cav1 regulates both lipid uptake and lipolysis. For example, Le Master et al. showed caveolae are a primary contributor to endothelial stiffening as a result of dyslipidemia and high fat diet (HFD).73 Loss of cav1 in mouse aortic ECs in vitro diminished the uptake of oxLDL and therefore decreased the vascular stiffening associated with oxLDL uptake. Interestingly, aortic endothelium lacking caveolin-1 were resistant to HFD induced aortic stiffening.73
Caveolae have also been shown to regulate lipid droplet metabolism in ECs via cAMP mediated lipolysis.74 There is impaired lipid droplet formation in mice lacking EC cav1 as seen with decreased lipid droplet staining in both the aorta of mice and ECs in culture. The authors attribute this difference to enhanced lipolysis and not fatty acid uptake, a previously underappreciated role of caveolae. Lipolysis is increased with loss of EC cav1 due to an increase in cAMP/PKA signaling which can be seen through elevated phosphorylation of hormone sensitive lipase. The authors also found an increase in EC produced prostacyclin, which can signal back to ECs in an autocrine manner to also increase cAMP mediated lipolysis. This was the first study to specifically highlight endothelial lipid droplet associated cav1 and show it is necessary for lipid droplet formation and can regulate cAMP-dependent lipolysis. The work presented by this group highlight two ideas in caveolar biology, an endocytosis role as well as a nonendocytic but rather signaling role for caveolae in ECs.
Conclusion
Caveolae are crucial for endothelial function. The deep invaginated structure of caveolae, primarily attributed to cav1 (Figure 2), provides the backbone for its cellular and physiological function (Figure 1). These invaginations form niche signaling hubs as they cluster proteins close together to facilitate signaling cascades. These signaling microdomains often regulate vascular function and subsequent vasoconstriction and dilation. Vasodilation can also be modulated through the regulation of eNOS by cav1, a well-established and vital role of caveolae (Figure 3). A less established and understudied function of caveolae is their molecular transport properties specifically in the lymphatic vasculature. Transport associated with caveolae has been extensively studied in the context of LDL and oxLDL uptake in atherosclerosis. The involvement of caveolae in both cardiovascular and metabolic disease continuous to be an area of investigation as caveolae prove to be more than just an entry point for lipids into endothelium.
Figure 3: Summary graphic.

Featured here are three roles for endothelial caveolae (left) focuses on the mechano-sensor function of caveolae in membrane tension with EHD2 serving as a transcription factor for more caveolae machinery. (center) signaling hub role of caveolae showing signaling microdomains with PANX1, P2Y2R, and TRPV4 all of which facilitate downstream vasodilation. (right) Cav1 can bind eNOS rendering it inactive until a phosphorylation event triggers NO production. eNOS can also become uncoupled to produce reactive oxygen species and vascular oxidative stress.
Here, distinct caveolar roles of mechanosensation, signaling, protein binding, and transport are presented. However, this is not an exhaustive list of caveolar function but a selection of work focused on understanding the specific roles of caveolae exclusively in the endothelium. There has been considerable progress made towards the understanding of caveolae in endothelium, however there are key questions in the field that remain under studied, these include:
Structure: How do post translational modifications of Cav1 (like phosphorylation and palmitoylation) effect the structure and therefore membrane placement of Cav1 and the microdomains it creates.
Mechanosenation and Signaling: The invaginations of caveolae can serve as a buffer for increases in cellular tension. It is also noted that crucial membrane proteins are specifically localized to caveolae for protection. What is not known is how these membrane proteins are localized there. What about the caveolae themselves helps create an environment where vital membrane proteins congregate and how do these domains different amongst EC subtypes (arterials, venous, capillary). In the same regard, it is known that structurally caveolae create signaling microdomains by facilitating close proximity between proteins in the same pathway. However, the mechanism localizing these groups of proteins to caveolae remains unknown.
eNOS: With advances in electron microscopy and molecular modeling, structural biologists do not agree where, or even if, cav1 and eNOS bind. Further investigation into this established protein pair’s physical interaction is needed to identify specific protein-protein binding sites.
