Abstract
Current therapy for leishmaniasis is unsatisfactory because parenteral antimonial salts and pentamidine are associated with significant toxicity and failure rates. We examined the efficacy of KY62, a new, water-soluble, polyene antifungal, against cutaneous infection with Leishmania amazonensis and against visceral infection with Leishmania donovani in susceptible BALB/c mice. Mice were infected with L. amazonensis promastigotes in the ear pinna and in the tail and were treated with KY62 or amphotericin B. The cutaneous lesions showed a remarkable response to therapy with KY62 at a dose of 30 mg per kg of body weight per day. At this dose, the efficacy of KY62 was equivalent to or better than that of amphotericin B at 1 to 5 mg/kg/day. Mice infected intravenously with 107 L. donovani promastigotes and treated with KY62 showed a 4-log reduction in the parasite burden in the liver and spleen compared to untreated mice. These studies indicate potent activity of KY62 against experimental cutaneous leishmaniasis caused by L. amazoniensis and against experimental visceral leishmaniasis caused by L. donovani.
The leishmaniases, caused by protozoa of the genus Leishmania, are distributed throughout the world. Cutaneous leishmaniasis, typically a slowly healing cutaneous ulcer, can result in deforming scars. Visceral leishmaniasis is characterized by fever, hepatosplenomegaly, pancytopenia, and cachexia and is associated with significant mortality (15). Current therapy for leishmaniasis is unsatisfactory (2). Antimonial compounds (sodium stibogluconate and meglumine antimoniate) are considered the drugs of first choice. These drugs are parenteral and are associated with significant side effects such as pancreatitis, myalgia, arthralgia, and, rarely, cardiac toxicity (6). Pentamidine, another alternative, causes renal toxicity and pancreatitis. These agents are also associated with significant failure and relapse rates, especially in the immunocompromised host (1).
Leishmania and fungi both have ergosterol-based sterols as essential components of their membrane structure. Antifungal agents, therefore, have potential in the treatment of leishmaniasis. The azole antifungals ketoconazole and itraconazole have been used to treat cutaneous leishmaniasis with variable success rates but have had no efficacy in cases of visceral disease (11, 16, 18). Amphotericin B interacts with leishmanial ergosterol and episterol, disrupting the membrane barrier and leading to loss of intracellular components and to cellular death (17). Amphotericin B has been successfully used in treating visceral leishmaniasis in animals and humans (9, 10). The use of amphotericin B is limited by substantial associated toxicity. Several recent reports also indicate success in treating leishmaniasis with lipid formulations of amphotericin B (4, 5, 21).
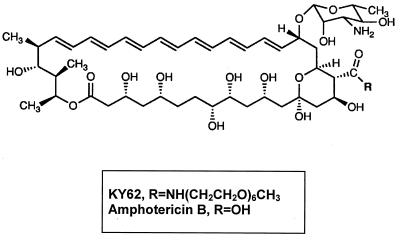
KY62 is a synthetic, water-soluble, polyene antifungal with structural resemblance to amphotericin B (Fig. 1). This drug has been administered to mice in high doses with no noticeable toxicity (7). We tested the activity of KY62 against Leishmania promastigotes in vitro, in experimental cutaneous leishmaniasis caused by Leishmania amazonensis, and in experimental visceral leishmaniasis caused by Leishmania donovani.
FIG. 1.
Chemical structure of KY62 and amphotericin B.
MATERIALS AND METHODS
Parasites.
The virulent strains L. donovani 1S (MHOM/SD/001S-2D) and L. amazonensis JOSEPHA were selected for the in vitro and in vivo studies. Additionally, L. major strain KK, L. mexicana strains 68 and 390 (gifts from F. Andrade-Narvaez, Merida, Mexico), and L. panamensis strains LS94 and L334 (gifts from B. Travi, CIDEIM, Cali, Colombia) were included in the in vitro testing. Prior to mouse infection, the parasites were washed in phosphate-buffered saline, counted, and resuspended in phosphate-buffered saline at the appropriate concentration.
Animals.
Age matched, 4- to 6-week-old female inbred BALB/c nu/+ mice (veterinary medical unit breeding colony; Audie Murphy Veterans Administration Hospital, San Antonio, Tex.) were used in these studies. Ten mice were included in each treatment and control group and were housed five mice per cage with free access to water and food.
