Abstract
Background and purpose
Diffusion MRI (dMRI) is sensitive to microstructural changes in white matter of people with relapse-remitting multiple sclerosis (pw-RRMS) that lead to progressive disability. The role of diffusion in assessing the efficacy of different therapies requires more investigation. This study aimed to evaluate selected dMRI metrics in normal-appearing white matter and white matter-lesion in pw-RRMS and healthy controls longitudinally and compare the effect of therapies given.
Material and methods
Structural and dMRI scans were acquired from 78 pw-RRMS (29 injectables, 36 fingolimod, 13 dimethyl fumarate) and 43 HCs at baseline and 2-years follow-up. Changes in dMRI metrics and correlation with clinical parameters were evaluated.
Results
Differences were observed in most clinical parameters between pw-RRMS and HCs at both timepoints (p ≤ 0.01). No significant differences in average changes over time were observed for any dMRI metric between treatment groups in either tissue type. Diffusion metrics in NAWM and WML correlated negatively with most cognitive domains, while FA correlated positively at baseline but only for NAWM at follow-up (p ≤ 0.05). FA correlated negatively with disability in NAWM and WML over time, while MD and RD correlated positively only in NAWM.
Conclusions
This is the first DTI study comparing the effect of different treatments on dMRI parameters over time in a stable cohort of pw-RRMS. The results suggest that brain microstructural changes in a stable MS cohort are similar to HCs independent of the therapies used.
Keywords: Multiple Sclerosis (MS), Diffusion tensor imaging (DTI), White Matter (WM), Clinical parameters, disease-modifying therapies (DMTs)
Introduction
Multiple sclerosis (MS) is a disease that affects the central nervous system, including the brain and spinal cord. The disease can be mild but often progresses to significant disability after a decade or two. The most common symptoms are fatigue, cognitive impairment and altered mental state. 1
Advanced quantitative MRI methods such as diffusion tensor imaging (DTI) have tremendous potential to monitor disease activity and treatment response due to their quantitative assessment of disease progression and visual representation of disease impact. 2
DTI is a specific extension of diffusion weighted imaging, allowing reconstruction of white matter 3 yielding several metrics; fractional anisotropy (FA), axial, radial and mean diffusivities (AD, RD and MD). 4 Studies focussing on lesions in people with MS have shown increased MD compared to normal white matter of healthy controls (HCs), suggesting that demyelinating inflammatory changes in MS can be detected through altered water diffusivity. 5
With the potential to detect microscopic features of demyelination, DTI enables us to evaluate disease progression. This can be achieved by calculating the change in values of different DTI parameters of lesions and normal-appearing white matter (NAWM). 6 It is suggested that the pathological damage reported in DTI in NAWM contributes significantly to disability and progression in MS. 7 DTI can be an ideal marker to track demyelination before atrophy, allowing personalized treatment decisions. However, evidence of the role of DTI in monitoring disease progression is limited. 8
Different disease-modifying therapies (DMTs) have different cellular and molecular therapeutic targets in MS. Hence, the efficacy of each DMT can be expected to vary based on the type and extent of interaction with the immune system. A recent review suggests that early initiation of high-efficacy DMTs showed better control of disease activity in some patients compared with delayed therapy. 9
In this study, patients on injectables, fingolimod or dimethyl fumarate (DMF) were recruited as a part of an ongoing longitudinal study. Here, we selected DTI metrics to evaluate NAWM and white matter-lesion (WML) changes over time in a large cohort on three different treatments and compared the metrics to age and sex-matched HCs. To compare disease progression, we assessed changes in cognition, disability, fatigue and mental health indices over time in all participants.
Materials and methods
Participants
78 people with relapse-remitting multiple sclerosis (pw-RRMS) (age mean and range, 43.4 ± 11.1; 23–54 years) (29 injectables, 36 fingolimod and 13 DMF) were age and sex-matched to 43 HCs (mean age, 40.8 ± 11.5 years). This was an open-label study and more fingolimod patients were recruited than patients on injectables or DMF and not all recruited patients completed the follow-up period. The inclusion criteria for patients were 1) fulfilling RRMS diagnosis according to McDonalds’ criteria, and 2) Expanded Disability Status Scale 10 (EDSS) score between 1 and 4. We excluded patients with recent relapses and comorbidities, which would affect any clinical assessments. The selection of HCs was based on 1) no prior/current psychiatric or neurological disorders and 2) not currently on any medications. All participants completed an MRI safety checklist and all the study components (including imaging and neuropsychological evaluations). Approval to conduct this study was obtained from the human research ethics committee of the local health care district (HREC no: 14/09/10/3.01 and 14/09/10/3.02).
Clinical assessments
The clinical assessment consisted of a comprehensive neurological examination for disability status by their treating neurologist using EDSS. 11 MS severity score (MSSS) 12 was calculated based on age and disease duration. The neuropsychological test for cognitive impairment was performed using audio recorded cognitive screen (ARCS) 13 which measures executive functioning/attention, memory, language, verbal fluency and visuospatial functioning. Symbol digit modalities test (SDMT) 14 was utilized for measuring information processing, speed and attention. Fatigue was assessed with the modified fatigue impact scale (MFIS). 15 All clinical assessments were carried out on the same day as the imaging session.
Imaging (structural and DTI)
The MRI data were obtained with a 3T Magnetom PRISMA system (Siemens Healthineers, Erlangen, Germany) equipped with a 64-channel receiver head coil.
The imaging protocol included three dimensional (3D) isotropic T1 weighted magnetization-prepared rapid gradient-echo (T1-MPRAGE), 3D T2 weighted fluid-attenuated spin-echo sequence (T2-FLAIR) and diffusion weighted sequence.
