Abstract
Nonalcoholic fatty liver disease (NAFLD) is a multisystem disease and is significantly associated with obesity, insulin resistance, type 2 diabetes mellitus, metabolic syndrome, and cardiovascular disease. NAFLD has become the most prevalent chronic liver disease in Western countries, and the proportion of NAFLD-related cirrhosis among patients on liver transplantation waiting lists has increased. In light of the accumulated data about NAFLD, and to provide a common approach with multi-disciplines dealing with the subject, it has become necessary to create new guidance for diagnosing and treating NAFLD. This guidance was prepared following an interdisciplinary study under the leadership of the Turkish Association for the Study of the Liver (TASL), Fatty Liver Special Interest Group. This new TASL Guidance is a practical application guide on NAFLD and was prepared to standardize the clinical approach to diagnosing and treating NAFLD patients. This guidance reflects many advances in the field of NAFLD. The proposals in this guidance are meant to aid decision-making in clinical practice. The guidance is primarily intended for gastroenterology, endocrinology, metabolism diseases, cardiology, internal medicine, pediatric specialists, and family medicine specialists.
Keywords: Nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, hepatosteatosis, noninvasive diagnostic tests, chronic hepatitis, cirrhosis
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a disease characterized by abnormal fat accumulation in hepatocytes without the association of significant alcohol consumption. NAFLD is one of the most common causes of chronic liver disease and cirrhosis in Western countries and Türkiye.[1] The estimated prevalence of NAFLD is 25% worldwide. NAFLD can be encountered in a broad clinical spectrum, including fatty liver (NAFL), nonalcoholic steatohepatitis (NASH), liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC).[2–4]
In light of the accumulated data and information about NAFLD, and to provide a common approach with multi-disciplines dealing with the subject, it has become necessary to create new guidelines for diagnosing and treating NAFLD. This guidance was prepared following an interdisciplinary study under the leadership of the Turkish Association for the Study of the Liver (TASL), Fatty Liver Special Interest Group. The study group consisted of specialists in gastroenterology, endocrinology and metabolism diseases, cardiology, nephrology, pathology, radiology, pediatrics, and general surgery, who were interested in the topic. The study group was organized into four subgroups, as follows;
Terminology, epidemiology, and natural course;
Associated metabolic disorders;
Diagnosis; and
Treatment approach.
NAFLD topics were distributed among the subgroups according to the group topics. Group members reviewed the current national and international data on their issues, studied the published guidelines, and reflected on their personal experiences; following this, initial report drafts were written. Finally, the group members discussed these texts, and the report was prepared. All group reports were then combined to form the NAFLD Clinical Practice Guidance.
This guidance is a practical application guide on NAFLD and were prepared to standardize the clinical approach to diagnosing and treating NAFLD patients. The proposes in this guidance are meant to aid decision-making in the clinical practice. The guidance is primarily intended for gastroenterology, endocrinology, metabolism diseases, cardiology, internal medicine, pediatric specialists, and family medicine specialists. Still, they can also aid all physicians and researchers interested in NAFLD.
Terminology, Epidemiology, and Natural Course
Terminology
NAFLD is characterized by abnormal fat accumulation in the liver without associated alcohol consumption (Table 1). To diagnose NAFLD, the following conditions must be met:
- Fatty liver should be demonstrated by one of the following methods:
- Radiologically, any degree of steatosis is seen through abdominal ultrasonography (US), or detection of steatosis above the threshold value determined in another imaging method: or
- Histologically, more than 5% of hepatocytes are fatty.
Alcohol consumption should not exceed the specified limits (20 g/day for women; 30 g/day for men) or no alcohol.
Causes that may lead to secondary fatty liver or other accompanying chronic liver diseases that may accompany should be excluded.
Table 1.
The Definition of the Clinical Spectrum of NAFLD
| NAFLD: Characterized by abnormal fat accumulation in the liver, unrelated to alcohol use, and can be encountered in a broad clinical spectrum ranging from fatty liver, steatohepatitis, liver cirrhosis, and hepatocellular cancer. NAFL: Can be caused by steatosis alone, or lobular inflammation may accompany steatosis without ballooning. Fibrosis may be present in some cases, especially in patients with lobular inflammation. NAFL has a shallow risk of progression to cirrhosis. NASH: A condition in which steatosis is accompanied by inflammation and ballooning with or without fibrosis. NASH is considered a progressive form of NAFLD. NASH Cirrhosis: Cirrhosis in patients with previously known or newly detected fatty liver/steatohepatitis. HCC: The development of HCC in NAFLD patients. It has been reported that developing HCC without cirrhosis in the context of NASH is possible. |
NAFLD: Nonalcoholic fatty liver disease; NAFL: Nonalcoholic fatty liver; NASH: Nonalcoholic steatohepatitis; HCC: Hepatocellular carcinoma.
The secondary reasons for fatty liver are summarized in Table 2. The diagnosis of NASH is histological and should not be used except in cases confirmed by liver biopsy.
Table 2.
Secondary Causes of Hepatic Steatosis
| Diseases |
| Alcohol-related liver disease (ALD) |
| Hepatitis C viral infection (genotype 3) |
| Wilson’s disease |
| Abetalipoproteinemia, hypobetalipoproteinemia |
| Lipodystrophy |
| Long-term starvation |
| Lecithin-cholesterol acyltransferase (LCAT) deficiency |
| Cholesterol ester storage disease |
| Drugs |
| Macrovesicular steatosis |
| Amiodarone |
| Corticosteroid |
| Methotrexate |
| Chemotherapeutic agents (Tamoxifen, 5-Fluorouracil, Irinotecan, Cisplatin, Asparaginase) |
| Total parenteral nutrition |
| Microvesicular steatosis |
| Tetracycline |
| Valproic acid |
| Nucleoside reverse transcriptase inhibitors |
| Corticosteroid |
| Cocaine |
| Steatohepatitis |
| Amiodarone |
| Methotrexate |
| Tamoxifen |
| Irinotecan |
ALD: Alcohol-related liver disease; LCAT: Lecithin-cholesterol acyltransferase.
Demonstrating the close relationship between NAFLD and metabolic disorders has prompted further discussion of the definition of NAFLD from a new perspective. In 2020, the consensus of a group of experts dealing with the subject from 22 countries suggested renaming NAFLD as “Metabolic Disease-Associated Fatty Liver Disease (MAFLD)”.[5,6] It is thought that the MAFLD definition reflects metabolic dysfunction and better explains the pathogenesis of fatty liver disease. According to this definition, for patients who have abnormal fat accumulation in hepatocytes detected by imaging methods and/or liver biopsy, the presence of at least one of the following criteria must be present for MAFLD to be diagnosed (Figure 1):
Being overweight or obese;
Type 2 Diabetes Mellitus (T2DM); or
Presence of at least two criteria of metabolic dysfunction.
Figure 1.

Diagnostic Scheme of Metabolic Disease-Associated Fatty Liver Disease (MAFLD).
The word alcoholic/alcohol consumption in NAFLD can lead to misunderstandings and stigmatization.[7,8] In addition, the definitions of “non” or “nonalcoholic” are not considered appropriate because they trivialize the disease.[7,8] For the NAFLD diagnostic criteria used to date, excluding other chronic liver diseases, including excessive alcohol use, is mandatory. MAFLD diagnostic criteria have been expanded to cover fatty liver associated with alcohol use and other chronic liver diseases. Thus, the definition of “patients with dual combined etiology” was created.
On the other hand, there still needs to be a consensus on replacing the term NAFLD with MAFLD. Instead of separating patients who underwent liver biopsy into NAFL and NASH, define the condition by specifying the degree of disease activity and fibrosis. Similarly, for the definition of “NASH cirrhosis,” it is more convenient to use “MAFLD-associated cirrhosis” in patients with cirrhosis who still meet the MAFLD diagnostic criteria currently or who did so in the past. The term “cryptogenic cirrhosis” in patients with cirrhosis who meet these criteria should be avoided.
Epidemiology and Risk Factors
NAFLD prevalence varies by geographic region and ethnicity. While the Middle East, South America, and Asia have the highest prevalence (>27%), the prevalence is lower (14%) in Africa.[1] Lifestyle changes (diet, physical activity, socioeconomic status) and environmental factors affect the disease’s frequency, severity, and course. Based on the limited data available, the prevalence of NASH is estimated that 3%–6% of the population in the United States (USA) have NASH.[9,10] NAFLD often coexists with; obesity, insulin resistance (IR), T2DM, metabolic syndrome (MetS), dyslipidemia, and cardiovascular diseases (CVD) (Table 3). NAFLD prevalence is higher in those with metabolic diseases than in the general population.[11–13] NAFLD is detected in 75%–80% of obese patients, 56%–70% of patients with T2DM, approximately 70% of patients with MetS, and 70% of those with dyslipidemia.[2,3,10] Furthermore, metabolic disorders are more common in those with NAFLD and NASH (Table 4).[1,11,13] Few studies have been conducted to determine the incidence of NAFLD, which differs in the method used for diagnosis. While NAFLD incidence is 20–86 per 1,000 person-years in patients with a US finding and/or high liver enzyme levels, with magnetic resonance imaging (MRI) imaging, the incidence is calculated as 34 per 1,000 person-years.[1,12]
Table 3.
NAFLD-Related Diseases and Risk Factors
| Major risk factors | Minor risk factors |
|---|---|
| Being overweight/obese | Hyperuricemia |
| Type 2 diabetes mellitus | Hypothyroidism |
| Insulin resistance | Intestinal microbiota dysbiosis |
| Hypertension | Sleep apnea syndrome |
| Metabolic syndrome | Polycystic ovary syndrome |
| Dyslipidemia | Genetic factors: PNPLA3, TM6SF2 |
| Epigenetic factors: DNA methylation, micro-RNA’s |
PNPLA3: Patatin-like phospholipase domain-containing protein; TM6SF2: Transmembrane 6 superfamily member 2.
Table 4.
Frequency of Metabolic Disorders in NAFLD and NASH Patients
| World (%) | Türkiye (%) | |||
|---|---|---|---|---|
| NAFLD | NASH | NAFLD | NASH | |
| Obesity | 51 | 82 | 41 | 61 |
| Dyslipidemia | 70 | 72 | 90 | 62 |
| Metabolic syndrome | 43 | 7 | 47 | 63 |
| Hypertension | 39 | 68 | 29 | 37 |
| Type 2 diabetes mellitus | 23 | 44 | 22 | 34 |
NAFLD: Nonalcoholic fatty liver disease; NASH: Nonalcoholic steatohepatitis.
According to the World Health Organization (WHO), Türkiye has the highest obesity rate in Europe, at 32%.[13] According to Turkish Diabetes Epidemiology (TURDEP) data from 1998 (TURDEP I) and 2010 (TURDEP II)-studies that were carried out in 540 centers to determine the prevalence and risk factors of obesity, diabetes, and hypertension in the adult population in Türkiye-within 12 years, Diabetes Mellitus (DM) frequency increased from 7.2% to 16.5%. Obesity prevalence increased from 22% to 36%.[13,14] In line with the increasing trend in obesity and DM prevalence, the prevalence of NAFLD is estimated to be more than 30% in Türkiye. In the screening studies conducted in Türkiye, the frequency of NAFLD was between 48% and 60%, placing Türkiye among the countries with the highest prevalence of NAFLD globally.[1,15]
In addition to metabolic disorders, age, sex, and ethnicity affect the development of NAFLD. The gender distribution differs in NAFLD, such that being male has been considered a risk factor; however, data are controversial. Patients are often diagnosed at the age of 40–50 years. The prevalence of NAFLD and the stage of the disease appear to increase with age. NAFLD is an insidious and progressive disease. The role of ethnicity and its impact on NAFLD has been discussed over the years. Hispanic individuals have a significantly higher prevalence of NAFLD. However, it suggested that the ethnic differences reported for NAFLD may be explained by the genetic variations related to the gene mutation.[2,4,16,17] Moreover, the at-risk groups for NAFLD should be identified (Table 3).
Natural Course of NAFLD
NAFLD patients have higher mortality rates and shorter survival times than the general population.[18–22] Mortality is related to cardiovascular disease (CVD) and nonhepatic malignancy, followed by liver disease.[20,21] Based on a previous statistical study, liver disease in the general population of the USA was the 12th most common cause of death, and it was ranked third among NAFLD patients.[22] Liver fibrosis level and steatohepatitis are reliable findings that can predict the clinical course of NAFLD and liver-related outcomes and mortality.[23–27] In cases in which the level of fibrosis was evaluated with control biopsies, it has been demonstrated that fibrosis can progress, regress, or remain stable among NAFL and NASH patients. It has been reported that in 30%–40% of NASH cases, fibrosis will progress, 40%–50% will remain stable, and 10%–20% will regress.[28–30] NASH patients and patients with stage 2 or higher fibrosis have a higher risk of liver-related morbidity and mortality.[24,28–30] Female gender, advanced age (>50 years), presence of T2DM, obesity, MetS, and high ferritin levels are defined as factors that negatively affect the natural course of NAFLD.[31,32] IR is universal in NAFLD patients. IR is present in the liver, muscle, and adipose tissue and promotes disease progression.[24,28,29] Genetic polymorphisms, such as PNPLA3 and TM6SF2 gene polymorphisms, have been linked to fibrosis progression, the severity of the disease, and the development of HCC; however, this information is not used in routine clinical practice today.[33,34] Systemic inflammation also contributes to disease progression.[24,28,29,31]
NAFLD has high morbidity and mortality rates.
NAFLD can progress, remain stable, or regress.
Liver fibrosis predicts disease progression and is influenced by the severity of baseline disease, genetic and environmental factors, and the presence and severity of concomitant metabolic disorders.
NAFLD and Concomitant Metabolic Diseases
Endocrine, metabolic, cardiac, renal, and other diseases can co-exist with NAFLD. These accompanying diseases affect NAFLD development, the disease’s natural course, and the treatment response. Here, recommendations for diagnosing, treating, and following conditions accompanying NAFLD are offered in light of the available scientific evidence.
Diabetes, Obesity, Dyslipidemia, and Hyperuricemia
IR, T2DM, MetS, hypertension, dyslipidemia, and hyperuricemia are associated with NAFLD.[2,3,35–40] T2DM is the most critical factor for developing NAFLD, disease progression, advanced fibrosis, and HCC.[36] Patients with T2DM have a higher prevalence of NAFLD. IR is the most critical responsible mechanism in the pathogenesis of T2DM and NAFLD.[35–37] The relationship between T2DM and NAFLD is bidirectional. It has been reported that NAFLD increases the risk of incident diabetes by 2–5 times and the risk of CVD by 2–3 times.[2,3,37–41] Increased secretion of diabetogenic hepatokines such as retinol-binding protein 4 (RBP-4), fetuin-A, and fibroblast growth factor 21 (FGF-21); increased inflammatory biomarkers, such as C-reactive protein, tumor necrosis factor-alpha, and interleukin 6; increased hepatic gluconeogenesis; and glycogen synthesis in NAFLD cause an increase in the risk of developing T2DM.[37–41] Moreover, hepatic lipid (diacylglycerol) accumulation also contributes to the development of DM by disrupting insulin signaling and causing IR.[37,41]
Today, an unhealthy diet and a sedentary lifestyle significantly increase fructose consumption and contribute to developing NAFLD in all age groups by increasing de novo lipogenesis in the liver.[3,41,42] Fructose is a factor that causes hepatosteatosis and accelerates the progression of the existing disease by increasing oxidative stress and causing mitochondrial dysfunction and endoplasmic reticulum stress. In addition, fructose metabolizes in the liver and contributes to the formation of uric acid. Hyperuripredisposesispose patients to developing NASH in association with IR.[3] Diabetic patients are at higher risk for NASH and advanced fibrosis. With NASH and T2DM, the risk of developing cirrhosis and HCC increases 2–4 times.[2,3,35,37,39,43,44] In T2DM patients, the risk of death from chronic liver disease is approximately three times higher than in the non-diabetic population.[36]
For the adult population in Türkiye, based on the data from the TURDEP-I and TURDEP-II studies, central obesity is defined according to waist circumference,
For the diagnosis of prediabetes, one of the following criteria must be met.
Impaired fasting glucose, fasting blood sugar is 100–125 mg/dl,
Impaired glucose tolerance, second-hour blood glucose level during the oral glucose tolerance test (OGTT) is 140–199 mg/dl; or
High-risk group (HbA1c value is 5.7%–6.4%)
MetS diagnostic criteria for the Turkish population are defined as follows. For the diagnosis of MetS, three or more of these criteria must be present:
Increased waist circumference (≥91 cm in women, ≥95 cm in men);
High blood pressure (≥130/85 mmHg) or taking antihypertensive therapy;
High fasting triglyceride (≥150 mg/dl) or taking specific drug therapy;
Decreased high-density lipoprotein (HDL) cholesterol (<50 mg/dl in women, <40 mg/dl in men),
Prediabetic; and
HOMA-IR score ≥2.5.
Metabolic Diagnostic Tests in Patients with NAFLD
Since the risk of MetS and T2DM increases in patients with NAFLD, MetS components, such as fasting serum triglyceride, total cholesterol, HDL cholesterol, creatinine, uric acid, and arterial blood pressure, should be investigated in these patients. Also, fasting blood glucose, HbA1c, and, if necessary, standard OGTT should also be monitored for the development of diabetes. Diabetes is diagnosed when fasting blood glucose exceeds 126 mg/dl at two-time points. In the standard OGTT test, a blood glucose level of ≥200 mg/dl at the second hour confirms the diagnosis. For individuals who have a complaint of polydipsia or polyuria, a blood glucose level of ≥200 mg/dl at any time is also diagnostic of diabetes. When T2DM is detected, NAFLD patients should be referred to the diabetes clinic for blood glucose regulation and follow-up.[2,3,45] Patients with prediabetes or T2DM should be investigated for the presence of NAFLD every 6–12 months.
In all NAFLD patients;
Waist circumference should be measured,
Blood pressure should be measured and monitored,
Fasting lipid profile should be checked,
HOMA-IR should be calculated,
Should be screened for the presence of T2DM, and
An evaluation should be made in terms of diagnosing MetS.
Obese patients, patients with IR, DM, and/or MetS should be screened for the presence of NAFLD.
Management of Metabolic Disorders
Since the CVD risk is higher in patients with NAFLD compared to the normal population, in the management of diabetes, CVD risk-reducing drugs should be initially preferred. Glucagon-like peptide-1A (GLP-1A) and sodium-glucose transporter-2 inhibitors (SGLT2i) can be prioritized over other agents in diabetic patients who cannot achieve adequate glycemic control with metformin therapy. Pioglitazone is a drug that positively affects hepatosteatosis and fibrosis; however, it should be used cautiously due to the risk of bone loss, bone fractures, fluid retention, and edema. Insulin is safe and should be considered first n patients with advanced liver disease.
The close relationship between NAFLD and hypertension is well established.[2–4] The risk of progression to fibrosis and liver-related morbidity increases among NAFLD patients with hypertension.[2,3] Strict blood pressure control is recommended in hypertensive NAFLD patients. The approach to treating hypertension is in line with current principles in the general population, beginning with lifestyle changes (regulation of diet and physical activity, salt restriction, alcohol restriction, not using cigarettes and other tobacco products).[27,29,39] Because the renin-angiotensin-aldosterone system (RAAS) may affect fibrosis as an antihypertensive agent, the renin-angiotensin system (RAS) blockers, such as angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor blockers, should be preferred.[27,39] Allopurinol may be recommended when lifestyle changes cannot improve serum uric acid levels.
In the treatment planning for T2DM patients, approaches that prevent the development and progression of NAFLD should be included.
Cardiovascular Disease
There is a bidirectional relationship between NAFLD and CVD.[46] Considering the close relationship between NAFLD and MetS components, an increased risk of CVD is expected in those with NAFLD. Furthermore, it is suggested that NAFLD may cause CVD independently of the classical cardiovascular risk factors.[47] It is thought that, in addition to conventional cardiovascular risks factors, such as diabetes, hypertension, and dyslipidemia, many secondary risk factors accompanying NAFLD, such as hyperuricemia, hypoadiponectinemia, and proinflammatory cytokines, may be responsible for the increase in the frequency of CVD in patients with NAFLD.[48] It should not be forgotten that NAFLD is closely related to cardiovascular mortality and associated with liver-related mortality. The probability of a nonfatal cardiovascular event or death from cardiac causes is increased twice in patients with NAFLD compared to the general population.[49] Cardiovascular events that can be seen in patients with NAFLD include:
Atherosclerosis (coronary artery disease, ischemic stroke, peripheral artery disease);
Arrhythmias (atrial fibrillation, conduction disturbances, QT prolongation, ventricular premature beats);[50]
Myocardial involvement (left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, heart failure with preserved ejection fraction),[51] and
Valve diseases (valve calcifications, aortic valve sclerosis, mitral annulus calcification).[52]
The first step in cardiovascular evaluation in patients with NAFLD is to question the patient for cardiac symptoms, such as chest pain, dyspnea, or palpitations. Patients with cardiac symptoms should receive a detailed cardiovascular evaluation. It should not be forgotten that subclinical atherosclerosis may be present in patients without cardiac symptoms.[51] Guidelines on preventing CVD are recommended for calculating absolute cardiovascular risk for all adults over 40 years of age or for those at risk regardless of age, using risk calculation tools.[53–55] This risk calculation should be repeated every five years. The purpose of risk classification is to be able to initiate risk-reducing interventions early. Therefore, it is recommended to calculate the baseline absolute cardiovascular risk independent of age in adult NAFLD patients. Various risk classification systems can be used to determine cardiovascular risk. Systematic Coronary Risk Estimation (SCORE) or Atherosclerotic Cardiovascular Disease (ASCVD), derived from the Pooled Cohort equation, is used today.
In one study, approximately one-half of NAFLD patients had a moderate or high ten-year risk of atherosclerotic CVD.[56] In addition, the degree of hepatosteatosis correlates with ASCVD risk scores.[57] The SCORE system, recommended by the European Society of Cardiology, predicts the ten-year cumulative risk for the first fatal cardiovascular event. The SCORE system offers different calculation options for various low- or high-risk regions. It has also been recalibrated using data from Türkiye and can be accessed at http://www.heartscore.org. The calculated SCORE risk is as follows:
<1% is low risk,
1% to <5% is moderate risk,
5% to <10% is a high risk, and
10% or more is considered very high risk.
Patients with known atherosclerotic CVD, target organ damage, long-term diabetes of more than ten years, moderate to severe chronic kidney disease, and familial hypercholesterolemia directly fall into the high- or very high-risk group.[54] The European Society of Cardiology guidelines consider NAFLD a “modifying factor” and suggest keeping in mind that NAFLD patients can be classified into a higher class than the SCORE risk calculated during treatment planning. Where the calculated risk is low or medium, additional imaging studies may be helpful for treatment planning. With coronary artery calcium (CAC) scoring, an Agatston score greater than 100 or detecting arterial plaque load (femoral or carotid) on the arterial US will increase the patient’s risk group.
The most crucial step in preventing atherosclerotic CVD, heart failure, and atrial fibrillation, independent of the risk level, is to adopt a healthy lifestyle that includes not smoking, eating a balanced diet, and engaging in regular exercise. The risk calculation guides the initiation of medical treatments. Different low-density lipoprotein (LDL) values are targeted at different risk levels to increase ease of application. Various lipid-lowering therapies, beginning with statins, are recommended to achieve these goals. In anti-lipidemic therapy, targeted LDL values are:
<55 mg/dl for very high-risk patients,
<70 mg/dl for high-risk patients,
<100 mg/dl for moderate-risk patients, and
<116 mg/dl for low-risk patients.