Drugs.
KY62, a polyene antifungal, was chemically synthesized in the laboratory of S. L. Regen (Lehigh University, Bethlehem, Pa.) (23). The drug was reconstituted from powder in sterile 5% dextrose in water (D5W); 0.2 ml of this solution was injected into the mice intraperitoneally (i.p.). Amphotericin B was purchased commercially (Bristol-Myers-Squibb, Princeton, N.J.) and was injected in a similar fashion.
In vitro susceptibility.
Leishmania promastigotes were maintained in Grace’s insect culture medium supplemented with 15% heat-inactivated fetal calf serum, 1 μg of biotin per ml, 0.1 mM adenine, 5 μg of hemin per ml, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. At log phase (3 days after passage in culture media), Leishmania promastigotes were harvested and counted with a hemacytometer. Promastigotes (5 × 106/ml) were incubated in microwell plates at 26°C in 250 μl of medium/well, with each well containing either a twofold dilution of one drug (0.125 to 8 μg/ml for KY62 and amphotericin B and 0.25 to 32 μg/ml for fluconazole) or control medium. The minimum protozoacidal concentration (MPC) was defined as the lowest concentration that reduced the number of viable promastigotes with respect to simultaneously growing controls by ≥90% after 18 h of incubation with the drug, as assessed by flagellar motility under indirect microscopy (14). In a few experiments, parasite death was also assessed by a [3H]thymidine incorporation assay. In these experiments, 1 μCi of [3H]thymidine was added to each well for the last 18 h of culture and the amount of incorporation was determined by scintillation counting.
Treatment of cutaneous L. amazonensis infection. (i) Two-week therapy.
Three groups of 10 female inbred BALB/c mice each were selected randomly to receive KY62 at a daily dose of 30 mg/kg of body weight, amphotericin B at a daily dose of 1 mg/kg, or D5W by i.p. injection. KY62 previously had shown no toxicity for mice treated for candidiasis at a dosage of 30 mg/kg/day (7). All mice were injected with 5 × 106 promastigotes of L. amazoniensis in 0.01 ml in the right ear pinna and with 5 × 106 promastigotes in 0.02 ml subcutaneously in the proximal third of the tail. This infective dose was selected to ensure development of significant lesions. Treatments were started on the third day postinfection and continued for 14 days. Measurements of the ear pinna thickness and tail lesion thicknesses, with a fine-scale caliper (Fowler Precision Tools; Lux Scientific Instrument Corp., New York, N.Y.), were started on the fourth day postinfection and repeated weekly for 6 weeks. Lesion size was considered the difference between the thicknesses of the infected and uninfected ear pinnae. The size of the tail lesion was obtained by subtracting the average of the measurements of the tail diameter at points just rostral and caudal to the lesion site from the maximal tail diameter at the lesion site. Histopathology was performed on ear and tail lesions of three mice from each group at 6 weeks postinfection.
(ii) One-week dose-ranging study.
Six groups of 10 BALB/c mice each were randomly selected to receive KY62 at daily doses of 30, 15, 5, or 1 mg/kg by i.p. injection for 7 days. A positive control group received amphotericin B at a daily dose of 5 mg/kg i.p., and the negative control group received D5W i.p. All mice were infected with L. amazonensis as described above. Treatments were started on the third day postinfection and continued for 7 days. Measurements of ear and tail lesions were started the first day postinfection and made weekly afterwards for 4 weeks.
(iii) Four-week therapy.
Two groups of 10 age-matched, randomly selected female BALB/c mice each were infected with L. amazonensis in the ear pinna and tail as described above. Treatments with KY62 at a daily dose of 15 mg/kg or with D5W were started on the third day postinfection and continued for 28 days. Measurement of lesions was done weekly for 5 weeks. At 37 days postinfection, eight mice per group were sacrificed, their external sacral lymph nodes (which drain the tail) were harvested, and the lymph node parasite burden was determined by quantitative limiting dilution (8). Lymph nodes were harvested and homogenized between the frosted ends of sterile slides in 1 ml of complete culture medium and diluted with the same medium to a final concentration of 1 mg/ml. Fourfold serial dilutions of the homogenized tissue were then plated in a 96-well tissue culture plate and cultured at 26°C for 3 weeks. The wells were examined for viable promastigotes at 3-day intervals, and the reciprocal of the highest dilution which was positive for parasites was considered to be the concentration of parasites per milligram of lymph nodes (8).
