Abstract
Objectives
Thrombi in cerebral large vessel occlusion associated with active cancer are often fibrin and platelet-rich white thrombi. However, evaluating the thrombus composition in a short time before thrombectomy is often ineffective. We sought to determine factors related to white thrombi in acute ischemic stroke due to large vessel occlusion in cancer patients.
Methods
Consecutive cancer patients undergoing thrombectomy for acute ischemic stroke due to large vessel occlusion between January 2018 and May 2022 were retrospectively reviewed. The patients were classified into white thrombus and red thrombus groups on the basis of the pathological findings of retrieved thrombi. Patient characteristics and laboratory findings were compared between the two groups.
Results
There were 12 patients in the white thrombus group and 11 patients in the red thrombus group. Active cancer was significantly more in the white thrombus group than in the red thrombus group (91.7% vs. 36.3%, p = 0.0094). Internal carotid artery occlusion was significantly less in the white thrombus group than in the red thrombus group (0% vs. 36.4%, p = 0.037). Among laboratory findings, D-dimer levels were an independent factor associated with white thrombi (odds ratio 8.97 [95% confidence interval 1.71–368.99], p < 0.0001). The cutoff value of D-dimer levels for predicting white thrombi was 3.5 μg/mL (83.3% sensitivity and 100% specificity).
Conclusions
In acute ischemic stroke in cancer patients, active cancer, no internal carotid artery occlusion, and higher D-dimer levels (≥3.5 μg/mL) may be associated with occlusion with fibrin and platelet-rich white thrombi.
Keywords: Cancer, ischemic stroke, thrombectomy, white thrombus
Introduction
Thrombi in cerebral large vessel occlusion associated with active cancer are often white thrombi, which are rich fibrin and platelets but have few red blood cells.1,2 Since frictional force against the vessel wall is greater in white thrombi than in red thrombi that are rich in red blood cells, traction of white thrombi with a stent retriever alone is often ineffective.3,4 However, evaluating the thrombus composition from cancer progression or other embolic sources in a short time before thrombectomy is difficult. Even after thrombectomy, determining whether the embolic thrombus is associated with cancer is difficult because there are various embolic sources. In this study, we sought to determine factors related to white thrombi in acute ischemic stroke due to large vessel occlusion in cancer patients.
Methods
Patients
This study was approved by our institutional review board. The requirement of written informed consent was waived due to the retrospective nature of this study. Consecutive cancer patients undergoing thrombectomy for acute ischemic stroke due to large vessel occlusion between January 2018 and May 2022 were retrospectively reviewed. Cancer patients were defined as those with a history of cancer, undergoing cancer treatment, or diagnosed with cancer during stroke treatment. Thrombi were collected from stent retrievers, aspiration catheters, guiding catheters, and syringes. The patients were classified into white thrombus and red thrombus groups on the basis of the pathological findings of retrieved thrombi. Patient characteristics and laboratory findings were compared between the two groups to determine factors related to white thrombi. Treatment and clinical outcome were also compared to evaluate the prognosis of cancer patients with cerebral large vessel occlusion.
Evaluation
The following patient information and laboratory data on admission were collected: age, sex, modified Rankin Scale score before stroke, initial scores of the National Institute of Health Stroke Scale (NIHSS), treatment with intravenous alteplase, occlusion site, hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, active cancer, hemoglobin levels, white blood cell counts, platelet counts, brain natriuretic peptide levels, C-reactive protein levels, prothrombin time, activated partial thromboplastin time, and D-dimer levels. Active cancer was defined as cancer that was diagnosed within 6 months, required chemotherapy or surgical treatment within 6 months, or was recurrent, metastatic, or inoperable on the basis of past reports.1,5 Recanalization status after thrombectomy was classified according to the modified thrombolysis in cerebral infarction (mTICI) scoring system; an mTICI score of 2b–3 indicates successful recanalization. Favorable clinical outcome was defined as a modified Rankin Scale score of 0–2 90 days after thrombectomy.
