Abstract
Objective.
To assess whether circulating levels of adiponectin, leptin, and fibroblast growth factor 21 (FGF-21) are associated with incident cardiovascular disease (CVD) in rheumatoid arthritis (RA).
Methods.
Adipokines were measured using banked enrollment serum from patients with RA and dichotomized above/below the median value. Incident CVD events (coronary artery disease [CAD], stroke, heart failure [HF] hospitalization, venous thromboembolism, CVD-related deaths) were identified using administrative data and the National Death Index. Covariates were derived from medical record, biorepository, and registry databases. Multivariable Cox models were generated to quantify associations between adipokine concentrations and CVD incidence. Five-year incidence rates were predicted.
Results.
Among 2,598 participants, 639 (25%) had at least 1 CVD event over 19,585 patient-years of follow-up. High adiponectin levels were independently associated with HF hospitalization (hazard ratio [HR] 1.39 [95% confidence interval (95% CI) 1.07–1.79], P = 0.01) and CVD-related death (HR 1.49 [95% CI 1.16–1.92], P = 0.002) but not with other CVD events. High leptin was independently associated with CVD-related death (HR 1.44 [95% CI 1.05–1.97], P = 0.02). High FGF-21 levels were independently associated with lower rates of CAD (HR 0.75 [95% CI 0.58–0.97], P = 0.03). In subgroup analyses, associations between high adiponectin and leptin levels with CVD-related death were driven by strong associations in nonobese patients.
Conclusion.
Adipokines are associated with HF hospitalization and CVD-related death in patients with RA, with stronger associations in nonobese participants. These findings suggest that adipokines effectively predict clinically important outcomes in RA perhaps through an association with body composition and metabolic health. Further study is needed to determine whether adipokine measures might augment existing tools to identify RA patients at increased risk of CVD.
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of mortality in rheumatoid arthritis (RA) (1,2), but current CVD risk prediction tools do not perform well in RA (3–5). For example, although obesity is associated with increased CVD risk in the general population, obesity appears to be paradoxically protective against cardiovascular mortality in RA (6). Poor outcome prediction using traditional risk factors may stem from disease-related changes in energy homeostasis and metabolism, resulting in pathologic weight loss and altered lipid profiles while also adversely affecting long-term risks. Weight loss and weight fluctuation have been linked to early mortality and cardiovascular events in RA (7–9). Improving CVD risk prediction using biomarkers associated with RA-related metabolic disturbances would be valuable.
Adipokines are protein hormones secreted by fat and muscle cells that function to regulate metabolism, appetite, and energy homeostasis. For example, adiponectin has been termed the “starvation signal” for its role in promoting energy metabolism in fasting conditions (10). Because of the relationship between metabolic health and long-term RA outcomes, adipokines have emerged as potential risk indicators (11,12). Adiponectin levels are generally higher in constitutionally thin individuals and increase with weight loss in obese patients (13,14). Adiponectin levels are associated with cachexia in chronic heart failure (HF) (15), and high adiponectin and low leptin are associated with protein–energy wasting in end-stage renal disease (16). Fibroblast growth factor 21 (FGF-21) levels are elevated in older adults with cachexia and in metabolic disease states such as obesity, insulin resistance, and type II diabetes mellitus (17–20). Higher adipokine levels are also associated with obesity and sarcopenia in RA (21,22).
Adipokines may thus be potentially useful biomarkers for identifying RA patients who have undergone unfavorable metabolic changes and therefore might help elucidate the link between RA and CVD risk. Adiponectin has been associated with cardiovascular mortality in a wide range of populations (23). In the general population, there have been mixed findings regarding whether high leptin is independently associated with CVD (24–26). One study in RA found a positive association between leptin and CVD incidence, even after adjusting for body mass index (BMI) (27). Higher FGF-21 has been found to independently predict coronary artery disease (CAD) in patients with type II diabetes mellitus and without preexisting CVD (28) but has not been studied in this context in RA. Taken together, evidence from other populations suggests adipokines might serve as helpful markers of metabolic stress, which may help anticipate cardiovascular events. While there is rationale to suggest a role for adipokines in inflammatory diseases, we are aware of few studies evaluating the predictive role of these metabolic markers in RA or other autoimmune disease.
In this study, we aimed to determine associations between adipokines (adiponectin, leptin, and FGF-21) and incident cardiovascular events independent of other comorbidities and RA disease characteristics to determine whether these biomarkers could augment CVD risk prediction. We hypothesized that higher adipokine levels would be associated with greater risks of cardiovascular events.
PATIENTS AND METHODS
Study design.
We conducted a cohort study in the Veterans’ Affairs Rheumatoid Arthritis (VARA) registry, an ongoing multicenter RA biosample and data repository active since 2003 (29). We followed patients from VARA enrollment until incident CVD event, death, or query date for CV outcomes (December 31, 2020). At the time of this study, 15 Veterans Affairs (VA) sites had contributed data. Clinicians at each site collected clinical data at enrollment and follow-up visits as part of routine care. Participants were >18 years old and satisfied the 1987 American College of Rheumatology RA classification criteria (30). We excluded those without baseline adipokine levels. Each site has institutional review board approval, and all participants provided written informed consent.
Adipokine measurements.
Adipokines (adiponectin, leptin, and FGF-21) were measured on serum collected at enrollment and stored at −70°C using a U-plex multianalyte panel (Meso Scale Discovery). Adipokine values were log-adjusted to fit a normal distribution and standardized to have the mean and SD set to 0 and 1, respectively. For analyses, we dichotomized each adipokine as high or low using median values (> or <12.4 μg/ml for adiponectin, > or <10.3 ng/ml for leptin, > or <608.4 pg/ml for FGF-21).
Cardiovascular outcomes.
Primary outcomes were: 1) CAD (myocardial infarction, percutaneous coronary intervention, or coronary artery bypass); 2) stroke (including transient ischemic attack); 3) HF hospitalization; 4) venous thromboembolism (VTE); and 5) CVD-related death. CAD, stroke, HF, and mortality outcomes were defined as previously described (31). Briefly, nonfatal CVD outcomes, including VTE (see Supplementary Table 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24885), were obtained from the VA Corporate Data Warehouse (CDW) using administrative algorithms incorporating previously validated International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes (31–33). The positive predictive value of these algorithms was >80–90% except for stroke, so all identified stroke events were confirmed by chart review. CVD-related deaths were determined using ICD-10 codes I00-I99 from the National Death Index.
Other covariates.
Demographic and disease-specific baseline characteristics were obtained from the VARA registry. Clinicians reported the presence of nodules and erosions at enrollment. Smoking status was reported at baseline and considered time invariant (presence or absence of smoking). BMI was obtained from the CDW, and the closest BMI value within 30 days of the visit was used. Multiple imputation was used to account for missing BMI values (5.5% of observations). Percent change from maximum BMI was calculated as the difference between enrollment BMI and the greatest pre-enrollment BMI available because pre-enrollment weight loss is associated with cardiovascular mortality in this cohort (8). Comorbidity was assessed using the Rheumatic Disease Comorbidity Index (RDCI) (34). Individual comorbidities were obtained from the CDW over 1 year preceding enrollment and categorized according to Healthcare Cost and Utilization Project Clinical Classification Software.