The initiation of antihyperlipidemic therapy is recommended in patients with serum LDL levels of more than 190 mg/dl, regardless of risk classification. Statins have been reported to reduce the likelihood of cardiovascular events in NAFLD patients.[58,59] Hypertriglyceridemia can be managed through lifestyle changes. However, aspirin reduces the risk of nonfatal myocardial infarction and increases the risk of severe bleeding and hemorrhagic stroke in individuals without known CVD. Therefore, its use is not recommended for the primary prevention of CVDs.[60]
Echocardiographic findings, such as left ventricular hypertrophy, deterioration in left ventricular geometry, left atrial dilatation, diastolic dysfunction, increased epicardial fat thickness, and valve calcifications, can be seen in patients with NAFLD.[52,61] However, indications for echocardiographic examination in NAFLD patients do not differ from the general population. An echocardiographic examination is indicated in patients with unexplained dyspnea, abnormal rhythm on electrocardiogram, patients with chest pain or dyspnea and a murmur heard, or when clinical evaluation indicates structural heart disease. Presenting with dyspnea, in the presence of other risk factors, such as hypertension and obesity, “heart failure with preserved ejection fraction” may exist in NAFLD patients. In an echocardiographic evaluation, diastolic and systolic functions should also be evaluated in detail.
Since there is a bidirectional relationship between NAFLD and CVD, NAFLD patients with symptoms and findings suggestive of CVD, and NAFLD patients with high or very high-risk scores, should be referred to a cardiologist. However, for patients presenting to the cardiology clinic with atherosclerosis or MetS components, it should be kept in mind by cardiologists should keep in mind that NAFLD may also be a factor, and these patients should be sent to a gastroenterologist to be evaluated for NAFLD.
Baseline cardiovascular risk using risk assessment systems, such as the SCORE risk score, should be determined in adult NAFLD patients.
In patients with low or moderate cardiovascular risk, CAC scoring or evaluation of carotid/femoral artery plaque load should be conducted to aid treatment decisions.
A detailed cardiovascular evaluation should be made in NAFLD patients with cardiac symptoms such as chest pain, dyspnea, or palpitations.
Statins are recommended for CVD risk reduction in NAFLD patients
The presence of NAFLD should be investigated in patients admitted to the cardiology outpatient clinic due to MetS or CVD.
Chronic Kidney Disease
It has been reported that the prevalence and incidence of chronic kidney disease (CKD) are increased in NAFLD patients.[62–64] This relationship is independent of the common risk factors for both diseases, such as diabetes, hypertension, and obesity.[62–64] The presence of NAFLD increases the prevalence of CKD in individuals with initially normal renal functions.[62] The prevalence of CKD was higher in patients with NASH than in patients with NAFL.[65,66] Metabolic risk factors, such as obesity, IR, DM, dyslipidemia, and hypertension, play an essential role in the development and progress of NAFLD and CKD. This situation makes revealing a direct causal relationship between the two diseases difficult.
Systemic mediators released from the fatty and inflamed liver (reactive oxygen radicals, advanced glycosylation end products, C-reactive protein, proinflammatory, pro-fibrogenic and anti-fibrinolytic molecules [fetuin-A, fibroblast growth factor-21, tumor necrosis factor-alpha (TNF) -α], transforming growth factor-β, and plasminogen activator inhibitor-1) play a role in the development of kidney and liver damage.[62,63,67] In addition, obesity-related mechanisms (lipotoxicity, oxidative stress, increased proinflammatory cytokine synthesis, and activation of the RAAS system) have been suggested as possible pathogenetic mechanisms.[67]
NAFLD patients, especially patients with steatohepatitis, should be screened for CKD. Many methods have been proposed for evaluating renal function in these patients. Measuring the estimated glomerular filtration rate (eGFR) and evaluating the albumin/creatinine ratio (ACR) in spot urine examinations to determine microalbuminuria are simple, inexpensive, practical, and beneficial methods for identifying renal dysfunction in such patients.
CKD Definition and Staging
CKD is defined by the degree of renal dysfunction and the presence of kidney damage, regardless of the type of kidney disease. CKD is defined as, for three months or longer, a GFR less than 60 ml/min/1.73 m2 or the presence of structural or functional abnormalities (normal or decreased GFR) of kidney damage.[68] The guidelines offered in “Kidney Diseases 2012: Improving Global Outcomes” (Kidney Disease; Improving Global Outcomes [KDIGO]) defined CKD as abnormalities in kidney structure and function that persist for more than three months and have health implications.[69] As markers of kidney damage, albuminuria, urinary sediment abnormalities, abnormalities due to tubular disorders, histologically detected abnormalities, structural abnormalities detected by imaging methods, and a history of kidney transplantation were determined (Table 5).
Table 5.
Chronic Kidney Disease Criteria According to 2012 KDIGO Guidelines
| CKD Criteria |
| At least one criterion must exist for more than three months |
| Markers of kidney damage |
|
| GFR reduction |
| GFR <60 ml/min/1.73 m2. |
KDIGO: Kidney Disease: Improving Global Outcomes; CKD: Chronic kidney disease; GFR: Glomerular filtration rate; AER: Albumin excretion ratio; ACR: Albumin/creatinine ratio.
GFR and albuminuria are independent and complementary factors of CKD progression, end-stage renal disease, acute kidney injury, cardiovascular mortality, and all-cause mortality.[69] This new classification proposed in the KDIGO guidelines may guide the prognosis prediction regarding progression rate and complication risks. Serum creatinine level alone is not sufficient to diagnose CKD. Because of high creatinine levels, approximately 50% of renal functions must be lost. Serum creatinine levels vary according to patient characteristics (age, gender, race, body weight, muscle mass, diet, etc.). Estimated GFR, adjusted for age, gender, race, and body weight, is reliable. GFR measurement is considered the best measure of kidney filtration ability in cases of sickness and health.[69] Frequently, three validated methods are used for the determination of GFR based on the measurement of serum creatinine levels: the Modification of Diet in Renal Disease (MDRD) equation,[68] the Cockcroft-Gault formula, and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. The CKD-EPI equation has been shown to provide better results, especially at higher-than-normal GFR values.[70]
Conversely, proteinuria is a sensitive marker of kidney damage in many CKD types, such as DM, hypertension, and glomerular disease. The Tamm-Horsfall glycoprotein, synthesized from the renal tubules, constitutes the plurality (40%) of the proteins normally found in the urine. Normal protein excretion is less than 150 mg/day in adults. Proteinuria is mentioned when daily urinary protein excretion exceeds this threshold. The determination of microalbuminuria is a sensitive test to indicate early kidney damage.[71] Since many factors affect urine albumin and protein concentration, such as the urine collection technique, exercise, and urinary tract infection, measuring the albumin/creatinine and protein/creatinine ratios in spot urine samples is recommended. The protein/creatinine ratio is compatible with 24-hour proteinuria if renal functions are stable. An albumin (mg)/creatinine (gr) ratio of less than 30 is considered normal, a 30–300 suggests microalbuminuria, and a value greater than 300 indicates macroalbuminuria.
Measuring GFR (using the MDRD or CKD-EPI formula) and evaluating the ACR to assess kidney function are recommended in patients with NAFLD. NAFLD patients with low GFR or microalbuminuria should be referred to a nephrologist to rule out other causes of CKD and to follow up on the disease. A correlation has been observed between microalbuminuria and a higher stage of fibrosis in patients with NAFLD.[72] A meta-analysis found that the presence of advanced fibrosis in NAFLD patients was associated with a higher CKD prevalence (odds ratio [OR] 5.20; 95% confidence interval [CI] 3.14–8.61) and incidence (OR 3.29; 95% CI 2.30–4.71), independent of other risk factors.[73] There was a positive relationship between NAFLD severity and CKD stage.[73,74] In long-term follow-up of NAFLD patients, it was found that the risk of CKD stage 3 or higher (eGFR <60 mL/min/1.73m2) increased 1.45 times.[74] RAAS blockade treatment is recommended in NAFLD patients with microalbuminuria if there is no contraindication.[67]
The frequency of CKD is increased in patients with NAFLD.
Microalbuminuria is an important diagnostic method for identifying early kidney damage. It is recommended to measure albumin/creatinine or protein/creatinine ratios in spot urine samples to screen for CKD in patients with NAFLD.
Lean NAFLD
NAFLD can also be seen in individuals with average weight (BMI <25 kg/m2 or <23 kg/m2 in Asian populations). In a recent systematic review and meta-analysis, the prevalence of normal-weight NAFLD cases was reported as 5% in the general population and 19% in the NAFLD population.[75] In two studies conducted in Türkiye, the corresponding ratios were 6.4% and 7.6%, respectively.[76,77] In another study, 4.3% of patients with NASH were found to be of average weight.[78] Among normal-weight individuals, the prevalence of NAFLD was 10.6%, and the incidence of NAFLD was 23.2 per 1,000 person-years (95% CI 7.3–48.0).[75]
Metabolic abnormalities similar to those of the obese have been described in some normal-weight people.[79] This group of patients is called metabolically obese but normal weight. These individuals show the characteristic metabolic profile of IR.[80,81] Normal weight NAFLD occurs at younger ages.[82] IR, T2DM, hypercholesterolemia, and hypertension were higher in normal-weight NAFLD patients than in normal-weight and non-fatty liver patients.[82] Approximately 13% of normal-weight NAFLD patients have IR.[82] MetS components are generally less common in normal-weight NAFLD patients than in obese or overweight NAFLD patients (2%–48% vs. 22%–64%).[83]
The PNPLA3 rs738409 GG allele was detected more frequently in Asian and non-MetS normal-weight NAFLD patients.[83–85] It has been suggested that this feature may be the reason for the similar prevalence among Caucasians, despite the lower metabolic load in Asian populations.[83–85] Genetic deficiency of phosphatidylethanolamine N-methyltransferase (PEMT) has increased the risk of NASH in normal-weight individuals.[84,86] Other genetic variants associated with normal-weight NAFLD are CETP rs12447924 and rs12597002, TM6SF2 rs58542926 C, and interferon lambda-4 rs368234815 TT polymorphisms.[86] In addition, it has been suggested that intestinal microbiota and metabolomic profiles may play a role in pathogenesis.[87–89] It is believed that pathways similar to those in obese NAFLD patients are activated in progression to NASH in normal-weight NAFLD patients.[86] No significant difference was found between the diets of normal weight and obese NAFLD patients.[90] Generally, it has been suggested that, among normal-weight people, those with NAFLD consume more total calories than those without.[91] However, there was no difference between normal-weight NAFLD patients and normal-weight individuals without steatosis regarding the amount of food and beverages consumed and the number of calories ingested.[82]
There are limited histological data available for normal-weight NAFLD patients. In two European studies, 50% and 65% of NAFLD patients of normal weight were found to have NASH. There were no significant differences between obese and non-obese patients regarding the severity of hepatic inflammation and fibrosis.[92,93] In some studies, the severity of steatosis and fibrosis was lower in the normal-weight NAFLD patient group than among overweight or obese patients.[94–96] Data on the prognosis of the disease are controversial. In some studies, normal-weight NAFLD patients have been found to have a higher risk of developing severe liver disease and have a shorter cumulative life span than overweight or obese NAFLD patients.[94,97–99] However, a study conducted in the USA reported that the prevalence of cirrhosis in NAFLD patients of average weight was lower than that among overweight or obese NAFLD patients.[100] A 19-year follow-up study in Japan found that NAFLD patients with and without normal weights did not differ in mortality and liver-related event rates.[101] A recently published systematic review and meta-analysis reported that hepatic and extra-hepatic comorbidities develop significantly in long-term follow-up among normal-weight patients. The investigators reported that NAFLD might have a progressive course in normal-weight patients.[75]
Diagnosis is usually made by detecting fatty liver in routine abdominal US. Non-invasive diagnostic methods and scores are used to predict fibrosis in NAFLD patients. These tests have not been separately validated for normal-weight NAFLD patients. There are limited data on the management of NAFLD in normal-weight patients. The general principles in the treatment approach are the same as those in overweight or obese NAFLD patients. Diet and lifestyle changes also have effective in normal-weight NAFLD patients.[102] The principles of using current and emerging drugs for overweight or obese NAFLD patients are also likely to apply to normal-weight NAFLD patients.
NAFLD And Combined Chronic Liver Disease
Due to the high prevalence of NAFLD, it is likely to be associated with other chronic liver diseases. This association may adversely affect the course and prognosis of the disease.[103–105] Patients with combined liver disease have a different natural course of disease and response to treatment than patients with chronic liver disease from a single cause.[105–107] NAFLD and alcohol can commonly be found in the same patient as two different etiologies.[103] The amount, duration, and type of alcohol use significantly affect the disease progression. Moderate and heavy alcohol consumption increases liver damage, disease progression, cirrhosis, HCC, extrahepatic malignancies, and death.[103,107] In the follow-up of patients with NAFLD and moderate alcohol use, more steatosis was detected, or a slower improvement in steatosis was observed than in those who did not use alcohol. At the same time, no difference was found in inflammation and fibrosis.[103] Moreover, alcohol intake should be assessed regularly in patients with NAFLD.
In patients with chronic viral hepatitis, NAFLD association is seen at least at the rate of NAFLD frequency in the community. Based on epidemiological studies conducted in the adult population in Türkiye, the prevalence of hepatitis B surface antigen (HBsAg) positivity is 4%.[108] In a study conducted on patients with chronic hepatitis B (CHB), the frequency of NAFL was 40%, and the rate of cirrhosis was significantly higher in NAFL patients.[109] NAFL independently increased the development of HBV-associated HCC by 7.3 times at a median follow-up of 80 months.[109] NASH was associated with HCC, liver transplantation (LT), and all-cause mortality.[105] It has been reported that the coexistence of NASH and CHB often results in advanced-stage fibrosis and death in a shorter timeframe.[105] Paradoxically, a lower incidence of cirrhosis and HCC and a higher clearance of HBsAg were associated with HBV-infected patients with radiological hepatic steatosis.[110] In another study, hepatitis B core antibody (anti-HBc) positivity was associated with cirrhosis, cirrhotic complications, and HCC in those with NAFLD.[111] In a study conducted in Türkiye, anti-HBc positivity was found to be a risk factor for cirrhosis in NAFLD.[112]
It is known that hepatic steatosis was seen in patients infected with the hepatitis C virus (HCV) genotype 3. Unlike NAFLD patients, hepatic steatosis is directly proportional to viral load, starting from the periportal area, not the centrilobular area.[113,114] This condition is unique to HCV genotype 3. However, fatty liver disease in patients infected with other HCV genotypes is associated with obesity, DM, and IR, as in patients with NAFLD. If these patients meet the diagnostic criteria for NAFLD, NAFLD and chronic hepatitis C should be considered a combined disease.[113,114] Follow-up and treatment for NAFLD should be continued after HCV eradication is achieved with antiviral therapy. It has been reported that fatty liver disease is associated with a poor prognosis in patients with HCV eradication.[115]
Diagnosis and Assessment of NAFLD
Noninvasive tests (NITs): Diagnostic tests for NAFLD are used to establish the diagnosis, determine the degree of inflammation, determine the fibrosis stage, and make a differential diagnosis. Diagnostic tests can be grouped as biochemical, radiological, and histopathological. Distinguishing NAFL and NASH is essential for evaluating the risk of developing fibrosis. Although there is no validated test to diagnose NASH, serum cytokeratin-18 fragments (M30, M65) can be used to diagnose steatohepatitis. Of these, M30 indicates hepatocyte apoptosis, and M65 shows cell death. The M30 level has 0.81, the area under the receiver operating characteristic (AUROC), 78% sensitivity, and 87% specificity for diagnosing NASH.[49,116,117] In addition, it has recently been suggested that the activated plasminogen activator inhibitor 1 (PAI1) can differentiate NAFL and NASH.[118]
A liver biopsy is accepted as a reference for evaluating the fibrosis stage. However, it should be used in selected cases due to sampling variability, inter/intra-observer variability, and procedural complications. NITs can usually be calculated from formulas created with biochemical data in routine practice (Table 6).[119–122] NITs are highly accurate in excluding advanced fibrosis (F3, F4) with a negative predictive value (NPV) greater than 95%.[119,121,123] Validation studies conducted in Türkiye supported these results.[124–126] NITs have similar diagnostic performance in NAFLD patients with diabetic or normal transaminase levels.[124–126] The enhanced liver fibrosis (ELF) test is associated with detecting extracellular matrix proteins, especially type III collagen deposition.[127] ADAPT, and ABC3D tests use Pro-C3 and show collagen III formation (Table 7).[128,129]
Table 6.
Scores Used to Determine the Degree of Fibrosis in Patients with NAFLD
| Test | Required parameters | AUROC |
|---|---|---|
| APRI | AST, platelet count | 0.77 |
| BARD | BMI, AST, ALT, DM | 0.76 |
| FIB-4 | Age, AST, ALT, platelet count | 0.84 |
| NFS | Age, BMI, DM, AST, ALT, albumin, platelet count | 0.84 |
APRI: Aspartate aminotransferase/platelet ratio index; AST: Aspartate aminotransferase; BMI: Body mass index; ALT: Alanine aminotransferase; NFS: NAFLD Fibrosis Score; DM: Diabetes mellitus; FIB-4: Fibrosis index-based 4.
Table 7.
Scores Calculated by Special Biochemical Tests to Determine the Degree of Fibrosis in NAFLD Patients
| Test | Required parameters | AUROC | |
|---|---|---|---|
| ELF | Hyaluronic acid, amino-terminal propeptide-type III procollagen, TIMP-1 | 0.77 | |
| Pro-C3 | Pro-C3 | 0.81 | |
| ADAPT | Pro-C3, age, DM, platelet count | 0.86 | |
| FIBC3, ABC3D | Pro-C3, BMI, DM, age, platelet count | 0.81 |
ELF: Enhanced liver fibrosis; BMI: Body mass index; DM: Diabetes mellitus; TIMP-1: Metalloprotease tissue inhibitor-1.
NITs can be used in primary care as first-line tests to detect NAFLD patients without advanced fibrosis because the tests are easy to administer and inexpensive. Patients with low test scores (FIB-4 score <1.3) are less likely to have advanced fibrosis. However, NITs have an overall low positive predictive value (PPV). The inadequacy of recognizing patients with advanced fibrosis is an important limitation of these tests. It should be noted that if the NIT result suggests advanced fibrosis, it must be confirmed with another test. However, it should be mentioned that since NITs are developed in clinics with a high number of patients with advanced fibrosis, the accuracy of the tests may decrease in primary care, where the patient frequency is lower. In addition, the accuracy of these tests with current threshold values decreases in individuals over 65 who are morbidly obese.[130,131] In the FIB-4 and NAFLD fibrosis score (NFS) tests, the “indeterminate group” rate in which the presence of advanced fibrosis cannot be determined is approximately 30%. The advantage of ADAPT and Pro-C3 tests are that there are no “indeterminate results.” Ultimately, there is a need for new markers to detect the fibrosis stage in NAFLD. Liquid biopsy and cell-free DNA methylation studies show promise in identifying patients with severe fibrosis.[132]
FIB-4 score as the first primary risk assessment test is recommended to evaluate fibrosis in NAFLD.
A liver biopsy is recommended in patients with anticipated advanced fibrosis.
Imaging Methods: Abdominal US, computed tomography (CT), and MRI are imaging modalities that can be used to diagnose NAFLD. The abdominal US is often the preferred imaging method because it is easily accessible and does not contain ionizing radiation. In the US, NAFLD is recognized as a diffuse increase in hepatic parenchymal echogenicity compared to the kidney or spleen. NAFLD grading by the US is as follows:
Grade 1: Mild diffuse increase in echogenicity;
Grade 2: Decreased visibility of portal vein wall and diaphragm with a moderate increase in echogenicity; and
Grade 3: Inability to visualize the portal vein wall, diaphragm, and posterior part of the liver with advanced echogenicity
However, abdominal US has limitations, such as being an operator-dependent subjective assessment, difficulty using it in obese patients, and low sensitivity in histological adiposity below 30%. The sensitivity of the US was 55%–100%, and the specificity was found to be in an extensive range, such as 26%–100%.[133] In detecting 20%–30% or more of histological hepatosteatosis, the mean sensitivity of the US was 85%, and the mean specificity was 94%.[133]
The US elastography methods of point shear-wave elastography (pSWE) and two-dimensional (2D) SWE have recently been used to evaluate accompanying fibrosis in NAFLD. Studies have found that 2D SWE and pSWE perform very well in diagnosing F2 and F3 fibrosis in NAFLD patients.[134–136]
Fibroscan® (Vibration-controlled transient elastography [VCTE]) predicts the fibrosis stage by measuring liver stiffness in a 3 cm3 liver volume with an ultrasound-based technique. This method is suggested to reflect the liver parenchyma better since this volume is at least 100 times larger than the tissue obtained by liver biopsy. The measurement depth varies between 15 and 75 mm depending on the probe. Liver stiffness measurements (LSM) are expressed in kilopascals (kPa). Measurements can be started with the M probe (3.5 MHz) and changed to the XL probe (5 MHz) by taking into account the recommendations for the device. For those with a BMI above 32 kg/m2, the measurement starts with the XL probe, and the size is completed with the final probe, considering the automatic probe selection recommendation of the device. In children, the probe selection is made according to the chest diameter. It is recommended that patients fast for at least three hours before the procedure. It should be noted that the results of the Fibroscan® must be interpreted in light of the clinical and laboratory findings and that the method is operator-dependent. The threshold values of the Fibroscan® are considered when AUROC is about 0.90; values greater than 8.2 kPa indicate significant fibrosis (≥F2), those greater than 9.7 kPa suggest advanced fibrosis (≥F3), and those 13.6 kPa and higher indicate cirrhosis (F4). LSM values less than 6 kPa are considered normal.
The controlled attenuation parameter (CAP) is a bedside test simultaneously measuring adiposity with liver stiffness in the Fibroscan® device. While the threshold value used to detect grade 2 or higher steatosis was 310 dB/m in one study,[137,138] it was 257 dB/m in studies from Türkiye.[138] In addition, a recent meta-analysis showed that the Magnetic Resonance Imaging-Proton Density Fat Fraction (MRI-PDFF) technique is more effective for quantifying hepatic steatosis in patients with NAFLD compared to Fibroscan® CAP.[139]
Fatty liver is recognized as a diffuse decrease in liver parenchymal density in a non-contrast CT examination. The reduction in the Hounsfield unit (HU) value obtained in the density measurement is related to the amount of steatosis. In determining steatosis, the values measured below 40 HU in the measurement made from the liver parenchyma can be classified as 30% or above steatosis.[140] However, in the comparative density measurement that can be made with the spleen, it has been reported that a liver/spleen density ratio below 0.8, or a difference between the two organs of more than 9 HU, indicates more than 30% fat in the liver.[141] However, ionizing radiation limits the use of CT examinations in clinical practice.