Pathological analysis
Retrieved thrombi were stained with hematoxylin and eosin. Stained specimens were quantitatively analyzed with ImageJ (National Institutes of Health, Bethesda, MD, USA). Thrombus components other than red and white blood cells were measured as fibrin and platelets. Three regions of interest representing thrombotic features in the sectioned thrombi were selected, and the mean fibrin/platelet percentage of the three regions of interest was measured. A white thrombus was defined as a thrombus in which the fibrin/platelet percentage is 80% or more, and a red thrombus as a thrombus in which the fibrin/platelet percentage is less than 80%.
Statistical analysis
Statistical analyses were performed using JMP version 11 (SAS Institute, Cary, NC, USA). Quantitative data are expressed as mean ± standard deviation or median [interquartile range]. Fisher’s exact test was used for categorical variables. The Mann–-Whitney U-test was used for continuous variables. Logistic regression analysis was performed to determine associations between laboratory findings and white thrombi. Multivariate logistic regression analysis was adjusted for age, sex, and significant influential factors identified in a univariate analysis. Receiver operating characteristic analysis was performed to assess D-dimer levels to predict white thrombi and to estimate the optimal cutoff value. A p < 0.05 was considered statistically significant.
Results
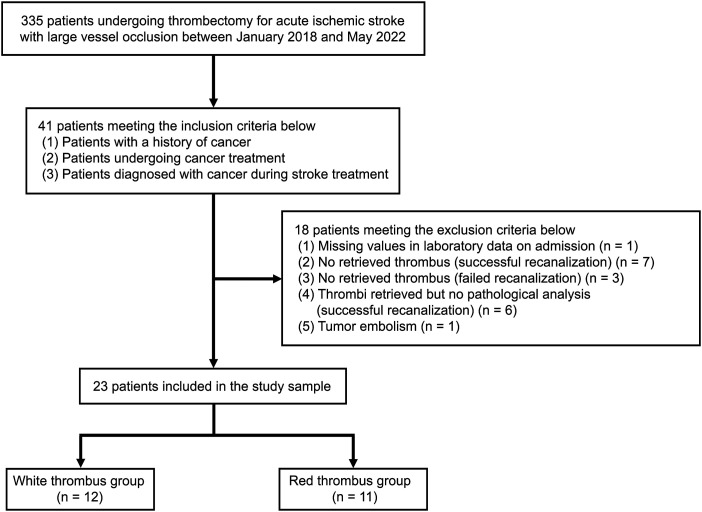
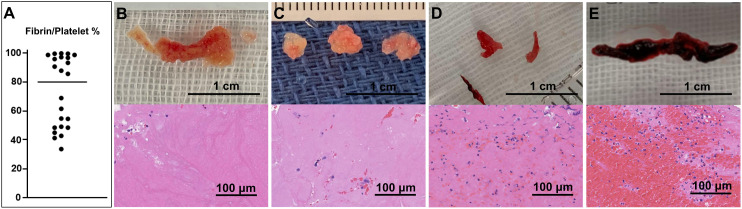
During the study period, 335 consecutive patients with acute ischemic stroke due to large vessel occlusion underwent thrombectomy, 41 of whom had cancer. Of the 41 cancer patients, 18 patients were excluded for the following reasons: missing values in laboratory data on admission (1 patient); successful recanalization but no retrieved thrombus (7 patients); failed recanalization and no retrieved thrombus (3 patients); failure to submit retrieved thrombi to pathologic analysis after successful recanalization (6 patients); and tumor embolus (1 patient). The remaining 23 patients were finally enrolled in this study (age 74.8 ± 9.5 years, men 56.5%). Median fibrin/platelet percentage in all retrieved thrombi was 84.9% (48.2–96.2). There were 12 patients in the white thrombus group (median fibrin/platelet percentage 96.2% (91.9–98.2)) and 11 patients in the red thrombus group (median fibrin/platelet percentage 47.9% (43.3–54.0), Figures 1 and 2(a)). White thrombi were whitish and hard (Figure 2(b)–(d)), while red thrombi were dark red and relatively soft (Figure 2(e)). No thrombi contained plaque, tumor, or bacterial components.
Figure 1.
Flow chart of patient selection.
Figure 2.
A plot of the fibrin/platelet percentage of each thrombus (a) and a gross appearance and a pathological image (hematoxylin and eosin staining) of representative thrombi (b–e). White thrombi with a fibrin/platelet percentage of 99.0% (b), 95.9% (c), and 84.9% (d), and a red thrombus with a fibrin/platelet percentage of 48.4% (e) are shown.