Baseline laboratory testing was performed for C-reactive protein (CRP) level (mg/liter), rheumatoid factor (RF), and anti–citrullinated protein antibody (ACPA) (35). Clinical assessments such as the Disease Activity Score in 28 joints (DAS28; range 0–28) and disability per the Multidimensional Health Assessment Questionnaire (MD-HAQ; range 0–3) (36) were extracted from the VARA registry at enrollment. To impute missing baseline DAS28 values (17.9%), we used the first nonmissing DAS28 scores for each participant and utilized multiple imputation for remaining missing values. RA treatments (methotrexate, hydroxychloroquine, tumor necrosis factor [TNF] inhibitors, and glucocorticoids) were extracted from VA pharmacy databases. Drug courses were defined from dispensing data using previously described algorithms (37). JAK inhibitors and non-TNF inhibitor biologic disease-modifying antirheumatic drugs were not included due to the small number of participants (n = 9, 0.3% and n = 30, 1.1%, respectively) with prior use at enrollment. Participants were considered exposed to methotrexate, hydroxychloroquine, and TNF inhibitors if the baseline visit occurred during a defined medication course. Glucocorticoid use was considered active if a course overlapped within 30 days of enrollment. This definition of glucocorticoid use had 85% accuracy compared to chart review (data not shown).
Statistical analysis.
Baseline participant characteristics were described by high versus low adiponectin, leptin, and FGF-21 levels. Differences between groups were assessed with Student’s t-tests (2-tailed), Wilcoxon’s rank sum tests, and Pearson’s chi-square tests.
Associations between each adipokine and time to each incident CVD outcome (CAD, stroke, HF hospitalization, VTE, and CVD-related death) were assessed using Cox proportional hazards models for each adipokine–outcome combination. To understand the impact of potential confounders and avoid overfitting models, we evaluated a minimally adjusted model and a final model. Minimally adjusted models included prehypothesized confounders such as age, age2, sex, race, BMI, BMI2 (for models predicting CVD-related death), DAS28 score, history of the outcome at enrollment, and calendar year at enrollment. Final models were developed by first including the aforementioned covariates and all variables at least moderately associated (P < 0.1) with each adipokine at baseline, then eliminating variables using stepwise deletion if they were not associated with the outcome (P > 0.05) and removal did not alter the hazard ratio (HR) for the exposure of interest by >15%. A breakdown of covariates included in each final model (diabetes mellitus, hypertension, etc.) is available in Supplementary Table 2, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24885. While we adjusted for a history of hyperlipidemia, we did not have quantitative data on lipids. Results of final and minimally adjusted models were similar, and thus only final model results are shown. As a sensitivity analysis, we ran final models excluding patients with prevalent disease; results were similar and therefore are not shown. All models employed clustering by VARA site. To evaluate prediction accuracy, Harrell C statistics were calculated for each final model.
We assessed prehypothesized interactions between adipokine groups (high versus low) and sex, obesity, and prevalent disease in final models for each adipokine–outcome combination. To test for interactions with obesity, BMI was dichotomized to obese (BMI >30 kg/m2) or nonobese (BMI ≤30 kg/m2). We also determined associations with continuous adipokine measures. Given the exploratory nature of these analyses and lack of independence of outcomes, no adjustment for multiple comparisons was performed.
Final models corresponding to each adipokine–outcome combination were used to calculate the predicted 5-year incidence at the means of all covariates for each adipokine group (high versus low). Proportional hazards assumptions were tested by visualizing the Schoenfeld residuals and were not violated (all P > 0.05 for proportional hazards test). Data were analyzed using Stata software, version 15.1, within the VA Informatics and Computing Infrastructure.
RESULTS
Baseline characteristics.
A total of 2,598 US veterans with RA had adipokines measured at baseline and were followed over ~19,585 person-years. Overall, patients were predominantly male (88.4%), non-Black (84.2%), non-current smokers (75.2%), had a mean age of 71.6 years, and were 77.1% RF positive and 77.6% ACPA positive. The mean DAS28 score was 3.6, with a mean MD-HAQ score of 0.88. For RA treatments, 52% were exposed to methotrexate, 32% to hydroxychloroquine, 25% to TNF inhibitors, and 34% to glucocorticoids.
The mean baseline RDCI score was 3.4. The 5 most common comorbidities were osteoarthritis (84%), hyperlipidemia (82%), hypertension (71%), spine disease (52%), and any neoplasm (50%). Approximately one-fifth (21%) of participants had prevalent CVD at enrollment, with 3% meeting criteria for prior VTE, 8% for HF hospitalization, 5% for stroke, and 10% for CAD.
Factors associated with baseline adipokine levels.
Baseline characteristics by adipokine group are shown in Table 1. Higher adiponectin was associated with older age, non-Black race, lower BMI, greater percent weight loss from maximum weight, higher proportion of radiographic damage, longer disease duration, lower CRP level, higher rates of hydroxychloroquine use, and enrollment before 2010. Those with higher adiponectin were also less likely to have prevalent diabetes mellitus, hypertension, and hyperlipidemia and more likely to have prevalent spine disease and osteoporosis (Table 1).
Table 1.