Treatment of visceral L. donovani infection.
Two groups of eight age-matched, randomly selected female BALB/c mice each were inoculated intravenously with 107 promastigotes of L. donovani in 0.2 ml through the lateral tail vein. One group was treated i.p. with KY62 at a daily dose of 15 mg/kg, and the other group received D5W alone. Treatment was started on the third day postinfection and continued for 14 days. Thirty days postinfection, all mice were terminated, and hepatic and splenic parasite burdens were determined by the quantitative limiting dilution method described above (an approximately 30-mg piece of organ was homogenized).
Statistical analysis.
For cutaneous infection, the means (± standard deviations) of the sizes of the ear and tail lesions between treatment groups and controls were compared by using the unpaired Student t test. For visceral infection, the mean log10 parasites per gram of liver and spleen of treatment groups and controls were compared by using the unpaired Student t test. A P value of <0.05 was considered statistically significant.
RESULTS
In vitro susceptibility to KY62, amphotericin B, and fluconazole.
In vitro assessment of the protozoacidal activity indicated potent activity of both KY62 and amphotericin B against Leishmania promastigotes of different species (Table 1). Fluconazole was not effective against Leishmania promastigotes in culture media, having an MPC of more than 32 μg/ml. KY62 and amphotericin B showed equivalent in vitro antileishmanial activities. The MPCs of the drugs as determined by visual inspection were confirmed by the loss of [3H]thymidine incorporation in the drug-treated parasites (data not shown).
TABLE 1.
Activity of antifungal agents against leishmania
| Species | MPC (μg/ml)a
|
||
|---|---|---|---|
| Amphotericin B | KY62 | Fluconazole | |
| L. donovani | 0.5 | 0.5 | >32 |
| L. amazonensis | 0.5 | 0.5 | >32 |
| L. major | 0.125 | 0.125 | >32 |
| L. mexicana 68 | 0.5 | 0.25 | >32 |
| L. mexicana 390 | 1.0 | 0.5 | >32 |
| L. panamensis L334 | 0.25 | 0.25 | >32 |
| L. panamensis LS94 | 0.25 | 0.125 | >32 |
The MPC is the concentration that results in 90% inhibition of promastigote motility after 18 h of incubation.
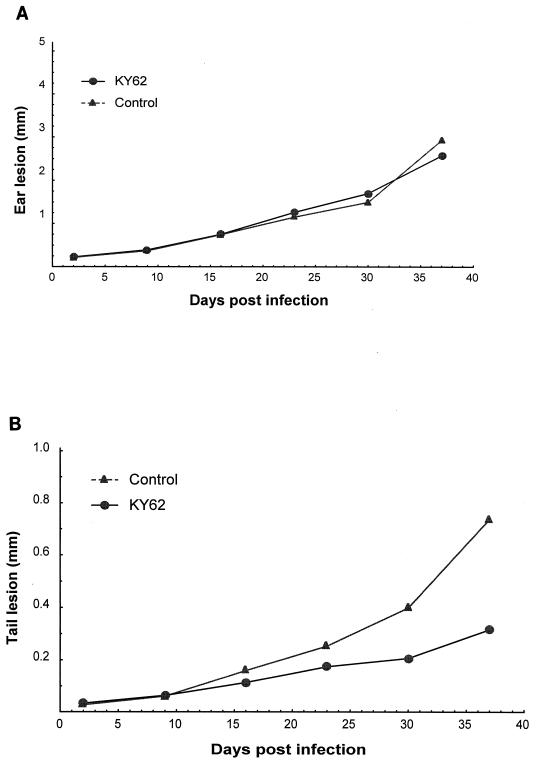
Efficacy of KY62 in experimental cutaneous leishmaniasis. (i) Two-week therapy.