Patient characteristics
Patient characteristics of the study sample are shown in Table 1. Between the two groups, there were no significant differences in age, sex, modified Rankin Scale score before stroke, initial NIHSS, intravenous alteplase, hypertension, diabetes mellitus, hyperlipidemia, and atrial fibrillation. Active cancer was significantly more in the white thrombus group than in the red thrombus group (91.7% vs. 36.3%, p = 0.0094). Internal carotid artery occlusion was significantly less in the white thrombus group than in the red thrombus group (0% vs. 36.4%, p = 0.037), while middle cerebral artery occlusion was significantly more in the white thrombus group than in the red thrombus group (91.7% vs. 45.5%, p = 0.027). Among laboratory findings, C-reactive protein and D-dimer levels were significantly higher in the white thrombus group than in the red thrombus group (1.7 [0.3–2.2] vs. 0.2 [0.1–0.4] mg/dL, p = 0.027; 7.0 [4.8–22.9] vs. 1.3 [0.9–1.8] μg/mL, p = 0.0004; respectively). Among laboratory findings, after adjusting for age and sex, D-dimer levels were an independent factor associated with white thrombi (odds ratio 8.97 [95% confidence interval 1.71–368.99], p < 0.0001; Table 2). According to the receiver operating characteristic analysis, the cutoff value of D-dimer levels for predicting white thrombi was 3.5 μg/mL (83.3% sensitivity and 100% specificity, area under the curve = 0.943).
Table 1.
Patient characteristics.
| Total (23 patients) | White thrombus group (12 patients) | Red thrombus group (11 patients) | p-value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, years | 74.8 ± 9.5 | 72.5 ± 9.1 | 77.3 ± 10.2 | 0.12 |
| Sex, men | 13 (56.5) | 6 (50.0) | 7 (63.6) | 0.68 |
| Modified rankin scale before stroke | 0 [0–0.5] | 0 [0–1] | 0 [0–0] | 0.33 |
| Initial NIHSS | 20 [12.5–26.5] | 20.5 [13.8–26.8] | 20 [11.5–24] | 0.64 |
| Intravenous alteplase | 9 (39.1) | 5 (41.7) | 4 (36.3) | 1 |
| Occlusion site | ||||
| ICA | 4 (17.4) | 0 (0.0) | 4 (36.4) | 0.037* |
| MCA | 16 (69.6) | 11 (91.7) | 5 (45.5) | 0.027* |
| MCA and ACA | 1 (4.3) | 1 (8.3) | 0 (0.0) | 1 |
| BA | 2 (8.7) | 0 (0.0) | 2 (18.2) | 0.22 |
| Clinical factors | ||||
| Hypertension | 16 (69.6) | 8 (66.7) | 8 (72.7) | 1 |
| Diabetes mellitus | 4 (17.4) | 1 (8.3) | 3 (27.3) | 0.32 |
| Hyperlipidemia | 4 (17.4) | 3 (25.0) | 1 (9.1) | 0.59 |
| Atrial fibrillation | 9 (39.1) | 3 (25.0) | 6 (54.5) | 0.21 |
| Active cancer | 15 (65.2) | 11 (91.7) | 4 (36.3) | 0.0094* |
| Laboratory findings | ||||
| Hemoglobin, g/dL | 12.4 [11.6–13.8] | 12.6 [11.6–13.8] | 12.4 [11.6–13.0] | 0.93 |
| White blood cells, 103/μL | 7.7 [5.4–10.2] | 8.0 [6.5–10.6] | 6.5 [4.7–9.2] | 0.39 |
| Platelets, 104/μL | 16.9 [14.0–20.4] | 16.4 [12.8–18.9] | 18.5 [16.5–23.7] | 0.16 |
| Brain natriuretic peptide, pg/mL | 93.8 [38.1–185.1] | 66.9 [38.2–106.8] | 147.3 [46.4–248.9] | 0.23 |
| C-reactive protein, mg/dL | 0.3 [0.2–2.0] | 1.7 [0.3–2.2] | 0.2 [0.1–0.4] | 0.027* |
| PT, INR | 1.1 [1.0–1.2] | 1.2 [1.0–1.2] | 1.1 [1.0–1.1] | 0.34 |
| APTT, seconds | 28.4 [25.9–35.4] | 27.4 [24.7–34.3] | 29.0 [26.6–35.4] | 0.37 |
| D-dimer, ng/mL | 3.3 [1.2–7.0] | 7.0 [4.8–22.9] | 1.3 [0.9–1.8] | 0.0004* |
Data are expressed in mean ± standard deviation, median [interquartile range], or number (percentage). NIHSS: National institutes of health stroke scale; ICA: Internal carotid artery; MCA: Middle cerebral artery; ACA: Anterior cerebral artery; BA: Basilar artery; PT: Prothrombin time; INR: International normalized ratio; APTT: Activated partial thromboplastin time.