The 3D T1-MPRAGE sequence was used for anatomical reference with the following parameters: repetition time (TR): 2000 ms; echo time (TE): 3.5 ms, inversion time (TI): 1100 ms and flip angle of 7°. Field of view (FOV) was 256 mm 2 with a voxel size of 1 mm 3 and acquisition time of 5 min and 38 s.
For assessment of lesion load, a 3D T2-FLAIR weighted sequence was used with TR/TE/TI = 5000/386/1800 ms, and flip angle of 120°. FOV was 256 mm 2 with a voxel size of 1 mm 3 and acquisition time of 6 min and 12 s.
Axial diffusion weighted MRI was obtained using a fat-suppressed single-shot echo-planar imaging (EPI) sequence. The MRI signal was sensitized to diffusion by application of a pair of bipolar trapezoidal gradient pulses with a duration of effective δ = 17.9 ms and effective diffusion time ∆ = 31.9 ms in 64 equally and spherically distributed directions; TR/TE: 9400/69 ms; number of slices:70; slice thickness: 2 mm; no gap; FOV: 240 mm 2 with a voxel size of 2 mm; 3 and b-value of 3000 s/mm.2 In addition, three non-diffusion weighted volumes (b-value = 0 s/mm2 and AP/PA directions) were acquired for distortion correction. The total acquisition time was 10 min and 18 s.
Data analysis, pre-processing and segmentation
Structural volumetric and diffusion data were exported from Siemens syngo in DICOM format. Brain and skull images were extracted from the whole brain 3D T1-MPRAGE data using SIENAX. 16 Total brain volume and partial volumes, including white matter (WM), grey matter (GM) and ventricular CSF volumes were calculated using FSL FAST.
SPM Lesion Segmentation Tool 17 was used to generate an initial binary lesion map from hyperintense T2-FLAIR image pixels resulting in a lesion probability map. This was followed by T1-MPRAGE lesion filling, using the binary lesion mask for each participant. 3D WM mask was generated using FSL FAST and overlaid on T2-FLAIR using Matlab. Consequently, fuzzy C-means clustering was applied to detect WM lesions using Matlab Software.
WM was segmented from gigamolar and CSF using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) segmentation as implemented in Matlab. A NAWM mask was created for each subject by inverting the predicted lesion maps and masking resultant WM segmentations from SPM12 in subject T1 space.
DTI pre-processing was performed by an in-house pipeline. A number of steps were performed to correct diffusion data for artefacts associated with the EPI sequence to enhance the image quality for visual, quantitative and statistical interpretation. The pipeline consisted of denoising 16 Gibbs ringing artefact removal 17 (MRtrix 3.0_RC2-61-g068b1398), followed by susceptibility-induced and eddy-current artefact correction using FSL topup and eddy (https://fsl.fmrib.ox.ac.uk/fsl/), and subsequently bias field correction 18 (ANTs 2.1.0). Finally, the corrected diffusion data were affine registered (12 degrees of freedom) to the anatomical T1 data to make use of WM and lesion segmentation. 19 After pre-processing, the DTI tensors were estimated using an iteratively reweighted linear least square fitting method implemented in MRtrix3 to identify and reject outliers. Then, using the DTI tensor values, FA, MD, RD and AD were calculated. We reported average FA, MD, RD and AD in both NAWM and WML for each subject.
Statistical analysis
The statistical analysis was performed using SPSS software. To investigate the significant differences between pw-RRMS and HCs groups, t-tests were applied (p ≤ 0.05) for independent and paired sample analyses. The level of significant change over time in DTI metrics associated with treatment efficacy was assessed using two-way repeated-measures ANOVA, adjusted for appropriate covariates, followed by post-hoc testing using Least Significant Difference (LSD). Finally, Pearson’s coefficient in R software (RStudio 2021.09.1 + 372 for Windows) was used to measure the correlations between clinical symptoms and DTI metrics.
Results
Demographics and clinical characteristics
Age and sex showed no statistical difference between HCs and pw-RRMS groups (p > 0.05). However, DMF cohort was significantly younger than other treatment groups (p ≤ 0.05). Although we did not find a significant difference at BL in the scores for total ARCS (tARCS), memory, visuospatial, language and attention (p > 0.05) between pw-RRMS and HC, pw-RRMS showed poorer performance in fluency and SDMT (p ≤ 0.05), as well as higher levels of anxiety, depression, stress and fatigue scores (p ≤ 0.01) than HCs, but no difference was found between treatment groups. The gap between HCs and pw-RRMS widened at the follow-up timepoint, showing significant differences in most scores apart from visuospatial and language. Detailed demographic characteristics and clinical details of both groups and pw-RRMS groups individually at two timepoints are shown in Table 1.
Table 1.
Longitudinal analysis of mean demographic scores and disease-related variables for pw-RRMS as combined and separated DMTs subgroups (injectables, fingolimod and DMF) and HCs at BL and 2-YFU.
