Abstract
Background
Hemorrhage is the most devastating complication of brain arteriovenous malformations (bAVMs), and to date, there is still concern about the needing for treatment in case of unruptured and asymptomatic bAVM. In fact, the morbidity and mortality of treatments may exceed that of the AVM’s natural history. None of the classifications and scores for bAVM allows to predict the risk of bleeding. In this study, we aimed to identify the angio-architectural characteristics of brain AVMs associated with bleeding.
Methods
We retrospectively evaluated all consecutive patients diagnosed with cerebral AVMs, between January 2010 and December 2019 from our prospective bAVM database. Univariate and multivariate logistic regression analysis were used to evaluate relationships between angio-architectural features of ruptured and unruptured bAVMs.
Results
Of the 143 retrieved bAVMs, 65 were unruptured and 78 were ruptured. The univariate logistic regression analysis demonstrated statistically significant differences into angio-architectural features of unruptured and ruptured bAVMs. The multivariate logistic regression analysis fitted well (p =.113) with a good discrimination capacity (ROC = 0.83) of three independent angio-architectural features mainly related to bleeding in bAVMs: a smaller diameter of the nidus (p < .001), the absence of venous drainage alterations (p = .047), of the presence of prenidal aneurysms (p = .005).
Conclusions
In our study, several features resulted related to an increased probability of rupture for bAVMs, among which the more relevant were a small diameter of the nidus, the absence of venous drainage alterations, and the presence of prenidal aneurysms.
Keywords: Arteriovenous malformations, angioarchitecture, ARUBA, AVM rupture
Introduction
Intracranial hemorrhage is the most devastating complication of brain arteriovenous malformations (bAVMs) and could lead to severe outcomes, from death to severe neurological deficits. Thus, the possibility to predict bAVMs prone to rupture is of great interest, to treat them promptly and avoid potentially fatal sequelae.
Although results of the ARUBA trial were criticized on various aspects, to date, it remains the highest available level of evidence regarding the management of patients with bAVMs.1,2 Its results states that for ruptured bAVMs, treatment is typically indicated,2–4 while for unruptured bAVMs the medical management alone is superior to medical management with treatment. To date, there is still concern about the needing for preventive treatment in patients with unruptured bAVMs, 1 as the morbidity and mortality of treatments may exceed that of AVM’s natural history.5,6
Previous articles have shown that the angioarchitecture of bAVMs evolves across time,7–11 and bleeding mainly occurs when the hemodynamical balance is lost. 12 Thus, there is the needing to identify features associated with higher probability of rupture, to prevent this potentially dramatical event.12–15
Despite several anatomic, hemodynamic, and angio-architectural features have been identified as being more prevalent in ruptured bAVMs, no scores or classification have been developed to consider the probability of bleeding. 16 In fact, none of the Spetzler and Martin (SM), 17 the Spetzler-Ponce, 18 the Lawton’s supplementary system, 19 the Buffalo, 20 and Toronto classifications and scores allow to predict the probability of bleeding for bAVMs. 21
In this study, we aimed to identify the angio-architectural characteristics of brain AVMs associated with bleeding.
Methods
In this retrospective study, we reviewed all consecutive patients diagnosed with cerebral AVMs, between January 2010 and December 2019 from our prospective bAVM database. The scientific and ethics committees of our institution approved the conduct of this research and we adhered to the Declaration of Helsinki.
Inclusion criteria were any patient with diagnosis of bAVM, and with at least one pre-operative diagnostic cerebral digital subtraction angiography (DSA) performed in a standardized fashion with biplane angiography, including injection of both internal carotid arteries (ICAs) and external carotid arteries, and of the vertebral arteries (VAs) bilaterally. Both ruptured and unruptured bAVM of any grade according to the Spetzler Martin classification were considered suitable for this study. No limits of age and sex were considered. Exclusion criteria were a bad quality angiography and previously treated bAVMs.