*p < 0.05.
Table 2.
Predictors of white thrombi among laboratory findings.
| Laboratory findings | Univariate OR (95% CI) | Multivariate adjusted OR (95% CI) | p-value |
|---|---|---|---|
| C-reactive protein, mg/dL | 2.01 (1.04–6.10) | 3.28 (0.42–45.64) | 0.24 |
| D-dimer, ng/mL | 3.66 (1.59–16.48) | 8.97 (1.71–368.99) | <0.0001* |
Adjusted for age and sex. OR: Odds ratio; CI: Confidence interval.
*p < 0.05.
Treatment and clinical outcome
Treatment and clinical outcome are shown in Table 3. Overall, successful recanalization was obtained in 95.7%, and favorite clinical outcome after 90 days in 30.4%. Between the two groups, there were no significant differences in the onset-to-treatment time, procedural time, combined technique as the first device pass, total number of passes, and successful recanalization. Large vessel reocclusion within 90 days occurred in 3 patients in the white thrombus group alone (1 day, 1 day, and 12 days, respectively), with no significant differences between the two groups. Favorable clinical outcome after 90 days was significantly lower in the white thrombus group than in the red thrombus group (8% vs. 55%, p = 0.027).
Table 3.
Treatment and clinical outcome.
| Total (23 patients) | White thrombus group (12 patients) | Red thrombus group (11 patients) | p-value | |
|---|---|---|---|---|
| Onset-to-treatment time, min | 209 [130–511] | 187 [113–359] | 209 [140–511] | 0.6 |
| Procedural time, min | 51 [35–70] | 58 [38–106] | 41 [31–60] | 0.16 |
| Combined technique as first device pass | 19 (82.6) | 11 (91.7) | 8 (72.7) | 0.32 |
| Total number of passes | 1 [1–3] | 2 [1–2.3] | 1 [1–3] | 0.53 |
| Successful recanalization | 22 (95.7) | 11 (91.7) | 11 (100.0) | 1 |
| Large vessel reocclusion within 90 days | 3 (13.0) | 3 (25.0) | 0 (0.0) | 0.22 |
| Modified rankin scale score 0–2 at 90 days | 7 (30.4) | 1 (8.3) | 6 (54.5) | 0.027* |
| Mortality at 90 days | 3 (13.0) | 2 (16.7) | 1 (9.1) | 1 |
Data are expressed in median [interquartile range] or number (percentage).
*p < 0.05.
Discussion
In this study, active cancer, no internal carotid artery occlusion, higher C-reactive protein levels, and higher D-dimer levels were shown to be factors related to white thrombi in acute ischemic stroke due to large vessel occlusion in cancer patients. Among laboratory findings, D-dimer levels were an independent factor associated with white thrombi, and its optimal cutoff value for predicting white thrombi was 3.5 μg/mL. Prognosis as part of clinical outcome was poorer in the white thrombus group than in the red thrombus group.