Lymphatics: Research on the lymphatic system has grown extensively in the last few years. In turn this has increased the fields knowledge on LEC cav1, however no signaling roles for cav1 in lymphatics have been established. Additionally, the morphology and spatiotemporal regulation of caveolae have not investigated in this vascular bed.
Metabolic disease: Cav1 has long been associated with the onset and progression of metabolic disease. Most of the literature focuses on cav1 facilitated oxLDL uptake into endothelium of large vessels like the aorta. However, little is known about what mechanism accomplishes lipid uptake via cav1 and how this might change in smaller vessels. Additionally, signaling roles for cav1, outside of transport, in the context of metabolic regulation have yet to be interrogated thoroughly.
For these reasons, continued rigorous investigation of endothelial caveola are required, which will by extension provide important insight into endothelial cell function.
Table 1:
List of the caveolar functions discussed in this manuscript with a brief description of the recent endothelial based literature covered.
| Caveolae function: | Description: |
|---|---|
| mechano-sensors |
|
| signaling hub |
|
| regulation of eNOS |
|
| lymphatic endothelium |
|
| metabolic disease |
|
Acknowledgments
This work was supported by: AHA predoctoral fellowship 915176 (MAL), NIH HL120840 (BEI), NIH HL137112 (BEI). We thank Dr. Luke Dunaway for critical feedback.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors of this manuscript have no disclosures or conflicting interests
References
- 1.Palade GE and Bruns R. Structural modulations of plasmalemmal vesicles. The Journal of cell biology. 1968;37:633–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamada E The fine structure of the gall bladder epithelium of the mouse. The Journal of biophysical and biochemical cytology. 1955;1:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parton RG, Joggerst B and Simons K. Regulated internalization of caveolae. The Journal of cell biology. 1994;127:1199–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelkmans L, Kartenbeck J and Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nature cell biology. 2001;3:473–483. [DOI] [PubMed] [Google Scholar]
- 5.Pelkmans L, Puntener D and Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. science. 2002;296:535–539. [DOI] [PubMed] [Google Scholar]
- 6.Zhou M, Shi SX, Liu N, Jiang Y, Karim MS, Vodovoz SJ, Wang X, Zhang B and Dumont AS. Caveolae-mediated endothelial transcytosis across the blood-brain barrier in acute ischemic stroke. Journal of Clinical Medicine. 2021;10:3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito A, Shiroto T, Godo S, Saito H, Tanaka S, Ikumi Y, Kajitani S, Satoh K and Shimokawa H. Important roles of endothelial caveolin-1 in endothelium-dependent hyperpolarization and ischemic angiogenesis in mice. American Journal of Physiology-Heart and Circulatory Physiology. 2019;316:H900–H910. [DOI] [PubMed] [Google Scholar]
- 8.Zhou HJ, Qin L, Jiang Q, Murray KN, Zhang H, Li B, Lin Q, Graham M, Liu X and Grutzendler J. Caveolae-mediated Tie2 signaling contributes to CCM pathogenesis in a brain endothelial cell-specific Pdcd10-deficient mouse model. Nature communications. 2021;12:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mylvaganam S, Plumb J, Yusuf B, Li R, Lu C-Y, Robinson LA, Freeman SA and Grinstein S. The spectrin cytoskeleton integrates endothelial mechanoresponses. Nature Cell Biology. 2022;24:1226–1238. [DOI] [PubMed] [Google Scholar]