Data acquisition
We retrospectively assessed the clinical, demographic, and imaging data (brain CT or MRI and, cerebral conventional DSA) of all included patients. Age at presentation, sex, and bleeding were included, as well as brain AVM’s angio-architectural features based on DSA evaluation. DSA images were carefully and independently reviewed by two neuroradiologists (a senior one with more than 15 years of experience and a junior one with 7 years of experience), to include numbers and characteristics of the arterial feeders (terminal, en-passage, pials, and the diameter of the major one, measured 1 cm before the nidus) (Figures 1, 2, and 3), the number of the major arterial supplies, the presence/absence of extracranial arterial supply, the diameter and volume of the nidus, the type of the nidus (compact, diffuse, or plurifocal), the presence/absence and characteristics of aneurysms (prenidal, intranidal, mixed), the number and type of venous drainage (superficial, deep, or mixed), the presence/absence of venous drainage alterations (ectasia, varices, stenosis, thrombosis, flow reversal), and the Buffalo and Spetzler Martin grades. The volume of the nidus was expressed in mm3 and calculated with the formula of an ellipsoid with different-axes lengths: . The diameter of the major arterial feeder measured 1 cm before the nidus was done on our DICOM image viewing software (CARESTREAM Vue PACS, Carestream Health Inc., USA). We used a curved line tool to measure 1 cm prior to the nidus on the major arterial arterial feeder and at that point we measured the diameter of the arterial feeders itself (Figures 1 and 2). All nomenclature and definitions of terms for the angio-architectural features of bAVMs were assessed basing on the joint writing group for the reporting terminology for Brain Arteriovenous Malformation. 22
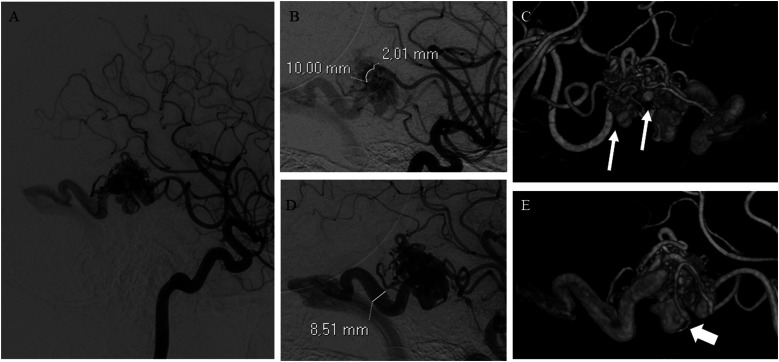
Figure 1.
(A) bAVM with a compact nidus receiving arterial supply mainly by terminal feeders arising from the right middle cerebral artery. (B) Demonstration of the measurement of the diameter of the major arterial feeder at 1 cm from the nidus. (C) Multiple intranidal aneurysms (arrows). (D) Single ectasic venous collector draining into the right transverse sinus (superficial venous drainage). (E) 3D DSA reconstruction demonstrating the presence of a venous varix (arrow) at the origin of the venous collector.
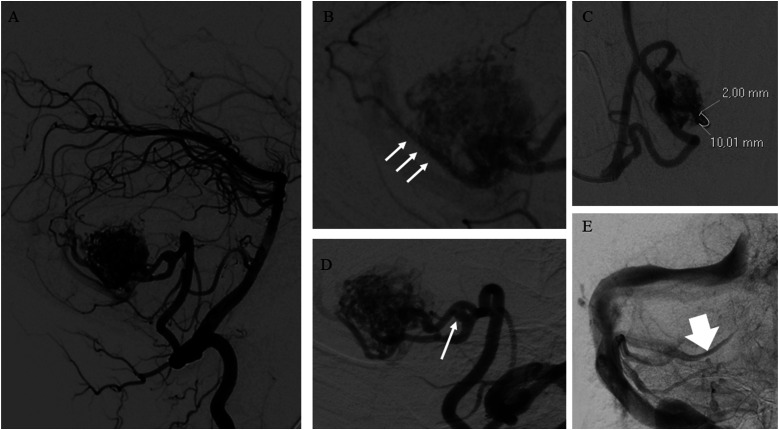
Figure 2.
(A) bAVM of the posterior fossa with a compact nidus. (B) The nidus receives arterial supply by multiple arterial feeders «en-passage» (arrows). (C) Demonstration of the measurement of the diameter of the major arterial feeder at 1 cm from the nidus. (D) A prenidal aneurysm (arrow). (E) Presence of venous reflux into one of the cerebellar cortical veins draining the bAVM (arrow).
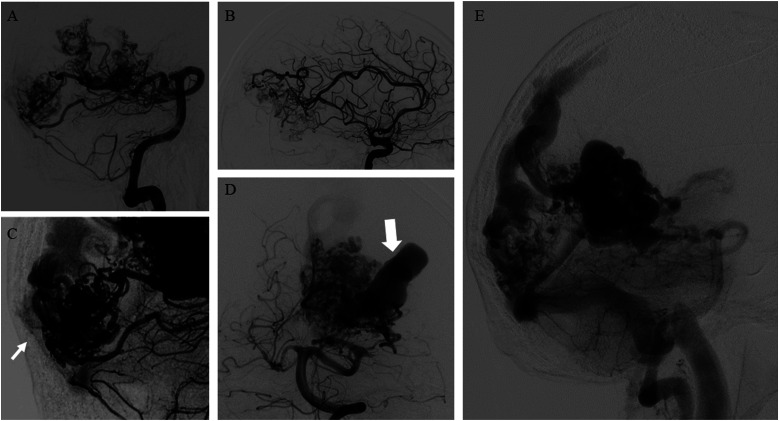
Figure 3.