MRI is a preferred imaging method in diagnosing and following NAFLD because it does not contain ionizing radiation and can show the amount of steatosis quantitatively. For this purpose, chemical shift-based methods, such as magnetic resonance spectroscopy (MRS) and Dixon, are used. MRS is an effective method for showing the amount of fatty liver. However, the critical limitations of MRS are that a limited volume, not the whole liver, can be evaluated; MRS examination cannot be performed in every MRI device; and special equipment is required to perform the evaluation.
The MRI-PDFF technique, which has recently become prominent among Dixon-based methods, effectively determines the amount of hepatic steatosis.[142,143] A meta-analysis revealed that the diagnostic accuracy in detecting the grades of steatosis in patients with NAFLD is very high and is highly compatible with the histological classification of fatty liver.[142] Therefore, MRI-PDFF has high sensitivity and specificity for grading fatty liver disease. According to the study of Tang et al.,[143] the MRI-PDFF threshold values for grading steatosis are as follows:
Grade 1: 6.4% (86% sensitivity and 83% specificity);
Grade 2: 17.4% (64% sensitivity and 96% specificity); and
Grade 3: 22.1% (71% sensitivity and 95% specificity).
In addition, in evaluating treatment responses in clinical studies, MRI-PDFF can reveal the change in fat content in the liver with high sensitivity. It has been reported that the MRI-PDFF technique shows liver fat changes with high sensitivity in adults and children.[144,145]
Liver fibrosis in patients with NAFLD can be evaluated using magnetic resonance elastography (MRE). In a meta-analysis involving NAFLD patients, threshold values of 2.88 kPa, 3.54 kPa, 3.77 kPa, and 4.09 kPa were determined for the F1, F2, F3, and F4 fibrosis diagnoses of MRE, respectively.[146] In this study, mean AUROC (95% CI) values were 0.86 (0.82–0.90), 0.87 (0.82–0.93), 0.90 (0.84–0.94), and 0.91 (0.76–0.95), respectively.[146]
•Abdominal US is the first-choice imaging method for diagnosing hepatic steatosis.
•MRI-PDFF can identify and quantify hepatic steatosis.
•Fibroscan® and MRE are reliable methods for the assessment of liver fibrosis. However, MRE more accurately assesses liver fibrosis than Fibroscan®.
Which Patients Should be Screened for NAFLD? How Should Primary Care Physicians Manage NAFLD?
NAFLD is mostly an asymptomatic disease. Since the prevalence of NAFLD is high, all population-based screening is not recommended. Obese patients with metabolic risk factors, such as IR, DM, MetS, or having fatty liver by the abdominal US, should be screened for NAFLD. The primary risk assessment determines NAFLD patients who are not likely to have advanced liver fibrosis. The FIB-4 score is often preferred for its easy use. The FIB-4 score can be easily calculated online at https://www.mdcalc.com/fibrosis-4-fib-4-index-liver-fibrosis. Patients with a FIB-4 score below 1.3 are defined as low-risk patients for advanced fibrosis. These patients can be followed up by their family physicians and should have the FIB-4 test repeated annually (Figure 2). When a patient is over 50 years old, the presence of thrombocytopenia (<150,000/ml) and an aspartate aminotransferase/alanine aminotransferase (AST/ALT) ratio greater than one predict fibrosis. Patients who have the following features are also at risk:
Patients with chronic liver disease findings on physical examination: and
Patients with a FIB-4 score greater than 1.3.
Figure 2.
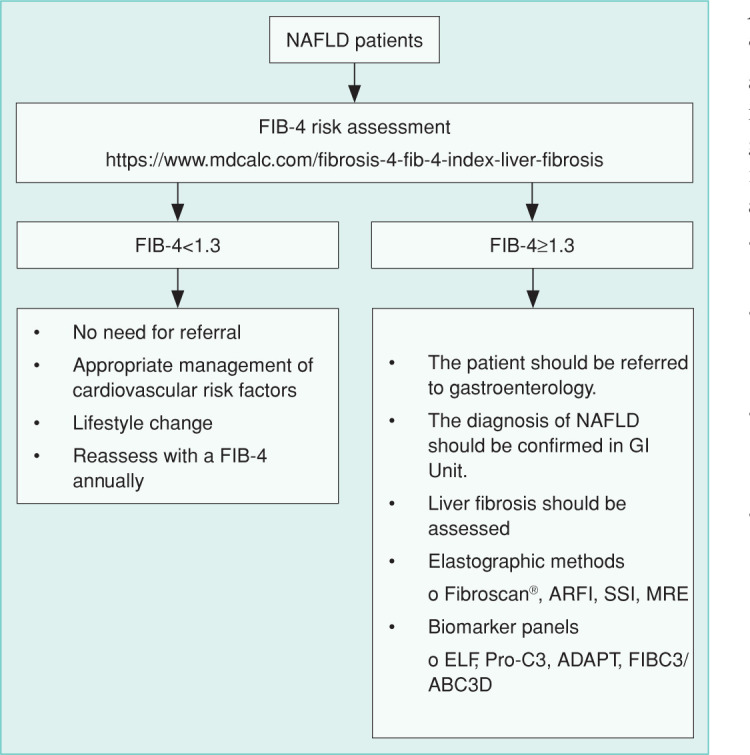
Scheme to Identify and Refer High-Risk Patients.
Patients with such characteristics should be referred to gastroenterology clinics for further evaluation and follow-up. High liver stiffness measures can predict the risk of advanced liver disease, hepatic decompensation, and mortality. Still, serum AST and ALT values within normal limits do not mean any liver damage. In addition, serum aminotransferase levels do not always correlate histologically with liver injury. Therefore, AST and ALT tests should not be used as prognostic markers.
Recommendations to family physicians, internists, and endocrinologists
Obese patients, patients with metabolic risk factors, or having fatty liver by the abdominal US should be screened for the presence of NAFLD. The FIB-4 score should be calculated in these patients. The FIB-4 risk assessment should be repeated every 1-2 years.
Patients with persistent high serum ALT-AST levels should be referred directly to the gastroenterology unit.
When Should a Liver Biopsy be Performed?
In clinical practice, liver biopsy is not a routine method for the diagnosis of NAFLD. The biopsy decision should be based on the patient’s clinical, laboratory, and radiological findings. Liver biopsy indications are as follows:
For the differential diagnosis of other possible etiologies;
For the diagnosis of concomitant liver diseases;
Detection of significant fibrosis (F≥2) by NIT in patients without clinical signs of advanced liver disease or when an indeterminate result is detected;
In case of incompatibility between two NITs; and
For approved studies.
A liver biopsy is not required if there are clinical, radiological, and laboratory findings of cirrhosis.
NAFLD Pathology
Macrosteatosis, the main morphological feature of NAFLD, is an adaptive response of hepatocytes.[147] Macrosteatosis may be accompanied by signs of hepatocellular injury, inflammation, and fibrosis (Table 8). Findings are predominantly parenchymal and characteristically start from acinar zone-3 (pericentral zone) and spread to other acinar zones.[148] However, in some NAFLD patients developing out of the MetS, especially in the pediatric age group, the morphology is predominant in the portal and periportal areas.[149] Therefore, when evaluating pediatric liver biopsies, it is essential to consider the unique (pediatric type) NAFLD morphology features in children to establish the diagnosis.
Table 8.
Histomorphological Features of NAFLD
| Morphology | Description | |
|---|---|---|
| Essential (guiding) morphological findings for diagnosis / classification | ||
| Steatosis | Macrosteatosis | • Should be present in ≥5% of hepatocytes for diagnosis. |
| • It is in the form of large lipid droplets (large vesicular) pushing the cell nucleus toward the cell membrane, and/or medium/small size (small vesicular) lipid droplets in which the nucleus is protected in the cell center. | ||
| Hepatocyte injury | Ballooning degeneration (BD) | • The cell is swollen and/or rounded. |
| • The nucleus is hyperchromatic and shrunken. | ||
| • The cytoplasm is loosely structured and pale, eosinophilic. | ||
| • Mild BD (non-classical BD), hepatocyte size was not changed. | ||
| • Pronounced BD (classical type BD), hepatocyte size increased at least two-fold. | ||
| Inflammation | Lobular Inflammation | • Focal necroses are usually mononuclear cell dominant or mixed type involving neutrophils. |
| • Microgranuloma and/or lipogranuloma may be seen. | ||
| Portal/Periportal Inflammation | •Limited to mild/moderate increase of mononuclear cells in the portal area and mild interface hepatitis activity. Portal/periportal area predominant inflammation should suggest non-NAFLD etiologies. | |
| • Usually in the foreground in the pediatric age group. | ||
| Fibrosis | Sinusoidal and pericellular fibrosis | •Starts around the central vein and has a reticulate appearance enveloping hepatocytes, along sinusoids. |
| • Usually starts in the portal/periportal area in the pediatric age group. | ||
| • Causes septa formation (central-central, central-portal, rarely porto-portal) during the fibrogenesis process and then incomplete and complete nodulation (advanced fibrosis). | ||
| Other morphological findings | ||
| Hepatocyte injury | Microsteatosis | • Due to loss of mitochondria function (fatty acid beta oxidation inhibition) |
| • Hepatocyte nucleus is in the cell center. It is observed mildly/focally. Its prevalence suggests different etiologies, the cytoplasm is pale and foamy. | ||
| • Observed mildly/focally. Its prevalence suggests different etiologies. | ||
| Mallory-Denk Bodies | • Develops due to cytoskeletal damage, is a cluster of keratins in the cytoplasm. | |
| Apoptosis | • Programmed cell death; hepatocyte nucleus is fragmented, cytoplasm is shrunken and dark eosinophilic. | |
| Megamitochondria | • Often accompanies microsteatosis. | |
| • Round or needle-shaped eosinophilic inclusions in hepatocyte cytoplasm. |
The main morphological finding leading to the diagnosis of NASH is the detection of ballooning degeneration reflecting cell damage. In the fatty liver inhibition of progression algorithm, lobular inflammation and ballooning are required to diagnose NASH.[150] NASH triggers fibrogenesis throughout the injury process and can result in advanced fibrosis (cirrhosis). As fibrogenesis becomes evident, the rate of fat in the liver decreases and may disappear entirely in the cirrhotic stage (“burned-out” NASH).[151]
Morphological Diagnostic Classification of NAFLD
The morphological findings observed reflect the ongoing liver damage in patients with NAFLD at the time of biopsy. Therefore, different morphological damage patterns can be seen in biopsy specimens in the gray zone in the morphological diagnosis classification.[151–153] NAFLD is classified according to the morphological findings in liver biopsies as follows:
Steatosis (NAFL): Pericentral (acinar zone-3) distribution of macrosteatosis (≥5%) is detected.
Steatosis accompanied by inflammation: Pericentral macrosteatosis is accompanied by lobular inflammation. Ballooning is not observed. Portal inflammation is usually absent or mild.
Steatohepatitis (Definite NASH): Macrosteatosis, inflammation, and ballooning degeneration are observed. Morphological findings show acinar zone-3 (pericentral) distribution.
Steatohepatitis (NASH), showing acinar zone-1 distribution: The characteristic morphology found in NASH cases seen in pediatric age groups. Findings are predominantly in the portal and periportal areas. Periportal macrosteatosis is accompanied by portal inflammation and portal fibrosis in some cases. Generally, ballooning degeneration is absent or mild and limited to the periportal extent. The presence of portal inflammation is essential in this pattern, which is also defined as “NASH Type 2” in the literature. It is accepted that portal inflammation and interface activity increase the risk of developing fibrosis in NAFLD cases.
-
Morphologies in the gray zone in morphological classification: These are controversial terminologies and require compliance with clinical findings for the diagnostic approach.
- Probable NASH (showing zone-3 distribution): In terms of pericentral macrosteatosis and NASH-associated fibrosis, typical pericentral (perivenular)/pericellular fibrosis is observed, but there is no ballooning. Lobular inflammation is present. Probable NASH patients are in the gray zone between steatosis accompanied by inflammation and NASH.
- Steatofibrosis: Defined for morphologies typical of pericentral fibrosis accompanied by macrosteatosis in the pericentral area. Ballooning and lobular inflammation are not observed.
Advanced-stage fibrosis associated with steatohepatitis (cirrhosis): Advanced-stage fibrosis (cirrhosis) with macrosteatosis and other diagnostic criteria is detected.
Cryptogenic cirrhosis: No macrosteatosis detected. It is observed in “burned-out” NASH patients where the steatosis completely disappears during the fibrogenesis process.[151–153]
In cases where ballooning degeneration is detected with a typical reticulated sinusoidal fibrosis pattern and other etiological factors are excluded, it can be interpreted that cryptogenic cirrhosis may have developed based on NAFLD. Still, it should not be given as a pathological diagnosis.
Grading, Staging, and Scoring
The grading evaluation is based on the extent of steatosis, ballooning, and inflammation. In contrast, staging assessment is made based on the presence of collagen and extracellular matrix deposition, distribution pattern, density, and liver roof disorder (septation, nodulation) caused by it. The NAFLD Activity Score (NAS) is applied for grading purposes (Table 9).[151,154] The system commonly used in staging is the “Nonalcoholic Steatohepatitis Clinical Research Network (NASH-CRN) Staging System” (Table 10).[154]
Table 9.
NAFLD Activity Score (NAS)
| Grade | Steatosis | Ballooning degeneration | Lobular inflammation (x200 magnification) |
|---|---|---|---|
| 0 | <5% | None | None |
| 1 | 5%–33% | Mild (few, pericentral) | <2 foci |
| 2 | 33%–66% | Prominent (many, scattered) | 2–4 foci |
| 3 | >66% | >4 foci |
NAS=Steatosis degree + Ballooning degree + Lobular inflammation degree. NAS Score has a value between 0 and 8.
Table 10.
Nonalcoholic Steatohepatitis Clinical Research Network Staging System (NASH-CRN)
| Stage | Description | |
|---|---|---|
| 1 | ||
| A | Pericentral sinusoidal fibrosis, mild (but detectable with collagen stains) | |
| B | Pericentral sinusoidal fibrosis, prominent (noticeable on Hematoxylin and Eosin stained sections) | |
| C | Portal fibrosis and/or periportal fibrosis | |
| 2 | Pericentral sinusoidal fibrosis (+) portal/periportal fibrosis | |
| 3 | Bridging fibrosis (central-central, central-portal, portal-portal) | |
| 4 | Cirrhosis (incomplete or complete) |
Different systems have been proposed for staging NAFLD patients due to the lack of clinical guidelines for the stage-1 subgroups in the NASH-CRN staging system, grouping the cases with fibrosis of different intensity in stage-3 and stage-4 within the same stage, and compatibility problems among pathologists.[150]
In NAFLD patients, the steatosis-activity-fibrosis score (SAF), where grading and staging can be given together, can be used.[150,155] Steatosis and fibrosis assessment in the SAF scoring system is similar to the NAS grading and NASH-CRN staging systems, respectively. The difference is in the way the activity score (ballooning + lobular inflammation) is determined (Table 11).[156,157]
Table 11.
Steatosis-Activity-Fibrosis (SAF) Score
| Grade | Steatosis | Ballooning degeneration | Lobular inflammation (LI) | Fibrosis |
|---|---|---|---|---|
| 0 | <5% | None | None | None |
| 1 | 5%–33% | Mild (non-classical) | 2 or fewer foci/lobules | Perisinusoidal or periportal |
| 2 | 34%–66% | Prominent (classic) | >2 foci/lobule | Perisinusoidal and portal/periportal |
| 3 | >66% | Bridging | ||
| 4 | Cirrhosis |
Mild BD (non-classical BD), hepatocyte size unchanged, but cell rounded; Prominent BD (classical BD), hepatocyte size increased at least twofold.
| SAF Score | Steatosis (S) | Activity (A) | Fibrosis (F) |
| S0,1,2,3 A0,1,2,3,4 F0,1,2,3,4 |
0–3 | BD (0–2)+ LI (0–2)=0–4 |
0-4 |
Pathology Report Content
Liver biopsies of NAFLD cases are evaluated with H&E, collagen (trichrome and/or reticulin), and iron stains. To detect ballooning degeneration, cytokeratin-18 immunohistochemistry staining can be applied (loss of immune expression in the ballooned cell). The adequacy of the biopsy should be stated in the report. Qualification criteria for biopsy length and the number of portal areas are greater than or equal to 1.5 cm and greater than or equal to ten portal areas, respectively. If the biopsy is subcapsular, the number of portal areas is low, and the sample is fragmented (in the form of small particles), it is defined as “biopsy inadequacy,” The content of the proposed report is summarized in Table 12.
Table 12.
NAFLD Pathology Report Example
| Biopsy length |
| •The number of portal areas: |
| •Biopsy adequacy (adequate/limited/inadequate) |
| Morphological findings |
| Morphological findings that must be included in the report: |
| •Macrosteatosis: No/Present |
| Ratio (%) |
| Zonal distribution pattern (zone-3 / zone-1 / azonal / panacinar) |
| •Ballooning degeneration: No/Present |
| Non-classical BD / classical BD |
| Few/many |
| Zonal distribution pattern (zone-3 / zone-1 / azonal) |
| •Lobular inflammation: No/Present |
| Number: Total number of apoptosis, focal necrosis, microgranuloma, and lipogranuloma lobule or x200 |
| Zonal distribution pattern |
| Microgranuloma and/or lipogranuloma: No / present |
| Apoptosis: No/yes |
| •Portal inflammation and interface hepatitis activity: No/Present* |
| Severity: Mild/moderate/pronounced |
| •Fibrosis: No/Present |
| Pericentral sinusoidal fibrosis: Mild/pronounced |
| Periportal sinusoidal fibrosis |
| Portal fibrosis |
| Septa formation: central-central/porto-central/porto-portal |
| Nodulation: Incomplete/complete |
| Suggested (optional) morphological findings to be included in the report |
| •Iron accumulation: None/Present |
| •Microsteatosis: No/Present |
| •Megamitochondria: Absent/Present |
| •Mallory-Denk bodies: None/Present |
| •Glycogenized nucleus: No/Present |
| Diagnosis |
| Fatty liver disease/NAFLD |
| Histological pattern |
| •Steatosis (NAFL) |
| •Steatosis with inflammation |
| •SH (NASH) |
| •SH (NASH), showing acinar zone-1 distribution |
| •Possible NASH (indicating Zone-3 distribution)** |
| •Steatofibrosis** |
| •Sthatohepatitis-related advanced fibrosis (Cirrhosis) |
| •Advanced fibrosis (Cirrhosis)*** |
| Grading and staging |
| Scoring systems that should be included in the report |
| •NAFLD Activity Score |
| •NAFL-CRN Staging System |
| •SAF Score |
| Concomitant pathology |
| •Dysplasia/early HCC: None/Present |
| •Chronic liver disease: None/Present |
Treatment responses should be specified in follow-up biopsies. *: Portal inflammation and interface hepatitis activity correlate with clinical course/fibrogenesis. Although not components of the NAS and/or SAF score, they must be specified in the report. **: Morphological patterns that require clinical correlation and can be interpreted with clinical data. ***: Although steatosis is not observed, if morphological findings suggest that it develops on the basis of NASH, the interpretation of “burned-out” NASH can be given in the notes section.
HCC screening
Patients with NASH have a high risk of developing liver cirrhosis and HCC.[158] Obesity increases the incidence of all malignancies, especially gastrointestinal cancers and HCC. In a population-based study conducted in the USA, the risk of death due to HCC was 4.5 times higher in men with a BMI above 35 kg/m2 compared to those with a BMI of 18.5–25 kg/m2.[159] In a meta-analysis of 11 studies, the risk of HCC increased by 17% in overweight and 89% in obese patients.[160] It has been reported that every 5 kg/m2 increase in BMI increases the risk of HCC by 25%, and obesity is associated with a 300% increased risk, especially in men.[160] The risk of developing HCC is higher in those with abdominal obesity than in generalized obesity.[161] The risk of malignancy is increased in T2DM patients independent of obesity. In a community-acquired longitudinal study, the risk of developing HCC was two times higher in patients with diabetes than those without diabetes (2.39 vs. 0.87 per 10,000 patient-years) in 10–15 years of follow-up.[162] HCC risk is more prominent, especially in elderly patients with DM (OR 2.87).[162] In a study conducted in England, it was found that the incidence of HCC increased 1.8 times between 2000 and 2010, while the incidence of HCC due to NAFLD increased ten times, and the underlying pathology was reported as NAFLD in 35% of those diagnosed with HCC in 2010.[163]
There are unanswered questions regarding NAFLD and HCC screening. First, should HCC screening be performed in cirrhosis developing based on NAFLD? HCC screening is recommended in risky patients with an annual cancer risk of 1.5% or more. Regardless of the etiology, the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) recommend HCC screening for cirrhosis with abdominal US for six months or once a year. The Asian Pacific Association for the Study of the Liver (APASL) recommends HCC screening with US and alpha-fetoprotein (AFP).[164] The incidence of HCC development in the background of NASH has been reported as 6.7% at five years and 15% at ten years.[165] In cohort studies, the cumulative incidence of HCC in cirrhosis due to NASH was reported to be 2.4%–12.8% at a mean follow-up of 3–7 years.[166]
Second, should patients with advanced fibrosis or cirrhosis detected by NITs be screened for HCC? In patients of NASH without a clinical or radiological diagnosis of cirrhosis, a high NFS (adjusted hazard ratio [HR] 5.64; 95% CI 1.49–21.44; p<0.01) or a high FIB-4 score (adjusted HR 13.99; 95% CI 3.00–65.23; p<0.001) was associated with the development of HCC.[167] The risk of HCC increases approximately seven times in patients with advanced fibrosis detected with Fibroscan®.[168] Therefore, the American Gastroenterological Association recommends that these patients be screened for HCC if there are signs of advanced fibrosis in at least two NITs (FIB-4, NFS, Fibroscan®, US, or MRE).[169]
Third, if scanning is to be done, what should be the scanning method? While abdominal US scanning is recommended for HCC screening in cirrhosis, most NAFLD patients are likely obese, making detecting early HCC with the US difficult. A retrospective review of 1,500 HCC patients found that fewer screenings were performed in HCC cases developing based on NAFLD compared to HCC cases developing due to alcohol and HCV.[170] In the screening examinations of patients diagnosed with HCC, up to three years before the diagnosis, it was observed that 57% of NAFLD-related cirrhosis, 40% of alcohol-related cirrhosis, and 13% of HCV-related cirrhosis patients were not screened for HCC.[170]
MRI and CT are more reliable tests in the early diagnosis of HCC. However, CT is unsuitable for HCC screening due to radiation exposure. Due to the low cost of MRIs in Türkiye compared to other countries, MRIs can be used in HCC screening in limited patients.
Fourth, is screening necessary in NAFLD patients without advanced fibrosis? HCC can develop without cirrhosis in patients with NAFLD. Since the frequency of HCC in non-cirrhotic patients is not known clearly, screening was not recommended in these patients. In many studies conducted in recent years, HCC cases developing without cirrhosis have been reported.[171–174] In a community-based survey examining SEER-Medicare data, MetS was higher among 3,649 HCC patients than the control group (37.1% vs. 17.1%; p<0.0001). In logistic regression analysis, it was shown that MetS significantly increased the risk of developing HCC (OR 2.13; 95% CI 1.96–2.31; p<0.001).[173] According to other etiologies, the prevalence of HCC development in patients without cirrhosis was higher in NASH cases (38.0% vs. 14.2%).[173] Another study reported that the risk of developing HCC without cirrhosis in NASH cases increased three times compared to other chronic hepatitis.[174] In NAFLD cases with obesity, T2DM, and MetS, genetic, epigenetic, systemic, and liver-specific lipid metabolism disorders, IR increased the risk of HCC by making changes in many different pathways of carcinogenesis pathway. Previous studies showed that PNPLA3 rs738409 C > G polymorphism positivity increased the risk of advanced fibrosis and HCC development.[175] However, routine screening of NAFLD patients with these tests is not practical. To recommend screening for NAFLD patients without advanced fibrosis, it is necessary to know the factors that predict the risk of developing HCC.
