Abstract
Exposure science is evolving from its traditional “after the fact” and “one chemical at a time” approach to forecasting chemical exposures rapidly enough to keep pace with the constantly expanding landscape of chemicals and exposures. In this article, we provide an overview of the approaches, accomplishments, and plans for advancing computational exposure science within the U.S. Environmental Protection Agency’s Office of Research and Development (EPA/ORD). First, to characterize the universe of chemicals in commerce and the environment, a carefully curated, web-accessible chemical resource has been created. This DSSTox database unambiguously identifies >1.2 million unique substances reflecting potential environmental and human exposures and includes computationally accessible links to each compound’s corresponding data resources. Next, EPA is developing, applying, and evaluating predictive exposure models. These models increasingly rely on data, computational tools like quantitative structure activity relationship (QSAR) models, and machine learning/artificial intelligence to provide timely and efficient prediction of chemical exposure (and associated uncertainty) for thousands of chemicals at a time. Integral to this modeling effort, EPA is developing data resources across the exposure continuum that includes application of high-resolution mass spectrometry (HRMS) non-targeted analysis (NTA) methods providing measurement capability at scale with the number of chemicals in commerce. These research efforts are integrated and well-tailored to support population exposure assessment to prioritize chemicals for exposure as a critical input to risk management. In addition, the exposure forecasts will allow a wide variety of stakeholders to explore sustainable initiatives like green chemistry to achieve economic, social, and environmental prosperity and protection of future generations.
Keywords: Chemical curation, Computational exposure science, Predictive exposure modeling, High-throughput, Non-targeted analysis (NTA), Machine learning, Uncertainty
1. Background/introduction
Computational exposure science represents a line of research that is both quickly advancing and critically needed for protecting human health and the environment (Cohen Hubal et al., 2010; Egeghy et al., 2016). Traditional single-chemical, retrospective exposure assessment approaches are no longer adequate to manage the enormous number of existing and new chemicals that are in commerce. Accordingly, the U.S. Environmental Protection Agency’s Office of Research and Development (EPA/ORD) is working to transform its exposure research portfolio to achieve a vision that provides chemical exposure foresight for thousands of chemicals at a time in an efficient and timely fashion. This vision recognizes regulatory needs for exposure screening, prioritization, and timely decision-making to manage the vast number of existing unevaluated chemicals along with new chemical registration applications. Furthermore, this vision will position exposure science to enable chemical management priorities related to environmental justice and cumulative impacts assessment and for a future of sustainability and environmental, social, and economic prosperity whereby exposure foresight is applied in a “green chemistry context” to inform chemical design and use (NAS, 2022; Anastas et al., 2021; Ganesh et al., 2021).
EPA/ORD’s computational research employs and further develops New Approach Methods (NAMs) for exposure to equip regulatory partners with state-of-the-art tools to support chemical safety decisions (Kavlock et al., 2018; Wambaugh et al., 2019). The stakes are high for public health and the environment due to the need to balance societal interests for chemical production and protection. Chemistry, engineering, and manufacturing play a large and critically beneficial role in the modern economy and society for disease prevention (e.g., Serafini et al 2020), food security (e.g., Popp et al 2013), and enhancing human well-being (e.g., Matlin & Abegaz, 2011). The chemical sector makes up a sizeable portion of the U.S. economy estimated at $5.2 trillion, representing 25 % of the U.S. Gross Domestic Product (GDP), and supporting 4.1 million jobs (NAS, 2022). Even so, the U.S. ranks third behind the European Union and China in chemical sales (The European Chemical Industry Facts and Figures, 2023). These beneficial interests need to be managed while also considering the threat that chemical pollution poses to ecosystems (Saaristo et al., 2018), biodiversity (Groh et al., 2022), environmental justice communities (Mah, 2016) and public health (Landrigan et al., 2018; Naidu et al., 2021; Rappaport, 2016), and a habitable planet (Rockström et al., 2009, 2023; Persson et al., 2022; Cousins et al., 2022). Although these consequences tend to be studied separately, there is growing appreciation for a one-health perspective that considers their interconnectedness (Prata et al., 2021).
EPA has various regulatory authorities for managing chemical environmental risks, e.g., Toxic Substances Control Act (Wilson and Schwarzman, 2009). Similarly, the European Union manages chemical risks under its Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) program (Williams et al., 2009) and more recently by establishing an assessment framework for ‘safe and sustainable by design’ chemicals and materials. Under these programs, Fantke et al., 2022, and Bruinen de Bruin et al., 2022 describe a supporting exposure science strategy. Chemical manufacturing in China has burgeoned over the last 20 years and has been associated with accidental releases resulting in increased regulation (e.g., “Law on Hazardous Chemical Safety of PRC”) (Wang et al., 2018). Considering the scale of chemical production, management requires transparent, systematic, timely, evidence-based decisions where computational approaches provide the only practical means of success.
To support these high stakes public policy decisions, computational exposure research must account for inherent complexities of chemical exposure. Exposure is a multifaceted phenomenon that spans diverse and intertwined domains of chemical, biological, physical, and social sciences traditionally represented as a continuum from source to dose and ultimately effect (Lioy, 2010). This traditional depiction ignores the compounded complexity of an exposure reality that encompasses an enormous and dynamic anthropogenic chemical space where little or no information is available for the vast majority of chemicals. The traditional exposure continuum represents one dimension of computational exposure science with the second dimension being one of enormous and highly diverse chemical space and the computational tools needed to integrate and process these dimensions efficiently and credibly. This complexity is well exemplified by the human and ecology health-relevant diverse range of chemicals in consumer products that numbers in the tens of thousands (Dionisio et al., 2018) with exposure to chemicals from near- and far-field sources occuring via multiple pathways, and with a strong social/behavioral determinant (Stanfield et al., 2021). The true number of chemicals and transformation products to which humans are exposed is unknown but likely is in the hundreds of thousands considering global trade and chemicals in commerce (Grulke et al., 2019; Wang et al., 2020). As such, EPA’s computational exposure science is evolving and includes the Exposure Forecasting (ExpoCast) program that was initiated in 2009 (Kavlock and Dix, 2010) to complement Toxicity Forecasting (ToxCast) in providing screening-level, high-throughput predictions to support next-generation risk assessment methods (Cohen Hubal et al., 2010; NRC, 2007, 2012; Egeghy et al., 2016; Wambaugh et al., 2019). This evolution has been leveraged by complementary computational exposure research occurring outside the Agency including the work of Csiszar et al., 2016 and Jolliet et al., 2021.
Scientific, public health, and regulatory interests strongly align in the continued development and advancement of computational exposure science (Vandenberg et al., 2023). Interests and research development efforts parallel those of computational toxicology (Thomas et al., 2019). The objective of this article is to provide: 1) our strategic research themes for advancing computational exposure science; 2) an overview of accomplishments; and 3) research plans extending over the next 5 years. Our working definition of computational exposure science incorporates three defining characteristics: predictive, rapid (e.g., conducted in hours or days rather than months or years), and high-throughput (e.g., considers thousands or tens of thousands of chemicals). These characteristics allow the Agency to assess exposure more comprehensively and at a pace and scale proportionate to the realities with which chemicals are placed in commerce with potential for release.
2. Computational exposure science at the cutting edge
The research themes defining the cutting edge of exposure science represent an evolution of the traditional elements of methods development, measurements, and models described a few decades ago (Ott, 1990). Technological advances in analytical instrumentation, data availability, and computing together with the maturation of machine learning algorithms, have transformed these elements into their computational equivalents.
There are three broad strategic themes to EPA’s computational exposure research program: 1) chemical data curation; 2) data acquisition and integration, including the application of measurement methods employing non-targeted analysis; and 3) model development. Complementing and cutting across these scientific advances are efforts to make the research readily useable to decision makers and the public, explicitly quantifying uncertainty, providing transparency, and ensuring reproducibility. There is an order to these efforts with chemical data curation serving a “core” role, feeding into data development efforts and ultimately culminating in models that deliver high-throughput exposure predictions (Fig. 1). We provide below an overview description of EPA’s research advances on these fronts and future plans, followed by a case study related to chemicals in consumer products to illustrate application, integration, and policy relevance.
Fig. 1.
Major components of computational exposure research program and their relation to one another.
2.1. Chemical data curation
Exposure science relating to chemicals relies upon knowledge of chemicals and associated properties that define interactions occurring across physical, environmental, and biological systems. Hence, a high-quality database of chemical substances, structures, and associated data, supported by chemical curation and enabling accurate property and data associations, is foundational to research in computational exposure and toxicology (Thomas et al., 2019). There are three defining characteristics of this effort: 1) a comprehensive accounting of the anthropogenic environmental chemical landscape; 2) high-level confidence in the accuracy with which each chemical, structure, and related meta data are specified; and 3) integration and accessibility of chemical lists and associated data for use in computational modeling, decision support, and as a public resource.
EPA’s Distributed Structure-Searchable Toxicity (DSSTox) chemical database project launched over 20 years ago with the goal of providing curated, high-quality structure-data associations to support predictive modeling in toxicology (Richard and Williams, 2002; Grulke et al., 2019). In the intervening decades, the database has expanded considerably to over 1.2 million substances (Grulke et al., 2019; Williams et al., 2017). DSSTox represents one of the highest quality sources of chemical substance and structure linked to exposure-related data. It has grown to be one of the most comprehensive collections of substances of anthropogenic origin potentially released to the environment. It includes chemicals from across a wide range of source categories with potential for environmental release or exposure (e.g., industrial, consumer products, food packaging, pharmaceuticals, biosolids) and includes environmental degradants and metabolites. Critical to its success has been the attention to ensuring quality associations of chemical identifiers (such as Chemical Abstract Services Registry Numbers–CAS-RN, chemical names, and structures) to corresponding data and chemical lists. Inconsistencies in chemical identifiers and errors in chemical-data associations, which propagate through models, are pervasive across public internet chemical resources (Williams et al., 2017). In contrast, the close coupling of expert manual curation with automated quality checks, and a strict enforcement of 1:1:1 mappings of CAS-RN, chemical name, and structure to a unique substance ID (DTXSID), sets the DSSTox project apart (Grulke et al., 2019). This focus on quality chemical substance annotations and data associations is essential to the foundational role DSSTox plays in supporting computational exposure and toxicology research and downstream modeling efforts. Plans over the next 5 years are to continue curating DSSTox records for chemicals and lists, as well as expand curation of chemical relationships (e.g., for parent compounds, metabolites, and transformation products) and chemical groupings of importance to EPA’s program offices and researchers (e.g., per/polyfluorinated alkyl substances, or PFAS). In addition, there will be increased focus on registration of polymers, mixtures, and ill-defined substances, adding more detailed annotation and linkages to defined structure substances, as well as on emerging contaminants identified through non-targeted analysis (NTA). This work will be accomplished using existing strategies and processes that have been successful in achieving the current listing (Grulke et al., 2019).