(A–B) bAVM with a plurifocal and diffuse nidus, receiving arterial supply by both anterior and posterior circulations. (C) Pials arterial feeders (arrow) also supply the plurifocal nidus of bAVM. (D) Presence of an intranidal giant aneurysm (arrow). (E) Mixed (superficial and deep) venous drainage draining the bAVM through cortical venous system and deep veins (the internal cerebral veins and the basal veins).
Statistics
Categorical variables were expressed as absolute numbers (n) and percentages (%), while continuous variables were expressed as mean and standard deviation (SD) or median and interquartile range (IQR) as appropriate. Variables differences were analyzed in the groups of unruptured and ruptured AVMs, with radiological evaluation of bleeding. The differences between the two groups were assessed by Mann–Whitney U test for continuous variables and by Pearson chi-square test or Fisher’ exact test for categorical ones.
The association between each demographic and clinical characteristic and the rupture of bAVM was assessed by univariate logistic regression models. Then, variables associated to the bAVM’s rupture were included in a multivariate logistic regression analysis; the final model was the one that best fit the data and with the greatest discrimination ability based on Hosmer and Lemeshow test and the area under the receiver-operating characteristic (ROC) curve, respectively. All statistical analyses were performed using Stata SE version 14.2 (StataCorp LLC).
Results
Patient population and angio-architectural baseline characteristics
A total of 143 patients (mean age 46.1 year (sd: 19 years)) were included in the analysis (Table 1). Of them, 65 were patients with unruptured bAVMs, while 78 were patients with ruptured bAVMs. One patient presented two separate bAVMs with different arterial supply, nidus, and venous drainage; only one of the bAVMs was ruptured due to the distribution of blood in the CT scan. Thus, as suggested by the joint writing group for the reporting terminology for Brain Arteriovenous Malformation, 22 we characterized separately each cerebral AVM.
Table 1.
Angio-architectural baseline characteristics compared between ruptured and unruptured bAVMs groups.
| Unruptured bAVM (N = 65) | Ruptured bAVM (N = 78) | p-value | ||
|---|---|---|---|---|
| Age at diagnosis [years] | median (IQR) | 43 (30–57) | 50 (35–65) | 0.039 |
| Diameter of nidus (LL horizontal) [mm] | median (IQR) | 2.88 (2.15–4.3) | 1.64 (1.13–2.59) | <0.001 |
| Diameter of nidus (LL vertical) [mm] | median (IQR) | 2.84 (1.96–3.8) | 1.37 (1.02–2.23) | <0.001 |
| Diameter of nidus (AP transverse) [mm] | median (IQR) | 2.68 (2.11–3.69) | 1.4 (0.93–1.8) | <0.001 |
| Volume of nidus [mm3] | median (IQR) | 12.21 (5.97–26.27) | 1.42 (0.63–4.73) | <0.001 |
| N of arterial axes | median (IQR) | 2 (1–2) | 1 (1–2) | 0.106 |
| N of arterial feeders | median (IQR) | 5 (3–7) | 4 (3–5) | 0.018 |
| N° of terminal arterial feeders | median (IQR) | 2 (1–4) | 2 (0–3) | 0.106 |
| N° of “en-passage” arterial feeders | median (IQR) | 3 (1–4) | 2 (1–3) | 0.299 |
| N° of pial arterial feeders | median (IQR) | 0 (0–0) | 0 (0–0) | 0.432 |
| N of draining veins | median (IQR) | 2 (1–3) | 1 (1–2) | 0.005 |
| Maximal diameter of nidus [mm] | median (IQR) | 3.31 (2.53–4.53) | 1.89 (1.37–2.75) | <0.001 |
| Diameter of the major arterial feeder measured 1 cm before nidus [mm] | median (IQR) | 2.08 (1.62–2.6) | 1.4 (1–2.03) | <0.001 |