| Characteristics | HCs | Combined pw-RRMS | INJ | FING | DMF | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample size | 43 | 78 | 29 | 36 | 13 | |||||
| Sex (% female) | 72.1% | 76.9% | 86.2% | 69.4% | 76.9% | |||||
| Age at baseline (years) | 40.8 ± 11 | 43.4 ± 11 | 45.1 ± 2 | 44.7 ± 2 | 35.9 ± 2 | |||||
| Timepoint | BL | 2-YFU | ||||||||
| Sample type | HCs | Combined pw-RRMS | INJ | FING | DMF | HCs | Combined pw-RRMS | INJ | FING | DMF |
| Disease duration (years) | - | 7 ± 1 | 7 ± 1 | 9 ± 1 | 5 ± 2 | - | 9 ± 1 | 9 ± 1 | 11 ± 1 | 7 ± 1 |
| EDSS | - | 1.9 ± 0.1 | 1.7 ± 0.2 | 2.1 ± 0.2 | 2.1 ± 0.2 | - | 2.1 ± 0.1 | 2.1 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 |
| MSSS | - | 3 ± 0.2 | 3 ± 0.3 | 3 ± 0.4 | 4 ± 0.3 | - | 3 ± 0.2 | 3 ± 0.2 | 3 ± 0.3 | 3 ± 0.6 |
| tARCS | 94 ±2 | 88 ± 2 | 91 ±3 | 88 ± 3 | 82 ± 4 | 97 ± 2 | 87 ± 2*** | 88 ± 3 | 87 ± 3* | 83 ± 5* |
| Memory | 96 ± 2 | 92 ± 2 | 93 ± 3 | 91 ± 4 | 87 ± 5 | 98 ± 2 | 88 ± 9** | 91 ± 4 | 88 ± 4 | 83 ± 8 |
| Fluency | 95 ± 3 | 84 ± 2** | 85 ± 2 | 85 ± 3 | 80 ± 4* | 95 ± 2 | 84 ± 2*** | 85 ± 2 | 83 ± 3* | 84 ± 6 |
| Visuospatial | 101 ± 1 | 102 ± 1 | 101 ± 1 | 102 ± 1 | 102 ± 1 | 101 ± 1 | 101 ± 1 | 99 ± 3 | 102 ± 1 | 101 ± 1 |
| Language | 91 ± 4 | 89 ± 2 | 93 ± 4 | 87 ± 4 | 80 ± 3 | 93 ± 4 | 90 ± 2 | 92 ± 4 | 91 ± 3 | 80 ± 4 |
| Attention | 98 ± 2 | 96 ± 2 | 97 ± 3 | 95 ± 2 | 92 ± 4 | 103 ± 2 | 93 ± 1*** | 92 ± 2* | 92 ± 3* | 97 ± 5 |
| SDMT | 59 ± 2 | 51 ± 1*** | 50 ± 2** | 49 ± 2*** | 54 ± 3 | 61 ± 2 | 50 ± 1*** | 49 ± 2*** | 48 ± 2*** | 53 ± 3 |
| DASS-21 | 12 ± 2 | 23 ± 2** | 17 ± 4 | 23 ± 4* | 30 ± 6* | 10 ± 1 | 22 ± 2*** | 19 ± 4 | 23 ± 3** | 23 ± 5 |
| Stress | 7 ± 1 | 11 ± 1** | 8 ± 2 | 11 ± 2 | 16 ± 3** | 5 ± 1 | 11 ± 1*** | 8 ± 1 | 11 ± 2** | 14 ± 3** |
| Anxiety | 3 ± 1 | 6 ± 1** | 4 ± 1 | 6 ± 1* | 7 ± 2 | 2 ± 0.4 | 5.1 ± 1*** | 4 ± 1 | 5 ± 1** | 5 ± 1 |
| Depression | 3 ± 1 | 6 ± 1** | 4 ± 1 | 7 ± 1* | 7 ± 2 | 3 ± 1 | 7 ± 1*** | 6 ± 2 | 6 ± 1 | 6 ± 2 |
| MFIS | 14 ± 2 | 33 ± 2*** | 25 ± 3* | 36 ± 3*** | 37 ± 5*** | 11 ± 2 | 31 ± 2*** | 27 ± 3*** | 33 ± 3*** | 29 ± 4** |
| Physical fatigue | 6 ± 1 | 17 ± 1*** | 12 ± 2** | 19 ± 2*** | 18 ± 3*** | 5 ± 1 | 15 ± 1*** | 13 ± 2*** | 16 ± 2*** | 13 ± 2* |
| Cognitive fatigue | 8 ± 1 | 16 ± 1*** | 12 ± 2 | 17 ± 2*** | 19 ± 3*** | 6 ± 1 | 16 ± 1*** | 13 ± 2** | 16 ± 2*** | 16 ± 2** |
Values are expressed in mean ± standard error of measurement. HCs: healthy controls; pw-RRMS: people with relapse-remitting MS; INJ: injectables; FING: fingolimod; DMF: dimethyl fumarate; BL: baseline timepoint; 2-YFU: 2-years follow-up timepoint; EDSS: expanded disability status scale; MSSS: multiple sclerosis severity score; tARCS: total audio recorded cognitive screen; SDMT: symbol digit modalities test; DASS: depression, anxiety, stress scale; MFIS: modified fatigue impact scale. *p-value ≤ 0.05, **p-value ≤ 0.01, ***p-value ≤ 0.001 refer to HCs versus combined, injectables, fingolimod or DMF at BL or 2-FYU.
DTI-NAWM differences over time between pw-RRMS and HCs
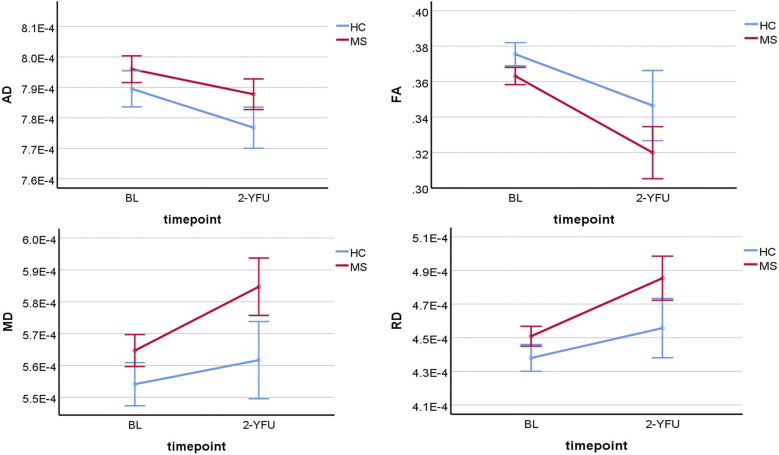
There was a statistically significant decline in average AD and FA of NAWM over time for HCs and pw-RRMS (p ≤ 0.001). However, there was no statistically significant difference in the average change in AD or FA of NAWM over time between groups (p = 0.19 and 0.24), as shown in Figure 1.