Acute ischemic stroke are possibly precipitated by hypercoagulability associated with cancer. In particular, cerebral large vessel occlusion is considered to be mainly due to vegetation caused by nonbacterial thrombotic endocarditis (NBTE).1,6 Pathological examinations of NBTE vegetation on cardiac valves show features of plate-rich thrombi.7,8 There are four possible causes of plate-rich thrombi as follows: (1) platelet adhesion to and accumulation in damaged endothelium due to high shear stress, (2) damage to endothelial cells and platelet adhesion due to elevated levels of circulating cytokines, (3) activation of platelet accumulation in high flow condition, (4) and platelet activation and thrombin production due to interaction between tumor cells and macrophages. 1
Inactive cancer is considered to have no clinical and radiological evidence of cancer activity for more than 5 years after completion of cancer treatment. 9 In clinical practice, we often encounter patients with cancer that cannot be classified as either active or inactive, and therefore established extensive inclusion criteria for cancer patients. Retrieved thrombi associated with active cancer tend to be rich in fibrin and platelets.1,2 In this study, white thrombi that were rich in fibrin and platelets were also associated with active cancer; however, since 36.3% of the red thrombus group had active cancer and 25.0% of the white thrombus group had atrial fibrillation, it was difficult to determine whether the embolic source was active cancer or atrial fibrillation. We therefore compared the two groups on the basis of pathological findings of retrieved thrombi.
D-dimer is a degradation product of fibrin thrombi and is a highly sensitive indicator of thrombus formation. 10 Ischemic stroke associated with active cancer has a significantly higher D-dimer level than that associated with inactive cancer.2,11 D-dimer levels are a highly sensitive marker of hypercoagulability associated with active cancer. 12 The optimal cutoff value of D-dimer levels to distinguish between cryptogenic stroke and conventional stroke in active cancer patients with acute ischemic stroke was 1.11 μg/mL (78.8% sensitivity and 71.8% specificity), 13 while that for determining whether cancer is the cause of ischemic stroke due to large vessel occlusion is unclear. In this study, the optimal cutoff value of D-dimer levels to predict white thrombi was 3.5 μg/mL, with 100% specificity. We determined ischemic stroke associated with cancer on the basis of pathological findings of retrieved thrombi. This method can be more accurate than that based on patient background, laboratory findings, and other embolic sources. The optimal cutoff value of D-dimer levels as a predictor of death after ischemic stroke associated with cancer has been reported to be 3.17 μg/mL and 3.95 μg/mL,14,15 to which the result of this study is close, indicating that it is appropriate as an index to predict acute ischemic stroke due to large vessel occlusion in cancer patients.
NBTE vegetations are generally small (<3 mm) and friable, which makes them difficult to detect on clinical imaging.6,16,17 Ischemic stroke due to NBTE vegetations has been reported to cause multifocal and multiple vascular territory infarctions.18,19 In this study, the white thrombus group had significantly less internal carotid artery occlusion and significantly more middle cerebral artery occlusion than the red thrombus group. Regarding the occlusion sites of large vessel occlusion associated with active cancer, Fu et al. did not examine them in detail, 2 and Park et al. and Jeon et al. reported that internal carotid artery occlusion accounted for 12.5% and 14.5%, respectively.1,4 In a report by the HERMES collaborators, internal carotid artery occlusion accounted for 21.5%. 20 Because NBTE vegetations are small, occlusion may be less likely to occur in the internal carotid arteries that have a large blood vessel diameter.
A study by Sgreccia et al. 21 on the macroscopic appearance of retrieved thrombi showed excellent agreement between two interventional neuroradiologists when the thrombi were visually classified as red/black or white thrombi. In their study, white thrombi were significantly associated with atypical etiologies such as cancer, infectious endocarditis, and heart valve thrombosis. Thus, the importance of the macroscopic appearance of retrieved thrombi is suggested, but it is difficult to determine the underlying cause, especially cancer or infectious endocarditis, from the macroscopic appearance alone. We therefore evaluated the retrieved thrombi by pathological findings and confirmed that they contained no bacterial components.