- 10.Pandit R, Koh WK, Sullivan RK, Palliyaguru T, Parton RG and Götz J. Role for caveolinmediated transcytosis in facilitating transport of large cargoes into the brain via ultrasound. Journal of Controlled Release. 2020;327:667–675. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J, Li Z, Ji Z, Wu Y, He Y, Liu K, Chang Y, Peng Y, Lin Z and Wang S. Glycocalyx is critical for blood-brain barrier integrity by suppressing caveolin1-dependent endothelial transcytosis following ischemic stroke. Brain Pathology. 2022;32:e13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothberg KG, Heuser JE, Donzell WC, Ying Y-S, Glenney JR and Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto T, Kogo H, Nomura R and Une T. Isoforms of caveolin-1 and caveolar structure. Journal of cell science. 2000;113:3509–3517. [DOI] [PubMed] [Google Scholar]
- 14.Scherer PE, Tang Z, Chun M, Sargiacomo M, Lodish HF and Lisanti MP. Caveolin isoforms differ in their N-terminal protein sequence and subcellular distribution. Identification and epitope mapping of an isoform-specific monoclonal antibody probe. Journal of Biological Chemistry. 1995;270:16395–16401. [DOI] [PubMed] [Google Scholar]
- 15.Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF and Lisanti MP. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proceedings of the National Academy of Sciences. 1996;93:131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galbiati F, Razani B and Lisanti MP. Caveolae and caveolin-3 in muscular dystrophy. Trends in molecular medicine. 2001;7:435–441. [DOI] [PubMed] [Google Scholar]
- 17.Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, Walser P, Abankwa D, Oorschot VM and Martin S. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Brown D, McKee M, LeBrasseur NK, Yang D, Albrecht KH, Ravid K and Pilch PF. Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell metabolism. 2008;8:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS and Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells: caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. Journal of Biological Chemistry. 1996;271:15160–15165. [DOI] [PubMed] [Google Scholar]
- 20.Stan RV. Structure and function of endothelial caveolae. Microscopy research and technique. 2002;57:350–364. [DOI] [PubMed] [Google Scholar]
- 21.Xu H, Zhang L, Chen W, Xu J, Zhang R, Liu R, Zhou L, Hu W, Ju R and Lee C. Inhibitory effect of caveolin-1 in vascular endothelial cells, pericytes and smooth muscle cells. Oncotarget. 2017;8:76165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musial DC, Miranda-Ferreira R, Padin JF, Arranz-Tagarro JA, Parra-Vitela AJ, Jurkiewicz A, Garcia AG and Jurkiewicz NH. Function of AT1 and AT2 receptors in atrial contractions from spontaneous hypertensive and diabetic-induced streptozotocin rats. Clinical and Experimental Pharmacology and Physiology. 2018;45:1274–1285. [DOI] [PubMed] [Google Scholar]
- 23.Touyz R and Berry C. Recent advances in angiotensin II signaling. Brazilian Journal of Medical and Biological Research. 2002;35:1001–1015. [DOI] [PubMed] [Google Scholar]
- 24.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F and Luft FC. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. science. 2001;293:2449–2452. [DOI] [PubMed] [Google Scholar]
- 25.Porta JC, Han B, Gulsevin A, Chung JM, Peskova Y, Connolly S, Mchaourab HS, Meiler J, Karakas E and Kenworthy AK. Molecular architecture of the human caveolin-1 complex. Science Advances. 2022;8:eabn7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han B, Wilson LF, Gulsevin A, Meiler J, Karakas E and Kenworthy AK. Design principles of caveolins across metazoa and beyond. bioRxiv. 2022. [Google Scholar]
- 27.Wong TH, Khater IM, Joshi B, Shahsavari M, Hamarneh G and Nabi IR. Single molecule network analysis identifies structural changes to caveolae and scaffolds due to mutation of the caveolin-1 scaffolding domain. Scientific reports. 2021;11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Couet J, Li S, Okamoto T, Ikezu T and Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain: implications for the interaction of caveolin with caveolae-associated proteins. Journal of Biological Chemistry. 1997;272:6525–6533. [DOI] [PubMed] [Google Scholar]