Figure 1.
Line graphs showing the four DTI-NAWM parameters change differences over time between pw-RRMS and HCs. BL: baseline; 2-YFU: 2-years follow-up; FA: fractional anisotropy; RD: radial diffusivity; AD: axial diffusivity; MD: mean diffusivity; NAWM: normal-appearing white matter; FA is unitless, while AD, MD and RD are in mm2/s.
There was a statistically significant increase in average MD and RD of NAWM over time for HCs and pw-RRMS (p ≤ 0.001). However, there was no statistically significant difference in the average change in MD or RD of NAWM over time between groups (p = 0.09 and 0.12) (Figure 1).
DTI-NAWM differences in pw-RRMS over time between injectables, fingolimod and DMF
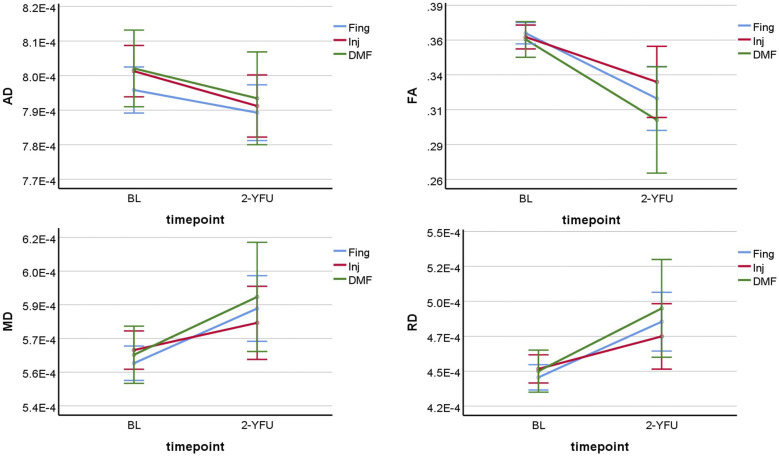
There was a statistically significant decline in average AD and FA of NAWM over time in all three groups (p ≤ 0.001). However, there was no statistically significant difference in the average change in AD or FA of NAWM over time between the treatment groups of pw-RRMS (p = 0.74 and 0.44), as shown in Figure 2.
Figure 2.
Line graphs showing the four DTI-NAWM parameters change differences in pw-RRMS over time between injectables, fingolimod and DMF. BL: baseline; 2-YFU: 2-years follow-up; FA: fractional anisotropy; RD: radial diffusivity; AD: axial diffusivity; MD: mean diffusivity; NAWM: normal-appearing white matter. FA is unitless, while AD, MD and RD are in mm2/s.
There was a statistically significant increase in average MD and RD of NAWM over time in all three groups (p ≤ 0.001). However, there was no statistically significant difference in the average change in MD or RD of NAWM over time between the treatment groups of pw-RRMS (p = 0.41 and 0.42) (Figure 2).
DTI-WML differences in pw-RRMS over time between injectables, fingolimod and DMF
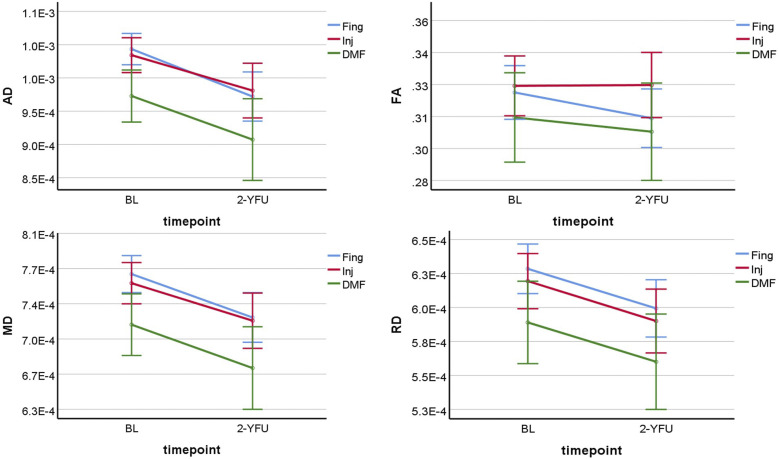
There was a statistically significant decline in average AD and FA of WML over time in all three treatment groups (p ≤ 0.001). However, there was no statistically significant difference in the average change in AD or FA of WML over time between the treatment groups of pw-RRMS (p = 0.78 and 0.46). AD and FA were significantly lower in the DMF group at BL and 2-YFU than in fingolimod and injectables (p ≤ 0.05), as seen in Figure 3.
Figure 3.
Line graphs showing the four DTI-WML parameters change differences in pw-RRMS over time between injectables, fingolimod and DMF. BL: baseline; 2-YFU: 2-years follow-up; FA: fractional anisotropy; RD: radial diffusivity; AD: axial diffusivity; MD: mean diffusivity; WML: white matter-lesion. FA is unitless, while AD, MD and RD are in mm2/s.
There was a statistically significant decline in average MD and RD in WML over time in all three groups (p ≤ 0.001). However, there was no statistically significant difference in the average change in MD or RD of WML over time between the treatment groups of pw-RRMS (p = 0.94 and 0.99). The DMF group was significantly lower in MD and RD at BL and 2-YFU than fingolimod and injectables (p ≤ 0.05) (Figure 3).