The DSSTox chemical substance database underpins EPA’s CompTox Chemicals Dashboard (hereafter referred to as “Dashboard”; Williams et al., 2017), where chemical structures and associated identifiers serve as primary integrators of multiple data streams also supported in the Dashboard. The Dashboard provides a portal to a wide range of chemical lists (currently > 420) spanning regulatory, chemical use, literature, and structure-based groupings. Chemical identifiers input into the batch search enables download of related content, including structures, predicted properties, toxicity, bioactivity data, and list associations (Lowe and Williams, 2021). The Dashboard also provides access to a wide range of chemical substance-linked resources and tools within EPA and across the Internet. The Dashboard provides uniformity and specificity in the representation of chemicals and serves a critical linking function across measurements, data development, and modeling research efforts. We expect exposure-relevant curation and linkages to continue to expand over the next 5 years meeting the needs of EPA program offices as exemplified by recent efforts related to biosolids (Richman et al., 2022) and organohalogen flame retardants (Bevington et al., 2022).
2.2. Acquisition and integration of exposure-relevant data
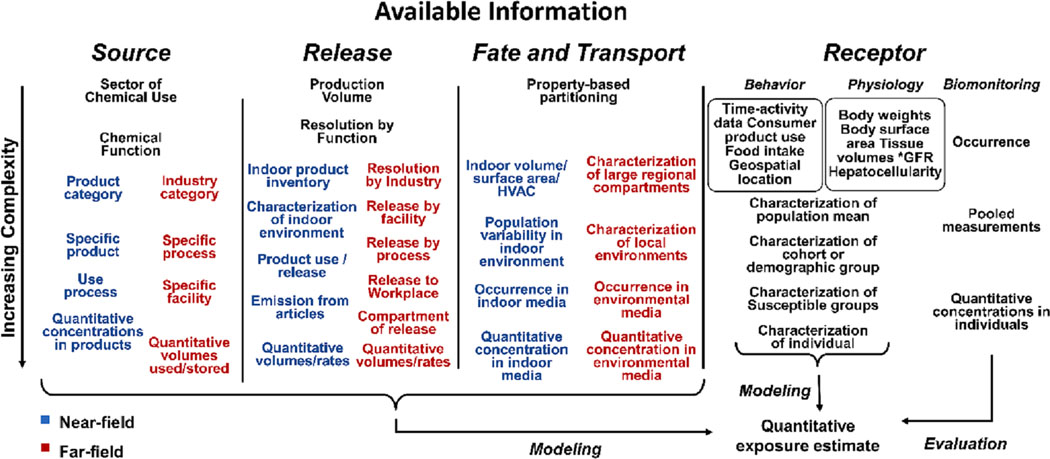
The data requirements for assessing chemical environmental exposures reflect its complexity in spanning chemical, physical, biological, environmental, and social sciences. These sciences all factor into the source to dose continuum conceptual framework representing exposure science (Fig. 2). The complexity represented here is magnified by an exposure-relevant chemical landscape that is very large, poorly characterized, and of widely varying properties.
Fig. 2.
Varying complexity in exposure-relevant data streams. The simplest data may be very general, while more detailed data may be specifically relevant to near-field and far-field exposure sources. Data availability dictates for which exposure pathways, exposure scenarios, and populations defensible exposure predictions can be developed. *GFR = Glomerular Filtration Rate.
Our data development efforts are strongly integrated with modeling. Data form the basis of model development and models inform data collection needs. Data streams are being developed in a manner consistent with recommendations for standardized organizing infrastructures that support increasing useability and accessibility of data (NAS, 2018). Our efforts are employing informatics, data mining, and data infrastructure technologies to support collection, organization, and integration of existing and emerging exposure data streams. These efforts are increasing the volume, quality, and accessibility of exposure-relevant data while enhancing the reproducibility and defensibility of the risk-based decisions they support allowing the rapid incorporation of new data into decision support frameworks and tools. Finally, these efforts enable the development of machine-learning models for filling gaps for data-poor chemicals. The data streams across the exposure continuum are depicted in Fig. 2 and described below.
2.2.1. Chemical source and release
Quantitative estimates of the amount of chemicals released or emitted from near-field or far-field sources is a key driver of receptor exposure (Arnot et al., 2012; Isaacs et al., 2014). Sources are generally classified in two ways: 1) commercial sector use, i.e., consumer, industrial, or agricultural; and 2) proximity to the receptor, i.e., near-field or far-field (Jayjock et al., 2009). Consumer product releases drive near-field (indoor) exposures as well as serve as a source for down-the-drain and out-the-window flows and are informed by quantitative information on chemical concentrations in products and chemical emission processes (e.g., release from articles or the releases from the use of consumer products). Over the last decade, we have focused efforts on the consumer product source category. This has included the curation of chemical ingredients in consumer products developed from public documents and classified by use (Dionisio et al., 2015, 2018; Goldsmith et al., 2014). These data have been used as a basis for estimating median population human exposure (Isaacs et al., 2014; Wambaugh et al., 2014; Ring et al., 2019). Future work is planned that will address two important data gaps: 1) the application of computational exposure strategies to access data poor chemical exposures occurring within the workplace (Minucci et al., 2023); and 2) chemical exposures that result from consumer articles such as furnishings and building materials.
There are far-field source data gaps associated with specific facilities, unique industrial processes, and agricultural applications. Current approaches rely on very crude surrogates such as production volume (Arnot et al., 2012; Ring et al., 2019) and property-based partitioning into compartments of release. To address this data need, we are developing approaches that use chemical function to inform releases (Li et al., 2021). Furthermore, we are working to develop models that will integrate available release information and downstream monitoring data (e. g., air or water) to develop quantitative estimates of loadings into different environmental compartments. Data models and databases that organize release information from different sources (Meyer et al., 2020) will be expanded to include predicted releases and integrated with chemical curation workflows as was done for surface water in Sayre et al., 2022.
2.2.2. Chemical fate and transport
Fate and transport are governed by chemical properties and environmental conditions. Data that characterize both the residential/indoor and outdoor environments are needed for characterizing movement and ultimate partitioning of chemicals. Environmental monitoring data provide the gold-standard fate information for use in assessments and for evaluating models of chemical fate and transport. There exist dozens of potentially relevant environmental media including ambient and indoor air, soil, surface-, ground-. waste-, and drinking- water, food, biosolids, precipitation, house dust, and sediment. We have made a major advance in support of fate and transport characterization and modeling by developing a harmonized database comprised of large amounts of monitoring data linked to media and chemical identifiers (Isaacs et al., 2022).
2.2.3. Human receptors
Human exposure is influenced by both behaviors and physiology. Behavioral information captures receptor activities that impact what sources, releases, and contaminated environmental media are encountered. This includes time-activity data that quantifies time spent in different microenvironments (McCurdy et al., 2000), exposure-relevant microactivities (e.g., bathing, smoking), food and drinking water intakes (Vieux et al., 2020), and consumer product use patterns (Isaacs et al., 2014). These consumer and household “habits and practices” data quantify the use of consumer products (e.g., detergent, paint, disinfectants) within the home including the nature and frequency of use, as well as the possession of furnishings, consumer articles, or building materials relevant to indoor sources (e.g., vinyl flooring or wallcoverings, foam-containing furniture, large electronics). These data traditionally come from cross-sectional surveys that can be expensive and quickly become dated. There is a recognized need to acquire data that are more dynamic and contemporary such as information inferred from social media or alternative “big data” sources (Ben et al., 2019). Physiological information is required to quantify exposure intakes (such as those impacted by breathing rates), assess their magnitudes relative to body weight or body surface areas, and predict fate and transport of chemicals within the body (i.e., toxicokinetics). Data relevant to understanding variability in absorption, distribution, metabolism, and elimination (ADME) of chemicals (and thus ultimate tissue concentrations) include tissue or organ volumes and blood flows, glomerular filtration rate (GFR), hepatocellularity, and cytochrome P450 enzyme expression (Wetmore et al., 2014).
2.2.4. Emerging technologies and data sources
The previous sections specify multiple domains along the source-to-dose continuum for which critical data gaps continue to exist. To address these gaps, we are developing and employing new informatic, computational, and analytical tools to support the collection and organization of exposure relevant data, as well as sustainable infrastructures for providing these data to the public and decision-makers. This includes informatics tools for identifying, extracting, and curating data from public documents including innovative literature retrieval systems (Baker et al., 2018) and sustainable applications for document management, data curation (Mansouri et al., 2016), and quality tracking. Non-traditional survey instruments take advantage of smart-phone technology and “the quantified self” to provide data related to consumer habits and practices (Von Goetz, N et al., 2018) activity patterns (Straczkiewicz et al., 2021), or microactivities (Myslín et al., 2013). In addition, other non-traditional data streams such as consumer product purchase information are being used to augment traditional field- or panel-study information on behavior (Tornero-Velez et al., 2021; Stanfield et al., 2021). Advanced/emerging monitoring technologies include sensor technologies (Peltier and Buckley, 2020) and non-targeted analysis (see below) that provide expanded capabilities for individual-based measurements and for the measurement of much larger chemical space, respectively. Machine learning methods are being used for data extrapolation and read-across using available chemical-specific property, use, and structure descriptors to fill gaps for thousands of data-poor chemicals. Lastly, sustainable data infrastructure and delivery systems such as the Dashboard will continue to be developed so that data are readily accessible to stakeholders in a timely, transparent, and usable manner.
2.2.5. Measurement at a scale of chemical exposure reality
High-resolution mass spectrometry (HRMS) and NTA methods are providing cutting-edge measurement capabilities that are enabling advances in computational exposure science. NTA methods rely on HRMS to rapidly acquire data on thousands of molecular features and then implement sophisticated workflows to assign likely chemical structures. These methods are cutting-edge in two ways: 1) the measurable chemical space is on par with the number of anthropogenic chemicals present in products and the environment; and 2) it is possible to detect chemicals without a priori knowledge of their presence in a sample or the availability of a standard (Guo et al., 2020). NTA provides a means to reveal the vast unknown of chemical environmental contaminants suggested by Landrigan et al., 2018, and expand interdependent quantitative modelling (Sobus et al., 2018). Examples of NTA applications and impact include chemical surveillance and discovery in house dust (Rager et al., 2016; Panagopoulos Abrahamsson et al., 2021), drinking water (Newton et al., 2018), consumer and personal care products (Phillips et al., 2018), recycled materials (Lowe et al., 2021a), human biological samples (Chao et al., 2022), and PFAS source attribution (Washington et al., 2020). Building on these successes, efforts are now being made toward applications for disaster and emergency response (Phillips et al., 2022).
The application of NTA to computational exposure science is constrained by technical challenges to broad adoption. Technical challenges stem from a lack of standardized methods and tools for performing NTA studies, as well as a lack of available training resources for lab personnel that are new to NTA. Indeed, successful NTA projects require combined expertise in analytical chemistry, HRMS, cheminformatics, and data processing/statistical analysis procedures. We are therefore developing and evaluating tools to overcome these constraints thereby enabling broader adoption of NTA by Agency clients and partners. This includes the development of HRMS reference data for the universe of anthropogenic chemicals and their degradants potentially present in the environment. DSSTox supports this activity by containing: 1) a growing inventory of curated chemical substances; 2) MS-ready structure (McEachran et al., 2018) mappings between registered substances and measurable (via HRMS) structures; and 3) metadata to help prioritize chemical candidates (McEachran et al., 2017) including predicted insilico spectra and prevalence on chemical lists such as ExpoCast and ToxCast (McEachran et al., 2019). Chemistry web applications, such as the Dashboard, allow easy access to these data through advanced batch and structure searching capabilities. Future developments will include the aggregation and harmonization of public domain experimental spectra generated within the agency or provided by collaborators. The Dashboard provides access to tools that aid in chemical identification (McEachran et al., 2020) based on the method of analysis (Lowe et al., 2021b) and retention time indices (Aalizadeh et al., 2021). DSSTox content is being expanded to enable NTA application to PFAS measurement as a chemical class of interest to many stakeholders (Williams et al., 2022). It is further being developed to support collaboration with other researchers who are developing specialized tools for chemical identification (e.g., FluoroMatch described in Koelmel et al., 2022).