| Aneurysm bAVM | median (IQR) | 1 (0–2) | 1 (0–2) | 0.828 |
| Sex | 0.999 | |||
| Males | n (%) | 30 (46.15) | 36 (46.15) | |
| Females | n (%) | 35 (53.85) | 42 (53.85) | |
| Type of nidus | 0.518 | |||
| Compact | n (%) | 2 (3.08) | 4 (5.13) | |
| Diffuse | n° (%) | 63 (96.92) | 72 (92.31) | |
| Pluri-focal | n (%) | 0 (0) | 2 (2.56) | |
| N° of arterial axes | 0.036 | |||
| 1 | n (%) | 32 (49.23) | 50 (64.10) | |
| 2 | n (%) | 26 (40.00) | 21 (26.92) | |
| 3 | n (%) | 6 (9.23) | 3 (3.85) | |
| 4 | n (%) | 0 (0) | 4 (5.13) | |
| 5 | n (%) | 1 (1.54) | 0 (0) | |
| External carotid artery supply | 0.251 | |||
| No | n (%) | 65 (100) | 75 (96.15) | |
| Yes | n° (%) | 0 (0) | 3 (3.85) | |
| Type of venous drainage | 0.049 | |||
| Superficial | n (%) | 31 (47.69) | 45 (57.69) | |
| Deep | n (%) | 16 (24.61) | 24 (30.77) | |
| Mixed | n (%) | 18 (27.60) | 9 (11.54) | |
| Venous drainage alterations | <0.001 | |||
| No | n (%) | 14 (21.54) | 40 (51.28) | |
| Yes | n (%) | 51 (78.46) | 38 (48.72) | |
| Venous ectasia | <0.001 | |||
| No | n (%) | 22 (33.85) | 52 (66.67) | |
| Yes | n (%) | 43 (66.15) | 26 (33.33) | |
| Venous varix | 0.105 | |||
| No | n (%) | 51 (78.46) | 69 (88.46) | |
| Yes | n (%) | 14 (21.54) | 9 (11.54) | |
| Venous stenosis | 0.857 | |||
| No | n (%) | 56 (86.15) | 68 (87.18) | |
| Yes | n (%) | 9 (13.85) | 10 (12.82) | |
| Venous thrombosis | 0.501 | |||
| No | n (%) | 65 (100) | 76 (97.44) | |
| Yes | n (%) | 0 (0) | 2 (2.56) | |
| Venous flow reversal | 0.999 | |||
| No | n (%) | 63 (96.92) | 75 (96.15) | |
| Yes | n (%) | 2 (3.08) | 3 (3.85) | |
| Eloquent area | 0.614 | |||
| No | n (%) | 39 (60) | 50 (64.10) | |
| Yes | n (%) | 26 (40) | 28 (35.90) | |
| Spetzler Martin grade | 0.015 | |||
| 1 | n (%) | 10 (15.38) | 23 (30.67) | |
| 2 | n (%) | 20 (30.77) | 29 (38.67) | |
| 3 | n (%) | 23 (35.38) | 18 (24.00) | |
| 4 | n (%) | 11 (16.92) | 3 (4.00) | |
| 5 | n (%) | 1 (1.54) | 2 (2.67) | |
| Buffalo grade | 0.997 | |||
| 1 | n (%) | 8 (12.31) | 9 (12.00) | |
| 2 | n° (%) | 14 (21.54) | 16 (21.33) | |
| 3 | n (%) | 24 (36.92) | 29 (38.67) | |
| 4 | n (%) | 19 (29.23) | 21 (28.00) | |
| Atypical feature of bAVM | 0.473 | |||
| No | n (%) | 28 (43.08) | 29 (37.18) | |
| Yes | n (%) | 37 (56.92) | 49 (62.82) | |
| Type of aneurysms | 0.010 | |||
| Intranidal | n (%) | 15 (23.08) | 13 (16.67) | |
| Prenidal | n (%) | 9 (13.85) | 29 (37.18) | |
| Mixed | n (%) | 13 (20.00) | 7 (8.97) | |
| No aneurysm | n (%) | 28 (43.08) | 29 (37.18) | |
The female proportion in both unruptured and ruptured bAVM was the same (53.85%). Ruptured bAVMs were more frequently detected in older patients (median age 50, IQR 35–65) compared to unruptured bAVMs in which patients were younger (median age 43, IQR 30–57) (p = .039). Between the two groups, statistical significant differences were present in the number of the arterial feeders (p = 0.018), in the diameter of the major arterial feeder measured 1 cm before the nidus (p < 0.001), in the number of major arterial supply (p = 0.036), in the 3 diameters (p < 0.001) and in the volume of the nidus (p < 0.001), in the number of draining veins (p = 0.005) and in the type of venous drainage (p = 0.049), as well as the presence of alterations of the venous drainage (p < 0.001), particularly venous ectasia (p < 0.001). Moreover, also the Spetzler and Martin grade (p = 0.015) and the type of aneurysms (p = 0.010) were significantly different among ruptured and unruptured bAVMs.