HCC screening is recommended for NAFLD-related cirrhosis.
If at least two of the NITs (FIB-4, Fibroscan, or MR elastography) or liver biopsy have signs of advanced fibrosis (≥F3), HCC screening may be recommended for these patients.
HCC screening may be recommended in NAFLD patients without advanced fibrosis who have T2DM, MetS, and those with a family history of HCC.
HCC screening should be performed with the abdominal US and AFP at six-month intervals in NAFLD-related cirrhosis cases.
Treatment
Lifestyle Intervention
A healthy diet and regular exercise are the cornerstone of the management of NAFLD.
Diet: Ideal treatment for NAFLD should reduce liver fat and damage and improve the metabolic and cardiovascular risks associated with NAFLD. Therefore, lifestyle changes in a moderate-intensity exercise program and a hypocaloric diet (reduction of 500–1,000 kcal per day) are currently the basis of NAFLD treatment.[4,176] In addition, gradual weight loss of 500–1,500 grams per week should be sought with a hypocaloric diet. Achieving weight loss also facilitates treating accompanying MetS, T2DM, hypertension, and CVD.
In a biopsy-controlled, 52-week diet and lifestyle adjustment study involving 293 overweight and obese patients, a correlation was shown between the degree of weight loss and the improvement in histological parameters of NASH.[177] Thirty percent of the patients lost 5% or more of their current weight, of which 58% had improvement in steatosis and inflammation (a two-point reduction in NAS score). In those with at least 10% or more weight loss, 90% of patients with NASH showed improvement, while 45% had a regression in fibrosis.[177] In another study investigating the role of a hypocaloric diet in NAFLD patients, a similar degree of weight loss was observed in both normal-weight and obese patients (5.4% vs. 5.7%) with an eight-week diet, and adiposity improved in 57% of normal-weight patients.[102] In this study, 5% weight loss effectively improved steatosis in normal-weight and obese patients.[102] In conclusion, at least 5% weight loss should be aimed at improving the fatty liver, and at least 10% weight loss should be targeted for a significant improvement in accompanying fibrosis.
There is no strong evidence to support a single type of diet specifically for patients with NAFLD. However, diets low in refined carbohydrates, low-carbohydrate ketogenic diets, and low-fat, Mediterranean-type diets should be encouraged. Vegetables, fruits, nuts, legumes, and grains provide large amounts of fiber for the Mediterranean diet. However, it is known that the Mediterranean diet, which is rich in polyunsaturated (PUFA) and monounsaturated (MUFA) fatty acids, also reduces cardiovascular risk. In a study conducted on diabetic NAFLD patients, adherence to the Mediterranean diet was shown to be a protective factor from the development of fibrosis.[178] The intermittent fasting diet (IF) is a new weight-loss diet that has become popular recently. The alternative fasting diet (AAD) is an innovative form of IF that alternates between a food-free eating day and a 75% energy-restricted fasting day. Improvement was found in BMI and blood lipid profiles with AAD.[179] There has yet to be a consensus on the safety, mode of administration, duration, and effects of these special diets. Therefore, they should not be recommended for treating NAFLD until new robust studies are concluded.
Diets high in protein are still popular and preferred diets in society as they provide weight loss in a short time. Protein ratios in these diets are 35%–40%, and animal- and vegetable-origin proteins are preferred. Although success is achieved in the short term, the success of such diets is low in the long term. Many side effects of these diets have been described.
NAFLD patients should avoid high-energy, processed meat, fructose-containing, packaged foods, and sweetened beverages. High fructose consumption is associated with changes in the gut microbiota, increased intestinal permeability, endotoxemia, lipid peroxidation, copper deficiency, tumor necrosis factor (TNF) production in the liver, and increased advanced glycolysis end products.[42] All these effects contribute to NAFLD development and its progression.[42] For this reason, patients with NAFLD should avoid consuming large amounts of high-fructose-containing foods and beverages.
Regularly consuming two cups or more per day of filter coffee is associated with lower liver fibrosis in patients with NAFLD.[180] The amount of coffee in one cup is around 5 gr for dry ground coffee and 20–30 gr for filtered coffee. On the other hand, it is important to limit alcohol intake in patients who use alcohol. Conclusions on the effects of limited alcohol use by NAFLD patients are controversial. In a study investigating what amount of alcohol use is safe, it was suggested that no alcohol is safest for health.[181]
Nutrition is essential in managing the underlying disease and its complications in NAFLD-related cirrhosis. Nutrition benefits the clinical course of patients with cirrhosis, including preventing infections and reducing mortality. Patients with cirrhosis have difficulty meeting their caloric and protein requirements due to increased nutritional needs, decreased oral intake, malabsorption, and changes in protein and glucose metabolism. Malnutrition, especially protein-energy malnutrition (PEM), is prevalent in patients with cirrhosis. Malnutrition is detected in 65%–90% of patients with decompensated cirrhosis.[182,183] Nutritional requirements in patients with cirrhosis should be calculated as follows:
Energy requirement: 1.2–1.4 × REE (Resting energy expenditure)
Protein requirement: 1.0–1.5 g/kg
Another critical issue for patients with cirrhosis is the ideal composition of nutritional content and regulation of meal and snack intake frequency. Late-evening snacking has improved sarcopenia and quality of life.[184] Given the changes in the liver’s glycogen storage capacity, the intake of carbohydrates along with protein is essential for muscle preservation and regeneration. Patients can benefit from a higher rate of plant-based and dairy-based protein. Increased intake of branched-chain amino acids may increase appetite and muscle synthesis and may be effective in improving quality of life.[185] PEM and some micronutrient deficiencies often develop in patients with cirrhosis due to digestive disorders, nutrient absorption problems, and poor oral intake. In cirrhotic patients, checking the serum levels of fat-soluble vitamins, B vitamins, zinc, copper, and magnesium is essential. Early nutrition education and interventions are imperative to prevent the development of malnutrition, sarcopenia, and friability in cirrhotic patients. Therefore, it is recommended to identify high-risk patients and routinely use validated “malnutrition screening tools” in these patients to apply nutrition and exercise interventions in the early period.[183,186]
Physical Activity
The physical act, activity guidelines for the general adult population are as follows:
Five days a week, 30 minutes/day, moderate-intensity exercise, or
Three days a week, ≥20 minutes/day, heavy-intensity exercise.[187]
Both aerobic and resistance exercises can reduce hepatic steatosis, NASH, and fibrosis. To ensure long-term compliance, the type of exercise should be tailored to the patient’s preferences and physical capacity. A meta-analysis showed that regular physical activity could improve liver tests, serum lipid, and intrahepatic fat in nondiabetic NAFLD patients, but the magnitude of the effect is generally small.[177] Maintaining regular physical activity for more than four months, regardless of the type of physical activity, significantly improved liver tests.[188] Another meta-analysis showed that exercise could reduce adiposity independent of diet.[189] Moderately intense and vigorous exercise had similar efficacy in reducing intrahepatic fat, which is associated with weight loss.[190]
Lifestyle modification consisting of a hypocaloric diet with limited carbohydrates and saturated fat, moderate-intensity exercise, and weight loss has been recommended for patients with NAFLD.
The Mediterranean diet is recommended as the ideal diet.
Current Medical Treatments
Since NAFLD is the hepatic reflection of a systemic disorder, it is clear that correcting the metabolic disease rather than utilizing liver-specific therapy is essential. However, since a liver-related condition is mentioned, it should also be shown that the liver improves with the treatment or at least prevents its deterioration. Currently, no drug therapy is approved for use in the treatment of NAFLD.
NAFLD without NASH is generally benign, and the risk of developing liver complications is low. For this, the patient must have NASH if drug treatment is to be considered. International guidelines state that significant fibrosis (≥F2 fibrosis) accompanying NASH should also be present for treatment indication.[2,3] The most critical problem in the treatment of NAFLD is the lack of a marker to follow the treatment response. Transaminase values are expected in 80% of patients with advanced liver disease. Although the level of fat in the liver can be followed by methods such as Fibroscan® and MRI, there is no ideal diagnostic method other than biopsy to follow inflammation and ballooning.
Today, lifestyle changes, diet, and exercise are most likely to improve accompanying morbidities. Numerous drugs have been and are being tried in the treatment of NAFLD. In international guidelines, only thiazolidinediones and vitamin E are recommended as drugs. Here, drugs that are in use with different indications and that have been tried in the treatment of NAFLD (rather than newly developed drugs) will be briefly mentioned.
1. Metabolic Regulator Drugs
Metformin has many effects, such as increasing catabolism, disrupting fatty acid synthesis, reducing glucose absorption, and increasing glucose metabolism. It has been shown to have many beneficial effects, such as anti-tumor effects, cardiovascular benefits, dementia inhibitory effects, and prolonged life expectancy.[191] Metformin has been tried in the treatment of NAFLD, but conflicting results have been obtained in studies conducted to date. In the first randomized controlled trial, metformin successfully normalized serum ALT and improved metabolic parameters.[192] In patients with histological control, metformin decreased the amount of fat, necroinflammatory activity, and fibrosis in the liver.[192] However, in a recent meta-analysis of nine randomized controlled trials, metformin produced a borderline reduction in serum AST, ALT, reduced HOMA score, and BMI. Still, there was no improvement in histological findings.[193] Another meta-analysis found that metformin did not change histological and biochemical parameters in patients with NAFLD.[194] Therefore, metformin is not recommended for the treatment of NAFLD. However, it is stated that metformin reduces the risk of HCC in diabetic patients.[158,192,194]
Thiazolidinediones are peroxisome proliferator-activated receptor gamma (PPAR-γ) agonists with insulin-sensitizing effects. They regulate many genes by activating PPAR-γ. In this way, lipolysis in adipose tissue decreases, fatty acid storage increases, IR caused by lipotoxicity improves, adiponectin secretion increases, fibroblast growth factor-21 is activated, inflammation is suppressed, hepatic glucose uptake increases, and gluconeogenesis is suppressed. Increased insulin sensitivity in muscles and adipose tissue increases glucose uptake. Thiazolidinediones stimulate the differentiation of preadipocytes into small adipocytes and cause an increase in subcutaneous adipose tissue, in particular, and can reduce visceral adipose tissue.[195–197] Pioglitazone has a partial PPAR-α agonist effect, thereby decreasing triglyceride production and increasing HDL cholesterol. In addition, Pioglitazone significantly reduces plasma-free fatty acid levels. There are many studies with pioglitazone in the treatment of NAFLD. A meta-analysis of five randomized controlled trials calculated ORs for pioglitazone to reduce fibrosis by one point or more and improve steatohepatitis were 10.2, 1.8, and 3.7, respectively.[198] Its beneficial effect is proven in diabetic and nondiabetic NAFLDs.[199–201] Pioglitazone causes weight gain, partly because it increases appetite but mainly because it increases the amount of subcutaneous adipose tissue. The weight gain effect is dose-dependent. In addition, pioglitazone can increase bone resorption by promoting the transformation of osteoblasts into adipocytes and activating osteoclasts. It can increase the risk of bone fractures, especially in postmenopausal women.[202–204] Pioglitazone may lead to edema and heart failure by increasing Na+ uptake from the kidneys.[202–204]
Glucagon-like peptide-1 receptor agonist (GLP-1RA), such as liraglutide, with a glucose-dependent mechanism, increases insulin secretion, decreases glucagon secretion, and corrects hyperglycemia. It also reduces appetite and energy intake, delaying gastric emptying and causing weight loss. In addition, weight loss occurs due to the suppression of the appetite center in the brain and the suppression of ghrelin secretion. Liraglutide reduced the risk of CVD in T2DM patients. Liraglutide resulted in weight reduction, serum ALT and gamma-glutamyl transferase improvement, and reduction in liver fat compared with placebo or other antidiabetics.[205,206] In the biopsy and randomized controlled trial of the liraglutide study (LEAN study), 39% of patients in the liraglutide arm and 9% of patients in the placebo arm experienced improvement in steatohepatitis. Gastrointestinal side effects were observed in 80% of the patients included in the study. The positive impact of liraglutide in NAFLD may be the natural consequence of weight reduction. Currently, liraglutide is not recommended for the treatment of NAFLD.
Promising results have been obtained in the treatment of NASH with semaglutide, another GLP-1RA analog. In a double-blind, placebo-controlled 72-week study, treatment with daily 0.4 mg subcutaneous semaglutide achieved 59% resolution of NASH without worsening fibrosis, compared to only 17% in the placebo arm (p<0.001). No significant improvement was observed in fibrosis compared to the placebo (43% vs. 33%, p=0.48). A 13% weight loss was observed in the semaglutide group, compared to an average of only 1% for the placebo. Gastrointestinal side effects (nausea, vomiting, constipation) were observed more frequently in semaglutide treatment.[207] A phase-3 study with semaglutide is ongoing.
2. Antioxidant Drugs
During the presence of IR, when the excessive fatty acid load in the liver increases the beta-oxidation load in mitochondria and peroxisomes, electron leakage increases, and reactive oxygen species (ROS) can grow at a pathological level and disrupt the structure of many macromolecules. Impairment in mitochondrial functions also plays a role in the increase in ROS. Increased oxidative stress in NAFLD affects the development of steatohepatitis. Therefore, it is recommended to use antioxidant drugs in the treatment of NASH.
Vitamin E studies offer conflicting results due to the use of different forms and doses of the vitamin and the lack of standard criteria and designs. In the PIVENS study of nondiabetic patients, vitamin E improved steatohepatitis significantly more than the placebo but did not improve fibrosis.[158] In a study of diabetic patients, the improvement of steatohepatitis without worsening fibrosis was found to be higher in the vitamin E group compared to the placebo group (33% vs. 12%, p=0.04), but no significant difference was found when the steatohepatitis components were examined individually and in fibrosis.[208] The vitamin E group alone did not differ from the placebo group in reaching the study’s primary endpoint. Generally, when the vitamin E studies are combined, more homogeneous findings have been revealed that it can improve transaminases in NASH, but different results have been obtained for fibrosis.[208–210] There are doubts about the safety of vitamin E in long-term use; specifically, studies report that it increases the risk of hemorrhagic stroke,[211,212] increases mortality,[213] and increases the risk of prostate cancer.[214] In our personal experience, vitamin E should not be used as a long-term therapy in the treatment of NAFLD.
3. Lipid-Lowering Agents
Statins block mevalonate synthesis in the cholesterol synthesis pathway by inhibiting the enzyme 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. As a result, decreased cholesterol levels in the liver increase the expression of LDL receptors on the hepatocyte surface, and very low-density lipoprotein (VLDL), LDL, and intermediate-density lipoprotein levels in the blood decrease. In addition, it has effects such as suppression of inflammation associated with suppression of liver X receptor (LXR) activity,[215] inhibition of SREBC-1c,[216] prevention of inflammation caused by oxidation of LDL cholesterol,[217] suppression of prenylation proceeding through the mevalonate pathway, improvement in endothelial function, increase in nitric oxide level, and suppression of the RAAS. Statins are used in patients with high cholesterol, which is present in most NAFLD patients. Studies have shown that statins reduce the level of transaminases in NAFLD.[218–220] However, it does not improve histology in patients with NASH.[221] Therefore, it is not recommended in NAFLD unless metabolic disorders require statins.
CVD risk is very high in patients with NAFLD, and CVD is the major cause of death. It is generally accepted that statins reduce the risk of CVD and death, especially among individuals with diabetes. For this purpose, statins are frequently used in patients with NAFLD. Statins may have elevated transaminases in a more than 15% of the patients. However, the risk of increasing serum ALT levels more than three times is less than 1%. Therefore, discontinuing the drug is not required unless the ALT level exceeds three times the upper limit of normal. Routine periodic transaminase monitoring is not necessary during statin treatment.[222] The use of statins is contraindicated in decompensated cirrhosis and acute liver failure. It has also been reported that there may be a risk of developing myopathy and the onset of T2DM during statin treatment.[223]
Fibrates, as PPAR-α agonists, decrease fatty acid synthesis and thus triglyceride and VLDL synthesis, and increase triglyceride catabolism by activating lipoprotein lipase. Fenofibrate, the most widely used option, improves IR, uric acid levels, and hypertension in patients with MetS.[224] In a study, using 200 mg/day of fenofibrate for 48 weeks improved liver enzymes and IR. Histological controls showed improvement in ballooning but no change in fat ratio, lobular inflammation, or fibrosis.[225] Transaminase elevations are seen in approximately 20% of fenofibrate users.[226] There is insufficient evidence for using fenofibrate in treating patients with NAFLD other than its indication.
4. Antihypertensives
RAAS is responsible for many effects that increase inflammation, oxidation, and thrombosis, as well as the classically known effects of renal sodium retention, abnormal contraction of vascular smooth muscles, and increasing aldosterone secretion.[227] In addition to blocking the negative effects of RAAS on metabolism and inflammation, angiotensin II receptor blockers can also provide metabolic improvement by increasing the conversion of increased angiotensin 2 to angiotensin 1–7 via the angiotensin-converting enzyme (ACE) 2.
Telmisartan is more likely to lower IR, improve body fat composition, reduce liver fat, and improve histology than other angiotensin 2 receptor blockers in patients with NAFLD.[228] This effect is explained by the partial PPAR-γ agonistic effect.[228] Previous studies showed that telmisartan improved IR, reduced liver fat, and improved liver histology in NAFLD patients with hypertension.[228–230] However, it is not recommended for use in the treatment of NAFLD since no robust randomized controlled trials have been conducted. However, it may be preferred as an antihypertensive in patients with hypertensive MetS and NAFLD patients with type T2DM.
5. Drugs with Multiple Effects
Ursodeoxycholic acid (UDCA), with the effect of the pregnane X receptor agonist, increases the expression of bile acid transport proteins, facilitates the excretion of bile acids from hepatocytes to the bile and plasma, and increases excretion from the urine. By increasing bicarbonate secretion from cholangiocytes, UDCA strengthens the bicarbonate’s protective effect and reduces the damage of bile acids to cholangiocytes. It changes the intracellular localization of bile acid transport proteins and makes them functional. UDCA reduces the membrane-dissolving effect of hydrophobic bile acids and blocks the apoptotic effects of primary bile acids. In three randomized placebo-controlled studies in the treatment of NAFLD, UDCA was not found to be different from the placebo in improving serum transaminase levels and histology.[231–233] Currently, UDCA is not recommended in the treatment of NAFLD.
Omega-3 fatty acids have been investigated in numerous studies as a treatment for NAFLD.[234,235] They have been found to provide a borderline decrease in serum ALT levels and can decrease the amount of liver fat. However, no studies show improvement in histopathological findings. Therefore, omega-3 fatty acids are not recommended therapeutically in the treatment of NAFLD.
Silymarin is a combination of flavonolignans (silybin A, silybin B, isosilybin A, isosilybin B, silychristin, isosilychristin, silydianin) and flavonoids (taxifolin) extracted from the seeds and fruit of the milk thistle plant. It is reported to have antioxidant, antifibrotic, and anti-inflammatory effects. In a meta-analysis combining randomized controlled trials in NAFLD patients, silymarin was shown to reduce serum AST and ALT levels.[236] Histologically, there was no difference between the placebo and silymarin regarding steatosis, lobular inflammation, and ballooning.[236]
Ongoing AND Recently Terminated Clinical Trials
Numerous phase studies are ongoing in the treatment of NAFLD. We see that each of the steps in the pathogenesis of NAFLD has become a target, and many agents affecting these steps are in the trial phase. When broadly classified, agents in phase studies can be grouped into metabolic modulators, anti-inflammatories, and antifibrotics. Some agents may also influence more than one pathogenetic mechanism. These agents can be evaluated as targeting IR or lipid metabolism, lipotoxicity and oxidative stress, inflammation, immune activation, apoptosis and necrosis, fibrogenesis, and collagen turnover. Here, we discuss agents with phase-3 studies (Table 13).
Table 13.
Drugs with Phase 3 Studies in the Treatment of NASH
| Active ingredient | Abbreviation | Name of the study | The company name | Mechanism of action |
|---|---|---|---|---|
| Obeticholic acid | OCA (Ocaliva) | REGENERATE | INTERCEPT | FXR agonist |
| Elafibranor | GFT-505 | GOLDEN-505 | GENFIT | PPAR-α/δ agonist |
| RESOLVE-IT | ||||
| Cenicriviroc | CVC | AURORA | ALLERGAN | CCR2/CCR5 inhibitor |
| Selonsertib | SEL | STELLAR 3 | GILEAD | ASK1 inhibitor |
| GS-4997 | STELLAR 4 | |||
| Aramchol | ARMOR | GALMED | SCD1 inhibitor | |
| Semaglutide | ESSENCE | NOVO NORDISK | GLP-1 receptor agonist | |
| Resmetirom | MGL-3196 | MAESTRO-NASH | MADRIGAL | THR-β agonist |
| Belapectin | GR-MD-02 | NAVIGATE | GALECTIN THERA PEUTICS | Galectin-3 inhibitor |
FXR: Farnesoid X receptor; PPAR: Peroxisome proliferator-activated receptor; CCR2, CCR5: C chemokine receptor type 2 and 5; ASK1: Apoptosis signal-regulating kinase 1; SCD1: Stearoyl -coenzyme A desaturase -1; GLP-1: Glucagon-like peptide-1; THR-β: Thyroid-hormone receptor-beta.
Obeticholic acid (OCA) is a farnesoid X receptor (FXR) agonist. FXRs participate in many metabolic events. They lower portal pressure in the liver, reduce inflammation and fibrosis, reduce cholesterol conversion to bile acids via CYP7A1, and decrease triglyceride levels in the liver. In a placebo-controlled study, FLINT, a phase-2 study, was conducted in non-cirrhotic NASH patients. Primary endpoints related to NAS score and secondary endpoints related to fibrosis were reached in the 72nd week of the study at rates of 45% and 35%, respectively. However, 23% of patients experienced significant itching in the OCA arm, and one patient required discontinuation of therapy. In addition, it was observed that total cholesterol and LDL cholesterol values increased and regressed when treatment was stopped.[237] REGENERATE, a phase-3 study, was conducted in NASH patients with fibrosis of stages 2–3. While improvement in fibrosis (>1 stage regression) was significant at the 25 mg dose, there was no difference in NASH resolution compared to the placebo. However, side effects were three times more common with OCA than with the placebo group. The most common side effects were itching, gallstones and their complications, and increased LDL levels.[238]
Elafibranor is a PPAR α/δ dual agonist.[239] Elafibranor did not reach established endpoints for NASH recovery and fibrosis.
