Abstract
In pregnant people colonized with group B Streptococcus (GBS) in Botswana, we report the presence/expansion of sequence types 223 and 109, a low rate of erythromycin resistance, and 3 novel sequence types. These data highlight the importance of local epidemiologic studies of GBS, a significant source of neonatal disease.
Group B Streptococcus (GBS) is a major cause of neonatal sepsis and meningitis and a leading cause of death in infants globally [1, 2]. Rectovaginal colonization in late pregnancy can lead to transmission to newborns and is the major risk factor for early-onset GBS disease. Sub-Saharan African countries carry much of the burden of invasive GBS disease [1]. In the United States and some other high-income countries, pregnant people are routinely screened for rectovaginal colonization in late gestation, and targeted intrapartum antibiotic prophylaxis (IAP) is highly effective for prevention of early-onset GBS disease [3]. However, IAP has no significant effect in preventing late-onset disease and has not been implemented widely in low- and middle-income countries. Furthermore, rates of GBS resistance to second-line drugs such as erythromycin and clindamycin have increased, and strains with reduced β-lactam susceptibility (RBLS), though rare, have become more common [4, 5].
Vaccines targeting GBS capsular polysaccharide are in development and offer a promising alternative to IAP. However, there are 10 known GBS serotypes (Ia, Ib, and II–IX), and current protein–capsular polysaccharide conjugate vaccine candidates cover only a subset of these [6]. In addition, immunogenicity of multivalent GBS vaccines may vary by serotype [7]. There is significant geographic variability in GBS serotype distribution, strain backgrounds as determined by multilocus sequence typing (MLST), clonal complex (CC) assignment, and antibiotic resistance [1, 8]. Therefore, local epidemiologic studies are important for understanding the potential utility of candidate vaccines. Our group previously reported GBS rectovaginal colonization rate and serotype distribution in a cohort of pregnant people in Botswana, demonstrating local predominance of serotype V (>45% of isolates compared with approximately 20% worldwide) [9, 10]. Here, we further characterize GBS isolates from that cohort by whole-genome sequencing (WGS) to determine MLST/CC, presence of specific bacterial virulence determinants, and antimicrobial susceptibility.
METHODS
Streptococcus agalactiae isolates were grown on selective agar (CHROMagar StrepB) at 37°C for 18–24 hours. Purple colonies, indicating GBS growth, were used to inoculate overnight cultures in 5 ml of tryptic soy broth. Bacteria were pelleted, resuspended in 300 mL of Tissue & Cell Lysis Solution (LGC Biosearch), and underwent bead beating with 0.1-mm zirconia/silica beads for 6 minutes. Genomic DNA was extracted using the MagMAX Viral/Pathogen Ultra Nucleic Acid Isolation Kit (Applied Biosystems) on a KingFisher Flex platform. Library preparation and WGS were performed on the Illumina NovaSeq 6000 platform (paired end; 150–base pair reads). Trimmomatic 0.36 software was used for adapter sequence removal and quality trimming, with a minimum read length of 120 base pairs.
We used SRST2 0.2.0 software for read mapping to assign genomic serotype using the GBS-SBG database [11], MLST/CC using S agalactiae allele sequences and MLST definition files from PubMLST [12], and antimicrobial resistance and protein profiles using databases from Metcalf et al [13]. We detected the presence of AlpST-1, a more distantly related member of the Alp protein family, by read-mapping to the AlpST-1 open reading frame from GBS SS1 [14]. Phenotypic determination of antimicrobial susceptibility was not performed. We used Mashtree 1.2.0 software to calculate a kmer distance tree and visualized it on the Microreact web server [15, 16]. Raw sequencing reads are available in the National Center for Biotechnology Information Sequence Read Archive (under BioProject identifier PRJNA986888). Isolate data are in the PubMLST database (PubMLST.org; accession nos. 25868–25908). This study of deidentified samples did not include factors necessitating patient consent.