Matsumoto et al. 22 showed that two white thrombi associated with Trousseau’s syndrome contained a higher proportion of fibrin (>90%) than red thrombi from other stroke etiologies. Park et al. 1 showed that thrombi in active cancer patients with NBTE contained a large proportion of platelets (median 68.9% [55.2–78.7]) and a small proportion of erythrocyte fractions (median 1.6% [1.1–2.5]), and the median fibrin/platelet percentage in thrombi of the active cancer group was >70%. Fu et al. 2 showed that the optimal cutoff value of the fibrin/platelet percentage for distinguishing active cancer from other stroke etiologies was 65% (no sensitivity and specificity data available); however, the median fibrin/platelet percentage in cardioembolism cases in their study was 43.9% [31.0–65.3]. If the cutoff value of white thrombi in this study were set to 65%, the white thrombus group would have contained a certain number of cardioembolism cases. In the plot of the fibrin/platelet percentage in this study, the thrombus composition was distinctly different around the boundary of 70%–80% (Figure 2(a)). Gunning et al. 3 showed that fibrin-rich clots (red blood cell content <20%) have a significantly higher coefficient of friction against the arterial wall than clots with a red blood cell content >20%. Since we also considered the possibility of leading to indication and method of mechanical thrombectomy, we defined a white thrombus as a thrombus with a fibrin/platelet percentage of 80% or more, that is, a red blood cell percentage of <20% on the basis of its potential to lead to indications and procedure selection for mechanical thrombectomy.
The efficacy of mechanical thrombectomy depends on the thrombus composition. 23 Complete recanalization may be more difficult to achieve in cancer-related stroke patients who have more fibrin and platelet-rich white thrombi than in simple stroke patients. 24 Jeon et al. 4 showed that in the treatment of cancer-related stroke, contact aspiration alone or that in combination with a stent retriever as the first-line mechanical thrombectomy was more effective for achieving rapid and successful recanalization than using a stent retriever alone. In this study, there was no significant difference in successful recanalization between the white and red thrombus groups. The reason why both groups enjoyed high successful recanalization rates may be that we basically opt for thrombectomy using the combined technique as the first-line choice.
Both the hyperdense vessel sign on CT and the susceptibility vessel sign on T2* weighted imaging represent erythrocyte-dominant thrombi.25,26 On the other hand, the susceptibility vessel sign has been reported to be negative in all 19 cases of large vessel occlusion associated with active cancer. 24 MRI is often not performed before thrombectomy to shorten the time until recanalization. At our institution, we do not perform MRI before thrombectomy within 6 hours of onset. In that case, predicting white thrombi with laboratory findings on arrival is highly useful. At our institution, D-dimer levels can be reported in about 20 minutes from the start of blood testing. If white thrombi are predicted on the basis of D-dimer levels before thrombectomy, the procedure with contact aspiration or the combined technique may be useful for achieving early recanalization.
Favorable clinical outcome 90 days after thrombectomy has been reported to be not significantly different between cancer-related stroke and those with other etiologies.2,27 On the other hand, outcome after thrombectomy for cancer-related stroke has been reported to be relatively poor.24,28 Lee et al. 28 showed that elevated D-dimer levels and higher baseline NIHSS scores were independent predictors of unfavorable outcome. In this study, there was no significant difference in baseline NIHSS scores between the two groups, but D-dimer levels were significantly higher in the white thrombus group than in the red thrombus group. Hypercoagulability is thought to affect cancer prognosis by promoting cancer growth and metastasis. 29 In this study, large vessel reocclusion within 90 days after thrombectomy occurred in 25% of the white thrombus group alone although there was no significant difference between the two groups. There is a possibility of large vessel reocclusion relatively early after thrombectomy due to cancer-related stroke.30–32 Large vessel reocclusion may contribute to poor outcome after thrombectomy due to cancer-related stroke.
There are three major limitations in this study. First, it has a single-center retrospective design, and its sample size was relatively small. This is partly because quite a few of cancer patients met the exclusion criteria (18/41 patients [43.9%]). Prospective accumulation of retrieved thrombi is needed to enable larger scale studies. Second, although 7 patients enjoyed success in recanalization, no definite thrombi were retrieved from them. This may have been associated with the nature of white thrombi, which is prone to distal emboli. In 3 patients whose recanalization was unsuccessful and from whom no thrombi were retrieved, in whom retrieval of white thrombi may have been more difficult. In 6 patients who had successful recanalization, thrombi were retrieved but not submitted to pathologic analysis. This may have been due to the small size of their specimens and the fact that they underwent emergency treatment. Finally, the fibrin/platelet percentage was evaluated on the basis of hematoxylin and eosin staining alone. Evaluation of the percentages of fibrin and platelets separately using immunohistological staining is needed in future studies.