Factors associated with ruptured bAVMs
The univariate logistic regression analysis (Table 2) demonstrates that for every one unit increase of the diameter of the major arterial feeders measured 1 cm before the nidus (p < 0.001, OR = 0.42, 95% CI 0.26–0.67), of the maximum diameter of the nidus of the nidus (p = 0.002, OR = 0.71, 95% CI 0.58–0.89), of the volume of the nidus (p = 0.031, OR = 0.98, 95% CI 0.97–1.00) and of the number of the draining veins (p = 0.005, OR = 0.64, 95% CI 0.46–0.87) the odds of bAVMs rupture decrease.
Table 2.
Univariate logistic regression analysis.
| Odds ratio | 95% Conf. Interval | p-value | ||
|---|---|---|---|---|
| Age | 1.02 | 1.00 | 1.04 | 0.036 |
| Diameter of nidus (LL horizontal) [mm] | 0.67 | 0.54 | 0.85 | 0.001 |
| Diameter of nidus (LL vertical) [mm] | 0.60 | 0.46 | 0.79 | <0.001 |
| Diameter of nidus (AP transverse) [mm] | 0.48 | 0.35 | 0.66 | <0.001 |
| Volume of nidus [mm3] | 0.98 | 0.97 | 1.00 | 0.031 |
| N° of arterial axes | 0.79 | 0.52 | 1.21 | 0.274 |
| N° of arterial feeders | 0.88 | 0.78 | 1.00 | 0.059 |
| N° of terminal arterial feeders | 0.86 | 0.73 | 1.02 | 0.079 |
| N° of “en-passage” arterial feeders | 0.93 | 0.77 | 1.11 | 0.415 |
| N° of pial arterial feeders | 0.99 | 0.53 | 1.87 | 0.977 |
| N° of draining veins | 0.64 | 0.46 | 0.87 | 0.005 |
| Maximal diameter of nidus [mm] | 0.71 | 0.58 | 0.89 | 0.002 |
| Diameter of the major arterial feeder measured 1 cm before nidus [mm] | 0.42 | 0.26 | 0.67 | <0.001 |
| Aneurysm bAVM | 1.02 | 0.79 | 1.32 | 0.878 |
| Female sex | 1.00 | 0.52 | 1.94 | 0.999 |
| Type of nidus | ||||
| Compact | 1.00 (base) | |||
| Diffuse | 0.57 | 0.10 | 3.23 | 0.526 |
| Pluri-focal | 1.00 | |||
| N° of arterial axes | ||||
| 1 | 1.00 (base) | |||
| 2 | 0.52 | 0.25 | 1.07 | 0.075 |
| 3 | 0.32 | 0.07 | 1.37 | 0.125 |
| 4 | 1.00 (empty) | |||
| 5 | 1.00 (empty) | |||
| External carotid artery supply | 1.00 (empty) | |||
| Type of venous drainage | ||||
| Superficial | 1.00 | |||
| Deep | 1.03 | 0.47 | 2.26 | 0.934 |
| Mixed | 0.34 | 0.14 | 0.87 | 0.023 |
| Venous drainage alterations | 0.26 | 0.12 | 0.55 | <0.001 |
| Venous ectasia | 0.26 | 0.13 | 0.51 | <0.001 |
| Venous varix | 0.48 | 0.19 | 1.18 | 0.110 |
| Venous stenosis | 0.92 | 0.35 | 2.41 | 0.857 |
| Venous thrombosis | 1.00 (empty) | |||
| Venous flow reversal | 1.26 | 0.20 | 7.78 | 0.803 |
| Eloquent area | 0.84 | 0.43 | 1.66 | 0.614 |
| Spetzler Martin grade | ||||
| 1 | 1.00 (base) | |||
| 2 | 0.63 | 0.25 | 1.61 | 0.334 |
| 3 | 0.34 | 0.13 | 0.89 | 0.029 |
| 4 | 0.12 | 0.03 | 0.52 | 0.005 |
| 5 | 0.87 | 0.07 | 10.73 | 0.913 |
| Buffalo grade | ||||
| 1 | 1.00 (base) | |||
| 2 | 1.02 | 0.31 | 3.35 | 0.979 |
| 3 | 1.07 | 0.36 | 3.21 | 0.898 |
| 4 | 0.98 | 0.32 | 3.06 | 0.976 |
| Atypical feature of bAVM | 1.28 | 0.65 | 2.50 | 0.474 |
| Type of aneurysms | ||||
| Intranidal | 0.84 | 0.34 | 2.07 | 0.700 |
| Prenidal | 3.11 | 1.25 | 7.73 | 0.015 |
| Mixed | 0.52 | 0.18 | 1.49 | 0.224 |
| No aneurysm | 1.00 (base) | |||
Mixed venous drainage (p = 0.023, OR = 0.34, 95% CI 0.14–0.87), as well as the presence of alterations in the venous drainage (p < 0.001, OR = 0.26, 95% CI 0.12–0 0.55), as venous ectasia (p < 0.001, OR = 0.26, 95% CI 0.13–0.51) were found to be protective factors for the probability of bAVMs rupture. Instead, the presence of a prenidal aneurysm was found to be a factor strongly associated with the probability of bAVMs rupture (p = 0.015, OR = 3.11, 95% CI 1.25–7.73).