Enrollment characteristics by adipokine levels above and below the median value (n = 1,299)*
| Characteristic | Low adiponectin | High adiponectin | Low leptin | High leptin | Low FGF-21 | High FGF-21 |
|---|---|---|---|---|---|---|
| Age, mean ± SD years | 70.8 ± 10.7† | 72.5 ± 10.2† | 72.2 ± 10.3† | 71.1 ± 10.6† | 71.5 ± 11.0 | 71.8 ± 9.9 |
| Female | 147 (11) | 153 (12) | 65 (5)† | 235 (18)† | 166 (13)† | 134 (10)† |
| Black | 233 (18)† | 177 (14)† | 175 (13)† | 235 (18)† | 253 (20)† | 157 (12)† |
| Current smoking | 311 (25) | 313 (25) | 405 (32)† | 219 (18)† | 280 (22)† | 344 (27)† |
| BMI, kg/m2 | 29.5 (5.9)† | 28.2 (5.7)† | 26.1 (4.3)† | 31.6 (5.8)† | 28.3 (5.5)† | 29.4 (6.1)† |
| % change max BMI, median (IQR) | 5.9 (1.9–12.1)† | 6.9 (2.8–14.7)† | 7.5 (3.2–15.2)† | 5.2 (1.6–11.5)† | 6.5 (2.4–13.2) | 6.2 (2.2–13.4) |
| RA characteristics | ||||||
| DAS28 score | 3.7 (1.5) | 3.6 (1.4) | 3.7 (1.5)† | 3.5 (1.4)† | 3.5 (1.4)† | 3.7 (1.5)† |
| MD-HAQ score, median (IQR) | 0.8 (0.4–1.3) | 0.8 (0.4–1.3) | 0.8 (0.4–1.3) | 0.8 (0.4–1.3) | 0.8 (0.3–1.3)† | 0.9 (0.4–1.3)† |
| Erosive disease | 434 (47)† | 546 (55)† | 510 (52) | 470 (50) | 479 (51) | 501 (52) |
| Nodules | 299 (35) | 343 (35) | 345 (38)† | 297 (32)† | 296 (33)† | 346 (37)† |
| RF positive | 872 (78) | 962 (77) | 937 (79)† | 897 (75)† | 876 (75)† | 958 (79)† |
| ACPA positive | 871 (78) | 972 (78) | 914 (77) | 929 (78) | 888 (76) | 955 (79) |
| Disease duration, median (IQR) years | 6.4 (1.7–14.6)† | 9.3 (2.9–19.2)† | 7.7 (2.3–17.2) | 7.6 (2.4–16.2) | 6.9 (2.0–15.9)† | 8.5 (2.6–18.0)† |
| hsCRP, median (IQR) mg/dl | 5.4 (2.3–13.8)† | 5.0 (1.9–10.8)† | 5.2 (1.9–13.7) | 5.3 (2.2–10.9) | 4.7 (1.8–11.2)† | 5.7 (2.3–12.9)† |
| Methotrexate | 667 (51) | 673 (52) | 674 (52) | 666 (51) | 719 (55)† | 621 (48)† |
| Glucocorticoids | 420 (32) | 466 (36) | 440 (34) | 446 (34) | 448 (35) | 438 (34) |
| TNF inhibitors | 315 (24) | 343 (26) | 326 (25) | 332 (26) | 331 (26) | 327 (25) |
| Hydroxychloroquine | 372 (29)† | 471 (36)† | 375 (29)† | 468 (36)† | 455 (35)† | 388 (30)† |
| Enrolled after 2010 | 708 (55)† | 568 (44)† | 698 (54)† | 578 (45)† | 653 (50) | 623 (50) |
| Comorbidity | ||||||
| RDCI score, median (IQR) | 3 (2–5) | 3 (2–5) | 3 (2–5)† | 4 (2–5)† | 3 (2–5)† | 4 (2–5)† |
| VTE | 38 (3) | 47 (4) | 35 (3) | 50 (4) | 38 (3) | 47 (4) |
| HF hospitalization | 97 (7) | 103 (8) | 79 (6)† | 121 (9)† | 81 (6)† | 119 (9)† |
| Stroke | 63 (5) | 77 (6) | 69 (5) | 71 (5) | 67 (5) | 73 (6) |
| CAD | 146 (11) | 121 (9) | 124 (10) | 143 (11) | 121 (9) | 146 (11) |
| Any CVD‡ | 279 (21) | 274 (21) | 249 (19)† | 304 (23)† | 250 (19)† | 303 (23)† |
| COPD/asthma | 444 (34) | 463 (36) | 429 (33)† | 478 (37)† | 418 (32)† | 489 (38)† |
| Diabetes mellitus | 478 (37)† | 384 (30)† | 371 (29)† | 491 (38)† | 396 (31)† | 466 (36)† |
| Osteoarthritis | 1,081 (83) | 1,089 (84) | 1,050 (81)† | 1,120 (86)† | 1,085 (84) | 1,085 (84) |
| Hypertension | 951 (73)† | 897 (69)† | 848 (65)† | 1,000 (77)† | 873 (67)† | 975 (75)† |
| Hyperlipidemia | 1,076 (83)† | 1,020 (80)† | 988 (77)† | 1,108 (86)† | 1,014 (79)† | 1,082 (84)† |
| Vascular disease | 174 (13) | 174 (13) | 165 (13) | 183 (14) | 154 (12)† | 194 (15)† |
| Liver disorder | 185 (14) | 196 (15) | 157 (12)† | 224 (17)† | 171 (13)† | 210 (16)† |
| Any neoplasm | 630 (49) | 663 (51) | 606 (47)† | 687 (53)† | 609 (47)† | 684 (53)† |
| Spine disease | 641 (49)† | 705 (54)† | 627 (48)† | 719 (55)† | 666 (51) | 680 (52) |
| Osteoporosis | 256 (20)† | 302 (23)† | 279 (21) | 279 (21) | 270 (21) | 288 (22) |
Values are the number (%) unless indicated otherwise. Each column represents 50% (n = 1,299) of the total number of veterans who had adipokines measured at baseline. ACPA = anti–citrullinated protein antibody; BMI = body mass index; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; CVD = cardiovascular disease; DAS28 = Disease Activity Score in 28 joints; FGF-21 = fibroblast growth factor 21; HF = heart failure; hsCRP = high-sensitivity C-reactive protein; IQR = interquartile range; MD-HAQ = Multidimensional Health Assessment Questionnaire; RA = rheumatoid arthritis; RDCI = Rheumatic Disease Comorbidity Index; RF = rheumatoid factor; TNF = tumor necrosis factor; VTE = venous thromboembolism.
Significant difference (P < 0.05) between high/low adipokine groups (see Supplementary Tables 3–5, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24885, for exact P values).
Composite CVD prevalence corresponds to the prevalence of at least 1 of VTE, HF hospitalization, stroke, and/or CAD.
Higher leptin was associated with younger age, female sex, Black race, non-smoking, higher BMI, lower percent weight loss from maximum weight, lower DAS28 score, absence of nodules, lower RF seropositivity rates, higher rates of hydroxychloroquine use, and enrollment before 2010. Compared to those with low leptin levels, those with high leptin had a mean BMI that was 5.4 kg/m2 higher. Those with higher leptin also had more comorbidities and were more likely to have prevalent HF, composite CVD, chronic obstructive pulmonary disease (COPD)/asthma, diabetes mellitus, osteoarthritis, hypertension, hyperlipidemia, liver disorder, any neoplasm, and spine disease (Table 1).
Higher FGF-21 was associated with male sex, non-Black race, smoking, higher BMI, higher DAS28 and MD-HAQ scores, presence of nodules, higher RF seropositivity rates, longer disease duration, higher CRP level, and lower methotrexate and hydroxychloroquine use. Those with higher FGF-21 also had more comorbidities and were more likely to have prevalent HF, composite CVD, COPD/asthma, diabetes mellitus, hypertension, hyperlipidemia, vascular disease, liver disorder, and any neoplasm (Table 1).
Associations between adipokines and incident CVD.
We identified 639 subjects (21%) with at least 1 incident CVD event. The overall incidence of VTE, HF hospitalization, stroke, CAD, and CVD-related deaths was 4.3, 6.2, 4.8, 11.2, and 11.1 per 1,000 person-years, respectively (Table 2).