The need for standard or consensus NTA methods is being addressed through EPA/ORD’s Non-Targeted Analysis Collaborative Trial (ENTACT), which was initiated to characterize differences across laboratories, identify best practices, and harmonize approaches (Ulrich et al., 2019). ENTACT prompted an international collaborative workgroup called Benchmarking and Publications for Non-Targeted Analysis (BP4NTA) (Place et al., 2021). The BP4NTA workgroup is a part of the cutting-edge effort in promoting and coordinating NTA efforts across government, academia, and industry thereby accelerating the pace with which standardized NTA methods are developed and adopted (Peter et al., 2021; Fisher et al., 2022; see resources at https://nontargetedanalysis.org/).
NTA is central to our computational exposure science efforts in identifying understudied or previously unknown chemicals in products and environmental and biological media at a scale comparable to chemicals in commerce. Advances are being made in mapping the relevant chemical space to various NTA methods (Black et al., 2022). In the next 5 years, we plan to expand NTA in multiple ways including: 1) development of methods to support quantitative non-targeted analysis (qNTA) and procedures for estimating quantitative uncertainty, building on the work of McCord et al., 2022 and Groff et al., 2022; and 2) expansion of NTA laboratory capabilities through technology transfer to state and regional stakeholders greatly extending chemical monitoring efforts supporting local public health interests. The availability of quantitative NTA and associated methodologies and tools is necessary for NTA to be fully utilized within a risk assessment context. This is important because risk assessment underlies the mission of many government laboratories. Because NTA encompasses a large and data-poor chemical space, the computational exposure science methods described here are necessary and relevant. Accordingly, there is a synergy and interdependence between between qNTA and computational exposure science methods in support of risk assessment.
2.3. Development of high throughput exposure models
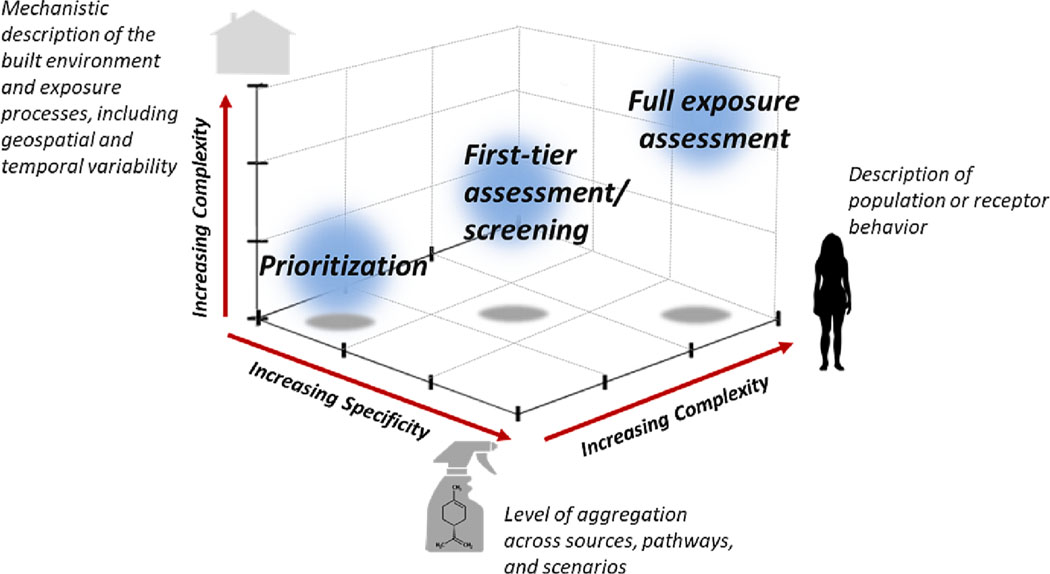
Modeling takes on critical importance for computational exposure science as it is the only practical means by which chemical exposures across an inherently large and poorly characterized chemical space can be estimated. Although exposure modeling is a well-established field with a long history (Jayjock et al., 2007), high-throughput exposure (HTE) model development efforts have largely occurred only over the past decade. There are three defining characteristics to HTE models and derived estimates: 1) large numbers of chemicals, i.e., ten-to-hundreds of thousands; 2) data-poor chemicals; and 3) greater uncertainty relative to traditional modeling approaches making them more suitable for screening or prioritization (Csiszar et al., 2016; Jolliet et al., 2021; Arnot et al., 2006; Isaacs et al., 2014; Wambaugh et al., 2013). As illustrated in Fig. 3, data requirements are partially dictated by the complexity and specificity of the needed predictions. HTE models trade precision for capacity (Wetmore et al., 2015; Sipes et al., 2017; Ring et al., 2017). For appropriate decision-making contexts such as prioritization, we aim to tailor the complexity of the models to the granularity of the application (e.g., days vs. hours or individuals vs. average population) and the availability of data to parameterize that model. The quantification of uncertainty is a key consideration in model development and application and is discussed as a cross-cutting issue below.
Fig. 3.
Fit-for-purpose exposure models. As decision contexts move from priority-setting to screening to exposure assessment, descriptions of receptors and the built environment become more complex, while the level of aggregation of exposures may decrease, with all potential exposures considered in prioritization, whereas specific statute-based scenarios are considered in full assessments.
Computational exposure modeling has made enormous progress relying on the National Health and Nutrition Examination Survey (NHANES) biomonitoring data in an approach termed the Systematic Empirical Evaluation of Models (SEEM). This approach has been used to estimate exposure for nearly 8,000 chemicals based on data from 106 NHANES biomarkers (Wambaugh et al., 2014) and again more recently using a model ensemble approach to screen exposure for nearly 700,000 chemicals (Ring et al., 2019). This current version of the SEEM framework incorporates multiple predictors of exposure, including HT models for consumer (Isaacs et al., 2014; Shin et al., 2017; Li et al., 2018) and far-field pathways (Arnot et al., 2012). However, the current version does not take location into account or address specific cohorts and has limitations in how existing exposure predictors are extrapolated to data-poor chemicals. Research is underway to address these limitations as well as to identify and characterize exposure pathways and chemicals outside the domain of the current model. In addition to these updates to the SEEM framework, research will address the need for geospatial resolution in HTE models such as the Stochastic Human Exposure and Dose Simulation High Throughput (SHEDS-HT) model, to allow for the integration of population and/or spatial variability in near-field and far-field sources as Wodtke et al. (2022) have recently shown to be important.
2.3.1. High throughput toxicokinetics
A primary application of computational exposure science is for the purpose of exposure screening or prioritization requiring comparison to the target dose that results in biological activity or toxicity. This comparison is achieved through toxicokinetic modelling. Chemical-specific toxicokinetic data are unavailable for most chemicals in commerce (Bell et al., 2018) although this is beginning to change as in vitro assays are developed to characterize key aspects of toxicokinetics (Rotroff et al., 2010; Breen et al., 2021a). Over the past decade, an extensive library of chemical-specific in vitro measurements has been developed for chemicals in commerce and the environment (Wetmore et al., 2012; Paini et al., 2019). Generic (chemical independent) toxicokinetic models have been developed for use with in vitro toxicokinetic data to couple exposure predictions with in vitro screening data (Wetmore et al., 2015; Breen et al., 2021b). These models aim to cover key routes of exposure (Linakis et al., 2020) and can include key aspects of population variability in physiological processes that drive toxicokinetics (Ring et al., 2017). Future research will build on work of Ring et al., 2017 with an emphasis on reducing key sources of model uncertainty (e.g., route specific bioavailability, restricted vs. non-restricted clearance) and evaluating variability within sensitive or highly exposed populations including those occupationally exposed.
2.3.2. Integration of data and models
Models and measurements are intertwined and iterative. We build and evaluate models based on what we can measure, make models to predict what we cannot measure, and generate measurements for what we cannot model. The more chemical space covered by measured data, the more model predictions can be made. The simpler the model, the easier it is to get chemical-specific data, e.g., Wambaugh et al., 2014. The importance of near-field sources to HTE modeling (Wambaugh et al., 2013) led to new, high-throughput consumer exposure models including SHEDS-HT (Isaacs et al.,2014), Ex-Priori (Hubbard et al., 2022), and RAIDAR-ICE (Li et al., 2018) as well as monitoring databases (Dionisio et al., 2018). The availability of the extensive DSSTox chemical structure database coupled with new structure-based models for predicting physio-chemical properties (Mansouri et al., 2018, 2019) accessed from the Dashboard, led to the third generation SEEM model making predictions of general population daily intake rate for nearly half a million chemical structures (Ring et al., 2019).
Human exposure is highly dynamic. However, the available data and models used to represent exposure tend to be static and incremental so that model estimates lag contemporary reality by years or decades. Recognizing trade-offs of uncertainty, duration, and cost of chemical assessments (Hagiwara et al., 2022), our research efforts optimize workflow efficiency related to aggregation, curation, and harmonization of data inputs into model runs. For example, improved approaches for the extraction, harmonization and curation of data have been developed (Mansouri et al., 2016) and are being used to extract physiochemical property data from public domain resources. A model registration system and workflow managing the relevant aspects of modeling are being developed to include: 1) automated splitting of training and test sets; 2) standardized description and methodology for generation of descriptors; and 3) automated approaches to make new predictions with an appropriate model version.
Workflows to integrate exposure data and model predictions into decision-making for risk assessment are also critical. We are actively developing workflows to integrate exposure information with hazard/toxicity information for risk-related decision-making. Efforts include the Public Information Curation and Synthesis (PICS) workflow for chemical prioritization under TSCA (US EPA, 2021) and RapidTox workflows for decision-making in contexts including emergency response (Mumtaz et al., 2021). In the next five years, we plan to continue the development of these workflows for high-priority applications of risk-related decision-making, including: 1) a workflow for the rapid evaluation of potential hazards and exposures to children and women of child-bearing age using Toxicity Estimation Software Tool (T.E.S.T.); 2) an application of NTA as part of a multi-omics evaluation of exposure and health; 3) workflows specific to the process of evaluating exposure potential for new chemicals under TSCA; 4) exposure scenarios of regulatory interest including occupational exposures and aggregate exposures within specific locations or demographic groups believed to be at risk; and 5) workflows developed in collaboration with EPA’s Office of Water for risk screening of chemical contaminants in biosolids.
2.4. Cross-cutting considerations
The elements making up computational exposure science described above all constitute lines of quantitative evidence. The goal is to develop and provide this quantitative evidence in a way that informs transparent, reproducible decision-making in rapid, predictive, and health-protective risk assessment (NAS, 2017). This goal requires quantitative evaluation of uncertainty and variability in both measured data and model predictions. It also requires publicly available software tools so that analysis workflows can be integrated and reproduced. Finally, it involves scientific review and effective communication about the data, models, and software tools to enable decision-makers to use them easily and confidently. These considerations are adopted from computational toxicology as described in Thomas et al., 2019 and more recently van der Zalm et al., 2022.