Conclusion
In acute ischemic stroke due to large vessel occlusion in cancer patients, active cancer, no internal carotid artery occlusion, and higher D-dimer levels may be associated with occlusion with fibrin and platelet-rich white thrombi. If a white thrombus is predicted from a D-dimer level of >3.5 μg/mL before thrombectomy, a procedure with contact aspiration or the combined technique may be useful for early recanalization.
Acknowledgements
We would like to thank the following doctors for the time they devoted to this investigation: Mai Tanimura, Yasunori Yokochi, Genki Kimura, Natsuki Akaike, Makoto Wada, Takuya Osuki, and Toshio Fujiwara. The authors would like to thank Ms Miho Kobayashi (Kurashiki Central Hospital) for the English language review.
Appendix.
Abbreviation
- ACA
Anterior cerebral artery
- APTT
Activated partial thromboplastin time
- BA
Basilar artery
- CI
Confidence interval
- CT
Computed tomography
- ICA
Internal carotid artery
- INR
International normalized ratio
- MCA
Middle cerebral artery
- mTICI
Modified thrombolysis in cerebral infarction
- NBTE
Nonbacterial thrombotic endocarditis
- NIHSS
National institute of health stroke scale
- MRI
Magnetic resonance imaging
- OR
Odds ratio
- PT
Prothrombin time
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This study was approved by our institutional review board.
Data availability: Data are available upon reasonable request; please contact rocky@kuhp.kyoto-u.ac.jp.
ORCID iDs
Hiroyuki Ikeda https://orcid.org/0000-0001-5710-7456
Ryota Ishibashi https://orcid.org/0000-0002-1646-9044
Ryosuke Kaneko https://orcid.org/0000-0002-6864-5628
Yoshitaka Kurosaki https://orcid.org/0000-0003-0738-760X
References
- 1.Park H, Kim J, Ha J, et al. Histological features of intracranial thrombi in stroke patients with cancer. Ann Neurol 2019; 86: 143–149. [DOI] [PubMed] [Google Scholar]
- 2.Fu CH, Chen CH, Lin YH, et al. Fibrin and platelet-rich composition in retrieved thrombi hallmarks stroke with active cancer. Stroke 2020; 51: 3723–3727. [DOI] [PubMed] [Google Scholar]
- 3.Gunning GM, McArdle K, Mirza M, et al. Clot friction variation with fibrin content; implications for resistance to thrombectomy. J Neurointerv Surg 2018; 10: 34–38. [DOI] [PubMed] [Google Scholar]
- 4.Jeon Y, Baik SH, Jung C, et al. Mechanical thrombectomy in patients with acute cancer-related stroke: Is the stent retriever alone effective? J Neurointerv Surg 2021; 13: 318–323. [DOI] [PubMed] [Google Scholar]
- 5.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003; 349: 146–153. [DOI] [PubMed] [Google Scholar]
- 6.Lopez JA, Ross RS, Fishbein MC, et al. Nonbacterial thrombotic endocarditis: a review. Am Heart J 1987; 113: 773–784. [DOI] [PubMed] [Google Scholar]
- 7.Biller J, Challa VR, Toole JF, et al. Nonbacterial thrombotic endocarditis: a neurologic perspective of clinicopathologic correlations of 99 patients. Arch Neurol 1982; 39: 95–98. [DOI] [PubMed] [Google Scholar]
- 8.Rogers LR, Cho ES, Kempin S, et al. Cerebral infarction from non-bacterial thrombotic endocarditis: clinical and pathological study including the effects of anticoagulation. Am J Med 1987; 83: 746–756. [DOI] [PubMed] [Google Scholar]
- 9.Lee D, Lee DH, Suh DC, et al. Intra-arterial thrombectomy for acute ischaemic stroke patients with active cancer. J Neurol 2019; 266: 2286–2293. [DOI] [PubMed] [Google Scholar]
- 10.Tripodi A. D-dimer testing in laboratory practice. Clin Chem 2011; 57: 1256–1262. [DOI] [PubMed] [Google Scholar]
- 11.Schwarzbach CJ, Schaefer A, Ebert A, et al. Stroke and cancer: The importance of cancer-associated hypercoagulation as a possible stroke etiology. Stroke 2012; 43: 3029–3034. [DOI] [PubMed] [Google Scholar]
- 12.Kim K, Lee JH. Risk factors and biomarkers of ischemic stroke in cancer patients. J Stroke 2014; 16: 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SG, Hong JM, Kim HY, et al. Ischemic stroke in cancer patients with and without conventional mechanisms: a multicenter study in Korea. Stroke 2010; 41: 798–801. [DOI] [PubMed] [Google Scholar]