RESULTS
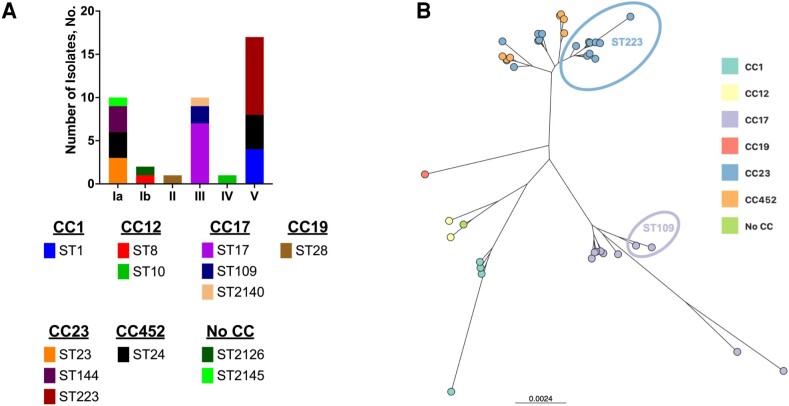
Of 53 GBS-positive samples from the original study, we were able to recover and generate high-quality genome sequences from 41 (77.4%). Serotype and MLST/CC data are presented in Figure 1A. Predicted serotypes were consistent with prior polymerase chain reaction–based serotyping [9]. We noted that sequence type [ST] 223 was the predominant ST [9 of 41 [22.0%]], followed by ST24 [7 of 41 [17.1%]] and ST17 [7 of 41 [17.1%]). A tree based on genome-wide genetic distances demonstrates a high degree of relatedness of the ST223 strains from this study (Figure 1B). The most common CCs were CC23 (15 of 41 [36.6%]), CC17 (10 of 41 [24.4%]), and CC452 (7 of 41 [17.1%]). A minority of isolates (3 of 41 [7.3%]) had MLST profiles not previously described. These were deposited in the PubMLST database and assigned STs 2126 (strain AR1534), 2140 (strain AR1537), and 2145 (strain AR1563). Complete isolate-level data appear in Table 1.
Figure 1.
A, Serotype, sequence type (ST), and clonal complex (CC) of group B Streptococcus (GBS) isolates from pregnant people in Botswana. B, GBS whole-genome similarity was assessed using Mashtree 1.2.0 and visualized using the Microreact web server. Nodes are shaded by CC. Branch lengths and scale bar represent Mash distances. Specific ST groupings discussed in the text (ST109 and ST223) are indicated.
Table 1.
Sequence Types, Clonal Complexes, Serotypes, Antibiotic Susceptibility Markers, and Surface Protein Genes of Group B Streptococcus Isolates as Determined by Whole-Genome Sequencing
| Strain | ST | CC | Serotype | MLS Resistance | Tetracycline Resistance | PBP2x Mutations | Alp | Pilus | Srr1/2 | HvgA |
|---|---|---|---|---|---|---|---|---|---|---|
| AR1515 | 24 | CC452 | V | … | tetM | T175A; I377V; V510I | Alpha | PI-2a | Srr1 | … |
| AR1516 | 17 | CC17 | III | … | tetM | I377V; G627V | Rib | PI-1; PI-2b | Srr2 | HvgA |
| AR1517 | 144 | CC23 | Ia | … | tetM | I377V; V510I | Rib | PI-1; PI-2a | Srr1 | - |
| AR1518 | 109 | CC17 | III | … | tetM | I377V; G398A; G627V | Rib | PI-1; PI-2b | Srr2 | HvgA |
| AR1519 | 223 | CC23 | V | … | tetM | I377V; G627V | Rib | PI-2a | Srr1 | … |
| AR1521 | 1 | CC1 | V | ermTR | … | … | Alp2/3; AlpST-1 | PI-1; PI-2a | Srr1 | … |
| AR1522 | 223 | CC23 | V | … | tetM | I377V; G627V | Rib | PI-2a | Srr1 | … |
| AR1523 | 223 | CC23 | V | … | tetM | I377V; G627V | Rib | PI-2a | Srr1 | … |
| AR1525 | 223 | CC23 | V | … | tetM | I377V; G627V | Rib | PI-2a | Srr1 | … |
| AR1526 | 223 | CC23 | V | … | tetM | I377V; G627V | Rib | PI-2a | Srr1 | … |
| AR1527 | 17 | CC17 | III | … | tetM | I377V; G627V | Rib | PI-1; PI-2b | Srr2 | HvgA |
| AR1528 | 28 | CC19 | II | … | tetM | … | Rib | PI-1; PI-2a | … | … |
| AR1529 | 223 | CC23 | V | … | tetM | I377V; G627V | Rib | PI-2a | Srr1 | … |
| AR1531 | 10 | CC12 | IV | … | tetM | … | Alpha | PI-1; PI-2b | Srr2 | … |
| AR1534 | 2126 | No CC | Ib | … | tetM | … | Alpha | PI-1; PI-2a | Srr1 | … |
| AR1535 | 8 | CC12 | Ib | … | tetM | … | Alpha | PI-1; PI-2a | Srr1 | … |