Data development tools are being designed for transparency, reproducibility, and flexibility. These tools collate data and meta data from available non-standardized, widely varying formats, applying transparent, reproducible workflows to clean, standardize, and harmonize the data format. This is achieved while maintaining a clear “chain of custody” back to the original data (i.e., no destructive editing). Furthermore, a link to the original reporting context is maintained. In the next five years, we plan to expand and refine our existing data informatics and infrastructure tools for curation, data development, and modeling as described above. Network modeling is of particular interest as an approach to relate and link heterogeneous data streams. In all applications, data architecture will be carefully designed for transparency, reproducibility, and flexibility, and planned for ultimate integration with existing tools such as the Dashboard.
Transparent quantification of uncertainty and variability is a key consideration in model development efforts. The capture and transparent portrayal of uncertainty and variability is a key consideration in model development efforts. This is accomplished by forward propagation of uncertainty and variability in model parameters to predictions using a Monte Carlo approach where estimates are derived by trying different randomly sampled combinations of parameters to map out a range of possible solutions (Bauer, 1958). Examples include SHEDS-HT (Isaacs et al., 2014), and HTTK-Pop (Ring et al., 2017; Breen et al., 2022). For model parameter values derived from data, uncertainty is estimated using statistical modeling approaches. For example, median population exposures and their 95 % credible intervals are inferred from urine biomonitoring data using Bayesian methods (Stanfield et al., 2022). Bayesian methods refer to inferring distributions of plausible parameter values that are consistent with available data and prior assumptions (Eddy, 2004). Uncertainty in toxicokinetic parameters estimated from a database of in vivo concentration vs. time data (CvTdb) is quantified using meta-regression methods where disparate but potentially informative sources of information are combined to derive a consensus model to determine a range of plausible answers or predictions (Van Houwelingen et al., 2002; Sayre et al., 2020; Wambaugh et al., 2019a). Transparency and reproducibility are achieved by publishing model and analysis code in peer-reviewed papers (Groff et al., 2022; Ring et al., 2019), on GitHub, and on platform-specific repositories such as the Comprehensive R Archive Network (CRAN). Furthermore, we plan to build on existing work to apply statistical modeling to estimate uncertainty and evaluate confidence in NTA and toxicokinetic results.
Our research delivery is consistent with FAIR principles (Findability, Accessibility, Interoperability, and Reuse of digital assets; Wilkinson et al., 2016). Data and models, as well as examples for use and descriptions of their development and functionality, are downloadable via EPA’s website (https://www.epa.gov/chemical-research/downloadable-computational-toxicology-data), hosted on figshare, GitHub, CRAN, and other easily accessible sites. An increasing amount of data is also available for incorporation in models via our APIs (https://api-ccte.epa.gov/docs/index.html). Development of research products is tailored toward maximizing data for multiple uses, in part by adopting widely available terminology ontologies such as the UMLS Metathesaurus, which links millions of terms and term relationships (Bodenreider, 2004).
Despite compelling justification for the need of computational exposure science and enormous progress in its advancement, there remain substantive challenges to adoption within a risk management decision context. These challenges fall into three general and related categories: 1) chemicals tend to enter the regulatory pipeline one at a time favoring traditional exposure assessment approaches; 2) there is appreciable inertia that needs to be overcome and associated lag in adoption and incorporation of new computational tools and data resources into existing decision work flows; and 3) the need to develop data science expertise within organizations responsible for risk management decisions facilitating the adoption and use of computational exposure tools and data resources within existing decision workflows.
3. Chemicals in consumer products case study
We have chosen the consumer pathway based on its relative importance among exposure pathways and the diversity of relevant chemicals. We highlight propylparaben (PP) as an example to show the relevance and integration of research strategy elements across a variety of efforts. PP is a preservative found in many water-based cosmetics including creams, lotions, shampoos, and bath products. It is also used as a food additive. It functions as an antifungal and antimicrobial agent. PP is currently being evaluated for endocrine receptor activity under EPA’s Endocrine Disruptor Screening Program and has been included on the TSCA Workplan for existing chemicals. Unambiguous chemical identification is fundamental to efforts to evaluate exposure. Chemicals in consumer products were initially identified by mining publicly available Material Safety Data Sheets (MSDS) from a large consumer product retailer where 1,797 unique chemicals in 8,921 products were identified (Goldsmith et al., 2014). The database was further developed and expanded to include >20,000 unique chemicals from >61,000 consumer products (Dionisio et al., 2018; Williams 2017). These data complement European efforts to inventory chemicals in cosmetics (n = 2,878; 2006, Decision 2006/257/EC). In CPDat V3, data for PP was curated from 805 public documents, reflecting 1040 different products in 79 unique product categories. Products that were reported to contain PP included 65 categories of personal care products (including many cosmetics), several arts and crafts products (e.g., adhesives), children’s products (e.g., bubble solution) as well as air fresheners, carpet and floor cleaners, and pet products.
NTA of consumer products made with virgin and recycled materials (Phillips et al., 2018; Lowe et al., 2021) identified PP even in products for which it was not reported by the manufacturer, including paper products, fabric products, and plastic children’s toys. This demonstrated the utility of NTA in augmenting reported ingredient information. Future research is planned to analyze larger samples of representative products with greater potential for human and ecological exposures.
The chemical curation effort of mapping chemicals to products addresses a fundamental need by establishing the near-field source term for source-to-dose models. The next key data needs for the consumer product scenario relates to better identification of those who are purchasing and using specific consumer products and a better understanding of their “habits and practices” when using the products. We have evaluated demographic patterns of product use through access to nationally representative longitudinal market research data (Tornero-Velez et al., 2021). Further, by linking products with the chemicals they contain via DSSTox, we evaluated chemical co-exposures, and highly exposed subpopulations (Stanfield et al., 2021). This analysis identified PP as the second most prevalent endocrine-active chemical brought into homes via purchased consumer products. PP is also a key component of chemical combinations whose prevalence varied by population group. These results informed the selection of PP-containing mixtures for novel in vitro toxicity testing of mixtures. A data mining approach similar to that used in the Nielsen analyses has been applied to NHANES biomonitoring data to identify demographic patterns of co-exposure to consumer product-related chemicals (Kapraun et al., 2017). In that analysis, PP was not only found to co-occur with other parabens among at least a third of the U.S. population, but the prevalence of the combinations varied by demographic group. As both Nielsen and NHANES data lag current exposures by several years, we are exploring a dynamic data pipeline for contemporary product use information relying on publicly available social media such as Google Trends search data. Quantitative data on chemical concentrations in environmental or biological media have also been curated and harmonized within the Multimedia Monitoring Database (Isaacs et al. 2022). Monitoring input has included NTA to identify consumer product chemicals in residential environmental media including dust, air, and drinking water. In MMDB, PP concentrations were reported in several media including surface water and fish, as well as human urine, blood, breastmilk, and amniotic fluid.
Research advances relating to modeling chemicals in consumer products includes the development of a HTE model for consumer pathways (SHEDS-HT, Isaacs et al., 2014) and integration of SHEDS-HT and other HT consumer models into consensus aggregate exposure predictions within the Systematic Empirical Evaluation of Models (SEEM) framework for thousands of chemicals (Ring et al., 2019). SHEDS-HT was parameterized with the quantitative data from CPDat for over 1000 chemicals to generate SEEM exposure predictions. PP was one of 114 chemicals with available NHANES biomonitoring data that enabled calibration of the SEEM model estimates (mg/kg-BW/day intake of chemical) and quantification of uncertainty. Plans to expand and improve SEEM predictions includes the application of machine learning for extrapolating existing consumer exposure predictions from SHEDS-HT to data-poor chemical-product combinations and integration with NTA to improve characterization of consumer exposure sources. Ultimately this work can support identification of priority chemical mixtures for risk evaluation and the characterization of unique exposure sources among sensitive or underrepresented populations building on the work of Tornero-Velez et al., 2021 and Kapraun et al., 2017.
A principal goal of these efforts is to provide EPA regulators and other stakeholders with timely, transparent, and credible exposure-related information to support evaluations or management decisions concerning chemicals used in consumer products. Examples include data extraction, curation, and measurement efforts related to chemicals in children’s consumer products supporting chemical pre-prioritization under TSCA, and evaluation of chemicals under the Minnesota Department of Health’s (MDH) Toxic Free Kids’ program (https://www.health.state.mn.us/communities/environment/childenvhealth/tfka/reports.html). These resources have also been used to score nominees for the MDH Chemicals of Emerging Concern (CEC) initiative (Isaacs et al., 2023) and have been used in collaborative efforts to support the identification and prioritization of breast-cancer related chemicals for further study based on mixture exposure potential (Koval et al., 2022). Other collaborative efforts include the use of Quantitative Structure Use Relationship (QSUR) models to support the development of a comprehensive inventory of flame-retardant chemicals to support assessments by the Consumer Product Safety Commission (Bevington et al., 2022). At the same time, chemical manufacturers are using computational exposure modeling approaches for evaluating risk to consumers and workers from exposure to mixtures (Sauer et al., 2020) and informing formulation decisions (Sunger et al., 2020).
4. Conclusions
There are numerous challenges to managing chemical risks within modern society. In the U.S. and across the globe, there are many historical examples of lapses resulting in harms to human health and the environment that became apparent after the fact and after great cost to public health had occurred (e.g., lead, asbestos, dichlorodiphenyltrichloroethane (DDT), polychlorinated biphenyls (PCBs), and the ozone destroying chlorofluorocarbons) (Landrigan et al., 2018). A critical aspect of the challenge to chemical management is the enormous gap in available information relating to exposure, hazard, and risk within the timeframe of regulatory and commercialization decisions, especially considering the many thousands of chemicals that are already in commerce (Woodruff et al., 2023). EPA’s computational exposure research complements its computational toxicology efforts and together are designed to substantively close this gap in information and time. There are three strategic components to this program including chemical curation, data development, and modeling. Further, the data and tools developed follow FAIR principles (Findability, Accessibility, Interoperability, and Reuse of digital assets; Wilkinson et al., 2016) in being public, transparent, as well as tailored to EPA program office priorities and workflows for ready incorporation into management decisions. The resulting research is transformative toward achieving a goal of providing exposure foresight at a scale and within a timeframe commensurate with chemicals being placed in commerce and human and ecological receptors exposed. This research provides a critical piece to the environmental health puzzle that is needed not only to protect human health and the environment, but to address environmental injustice (Landrigan et al., 2018), “reinvent our relationship with planet Earth,” and “become effective stewards of the global commons — the climate, ice, land, ocean, freshwater, forests, soils, and rich diversity of life that regulate the state of the planet and combine to create a unique and harmonious life-support system” (NAS, 2021).
Acknowledgements
The authors are grateful to Nicolle Tulve and Dan Vallero for their constructive and insightful internal technical review.
Funding source
The U.S. Environmental Protection Agency funded the research outlined in this manuscript.
Footnotes
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: On behalf of my coauthors and I, I hereby declare that we have no competing financial or personal relationship conflicts of interest that would bias the research presented in our article “Cutting-Edge Computational Chemical Exposure Research at the U.S. Environmental Protection Agency.”