- 29.Shin H, Haga J, Kosawada T, Kimura K, Li Y, Chien S and Schmid-Schönbein G. Fine control of endothelial VEGFR-2 activation: caveolae as fluid shear stress shelters for membrane receptors. Biomechanics and modeling in mechanobiology. 2019;18:5–16. [DOI] [PubMed] [Google Scholar]
- 30.Sinha B, Köster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler-Browne G, Vedie B and Johannes L. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell. 2011;144:402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV and Sessa WC. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. The Journal of clinical investigation. 2006;116:1284–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torrino S, Shen W-W, Blouin CM, Mani SK, Viaris de Lesegno C, Bost P, Grassart A, Köster D, Valades-Cruz CA and Chambon V. EHD2 is a mechanotransducer connecting caveolae dynamics with gene transcription. Journal of Cell Biology. 2018;217:4092–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michell DL, Shihata WA, Andrews KL, Abidin NAZ, Jefferis A-M, Sampson AK, Lumsden NG, Huet O, Parat M-O and Jennings GL. High intraluminal pressure promotes vascular inflammation via caveolin-1. Scientific reports. 2021;11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W, Song H, Xu J, Guo Y, Zhang C, Yao Y, Zhang H, Liu Z and Li Y-C. Low shear stress inhibits endothelial mitophagy via caveolin-1/miR-7–5p/SQSTM1 signaling pathway. Atherosclerosis. 2022;356:9–17. [DOI] [PubMed] [Google Scholar]
- 35.Leo F, Hutzler B, Ruddiman CA, Isakson BE and Cortese-Krott MM. Cellular microdomains for nitric oxide signaling in endothelium and red blood cells. Nitric Oxide. 2020;96:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu T, Wang X-L, Chai Q, Sun X, Sieck GC, Katusic ZS and Lee H-C. Role of the endothelial caveolae microdomain in shear stress–mediated coronary vasorelaxation. Journal of Biological Chemistry. 2017;292:19013–19023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hashimoto T, Isaji T, Hu H, Yamamoto K, Bai H, Santana JM, Kuo A, Kuwahara G, Foster TR and Hanisch JJ. Stimulation of caveolin-1 signaling improves arteriovenous fistula patency. Arteriosclerosis, thrombosis, and vascular biology. 2019;39:754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bucci M, Gratton J-P, Rudic RD, Acevedo L, Roviezzo F, Cirino G and Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nature medicine. 2000;6:1362–1367. [DOI] [PubMed] [Google Scholar]
- 39.Khater IM, Meng F, Wong TH, Nabi IR and Hamarneh G. Super resolution network analysis defines the molecular architecture of caveolae and caveolin-1 scaffolds. Scientific reports. 2018;8:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martens JR, O’Connell K and Tamkun M. Targeting of ion channels to membrane microdomains: localization of KV channels to lipid rafts. Trends in pharmacological sciences. 2004;25:16–21. [DOI] [PubMed] [Google Scholar]
- 41.Morris JB, Huynh H, Vasilevski O and Woodcock EA. α1-Adrenergic receptor signaling is localized to caveolae in neonatal rat cardiomyocytes. Journal of molecular and cellular cardiology. 2006;41:17–25. [DOI] [PubMed] [Google Scholar]
- 42.Balijepalli RC, Foell JD, Hall DD, Hell JW and Kamp TJ. Localization of cardiac L-type Ca2+ channels to a caveolar macromolecular signaling complex is required for β2-adrenergic regulation. Proceedings of the National Academy of Sciences. 2006;103:7500–7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeLalio LJ, Keller AS, Chen J, Boyce AK, Artamonov MV, Askew-Page HR, Keller IV TS, Johnstone SR, Weaver RB and Good ME. Interaction between pannexin 1 and caveolin-1 in smooth muscle can regulate blood pressure. Arteriosclerosis, thrombosis, and vascular biology. 2018;38:2065–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daneva Z, Ottolini M, Chen YL, Klimentova E, Kuppusamy M, Shah SA, Minshall RD, Seye CI, Laubach VE and Isakson BE. Endothelial pannexin 1–TRPV4 channel signaling lowers pulmonary arterial pressure in mice. Elife. 