Correlation of DTI and clinical measures in BL and 2-YFU using Pearson’s correlation
Clinical correlations at BL
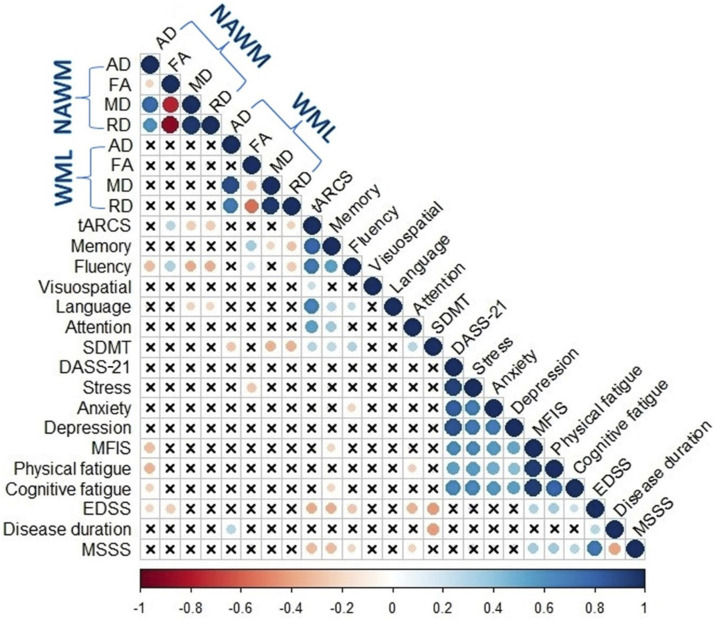
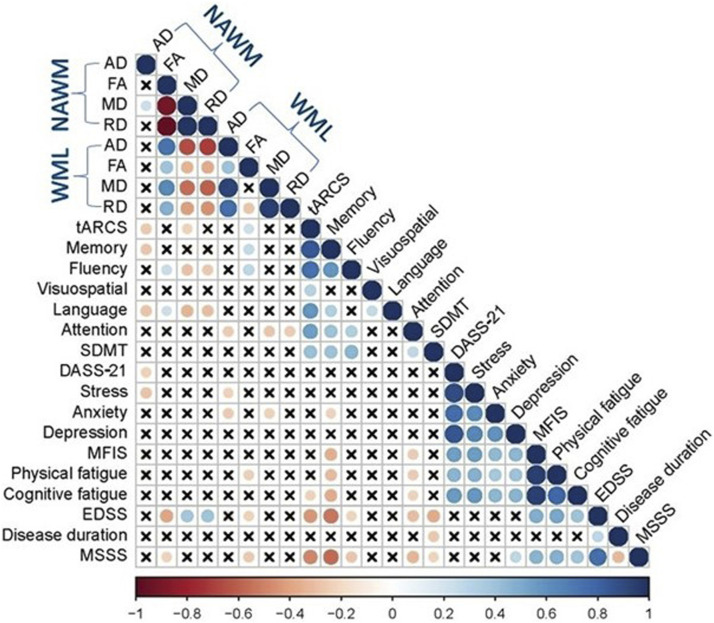
At BL: FANAWM was positively correlated with tARCS and fluency (r = 0.28 and 0.32, p ≤ 0.01), while negative correlations were noted for MDNAWM and RDNAWM with tARCS, fluency and language (p ≤ 0.01). ADNAWM was negatively correlated with MFIS, physical and cognition fatigues and disability scores (r ≥ −0.35, p ≤ 0.01). FAWML correlated positively with memory (r = 0.35, p ≤ 0.01). RDWML also negatively correlated with tARCS, memory, fluency and SDMT (r ≥ −0.36, p ≤ 0.01), while MDWML correlated with memory and SDMT (r ≥ −0.35, p ≤ 0.01). The strongest correlation value was found between MDNAWM and fluency (r = −0.38, p ≤ 0.01), while the lowest correlation value was noted between FAWML and fluency (r = 0.23, p ≤ 0.05) and between MDWML and memory (r = −0.23, p ≤ 0.05) as shown in Figure 4.
Figure 4.
Heat map showing Pearson’s correlation coefficients between DTI metrics of NAWM and WML with clinical parameters in pw-RRMS at BL. Self-self correlations are identified in dark blue circles. Significant correlations (p ≤ 0.05) are coloured either in blue (positive) or red (negative) hues, while correlations that were not significant (p > 0.05) are shown in (x). pw-RRMS: people with relapse-remitting multiple sclerosis; DTI: diffusion tensor imaging; FA: fractional anisotropy; MD: mean diffusivity; RD: radial diffusivity; AD: axial diffusivity; NAWM: normal-appearing white matter; WML: white matter-lesion; tARCS: total audio recorded cognitive screen; SDMT: symbol digit modalities test; MFIS: modified fatigue impact scale EDSS: expanded disability status scale.
Clinical correlations at 2-YFU
At 2-YFU: FANAWM negatively correlated with EDSS (r = −0.39, p ≤ 0.01). ADNAWM, MDNAWM and RDNAWM were negatively correlated with most cognition scores, including tARCS, memory, fluency and language (r≥−0.35, p ≤ 0.01). ADNAWM was negatively correlated with mood scores (r = -0.35, p ≤ 0.01). MDNAWM and RDNAWM were also correlated positively with EDSS (r ≥ 0.37, p ≤ 0.01). The strongest correlation value was found between FANAWM and EDSS (r = −0.39, p ≤ 0.01), while the lowest correlation value was noted between FANAWM and language (r = 0.22, p ≤ 0.05), as can be seen in Figure 5.
Figure 5.
Heat map showing Pearson’s correlation coefficients between DTI metrics of NAWM and WML with clinical parameters in pw-RRMS at 2-YFU. Self-self correlations are identified in dark blue circles. Significant correlations (p ≤ 0.05) are coloured either in blue (positive) or red (negative) hues, while correlations that were not significant (p > 0.05) are shown in (x). pw-RRMS: people with relapse-remitting multiple sclerosis; DTI: diffusion tensor imaging; FA: fractional anisotropy; MD: mean diffusivity; RD: radial diffusivity; AD: axial diffusivity; NAWM: normal-appearing white matter; WML: white matter-lesion; tARCS: total audio recorded cognitive screen; DASS-21: depression, anxiety and stress Scale; EDSS: expanded disability status scale; MSSS: MS severity score.