Cenicriviroc is a C chemokine receptor type 2 and 5 (CCR2, CCR5) inhibitor.[240]
Selonsertib ASK1 is an “apoptosis signal-regulating kinase” activated by oxidative stress. In the selonsertib Phase-2 study, simtuzumab and selonsertib were compared. The positive effect of selonsertib on fibrosis and MRE and MRI-PDFF results were found to be higher than simtuzumab but were not statistically significant.[241] In the phase-3 studies STELLAR-3 and STELLAR-4, the goal of a one-point or greater improvement in fibrosis without worsening in NASH were not achieved.[242,243]
Aramchol is a fatty acid-bile acid conjugate and is a “stearoyl-coenzyme A desaturase-1 (SCD1)” inhibitor. SDC1 plays a significant role in energy metabolism, directing lipid substrates for utilization or storage. With SCD1 inhibition, fatty acids enter oxidation pathways instead of being stored. Aramchol has positive effects on apoptosis, inflammation, and fibrogenesis, as well as steatosis.
Resmetirom is a selective “thyroid-hormone receptor-beta (THR-β)” agonist. It acts on THR-β receptors in the liver. THR-β is the predominant T4 receptor in the liver. When this receptor is stimulated, there is an increase in cholesterol metabolism and biliary excretion of cholesterol. Resmetirom reduces the level of serum and liver triglycerides levels as well as cholesterol levels. In the phase-2 study of resmetirom, it was found to be superior to the placebo in reducing liver fat at 12 and 36 weeks (respectively, resmetirom 32.9% vs. placebo 10.4%, p<0.001; resmetirom 37.3% vs. placebo 8.5%, p<0.001) in patients with NASH.[244] Moreover, decreases of two points or greater in NAS score and improvement in NASH at 36 weeks from the baseline were more significant in the resmetirom arm than in the placebo arm.[244] It has been reported that the effect of resmetirom on fibrosis regression was insignificant, but fibrosis disappeared completely in half of the patients with NASH resolution. Resmetirom also corrected serum transaminase levels.[244] No significant side effects were detected in the resmetirom group except mild diarrhea and nausea.
Phase studies of many drugs to treat NASH have been completed or are ongoing. Studies examining the efficacy of sequential or combined treatment modalities of new drugs will be needed since phase-2 and -3 studies of many molecules cannot provide sufficient efficacy alone.
Endoscopic Management
Sustainable weight loss after lifestyle changes, diet, exercise, and pharmacotherapy can be achieved in less than 5% of patients. Although more effective and more permanent weight loss can be achieved with bariatric surgery. Early and late complications of bariatric surgery affect up to 30% of patients.[245,246] Endoscopic bariatric and metabolic treatments (EBMT) can be applied to patients with a BMI between 30 and 40 kg/m2, who cannot achieve adequate weight loss with diet and exercise, and who do not accept bariatric surgery. Potentially applicable methods are summarized in Figure 3.[247] The advantages of endoscopic treatments are loss of body weight by over 10%, low cost, fewer complications compared to surgery, reversibility, and anatomical preservation. However, the disadvantages of endoscopic treatment are that the weight loss is less permanent, especially in those treated with a removable device; frequent weight regains; the need for behavioral treatments to maintain the patient’s weight; and the need for continuation of therapy with pharmacotherapy in some cases.
Figure 3.
Current Endoscopic Bariatric and Metabolic Treatments.
The intragastric balloon is the most widely used safe method approved by the FDA. Different intragastric balloons are available (Reshape duo, Orbera, Obalon, Spatz 3, Elipse Balloon). Balloon therapy is contraindicated in patients with hiatal hernia, gastroesophageal reflux, motility disorders, patients taking anticoagulant therapy, and those with previous upper gastrointestinal system surgery.[247]
Orbera, one of the intragastric balloons, is controlled endoscopically after insertion and inflated with 450–700 ml/saline and methylene blue. It is removed endoscopically after six months. Nausea and abdominal pain are the most common side effects (29–34%). Most patients adapt to these side effects after the first week. Migration (1%–4%) and perforation (0.1%) are less frequently seen.
Endoscopy is not required for the Obalon placement. The balloon is provided in the form of a gelatin capsule. After the balloon is swallowed, its location is checked with fluoroscopy and inflated with nitrogen gas. Up to three balloons can be used in the same session. Until the weight loss plateaus, a new one can be added. The balloon can be removed endoscopically after 12–36 weeks.
The Spatz balloon is placed endoscopically. After the balloon is set, the volume of the balloon can be adjusted endoscopically by fluid injection or aspiration. Second-generation balloons have been developed because of the 20% risk of ulcer development in the first-generation balloons and the catheter not getting through.
Botox treatment is very controversial in patients with NAFLD. The effectiveness of treatment lasts for six months. Complications such as severe bloating, gas, fibrosis in the stomach wall, perforation, and bacterial overgrowth may develop.
All patients must be consulted by the gastroenterology, endocrine, and psychiatry departments before EBMTs. All endoscopic procedures should be follow-ups, and possible complication management should be performed in experienced centers.
Endoscopic bariatric and metabolic treatments are an effective and safe option for morbidly obese patients for whom a surgical approach is not planned.
Bariatric Surgery
According to the results of the Türkiye Nutrition and Health Survey conducted by the Ministry of Health, the prevalence of obesity in Türkiye was 30%.[248] Obesity is the most significant factor in developing NAFLD. The fight against obesity aims to reduce the weight of the patients, as well as to place comorbid diseases in remission and improve the general health picture. Although there are diet, exercise, behavioral treatments, and medical treatments in the fight against obesity, bariatric surgery has been accepted as the most effective treatment method that provides long-term sustainable weight loss.[248–250] It is known that bariatric surgery has beneficial metabolic effects independent of weight loss, includes remission in diabetes, and reduces CVD risk factors in obese and T2DM patients with a BMI of 35 kg/m2 or greater who do not respond adequately to medical treatment.[248–251]
At the Diabetes Surgery Summit held in Rome in 2007, it was suggested to use the term “Metabolic Surgery” instead of the words “Obesity Surgery” or “Bariatric Surgery” to emphasize that these surgeries provide weight loss as well as remission in severe comorbid diseases.[252] Indications for metabolic surgery are given in Table 14.
Table 14.
Indications for Metabolic Surgery According to the International Federation for the Surgery of Obesity and Metabolic Disorders
| 45 kg more than the ideal body weight calculated according to the patient’s gender and height BMI >40 kg/m2 without comorbid illness or In case of BMI >35 kg/m2 and at least one of the obesity-related comorbidities, including NAFLD BMI values can be stretched to the range of 30–35 kg/m2 considering comorbid diseases* Age range of 18–65 years Appropriate psychiatric status No drug addiction The patient has the capacity to understand the risks associated with the surgery and what to do after the surgery |
: In conditions involving multiple comorbidities such as metabolic syndrome; BMI: Body mass index.
Before metabolic surgery, it should be determined whether the patient has had at least two professionally assisted weight-loss attempts. Metabolic surgery should not be used as a first-line treatment in individuals who have never attempted to lose weight. The most important reason for this is the prediction that the patient may be incompatible with the diet and lifestyle changes that will be given after the surgery. Therefore,
Metabolic surgery procedures should be performed in approved obesity centers;
Before metabolic surgery, a multidisciplinary evaluation of the patient should be conducted by specialists in internal medicine, gastroenterology, cardiology, physical medicine and rehabilitation, general surgery, psychiatry; and
Physicians should plan a treatment of approximately six months that gradually introduces cognitive training, behavioral and environmental changes, and diet practices to patients in modules designed by the Republic of Türkiye’s Ministry of Health in the circular numbered 19602659.
It has been shown that obese patients with NAFLD undergoing metabolic surgery lost an average of 55% of their excess weight in the sixth month, and there was a 22% decrease in liver volumes, 84% decrease in steatosis, and a 50% regression in fibrosis in MRI follow-ups.[253] It has been reported that, while the decrease in liver volume reaches its peak in the first-month post-operation, hepatic steatosis continues to decrease in parallel with weight loss in the ongoing process. In the AASLD guidelines, sleeve gastrectomy is recommended as the surgical method in obese patients with NAFLD.[2] Since these patients can regain weight after surgery, they should be followed up regularly in the post-surgical period. The need for surgical revision after metabolic surgery is reported to be between 10% and 20%.[254]
Obesity has a negative effect on the natural history of compensated cirrhosis. It has been suggested that weight loss is an essential therapeutic intervention in these patients.[255] Obese patients with compensated cirrhosis may benefit from metabolic surgery for this purpose. The incidence of incidental cirrhosis during metabolic surgery is reported to be between 1% and 4%.[256] Elective surgeries in patients with cirrhosis are high-risk procedures. While the metabolic surgery mortality rate is 0.3% in patients without cirrhosis, this rate increases to 0.9% in cases of compensated cirrhosis and 16.3% in those with decompensated cirrhosis.[257] A systematic review reported that complications were seen in approximately 21% of patients after bariatric surgery, decompensation developed in 7% of patients, and 1.6% and 2.5% mortality were observed in the early and late periods, respectively.[258] In addition, rapid weight loss after surgery may also cause decompensation. Surgical procedures should be performed by an experienced bariatric surgeon and follow the decision of a board of physicians who are experts in their field. It is recommended that obese patients should be evaluated for cirrhosis by gastroenterology specialists before metabolic surgery and that they should be followed up regularly after surgery.
Sleeve gastrectomy is frequently preferred in cirrhotic patients because of the rapid procedure, low complication rates, no anatomical changes, and no impediment to LT.[259,260] In addition, it has been reported that sleeve gastrectomy can be performed as a safe surgical procedure with or after LT in these patients.[259,260] Sleeve gastrectomy should be performed in experienced centers in compensated cirrhotic patients. Sleeve gastrectomy may rarely increase the risk of gastric variceal bleeding.
Malabsorptive procedures (Roux en Y gastric bypass and biliopancreatic diversion) are rarely applied and should be preferred in patients with high-calorie liquid food addiction. These approaches increase hepatic decompensation. Moreover, due to anatomical changes, endoscopic interventions in the remnant stomach and biliary system become impossible in cases such as gastrointestinal bleeding and biliary obstruction.
If weight loss cannot be achieved with lifestyle changes, diet, and exercise in obese NAFLD patients, bariatric surgery should be considered. The benefits and risks of the surgery should be evaluated with a multidisciplinary approach, and a joint decision should be made.
Liver Transplantation and Follow-Up
The frequency of LT with indications for NAFLD-related cirrhosis and HCC is increasing in Türkiye and worldwide. NAFLD-related cirrhosis is the second most common reason for LT in the United States.[1–3,261] NAFLD-related cirrhosis and HCC is the second most common etiologic factor for liver recipients on the waiting list.[1–3,262] While the annual rate of liver transplant patients for NAFLD-related cirrhosis in the UK was 4% in 1995, it was 12% in 2013.[43] Indication of LT was not different from the cases of liver cirrhosis and HCC due to other causes in the NAFLD group.
Patients with NAFLD on the LT waiting list are generally older and more likely to experience MetS, kidney failure, and lower GFR. Post-transplant survival is usually reported at similar rates to LTs with other etiologies. However, according to the United Network of Organ Sharing data, ten-year survival in liver transplant patients due to NAFLD cirrhosis is lower than in patients with autoimmune liver diseases, chronic hepatitis B, and prolonged hospital stays have been reported due to comorbidities.[2,38,263–265] The presence of MetS and CVD is a determinant in terms of morbidity and mortality before and after LT (Table 15).[264–266] For this reason, it is recommended that this group of patients should be evaluated in detail before LT.[3,262,266]
Table 15.
Risk Factors for the Development of NAFLD After Liver Transplantation
| Diabetes mellitus and insulin resistance | Hyperlipidemia |
| Weight gain | Fatty donor liver |
| Hypertension | Hepatitis C virus infection |
Different rates of disease recurrence or newly developed NAFLD are reported by transplant centers after LT.[264,267–270] Histopathologically, steatosis is observed in 18%–40% of protocol biopsies performed after LT, regardless of recurrent disease.[267–269] Steatohepatitis in recipients is observed in 1%–13% of cases, and steatosis is usually macrovesicular and moderate.
The distinction between disease recurrence or newly developed NAFLD after LT is often difficult. If there is NAFLD development following LT in patients with cryptogenic cirrhosis, obesity, diabetes, hyperlipidemia, hypertension, or MetS risk factors in the pre-transplantation period, recurrence of NAFLD should be considered primarily.[267–270] It has been reported that the disease progresses more slowly in newly developed NAFLD cases. Hepatosteatosis is prominent, and fibrosis develops more slowly. However, fibrosis development and progression to cirrhosis are faster in recurrent NAFLD cases.[267–270] MetS, obesity, renal failure, and CVD are more common in recurrent cases.[267–270]
The diagnosis and treatment approach in patients who develop NAFLD following LT is similar to that of patients without transplantation. Diet and exercise, blood glucose regulation, control of hypertension, and lipid levels should be targeted.[267–271] In addition, immunosuppressive therapy should be individualized.
Combined LT and metabolic surgery after LT are recommended in morbidly obese cases.[272,273] However, since living donor LT is frequently performed in Türkiye, it would be a more appropriate approach to perform metabolic surgery electively after LT instead of combining the two. On the other hand, finding suitable living donors for patients with NAFLD-related cirrhosis is an important problem in clinical practice.
Liver transplantation is recommended in the presence of decompensated cirrhosis with/without HCC due to NAFLD.
The presence of MetS and CVD are important causes of morbidity and mortality after transplantation.
NAFLD in Children
NAFLD develops in children aged 18 years and under. Genetic or metabolic diseases, infections, drug use, alcohol consumption, or malnutrition are not responsible for the etiology of NAFLD.[274] NAFLD is often closely associated with obesity, IR, and dyslipidemia.[2,3,274] As in adults, because of the increasing prevalence of obesity worldwide, NAFLD has become the most common pediatric liver disease in Western countries.[274,275] The spectrum of pediatric NAFLD is similar to that of adults and ranges from simple fatty liver to steatohepatitis, cirrhosis, and HCC. NAFLD-related advanced liver failure rarely develops in children.[2,3,274,276] Cirrhosis has been reported as early as two years and at 8–9 years of age.[277–279] Adults with NAFLD onset in childhood are at increased risk for early or severe complications from NAFLD.[2] Therefore, early detection and identifying a practical approach to treating NAFLD are important to prevent future complications.
Prevalence and Risk Factors
The incidence and prevalence of NAFLD in children vary due to differences in the diagnostic method used and the heterogeneity in the population studied (e.g., geographic region, ethnicity, and race). In a study from the USA that included 742 autopsies of children aged 2–19 years, the prevalence of NAFLD was 9.6% in the entire study group. The prevalence has been reported as 5% in normal-weight, 16% in overweight (BMI 85–94th percentile), and 38% in obese (BMI ≥95th percentile) children.[278] In the same study, while the frequency of NAFLD was 0.7% between the ages of 0 and 2 years, it was observed that the frequency gradually increased with age and reached 17% between the ages of 15 and 19.[278] In a meta-analysis involving children aged 1–19 years in 76 different populations, the frequency of NAFLD was 7.6% in the general pediatric population and 34.2% among obese children. However, there was significant heterogeneity between studies.[280] In a recent study involving 407 obese children aged 9–17, NAFLD was detected in 26% of patients with MRI.[281]
Several factors, such as genetics, environmental factors, high-calorie diet, excess (saturated) fat, refined carbohydrates, sugar-sweetened beverage consumption, excess fructose intake, and the Western diet, are all associated with the development of obesity and NAFLD in children.[283,284] Obesity is the most common and best-documented risk factor for NAFLD.[2] While MetS components, especially T2DM, are frequently seen in NAFLD patients. The risk of developing NAFLD is higher in those with MetS components.[1,3,37] Other risk factors, including puberty, male gender, ethnicity (higher in Hispanics and Asians, lower in Africans), and race (higher in Caucasians), are associated with pediatric NAFLD.[2,278] Studies conducted in the USA have reported that the risk of hepatic steatosis is four times higher in Hispanic adolescents than in non-Hispanic adolescents.[285] Many studies have reported that NAFLD is more common in boys.[278,286,287] PNPLA3 I148M polymorphism, TM6SF2 rs5854292624 gene, and glucokinase regulatory protein (GCKR) rs126032623 gene variants are mutations known as significant risk factors for the development of NASH and fibrosis in the pediatric group.[3,282] However, today, genotype analysis is only sometimes recommended for NAFLD.[3] Some prenatal factors, such as maternal obesity, MetS during pregnancy, gestational diabetes, and intrauterine growth retardation, have also been shown to play a role in developing pediatric NAFLD.[288–290]
Natural Course of NAFLD
The natural course and consequences of pediatric NAFLD are not yet clear. However, it is well known that the entire spectrum of NAFLD can occur in childhood.[2,3,274,276] HCC is very rare in children.[291–293] NAFLD diagnosed in childhood, appears to be associated with increased morbidity and mortality in early adulthood.[292,293] In a study conducted between 2005 and 2015, control biopsy data of children diagnosed through NAFLD biopsy and followed only with lifestyle change recommendations were evaluated.[293] The investigators reported that fibrosis improved in 34% of patients and progressed in 23%, regardless of age and gender.[294] The disease improved in 29% of children with borderline or definitive NASH and progressed to classic NASH in 18% of patients between simple fatty liver and NASH. Overall, the disease progressed to fibrosis or NASH grade in 28% of the patients, and both fibrosis and NASH grades progressed in 7% of the patients. T2DM developed in 8% of patients.[294] The cause of the earliest morbidity of pediatric NAFLD is the development of T2DM.[282] In addition, pediatric NAFLD is associated with developing dyslipidemia and hypertension.[274]
Screening for NAFLD
Children with NAFLD are often asymptomatic in children. Since it can be a progressive disease, screening the risk group for NAFLD is appropriate. Overweight and obese children are at increased risk for NAFLD. The risk of developing NAFLD is higher in the presence of cardiometabolic risk factors such as IR, prediabetes, diabetes, dyslipidemia, and central adiposity. Children with cardiometabolic risk factors who are not overweight are also at risk for NAFLD.[274] Siblings and parents who are overweight due to genetic predisposition are also at high risk of developing NAFLD.[295] The North American Society for Pediatric Gastroenterology, Hepatology & Nutrition (NASPGHAN) practice guideline recommends screening for NAFLD between the ages of 9 and 11 in all obese children and overweight children with additional risk factors (central adiposity, IR, prediabetes or diabetes, dyslipidemia, sleep apnea or a family history of NAFLD/NASH).[274] It has been stated that screening at a younger age is appropriate in children in high-risk groups, such as those with severe obesity or panhypopituitarism.
Diagnosis and Assessment
Biochemical tests and imaging methods are frequently used in the diagnosis of NAFLD. However, it is known that advanced fibrosis may occur in cases where ALT is normal or slightly increased.[279,296,297] The NASPGHAN guidelines recommend using serum ALT levels in NAFLD screening, although this has limitations. Specifically, it is emphasized that the age- and gender-specific upper limits of average (22 U/L in girls, 26 U/L in boys) should be taken into consideration, and cases should be screened for three months when the serum ALT level is two times higher than the upper limit of normal. Although serum ALT levels are not sensitive enough to determine the NAFLD phenotype, it has been reported that children with high serum ALT levels (≥80 U/L) are more likely to have NASH than those with lower levels (<80 U/L).[298] International guidelines have suggested that abdominal US is the first imaging modality of choice in diagnosing NAFLD, as it provides additional information.[3]
Before the diagnosis of NAFLD is made, causes that increase liver damage, such as viral hepatitis, autoimmune liver disease, congenital metabolic diseases (fatty acid or carnitine metabolism disorders, monogenic diseases such as peroxisomal diseases, cystic fibrosis, lysosomal storage diseases), celiac disease, and hepatotoxic drug use, should be excluded.[2,274] In many children, serum autoantibodies may be positive at low titer. In cases with high autoantibody positivity and especially with increased serum globulin values, a liver biopsy is required to differentiate autoimmune hepatitis from NAFLD.[2,274,296]
The degree of fibrosis in patients with NAFLD is an important prognostic factor for the long-term outcome of the disease. It has been observed that fibrosis scoring systems such as AST/ALT ratio, NFS, APRI, and FIB-4 score, which are used to determine advanced fibrosis in adult patients with NAFLD, do not provide satisfactory results in determining advanced fibrosis in children.[299–303] The “Pediatric NAFLD fibrosis index”[299] developed for pediatric patients was not found to be sufficient in the external validation study.[300] Similarly, although serological tests, such as ELF test and pediatric NAFLD fibrosis score, have been reported to have high accuracy in children, it has been emphasized 34 that external validation studies are needed for their routine use.[301,302] Good results have been reported in ongoing studies of the validity and accuracy of imaging techniques, such as TE and MRE, in children, but validation studies are needed.[274,303,304] Liver biopsy remains the gold standard for diagnosing and grading NAFLD.[305,306] In international guidelines, liver biopsy is recommended in children with suspected NAFLD when another or a concurrent curable disease is suspected, when clinically advanced NAFLD is suspected, and when pharmacological treatment or surgical intervention is considered for NAFLD.[2,307] Pathologists interpreting pediatric liver biopsies should recognize and report specific (pediatric type) NAFLD features in children.
During the examination of a patient with suspected pediatric NAFLD, it is mandatory to exclude other etiological factors that may cause elevated serum aminotransferase or hepatic steatosis and to investigate coexisting chronic liver diseases.
A liver biopsy is still the gold standard for the diagnosis and determination of the grade of NAFLD.
Treatment
The primary goal in the treatment of NAFLD is reducing hepatic adiposity, the severity of inflammation, and the fibrosis stage. Another treatment goal is the reduction of excess adipose tissue to correct dyslipidemia, IR, high blood pressure, and central adiposity. It is essential to manage diabetes, CVD, and hypertension, which are comorbidities of NAFLD in children, to prevent undesirable clinical results that may occur in the future.[274]
Lifestyle Intervention
Lifestyle changes should be implemented in all overweight and obese children with NAFLD.[2] It has been demonstrated that weight loss significantly improves metabolic and hepatic findings in these children.[177] Today, lifestyle modification, diet, and increasing physical activity are the primary treatment approach for pediatric NAFLD.[274] However, it is unknown how much weight loss is required to recover NASH histology in children.[2,274,308] The NASPGHAN guidelines recommend avoiding sugar-sweetened beverages, consuming a healthy and balanced diet, moderate and high-intensity daily exercise, and less than two hours of screen time per day as lifestyle modifications.[274]
Medical Treatment
Currently, no drug is approved for NAFLD in children within the indication. As in adults, clinical studies on the treatment of pediatric NAFLD have focused on IR and oxidative stress. Bariatric surgery is not a specifically recommended treatment for pediatric NAFLD patients.[274] In the NASPGHAN guidelines, metabolic surgery can be performed in children with a BMI of 35 kg/m2 or above without cirrhosis and in selected patients with serious comorbidities such as T2DM, severe sleep apnea, and idiopathic intracranial hypertension.[274]
The appropriate long-term follow-up frequency of children with NAFLD needs to be more precise. The frequency of follow-up is related to the severity of the disease. It has been demonstrated that frequent visits are effective in achieving and maintaining the intended weight loss in overweight and obese children.[309,310] Pediatric NAFLD is frequently associated with CVD risk factors, particularly dyslipidemia.[311] Children with NAFLD often have early signs of atherosclerosis.[312,313] In the NASPGHAN guidelines, it is recommended that children with NAFLD be screened for dyslipidemia at the time of diagnosis and periodically, but there is no recommendation for treating dyslipidemia.[274] Children with NAFLD are at higher risk of hypertension than obese children without NAFLD, and this risk persists over time.[314] Therefore, guidelines recommend monitoring and treating overweight and obese children for hypertension.[315] Studies on the prevalence of prediabetes and T2DM in children with NAFLD are limited. A study conducted on children with biopsy-proven NAFLD found that the probability of NASH is higher in children with prediabetes and diabetes.[316] Another study found that the prevalence of prediabetes and MetS increased with the hepatic fat content determined by MRI.[317] It was reported that glucose and insulin sensitivity indicators were associated with basal hepatic fat content in children.[318] The NASPGHAN guidelines recommend that children with NAFLD should be followed up by measuring fasting serum glucose level or HbA1c level at least once a year during and after diagnosis. A glucose tolerance test should be performed if these test values are at prediabetic levels.[274]
Lifestyle modification, including dietary and exercise, is the first treatment approach in children with NAFLD.