| AR1537 | 2140 | CC17 | III | … | tetM | I377V; G627V | Rib | PI-1; PI-2b | Srr2 | HvgA |
| AR1538 | 24 | CC452 | V | … | tetM | V510I | Alpha | PI-2a | Srr1 | … |
| AR1539 | 1 | CC1 | V | ermTR | tetM | … | Alp2/3; AlpST-1 | PI-1; PI-2a | Srr1 | … |
| AR1540 | 24 | CC452 | V | … | tetM | I377V; V510I | Alpha | PI-2a | Srr1 | … |
| AR1541 | 24 | CC452 | V | … | tetM | I377V; V510I | Alpha | PI-2a | Srr1 | … |
| AR1542 | 223 | CC23 | V | … | tetM | I377V; G627V | Rib | PI-2a | Srr1 | … |
| AR1543 | 24 | CC452 | Ia | … | tetM | I377V; V510I | Alpha | PI-2a | Srr1 | … |
| AR1544 | 17 | CC17 | III | … | tetM | I377V; G627V | Rib | PI-1; PI-2b | Srr2 | HvgA |
| AR1545 | 144 | CC23 | Ia | … | tetM | I377V; V510I | Rib | PI-2a | Srr1 | … |
| AR1553 | 24 | CC452 | Ia | … | tetM | I377V; V510I | Alpha | PI-2a | Srr1 | … |
| AR1555 | 223 | CC23 | V | … | … | I377V; G627V | Rib | PI-2a | Srr1 | … |
| AR1558 | 23 | CC23 | Ia | … | tetM | I377V; V510I | Alp1 | PI-2a | Srr1 | … |
| AR1560 | 17 | CC17 | III | … | tetM | I377V; G627V | Rib | PI-1; PI-2b | Srr2 | HvgA |
| AR1561 | 24 | CC452 | Ia | … | tetM | I377V; V510I | Alpha | PI-2a | Srr1 | … |
| AR1563 | 2145 | No CC | Ia | … | tetM | I377V; V510I | Alpha | PI-2a | Srr1 | … |
| AR1723 | 23 | CC23 | Ia | … | tetM | I377V; V510I | Alp1 | PI-2a | Srr1 | … |
| AR1724 | 17 | CC17 | III | … | tetM | I377V; G627V | Rib | PI-1; PI-2b | Srr2 | HvgA |
| AR1753 | 223 | CC23 | V | … | tetM | I377V; G627V | Rib | PI-2a | Srr1 | … |
| AR1754 | 17 | CC17 | III | … | tetM | I377V; G627V | Rib | PI-1; PI-2b | Srr2 | HvgA |
| AR1755 | 1 | CC1 | V | ermTR | tetM | … | Alp2/3; AlpST-1 | PI-1; PI-2a | Srr1 | … |
| AR1765 | 23 | CC23 | Ia | … | tetM | I377V; V510I | Alp1 | PI-2a | Srr1 | … |
| AR1771 | 144 | CC23 | Ia | … | tetM | I377V; V510I | Rib | PI-2a | Srr1 | … |
| AR2184 | 17 | CC17 | III | … | tetM | I377V; G627V | Rib | PI-1; PI-2b | Srr2 | HvgA |
| AR2189 | 1 | CC1 | V | ermTR | tetM | … | Alp2/3; AlpST-1 | PI-1; PI-2a | Srr1 | - |
| AR2287 | 109 | CC17 | III | … | tetM | I377V; G398A; G627V | Rib | PI-1; PI-2b | Srr2 | HvgA |
Abbreviations: Alp, Alpha-like protein; CC, clonal complex; HvgA, hypervirulent group B Streptococcus adhesin; MLS, macrolide-lincosamide-streptogramin; PBP, penicillin-binding protein; Srr, serine-rich repeat protein.; ST, sequence type.
Among the serotype III GBS isolates, 10 of 10 (100%) were members of the hypervirulent CC17 group. Most of these (7 of 10 [70%]) were ST17, but 2 were ST109, a GBS type previously described as a cause of invasive disease among children in Mozambique and Angola [17, 18]. ST109 has been reported to have an RBLS phenotype owing to the presence of a G398A mutation in penicillin-binding protein 2x [18]. Like the ST109 strains from the Mozambique study, both ST109 strains described here were found to have the G398A mutation. No other strains from the current study had G398A or other penicillin-binding protein 2x mutations known to be associated with a RBLS phenotype.