Disclaimer: The United States Environmental Protection Agency (U.S. EPA) through its Office of Research and Development has subjected this article to Agency administrative review and approved it for publication. Mention of trade names or commercial products does not constitute endorsement for use. The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the US EPA.
Data availability
No data were used for the research described in the article.
References
- Aalizadeh R, Alygizakis NA, Schymanski EL, Krauss M, Schulze T, Ibáñez M, McEachran AD, Chao A, Williams AJ, Gago-Ferrero P, Covaci A, Moschet C, Young TM, Hollender J, Slobodnik J, Thomaidis NS, 2021. Development and application of liquid chromatographic retention time indices in HRMS-based suspect and nontarget screening. Anal. Chem 93 (33), 11601–11611. 10.1021/acs.analchem.1c02348. Epub 2021 Aug 12 [DOI] [PubMed] [Google Scholar]
- Anastas PT, Zimmerman JB, 2021. Moving from protection to prosperity: evolving the U.S. Environmental Protection Agency for the next 50 years. Environ. Sci. Tech 55 (5), 2779–2789. 10.1021/acs.est.0c07287. Epub 2021 Feb 15. [DOI] [PubMed] [Google Scholar]
- Arnot JA, Brown TN, Wania F, Breivik K, McLachlan MS, 2012. Prioritizing chemicals and data requirements for screening-level exposure and risk assessment. Environ. Health Perspect 120(11), 1565–1570. doi: 10.1289/ehp.1205355. Epub 2012 Sep 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnot JA, Mackay D, Webster E, Southwood JM, 2006. Screening level risk assessment model for chemical fate and effects in the environment. Environ. Sci. Tech 40 (7), 2316–2323. 10.1021/es0514085. [DOI] [PubMed] [Google Scholar]
- Baker NC, Ekins S, Williams AJ, Tropsha A, 2018. A bibliometric review of drug repurposing. Drug Discov. Today 23 (3), 661–672. 10.1016/j.drudis.2018.01.018. Epub 2018 Jan 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer WF, 1958. The monte carlo method. J. Soc. Ind. Appl. Math 6 (4), 438–451. [Google Scholar]
- Bell SM, Chang X, Wambaugh JF, Allen DG, Bartels M, Brouwer KLR, Casey WM, Choksi N, Ferguson SS, Fraczkiewicz G, Jarabek AM, Ke A, Lumen A, Lynn SG, Paini A, Price PS, Ring C, Simon TW, Sipes NS, Sprankle CS, Strickland J, Troutman J, Wetmore BA, Kleinstreuer NC, 2018. In vitro to in vivo extrapolation for high throughput prioritization and decision making. Toxicol. In Vitro 47, 213–227. 10.1016/j.tiv.2017.11.016. Epub 2017 Dec 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Y, Ma F, Wang H, Hassan MA, Yevheniia R, Fan W, Li Y, Dong Z, 2019. A spatio-temporally weighted hybrid model to improve estimates of personal PM2.5 exposure: incorporating big data from multiple data sources. Environ. Pollut 253, 403–411. 10.1016/j.envpol.2019.07.034. Epub 2019 Jul 11. [DOI] [PubMed] [Google Scholar]
- Bevington C, Williams AJ, Guider C, et al. , 2022. Development of a flame retardant and an organohalogen flame retardant chemical inventory. Sci. Data 9, 295. 10.1038/s41597-022-01351-0. [DOI] [Google Scholar]
- Black G, Lowe C, Anumol T, et al. , 2022. Exploring chemical space in non-targeted analysis: a proposed ChemSpace tool. Anal. Bioanal. Chem 10.1007/s00216-022-04434-4. [DOI] [PMC free article] [PubMed]
- Bodenreider O, 2004. The Unified Medical Language System (UMLS): integrating biomedical terminology. Nucleic Acids Res. 32 (Database issue), D267–D270. 10.1093/nar/gkh061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen MS, Isakov V, Prince S, McGuinness K, Egeghy PP, Stephens B, Arunachalam S, Stout D, Walker R, Alston L, Rooney AA, Taylor KW, Buckley TJ, 2021a. Integrating personal air sensor and GPS to determine microenvironment-specific exposures to volatile organic compounds. Sensors (Basel) 21 (16), 5659. 10.3390/s21165659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen M, Ring CL, Kreutz A, Goldsmith MR, Wambaugh JF, 2021b. High-throughput PBTK models for in vitro to in vivo extrapolation. Expert Opin. Drug Metab. Toxicol 17 (8), 903–921. 10.1080/17425255.2021.1935867. Epub 2021 Jun 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen M, Wambaugh JF, Bernstein A, Sfeir M, Ring CL, 2022. Simulating toxicokinetic variability to identify susceptible and highly exposed populations. J. Eposure Sci. Environ. Epidemiol 32 (6), 855–863. 10.1038/s41370-022-00491-0. Epub 2022 Nov 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruinen de Bruin Y, Franco A, Ahrens A, et al. , 2022. Enhancing the use of exposure science across EU chemical policies as part of the European Exposure Science Strategy 2020–2030. J. Eposure Sci. Environ. Epidemiol 32, 513–525. 10.1038/s41370-021-00388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A, Grossman J, Carberry C, Lai Y, Williams AJ, Minucci JM, Thomas Purucker S, Szilagyi J, Lu K, Boggess K, Fry RC, Sobus JR, Rager JE, 2022. Integrative exposomic, transcriptomic, epigenomic analyses of human placental samples links understudied chemicals to preeclampsia. Environ. Int 167, 107385 10.1016/j.envint.2022.107385. Epub 2022 Jun 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Hubal EA, Richard A, Aylward L, Edwards S, Gallagher J, Goldsmith MR, Isukapalli S, Tornero-Velez R, Weber E, Kavlock R, 2010. Advancing exposure characterization for chemical evaluation and risk assessment. J. Toxicol. Environ. Health B Crit. Rev 13 (2–4), 299–313. 10.1080/10937404.2010.483947. [DOI] [PubMed] [Google Scholar]
- Cousins IT, Johansson JH, Salter ME, Sha B, Scheringer M, 2022. Outside the safe operating space of a new planetary boundary for per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Technol 56 (16), 11172–11179. 10.1021/acs.est.2c02765. Epub 2022 Aug 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar SA, Ernstoff AS, Fantke P, Meyer DE, Jolliet O, 2016. High-throughput exposure modeling to support prioritization of chemicals in personal care products. Chemosphere 163, 490–498. 10.1016/j.chemosphere.2016.07.065. Epub 2016 Aug 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisio KL, Frame AM, Goldsmith MR, Wambaugh JF, Liddell A, Cathey T, Smith D, Vail J, Ernstoff AS, Fantke P, Jolliet O, Judson RS, 2015. Exploring consumer exposure pathways and patterns of use for chemicals in the environment. Toxicol. Rep 2 (2), 228–237. 10.1016/j.toxrep.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisio KL, Phillips K, Price PS, Grulke CM, Williams A, Biryol D, Hong T, Isaacs KK, 2018. The Chemical and Products Database, a resource for exposure-relevant data on chemicals in consumer products. Sci. Data 10 (5), 180125. 10.1038/sdata.2018.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy SR, 2004. What is Bayesian statistics? Nat. Biotechnol 22 (9), 1177–1178. [DOI] [PubMed] [Google Scholar]
- Egeghy PP, Sheldon LS, Isaacs KK, ¨ zkaynak H, Goldsmith MR, Wambaugh JF, Judson RS, Buckley TJ, 2016. Computational exposure science: an emerging approach for 21st century risk assessment. Environ. Health Perspect 124 (6), 697–702. 10.1289/ehp.1509748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantke P, Bruinen de Bruin Y, Schlüter U, Connolly A, Bessems J, Kephalopoulos S, Zare Jeddi M, van Nieuwenhuyse A, Dudzina T, Scheepers PTJ, von Goetz N, 2022. The European exposure science strategy 2020–2030. Environ. Int 170, 107555 10.1016/j.envint.2022.107555. Epub 2022 Sep 30. [DOI] [PubMed] [Google Scholar]
- Fisher CM, Peter KT, Newton SR, Schaub AJ, Sobus JR, 2022. Approaches for assessing performance of high-resolution mass spectrometry-based non-targeted analysis methods. Anal. Bioanal. Chem 414 (22), 6455–6471. 10.1007/s00216-022-04203-3. Epub 2022 Jul 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh KN, Zhang D, Miller SJ, Rossen K, Chirik PJ, Kozlowski MC, Zimmerman JB, Brooks BW, Savage PE, Allen DT, Voutchkova-Kostal AM, 2021. Green chemistry: a framework for a sustainable future. Org. Lett 23 (13), 4935–4939. 10.1021/acs.orglett.1c01906. Epub 2021 Jun 15 [DOI] [PubMed] [Google Scholar]
- Goldsmith MR, Grulke CM, Brooks RD, Transue TR, Tan YM, Frame A, Egeghy PP, Edwards R, Chang DT, Tornero-Velez R, Isaacs K, Wang A, Johnson J, Holm K, Reich M, Mitchell J, Vallero DA, Phillips L, Phillips M, Wambaugh JF, Judson RS, Buckley TJ, Dary CC, 2014. Development of a consumer product ingredient database for chemical exposure screening and prioritization. Food Chem. Toxicol 65, 269–279. 10.1016/j.fct.2013.12.029. Epub 2013 Dec 27 [DOI] [PubMed] [Google Scholar]
- Groff LC 2nd, Grossman JN, Kruve A, Minucci JM, Lowe CN, McCord JP, Kapraun DF, Phillips KA, Purucker ST, Chao A, Ring CL, Williams AJ, Sobus JR, 2022. Uncertainty estimation strategies for quantitative non-targeted analysis. Anal. Bioanal. Chem 414 (17), 4919–4933. 10.1007/s00216-022-04118-z. Epub 2022 Jun 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh K, Vom Berg C, Schirmer K, Tlili A, 2022. Anthropogenic chemicals as underestimated drivers of biodiversity loss: scientific and societal implications. Environ. Sci. Tech 56 (2), 707–710. 10.1021/acs.est.1c08399. Epub 2022 Jan 2 [DOI] [PubMed] [Google Scholar]
- Grulke CM, Williams AJ, Thillanadarajah I, Richard AM, 2019. EPA’s DSSTox database: history of development of a curated chemistry resource supporting computational toxicology research. Comput. Toxicol 12, 100096 10.1016/j.comtox.2019.100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Huang S, Wang J, Feng YL, 2020. Recent advances in non-targeted screening analysis using liquid chromatography - high resolution mass spectrometry to explore new biomarkers for human exposure. Talanta 1 (219), 121339. 10.1016/j.talanta.2020.121339. Epub 2020 Jul 7. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Paoli GM, Price PS, Gwinn MR, Guiseppi-Elie A, Farrell PJ, Hubbell BJ, Krewski D, Thomas RS, 2022. A value of information framework for assessing the trade-offs associated with uncertainty, duration, and cost of chemical toxicity testing. Risk Anal. 10.1111/risa.13931. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- Hubbard HF, Ring CL, Hong T, Henning CC, Vallero DA, Egeghy PP, Goldsmith MR, 2022. Exposure prioritization (ex priori): a screening-level high-throughput chemical prioritization tool. Toxics 10 (10), 569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs KK, Glen WG, Egeghy P, Goldsmith MR, Smith L, Vallero D, Brooks R, Grulke CM, Ö zkaynak H, 2014. SHEDS-HT: an integrated probabilistic exposure model for prioritizing exposures to chemicals with near-field and dietary sources. Environ. Sci. Tech 48 (21), 12750–12759. 10.1021/es502513w. Epub 2014 Oct 21 [DOI] [PubMed] [Google Scholar]