Currently, there is no approved drug for the treatment of NAFLD in children.
Preventive Strategies and Health Policies
The proportions of overweight and obese individuals in the Turkish population were 32% and 15%, respectively, in 2008. These rates increased to 35% and 21%, respectively, in 2019, based on data from the Turkish Statistical Institute (TUIK). It is known that the median age of those diagnosed with NAFLD is 55 years; 40% of the patients were diagnosed in their very productive years, such as 35–55 years. The rate among individuals under 35 years old is approximately 9%, and they were followed up for about 2.6 years after diagnosis.[319] Hospital visits of these patients increased after they were diagnosed with NAFLD. This increase in visits started from primary health centers and reflected all other clinics, especially gastroenterology, endocrine, and cardiology. The initial diagnosis costs of these patients were relatively high. Unfortunately, studies on the disease burden and the cost of NAFLD in Türkiye are limited. In the United States, approximately $100 billion of annual medical expenses (60 million patients, $1,613 per patient) are thought to result from NAFLD.[319,320] In European countries, 35 billion euros of annual medical costs (50 million patients, 354–1,163 euros per patient) are due to NAFLD.[320] Prices are highest in the 45–65 age group, as expected. The disease burden increases significantly when social and economic fees other than direct health expenditures are included. According to studies, NAFLD, NASH, and advanced liver disease patients are admitted to the hospital on average 2.9, 4.2, and 4.4 times a year, respectively. It is reported that the annual health expenditures of these patients are 10,576 euros in patients without advanced liver disease, 19,681 euros in patients with cirrhosis, 26,220 euros in patients with HCC, and 65,137 euros in those who undergo LT.[321] In addition, due to the high prevalence of diabetes, hypertension, and other cardiovascular comorbidities, the disease burden increases even more.[1–3,261]
According to the 2020 report by the WHO, approximately 71% of annual deaths are due to non-communicable diseases. About 30 million people between 30 and 69 die yearly.[322] Following the global impact of COVID-19 on the whole world, the living conditions of individuals have changed, the length of stay at home has increased, physical activities have decreased, and there have been delays and disruptions in the provision of non-COVID-19 healthcare services. Curfews, disturbances in the diagnosis and treatment of chronic diseases, psychological stress, increased anxiety, and changes in nutritional profile negatively affected the process. Likewise, the decrease in the physical activities of children, being away from school, decrease in social activities, and increase in the time spent in front of the computer will soon negatively affect the incidence of obesity and related diseases in this age group. According to the “Food Supplement Use and Nutritional Habits Measurement” survey conducted by the Food Supplement and Nutrition Association, food supplements have increased during the COVID-19 pandemic. Looking at an analysis of food sales analysis, there has been a change in nutrition habits during this pandemic. This situation is expected to worsen in Türkiye and gradually become essential in health expenditures.
Lifestyle changes, deterioration of nutritional habits, uncontrolled and unconscious supplementation, and drug use adversely affect the NAFLD process.
It is essential to determine cost-effective screening programs for NAFLD, establish early diagnostic methods, and analyze effectiveness and efficiency in developing preventive strategies and health policies for NAFLD.
Conclusion
NAFLD is a chronic liver disease with increasing incidence in the pediatric and adult populations. It is characterized by abnormal fat accumulation in the liver without significant alcohol use. NAFLD is more common in individuals with obesity, IR, T2DM, and MetS. NASH is a progressive disease, and accompanying metabolic disorders accelerate its progression. Therefore, individuals with metabolic abnormalities should be evaluated for NAFLD. Abdominal US is the first method to be used in diagnosis and screening. Transient elastography and MRE accurately identify liver fibrosis if access is possible. A liver biopsy remains the gold standard for diagnosing and assessing NAFLD. Diet, exercise, and improvement of accompanying metabolic disorders are currently the most effective approaches in the treatment of NAFLD.
Abbreviations
AASLD: American Association for the Study of Liver Diseases
ACE-2: Angiotensin-converting enzyme
ACEI: Angiotensin-converting enzyme inhibitors
ACR: Albumin/creatinine ratio
AFP: Alpha-fetoprotein
ALD: Alcohol-related liver disease
ALT: Alanine aminotransferase
APASL: Asian Pacific Association for the Study of the Liver
APRI: Aspartate Aminotransferase/Platelet Ratio Index
ARB: Angiotensin receptor blockers
ARFI: Acoustic Radiation Force Impulse
ASCVD: Atherosclerotic Cardiovascular Disease
ASK1: Apoptosis Signal-Regulating Kinase 1
AST: Aspartate aminotransferase
BD: Ballooning Degeneration
BMI: Body mass index
CAC: Coronary artery calcium
CAP: Controlled-attenuation parameter
CCR2/CCR5: Chemokine receptor-2, 5
CETP: Cholesterol ester transferrin protein
CHB: Chronic hepatitis B
CKD: Chronic kidney disease
CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration
CT: Computed tomography
CVD: Cardiovascular diseases
EASL: European Association for the Study of Liver
EBMT: Endoscopic bariatric and metabolic treatments
eGFR: Estimated glomerular filtration rate
ELF: Enhanced liver fibrosis
ER: Endoplasmic reticulum
FGF-21: Fibroblast growth factor 21
FIB-4: Fibrosis index-based 4
FLIP: Fatty liver inhibition of progression
FXR: Farnesoid X receptor
GCKR: Glucokinase regulatory protein
GGT: Gamma-glutamyl transferase
GLP-1: Glucagon-like peptide-1
HBsAg: Hepatitis B surface antigen
HCC: Hepatocellular carcinoma
HCV: Hepatitis C virus
HDL: High-density lipoprotein
HMG-CoA: Hydroxy-3-methylglutaryl-coenzyme A
HOMA: Homeostasis model assessment
HU: Hounsfield unit
IFG-I: Impaired fasting glucose
IFSO: International Federation for the Surgery of Obesity and Metabolic Disorders
IGT: Impaired glucose tolerance
IR: Insulin resistance
KDIGO: Kidney Disease Improving Global Outcomes
LCAT: Lecithin-cholesterol acyltransferase
LDL: Low-density lipoprotein
LSM: Liver stiffness measurements
LT: Liver transplantation
LXR: Liver X receptor
MAFLD: Metabolic Disease-Associated Fatty Liver Disease
MDRD: Modification of Diet in Renal Disease
MetS: Metabolic syndrome
MONW: Metabolically obese but normal weight
MR: Magnetic resonance
MRE: MR elastography
MRI: MR imaging
MRI-PDFF: Magnetic Resonance- Proton Density Fat Fraction
MRS: MR spectroscopy
MUFA: Monounsaturated fatty acid
NAFL: Nonalcoholic fatty liver
NAFLD: Nonalcoholic fatty liver disease
NAS: NAFLD Activity Score
NASH: Nonalcoholic steatohepatitis
NASPGHAN: North American Society for Pediatric Gastroenterology, Hepatology & Nutrition
NASH-CRN: Nonalcoholic Steatohepatitis Clinical Research Network
NFS: NAFLD fibrosis score
NITs: Noninvasive tests
OGTT: Oral glucose tolerance test
PEM: Protein-energy malnutrition
PEMT: Phosphatidylethanolamine N-methyltransferase
PPAR-γ: Peroxisome proliferator-activated receptor gamma
Pro-C3: Pro-peptide of type 3 collagen
PNPLA3: Patatin-like phospholipase domain-containing protein
PPV: Positive predictive value
pSWE: Point shear-wave elastography
PUFA: Polyunsaturated fatty acid
RAAS: Renin-angiotensin-aldosterone system
RBP-4: Retinol-binding protein 4
REE: Resting energy expenditure
ROS: Reactive oxygen species
SAF: Steatosis, activity fibrosis score
SCD1: Stearoyl-coenzyme A desaturase-1
SCORE: Systematic Coronary Risk Estimation
SEER: Surveillance Epidemiology and End Results
SGLT2i: Sodium-glucose transporter-2 inhibitors
SSI: Supersonic Shear Imaging
TASL: Turkish Association for the Study of the Liver
T2DM: Type 2 Diabetes Mellitus
THR-β: Thyroid-hormone receptor-beta
TIMP-1: Metalloprotease tissue inhibitor-1
TM6SF2: Transmembrane 6 superfamily member 2.
TNF: Tumor necrosis factor
TNF-α: Tumor necrosis factor-alpha
TURDEP: Turkish Diabetes Epidemiology
TUIK: Turkish Statistical Institute
UDCA: Ursodeoxycholic acid
UNOS: United Network of Organ Sharing
US: Ultrasonography
VLDL: Very low-density lipoprotein
VCTE: Vibration-controlled transient elastography
WHO: World Health Organization
Author Contributions
Yusuf Yilmaz: Design of the study, writing, analysis, and interpretation of the data, revising the manuscript critically for important intellectual content. Mujdat Zeybel: Design of the study, writing, analysis, and interpretation of the data, revising the manuscript critically for important intellectual content. Gupse Adali: Design of the study, analysis, and interpretation of the data, revising the manuscript critically for important intellectual content. Arif Mansur Cosar: Design of the study, analysis, and interpretation of the data, revising the manuscript critically for important intellectual content. Elif Sertesen: Acquisition of data for the manuscript and English editing. Hale Gokcan: Design of the study, writing, analysis, and interpretation of the data, revising the manuscript critically for important intellectual content. Halil Ibrahim Bahcecioglu: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Mustafa Sahin: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Cansin Tulunay: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Ihsan Ergun: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Ilker Turan: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Ilkay Sedakat Idilman: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Cigdem Celikel: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Hale Kirimlioglu: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Gulen Akyol: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Funda Yilmaz: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Cenk Sokmensuer: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Hakan Guveli: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Ulus Salih Akarca: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Umit Akyuz: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Volkan Genc: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Murat Akyildiz: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Nuray Yazihan: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Engin Tutar: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Fehmi Ates: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Dinc Dincer: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Yasemin Balaban: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Murat Kiyici: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Meral Akdogan: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Abdullah Sonsuz: Acquisition of data for the manuscript, writing, and revising the manuscript critically for important intellectual content. Ramazan Idilman: Design of the study, analysis, and interpretation of the data, revising the manuscript critically for important intellectual content. Members of TASL Fatty Liver Special Interest Group: Revising the manuscript critically for important intellectual content.
Footnotes
How to cite this article: Yilmaz Y, Zeybel M, Adali G, Cosar AM, Sertesen E, Gokcan H, et al. TASL Practice Guidance on the Clinical Assessment and Management of Patients with Nonalcoholic Fatty Liver Disease. Hepatology Forum 2023; Suppl 1:1–32.
Conflict of Interest
The authors have no conflict of interest to declare.
Financial Disclosure
The authors declared that this study has received no financial support.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the L, European Association for the Study of D, European Association for the Study of O EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The Diagnosis and Management of Non-alcoholic Fatty Liver Disease: Practice Guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 6.Eslam M, Sanyal AJ, George J, International Consensus P. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014 e1991. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 7.Demirtaş CO, Yilmaz Y. Metabolic-associated Fatty Liver Disease: Time to integrate ground-breaking new terminology to our clinical practice? Hepatology Forum. 2020;3:79–81. doi: 10.14744/hf.2020.2020.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yilmaz Y, Byrne CD, Musso G. A single-letter change in an acronym: signals, reasons, promises, challenges, and steps ahead for moving from NAFLD to MAFLD. Expert Rev Gastroenterol Hepatol. 2021;4:345–352. doi: 10.1080/17474124.2021.1860019. [DOI] [PubMed] [Google Scholar]
- 9.Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69:2672–2682. doi: 10.1002/hep.30251. [DOI] [PubMed] [Google Scholar]
- 10.Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic Steatohepatitis: A Review. JAMA. 2020;323:1175–1183. doi: 10.1001/jama.2020.2298. [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz Y, Yilmaz N, Ates F, Karakaya F, Gokcan H, Kaya E, et al. The prevalence of metabolic associated fatty liver disease in the Turkish population: A multicenter study. Hepatology Forum. 2021;2:37–42. doi: 10.14744/hf.2021.2020.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchesini G, Mazzotti A. NAFLD incidence and remission: only a matter of weight gain and weight loss? J Hepatol. 2015;62:15–17. doi: 10.1016/j.jhep.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Satman I KS, Salman S, Sengul A, Sargin M, Karsidag K, Dinccag N, et al. In: 89th Annual Meeting ENDO 07, June 2-5, 2007, Toronto, Canada. 2007. Defining a population-specific waist circumference in Turkish population. [Google Scholar]
- 14.Dinccag N SI, Kalaca S, Omer B, Karsidag K, Yilmaz T, Tutuncu Y, et al. the TURDEP-II Study Group . 2011. T. The prevalence of the metabolic syndrome is comparable using the nation-specific IDF, WHO and NECPATP III criteria in Turkey. In: 47th EASD Annual Meeting. Lisbon, Portugal Diabetologia Diabetologia; p. 2544. [Google Scholar]
- 15.Kaya E, Yilmaz Y. Türkiye’de ve dünyada nonalkolik yağlı karaciğer hastalığı epidemiyolojisi. In: Sonsuz A, editor. Nonalkolik Yağlı Karaciğer Hastalığı, 1. Baskı Ankara: Türkiye Klinikleri; 2019. pp. 1–7. [Google Scholar]
- 16.Sheth SG CS. Epidemiology, clinical features, and diagnosis of nonalcoholic fatty liver disease in adults. In: Post TW (Ed), editor. Waltham, MA: 2020. In. UpToDate. [Google Scholar]
- 17.Falck-Ytter Y, Younossi ZM, Marchesini G, McCullough AJ. Clinical features and natural history of nonalcoholic steatosis syndromes. Semin Liver Dis. 2001;21:17–26. doi: 10.1055/s-2001-12926. [DOI] [PubMed] [Google Scholar]
- 18.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Soderberg C, Stal P, Askling J, Glaumann H, Lindberg G, Marmur J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 20.Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69:1691–1705. doi: 10.1136/gutjnl-2020-320622. [DOI] [PubMed] [Google Scholar]
- 21.Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. doi: 10.1136/bmj.d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Younossi Z, Henry L. Contribution of Alcoholic and Nonalcoholic Fatty Liver Disease to the Burden of Liver-Related Morbidity and Mortality. Gastroenterology. 2016;150:1778–1785. doi: 10.1053/j.gastro.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Metwally M, et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology. 2018;155:443–457.e417. doi: 10.1053/j.gastro.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 25.Castera L. Non-invasive tests for liver fibrosis in NAFLD: Creating pathways between primary healtcare and liver clinics. Liver Intern. 2020;S1:77–81. doi: 10.1111/liv.14347. [DOI] [PubMed] [Google Scholar]
- 26.Tovo CV, Villela-Nogueira CA, Leite NC, Panke CL, Port GZ, Fernandes S, et al. Transient hepatic elastography has the best performance to evaluate liver fibrosis in non-alcoholic fatty liver disease (NAFLD) Ann Hepatol. 2019;18:445–449. doi: 10.1016/j.aohep.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Mustapic S, Ziga S, Matic V, Bokun T, Radic B, Lucijanic M, et al. Ultrasound Grade of Liver Steatosis Is Independently Associated with the Risk of Metabolic Syndrome. Can J Gastroenterol Hepatol. 2018;2018:8490242. doi: 10.1155/2018/8490242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy YK, Marella HK, Jiang Y, Ganguli S, Snell P, Podila PSB, et al. Natural history of non-alcoholic fatty liver disease: A study with paired liver biopsies. J Clin Exp Hepatol. 2020;10:245–54. doi: 10.1016/j.jceh.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanyal AJ, Harrison SA, Ratziu V, Abdelmalek MF, Diehl AM, Caldwell S, et al. The Natural History of Advanced Fibrosis Due to Nonalcoholic Steatohepatitis: Data From the Simtuzumab Trials. Hepatology. 2019;70:1913–1927. doi: 10.1002/hep.30664. [DOI] [PubMed] [Google Scholar]
- 30.Pais R, Charlotte F, Fedchuk L, Bedossa P, Lebray P, Poynard T, et al. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol. 2013;59:550–556. doi: 10.1016/j.jhep.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 31.Dam-Larsen S, Franzmann M, Andersen IB, Christoffersen P, Jensen LB, Sorensen TI, et al. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004;53:750–755. doi: 10.1136/gut.2003.019984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valenti L, Dongiovanni P, Fargion S. Diagnostic and therapeutic implications of the association between ferritin level and severity of nonalcoholic fatty liver disease. World J Gastroenterol. 2012;18:3782–3786. doi: 10.3748/wjg.v18.i29.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dongiovanni P, Donati B, Fares R, Lombardi R, Mancina RM, Romeo S, et al. PNPLA3 I148M polymorphism and progressive liver disease. World J Gastroenterol. 2013;19:6969–6978. doi: 10.3748/wjg.v19.i41.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Severson TJ, Besur S, Bonkovsky HL. Genetic factors that affect nonalcoholic fatty liver disease: A systematic clinical review. World J Gastroenterol. 2016;22:6742–6756. doi: 10.3748/wjg.v22.i29.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jarvis H, Craig D, Barker R, Spiers G, Stow D, Anstee QM, et al. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of population-based observational studies. PLoS Med. 2020;17:e1003100. doi: 10.1371/journal.pmed.1003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 37.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47–64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Tsochatzis E, Coilly A, Nadalin S, Levistky J, Tokat Y, Ghobrial M, et al. International Liver Transplantation Consensus Statement on End-stage Liver Disease Due to Nonalcoholic Steatohepatitis and Liver Transplantation. Transplantation. 2019;103:45–56. doi: 10.1097/TP.0000000000002433. [DOI] [PubMed] [Google Scholar]
- 39.Alswat KA, Fallatah HI, Al-Judaibi B, Elsiesy HA, Al-Hamoudi WK, Qutub AN, et al. Position statement on the diagnosis and management of non-alcoholic fatty liver disease. Saudi Med J. 2019;40:531–540. doi: 10.15537/smj.2019.6.23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong VW, Chan WK, Chitturi S, Chawla Y, Dan YY, Duseja A, et al. Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 1: Definition, risk factors and assessment. J Gastroenterol Hepatol. 2018;33:70–85. doi: 10.1111/jgh.13857. [DOI] [PubMed] [Google Scholar]
- 41.Jegatheesan P, De Bandt JP. Fructose and NAFLD: The Multifaceted Aspects of Fructose Metabolism. Nutrients. 2017;9 [Google Scholar]
- 42.Yilmaz Y. Review article: fructose in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2012;35:1135–1144. doi: 10.1111/j.1365-2036.2012.05080.x. [DOI] [PubMed] [Google Scholar]
- 43.Williams R, Alexander G, Armstrong I, Baker A, Bhala N, Camps-Walsh G, et al. Disease burden and costs from excess alcohol consumption, obesity, and viral hepatitis: fourth report of the Lancet Standing Commission on Liver Disease in the UK. Lancet. 2018;391:1097–1107. doi: 10.1016/S0140-6736(17)32866-0. [DOI] [PubMed] [Google Scholar]
- 44.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 45.Lai LL, Wan Yusoff WNI, Vethakkan SR, Nik Mustapha NR, Mahadeva S, Chan WK. Screening for non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus using transient elastography. J Gastroenterol Hepatol. 2019;34:1396–1403. doi: 10.1111/jgh.14577. [DOI] [PubMed] [Google Scholar]
- 46.Ma J, Hwang SJ, Pedley A, Massaro JM, Hoffmann U, Chung RT, et al. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J Hepatol. 2017;66:390–397. doi: 10.1016/j.jhep.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013;230:258–267. doi: 10.1016/j.atherosclerosis.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 48.Velarde-Ruiz Velasco JA, Garcia-Jimenez ES, Garcia-Zermeno KR, Morel-Cerda EC, Aldana-Ledesma JM, Castro-Narro GE, et al. Extrahepatic complications of non-alcoholic fatty liver disease: Its impact beyond the liver. Rev Gastroenterol Mex. 2019;84:472–481. doi: 10.1016/j.rgmx.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 50.Whitsett M, Wilcox J, Yang A, Zhao L, Rinella M, VanWagner LB. Atrial fibrillation is highly prevalent yet undertreated in patients with biopsy-proven nonalcoholic steatohepatitis. Liver Int. 2019;39:933–940. doi: 10.1111/liv.14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.VanWagner LB, Wilcox JE, Ning H, Lewis CE, Carr JJ, Rinella ME, et al. Longitudinal Association of Non-Alcoholic Fatty Liver Disease With Changes in Myocardial Structure and Function: The CARDIA Study. J Am Heart Assoc. 2020;9:e014279. doi: 10.1161/JAHA.119.014279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Markus MR, Baumeister SE, Stritzke J, Dorr M, Wallaschofski H, Volzke H, et al. Hepatic steatosis is associated with aortic valve sclerosis in the general population: the Study of Health in Pomerania (SHIP) Arterioscler Thromb Vasc Biol. 2013;33:1690–1695. doi: 10.1161/ATVBAHA.112.300556. [DOI] [PubMed] [Google Scholar]
- 53.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Rev Esp Cardiol (Engl Ed) 2016;69:939. doi: 10.1016/j.rec.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 54.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 55.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:1376–1414. doi: 10.1016/j.jacc.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Golabi P, Fukui N, Paik J, Sayiner M, Mishra A, Younossi ZM. Mortality Risk Detected by Atherosclerotic Cardiovascular Disease Score in Patients With Nonalcoholic Fatty Liver Disease. Hepatol Commun. 2019;3:1050–1060. doi: 10.1002/hep4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pisetta C, Chille C, Pelizzari G, Pigozzi MG, Salvetti M, Paini A, et al. Evaluation of Cardiovascular Risk in Patient with Primary Non-alcoholic Fatty Liver Disease. High Blood Press Cardiovasc Prev. 2020;27:321–330. doi: 10.1007/s40292-020-00389-8. [DOI] [PubMed] [Google Scholar]