Nearly all Botswana GBS isolates (39/41 [95.1%]) harbored a tetM gene tetracycline resistance allele. Four isolates (4 of 41[9.8%]), all of which were serotype V/ST1, contained an ermTR gene that confers resistance to macrolides, lincosamides, and streptogramin B (MLS). No other MLS resistance determinants were identified, and no mutations in the 23S ribosomal subunit or the rpo genes were found. No changes in predicted quinolone resistance determinants were predicted by the analysis pipeline.
All strains contained ≥1 gene encoding a member of the alpha-like family of proteins (Rib, Alp1, Alp2/3, AlpST-1, or Alpha). AlpST-1 was detected in the 4 serotype V/ST1 strains that also encoded ermTR and tetM [14]. That combination of genotypic features is consistent with the ST1 subclade 2, previously reported by Cubria et al [19]. Consistent with prior data, CC17 strain genomes all had genes encoding Rib, pilus type 1, pilus type 2b, serine-rich repeat protein Srr2, and hypervirulent group B Streptococcus adhesin (HvgA). Non-CC17 strains were predicted to express Srr1, with 2 exceptions: strain AR1528 (II/CC19), which was not predicted to express either Srr1 or Srr2, and strain AR1531 (Ib/CC12), predicted to express Srr2.
DISCUSSION
Africa has the highest estimated burden of invasive neonatal GBS cases, but epidemiologic data on circulating strains are still limited [1, 20]. GBS capsular serotype and MLST distributions differ among countries, with implications for potential efficacy of candidate vaccines. Here, we used bacterial WGS to determine serotypes, MLST, and the presence of genes encoding potential virulence factors and antimicrobial resistance genes in GBS strains from a previously described cohort of pregnant people in Botswana [9]. We noted an abundance (>20%) of serotype V, ST223 GBS strains. These findings differ from those of a prior study in coastal Kenya, in which <1% of GBS isolates from pregnant people were ST223 [21]. Similarly, in a GBS pangenome-wide association study, 6% of Malawian isolates were identified as ST223 [22]. In a sample of >6000 invasive GBS isolates from the United States, only 3 (<0.1%) were ST223 [23]. Notably, all of those US-based ST223 isolates were serotype Ia [23]. Local expansion of specific GBS types has been noted, including emergence of ST283 as a cause of invasive infections in humans and fish in southeast Asia and of ST459 in North America [24,25–26].Of the 6 major GBS CCs, CC17 is highly associated with sepsis and bacterial meningitis in infants in the first 90 days of life. Within CC17, ST109 strains are not commonly found but are of potential concern, given their association with both invasive disease and RBLS [17, 18]. Two ST109 isolates were identified in the current study, representing approximately 5% of GBS strains and raising the possibility that this GBS type may cause neonatal disease in Botswana and could be more widespread in the region than previously appreciated. We found that a low percentage of colonizing isolates (<10%) were predicted to be resistant to erythromycin, a rate lower than that reported in a recent meta-analysis from African nations (25.1%) [27]. We speculate that this finding may reflect a low rate of exposure to this class of antibiotics among the sampled population. In the United States, more than half of GBS strains are resistant to erythromycin [13, 23]. Fluoroquinolone resistance, which was not detected in this cohort, is rare in the United States (<2% of strains) [23]. The high rate of tetracycline resistance noted is consistent with findings that most human colonizing and invasive GBS isolates derive from tetracycline-resistant clones [28].
Taken together, these data carry messages of both hope and caution. All of the rectovaginal colonization isolates from this cohort of pregnant people in Botswana have capsular serotypes that are contained in the hexavalent vaccine candidate currently in clinical trials [7, 29]. In addition, rates of GBS resistance to erythromycin appear to be low in Botswana. However, local proliferation of the CC17/ST223 strain and detection of 2 ST109 isolates in this cohort underscore the need for expanded molecular epidemiologic studies of GBS in this region and for assessment of the role of emerging local strains in neonatal disease.
Acknowledgments
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding bodies.
Financial support. This work was supported by the Neonatology Division (B. T.) and the Melissa Ketunuti Basselier Endowment (B. T. and A. P. S.) of the Children's Hospital of Philadelphia, which also provided support for open access fees; the University of Pennsylvania Center for AIDS Research, funded by the National Institutes of Health (grant P30 AI045008); the Pediatric Infectious Diseases Society SUMMERS program (support to A. C. B.); and the Medical Student Summer Research Fellowship program at the Department of Pediatrics, New York University Grossman School of Medicine (support to A. T.).