- Isaacs KK, Wall JT, Williams AR, Hobbie KA, Sobus JR, Ulrich E, Lyons D, Dionisio KL, Williams AJ, Grulke C, Foster CA, McCoy J, Bevington C, 2022. A harmonized chemical monitoring database for support of exposure assessments. Sci. Data 9 (1), 314. 10.1038/s41597-022-01365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs KK, Wall JT, Paul Friedman K, Franzosa J, Goeden H, Williams AJ, Dionisio K, Lambert J, Linnenbrink M, Singh A, Wambaugh J, Bogdan AR, Greene C, 2023. Screening for drinking water contaminants of concern using an automated exposure-focused workflow. J. Eposure Sci. Environ. Epidemiol 10.1038/s41370-023-00552-y. [DOI] [PMC free article] [PubMed]
- Jayjock MA, Chaisson CF, Arnold S, Dederick EJ, 2007. Modeling framework for human exposure assessment. J. Eposure Sci. Environ. Epidemiol 17 (Suppl. 1), S81–S89. 10.1038/sj.jes.7500580. Epub 2007 May 16 [DOI] [PubMed] [Google Scholar]
- Jayjock MA, Chaisson CF, Franklin CA, Arnold S, Price PS, 2009. Using publicly available information to create exposure and risk-based ranking of chemicals used in the workplace and consumer products. J. Eposure Sci. Environ. Epidemiol 19 (5), 515–524. 10.1038/jes.2008.43. Epub 2008 Aug 6 [DOI] [PubMed] [Google Scholar]
- Jolliet O, Huang L, Hou P, Fantke P, 2021. High throughput risk and impact screening of chemicals in consumer products. Risk Anal. 41, 627–644. 10.1111/risa.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapraun DF, Wambaugh JF, Ring CL, Tornero-Velez R, Setzer RW, 2017. A method for identifying prevalent chemical combinations in the U.S. population. Environ. Health Perspect 125 (8), 087017 10.1289/EHP1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock RJ, et al. , 2018. Accelerating the pace of chemical risk assessment. Chem. Res. Toxicol 31 (5), 287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock R, Dix D, 2010. Computational toxicology as implemented by the U.S. EPA: providing high throughput decision support tools for screening and assessing chemical exposure, hazard and risk. J. Toxicol. Environ. Health B Crit. Rev 13 (2–4), 197–217. 10.1080/10937404.2010.483935. [DOI] [PubMed] [Google Scholar]
- Koelmel JP, Stelben P, McDonough CA, et al. , 2022. FluoroMatch 2.0—making automated and comprehensive non-targeted PFAS annotation a reality. Anal. Bioanal. Chem 414, 1201–1215. 10.1007/s00216-021-03392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval LE, Dionisio KL, Friedman KP, Isaacs KK, Rager JE, 2022. Environmental mixtures and breast cancer: identifying co-exposure patterns between understudied vs breast cancer-associated chemicals using chemical inventory informatics. J. Eposure Sci. Environ. Epidemiol 32 (6), 794–807. 10.1038/s41370-022-00451-8. Epub 2022 Jun 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu NN, Baldé AB, Bertollini R, Bose-O’Reilly S, Boufford JI, Breysse PN, Chiles T, Mahidol C, Coll-Seck AM, Cropper ML, Fobil J, Fuster V, Greenstone M, Haines A, Hanrahan D, Hunter D, Khare M, Krupnick A, Lanphear B, Lohani B, Martin K, Mathiasen KV, McTeer MA, Murray CJL, Ndahimananjara JD, Perera F, Potočnik J, Preker AS, Ramesh J, Rockström J, Salinas, Samson LD, Sandilya K, Sly PD, Smith KR, Steiner A, Stewart RB, Suk WA, van Schayck OCP, Yadama GN, Yumkella K, Zhong M, 2018. The Lancet Commission on pollution and health. Lancet. 391 (10119), 462–512. 10.1016/S0140-6736(17)32345-0. Epub 2017 Oct 19. Erratum in: Lancet. 2018 Feb 3;391(10119):430. [DOI] [PubMed] [Google Scholar]
- Li L, Westgate JN, Hughes L, Zhang X, Givehchi B, Toose L, Armitage JM, Wania F, Egeghy P, Arnot JA, 2018. A model for risk-based screening and prioritization of human exposure to chemicals from near-field sources. Environ. Sci. Tech 52 (24), 14235–14244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Sangion A, Wania F, Armitage JM, Toose L, Hughes L, Arnot JA, 2021. Development and evaluation of a holistic and mechanistic modeling framework for chemical emissions, fate, exposure, and risk. Environ. Health Perspect 129 (12), 127006 10.1289/EHP9372. Epub 2021 Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linakis MW, Sayre RR, Pearce RG, Sfeir MA, Sipes NS, Pangburn HA, Gearhart JM, Wambaugh JF, 2020. Development and evaluation of a high throughput inhalation model for organic chemicals. J. Expo. Sci. Environ. Epidemiol 30 (5), 866–877. 10.1038/s41370-020-0238-y. Epub 2020 Jun 16. Erratum in: J. Expo. Sci. Environ. Epidemiol. 2020 Jul 9;: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioy PJ, 2010. Exposure science: a view of the past and milestones for the future. Environ. Health Perspect 118 (8), 1081–1090. 10.1289/ehp.0901634. Epub 2010 Mar 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe CN, Phillips KA, Favela KA, Yau AY, Wambaugh JF, Sobus JR, Isaacs KK, 2021a. Chemical characterization of recycled consumer products using suspect screening analysis. Environ. Sci. Tech 55 (16), 11375–11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe CN, Williams AJ, 2021. Enabling high-throughput searches for multiple chemical data using the U.S.-EPA CompTox chemicals dashboard. J. Chem. Inf. Model 61 (2), 565–570. 10.1021/acs.jcim.0c01273. Epub 2021 Jan 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe CN, Isaacs KK, McEachran A, Grulke CM, Sobus JR, Ulrich EM, Richard A, Chao A, Wambaugh J, Williams AJ, 2021b. Predicting compound amenability with liquid chromatography-mass spectrometry to improve non-targeted analysis. Anal. Bioanal. Chem 413 (30), 7495–7508. 10.1007/s00216-021-03713-w. Epub 2021 Oct 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah A, 2016. Environmental justice in the age of big data: challenging toxic blind spots of voice, speed, and expertise. Environ. Sociol 3 (2), 122–133. 10.1080/23251042.2016.1220849. [DOI] [Google Scholar]
- Mansouri K, Grulke CM, Richard AM, Judson RS, Williams AJ, 2016. An automated curation procedure for addressing chemical errors and inconsistencies in public datasets used in QSAR modelling. SAR QSAR Environ. Res 27 (11), 939–965. 10.1080/1062936X.2016.1253611. [DOI] [PubMed] [Google Scholar]
- Mansouri K, Grulke CM, Judson RS, et al. , 2018. OPERA models for predicting physicochemical properties and environmental fate endpoints. J. Cheminform 10, 10. 10.1186/s13321-018-0263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri K, Cariello NF, Korotcov A, et al. , 2019. Open-source QSAR models for pKa prediction using multiple machine learning approaches. J. Cheminform 11, 60. 10.1186/s13321-019-0384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin SA, Abegaz BM, 2011. Chapter 1 Chemistry for development in The Chemical Element: Chemistry’s Contribution to Our Global Future, First Edition. Edited by Garcia-Martinez Javier, Serrano-Torregrosa Elena © 2011 Wiley-VCH Verlag GmbH & Co. KGaA. Published 2011 by Wiley-VCH Verlag GmbH & Co. KGaA. https://application.wiley-vch.de/books/sample/3527328807_c01.pdf. [Google Scholar]
- McCord JP, Groff LC 2nd, Sobus JR, 2022. Quantitative non-targeted analysis: bridging the gap between contaminant discovery and risk characterization. Environ. Int 158, 107011 10.1016/j.envint.2021.107011. Epub 2021 Dec 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy T, Glen G, Smith L, Lakkadi Y, 2000. The national exposure research laboratory’s consolidated human activity database. J. Expo. Anal. Environ. Epidemiol 10 (6 Pt 1), 566–578. 10.1038/sj.jea.7500114. [DOI] [PubMed] [Google Scholar]
- McEachran AD, Sobus JR, Williams AJ, 2017. Identifying known unknowns using the US EPA’s CompTox Chemistry Dashboard. Anal. Bioanal. Chem 409, 1729–1735. 10.1007/s00216-016-0139-z. [DOI] [PubMed] [Google Scholar]
- McEachran AD, Mansouri K, Grulke C, Schymanski EL, Ruttkies C, Williams AJ, 2018. “MS-Ready” structures for non-targeted high-resolution mass spectrometry screening studies. J. Cheminform 10 (1), 45. 10.1186/s13321-018-0299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachran AD, Balabin I, Cathey T, et al. , 2019. Linking in silico MS/MS spectra with chemistry data to improve identification of unknowns. Sci. Data 6, 141. 10.1038/s41597-019-0145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachran AD, Chao A, Al-Ghoul H, Lowe C, Grulke C, Sobus JR, Williams AJ, 2020. Revisiting five years of CASMI contests with EPA identification tools. Metabolites 10 (6), 260. 10.3390/metabo10060260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DE, Bailin SC, Vallero D, Egeghy PP, Liu SV, Cohen Hubal EA, 2020. Enhancing life cycle chemical exposure assessment through ontology modeling. Sci. Total Environ 712, 136263 10.1016/j.scitotenv.2019.136263. Epub 2019 Dec 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minucci JM, Purucker ST, Isaacs KK, Wambaugh JF, Phillips KA, 2023. A data-driven approach to estimating occupational inhalation exposure using workplace compliance data. Environ. Sci. Technol 57 (14), 5947–5956. 10.1021/acs.est.2c08234. Epub 2023 Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumtaz MM, Nickle RA, Lambert JC, Johnson MS, 2021. Advances in assessing hazard and risk to emerging threats and emergency response: comparing and contrasting efforts of 3 federal agencies. Toxicol. Sci 185 (1), 1–9. 10.1093/toxsci/kfab126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myslín M, Zhu SH, Chapman W, Conway M, 2013. Using twitter to examine smoking behavior and perceptions of emerging tobacco products. J. Med. Internet Res 15 (8), e174 10.2196/jmir.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu R, Biswas B, Willett IR, Cribb J, Kumar Singh B, Paul Nathanail C, Coulon F, Semple KT, Jones KC, Barclay A, Aitken RJ, 2021. Chemical pollution: a growing peril and potential catastrophic risk to humanity. Environ. Int 156, 106616 10.1016/j.envint.2021.106616. Epub 2021 May 12 [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine, 2017. Using 21st Century Science to Improve Risk-Related Evaluations. The National Academies Press, Washington, DC. doi: 10.17226/24635. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine 2018. Informing Environmental Health Decisions through Data Integration: Proceedings of a Workshop in Brief. The National Academies Press, Washington, DC. doi: 10.17226/25139. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine 2022. Our Planet, Our Future, An Urgent Call for Action. Summit Statement of Nobel Prize Laureates and Other Experts. The National Academies Press, Washington, DC. https://www.nationalacademies.org/news/2021/04/nobel-prize-laureates-and-other-experts-issue-urgent-call-for-action-after-our-planet-our-future-summit. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine, 2022. The Importance of Chemical Research to the U.S. Economy. Washington, DC: The National Academies Press. doi: 10.17226/26568. [DOI] [Google Scholar]