Table 2.
Summary data and hazard ratios (HRs) from Cox proportional hazards models to predict cardiovascular disease (CVD) incidence by adipokine group*
| Model | No.† | Total no. of events† | Total time at risk, person-years† | IR per 1,000 personyears† | Fully-adjusted model for high adipokine level, HR (95% CI)‡ | P | Harrell C statistic, without/with adipokine |
|---|---|---|---|---|---|---|---|
| VTE | |||||||
| Adiponectin | 2,583 | 84 | 19,360 | 4.34 | 1.00 (0.65–1.55) | 1.00 | 0.69/0.69 |
| Leptin | 2,580 | 84 | 19,352 | 4.34 | 1.71 (0.94–3.09) | 0.08 | 0.74/0.74 |
| FGF-21 | 2,580 | 84 | 19,352 | 4.34 | 1.25 (0.85–1.84) | 0.26 | 0.71/0.71 |
| HF hospitalization | |||||||
| Adiponectin | 2,583 | 120 | 19,295 | 6.22 | 1.39 (1.07–1.79) | 0.01 | 0.76/0.76 |
| Leptin | 2,583 | 120 | 19,295 | 6.22 | 0.95 (0.65–1.38) | 0.78 | 0.76/0.75 |
| FGF-21 | 2,583 | 120 | 19,295 | 6.22 | 0.81 (0.56–1.18) | 0.27 | 0.77/0.77 |
| Stroke | |||||||
| Adiponectin | 2,583 | 91 | 19,170 | 4.75 | 0.95 (0.74–1.22) | 0.68 | 0.66/0.66 |
| Leptin | 2,583 | 91 | 19,170 | 4.75 | 0.86 (0.59–1.26) | 0.44 | 0.66/0.66 |
| FGF-21 | 2,583 | 91 | 19,170 | 4.75 | 1.06 (0.68–1.64) | 0.80 | 0.69/0.69 |
| CAD | |||||||
| Adiponectin | 2,583 | 209 | 18,626 | 11.22 | 0.88 (0.75–1.03) | 0.11 | 0.68/0.68 |
| Leptin | 2,580 | 209 | 18,618 | 11.23 | 0.79 (0.59–1.07) | 0.13 | 0.69/0.69 |
| FGF-21 | 2,580 | 209 | 18,618 | 11.23 | 0.75 (0.58–0.97) | 0.03 | 0.70/0.70 |
| CVD-related death | |||||||
| Adiponectin | 2,583 | 218 | 19,585 | 11.13 | 1.49 (1.16–1.92) | 0.002 | 0.77/0.79 |
| Leptin | 2,580 | 218 | 19,577 | 11.14 | 1.44 (1.05–1.97) | 0.02 | 0.77/0.79 |
| FGF-21 | 2,369 | 215 | 19,142 | 11.23 | 1.20 (0.79–1.82) | 0.39 | 0.79/0.80 |
95% CI = 95% confidence interval; CAD = coronary artery disease; FGF-21 = fibroblast growth factor 21; HF = heart failure; HR = hazard ratio; IR = incidence rate; VTE = venous thromboembolism.
Number, total number of events, total time at risk, and IR differ slightly between some models predicting the same CVD outcome due to different covariates and missing data within corresponding final models.
Final models were developed by first including the covariates from minimally adjusted models and all variables found to be moderately associated (P < 0.1) with each adipokine at baseline, and then eliminating variables using stepwise deletion. In the stepwise deletion process, covariates were sequentially removed from final models if they were not associated with the outcome (P > 0.05) and their removal did not alter the HR for the exposure of interest by >15% (see Supplementary Table 2, available at http://onlinelibrary.wiley.com/doi/10.1002/acr.24885).
In final models, higher adiponectin was independently associated with higher HF hospitalization rates (HR 1.39 [95% confidence interval (95% CI) 1.07–1.79], P = 0.01) and CVD-related death (HR 1.49 [95% CI 1.16–1.92], P = 0.002). However, adiponectin was not significantly associated with VTE, CAD, or stroke events. Higher leptin was independently associated with higher CVD-related death rates (HR 1.44 [95% CI 1.05–1.97], P = 0.02) and with numerically, but not statistically greater VTE risk (HR 1.71 [95% CI 0.94–3.09], P = 0.08). However, leptin was not associated with HF hospitalization, CAD, or stroke events. Higher FGF-21 was independently associated with lower CAD rates (HR 0.75 [95% CI 0.58–0.97], P = 0.03) (Table 2) but not with other cardiovascular outcomes. Harrell C statistics for final models, with and without adipokine levels included, are presented in Table 2. Overall, model prediction was not significantly improved with the addition of adipokines.
Predicted 5-year incidence rates per 100 person-years for each CVD outcome by adipokine group are shown in Figure 1. For example, the predicted 5-year incidence rate for HF hospitalization was significantly higher in the high adiponectin group compared to the low adiponectin group (2.63 versus 1.90; P = 0.01).
Figure 1.