Contributor Information
Karen L Hanze Villavicencio, Division of Infectious Diseases, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, USA; Department of Pediatrics, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA.
Megan J Job, Department of Pediatrics, NewYork University Grossman School of Medicine, New York, New York, USA.
Anne Claire Burghard, Department of Pediatrics, NewYork University Grossman School of Medicine, New York, New York, USA; Renaissance School of Medicine, Stony Brook University, Stony Brook, NewYork, USA.
Allison Taffet, Department of Pediatrics, NewYork University Grossman School of Medicine, New York, New York, USA.
Francis M Banda, Department of Pediatrics & Adolescent Health, Faculty of Medicine, University of Botswana, Gaborone, Botswana.
Moses Vurayai, School of Allied Health Professionals, Faculty of Health Sciences, University of Botswana, Gaborone, Botswana.
Margaret Mokomane, School of Allied Health Professionals, Faculty of Health Sciences, University of Botswana, Gaborone, Botswana.
Tonya Arscott-Mills, Department of Pediatrics & Adolescent Health, Faculty of Medicine, University of Botswana, Gaborone, Botswana; Botswana-UPenn Partnership, Faculty of Health Sciences, University of Botswana, Gaborone, Botswana; Global Health Center, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, USA.
Tiny Mazhani, Department of Pediatrics & Adolescent Health, Faculty of Medicine, University of Botswana, Gaborone, Botswana.
Seeletso Nchingane, Department of Pediatrics, Princess Marina Hospital, Gaborone, Botswana.
Brady Thomas, Department of Pediatrics, Stead Family Children's Hospital, University of Iowa, Iowa City, Iowa, USA.
Andrew P Steenhoff, Division of Infectious Diseases, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, USA; Department of Pediatrics, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA; Department of Pediatrics & Adolescent Health, Faculty of Medicine, University of Botswana, Gaborone, Botswana; Botswana-UPenn Partnership, Faculty of Health Sciences, University of Botswana, Gaborone, Botswana; Global Health Center, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, USA.
Adam J Ratner, Department of Pediatrics, NewYork University Grossman School of Medicine, New York, New York, USA; Department of Microbiology, NewYork University Grossman School of Medicine, New York, New York, USA.
References
- 1. Goncalves BP, Procter SR, Paul P, et al. . Group B Streptococcus infection during pregnancy and infancy: estimates of regional and global burden. Lancet Glob Health 2022; 10:e807–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Madrid L, Seale AC, Kohli-Lynch M, et al. . Infant group B streptococcal disease incidence and serotypes worldwide: systematic review and meta-analyses. Clin Infect Dis 2017; 65:S160–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Puopolo KM, Lynfield R, Cummings JJ; Committee on Fetus and Newborn; Committee on Infectious Diseases . Management of infants at risk for group B streptococcal disease. Pediatrics 2019; 144:e20191881. [DOI] [PubMed] [Google Scholar]
- 4. Hayes K, O'Halloran F, Cotter L. A review of antibiotic resistance in group B Streptococcus: the story so far. Crit Rev Microbiol 2020; 46:253–69. [DOI] [PubMed] [Google Scholar]
- 5. Kobayashi M, McGee L, Chochua S, et al. . Low but increasing prevalence of reduced beta-lactam susceptibility among invasive group B streptococcal isolates, US population-based surveillance, 1998–2018. Open Forum Infect Dis 2021; 8:ofaa634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Absalon J, Simon R, Radley D, et al. . Advances towards licensure of a maternal vaccine for the prevention of invasive group B Streptococcus disease in infants: a discussion of different approaches. Hum Vaccin Immunother 2022; 18:2037350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Madhi SA, Anderson AS, Absalon J, et al. . Potential for maternally administered vaccine for infant group B Streptococcus. N Engl J Med 2023; 389:215–27. [DOI] [PubMed] [Google Scholar]
- 8. Paul P, Gonçalves BP, Le Doare K, Lawn JE. 20 Million pregnant women with group B Streptococcus carriage: consequences, challenges, and opportunities for prevention. Curr Opin Pediatr 2023; 35:223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. A'Hearn-Thomas B, Khatami A, Randis TM, et al. . High rate of serotype V Streptococcus agalactiae carriage in pregnant women in Botswana. Am J Trop Med Hyg 2019; 100:1115–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Russell NJ, Seale AC, O'Driscoll M, et al. . Maternal colonization with group B Streptococcus and serotype distribution worldwide: systematic review and meta-analyses. Clin Infect Dis 2017; 65:S100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tiruvayipati S, Tang WY, Barkham TMS, Chen SL. GBS-SBG—GBS serotyping by genome sequencing. Microb Genom 2021; 7:000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jolley KA, Bray JE, Maiden MCJ. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res 2018; 3:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Metcalf BJ, Chochua S, Gertz RE Jr, et al. . Short-read whole genome sequencing for determination of antimicrobial resistance mechanisms and capsular serotypes of current invasive Streptococcus agalactiae recovered in the USA. Clin Microbiol Infect 2017; 23:574.e7–e14. [DOI] [PubMed] [Google Scholar]