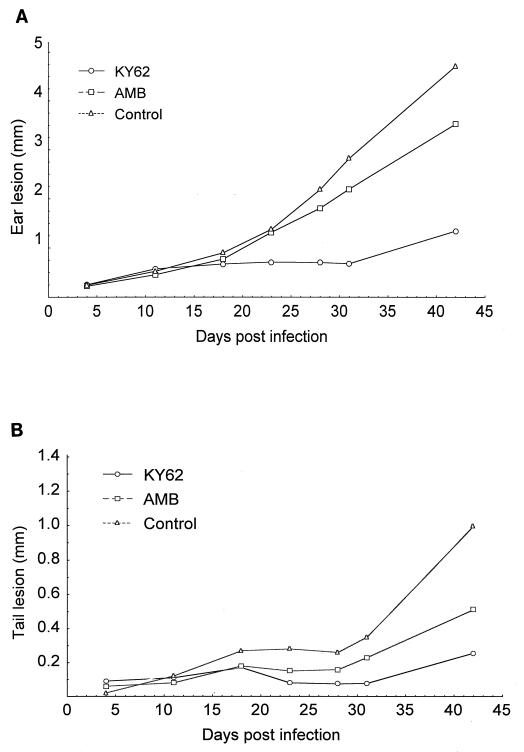
Six weeks postinfection, ear lesions of the control group were remarkably larger than those of the groups receiving KY62 or amphotericin B. The lesion sizes were 4.51 ± 1.40 mm, 1.15 ± 0.81 mm, and 3.33 ± 1.45 mm for controls, KY62 recipients, and amphotericin B recipients, respectively. The differences were significant for the KY62- treated group compared to controls (P = 0.00008) (Fig. 2A). At the doses used, KY62 was significantly more effective than amphotericin B in reducing lesion size (P = 0.001).
FIG. 2.
Efficacy of a 14-day treatment course with KY62 (30 mg/kg/day) or amphotericin B (1 mg/kg/day) in experimental cutaneous leishmaniasis caused by L. amazonensis. Sizes of ear pinna lesions (A) and tail lesions (B) in the treated and untreated control mice are shown. Treatments were started on the third day postinfection and continued for 14 days. The ear lesion size was considered the difference between the thicknesses of the infected and uninfected ear pinna. The size of the tail lesion was obtained by subtracting the average of the measurements of the tail diameter at points just rostral and caudal to the lesion site from the maximal tail diameter at the lesion site.
The tail lesions at 6 weeks postinfection were smaller in the KY62 (0.25 ± 0.23 mm) and amphotericin B (0.50 ± 0.17 mm) groups compared to controls (0.98 ± 0.41 mm). These differences were significant (P = 0.0002 for KY62 and P = 0.003 for amphotericin B compared to controls) (Fig. 2B). The tail lesions in the KY62-treated mice were also significantly smaller than those in the amphotericin B-treated mice (P = 0.015).
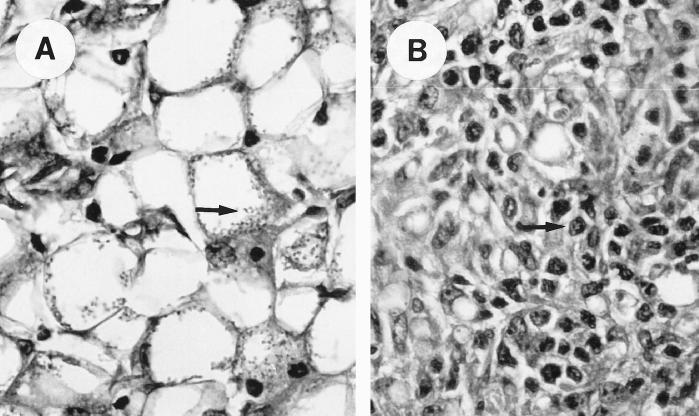
Histopathologic analysis of the ear lesions revealed lymphocytic infiltration with few parasites in mice treated with KY62 but abundant, foamy macrophages with intracellular amastigotes in the untreated mice (Fig. 3).
FIG. 3.
Wright’s stain of sections of an ear lesion in the control (A) and KY62-treated (B) mice. The section from the control mouse shows a predominance of the large, foamy macrophages with abundant amastigotes (arrow), whereas the section from the treated mouse shows a more lymphocytic predominance (arrow) and a markedly reduced number of amastigotes. Magnification, ×40 (for both panels).