- 58.Athyros VG, Giouleme O, Ganotakis ES, Elisaf M, Tziomalos K, Vassiliadis T, et al. Safety and impact on cardiovascular events of long-term multifactorial treatment in patients with metabolic syndrome and abnormal liver function tests: a post hoc analysis of the randomised ATTEMPT study. Arch Med Sci. 2011;7:796–805. doi: 10.5114/aoms.2011.25554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376:1916–1922. doi: 10.1016/S0140-6736(10)61272-X. [DOI] [PubMed] [Google Scholar]
- 60.Zheng SL, Roddick AJ. Association of Aspirin Use for Primary Prevention With Cardiovascular Events and Bleeding Events: A Systematic Review and Meta-analysis. JAMA. 2019;321:277–287. doi: 10.1001/jama.2018.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.VanWagner LB, Wilcox JE, Colangelo LA, Lloyd-Jones DM, Carr JJ, Lima JA, et al. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: A population-based study. Hepatology. 2015;62:773–783. doi: 10.1002/hep.27869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Targher G, Chonchol MB, Byrne CD. CKD and nonalcoholic fatty liver disease. Am J Kidney Dis. 2014;64:638–652. doi: 10.1053/j.ajkd.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 63.Targher G, Byrne CD. Non-alcoholic fatty liver disease: an emerging driving force in chronic kidney disease. Nat Rev Nephrol. 2017;13:297–310. doi: 10.1038/nrneph.2017.16. [DOI] [PubMed] [Google Scholar]
- 64.Arase Y, Suzuki F, Kobayashi M, Suzuki Y, Kawamura Y, Matsumoto N, et al. The development of chronic kidney disease in Japanese patients with non-alcoholic fatty liver disease. Intern Med. 2011;50:1081–1087. doi: 10.2169/internalmedicine.50.5043. [DOI] [PubMed] [Google Scholar]
- 65.Yasui K, Sumida Y, Mori Y, Mitsuyoshi H, Minami M, Itoh Y, et al. Nonalcoholic steatohepatitis and increased risk of chronic kidney disease. Metabolism. 2011;60:735–739. doi: 10.1016/j.metabol.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 66.Targher G, Bertolini L, Rodella S, Lippi G, Zoppini G, Chonchol M. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol. 2010;5:2166–2171. doi: 10.2215/CJN.05050610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kiapidou S, Liava C, Kalogirou M, Akriviadis E, Sinakos E. Chronic kidney disease in patients with non-alcoholic fatty liver disease: What the Hepatologist should know? Ann Hepatol. 2020;19:134–144. doi: 10.1016/j.aohep.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 68.National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 69.Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85:49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 70.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murphree DD, Thelen SM. Chronic kidney disease in primary care. J Am Board Fam Med. 2010;23:542–550. doi: 10.3122/jabfm.2010.04.090129. [DOI] [PubMed] [Google Scholar]
- 72.Yilmaz Y, Alahdab YO, Yonal O, Kurt R, Kedrah AE, Celikel CA, et al. Microalbuminuria in nondiabetic patients with nonalcoholic fatty liver disease: association with liver fibrosis. Metabolism. 2010;59:1327–1330. doi: 10.1016/j.metabol.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 73.Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001680. doi: 10.1371/journal.pmed.1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mantovani A, Petracca G, Beatrice G, Csermely A, Lonardo A, Schattenberg JM, et al. Gut. 2020. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta-analysis. [DOI] [PubMed] [Google Scholar]
- 75.Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:739–752. doi: 10.1016/S2468-1253(20)30077-7. [DOI] [PubMed] [Google Scholar]
- 76.Yilmaz Y, Kani HT, Demirtas CO, Kaya E, Sapmaz AF, Qutranji L, et al. Growing burden of nonalcoholic fatty liver disease in Turkey: A single-center experience. Turk J Gastroenterol. 2019;30:892–898. doi: 10.5152/tjg.2019.19072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akyuz U, Yesil A, Yilmaz Y. Characterization of lean patients with nonalcoholic fatty liver disease: potential role of high hemoglobin levels. Scand J Gastroenterol. 2015;50:341–346. doi: 10.3109/00365521.2014.983160. [DOI] [PubMed] [Google Scholar]
- 78.Uslusoy HS, Nak SG, Gulten M. Noninvasive predictors for liver fibrosis in patients with nonalcoholic steatohepatitis. World J Hepatol. 2011;3:219–227. doi: 10.4254/wjh.v3.i8.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 80.Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab. 2004;89:2569–2575. doi: 10.1210/jc.2004-0165. [DOI] [PubMed] [Google Scholar]
- 81.Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47:699–713. doi: 10.2337/diabetes.47.5.699. [DOI] [PubMed] [Google Scholar]
- 82.Younossi ZM, Stepanova M, Negro F, Hallaji S, Younossi Y, Lam B, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012;91:319–327. doi: 10.1097/MD.0b013e3182779d49. [DOI] [PubMed] [Google Scholar]
- 83.Wei JL, Leung JC, Loong TC, Wong GL, Yeung DK, Chan RS, et al. Prevalence and Severity of Nonalcoholic Fatty Liver Disease in Non-Obese Patients: A Population Study Using Proton-Magnetic Resonance Spectroscopy. Am J Gastroenterol. 2015;110:1306–1314. doi: 10.1038/ajg.2015.235. quiz 1315. [DOI] [PubMed] [Google Scholar]
- 84.Hu X, Huang Y, Bao Z, Wang Y, Shi D, Liu F, et al. Prevalence and factors associated with nonalcoholic fatty liver disease in Shanghai work-units. BMC Gastroenterol. 2012;12:123. doi: 10.1186/1471-230X-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chan WK, Treeprasertsuk S, Imajo K, Nakajima A, Seki Y, Kasama K, et al. Clinical features and treatment of nonalcoholic fatty liver disease across the Asia Pacific region-the GO ASIA initiative. Aliment Pharmacol Ther. 2018;47:816–825. doi: 10.1111/apt.14506. [DOI] [PubMed] [Google Scholar]
- 86.Albhaisi S, Chowdhury A, Sanyal AJ. Non-alcoholic fatty liver disease in lean individuals. JHEP Rep. 2019;1:329–341. doi: 10.1016/j.jhepr.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duarte SMB, Stefano JT, Miele L, Ponziani FR, Souza-Basqueira M, Okada L, et al. Gut microbiome composition in lean patients with NASH is associated with liver damage independent of caloric intake: A prospective pilot study. Nutr Metab Cardiovasc Dis. 2018;28:369–384. doi: 10.1016/j.numecd.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 88.Yun Y, Kim HN, Lee EJ, Ryu S, Chang Y, Shin H, et al. Fecal and blood microbiota profiles and presence of nonalcoholic fatty liver disease in obese versus lean subjects. PLoS One. 2019;14:e0213692. doi: 10.1371/journal.pone.0213692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feldman A, Eder SK, Felder TK, Kedenko L, Paulweber B, Stadlmayr A, et al. Clinical and Metabolic Characterization of Lean Caucasian Subjects With Non-alcoholic Fatty Liver. Am J Gastroenterol. 2017;112:102–110. doi: 10.1038/ajg.2016.318. [DOI] [PubMed] [Google Scholar]
- 90.Li C, Guo P, Okekunle AP, Ji X, Huang M, Qi J, et al. Lean non-alcoholic fatty liver disease patients had comparable total caloric, carbohydrate, protein, fat, iron, sleep duration and overtime work as obese non-alcoholic fatty liver disease patients. J Gastroenterol Hepatol. 2019;34:256–262. doi: 10.1111/jgh.14360. [DOI] [PubMed] [Google Scholar]
- 91.Sharp KPH, Schultz M, Coppell KJ. Is non-alcoholic fatty liver disease a reflection of what we eat or simply how much we eat? JGH Open. 2018;2:59–74. doi: 10.1002/jgh3.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 93.Margariti A, Deutsch M, Manolakopoulos S, Tiniakos D, Papatheodoridis GV. The severity of histologic liver lesions is independent of body mass index in patients with nonalcoholic fatty liver disease. J Clin Gastroenterol. 2013;47:280–286. doi: 10.1097/MCG.0b013e31826be328. [DOI] [PubMed] [Google Scholar]
- 94.Dela Cruz A BE, George J, Bugianesi E, Day C, Liaquat H, Charatcharoenwitthaya P, et al. Characteristics and long-term prognosis of lean patients with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:S909. [Google Scholar]
- 95.Leung JC, Loong TC, Wei JL, Wong GL, Chan AW, Choi PC, et al. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology. 2017;65:54–64. doi: 10.1002/hep.28697. [DOI] [PubMed] [Google Scholar]
- 96.Sookoian S, Pirola CJ. Systematic review with meta-analysis: the significance of histological disease severity in lean patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2018;47:16–25. doi: 10.1111/apt.14401. [DOI] [PubMed] [Google Scholar]
- 97.Wang AY, Dhaliwal J, Mouzaki M. Lean non-alcoholic fatty liver disease. Clin Nutr. 2019;38:975–981. doi: 10.1016/j.clnu.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 98.Bernhardt P, Kratzer W, Schmidberger J, Graeter T, Gruener B, EMIL Study Group Laboratory parameters in lean NAFLD: comparison of subjects with lean NAFLD with obese subjects without hepatic steatosis. BMC Res Notes. 2018;11:101. doi: 10.1186/s13104-018-3212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hagstrom H, Nasr P, Ekstedt M, Hammar U, Stal P, Hultcrantz R, et al. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: A long-term follow-up study. Hepatol Commun. 2018;2:48–57. doi: 10.1002/hep4.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weinberg EM, Trinh HN, Firpi RJ, Bhamidimarri KR, Klein S, Durlam J, et al. Lean Americans With Nonalcoholic Fatty Liver Disease Have Lower Rates of Cirrhosis and Co-morbid Diseases. Clin Gastroenterol Hepatol. 2020. [DOI] [PubMed]
- 101.Hirose S, Matsumoto K, Tatemichi M, Tsuruya K, Anzai K, Arase Y, et al. Nineteen-year prognosis in Japanese patients with biopsy-proven nonalcoholic fatty liver disease: Lean versus overweight patients. PLoS One. 2020;15:e0241770. doi: 10.1371/journal.pone.0241770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hamurcu Varol P, Kaya E, Alphan E, Yilmaz Y. Role of intensive dietary and lifestyle interventions in the treatment of lean nonalcoholic fatty liver disease patients. Eur J Gastroenterol Hepatol. 2020;32:1352–1357. doi: 10.1097/MEG.0000000000001656. [DOI] [PubMed] [Google Scholar]
- 103.Boyle M, Masson S, Anstee QM. The bidirectional impacts of alcohol consumption and the metabolic syndrome: Cofactors for progressive fatty liver disease. J Hepatol. 2018;68:251–267. doi: 10.1016/j.jhep.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 104.Brunt EM, Ramrakhiani S, Cordes BG, Neuschwander-Tetri BA, Janney CG, Bacon BR, et al. Concurrence of histologic features of steatohepatitis with other forms of chronic liver disease. Mod Pathol. 2003;16:49–56. doi: 10.1097/01.MP.0000042420.21088.C7. [DOI] [PubMed] [Google Scholar]
- 105.Choi HSJ, Brouwer WP, Zanjir WMR, de Man RA, Feld JJ, Hansen BE, et al. Nonalcoholic Steatohepatitis Is Associated With Liver-Related Outcomes and All-Cause Mortality in Chronic Hepatitis B. Hepatology. 2020;71:539–548. doi: 10.1002/hep.30857. [DOI] [PubMed] [Google Scholar]
- 106.Chiang DJ, McCullough AJ. The impact of obesity and metabolic syndrome on alcoholic liver disease. Clin Liver Dis. 2014;18:157–163. doi: 10.1016/j.cld.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ceballos N, Babor TF. Editor’s Corner: Binge Drinking and the Evolving Language of Alcohol Research. J Stud Alcohol Drugs. 2017;78:488–490. doi: 10.15288/jsad.2017.78.488. [DOI] [PubMed] [Google Scholar]
- 108.Tozun N, Ozdogan O, Cakaloglu Y, Idilman R, Karasu Z, Akarca U, et al. Seroprevalence of hepatitis B and C virus infections and risk factors in Turkey: a fieldwork TURHEP study. Clin Microbiol Infect. 2015;21:1020–1026. doi: 10.1016/j.cmi.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 109.Chan AW, Wong GL, Chan HY, Tong JH, Yu YH, Choi PC, et al. Concurrent fatty liver increases risk of hepatocellular carcinoma among patients with chronic hepatitis B. J Gastroenterol Hepatol. 2017;32:667–676. doi: 10.1111/jgh.13536. [DOI] [PubMed] [Google Scholar]
- 110.Li J, Yang HI, Yeh ML, Le MH, Le AK, Yeo YH, et al. Association between fatty liver and cirrhosis, hepatocellular carcinoma, and HBsAg seroclearance in chronic hepatitis B. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa739. [DOI] [PubMed] [Google Scholar]
- 111.Chan TT, Chan WK, Wong GL, Chan AW, Nik Mustapha NR, Chan SL, et al. Positive Hepatitis B Core Antibody Is Associated With Cirrhosis and Hepatocellular Carcinoma in Nonalcoholic Fatty Liver Disease. Am J Gastroenterol. 2020;115:867–875. doi: 10.14309/ajg.0000000000000588. [DOI] [PubMed] [Google Scholar]
- 112.Ergenc I GP, Adali G, Kani HT, Ozer Demirtas C, Gunduz F, Ataizi Celikel C, et al. High incidence of hepatitis B core antibody positivity in metabolic-associated fatty liver disease-related cirrhosis. Hepatology Forum. 2021;1:20–25. doi: 10.14744/hf.2020.2020.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Patton HM, Patel K, Behling C, Bylund D, Blatt LM, Vallee M, et al. The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C patients. J Hepatol. 2004;40:484–490. doi: 10.1016/j.jhep.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 114.Pazienza V, Clement S, Pugnale P, Conzelman S, Foti M, Mangia A, et al. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology. 2007;45:1164–1171. doi: 10.1002/hep.21634. [DOI] [PubMed] [Google Scholar]
- 115.Peleg N, Issachar A, Sneh Arbib O, Cohen-Naftaly M, Harif Y, Oxtrud E, et al. Liver steatosis is a major predictor of poor outcomes in chronic hepatitis C patients with sustained virological response. J Viral Hepat. 2019;26:1257–1265. doi: 10.1111/jvh.13167. [DOI] [PubMed] [Google Scholar]
- 116.Kwok R, Tse YK, Wong GLH, Ha Y, Lee AU, Ngu MC, et al. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease--the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther. 2014;39:254–269. doi: 10.1111/apt.12569. [DOI] [PubMed] [Google Scholar]
- 117.Yilmaz Y. Systematic review: caspase-cleaved fragments of cytokeratin 18-the promises and challenges of a biomarker for chronic liver disease. Aliment Pharmacol Ther. 2009;30:1103–1109. doi: 10.1111/j.1365-2036.2009.04148.x. [DOI] [PubMed] [Google Scholar]
- 118.Ajmera V, Perito ER, Bass NM, Terrault NA, Yates KP, Gill R, et al. Novel plasma biomarkers associated with liver disease severity in adults with nonalcoholic fatty liver disease. Hepatology. 2017;65:65–77. doi: 10.1002/hep.28776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Adler M, Gulbis B, Moreno C, Evrard S, Verset G, Golstein P, et al. The predictive value of FIB-4 versus FibroTest, APRI, FibroIndex and Forns index to noninvasively estimate fibrosis in hepatitis C and nonhepatitis C liver diseases. Hepatology. 2008;47:762–763. doi: 10.1002/hep.22085. author reply 763. [DOI] [PubMed] [Google Scholar]
- 120.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ, Nash Clinical Research N. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265–1269. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 122.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 123.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology. 2017;66:1486–1501. doi: 10.1002/hep.29302. [DOI] [PubMed] [Google Scholar]
- 124.Kaya E, Bakir A, Eren F, Yilmaz Y. The utility of noninvasive scores in non-alcoholic fatty liver disease patients with normal and elevated serum transaminases. Hepatology Forum. 2020;1:8–13. doi: 10.14744/hf.2020.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Alkayyali T, Qutranji L, Kaya E, Bakir A, Yilmaz Y. Clinical utility of noninvasive scores in assessing advanced hepatic fibrosis in patients with type 2 diabetes mellitus: a study in biopsy-proven non-alcoholic fatty liver disease. Acta Diabetol. 2020;57:613–618. doi: 10.1007/s00592-019-01467-7. [DOI] [PubMed] [Google Scholar]
- 126.Kaya E, Bakir A, Kani HT, Demirtas CO, Keklikkiran C, Yilmaz Y. Simple Noninvasive Scores Are Clinically Useful to Exclude, Not Predict, Advanced Fibrosis: A Study in Turkish Patients with Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gut Liver. 2020;14:486–491. doi: 10.5009/gnl19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008;47:455–460. doi: 10.1002/hep.21984. [DOI] [PubMed] [Google Scholar]
- 128.Daniels SJ, Leeming DJ, Eslam M, Hashem AM, Nielsen MJ, Krag A, et al. ADAPT: An Algorithm Incorporating PRO-C3 Accurately Identifies Patients With NAFLD and Advanced Fibrosis. Hepatology. 2019;69:1075–1086. doi: 10.1002/hep.30163. [DOI] [PubMed] [Google Scholar]
- 129.Boyle M, Tiniakos D, Schattenberg JM, Ratziu V, Bugianessi E, Petta S, et al. Performance of the PRO-C3 collagen neo-epitope biomarker in non-alcoholic fatty liver disease. JHEP Rep. 2019;1:188–198. doi: 10.1016/j.jhepr.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am J Gastroenterol. 2017;112:740–751. doi: 10.1038/ajg.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eren F, Kaya E, Yilmaz Y. Accuracy of Fibrosis-4 index and non-alcoholic fatty liver disease fibrosis scores in metabolic (dysfunction) associated fatty liver disease according to body mass index: failure in the prediction of advanced fibrosis in lean and morbidly obese individuals. Eur J Gastroenterol Hepatol. 2022;34:98–103. doi: 10.1097/MEG.0000000000001946. [DOI] [PubMed] [Google Scholar]
- 132.Yigit B, Boyle M, Ozler O, Erden N, Tutucu F, Hardy T, et al. Plasma cell-free DNA methylation: a liquid biomarker of hepatic fibrosis. Gut. 2018;67:1907–1908. doi: 10.1136/gutjnl-2017-315668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yoneda M, Suzuki K, Kato S, Fujita K, Nozaki Y, Hosono K, et al. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010;256:640–647. doi: 10.1148/radiol.10091662. [DOI] [PubMed] [Google Scholar]
- 135.Cui J, Heba E, Hernandez C, Haufe W, Hooker J, Andre MP, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the Diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: A prospective study. Hepatology. 2016;63:453–461. doi: 10.1002/hep.28337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cassinotto C, Boursier J, de Ledinghen V, Lebigot J, Lapuyade B, Cales P, et al. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817–1827. doi: 10.1002/hep.28394. [DOI] [PubMed] [Google Scholar]
- 137.de Ledinghen V, Wong GL, Vergniol J, Chan HL, Hiriart JB, Chan AW, et al. Controlled attenuation parameter for the diagnosis of steatosis in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016;31:848–855. doi: 10.1111/jgh.13219. [DOI] [PubMed] [Google Scholar]
- 138.Yilmaz Y, Yesil A, Gerin F, Ergelen R, Akin H, Celikel CA, et al. Detection of hepatic steatosis using the controlled attenuation parameter: a comparative study with liver biopsy. Scand J Gastroenterol. 2014;49:611–616. doi: 10.3109/00365521.2014.881548. [DOI] [PubMed] [Google Scholar]
- 139.Gu Q, Cen L, Lai J, Zhang Z, Pan J, Zhao F, et al. A meta-analysis on the diagnostic performance of magnetic resonance imaging and transient elastography in nonalcoholic fatty liver disease. Eur J Clin Invest. 2021;51:e13446. doi: 10.1111/eci.13446. [DOI] [PubMed] [Google Scholar]
- 140.Kodama Y, Ng CS, Wu TT, Ayers GD, Curley SA, Abdalla EK, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188:1307–1312. doi: 10.2214/AJR.06.0992. [DOI] [PubMed] [Google Scholar]
- 141.Rogier J, Roullet S, Cornelis F, Biais M, Quinart A, Revel P, et al. Noninvasive assessment of macrovesicular liver steatosis in cadaveric donors based on computed tomography liver-to-spleen attenuation ratio. Liver Transpl. 2015;21:690–695. doi: 10.1002/lt.24105. [DOI] [PubMed] [Google Scholar]
- 142.Gu J, Liu S, Du S, Zhang Q, Xiao J, Dong Q, et al. Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: a meta-analysis. Eur Radiol. 2019;29:3564–3573. doi: 10.1007/s00330-019-06072-4. [DOI] [PubMed] [Google Scholar]
- 143.Tang A, Desai A, Hamilton G, Wolfson T, Gamst A, Lam J, et al. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology. 2015;274:416–425. doi: 10.1148/radiol.14140754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930–1940. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Middleton MS, Heba ER, Hooker CA, Bashir MR, Fowler KJ, Sandrasegaran K, et al. Agreement Between Magnetic Resonance Imaging Proton Density Fat Fraction Measurements and Pathologist-Assigned Steatosis Grades of Liver Biopsies From Adults With Nonalcoholic Steatohepatitis. Gastroenterology. 2017;153:753–761. doi: 10.1053/j.gastro.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Singh S, Venkatesh SK, Loomba R, Wang Z, Sirlin C, Chen J, et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol. 2016;26:1431–1440. doi: 10.1007/s00330-015-3949-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brunt EM, Wong VW, Nobili V, Day CP, Sookoian S, Maher JJ, et al. Nonalcoholic fatty liver disease. Nat Rev Dis Primers. 2015;1:15080. doi: 10.1038/nrdp.2015.80. [DOI] [PubMed] [Google Scholar]
- 148.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 149.Takahashi Y, Fukusato T. Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:15539–15548. doi: 10.3748/wjg.v20.i42.15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56:1751–1759. doi: 10.1002/hep.25889. [DOI] [PubMed] [Google Scholar]
- 151.Drescher HK, Weiskirchen S, Weiskirchen R. Current Status in Testing for Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH) Cells. 2019;8 doi: 10.3390/cells8080845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis. 2012;32:3–13. doi: 10.1055/s-0032-1306421. [DOI] [PubMed] [Google Scholar]
- 153.Wang L SY, Chan AWH. Pathology of Non-lcoholic fatty liver disease. Int J Dig Dis. 2016;2:1. [Google Scholar]
- 154.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 155.Bedossa P AJ, Davies S, et al. The EPoS staging system is a reproducible 7-tier fibrosis score for NAFLD adapted both to glass slides and digitized images (e-slides) J Hepatol. 2018;68:S553. [Google Scholar]
- 156.Bedossa P, Consortium FP. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. 2014;60:565–575. doi: 10.1002/hep.27173. [DOI] [PubMed] [Google Scholar]
- 157.Bedossa P. Pathology of non-alcoholic fatty liver disease. Liver Int. 2017;37(Suppl 1):85–89. doi: 10.1111/liv.13301. [DOI] [PubMed] [Google Scholar]
- 158.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 160.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005–1008. doi: 10.1038/sj.bjc.6603932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schlesinger S, Aleksandrova K, Pischon T, Fedirko V, Jenab M, Trepo E, et al. Abdominal obesity, weight gain during adulthood and risk of liver and biliary tract cancer in a European cohort. Int J Cancer. 2013;132:645–657. doi: 10.1002/ijc.27645. [DOI] [PubMed] [Google Scholar]
- 162.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: A systematic review of epidemiologic evidence. Clinical Gastroenterology and Hepatology. 2006;4:369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 163.Dyson J, Jaques B, Chattopadyhay D, Lochan R, Graham J, Das D, et al. Hepatocellular cancer: The impact of obesity, type 2 diabetes and a multidisciplinary team. Journal of Hepatology. 2014;60:110–117. doi: 10.1016/j.jhep.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 164.Bruix J, Sherman M. Management of Hepatocellular Carcinoma: An Update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Reig M, Gambato M, Man NK, Roberts JP, Victor D, Orci LA, et al. Should Patients With NAFLD/NASH Be Surveyed for HCC? Transplantation. 2019;103:39–44. doi: 10.1097/TP.0000000000002361. [DOI] [PubMed] [Google Scholar]