- National Research Council, 2007. Toxicity Testing in the 21st Century: A Vision and a Strategy. The National Academies Press, Washington, DC. doi: 10.17226/11970. [DOI] [Google Scholar]
- National Research Council, 2012. Exposure Science in the 21st Century: A Vision and a Strategy. The National Academies Press, Washington, DC. doi: 10.17226/13507. [DOI] [PubMed] [Google Scholar]
- Newton SR, McMahen RL, Sobus JR, Mansouri K, Williams AJ, McEachran AD, Strynar MJ, 2018. Suspect screening and non-targeted analysis of drinking water using point-of-use filters. Environ. Pollut 234, 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott WR, 1990. Total human exposure: basic concepts, EPA field studies, and future research needs. J. Air Waste Manag. Assoc 40 (7), 966–975. 10.1080/10473289.1990.10466747. [DOI] [PubMed] [Google Scholar]
- Paini A, Leonard JA, Joossens E, Bessems JGM, Desalegn A, Dorne JL, Gosling JP, Heringa MB, Klaric M, Kliment T, Kramer NI, Loizou G, Louisse J, Lumen A, Madden JC, Patterson EA, Proença S, Punt A, Setzer RW, Suciu N, Troutman J, Yoon M, Worth A, Tan YM, 2019. Next generation physiologically based kinetic (NG-PBK) models in support of regulatory decision making. Comput. Toxicol 9, 61–72. 10.1016/j.comtox.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagopoulos Abrahamsson D, Sobus JR, Ulrich EM, Isaacs K, Moschet C, Young TM, Tulve NS, 2021. A quest to identify suitable organic tracers for estimating children’s dust ingestion rates. J. Eposure Sci. Environ. Epidemiol 31 (1), 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier RE, Buckley TJ, 2020. Sensor technology: a critical cutting edge of exposure science. J. Expo. Sci. Environ. Epidemiol 30 (6), 901–902. 10.1038/s41370-020-00268-3. Epub 2020 Sep 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson L, Carney Almroth BM, Collins CD, Cornell S, de Wit CA, Diamond ML, Fantke P, Hassellöv M, MacLeod M, Ryberg MW, Søgaard Jørgensen P, Villarrubia-Gómez P, Wang Z, Hauschild MZ, 2022. Outside the safe operating space of the planetary boundary for novel entities. Environ. Sci. Technol 56(3), 1510–1521. doi: 10.1021/acs.est.1c04158. Epub 2022 Jan 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter KT, Phillips AL, Knolhoff AM, Gardinali PR, Manzano CA, Miller KE, Pristner M, Sabourin L, Sumarah MW, Warth B, Sobus JR, 2021. Nontargeted analysis study reporting tool: a framework to improve research transparency and reproducibility. Anal. Chem 93 (41), 13870–13879. 10.1021/acs.analchem.1c02621. Epub 2021 Oct 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AL, Williams AJ, Sobus JR, Ulrich EM, Gundersen J, Langlois-Miller C, Newton SR, 2022. A framework for utilizing high-resolution mass spectrometry and nontargeted analysis in rapid response and emergency situations. Environ. Toxicol. Chem 41 (5), 1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Yau A, Favela KA, Isaacs KK, McEachran A, Grulke C, Wambaugh JF, 2018. Suspect screening analysis of chemicals in consumer products. Environ. Sci. Tech 52 (5), 3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place BJ, Ulrich EM, Challis JK, Chao A, Du B, Favela K, Feng YL, Fisher CM, Gardinali P, Hood A, Knolhoff AM, McEachran AD, Nason SL, Newton SR, Ng B, Nuñez J, Peter KT, Phillips AL, Quinete N, Renslow R, Sobus JR, Sussman EM, Warth B, Wickramasekara S, Williams AJ, 2021. An introduction to the benchmarking and publications for non-targeted analysis working group. Anal. Chem 93 (49), 16289–16296. 10.1021/acs.analchem.1c02660. Epub 2021 Nov 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp J, Pető K, Nagy J, 2013. Pesticide productivity and food security: a review. Agron. Sustain. Dev 33, 243–255. 10.1007/s13593-012-0105-x. [DOI] [Google Scholar]
- Prata JC, da Costa JP, Lopes I, Andrady AL, Duarte AC, Rocha-Santos T, 2021. A One Health perspective of the impacts of microplastics on animal, human and environmental health. Sci. Total Environ 10 (777), 146094 10.1016/j.scitotenv.2021.146094. Epub 2021 Feb 27. [DOI] [PubMed] [Google Scholar]
- Rager JE, Strynar MJ, Liang S, McMahen RL, Richard AM, Grulke CM, Sobus JR, 2016. Linking high resolution mass spectrometry data with exposure and toxicity forecasts to advance high-throughput environmental monitoring. Environ. Int 88, 269–280. [DOI] [PubMed] [Google Scholar]
- Rappaport SM, 2016. Genetic factors are not the major causes of chronic diseases. PLoS ONE 11 (4), e0154387. 10.1371/journal.pone.0154387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard AM, Williams CR, 2002. Distributed structure-searchable toxicity (DSSTox) public database network: a proposal. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 499 (1), 27–52. [DOI] [PubMed] [Google Scholar]
- Richman T, Arnold E, Williams AJ, 2022. Curation of a list of chemicals in biosolids from EPA National Sewage Sludge Surveys & Biennial Review Reports. Sci. Data 9 (1), 180. 10.1038/s41597-022-01267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring CL, Pearce RG, Setzer RW, Wetmore BA, Wambaugh JF, 2017. Identifying populations sensitive to environmental chemicals by simulating toxicokinetic variability. Environ. Int 106, 105–118. 10.1016/j.envint.2017.06.004. Epub 2017 Jun 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring CL, Arnot JA, Bennett DH, Egeghy PP, Fantke P, Huang L, Isaacs KK, Jolliet O, Phillips KA, Price PS, Shin HM, Westgate JN, Setzer RW, Wambaugh JF, 2019. Consensus modeling of median chemical intake for the U.S. population based on predictions of exposure pathways. Environ. Sci. Technol 53 (2), 719–732. 10.1021/acs.est.8b04056. Epub 2018 Dec 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockström J, Steffen W, Noone K, Persson A, Chapin FS 3rd, Lambin EF, Lenton TM, Scheffer M, Folke C, Schellnhuber HJ, Nykvist B, de Wit CA, Hughes T, van der Leeuw S, Rodhe H, Sörlin S, Snyder PK, Costanza R, Svedin U, Falkenmark M, Karlberg L, Corell RW, Fabry VJ, Hansen J, Walker B, Liverman D, Richardson K, Crutzen P, Foley JA, 2009. A safe operating space for humanity. Nature 461 (7263), 472–475. 10.1038/461472a. [DOI] [PubMed] [Google Scholar]
- Rockström J, Gupta J, Qin D, et al. , 2023. Safe and just Earth system boundaries. Nature. 10.1038/s41586-023-06083-8. [DOI] [PMC free article] [PubMed]
- Rotroff DM, Wetmore BA, Dix DJ, Ferguson SS, Clewell HJ, Houck KA, Lecluyse EL, Andersen ME, Judson RS, Smith CM, Sochaski MA, Kavlock RJ, Boellmann F, Martin MT, Reif DM, Wambaugh JF, Thomas RS, 2010. Incorporating human dosimetry and exposure into high-throughput in vitro toxicity screening. Toxicol. Sci 117 (2), 348–358. 10.1093/toxsci/kfq220. Epub 2010 Jul 16. Erratum in: Toxicol Sci. 2014 Feb;137(2):499. Dosage error in article text. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaristo M, Brodin T, Balshine S, Bertram MG, Brooks BW, Ehlman SM, McCallum ES, Sih A, Sundin J, Wong BBM, Arnold KE, 1885. Direct and indirect effects of chemical contaminants on the behaviour, ecology and evolution of wildlife. Proc. Biol. Sci 2018. (285), 20181297 10.1098/rspb.2018.1297. [DOI] [PMC free article] [PubMed]
- Sauer UG, Barter RA, Becker RA, Benfenati E, Berggren E, Hubesch B, Hollnagel HM, Inawaka K, Keene AM, Mayer P, Plotzke K, 2020. 21st century approaches for evaluating exposures, biological activity, and risks of complex substances: workshop highlights. Regul. Toxicol. Pharm 1 (111), 104583 10.1016/j.yrtph.2020.104583. [DOI] [PubMed] [Google Scholar]
- Sayre RR, Wambaugh JF, Grulke CM, 2020. Database of pharmacokinetic time-series data and parameters for 144 environmental chemicals. Sci. Data 7 (1), 122. 10.1038/s41597-020-0455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayre RR, Setzer RW, Serre ML, Wambaugh JF, 2022. Characterizing surface water concentrations of hundreds of organic chemicals in United States for environmental risk prioritization. J. Expo. Sci. Environ. Epidemiol 10.1038/s41370-022-00501-1. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- Serafini M, Cargnin S, Massarotti A, Pirali T, Genazzani AA, 2020. Essential medicinal chemistry of essential medicines. J. Med. Chem 63 (18), 10170–10187. 10.1021/acs.jmedchem.0c00415. Epub 2020 May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HM, McKone TE, Bennett DH, 2017. Model framework for integrating multiple exposure pathways to chemicals in household cleaning products. Indoor Air 27 (4), 829–839. 10.1111/ina.12356. Epub 2016 Dec 17 [DOI] [PubMed] [Google Scholar]
- Sipes NS, Wambaugh JF, Pearce R, Auerbach SS, Wetmore BA, Hsieh JH, Shapiro AJ, Svoboda D, DeVito MJ, Ferguson SS, 2017. An intuitive approach for predicting potential human health risk with the Tox21 10k library. Environ. Sci. Technol 51 (18), 10786–10796. 10.1021/acs.est.7b00650. Epub 2017 Sep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobus JR, Wambaugh JF, Isaacs KK, et al. , 2018. Integrating tools for non-targeted analysis research and chemical safety evaluations at the US EPA. J. Eposure Sci. Environ. Epidemiol 28, 411–426. 10.1038/s41370-017-0012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield Z, Addington CK, Dionisio KL, Lyons D, Tornero-Velez R, Phillips KA, Buckley TJ, Isaacs KK, 2021. Mining of consumer product ingredient and purchasing data to identify potential chemical coexposures. Environ. Health Perspect 129 (6), 67006 10.1289/EHP8610. Epub 2021 Jun 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield Z, Setzer RW, Hull V, Sayre RR, Isaacs KK, Wambaugh JF, 2022. Bayesian inference of chemical exposures from NHANES urine biomonitoring data. J. Eposure Sci. Environ. Epidemiol 32 (6), 833–846. 10.1038/s41370-022-00459-0. Epub 2022 Aug 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straczkiewicz M, James P, Onnela JP, 2021. A systematic review of smartphone-based human activity recognition methods for health research. NPJ Digit Med. 4 (1), 148 10.1038/s41746-021-00514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunger N, Beasley A, Landenberger BD, Arnold SM, 2020. High-Throughput Exposure Assessment Tool (HEAT) for exposure-based prioritization of chemicals. Hum. Ecol. Risk Assess. Int. J 26 (4), 1076–1086. 10.1080/10807039.2018.1554993. [DOI] [Google Scholar]