2021;10:e67777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daneva Z, Marziano C, Ottolini M, Chen Y-L, Baker TM, Kuppusamy M, Zhang A, Ta HQ, Reagan CE and Mihalek AD. Caveolar peroxynitrite formation impairs endothelial TRPV4 channels and elevates pulmonary arterial pressure in pulmonary hypertension. Proceedings of the National Academy of Sciences. 2021;118:e2023130118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.García-Cardeña G, Fan R, Stern DF, Liu J and Sessa WC. Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. Journal of Biological Chemistry. 1996;271:27237–27240. [DOI] [PubMed] [Google Scholar]
- 47.Chidlow JH Jr and Sessa WC. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovascular research. 2010;86:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shu X, Keller T, Begandt D, Butcher JT, Biwer L, Keller AS, Columbus L and Isakson BE. Endothelial nitric oxide synthase in the microcirculation. Cellular and molecular life sciences. 2015;72:4561–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ju H, Zou R, Venema VJ and Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. Journal of Biological Chemistry. 1997;272:18522–18525. [DOI] [PubMed] [Google Scholar]
- 50.Garcıa-Cardeña G, Martasek P, Masters BSS, Skidd PM, Couet J, Li S, Lisanti MP and Sessa WC. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin: Functional significance of the NOS caveolin binding domainin vivo. Journal of Biological Chemistry. 1997;272:25437–25440. [DOI] [PubMed] [Google Scholar]
- 51.Chen Z, DS Oliveira S, Zimnicka AM, Jiang Y, Sharma T, Chen S, Lazarov O, Bonini MG, Haus JM and Minshall RD. Reciprocal regulation of eNOS and caveolin-1 functions in endothelial cells. Molecular biology of the cell. 2018;29:1190–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trane AE, Hiob MA, Uy T, Pavlov D and Bernatchez P. Caveolin-1 scaffolding domain residue phenylalanine 92 modulates Akt signaling. European journal of pharmacology. 2015;766:46–55. [DOI] [PubMed] [Google Scholar]
- 53.Piazza M, Dieckmann T and Guillemette JG. Structural studies of a complex between endothelial nitric oxide synthase and calmodulin at physiological calcium concentration. Biochemistry. 2016;55:5962–5971. [DOI] [PubMed] [Google Scholar]
- 54.Chen Z, Bakhshi FR, Shajahan AN, Sharma T, Mao M, Trane A, Bernatchez P, van Nieuw Amerongen GP, Bonini MG and Skidgel RA. Nitric oxide–dependent Src activation and resultant caveolin-1 phosphorylation promote eNOS/caveolin-1 binding and eNOS inhibition. Molecular biology of the cell. 2012;23:1388–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Li Q, Zhang Z, Peng K, Zhang D-M, Yang Q, Passerini AG, Simon SI and Sun C. mTOR contributes to endothelium-dependent vasorelaxation by promoting eNOS expression and preventing eNOS uncoupling. Communications biology. 2022;5:726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takaya T, Hirata K-i, Yamashita T, Shinohara M, Sasaki N, Inoue N, Yada T, Goto M, Fukatsu A and Hayashi T. A specific role for eNOS-derived reactive oxygen species in atherosclerosis progression. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:1632–1637. [DOI] [PubMed] [Google Scholar]
- 57.Santana MNS, Souza DS, Miguel-dos-Santos R, Rabelo TK, de Vasconcelos CML, Navia-Pelaez JM, de Jesus ICG, da Silva-Neto JA, Lauton-Santos S and Capettini LdSA. Resistance exercise mediates remote ischemic preconditioning by limiting cardiac eNOS uncoupling. Journal of molecular and cellular cardiology. 2018;125:61–72. [DOI] [PubMed] [Google Scholar]
- 58.Aoki T, Nomura R and Fujimoto T. Tyrosine phosphorylation of caveolin-1 in the endothelium. Experimental cell research. 1999;253:629–636. [DOI] [PubMed] [Google Scholar]