Predictive model
Multivariate logistic regression analysis (Table 3) suggested that there were four independent factors related to bAVMs rupture suggested that there were four independent factors related to bAVMs rupture: the major diameter of the nidus, the presence of venous drainage alterations, and the presence and type of aneurysms. This final model was the best predictive model, that is, the one that fitted well (Hosmer & Lemeshow test, p = 0.113) and had a good discrimination capacity (ROC = 0.83), allowing to detect three independent angio-architectural features mainly related to bleeding in bAVMs: a smaller diameter of the nidus (p < 0.001), the absence of venous drainage alterations (p = 0.047), and the presence of prenidal aneurysms (p = 0.005).
Table 3.
Multivariate logistic regression analysis.
| Odds ratio | 95% Conf. Interval | p-value | ||
|---|---|---|---|---|
| Major diameter of the nidus | 0.54 | 0.39 | 0.75 | <0.001 |
| Venous drainage alterations | 0.40 | 0.16 | 0.99 | 0.047 |
| Aneurysms type | ||||
| Intranidal | 1.16 | 0.42 | 3.21 | 0.611 |
| Prenidal | 4.69 | 1.58 | 13.89 | 0.005 |
| Mixed | 1.21 | 0.364 | 4.09 | 0.756 |
| No aneurysm | 1 (base) | |||
| Constant | 5.64 | 2.29 | 13.88 | < 0.001 |
Discussion
It should be first mentioned that the analysis of the differences of unruptured and ruptured bAVMs groups showed statistically significant differences into groups’ ages and lots of angio-architectural features (Table 1). In fact, in our series, ruptured bAVMs were more frequently detected in older patients, supporting the hypothesis that bAVMs are not static in their shape and in hemodynamic, but evolve during lifetime.7–10, 12 Among the angio-architectural features of bAVMs between ruptured and unruptured groups, we found a statistically significant differences into several features, both into the arterial, nidus, and venous portions. These statistically significant differences among ruptured and unruptured bAVMs support the hypothesis that angio-architectural features should be considered during treatment decision, both for newly discovered bAVMs and during follow-up.12–15
As reported in the results, the logistic regression analysis demonstrates that for every one unit increase of the diameter of the arterial feeder measured 1 cm before the nidus, of the maximum diameter and of the volume of the nidus, and of the number of the draining veins, the odds of bAVMs to being rupture decrease (Table 2). This could be explained by the fact that all those factors together are related to a hemodynamical stability into bAVMs. In fact, if the arterial feeders, the size/volume of the nidus and the number of draining veins increase, the system can support the increase amount of blood and flow enter in the bAVM. These results are in accordance with a study published by Sahlein et al., assessing that bAVMs with higher numbers of draining veins would be protected from hemorrhage, compared to those with single draining vein, because in the first group there is a lower outflow impedance. 15 We also found other venous features relates to the reduction of bAVM probability of rupture, as the presence of mixed venous drainage, alterations in the venous drainage, particularly venous ectasia (Table 2). These results can be explained by the fact that a greater venous drainage system of bAVMs can accommodate a greater amount of blood and flow, until a hemodynamical balance exists. However, in our study the only factor related to a greater probability of bleeding for AVMs was the presence of a prenidal aneurysm (Table 2). This result can be supported by the intrinsic weakness of a prenidal flow-related aneurysm, which lacks the arterial layers in a high-pressure system. 23
Finally, the logistic multivariate regression analysis demonstrated four independent factors related to AVMs rupture (Table 3). In our model, both a larger diameter of the nidus and the presence of venous drainage alterations were protective factors (respectively, OR = 0.54 and OR = 0.40), whereas the presence of prenidal aneurysms was a factor increasing the likelihood of bAVMs bleeding (OR = 4.69). These results corroborate the fact that some of the angio-architectural features of brain AVMs are associated with higher probability of rupture and there is the needing to develop a scoring system to estimate bleeding risk, as it has been done for intracranial aneurysms.