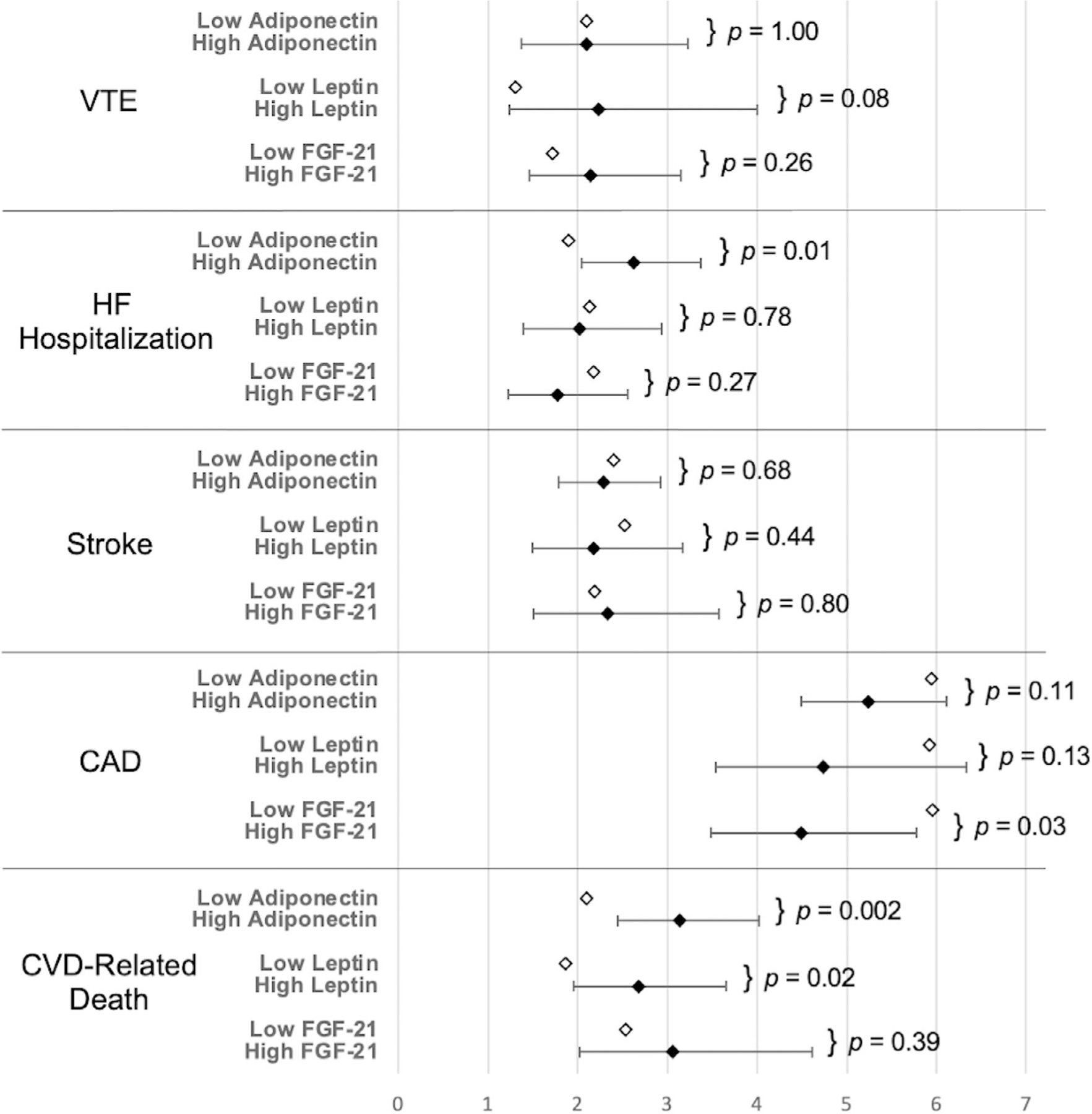
Predicted 5-year cardiovascular disease (CVD) incidence rates by adipokine group per 100 person-years for the low adipokine (reference) group (open diamonds) and the high adipokine group (solid diamonds). Bars indicate 95% confidence intervals for predicted 5-year incidence rates derived from final Cox proportional hazards models for each adipokine–outcome combination. No confidence interval is available for the low adipokine group, which serves as the reference 5-year incidence rate. P values correspond to adipokine hazard ratio significance in final Cox proportional hazards models. CAD = coronary artery disease; FGF-21 = fibroblast growth factor 21; HF = heart failure; VTE = venous thromboembolism.
Secondary analyses.
Prehypothesized subgroup analyses demonstrated 5 significant interactions (Figure 2), suggesting that associations between adipokines and cardiovascular events differed based on select covariates. For example, high adiponectin was strongly associated with CVD-related death among nonobese patients (HR 1.70 [95% CI 1.44–2.01], P < 0.001) but not in obese patients (HR 1.04 [95% CI 0.65–1.66], P = 0.87; P for interaction = 0.049). Similarly, high leptin was associated with CVD-related death in nonobese patients (HR 1.42 [95% CI 1.04–1.95], P = 0.03) but not in obese patients (HR 0.84 [95% CI 0.48–1.48], P = 0.55; P for interaction = 0.03). High leptin was also associated with CVD-related death among patients without prevalent CVD at enrollment (HR 1.72 [95% CI 1.25–2.37], P = 0.001) but not in those with prevalent CVD at enrollment (HR 1.13 [95% CI 0.77–1.67], P = 0.53; P for interaction = 0.01) (Figure 2). No other significant statistical interactions were identified from the predefined analyses.
Figure 2.
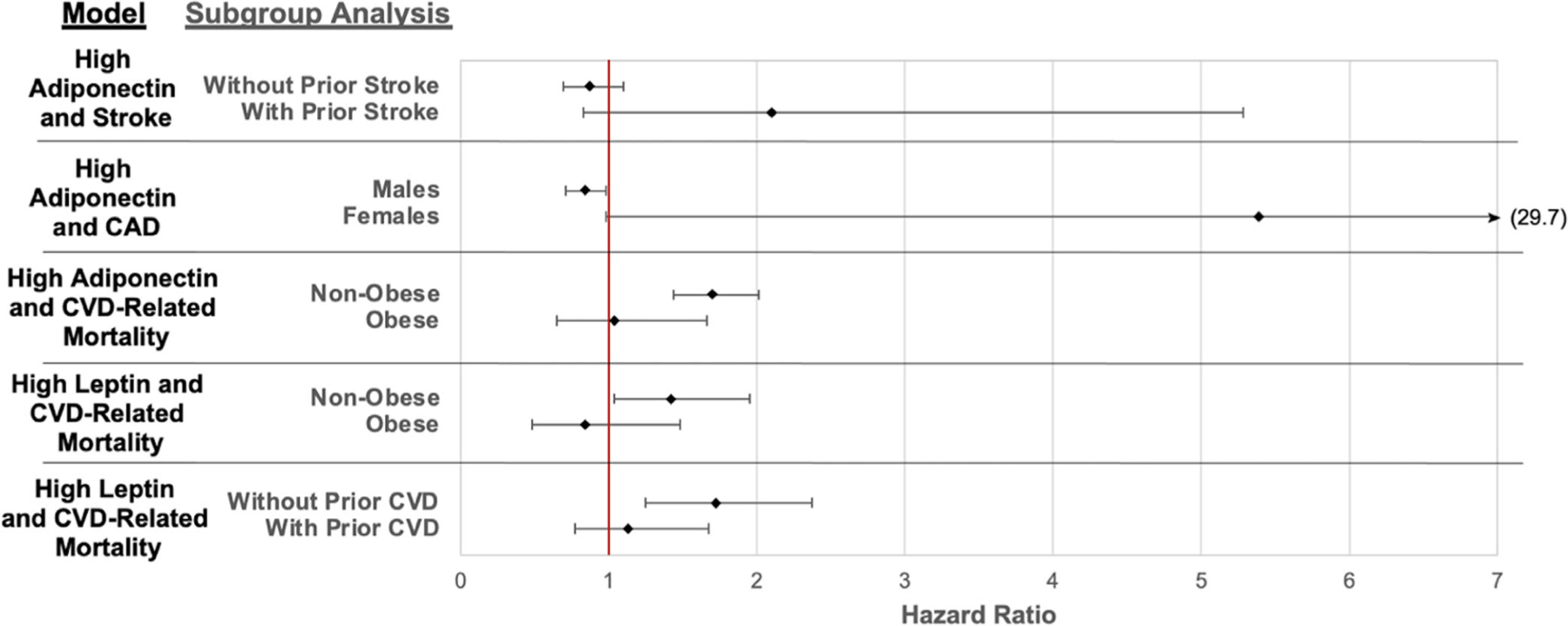
Subgroup analyses with significant interactions from Cox proportional hazards models to predict cardiovascular disease (CVD) incidence by adipokine group. Bars indicate 95% confidence intervals. Diamonds represent incidence rates. P < 0.05 for all statistical interaction (5 significant interactions of 45 tested). CAD = coronary artery disease.