Discussion
This is the first ever MS study that compares the effects of different treatments on clinical and DTI parameters over time in a stable cohort of pw-RRMS. We used DTI to measure the change of axonal and myelin integrity in NAWM and WML. We found strong evidence that changes in white matter translate to changes in clinical parameters, linking degeneration in myelin and axonal integrity directly with MS burden.
The major findings of this study highlight a marked reduction of FA and significantly increased diffusivities in NAWM and WML of people with RRMS compared to HCs, even after controlling for age and other covariates. This is in concordance with our previous findings in a subset of this group 20 and other studies 21 However, while this finding persists over time, DTI parameters do not change significantly faster in pw-RRMS than HCs when stable on DMTs. This indicates that decreases in FA and AD over time (and increases in RD and MD) for both groups likely reflect ageing processes 22 or variance inherent in test-retest studies of DTI. 23 This indicates that the widening gap of clinical parameters over 2 years in a stable cohort of treated patients is likely not due to deteriorating white matter but the result of ageing likely to reduce compensatory strategies.
The finding that pw-RRMS do not significantly progress in degeneration in NAWM confirms a previous study from Klistorner and others, although they identified an increase in their WMLs count followed over 3–4 years; 6 however, the treatment status of those patients was not clear, and they did not have a control group. The relative stability of DTI metrics in our study suggests a protective role of treatment on microstructure. Beta-IFN, fingolimod and DMF are indicated in the treatment of pw-RRMS and have demonstrated not only clinical benefit over placebo24,25 but also have shown beneficial effects on MRI markers of disease activity, especially fingolimod. 26 The mechanism of action of the drugs examined in this study implicates anti-inflammatory properties24,25 suggesting the suppression of inflammation forms a protection against axonal damage and results in clinical improvements. There is no drug at present approved with remyelinating or axonal protective properties.
The results from previous longitudinal studies are mixed and contradictory, possibly due to the duration of the study and the variability in their analyses of DTI data. While Harrison and others 27 reported similar results with decreased FA and RD in the corpus callosum, other groups found no changes in the global DTI assessment during their follow-up measurements. 28 It is plausible that the results we observed in our cohort could be due to their particular disease stability, treatment efficacy or that there was not marked enough change after 2 years. Our study had the advantage of having a healthy cohort to compare change over time. This allowed us to identify that decline in WM integrity may not be disease specific.
Our analysis revealed statistically significant changes in all DTI metrics over time in NAWM and WML when all groups were combined. However, when comparing treatment groups, we could not find any significant change in any of the DTI values. This was surprising as injectable treatments are generally considered less effective than oral treatments. Patients were stable before entering the study and remained stable during the follow-up. This indicates the overall efficacy of the different DMTs does not change the progression in the absence of relapses. A study of glatiramer acetate in pw-RRMS showed similar findings that DMTs are neuroprotective in the absence of relapses. 29 Patients experiencing relapses changed treatment and were excluded from the analysis. Further work by our group would investigate the role of DMTs on tract changes over longer periods of time, such as five or more years.
DMF showing lower average DTI metrics compared with injectables and fingolimod may be because the cohort was younger and had a shorter disease duration and shorter time since the last relapse. The positive effect of DMF on DTI metrics was also seen in another study 30 showing no change in DTI measures following 2-YFU DMF therapy, suggesting a neuroprotective role of DMF. Previous studies on the effect of DTI post fingolimod therapy exhibit varying results. Studies were conducted with a follow-up of fewer than 12 months post-starting fingolimod therapy and found improvements in all DTI values.31,32 As this was not compared to other therapies, it could have just represented the settling of disease activity. Our results corroborate the findings of a recent study by Bhattacharyya and others 33 in which the authors concluded DTI measures seem to stabilize after 1 year of fingolimod treatment.
Moderate clinical correlations of FA/MD/RDNAWM and FAWML with EDSS indicate that myelin-related degeneration processes and reduction in the specificity of WM, especially within lesion sites, lead to a higher disease burden. Others have found similar results in pw-RRMS 34 while the many significant correlations of DTI metrics with clinical parameters suggest redundancy in DTI metrics and measures of disease burden in MS. Conversely, Lipp and others 35 found that while DTI metrics are partially correlated with each other, each is sensitive to different dimensions of pathology. EDSS remained relatively stable over time, and while other studies have found that disability, absence of MRI activity and relapses are not indicative of disease worsening over time and 36 treatment may offer protection. Notably, we found no treatment-dependent changes in DTI metrics comparing injectables, fingolimod and DMF, indicating that treatment choice does not significantly affect tract changes over time in stable disease. Likewise, a strong correlation between components of the tARCS (fluency, memory and language) at 2-YFU with DTI metrics in NAWM suggests better cognitive performance is associated with higher WM integrity and decreased diffusivity across axons. This finding agrees with other recent work that found strong association of FA and AD with executive function in progressive MS. 37
Limitations
Limitations of our current study are 1) Lack of specificity to tracts that have a strong relationship to disease burden (such as corticospinal and corticothalamic tracts). Further work in our group will use region-of-interest analyses or cluster-based approaches to answer whether these DMTs act with specificity, and whether clinical correlations are restricted to certain regions that were not distinguished by a NAWM or WML-based average. 2) Lesion distribution was not examined in our groups, though lesion volume was accounted for in the analysis. A parity of disease burden across lesion sites (i.e. the analysis is agnostic to lesion location) is assumed for simplicity in this study, even though lesion location is likely to influence clinical parameters and disease subtype. 38 3) Patients were not randomized to their treatment, and therefore, it is likely that milder forms of MS were treated with injectables. Another aspect of this is the disease subtype of interest, RRMS, is not prescriptive of the entire pathology, nor is it considered highly prognosticative of disease outcome. 39 As such, our results cannot be generalized to progressive forms of MS. 4) Though our sample of pw-RRMS was decently sized, the different treatment groups were small comparatively. This could have affected the utility of our statistical models when examining pw-RRMS subgroups.