To date, none of the different scores proposed for bAVMs assess the risk of rupture and thus, the needing for treatment. Therefore, it is difficult to decide towards the needing for treatment in patients with brain AVMs, as far as the risk-benefit balance between invasive treatment and follow-up relies mainly on single physicians’ experience. Further prospective studies are needed to develop a scoring system on angio-architectural features for brain AVMs to help physician in the decision towards treatment or conservative management for brain AVMs, and also to distinguish patients at low risk from patients at high risk of bleeding.
Limitations
Because of its retrospective nature, our study has several limitations. First, the lack of hemodynamical evaluation of the angio-architectural features, which is of a certainty relevance. Another limitation of this study is the small sample size, although consistent with previously published series. Further prospective studies with grater sample size are needed to confirm our results and eventually find new angio-architectural features, to detect the risk of bleeding of bAVMs, and eventually create a scoring system
Conclusions
Our study evaluated the angio-architectural features of brain AVMs to assess the probability of bleeding, to underly the importance of identifying features associated with higher risk of rupture that would require early and aggressive treatments. In our study, several angio-architectural features were related to an increased probability of rupture for bAVMs, among which the more relevant resulted in a small diameter of the nidus, the absence of venous drainage alterations and the presence of prenidal aneurysms.
Supplemental Material
Supplemental Material for The angio-architectural features of brain arteriovenous malformations: is it possible to predict the probability of rupture? by Arianna Rustici, Francesca Vari, Carmelo Sturiale, Alfredo Conti, Antonino Scibilia, Carlo Bortolotti, Raffaele Agati, Caterina Tonon, Raffaele Lodi, Diego Mazzatenta, Matteo Zoli, Ciro Princiotta, Massimo Dall’Olio and Luigi Cirillo in The Neuroradiology Journal
Acknowledgments
All authors are sincerely grateful to Corrado Zenesini and Laura Belotti for their help in the statistical analysis of the data.
Author contribution: RA, FV, MZ, and CL equally contributed to this manuscript, designed, and drafted the study. RA, CL, and FV collected the data, while RA, ZC and BL analyzed the data. The rest of the authors revising this manuscript critically for important intellectual content. All authors read and approved the final version of the manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Arianna Rustici https://orcid.org/0000-0002-3753-6189
Matteo Zoli https://orcid.org/0000-0001-8838-5408
Ciro Princiotta https://orcid.org/0000-0001-7018-5280
References
- 1.Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet 2014; 383: 614–621. DOI: 10.1016/S0140-6736(13)62302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong CS, Peterson EC, Ding D, et al. Intervention for a randomized trial of unruptured brain arteriovenous malformations (ARUBA) - Eligible patients: an evidence-based review. Clin Neurol Neurosurg 2016; 150: 133–138. DOI: 10.1016/j.clineuro.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 3.Martinez JL, Macdonald RL. Surgical strategies for acutely ruptured arteriovenous malformations. Front Neurol Neurosci 2015; 37: 166–181, DOI: 10.1159/000437121 10.1159/000437121 [DOI] [PubMed] [Google Scholar]
- 4.van Rooij WJ, Jacobs S, Sluzewski M, et al. Endovascular treatment of ruptured brain AVMs in the acute phase of hemorrhage. AJNR Am J Neuroradiol 2012; 33: 1162–1166. DOI: 10.3174/ajnr.A2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutledge C, Cooke DL, Hetts SW, et al. Brain arteriovenous malformations. Handb Clin Neurol 2021; 176: 171–178. DOI: 10.1016/B978-0-444-64034-5.00020-1 [DOI] [PubMed] [Google Scholar]
- 6.Müller-Forell W, Valavanis A. How angioarchitecture of cerebral arteriovenous malformations should influence the therapeutic considerations. Minim Invasive Neurosurg 1995; 38(1): 32–40. DOI: 10.1055/s-2008-1053458. [DOI] [PubMed] [Google Scholar]
- 7.Sure U, Butz N, Schlegel J, et al. Endothelial proliferation, neoangiogenesis, and potential de novo generation of cerebrovascular malformations. J Neurosurg 2001; 94(6): 972–977. DOI: 10.3171/jns.2001.94.6.0972 [DOI] [PubMed] [Google Scholar]