Secondary analyses evaluating the effect of continuous adipokine levels (per 1 SD) to predict CVD outcomes demonstrated associations similar to those from primary analyses. Higher continuous adiponectin levels were independently associated with CVD-related death (HR 1.18 [95% CI 1.04–1.35], P = 0.01). Higher leptin was independently associated with both VTE risk (HR 1.26 [95% CI 1.05–1.52], P = 0.02) and CVD-related death (HR 1.23 [95% CI 1.03–1.48], P = 0.02). Higher FGF-21 was independently associated with lower CAD rates (HR 0.90 [95% CI 0.84–0.97], P = 0.003).
DISCUSSION
This prospective cohort study identified independent associations between circulating adipokine levels and incident CVD events in RA patients. Specifically, higher adiponectin was associated with increased risks of HF hospitalization and CVD-related death, higher leptin was associated with VTE and increased risk of CVD-related death, and higher FGF-21 was associated with lower risk of CAD events. Since pathologic weight loss and altered lipid profiles may adversely impact risk prediction accuracy in RA, it is important to identify markers of RA-related metabolic disturbances that might contribute to long-term risks. Results of this study provide support for adipokines as biomarkers of metabolic health that might help improve CVD risk prediction in RA. Overall, however, associations were modest; further study is needed to assess their role in risk prediction in key subgroups (i.e., nonobese).
Prespecified subgroup analyses demonstrated that associations between high adiponectin and leptin with CVD-related death were stronger in nonobese patients. Weight fluctuations have also been previously found to predict CVD more strongly in underweight patients (9). These findings together suggest the need for improved understanding of CVD risk in populations with lower BMI, who may be overlooked in traditional CVD risk prediction tools. Additionally, high leptin was only associated with increased CVD-related death in those without prevalent CVD. These observations suggest that the predictive value of adipokines might be modified by the clinical scenario and may be more predictive in subgroups appearing to be at lower risk. Therefore, while this study suggests that adipokines are unlikely to add substantially to risk prediction in RA populations overall, findings also provide initial evidence to support future study in particular subgroups within which risk might otherwise be underappreciated. Further exploration of effect modification identified in this study may also provide insights into biologic mechanisms underlying CVD risk in RA.
Heart failure has increasingly been recognized as an inflammatory state with disruption of metabolic pathways (38). The characterization of the interaction between adipokines and these inflammatory pathways is an ongoing area of research. While adiponectin has been proposed to be cardioprotective in healthy populations, prior studies have demonstrated associations between higher adiponectin and greater risk of both HF hospitalization and mortality in patients with existing CVD (39–41). Some have proposed that high adiponectin in HF might represent a compensatory mechanism for patients with vascular injury or underlying inflammatory states (40–42). Alternatively, adiponectin elevations may be linked to development of RA cachexia or cardiac cachexia. This hypothesis is supported by our study’s observation that adiponectin was more strongly associated with adverse outcomes in nonobese patients. A recent study found a positive association between adiponectin and disease activity in RA patients with low body weight, perhaps due to relationships between inflammatory cytokines, weight loss, and adiponectin production (43). Our group has previously demonstrated associations between adipokines and sarcopenia in RA (22). Our findings, in the context of these prior observations, suggest that adiponectin may serve as a marker of chronic illness and disease severity, helping to predict hospitalization and death, but not necessarily initial acute events such as coronary events, stroke, or VTE.
It has also been proposed that adiponectin may have different physiologic effects in different tissues or contexts. While largely considered an antiinflammatory molecule, adiponectin has been observed to stimulate production of vascular endothelial growth factor and other inflammatory mediators in chondrocytes from RA patients (23,44), suggesting that adiponectin may have proinflammatory effects in certain contexts. However, future mechanistic studies are needed to improve understanding of the physiological role of adiponectin in both cardiovascular homeostasis and underlying inflammatory states such as RA and HF.
We observed that leptin levels, in particular, predicted CVD-related death both in nonobese patients and among those without prevalent CVD. Because leptin is so strongly associated with adiposity, when adjusting for BMI, higher leptin levels imply a higher body mass proportion comprising fat. Associations between leptin and both CVD-related death and VTE events, particularly in nonobese patients, suggest a potential relationship between CVD outcomes and adverse body composition and metabolic health. Prior studies in non-RA populations observed that leptin levels and low muscle mass predict mortality in men without prevalent CAD or HF (45). The tendency for leptin to predict VTE events in our study conflicts somewhat with a prior study, which suggested that the association between leptin and VTE is explained by BMI (46). Overall, our findings support the hypothesis that body composition and circulating leptin may be important predictors of CVD risk independent of BMI at least for VTE and CVD-related death, particularly among thinner patients. In this large study, the lack of association between leptin and other key cardiovascular outcomes is also informative, as it suggests that leptin will not likely be a useful biomarker for these outcomes in this population.
Prior studies observed positive associations between FGF-21 and CVD risk in different patient populations with and without histories of CVD (28,47,48). However, findings have been mixed; a recent large study of patients without prior CVD found no association between FGF-21 and cardiovascular events (49). These prior findings suggest that relationships between FGF-21 and CVD may vary based on study populations and methods. In the current study, the first to our knowledge in RA, FGF-21 was not associated with incident VTE, HF hospitalization, stroke, or CVD-related death but was associated with incident CAD events after adjusting for other factors. This observation is novel but should be interpreted in the context of multiple comparisons. Taken together, this inconsistency in effect across studies and populations does not support a causal role of FGF-21 in promoting CVD.
This study is among the first to describe clinical characteristics of patients with high adiponectin, leptin, and FGF-21 levels. Of note, high adiponectin tended to be associated with select indicators of RA severity, such as radiographic damage and osteoporosis, whereas elevated leptin and FGF-21 were primarily observed in patients with obesity and obesity-related comorbidities. Characterizing these associations was not this study’s primary goal, and further study is needed to better understand clinical factors leading to elevated adipokines in this population.
There are several limitations worth noting, including that this is a predominantly male and older cohort of US veterans, which may limit generalizability. We did not observe significant effect modification by sex, except for adiponectin and CAD. Use of baseline adipokine measurements and covariates in longitudinal analyses did not allow for investigation of potentially important time-varying effects. Furthermore, dichotomization of adipokines around median values for this population may not be the optimal cutoff. Further research is needed to explore optimal risk prediction thresholds. Future study should consider how adipokines may improve risk prediction in key subgroups identified here after considering other cardiovascular biomarkers. For example, we did not have lipid biomarkers at the time of these analyses, although future studies aimed at understanding complex relationships between adipokines and lipids are of interest. While our study does not generally support the hypothesis that adipokines will meaningfully improve existing risk prediction tools, this was not formally tested in our study. Associations between adipokines and the development of cardiovascular risk factors such as diabetes mellitus and hypertension are also of interest and considered future aims.