Recent advances in MRI measures specific to WM microstructure can be utilized to distinguish disease-related processes in MS. Our work has shown that pw-RRMS have axonal changes that DTI can characterize, and these changes are associated with clinical parameters of disease burden, even in a stable, mildly disabled cohort. Future work should examine the role of individual tracts and their relationship to disease burden using cluster-based or region-of-interest approaches to further characterize the pathology of this pernicious disease.
Conclusion
Our findings support that DTI measures are sensitive in detecting microstructural alteration of white matter in MS, providing a valuable tool for evaluating treatment efficacy of DMTs. This study examining the effects of three treatments over time in reasonably sized cohorts showed correlations of DTI metrics with most clinical symptoms of MS over time, in particular cognition scores. More importantly, the lack of treatment-dependent effects on DTI metrics over time in the absence of relapses demonstrates the stabilizing effect of the examined DMTs. However, our findings suggest that the treatment choice does not significantly affect tract changes over time in stable disease. The increasing role of DTI as a valuable technique for evaluating treatment response of white matter disease warrants further investigation.
Acknowledgements
The authors acknowledge the facilities and scientific and technical assistance of the National Imaging Facility, a National Collaborative Research Infrastructure Strategy (NCRIS) capability, at the Hunter Medical Research Institute Imaging Centre, University of Newcastle. The authors acknowledge the patients and healthy controls who participated in this study, Imaging Centre (HMRI-IC) of the University of Newcastle and Hunter Medical Research Institute.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AA, OA, NK, IK, RL and SR have no competing interests. JLS: has accepted travel compensation from Novartis, Biogen and Merck Serono. Her institution receives the honoraria for talks and advisory board commitment and also clinic support from Bayer Health Care, Biogen Idec, CSL, Genzyme Sanofi, Merck Serono, Novartis and Teva.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by an independent grant provided by Novartis Pharmaceuticals Australia Pty Ltd. A. Alshehri was supported by a PhD scholarship with annual grant support from Imam Abdulrahman Bin Faisal University (IAU) in Saudi Arabia and the Saudi Arabian Cultural Mission (SACM) in Australia.
ORCID iDs
Abdulaziz Alshehri https://orcid.org/0000-0003-4893-223X
Saadallah Ramadan https://orcid.org/0000-0003-3874-7866
References
- 1.Kister I, Bacon TE, Chamot E, et al. Natural history of multiple sclerosis symptoms. Int Journal MS Care 2013; 15: 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ge Y. Multiple sclerosis: the role of MR imaging. Am J Neuroradiology 2006; 27: 1165–1176. [PMC free article] [PubMed] [Google Scholar]
- 3.Cauley KA, Filippi CG. Diffusion-tensor imaging of small nerve bundles: cranial nerves, peripheral nerves, distal spinal cord, and lumbar nerve roots--clinical applications. AJR Am J Roentgenol 2013; 201: W326–W335. DOI: 10.2214/AJR.12.9230. [DOI] [PubMed] [Google Scholar]
- 4.Curran KM, Emsell L, Leemans A. Diffusion Tensor Imaging. Springer, 2016, Berlin, Germany. [Google Scholar]
- 5.Elshafey R, Hassanien O, Khalil M. Diffusion tensor imaging for characterizing white matter changes in multiple sclerosis. Egypt J Radiol Nucl Med 2014; 45: 881–888, DOI: 10.1016/j.ejrnm.2014.04.006 10.1016/j.ejrnm.2014.04.006. [DOI] [Google Scholar]
- 6.Klistorner A, Wang C, Yiannikas C, et al. Evidence of progressive tissue loss in the core of chronic MS lesions: A longitudinal DTI study. NeuroImage: Clin 2018; 17: 1028–1035, DOI: 10.1016/j.nicl.2017.12.010 10.1016/j.nicl.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciccarelli O, Werring D, Wheeler–Kingshott C, et al. Investigation of MS normal-appearing brain using diffusion tensor MRI with clinical correlations. Neurology 2001; 56: 926–933. [DOI] [PubMed] [Google Scholar]
- 8.Ge Y, Law M, Grossman RI. Applications of diffusion tensor MR imaging in multiple sclerosis. Ann N Y Acad Sci 2005; 1064: 202–219. DOI: 10.1196/annals.1340.039. [DOI] [PubMed] [Google Scholar]
- 9.Merkel B, Butzkueven H, Traboulsee AL, et al. Timing of high-efficacy therapy in relapsing-remitting multiple sclerosis: a systematic review. Autoimmun Reviews 2017; 16: 658–665. [DOI] [PubMed] [Google Scholar]
- 10.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. DOI: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 11.Meyer-Moock S, Feng Y-S, Maeurer M, et al. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurology 2014; 14: 58–58. DOI: 10.1186/1471-2377-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roxburgh RHSR, Seaman SR, Masterman T, et al. Multiple Sclerosis Severity Score. Using Disability Disease Duration Rate Disease Severity 2005; 64: 1144–1151. DOI: 10.1212/01.Wnl.0000156155.19270.F8. [DOI] [PubMed] [Google Scholar]
- 13.Lechner-Scott J, Kerr T, Spencer B, et al. The Audio Recorded Cognitive Screen (ARCS) in patients with multiple sclerosis: a practical tool for multiple sclerosis clinics. Mult Scler 2010; 16: 1126–1133. DOI: 10.1177/1352458510374743. [DOI] [PubMed] [Google Scholar]