- 8.Redekop G, TerBrugge K, Montanera W, et al. Arterial aneurysms associated with cerebral arteriovenous malformations: classification, incidence, and risk of hemorrhage. J Neurosurg 1998; 89(4): 539–546. DOI: 10.3171/jns.1998.89.4.0539. [DOI] [PubMed] [Google Scholar]
- 9.Garzelli L, Shotar E, Blauwblomme T, et al. Risk factors for early brain AVM rupture: cohort study of pediatric and adult patients. AJNR Am J Neuroradiol 2020; 41: 2358–2363. DOI: 10.3174/ajnr.A6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hetts SW, Cooke DL, Nelson J, et al. Influence of patient age on angioarchitecture of brain arteriovenous malformations. AJNR Am J Neuroradiol 2014; 35: 1376–1380. DOI: 10.3174/ajnr.A3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin H, Lenck S, Krings T, et al. Interval angioarchitectural evolution of brain arteriovenous malformations following rupture. J Neurosurg 2018; 131(1): 96–103. DOI: 10.3171/2018.2.JNS18128. PMID: 30052159 [DOI] [PubMed] [Google Scholar]
- 12.Mosteiro A, Pedrosa L, Torne R, et al. Venous tortuosity as a novel biomarker of rupture risk in arteriovenous malformations: ARI score. J Neurointerv Surg 2021. Epub ahead of print. DOI: 10.1136/neurintsurg-2021-018181 [DOI] [PubMed] [Google Scholar]
- 13.Qureshi AM, Muthusami P, Krings T, et al. Clinical and angioarchitectural features of hemorrhagic brain arterio-venous malformations in adults and children: contrasts and implications on outcome. Neurosurgery 2021; 89(4): 645–652. DOI: 10.1093/neuros/nyab251 [DOI] [PubMed] [Google Scholar]
- 14.Mansmann U, Meisel J, Brock M, et al. Factors associated with intracranial hemorrhage in cases of cerebral arteriovenous malformation. Neurosurgery 2000; 46(2): 272–279. DOI: 10.1097/00006123-200002000-00004 [DOI] [PubMed] [Google Scholar]
- 15.Sahlein DH, Mora P, Becske T, et al. Features predictive of brain arteriovenous malformation hemorrhage: extrapolation to a physiologic model. Stroke 2014; 45(7): 1964–1970. DOI: 10.1161/STROKEAHA.114.005170 [DOI] [PubMed] [Google Scholar]
- 16.Shankar JJ, Menezes RJ, Pohlmann-Eden B, et al. Angioarchitecture of brain AVM determines the presentation with seizures: proposed scoring system. AJNR Am J Neuroradiol 2013; 34(5): 1028–1034. DOI: 10.3174/ajnr.A3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg 1986; 65(4): 476–483. DOI: 10.3171/jns.1986.65.4.0476 [DOI] [PubMed] [Google Scholar]
- 18.Spetzler RF, Ponce FA. A 3-tier classification of cerebral arteriovenous malformations. Clinical article. J Neurosurg 2011; 114(3): 842–849. DOI: 10.3171/2010.8.JNS10663 [DOI] [PubMed] [Google Scholar]
- 19.Lawton MT, Kim H, McCulloch CE, et al. A supplementary grading scale for selecting patients with brain arteriovenous malformations for surgery. Neurosurgery 2010; 66: 702–713. DOI: 10.1227/01.NEU.0000367555.16733.E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumont TM, Kan P, Snyder KV, et al. A proposed grading system for endovascular treatment of cerebral arteriovenous malformations: Buffalo score. Surg Neurol Int 2015; 6: 3PMC4310056. DOI: 10.4103/2152-7806.148847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padilla-Vazquez F, Zenteno MA, Balderrama J, et al. A proposed classification for assessing rupture risk in patients with intracranial arteriovenous malformations. Surg Neurol Int 2017; 8: 303PMC5764916. DOI: 10.4103/sni.sni_273_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkinson RP, Awad IA, Batjer HH, et al. Reporting terminology for brain arteriovenous malformation clinical and radiographic features for use in clinical trials. Stroke 2001; 32 pp. 1430–1442. DOI: 10.1161/01.str.32.6.1430. [DOI] [PubMed] [Google Scholar]
- 23.Meisel HJ, Mansmann U, Alvarez H, et al. Cerebral arteriovenous malformations and associated aneurysms: analysis of 305 cases from a series of 662 patients. Neurosurgery 2000; 46(4): 793–800. DOI: 10.1097/00006123-200004000-00004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for The angio-architectural features of brain arteriovenous malformations: is it possible to predict the probability of rupture? by Arianna Rustici, Francesca Vari, Carmelo Sturiale, Alfredo Conti, Antonino Scibilia, Carlo Bortolotti, Raffaele Agati, Caterina Tonon, Raffaele Lodi, Diego Mazzatenta, Matteo Zoli, Ciro Princiotta, Massimo Dall’Olio and Luigi Cirillo in The Neuroradiology Journal