During treatment, only one mouse (a KY62 recipient) died before the end of the study. Death was attributed to anesthesia with methoxyflurane during measurement of lesions.
(ii) One-week dose-ranging study.
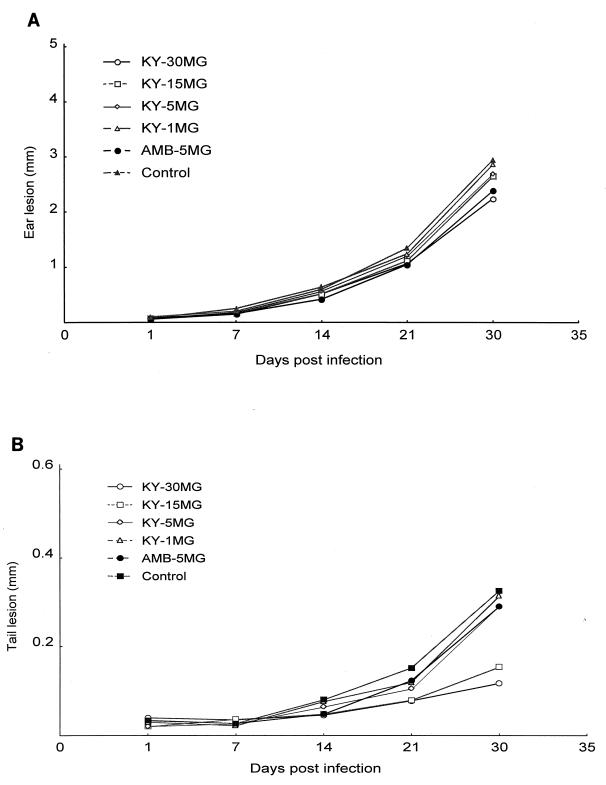
To determine if a shorter course of therapy and lower drug doses could be effective in the treatment of experimental cutaneous leishmaniasis, a study of KY62 and amphotericin B given at different doses for 7 days was conducted. At 30 days postinfection, smaller tail lesion sizes were found in mice treated with KY62 at dosages of 30 and 15 mg/kg/day, but not at dosages of 5 and 1 mg/kg/day. The mean diameters of the tail lesions were 0.32 ± 0.14 mm, 0.11 ± 0.07 mm, 0.14 ± 0.06 mm, 0.30 ± 0.10 mm, 0.29 ± 0.13 mm, and 0.28 ± 0.08 mm for controls, KY62 (30 mg/kg), KY62 (15 mg/kg), KY62 (5 mg/kg), KY62 (1 mg/kg), and amphotericin B (5 mg/kg) treatment groups, respectively. P values of 0.0005 and 0.0023 were calculated for KY62 at the 30- and 15-mg/kg doses, respectively, compared to controls (Fig. 4B). At 5 mg/kg/day for 7 days, amphotericin B was not significantly better than controls in either the ear or the tail infections. None of the treatment groups had significantly better outcomes of ear infections than control mice (Fig. 4A). A 7-day course of therapy was thus inferior to the 14-day course used in the first experiment.
FIG. 4.
Efficacy of a 7-day treatment course with various doses of KY62 or with amphotericin B (5 mg/kg/day) in experimental cutaneous leishmaniasis caused by L. amazonensis. Sizes of ear pinna lesions (A) and tail lesions (B) are shown. Treatments were started on the third day postinfection and continued for 7 days. Lesions were measured as described in the legend to Fig. 2.
(iii) Four-week therapy.
To determine if a lower dose of KY62 given for a longer duration could be effective, mice were treated for 28 days at 15 mg/kg/day. Five weeks postchallenge, the tail lesions were significantly smaller in KY62-treated mice than in controls (Fig. 5B) (mean, 0.31 ± 0.10 mm and 0.73 ± 0.12 mm, respectively [P = 0.00001]). There was no significant difference between treated mice and controls in ear infections (Fig. 5A). The parasite burden in the external sacral lymph nodes was markedly lower in the KY62-treated group (mean log10, 7.29 ± 1.0 and 4.43 ± 1.4 parasites per lymph node for controls and KY62-treated mice, respectively).