- 14.Benedict RH, DeLuca J, Phillips G, et al. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult Scler 2017; 23: 721–733. DOI: 10.1177/1352458517690821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larson RD. Psychometric properties of the modified fatigue impact scale. Int Journal MS Care 2013; 15: 15–20. DOI: 10.7224/1537-2073.2012-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veraart J, Novikov DS, Christiaens D, et al. Denoising of diffusion MRI using random matrix theory. NeuroImage 2016; 142: 394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kellner E, Dhital B, Kiselev VG, et al. Gibbs‐ringing artifact removal based on local subvoxel‐shifts. Magn Resonance Medicine 2016; 76: 1574–1581. [DOI] [PubMed] [Google Scholar]
- 18.Tustison NJ, Avants BB, Cook PA, et al. N4ITK: improved N3 bias correction. IEEE Transactions Medical Imaging 2010; 29: 1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain S, Sima DM, Ribbens A, et al. Automatic segmentation and volumetry of multiple sclerosis brain lesions from MR images. NeuroImage: Clin 2015; 8: 367–375, DOI: 10.1016/j.nicl.2015.05.003 10.1016/j.nicl.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alshehri A, Al-iedani O, Arm J, et al. Neural diffusion tensor imaging metrics correlate with clinical measures in people with relapsing-remitting MS. Neuroradiology J 2022; 35(5):592–599. DOI: 10.1177/19714009211067400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolasa M, Hakulinen U, Brander A, et al. Diffusion tensor imaging and disability progression in multiple sclerosis: A 4‐year follow‐up study. Brain Behavior 2019; 9: e01194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakun JG, Zhu Z, Brown CA, et al. Longitudinal alterations to brain function, structure, and cognitive performance in healthy older adults: A fMRI-DTI study. Neuropsychologia 2015; 71: 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahim P, Holleran L, Kim JH, et al. Test-retest reliability of high spatial resolution diffusion tensor and diffusion kurtosis imaging. Scientific Reports 2017; 7: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chun J, Hartung H-P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacology 2010; 33: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mills EA, Ogrodnik MA, Plave A, et al. Emerging Understanding of the Mechanism of Action for Dimethyl Fumarate in the Treatment of Multiple Sclerosis. Front Neurol 2018; 9: DOI: 10.3389/fneur.2018.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radue E-W, O’connor P, Polman CH, et al. Impact of fingolimod therapy on magnetic resonance imaging outcomes in patients with multiple sclerosis. Arch Neurology 2012; 69: 1259–1269. [DOI] [PubMed] [Google Scholar]
- 27.Harrison D, Caffo B, Shiee N, et al. Longitudinal changes in diffusion tensor–based quantitative MRI in multiple sclerosis. Neurology 2011; 76: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rashid W, Hadjiprocopis A, Davies G, et al. Longitudinal evaluation of clinically early relapsing-remitting multiple sclerosis with diffusion tensor imaging. J Neurology 2008; 255: 390–397. [DOI] [PubMed] [Google Scholar]
- 29.Kasindi A, Fuchs D-T, Koronyo Y, et al. Glatiramer Acetate Immunomodulation: Evidence of Neuroprotection and Cognitive Preservation. Cells 2022; 11: 1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zivadinov R, Hagemeier J, Bergsland N, et al. Effect of dimethyl fumarate on gray and white matter pathology in subjects with relapsing multiple sclerosis: a longitudinal study. Eur J Neurol 2018; 25. [DOI] [PubMed] [Google Scholar]
- 31.Gurevich M, Waknin R, Stone E, et al. Fingolimod‐improved axonal and myelin integrity of white matter tracts associated with multiple sclerosis‐related functional impairments. CNS Neuroscience Therapeutics 2018; 24: 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saraste M, Bezukladova S, Sucksdorff M, et al. Fingolimod treatment reverses signs of diffuse white matter damage in multiple sclerosis: A pilot study. Mult Scler Relat Disord 2021; 48: 102690. [DOI] [PubMed] [Google Scholar]
- 33.Bhattacharyya P, Lowe M, Sakaie K, et al. Changes in structural and functional connectivity during two years of fingolimod therapy for multiple sclerosis. Magn Reson Imaging 2020; 74: 113–120. [DOI] [PubMed] [Google Scholar]
- 34.Yu HJ, Christodoulou C, Bhise V, et al. Multiple white matter tract abnormalities underlie cognitive impairment in RRMS. Neuroimage 2012; 59: 3713–3722. [DOI] [PubMed] [Google Scholar]
- 35.Lipp I, Jones DK, Bells S, et al. Comparing MRI metrics to quantify white matter microstructural damage in multiple sclerosis. Hum Brain Mapp 2019; 40: 2917–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harel A, Sperling D, Petracca M, et al. Brain microstructural injury occurs in patients with RRMS despite ‘no evidence of disease activity. J Neurol Neurosurg Psychiatry 2018; 89: 977–982. [DOI] [PubMed] [Google Scholar]
- 37.Campanholo K, Pitombeira M, Rimkus C, et al. Myelin imaging measures as predictors of cognitive impairment in MS patients: A hybrid PET-MRI study. Mult Scler Relat Disord 2022; 57: 103331. [DOI] [PubMed] [Google Scholar]
- 38.Vellinga M, Geurts J, Rostrup E, et al. Clinical correlations of brain lesion distribution in multiple sclerosis. J Magn Reson Imaging Official J Int Soc Magn Reson Med 2009; 29: 768–773. [DOI] [PubMed] [Google Scholar]
- 39.Oh J, Vidal-Jordana A, Montalban X. Multiple sclerosis: clinical aspects. Curr Opinion Neurology 2018; 31: 752–759. [DOI] [PubMed] [Google Scholar]