- 59.Potje SR, Grando MD, Chignalia AZ, Antoniali C and Bendhack LM. Reduced caveolae density in arteries of SHR contributes to endothelial dysfunction and ROS production. Scientific reports. 2019;9:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oliveira SD, Castellon M, Chen J, Bonini MG, Gu X, Elliott MH, Machado RF and Minshall RD. Inflammation-induced caveolin-1 and BMPRII depletion promotes endothelial dysfunction and TGF-β-driven pulmonary vascular remodeling. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2017;312:L760–L771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Godo S, Sawada A, Saito H, Ikeda S, Enkhjargal B, Suzuki K, Tanaka S and Shimokawa H. Disruption of physiological balance between nitric oxide and endothelium-dependent hyperpolarization impairs cardiovascular homeostasis in mice. Arteriosclerosis, thrombosis, and vascular biology. 2016;36:97–107. [DOI] [PubMed] [Google Scholar]
- 62.Petrova TV and Koh GY. Biological functions of lymphatic vessels. Science. 2020;369:eaax4063. [DOI] [PubMed] [Google Scholar]
- 63.Triacca V, Güç E, Kilarski WW, Pisano M and Swartz MA. Transcellular pathways in lymphatic endothelial cells regulate changes in solute transport by fluid stress. Circulation research. 2017;120:1440–1452. [DOI] [PubMed] [Google Scholar]
- 64.Baranwal G, Creed HA, Cromer WE, Wang W, Upchurch BD, Smithhart MC, Vadlamani SS, Clark MCC, Busbuso NC and Blais SN. Dichotomous effects on lymphatic transport with loss of caveolae in mice. Acta Physiologica. 2021;232:e13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernández-Hernando C, Yu J, Suárez Y, Rahner C, Dávalos A, Lasunción MA and Sessa WC. Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell metabolism. 2009;10:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fernández-Hernando C, Yu J, Dávalos A, Prendergast J and Sessa WC. Endothelialspecific overexpression of caveolin-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. The American journal of pathology. 2010;177:998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang H, Wang AX and Barrett EJ. Caveolin-1 is required for vascular endothelial insulin uptake. American Journal of Physiology-Endocrinology and Metabolism. 2011;300:E134–E144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shamsaldeen YA, Ugur R, Benham CD and Lione LA. Diabetic dyslipidaemia is associated with alterations in eNOS, caveolin-1, and endothelial dysfunction in streptozotocin treated rats. Diabetes/Metabolism Research and Reviews. 2018;34:e2995. [DOI] [PubMed] [Google Scholar]
- 69.Li X, Sun X and Carmeliet P. Hallmarks of endothelial cell metabolism in health and disease. Cell metabolism. 2019;30:414–433. [DOI] [PubMed] [Google Scholar]
- 70.Kakava S, von Eckardstein A and Robert J. Regulation of low-density lipoprotein transport through endothelial cells by caveolae. Atherosclerosis. 2023. [DOI] [PubMed] [Google Scholar]
- 71.Zhang X, Gong P, Zhao Y, Wan T, Yuan K, Xiong Y, Wu M, Zha M, Li Y and Jiang T. Endothelial caveolin-1 regulates cerebral thrombo-inflammation in acute ischemia/reperfusion injury. EBioMedicine. 2022;84:104275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramírez CM, Zhang X, Bandyopadhyay C, Rotllan N, Sugiyama MG, Aryal B, Liu X, He S, Kraehling JR and Ulrich V. Caveolin-1 regulates atherogenesis by attenuating low-density lipoprotein transcytosis and vascular inflammation independently of endothelial nitric oxide synthase activation. Circulation. 2019;140:225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Le Master E, Paul A, Lazarko D, Aguilar V, Ahn SJ, Lee JC, Minshall RD and Levitan I. Caveolin-1 is a primary determinant of endothelial stiffening associated with dyslipidemia, disturbed flow, and ageing. Scientific Reports. 2022;12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuo A, Lee MY, Yang K, Gross RW and Sessa WC. Caveolin-1 regulates lipid droplet metabolism in endothelial cells via autocrine prostacyclin–stimulated, cAMP-mediated lipolysis. Journal of Biological Chemistry. 2018;293:973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]