FIG. 5.
Efficacy of a prolonged treatment course with KY62 (15 mg/kg/day) in experimental cutaneous leishmaniasis caused by L. amazonensis. Sizes of ear pinna lesions (A) and tail lesions (B) in the KY62-treated and control mice are shown. Treatments were started on the third day postinfection and continued for 28 days. Lesions were measured as described in the legend to Fig. 2.
Efficacy of KY62 in experimental visceral leishmaniasis.
L. donovani-infected mice were treated for 14 days with KY62 (15 mg/kg/day), and the visceral parasite burden was determined at 30 days postinfection. The mean hepatic parasite burden (log10 per gram of liver) in the KY62-treated mice was 3.51 log10 lower than the parasite burden in controls (5.96 ± 0.91 and 9.47 ± 1.09 for KY62-treated and control groups, respectively [P = 0.00001]). The mean splenic parasite burden (log10 per gram of spleen) was 4.21 logs lower in the KY62-treated mice than controls (5.49 ± 0.96 and 9.66 ± 0.81 for KY62-treated and control groups, respectively [P = 0.000001]).
DISCUSSION
Efforts to find new agents for the treatment of leishmaniasis have been ongoing for several decades. Derivatives of antimony have been used with reasonable success for more than 50 years to treat leishmaniasis. Recently, an increased number of reports of treatment failures and relapses with antimonial therapy have been published (1, 9, 10, 20). This is due partly to leishmanial infection in patients with AIDS and partly to acquired resistance to the antimonial compounds (9, 10).
In this study, we have identified a new polyene antifungal drug, KY62, which had high leishmanicidal activity in vitro. Although in vitro activity of a drug against the promastigote stage may not necessarily correlate with activity against intracellular amastigotes, our in vivo studies demonstrated that KY62 was highly efficacious in experimental models of cutaneous and visceral leishmaniasis. KY62, which differs from amphotericin B in one side chain, showed in vitro activity which was equivalent to that of amphotericin B. The mechanism of action of KY62 against Leishmania spp. is probably similar to that of amphotericin B, i.e., interaction with membrane sterols resulting in disruption in the cell membrane with leakage of intracellular components. KY62 has the advantage of having lower toxicity in mice than amphotericin B. The higher allowed doses may result in enhanced efficacy, but formal studies to address this issue are needed.
We studied the efficacy of KY62 in an experimental model of cutaneous leishmaniasis caused by a highly virulent strain of L. amazonensis. Mice were infected at both relatively permissive (ear pinna) and nonpermissive (tail) sites (12, 13), and the response to therapy was assessed by measurement of lesion size and parasite burden in the draining lymph nodes. KY62, when used at higher doses, was consistently superior to standard, tolerated doses of amphotericin B in reducing the size of cutaneous lesions. However, the determination of the advantage of KY62 over amphotericin B will have to await more formal toxicity testing. The lower parasite burdens present in the draining lymph nodes in KY62-treated mice confirmed the results of the clinical response. When a shorter duration of therapy was used, the more permissive ear pinna infection was less responsive to therapy. In humans, leishmanial infections involving the ear are more chronic and progressive and are more difficult to treat than those involving other cutaneous sites (22). This may be due to the lower vascularity of this cartilaginous structure. A longer duration of therapy (28 days) did not eradicate the infection, but it should be noted that a very large parasite inoculum was used in these studies. In a study of treatment of experimental L. major infections, it was observed that amphotericin B-treated mice had a noticeably smaller lesion size as early as the end of the 2-week drug treatment period (3). In our study of L. amazonensis infections, the difference in lesion size between the KY62-treated and untreated mice was not observed until 10 to 20 days after the treatment period. Although we cannot exclude a delayed antileishmanial immune response as a contributor to this apparent delay in response to treatment, it appears that the difference in lesion size was not noticeable earlier because the lesions in the untreated mice had not developed to a sufficient size to be significantly different than the lesions in the treated mice. Thus, the lower rate of lesion development in our model than in the L. major model probably accounts for some of the observed differences in response to drug treatment. Differences in parasite strain, site of inoculation, dose and route of delivery of the drugs, and mouse strain are also likely to contribute to the slightly different results.