- 14.Lee MJ, Chung JW, Ahn MJ, et al. Hypercoagulability and mortality of patients with stroke and active cancer: the oasis-cancer study. J Stroke 2017; 19: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuchihashi Y, Shimizu T, Akiyama H, et al. The risk factors for death within 6 months after ischemic stroke in patients with cancer. J Stroke Cerebrovas Dis 2020; 29: 105365. [DOI] [PubMed] [Google Scholar]
- 16.Dutta T, Karas MG, Segal AZ, et al. Yield of transesophageal echocardiography for nonbacterial thrombotic endocarditis and other cardiac sources of embolism in cancer patients with cerebral ischemia. Am J Cardiol 2006; 97: 894–898. [DOI] [PubMed] [Google Scholar]
- 17.Asopa S, Patel A, Khan OA, et al. Non-bacterial thrombotic endocarditis. Eur J Cardiothorac Surg 2007; 32: 696–701. [DOI] [PubMed] [Google Scholar]
- 18.Chen W, He Y, Su Y. Multifocal cerebral infarction as the first manifestation of occult malignancy: case series of Trousseau’s syndrome and literature review. Brain Circ 2018; 4: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nouh AM, Staff I, Finelli PF. Three territory sign: an MRI marker of malignancy-related ischemic stroke (Trousseau syndrome). Neurol Clin Pract 2019; 9: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 21.Sgreccia A, Duchmann Z, Desilles JP, et al. Association between acute ischemic stroke etiology and macroscopic aspect of retrieved clots: is a clot’s color a warning light for underlying pathologies? J Neurointerv Surg 2019; 11: 1197–1200. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto N, Fukuda H, Handa A, et al. Histological examination of Trousseau syndrome-related thrombus retrieved through acute endovascular thrombectomy: report of 2 cases. J Stroke Cerebrovas Dis 2016; 25: e227–e230. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto T, Hayakawa M, Funatsu N, et al. Histopathologic analysis of retrieved thrombi associated with successful reperfusion after acute stroke thrombectomy. Stroke 2016; 47: 3035–3037. [DOI] [PubMed] [Google Scholar]
- 24.Jung S, Jung C, Hyoung Kim J, et al. Procedural and clinical outcomes of endovascular recanalization therapy in patients with cancer-related stroke. Interv Neuroradiol 2018; 24: 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liebeskind DS, Sanossian N, Yong WH, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke 2011; 42: 1237–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinjikji W, Duffy S, Burrows A, et al. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: a systematic review. J Neurointerv Surg 2017; 9: 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sallustio F, Mascolo AP, Marrama F, et al. Safety and efficacy of reperfusion therapies for acute ischemic stroke patients with active malignancy. J Stroke Cerebrovas Dis 2019; 28: 2287–2291. [DOI] [PubMed] [Google Scholar]
- 28.Lee EJ, Bae J, Jeong HB, et al. Effectiveness of mechanical thrombectomy in cancer-related stroke and associated factors with unfavorable outcome. BMC Neurol 2021; 21: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nierodzik M, Karpatkin S. Hypercoagulability preceding cancer: does hypercoagulability awaken dormant tumor cells in the host? J Thromb Haemost 2005; 3: 577–580. [DOI] [PubMed] [Google Scholar]
- 30.Oki S, Kawabori M, Echizenya S, et al. Long-term clinical outcome and prognosis after thrombectomy in patients with concomitant malignancy. Front Neurol 2020; 11: 572589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cicilioni K, Cristiano B, Jacobson JP, et al. Multiple thrombectomies in the same patient within one month: case report of a patient with Trousseau syndrome and acute ischemic stroke. Brain Sci 2020; 10: 590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoue S, Fujita A, Mizowaki T, et al. Successful treatment of repeated bilateral middle cerebral artery occlusion by performing mechanical thrombectomy in a patient with Trousseau syndrome. No Shinkei Geka 2016; 44: 501–506. [In Japanese]. [DOI] [PubMed] [Google Scholar]