Because this was an observational study, the risk of residual confounding must be considered when interpreting a causal link between adipokines and CVD. For example, fat distribution was not measured and may be associated with both adipokines and CVD outcomes independent of BMI. Significant observations should be viewed in the context of the multiple adipokine–outcome comparisons evaluated in this study. A correction such as Bonferroni was considered overly conservative in the context of related cardiovascular outcomes. Cardiovascular events occurring outside the VA may have been missed due to reliance on administrative data, but efforts were made to systematically minimize outcome misclassification by using previously validated algorithms, or when those were unavailable, validating events through medical record review (31). Likewise, we queried for CVD events that occurred outside the VA that were billed to the VA. Prior studies have demonstrated relatively low rates of dual health care utilization in this RA population for chronic disease care, although greater use of non-VA care for acute events is likely (50).
Strengths of this study include comprehensively assessed adipokine measurements, validated cardiovascular outcomes, and thorough adjustment for comorbidities, clinical disease activity, and RA treatments. The large sample size and longitudinal nature of the study allowed us to assess temporal relationships important for understanding potential roles of adipokines as biomarkers to predict rare outcomes. Future study may help to clarify the utility of combining adipokine measurements with other risk tools or to identify subgroups for which these measurements are most clinically useful.
In conclusion, this study observed significant associations between adiponectin, leptin, and cardiovascular death, with stronger associations in nonobese patients. Further, high adiponectin levels predicted HF hospitalizations, and high leptin levels tended to predict VTE events. These associations were independent of baseline characteristics, BMI, RA disease activity, prevalent CVD, and other comorbidities and RA treatments. While the additive predictive role appears to be modest in the general RA population, these adipokines may add value to risk stratification for outcomes such as HF hospitalization and death in certain subgroups.
Supplementary Material
SIGNIFICANCE & INNOVATIONS.
Cardiovascular disease is the leading cause of mortality in patients with rheumatoid arthritis (RA), but current cardiovascular risk prediction tools do not perform well in this patient population.
Adipokines (adiponectin, leptin, and fibroblast growth factor 21 [FGF-21]) may help to identify metabolic disturbances in patients with RA, which could contribute to long-term cardiovascular risks.
High adiponectin and leptin levels are independently associated with cardiovascular death, with stronger associations in nonobese patients.
High adiponectin levels predicted heart failure hospitalizations, higher leptin levels predicted venous thromboembolic events, and high FGF-21 levels were associated with a lower risk of coronary artery disease events.
Acknowledgments
Supported by the Center of Excellence for Suicide Prevention, Joint Department of Veterans Affairs and Department of Defense Mortality Data Repository, National Death Index. Dr. England’s work was supported by the Veterans Affairs Career Development Award (grant IK2CX002203). Dr. Wysham’s work was supported by the Department of Veterans Affairs and the Rheumatology Research Foundation. Dr. George’s work was supported by the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases grant K23-AR-073931). Dr. Mikuls’ work was supported by the Department of Veterans Affairs (Merit Award grant I01BX0046000), the Department of Defense (grant PR200793), and the NIH (National Institute on Alcohol Abuse and Alcoholism grant R25-AA-020818, National Institute of General Medical Sciences grant U54-GM-115458, and National Institute of Arthritis and Musculoskeletal and Skin Diseases grant P50-AR-60772). Dr. Baker’s work was supported by the Department of Veterans Affairs (Clinical Science Research and Development Career Merit Award grant I01CX001703 and Rehabilitation Research and Development grants I21CX003157 and I01CX003644).
Footnotes
The contents herein do not represent the views of the Department of Veterans Affairs or the US government.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Facr.24885&file=acr24885-sup-0001-Disclosureform.pdf.
REFERENCES
- 1.Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol 2008;26:S35–61. [PubMed] [Google Scholar]
- 2.Kitas GD, Gabriel SE. Cardiovascular disease in rheumatoid arthritis: state of the art and future perspectives. Ann Rheum Dis 2011;70: 8–14. [DOI] [PubMed] [Google Scholar]
- 3.Crowson CS, Matteson EL, Roger VL, et al. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol 2012;110:420–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawai VK, Chung CP, Solus JF, et al. The ability of the 2013 American College of Cardiology/American Heart Association cardiovascular risk score to identify rheumatoid arthritis patients with high coronary artery calcification scores. Arthritis Rheumatol 2015;67:381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arts EE, Popa C, Den Broeder AA, et al. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis 2015;74:668–74. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012; 64:1471–9. [DOI] [PubMed] [Google Scholar]
- 7.Baker JF, Billig E, Michaud K, et al. Weight loss, the obesity paradox, and the risk of death in rheumatoid arthritis. Arthritis Rheumatol 2015;67:1711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.England BR, Baker JF, Sayles H, et al. Body mass index, weight loss, and cause-specific mortality in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2018;70:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker JF, Reed G, Kremer J. Weight fluctuation and the risk of cardiovascular events in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2022;74:229–35. [DOI] [PubMed] [Google Scholar]
- 10.Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett 2008;582:74–80. [DOI] [PubMed] [Google Scholar]
- 11.Gremese E, Ferraccioli G. The metabolic syndrome: the crossroads between rheumatoid arthritis and cardiovascular risk. Autoimmun Rev 2011;10:582–9. [DOI] [PubMed] [Google Scholar]
- 12.Fatel EC, Rosa FT, Simão AN, et al. Adipokines in rheumatoid arthritis. Adv Rheumatol 2018;58:25. [DOI] [PubMed] [Google Scholar]
- 13.Tagami T, Satoh N, Usui T, et al. Adiponectin in anorexia nervosa and bulimia nervosa. J Clin Endocrinol Metab 2004;89:1833–7. [DOI] [PubMed] [Google Scholar]
- 14.Wroblewski E, Swidnicka-Siergiejko A, Hady HR, et al. Variation in blood levels of hormones in obese patients following weight reduction induced by endoscopic and surgical bariatric therapies. Cytokine 2016;77:56–62. [DOI] [PubMed] [Google Scholar]
- 15.Araújo JP, Lourenço P, Rocha-Gonçalves F, et al. Adiponectin is increased in cardiac cachexia irrespective of body mass index. Eur J Heart Fail 2009;11:567–72. [DOI] [PubMed] [Google Scholar]