- The European Chemical Industry Facts and Figures, 2023. A vital part of Europe’s Future. URL: https://cefic.org/a-pillar-of-the-european-economy/facts-and-figures-of-the-european-chemical-industry/ (accessed June 2, 2023). [Google Scholar]
- Thomas RS, Bahadori T, Buckley TJ, Cowden J, Deisenroth C, Dionisio KL, Frithsen JB, Grulke CM, Gwinn MR, Harrill JA, Higuchi M, Houck KA, Hughes MF, Hunter ES, Isaacs KK, Judson RS, Knudsen TB, Lambert JC, Linnenbrink M, Martin TM, Newton SR, Padilla S, Patlewicz G, Paul-Friedman K, Phillips KA, Richard AM, Sams R, Shafer TJ, Setzer RW, Shah I, Simmons JE, Simmons SO, Singh A, Sobus JR, Strynar M, Swank A, Tornero-Velez R, Ulrich EM, Villeneuve DL, Wambaugh JF, Wetmore BA, Williams AJ, 2019. The next generation blueprint of computational toxicology at the U.S. Environmental Protection Agency. Toxicol. Sci 169 (2), 317–332. 10.1093/toxsci/kfz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero-Velez R, Isaacs K, Dionisio K, Prince S, Laws H, Nye M, Price PS, Buckley TJ, 2021. Data mining approaches for assessing chemical coexposures using consumer product purchase data. Risk Anal. 41 (9), 1716–1735. 10.1111/risa.13650. Epub 2020 Dec 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich EM, Sobus JR, Grulke CM, Richard AM, Newton SR, Strynar MJ, Mansouri K, Williams AJ, 2019. EPA’s non-targeted analysis collaborative trial (ENTACT): genesis, design, and initial findings. Anal. Bioanal. Chem 411 (4), 853–866. 10.1007/s00216-018-1435-6. Epub 2018 Dec 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA, 2021. A Proof-of-Concept Case Study Integrating Publicly Available Information to Screen Candidates for Chemical Pre-Prioritization under TSCA. June 2021 U.S. Environmental Protection Agency, Office of Chemical Safety and Pollution Prevention and Office of Research and Development, Washington, DC. EPA/600/R-21–106. [PubMed] [Google Scholar]
- van der Zalm AJ et al. , 2022. A framework for establishing scientific confidence in new approach methodologies. Arch. Toxicol 2022. doi: 10.1007/s00204-022-03365-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houwelingen HC, Arends LR, Stijnen T, 2002. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat. Med 21 (4), 589–624. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Rayasam SDG, Axelrad DA, Bennett DH, Brown P, Carignan CC, Chartres N, Diamond ML, Joglekar R, Shamasunder B, Shrader-Frechette K, Subra WA, Zarker K, Woodruff TJ, 2023. Addressing systemic problems with exposure assessments to protect the public’s health. Environ. Health 21 (Suppl. 1), 121. 10.1186/s12940-022-00917-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieux F, Maillot M, Rehm CD, Barrios P, Drewnowski A, 2020. Trends in tap and bottled water consumption among children and adults in the United States: analyses of NHANES 2011–16 data. Nutr. J 19 (1), 10 10.1186/s12937-020-0523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambaugh JF, Setzer RW, Reif DM, Gangwal S, Mitchell-Blackwood J, Arnot JA, Joliet O, Frame A, Rabinowitz J, Knudsen TB, Judson RS, Egeghy P, Vallero D, Cohen Hubal EA, 2013. High-throughput models for exposure-based chemical prioritization in the ExpoCast project. Environ. Sci. Tech 47 (15), 8479–8488. 10.1021/es400482g. Epub 2013 Jul 11 [DOI] [PubMed] [Google Scholar]
- Wambaugh JF, Wang A, Dionisio KL, Frame A, Egeghy P, Judson R, Setzer RW, 2014. High throughput heuristics for prioritizing human exposure to environmental chemicals. Environ. Sci. Tech 48 (21), 12760–12767. 10.1021/es503583j. Epub 2014 Oct 24 [DOI] [PubMed] [Google Scholar]
- Von Goetz N, Garcia-Hidalgo E, Balachandran C, Fuchs K, Frey R, Ilic A, 2018. Assessing exposure factors in the smartphone generation: Design and evaluation of a smartphone app that collects use patterns of cosmetics and household chemicals. Food Chem. Toxicol 118, 532–540. 10.1016/j.fct.2018.05.060. Epub 2018 May 31 [DOI] [PubMed] [Google Scholar]
- Wambaugh JF, Bare JC, Carignan CC, Dionisio KL, Dodson RE, Jolliet O, Liu X, Meyer DE, Newton SR, Phillips KA, Price PS, Ring CL, Shin H, Sobus JR, Tal T, Ulrich EM, Vallero DA, Wetmore BA, Isaacs KK, 2019a. New approach methodologies for exposure science. Curr. Opin. Toxicol 15, 76–92. 10.1016/j.cotox.2019.07.001. ISSN 2468–2020. [DOI] [Google Scholar]
- Wambaugh JF, Wetmore BA, Ring CL, Nicolas CI, Pearce RG, Honda GS, Dinallo R, Angus D, Gilbert J, Sierra T, Badrinarayanan A, Snodgrass B, Brockman A, Strock C, Setzer RW, Thomas RS, 2019b. Assessing toxicokinetic uncertainty and variability in risk prioritization. Toxicol. Sci 172 (2), 235–251. 10.1093/toxsci/kfz205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Walker GW, Muir DCG, Nagatani-Yoshida K, 2020. Toward a global understanding of chemical pollution: a first comprehensive analysis of national and regional chemical inventories. Environ. Sci. Tech 54 (5), 2575–2584. 10.1021/acs.est.9b06379. Epub 2020 Feb 14 [DOI] [PubMed] [Google Scholar]
- Wang B, Wu C, Reniers G, Huang L, Kang L, Zhang L, 2018. The future of hazardous chemical safety in China: opportunities, problems, challenges and tasks. Sci. Total Environ 1 (643), 1–11. 10.1016/j.scitotenv.2018.06.174. Epub 2018 Jun 20 [DOI] [PubMed] [Google Scholar]
- Washington JW, Rosal CG, McCord JP, Strynar MJ, Lindstrom AB, Bergman EL, Goodrow SM, Tadesse HK, Pilant AN, Washington BJ, Davis MJ, Stuart BG, Jenkins TM, 2020. Nontargeted mass-spectral detection of chloroperfluoropolyether carboxylates in New Jersey soils. Science 368 (6495), 1103–1107. 10.1126/science.aba7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmore BA, Wambaugh JF, Ferguson SS, Sochaski MA, Rotroff DM, Freeman K, Clewell HJ 3rd, Dix DJ, Andersen ME, Houck KA, Allen B, Judson RS, Singh R, Kavlock RJ, Richard AM, Thomas RS, 2012. Integration of dosimetry, exposure, and high-throughput screening data in chemical toxicity assessment. Toxicol. Sci 125 (1), 157–174. 10.1093/toxsci/kfr254. Epub 2011 Sep 26 [DOI] [PubMed] [Google Scholar]
- Wetmore BA, Allen B, Clewell HJ 3rd, Parker T, Wambaugh JF, Almond LM, Sochaski MA, Thomas RS, 2014. Incorporating population variability and susceptible subpopulations into dosimetry for high-throughput toxicity testing. Toxicol. Sci 142 (1), 210–224. 10.1093/toxsci/kfu169. Epub 2014 Aug 21 [DOI] [PubMed] [Google Scholar]
- Wetmore BA, Wambaugh JF, Allen B, Ferguson SS, Sochaski MA, Setzer RW, Houck KA, Strope CL, Cantwell K, Judson RS, LeCluyse E, Clewell HJ, Thomas RS, Andersen ME, 2015. Incorporating high-throughput exposure predictions with dosimetry-adjusted in vitro bioactivity to inform chemical toxicity testing. Toxicol. Sci 148 (1), 121–136. 10.1093/toxsci/kfv171. Epub 2015 Aug 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson M, Dumontier M, Aalbersberg I, et al. , 2016. The FAIR Guiding Principles for scientific data management and stewardship. Sci. Data 3, 160018. 10.1038/sdata.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A, 2017. The Chemical and Products Database (CPDat) MySQL Data File. The United States Environmental Protection Agency’s Center for Computational Toxicology and Exposure. Dataset. 10.23645/epacomptox.5352997. [DOI]
- Williams AJ, Grulke CM, Edwards J, McEachran AD, Mansouri K, Baker NC, Patlewicz G, Shah I, Wambaugh JF, Judson RS, Richard AM, 2017. The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. J. Cheminf 9 (1), 1–27. 10.1186/s13321-017-0247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AJ, Gaines LGT, Grulke CM, Lowe CN, Sinclair GFB, Samano V, Thillainadarajah I, Meyer B, Patlewicz G, Richard AM, 2022. Assembly and curation of lists of per- and polyfluoroalkyl substances (PFAS) to support environmental science research. Front. Environ. Sci 5 (10), 1–13. 10.3389/fenvs.2022.850019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ES, Panko J, Paustenbach DJ, 2009. The European Union’s REACH regulation: a review of its history and requirements. Crit. Rev. Toxicol 39 (7), 553–575. 10.1080/10408440903036056. [DOI] [PubMed] [Google Scholar]
- Wilson MP, Schwarzman MR, 2009. Toward a new U.S. chemicals policy: rebuilding the foundation to advance new science, green chemistry, and environmental health. Environ. Health Perspect 117 (8), 1202–1209. 10.1289/ehp.0800404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodtke GT, Ard K, Bullock C, White K, Priem B, 2022. Concentrated poverty, ambient air pollution, and child cognitive development. Sci. Adv 8 (48), eadd0285 10.1126/sciadv.add0285. Epub 2022 Nov 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Rayasam SDG, Axelrad DA, Koman PD, Chartres N, Bennett DH, Birnbaum LS, Brown P, Carignan CC, Cooper C, Cranor CF, Diamond ML, Franjevic S, Gartner EC, Hattis D, Hauser R, Heiger-Bernays W, Joglekar R, Lam J, Levy JI, MacRoy PM, Maffini MV, Marquez EC, Morello-Frosch R, Nachman KE, Nielsen GH, Oksas C, Abrahamsson DP, Patisaul HB,Patton S, Robinson JF, Rodgers KM, Rossi MS, Rudel RA, Sass JB, Sathyanarayana S, Schettler T, Shaffer RM, Shamasunder B, Shepard PM, Shrader-Frechette K, Solomon GM, Subra WA, Vandenberg LN, Varshavsky JR, White RF, Zarker K, Zeise L, 2023. A science-based agenda for health-protective chemical assessments and decisions: overview and consensus statement. Environ. Health 21 (Suppl. 1), 132. 10.1186/s12940-022-00930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used for the research described in the article.