Treatment with KY62 in the ear was associated with a predominantly lymphocytic infiltration with few amastigotes. In contrast, the untreated animals had heavily parasitized, foamy macrophages and a sparse lymphocytic infiltration. The importance of an intact cellular immune response in concert with antileishmanial therapy has previously been demonstrated (19). Whether the histopathological changes seen in the KY62-treated lesions are a direct effect of the drug or are due merely to the destruction of parasites with release of proinflammatory mediators remains to be determined.
In the model of visceral leishmaniasis caused by L. donovani, the efficacy of KY62 was equally impressive, with reduction in parasite burdens of approximately 4 logs in the liver and spleen compared to untreated controls. These findings are significant because visceral leishmaniasis is difficult to treat and is associated with high mortality. Amphotericin B was superior to antimony and pentamidine in treating Indian kala-azar (9, 10). It was also significantly better than pentavalent antimony in HIV-infected patients with visceral leishmaniasis in southern France (20). Lipid formulations of amphotericin B were also shown to be effective in the treatment of visceral leishmaniasis in several different regions where it is endemic (4, 5, 21). Our results indicate that KY62 has efficacy equal to, if not greater than, that of amphotericin B. Since KY62 can be given to mice at a much higher dose than amphotericin B, it may offer an improvement in the current therapy of leishmaniasis.
ACKNOWLEDGMENTS
This work was supported in part by funding from the Veteran Administration to P.C.M. and from DHHS (NIH AI28220) to S.L.R.
We are grateful to A. Fothergill (Fungus Testing Laboratory, San Antonio, Tex.) for her help in preparing drugs for in vitro testing and to Weigou Zhao for his help with preparation of the culture media.
REFERENCES
- 1.Alvar J, Cañavate C, Gutiérrez-Solar B, Jiménez M, Laguna F, López-Vélez R, Molina R, Moreno J. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin Microbiol Rev. 1997;10:298–319. doi: 10.1128/cmr.10.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berman J D. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis. 1997;24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 3.Bjorvatn B, Neva F A. Experimental therapy of mice infected with Leishmania tropica. Am J Trop Med Hyg. 1979;28:480–485. doi: 10.4269/ajtmh.1979.28.480. [DOI] [PubMed] [Google Scholar]
- 4.Castagnola E, Davidson R N, Fiore P, Tasso L, Rossi G, Mangraviti S, di Martino L, Scotti S, Cascio A, Pempinello R, Gradoni L, Giacchino R. Early efficacy of liposomal amphotericin B in the treatment of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1996;90:317–318. doi: 10.1016/s0035-9203(96)90270-9. [DOI] [PubMed] [Google Scholar]
- 5.Davidson R N, di Martino L, Gradoni L, Giacchino R, Gaeta G B, Pempinello R, Scotti S, Cascio A, Castagnola E, Maisto A, Gramiccia M, di Caprio D, Wilkinson R J, Bryceson A D. Short-course treatment of visceral leishmaniasis with liposomal amphotericin B (AmBisome) Clin Infect Dis. 1996;22:938–943. doi: 10.1093/clinids/22.6.938. [DOI] [PubMed] [Google Scholar]
- 6.Gasser R A, Jr, Magill A J, Oster C N, Franke E D, Grogl M, Berman J D. Pancreatitis induced by pentavalent antimonial agents during treatment of leishmaniasis. Clin Infect Dis. 1994;18:83–90. doi: 10.1093/clinids/18.1.83. [DOI] [PubMed] [Google Scholar]