- 16.Markaki A, Grammatikopoulou MG, Venihaki M, et al. Associations of adiponectin and leptin levels with protein-energy wasting, in end stage renal disease patients. Endocrinol Nutr 2016;63:449–57. [DOI] [PubMed] [Google Scholar]
- 17.Franz K, Ost M, Otten L, et al. Higher serum levels of fibroblast growth factor 21 in old patients with cachexia. Nutrition 2019;63–64:81–6. [DOI] [PubMed] [Google Scholar]
- 18.Tezze C, Romanello V, Sandri M. FGF21 as modulator of metabolism in health and disease. Front Physiol 2019;10:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Yeung DC, Karpisek M, et al. Serum FGF21 Levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008;57:1246–53. [DOI] [PubMed] [Google Scholar]
- 20.Chavez AO, Molina-Carrion M, Abdul-Ghani MA, et al. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 2009;32:1542–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gould PW, Zemel BS, Taratuta EG, et al. Circulating fibroblast growth factor-21 levels in rheumatoid arthritis: associations with disease characteristics, body composition, and physical functioning. J Rheumatol 2021;48:504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker JF, Katz P, Weber DR, et al. Adipocytokines and associations with abnormal body composition in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2021. doi: 10.1002/acr.24790. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menzaghi C, Trischitta V. The adiponectin paradox for all-cause and cardiovascular mortality. Diabetes 2018;67:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallace AM, McMahon AD, Packard CJ, et al. Plasma leptin and the risk of cardiovascular disease in the West of Scotland Coronary Prevention Study (WOSCOPS). Circulation 2001;104:3052–6. [DOI] [PubMed] [Google Scholar]
- 25.Romero-Corral A, Sierra-Johnson J, Lopez-Jimenez F, et al. Relationships between leptin and C-reactive protein with cardiovascular disease in the adult general population. Nat Rev Pract Cardiovasc Med 2008;5:418–25. [DOI] [PubMed] [Google Scholar]
- 26.Yang H, Guo W, Li J, et al. Leptin concentration and risk of coronary heart disease and stroke: a systematic review and meta-analysis. PLoS One 2017;12:e0166360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Xie Z, Bin Z. The association between serum leptin levels and cardiovascular events in patients with rheumatoid arthritis. Lab Med 2021;52:86–92. [DOI] [PubMed] [Google Scholar]
- 28.Lee CH, Woo YC, Chow WS, et al. Role of circulating fibroblast growth factor 21 measurement in primary prevention of coronary heart disease among Chinese patients with type 2 diabetes mellitus. J Am Heart Assoc 2017;6:e005344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikuls TR, Reimold A, Kerr GS, et al. Insights and implications of the VA Rheumatoid Arthritis Registry. Fed Pract 2015;32:24–9. [PMC free article] [PubMed] [Google Scholar]
- 30.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 31.Johnson TM, Sayles HR, Baker JF, et al. Investigating changes in disease activity as a mediator of cardiovascular risk reduction with methotrexate use in rheumatoid arthritis. Ann Rheum Dis 2021;80: 1385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SC, Schneeweiss S, Liu J, et al. Risk of venous thromboembolism in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65:1600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamariz L, Harkins T, Nair V. A systematic review of validated methods for identifying venous thromboembolism using administrative and claims data. Pharmacoepidemiol Drug Saf 2012;21 Suppl 1: 154–62. [DOI] [PubMed] [Google Scholar]
- 34.England BR, Sayles H, Mikuls TR, et al. Validation of the Rheumatic Disease Comorbidity Index. Arthritis Care Res (Hoboken) 2015;67: 865–72. [DOI] [PubMed] [Google Scholar]
- 35.Miriovsky BJ, Michaud K, Thiele GM, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis 2010;69:1292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pincus T, Yazici Y, Bergman M. Development of a Multi-Dimensional Health Assessment Questionnaire (MDHAQ) for the infrastructure of standard clinical care. Clin Exp Rheumatol 2005;5 Suppl 39:S19–28. [PubMed] [Google Scholar]
- 37.Cannon GW, Mikuls TR, Hayden CL, et al. Merging Veterans Affairs Rheumatoid Arthritis registry and pharmacy data to assess methotrexate adherence and disease activity in clinical practice. Arthritis Care Res (Hoboken) 2011;63:1680–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adamo L, Rocha-Resende C, Prabhu SD, et al. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol 2020;17:269–85. [DOI] [PubMed] [Google Scholar]
- 39.Beatty AL, Zhang MH, Ku IA, et al. Adiponectin is associated with increased mortality and heart failure in patients with stable ischemic heart disease: data from the Heart and Soul Study. Atherosclerosis 2012;220:587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu ZJ, Cheng YJ, Gu WJ, et al. Adiponectin is associated with increased mortality in patients with already established cardiovascular disease: a systematic review and meta-analysis. Metabolism 2014; 63:1157–66. [DOI] [PubMed] [Google Scholar]
- 41.Kamareddine L, Ghantous CM, Allouch S, et al. Between inflammation and autophagy: the role of leptin-adiponectin axis in cardiac remodeling. J Inflamm Res 2021;14:5349–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cavusoglu E, Ruwende C, Chopra V, et al. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur Heart J 2006;27: 2300–9. [DOI] [PubMed] [Google Scholar]
- 43.Minamino H, Katsushima M, Yoshida T, et al. Increased circulating adiponectin is an independent disease activity marker in patients with rheumatoid arthritis: a cross-sectional study using the KURAMA database. PLoS One 2020;15:e0229998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu D, Luo S, Li Z. Multifaceted roles of adiponectin in rheumatoid arthritis. Int Immunopharmacol 2015;28:1084–90. [DOI] [PubMed] [Google Scholar]
- 45.Wannamethee SG, Shaper AG, Whincup PH, et al. The obesity paradox in men with coronary heart disease and heart failure: the role of muscle mass and leptin. Int J Cardiol 2014;171:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frischmuth T, Hindberg K, Aukrust P, et al. Plasma levels of leptin and risk of future incident venous thromboembolism. Thromb Haemost 2022;122:560–9. [DOI] [PubMed] [Google Scholar]
- 47.Ong KL, Hui N, Januszewski AS, et al. High plasma FGF21 levels predicts major cardiovascular events in patients treated with atorvastatin (from the Treating to New Targets [TNT] Study). Metabolism 2019;93: 93–9. [DOI] [PubMed] [Google Scholar]
- 48.Wu L, Qian L, Zhang L, et al. Fibroblast growth factor 21 is related to atherosclerosis independent of nonalcoholic fatty liver disease and predicts atherosclerotic cardiovascular events. J Am Heart Assoc 2020;9:e015226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ong KL, Campbell S, Wu BJ, et al. Relationship of fibroblast growth factor 21 with subclinical atherosclerosis and cardiovascular events: multi-ethnic study of atherosclerosis. Atherosclerosis 2019;287: 46–53. [DOI] [PubMed] [Google Scholar]
- 50.Schwab P, Sayles H, Bergman D, et al. Utilization of care outside the Veterans Affairs health care system by US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2017;69:776–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