- 14. Flores AR, Galloway-Pena J, Sahasrabhojane P, et al. . Sequence type 1 group B Streptococcus, an emerging cause of invasive disease in adults, evolves by small genetic changes. Proc Natl Acad Sci U S A 2015; 112:6431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Argimon S, Abudahab K, Goater RJE, et al. . Microreact: visualizing and sharing data for genomic epidemiology and phylogeography. Microb Genom 2016; 2:e000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katz LS, Griswold T, Morrison SS, et al. . Mashtree: a rapid comparison of whole genome sequence files. J Open Source Softw 2019; 4:10.21105/joss.01762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Florindo C, Gomes JP, Rato MG, et al. . Molecular epidemiology of group B streptococcal meningitis in children beyond the neonatal period from Angola. J Med Microbiol 2011; 60:1276–80. [DOI] [PubMed] [Google Scholar]
- 18. Sigauque B, Kobayashi M, Vubil D, et al. . Invasive bacterial disease trends and characterization of group B streptococcal isolates among young infants in southern Mozambique, 2001–2015. PLoS One 2018; 13:e0191193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cubria MB, Vega LA, Shropshire WC, et al. . Population genomics reveals distinct temporal association with the emergence of ST1 serotype V group B Streptococcus and macrolide resistance in North America. Antimicrob Agents Chemother 2022; 66:e0071421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seale AC, Bianchi-Jassir F, Russell NJ, et al. . Estimates of the burden of group B streptococcal disease worldwide for pregnant women, stillbirths, and children. Clin Infect Dis 2017; 65:S200–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seale AC, Koech AC, Sheppard AE, et al. . Maternal colonization with Streptococcus agalactiae and associated stillbirth and neonatal disease in coastal Kenya. Nat Microbiol 2016; 1:16067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gori A, Harrison OB, Mlia E, et al. . Pan-GWAS of Streptococcus agalactiae highlights lineage-specific genes associated with virulence and niche adaptation. mBio 2020; 11:e00728-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McGee L, Chochua S, Li Z, et al. . Multistate, population-based distributions of candidate vaccine targets, clonal complexes, and resistance features of invasive group B streptococci within the United States, 2015–2017. Clin Infect Dis 2021; 72:1004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barkham T, Zadoks RN, Azmai MNA, et al. . One hypervirulent clone, sequence type 283, accounts for a large proportion of invasive Streptococcus agalactiae isolated from humans and diseased tilapia in Southeast Asia. PLoS Negl Trop Dis 2019; 13:e0007421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diedrick MJ, Flores AE, Hillier SL, Creti R, Ferrieri P. Clonal analysis of colonizing group B Streptococcus, serotype IV, an emerging pathogen in the United States. J Clin Microbiol 2010; 48:3100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Teatero S, McGeer A, Li A, et al. . Population structure and antimicrobial resistance of invasive serotype IV group B Streptococcus, Toronto, Ontario, Canada. Emerg Infect Dis 2015; 21:585–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wadilo F, Hailemeskel E, Kedir K, et al. . Prevalence of group B Streptococcus maternal colonization, serotype distribution, and antimicrobial resistance in Sub-Saharan Africa: a systematic review and meta-analysis. J Glob Antimicrob Resist 2023; 32:134–44. [DOI] [PubMed] [Google Scholar]
- 28. Da Cunha V, Davies MR, Douarre PE, et al. . Streptococcus agalactiae clones infecting humans were selected and fixed through the extensive use of tetracycline. Nat Commun 2014; 5:4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buurman ET, Timofeyeva Y, Gu J, et al. . A novel hexavalent capsular polysaccharide conjugate vaccine (GBS6) for the prevention of neonatal group B streptococcal infections by maternal immunization. J Infect Dis 2019; 220:105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]