- 7.Graybill J R, Najvar L K, Fothergill A, Hardin T, Rinaldi M, Lambros C, Regen S L. KY-62, a polyene analog of amphotericin B, for treatment of murine candidiasis. Antomicrob Agents Chemother. 1998;42:147–150. doi: 10.1128/aac.42.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melby P C, Yang Y-Z, Cheng J, Zhao W. Regional differences in the cellular immune response to experimental cutaneous or visceral infection with Leishmania donovani. Infect Immun. 1998;66:18–27. doi: 10.1128/iai.66.1.18-27.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishra M, Biswas U K, Jha A M, Khan A B. Amphotericin versus sodium stibogluconate in first-line treatment of Indian kala-azar. Lancet. 1994;344:1599–1600. doi: 10.1016/s0140-6736(94)90406-5. [DOI] [PubMed] [Google Scholar]
- 10.Mishra M, Biswas U K, Jha A M, Khan A B. Amphotericin versus pentamidine in antimony-unresponsive kala-azar. Lancet. 1992;340:1256–1257. doi: 10.1016/0140-6736(92)92952-c. [DOI] [PubMed] [Google Scholar]
- 11.Momeni A Z, Jalayer T, Emamjomeh M, Bashardost N, Ghassemi R L, Meghdadi M, Javadi A, Aminjavaheri M. Treatment of cutaneous leishmaniasis with itraconazole. Randomized double-blind study. Arch Dermatol. 1996;132:784–786. [PubMed] [Google Scholar]
- 12.Mortatti R C, Henriques A. Experimental cutaneous leishmaniasis by Leishmania amazonensis: course of fast-growth infection in the mouse ear. Parasitol Res. 1990;76:729–730. doi: 10.1007/BF00931097. [DOI] [PubMed] [Google Scholar]
- 13.Neal R A. Chemotherapy of cutaneous leishmaniasis: Leishmania tropica infection in mice. Ann Trop Med Parasitol. 1964;58:420–430. doi: 10.1080/00034983.1964.11686264. [DOI] [PubMed] [Google Scholar]
- 14.Pearson R D, Manian A A, Harcus J L, Hall D, Hewlett E L. Lethal effect of phenothiazine neuroleptics on the pathogenic protozoan Leishmania donovani. Science. 1982;217:369–371. doi: 10.1126/science.6124040. [DOI] [PubMed] [Google Scholar]
- 15.Pearson R D, Sousa A Q. Clinical spectrum of leishmaniasis. Clin Infect Dis. 1996;22:1–13. doi: 10.1093/clinids/22.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Pirson P, Leclef B, Trouet A. Activity of ketoconazole derivatives against Leishmania mexicana amazonensis within mouse peritoneal macrophages. Ann Trop Med Parasitol. 1990;84:133–139. doi: 10.1080/00034983.1990.11812446. [DOI] [PubMed] [Google Scholar]
- 17.Ramos H, Valdivieso E, Gamargo M, Dagger F, Cohen B E. Amphotericin B kills unicellular Leishmania by forming aqueous pores permeable to small cations and anions. J Membr Biol. 1996;152:65–75. doi: 10.1007/s002329900086. [DOI] [PubMed] [Google Scholar]
- 18.Rashid J R, Wasunna K M, Gachihi G S, Nyakundi P M, Mbugua J, Kirigi G. The efficacy and safety of ketoconazole in visceral leishmaniasis. East Afr Med J. 1994;71:392–395. [PubMed] [Google Scholar]
- 19.Reiner S L, Locksley R M. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–77. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 20.Rosenthal E, Marty P, Poizot-Martin I, Reynes J, Pratlong F, Lafeuillade A, Jaubert D, Boulat O, Dereure J, Gambarelli F. Visceral leishmaniasis and HIV-1 co-infection in southern France. Trans R Soc Trop Med Hyg. 1995;89:159–162. doi: 10.1016/0035-9203(95)90476-x. [DOI] [PubMed] [Google Scholar]
- 21.Seaman J, Boer C, Wilkinson R, de Jong J, de Wilde E, Sondorp E, Davidson R. Liposomal amphotericin B (AmBisome) in the treatment of complicated kala-azar under field conditions. Clin Infect Dis. 1995;21:188–193. doi: 10.1093/clinids/21.1.188. [DOI] [PubMed] [Google Scholar]
- 22.Walton B C. American cutaneous and mucocutaneous leishmaniasis. In: Peters W, Killick-Kendrick R, editors. The leishmaniases in biology and medicine. II. London, United Kingdom: Academic Press; 1987. p. 644. [Google Scholar]
- 23.Yarnasita K, Janout V, Bernard E M, Armstrong D, Regen S L. Micelle/monomer control over the membrane-disrupting properties of an amphiphilic antibiotic. J Am Chem Soc. 1995;117:6249–6253. [Google Scholar]