- 166.White DL, Kanwal F, El-Serag HB. Association Between Nonalcoholic Fatty Liver Disease and Risk for Hepatocellular Cancer, Based on Systematic Review. Clinical Gastroenterology and Hepatology. 2012;10:1342–1359. doi: 10.1016/j.cgh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim GA, Lee HC, Choe J, Kim MJ, Lee MJ, Chang HS, et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. Journal of Hepatology. 2018;68:140–146. doi: 10.1016/j.jhep.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 168.Younossi ZM, Loomba R, Anstee QM, Rinella ME, Bugianesi E, Marchesini G, et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology. 2018;68:349–360. doi: 10.1002/hep.29721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Loomba R, Lim JK, Patton H, El-Serag HB. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2020;158:1822–1830. doi: 10.1053/j.gastro.2019.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mittal S, Sada YH, El-Serag HB, Kanwal F, Duan Z, Temple S, et al. Temporal Trends of Nonalcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma in the Veteran Affairs Population. Clinical Gastroenterology and Hepatology. 2015;13:594–601. doi: 10.1016/j.cgh.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Younes R, Bugianesi E. Should we undertake surveillance for HCC in patients with NAFLD? Journal of Hepatology. 2018;68:326–334. doi: 10.1016/j.jhep.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 172.Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, et al. The Natural History of Nonalcoholic Fatty Liver Disease With Advanced Fibrosis or Cirrhosis: An International Collaborative Study. Hepatology. 2011;54:1208–1216. doi: 10.1002/hep.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic Syndrome Increases the Risk of Primary Liver Cancer in the United States: A Study in the SEER-Medicare Database. Hepatology. 2011;54:463–471. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan ZG, Temple S, et al. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans Is Associated With Nonalcoholic Fatty Liver Disease. Clinical Gastroenterology and Hepatology. 2016;14:124–31.e1. doi: 10.1016/j.cgh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu YL, Patman GL, Leathart JBS, Piguet AC, Burt AD, Dufour JF, et al. Carriage of the PNPLA3 rs738409 C > G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61:75–81. doi: 10.1016/j.jhep.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 176.Berna G, Romero-Gomez M. The role of nutrition in non-alcoholic fatty liver disease: Pathophysiology and management. Liver Int. 2020;40(Suppl 1):102–108. doi: 10.1111/liv.14360. [DOI] [PubMed] [Google Scholar]
- 177.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367–378.e365. doi: 10.1053/j.gastro.2015.04.005. quiz e314-365. [DOI] [PubMed] [Google Scholar]
- 178.Aller R, Siguenza R, Pina M, Laserna C, Antolin B, Burgueno B, et al. Insulin resistance is related with liver fibrosis in type 2 diabetic patients with non-alcoholic fatty liver disease proven biopsy and Mediterranean diet pattern as a protective factor. Endocrine. 2020;68:557–563. doi: 10.1007/s12020-020-02268-7. [DOI] [PubMed] [Google Scholar]
- 179.Cai H, Qin YL, Shi ZY, Chen JH, Zeng MJ, Zhou W, et al. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: a randomised controlled trial. BMC Gastroenterol. 2019;19:219. doi: 10.1186/s12876-019-1132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yesil A, Yilmaz Y. Review article: coffee consumption, the metabolic syndrome and non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;38:1038–1044. doi: 10.1111/apt.12489. [DOI] [PubMed] [Google Scholar]
- 181.GBD 2016 Alcohol Collaborators Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Theodoridis X, Grammatikopoulou MG, Petalidou A, Kontonika SM, Potamianos SP, Bogdanos DP. A Systematic Review of Medical Nutrition Therapy Guidelines for Liver Cirrhosis: Do We Agree? Nutr Clin Pract. 2020;35:98–107. doi: 10.1002/ncp.10393. [DOI] [PubMed] [Google Scholar]
- 183.Purnak T, Yilmaz Y. Liver disease and malnutrition. Best Pract Res Clin Gastroenterol. 2013;27:619–629. doi: 10.1016/j.bpg.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 184.Tsien CD, McCullough AJ, Dasarathy S. Late evening snack: exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol. 2012;27:430–441. doi: 10.1111/j.1440-1746.2011.06951.x. [DOI] [PubMed] [Google Scholar]
- 185.Moss O. Nutrition priorities: Diet recommendations in liver cirrhosis. Clin Liver Dis (Hoboken) 2019;14:146–148. doi: 10.1002/cld.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Perdomo CM, Fruhbeck G, Escalada J. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients. 2019;11 doi: 10.3390/nu11030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand Quantity and quality of exercise for developing and maintaining cardiorespiratory musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 188.Wang ST, Zheng J, Peng HW, Cai XL, Pan XT, Li HQ, et al. Physical activity intervention for non-diabetic patients with non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials. BMC Gastroenterol. 2020;20:66. doi: 10.1186/s12876-020-01204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Orci LA, Gariani K, Oldani G, Delaune V, Morel P, Toso C. Exercise-based Interventions for Nonalcoholic Fatty Liver Disease: A Meta-analysis and Meta-regression. Clin Gastroenterol Hepatol. 2016;14:1398–1411. doi: 10.1016/j.cgh.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 190.Zhang HJ, He J, Pan LL, Ma ZM, Han CK, Chen CS, et al. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: A randomized clinical Trial. JAMA Intern Med. 2016;176:1074–1082. doi: 10.1001/jamainternmed.2016.3202. [DOI] [PubMed] [Google Scholar]
- 191.Stoica RA, Rizzo M, Ştefan DS, Rizzo M, Suceveanu AI, Suceveanu AP, et al. Metformin Indications, Dosage, Adverse Reactions, and Contraindications. In: Stoian AMP, Rizzo RM, editors. Metformin. Rijeka: IntechOpen; 2020. [Google Scholar]
- 192.Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2013;1:57–64. doi: 10.3892/br.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Said A, Akhter A. Meta-Analysis of Randomized Controlled Trials of Pharmacologic Agents in Non-alcoholic Steatohepatitis. Ann Hepatol. 2017;16:538–547. doi: 10.5604/01.3001.0010.0284. [DOI] [PubMed] [Google Scholar]
- 194.Zhang X, Harmsen WS, Mettler TA, Kim WR, Roberts RO, Therneau TM, et al. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology. 2014;60:2008–2016. doi: 10.1002/hep.27199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Koenen TB, Tack CJ, Kroese JM, Hermus AR, Sweep FCG, Laak van der J, et al. Pioglitazone treatment enlarges subcutaneous adipocytes in insulin-resistant patients. J Clin Endocrinol Metab. 2009;94:4453–4457. doi: 10.1210/jc.2009-0517. [DOI] [PubMed] [Google Scholar]
- 196.McLaughlin TM, Liu T, Yee G, Abbasi F, Lamendola C, Reaven GM, et al. Pioglitazone increases the proportion of small cells in human abdominal subcutaneous adipose tissue. Obesity. 2010;18:926–931. doi: 10.1038/oby.2009.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kajita K, Mori I, Hanamoto T, Ikeda T, Fujioka K, Yamauchi M, et al. Pioglitazone enhances small-sized adipocyte proliferation in subcutaneous adipose tissue. Endocr J. 2012;59:1107–1114. doi: 10.1507/endocrj.ej12-0259. [DOI] [PubMed] [Google Scholar]
- 198.Musso G, Cassader M, Paschetta E, Gambino R. Pioglitazone for advanced fibrosis in nonalcoholic steatohepatitis: New evidence, new challenges. Hepatology. 2017;65:1058–1061. doi: 10.1002/hep.28960. [DOI] [PubMed] [Google Scholar]
- 199.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 200.Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 201.Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Lopez CO, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or Type 2 Diabetes Mellitus: A randomized trial. Ann Intern Med. 2016;165:305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 202.Portillo-Sanchez P, Bril F, Lomonaco R, Barb D, Orsak B, Bruder JM, et al. Effect of pioglitazone on bone mineral density in patients with nonalcoholic steatohepatitis: A 36-month clinical trial. J Diabetes. 2019;11:223–231. doi: 10.1111/1753-0407.12833. [DOI] [PubMed] [Google Scholar]
- 203.Bełtowski J, Rachańczyk J, Włodarczyk M. Thiazolidinedione-induced fluid retention: Recent insights into the molecular mechanisms. PPAR Res. 2013;2013:628628. doi: 10.1155/2013/628628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhu Z-N, Jiang Y-F, Ding T. Risk of fracture with thiazolidinediones: an updated meta-analysis of randomized clinical trials. Bone. 2014;68:115–123. doi: 10.1016/j.bone.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 205.Ohki T, Isogawa A, Iwamoto M, Ohsugi M, Yoshida H, Toda N, et al. The effectiveness of liraglutide in nonalcoholic fatty liver disease patients with type 2 diabetes mellitus compared to sitagliptin and pioglitazone. Sci World Journal. 2012;2012:496453. doi: 10.1100/2012/496453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mantovani A, Beatrice G, Petracca G, Pampagnin F, Sandri D, Targher G. GLP-1 receptor agonists for NAFLD treatment in patients with and without type 2 diabetes: an updated meta-analysis. Explor Med. 2020;1:1–108. [Google Scholar]
- 207.Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 208.Bril F, Biernacki DM, Kalavalapalli S, Lomonaco R, Subbarayan SK, Lai J, et al. Role of vitamin E for nonalcoholic steatohepatitis in patients withType 2 diabetes: A randomized controlled trial. Diabetes Care. 2019;42:1481–1488. doi: 10.2337/dc19-0167. [DOI] [PubMed] [Google Scholar]
- 209.Parikh P, Ingle M, Patel J, Bhate P, Pandey V, Sawant P. An openlabel randomized control study to compare the efficacy of Vitamin E versusursodeoxycholic acid in nondiabetic and noncirrhotic Indian NAFLD patients. Saudi J Gastroenterol. 2016;22:192–197. doi: 10.4103/1319-3767.182451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Caldwell S. NASH Therapy: Omega 3 Supplementation, vitamin E, insulin sensitizers and statin drugs. Clin Mol Hepatol. 2017;23:103–108. doi: 10.3350/cmh.2017.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Schürks M, Glynn RJ, Rist PM, Tzourio C, Kurth T. Effects of vitamin E on stroke subtypes: Meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702. doi: 10.1136/bmj.c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cheng P, Wang L, Ning S, Liu Z, Lin H, Chen S, et al. Vitamin E intake and risk of stroke: A meta-analysis. Br J Nutr. 2018;120:1181–1188. doi: 10.1017/S0007114518002647. [DOI] [PubMed] [Google Scholar]
- 213.Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: High-dosage vitamin E supplementation may increaseall-cause mortality. Ann Inter Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 214.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin ECancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Olkkonen VM. Cellular Lipid Metabolism. Berlin: Springer Berlin Heidelberg; 2009. Oxysterols and oxysterol-binding proteins in cellular lipid metabolism. [Google Scholar]
- 216.Pramfalk C, Parini P, Gustafsson U, Sahlin S, Eriksson M. Effects of high-dose statin on the human hepatic expression of genes involved incarbohydrate and triglyceride metabolism. J Intern Med. 2011;269:333–339. doi: 10.1111/j.1365-2796.2010.02305.x. [DOI] [PubMed] [Google Scholar]
- 217.Quist-Paulsen P. Statins and inflammation: an update. Curr Opin Cardiol. 2010;25:399–405. doi: 10.1097/HCO.0b013e3283398e53. [DOI] [PubMed] [Google Scholar]
- 218.Gomez-Dominguez E, Gisbert JP, Moreno-Monteagudo JA, Garcia-Buey L, Moreno-Otero R. A pilot study of atorvastatin treatment indyslipemid, non-alcoholic fatty liver patients. Aliment Pharmacol Ther. 2006;23:1643–1647. doi: 10.1111/j.1365-2036.2006.02926.x. [DOI] [PubMed] [Google Scholar]
- 219.Antonopoulos S, Mikros S, Mylonopoulou M, Kokkoris S, Giannoulis G. Rosuvastatin as a novel treatment of non-alcoholic fatty liver disease in hyperlipidemic patients. Atherosclerosis. 2006;184:233–234. doi: 10.1016/j.atherosclerosis.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 220.Abel T, Feher J, Dinya E, Eldin MG, Kovacs A. Safety and efficacy of combined ezetimibe/simvastatin treatment and simvastatin monotherapyin patients with non-alcoholic fatty liver disease. Med Sci Monit. 2009;15:MS6–MS11. [PubMed] [Google Scholar]
- 221.Ekstedt M, Franzen LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: A histopathological follow-up study. J Hepatol. 2007;47:135–141. doi: 10.1016/j.jhep.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 222.Bril F, Portillo Sanchez P, Lomonaco R, Orsak B, Hecht J, Tio F, et al. Liver safety of statins in prediabetes or T2DM and nonalcoholic steatohepatitis: Post Hoc analysis of a randomized trial. J Clin Endocrinol Metab. 2017;102:2950–2961. doi: 10.1210/jc.2017-00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.FDA FDA Drug Safety Communication: Important safety label changes to cholesterol-lowering statin drugs. Last Accessed Date: 18.04.2023, Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-important-safety-label-changes-cholesterol-lowering-statin-drugs.
- 224.Idzior-Walus B, Sieradzki J, Rostworowski W, Zdzienicka A, Kawalec E, Wojcik J, et al. Effects of comicronised fenofibrate on lipid and insulin sensitivity in patients with polymetabolic syndrome X. Eur J Clin Invest. 2000;30:871–878. doi: 10.1046/j.1365-2362.2000.00734.x. [DOI] [PubMed] [Google Scholar]
- 225.Fernandez-Miranda C, Perez-Carreras M, Colina F, Lopez-Alonso G, Vargas C, Heruzo JAS. A pilot trial of fenofibrate for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis. 2008;40:200–205. doi: 10.1016/j.dld.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 226.Gandhi N, Lenton R, Bhartia M, Abbas A, Raju J, Ramachandran S. Effect of fibrate treatment on liver function tests in patients with the metabolic syndrome. Springerplus. 2014;3:14. doi: 10.1186/2193-1801-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Borem LMA, Neto JFR, Brandi IV, Lelis DF, Santos SHS. The role of the angiotensin II type I receptor blocker telmisartan in the treatment of non-alcoholic fatty liver disease: a brief review. Hypertens Res. 2018;41:394–405. doi: 10.1038/s41440-018-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Santos RA, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: new players of the renin-angiotensin system. J Endocrinol. 2013;216:R1–R17. doi: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- 229.Alam S, Abrar M, Islam S, Kamal M, Hasan MJ, Khan AS, et al. Effect of telmisartan and vitamin E on liver histopathology with non-alcoholic steatohepatitis:A randomized, open-label, noninferiority trial. JGH Open. 2020;4:663–669. doi: 10.1002/jgh3.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Park JG, Mok JS, Han YI, Park TS, Kang KW, Choi CS, et al. Connectivity mapping of angiotensin-PPAR interactions involved in the amelioration of non-alcoholic steatohepatitis by Telmisartan. Sci Rep. 2019;9:4003. doi: 10.1038/s41598-019-40322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis:results of a randomized trial. Hepatology. 2004;39:770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 232.Leuschner UF, Lindenthal B, Herrmann G, Arnold JC, Rossle M, Cordes HJ, et al. High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis:a double-blind, randomized, placebo-controlled trial. Hepatology. 2010;52:472–479. doi: 10.1002/hep.23727. [DOI] [PubMed] [Google Scholar]
- 233.Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, et al. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4:1537–1543. doi: 10.1016/j.cgh.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 234.Musa-Veloso K, Venditti C, Lee HY, Darch M, Floyd S, West S, et al. Systematic review and meta-analysis of controlled intervention studies on the effectiveness of long-chain omega-3 fatty acids in patients with nonalcoholic fatty liver disease. Nutr Rev. 2018;76:581–602. doi: 10.1093/nutrit/nuy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.He XX, Wu XL, Chen RP, Chen C, Liu XG, Wu BJ, et al. Effectiveness of omega-3 polyunsaturated fatty acids in non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials. PLoS One. 2016;11:e0162368. doi: 10.1371/journal.pone.0162368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhong S, Fan Y, Yan Q, Fan X, Wu B, Han Y, et al. The therapeutic effect of silymarin in the treatment of nonalcoholic fatty disease:A meta-analysis (PRISMA) of randomized control trials. Medicine (Baltimore) 2017;96:e9061. doi: 10.1097/MD.0000000000009061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 239.Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Servaty L, et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150:1147–1159.e5. doi: 10.1053/j.gastro.2016.01.038. Erratum, Gastroenterology 2017,152:2084. [DOI] [PubMed] [Google Scholar]
- 240.Ratziu V, Sanyal A, Harrison SA, Wong VW, Francque S, Goodman Z, et al. Cenicriviroc treatment for adults with nonalcoholic steatohepatitis and fibrosis:Final analysis of the Phase 2b CENTAUR Study. Hepatology. 2020;72:892–905. doi: 10.1002/hep.31108. [DOI] [PubMed] [Google Scholar]
- 241.Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H, et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis A randomized, phase 2 trial. Hepatology. 2018;67:549–559. doi: 10.1002/hep.29514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gilead Announces Topline Data From Phase 3 STELLAR-3 Study of Selonsertib in Bridging Fibrosis (F3) Due to Nonalcoholic Steatohepatitis(NASH) Last Accessed Date: 18.04.2023. Available at: https://www.gilead.com/news-and-press/press-room/press-releases/2019/4/gilead-announces-topline-data-from-phase-3-stellar3-study-of-selonsertib-in-bridging-fibrosis-f3-due-to-nonalcoholic-steatohepatitis-nash.
- 243.Gilead Announces Topline Data From Phase 3 STELLAR-4 Study of Selonsertib in Compensated Cirrhosis (F4) Due to Nonalcoholic Steatohepatitis (NASH) Last Accessed Date: 18.04.2023. Available at: https://www.gilead.com/news-and-press/press-room/press-releases/2019/2/gilead-announces-topline-data-from-phase-3-stellar4-study-of-selonsertib-in-compensated-cirrhosis-f4-due-to-nonalcoholic-steatohepatitis-nash.
- 244.Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394:2012–2024. doi: 10.1016/S0140-6736(19)32517-6. [DOI] [PubMed] [Google Scholar]
- 245.Look A, HEAD Research Group Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring) 2014;22:5–13. doi: 10.1002/oby.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Daniel S, Soleymani T, Garvey WT. A complications-based clinical staging of obesity to guide treatment modality and intensity. Curr Opin Endocrinol Diabetes Obes. 2013;20:377–388. doi: 10.1097/01.med.0000433067.01671.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.ASGE Bariatric Endoscopy Task Force. Sullivan S, Kumar N, Edmundowicz SA, Abu Dayyeh BK, Jonnalagadda SS, Larsen M, et al. ASGE position statement on endoscopic bariatric therapies in clinical practice. Gastrointest Endosc. 2015;82:767–772. doi: 10.1016/j.gie.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 248.Türkiye Beslenme ve Sağlık Araştırması. 2010 Son Erişim Tarihi: 5.06.2020 https://hsgm.saglik.gov.tr/depo/birimler/saglikli-beslenme-hareketli-hayat-db/Yayinlar/kitaplar/diger-kitaplar/TBSA-Beslenme-Yayini.pdf. [Google Scholar]
- 249.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 250.Karlsson J, Taft C, Ryden A, Sjostrom L, Sullivan M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes (Lond) 2007;31:1248–1261. doi: 10.1038/sj.ijo.0803573. [DOI] [PubMed] [Google Scholar]
- 251.Carlsson LMS, Sjoholm K, Karlsson C, Jacobson P, Andersson-Assarsson JC, Svensson PA, et al. Long-term incidence of microvascular disease after bariatric surgery or usual care in patients with obesity, stratified by baseline glycaemic status: a post-hoc analysis of participants from the Swedish Obese Subjects study. Lancet Diabetes Endocrinol. 2017;5:271–279. doi: 10.1016/S2213-8587(17)30061-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.IFSO Son Erişim tarihi: 11.08.2020. https://www.ifso.com/are-you-a-candidate/
- 253.Luo RB, Suzuki T, Hooker JC, Covarrubias Y, Schlein A, Liu S, et al. How bariatric surgery affects liver volume and fat density in NAFLD patients. Surg Endosc. 2018;32:1675–1682. doi: 10.1007/s00464-017-5846-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yu Y, Klem ML, Kalarchian MA, Ji M, Burke LE. Predictors of weight regain after sleeve gastrectomy: an integrative review. Surg Obes Relat Dis. 2019;15:995–1005. doi: 10.1016/j.soard.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 255.Berzigotti A, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Morillas R, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54:555–561. doi: 10.1002/hep.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Shimizu H, Phuong V, Maia M, Kroh M, Chand B, Scauer PR, et al. Bariatric surgery in patients with liver cirrhosis. Surg Obes Relat Dis. 2013;9:1–6. doi: 10.1016/j.soard.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 257.Mosko JD, Nguyen GC. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:897–901. doi: 10.1016/j.cgh.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 258.Jan A, Narwaria M, Mahawar KK. A systematic review of bariatric surgery in patients with liver cirrhosis. Obes Surg. 2015;25:1518–1526. doi: 10.1007/s11695-015-1727-2. [DOI] [PubMed] [Google Scholar]
- 259.Heimbach JK, Watt KD, Poterucha JJ, Ziller NF, Cecco SD, Charlton MR, et al. Combined liver transplantation and gastric sleeve resection for patients with medically complicated obesity and end-stage liver disease. Am J Transplant. 2013;13:363–368. doi: 10.1111/j.1600-6143.2012.04318.x. [DOI] [PubMed] [Google Scholar]
- 260.Lin MY, Tavakol MM, Sarin A, Amirkiai SM, Rogers SJ, Carter JT, et al. Safety and feasibility of sleeve gastrectomy in morbidly obese patients following liver transplantation. Surg Endosc. 2013;27:81–85. doi: 10.1007/s00464-012-2410-5. [DOI] [PubMed] [Google Scholar]
- 261.Cholankeril G, Ahmed A. Alcoholic liver disease replaces hepatitis C virus infection as the leading indication for liver transplantation in the United States. Clin Gastroenterol Hepatol. 2018;16:1356–1358. doi: 10.1016/j.cgh.2017.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Martin P, DiMartini A, Feng S, Brown R, Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144–1165. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 263.Golabi P, Bush H, Stepanova M, Locklear CT, Jacobson IM, Mishra A, et al. Liver transplantation (LT) for cryptogenic cirrhosis (CC) and nonalcoholic steatohepatitis (NASH) cirrhosis: Data from the Scientific Registry of Transplant Recipients (SRTR): 1994 to 2016. Medicine (Baltimore) 2018;97:e11518. doi: 10.1097/MD.0000000000011518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Haldar D, Kern B, Hodson J, Armstrong MJ, Adam R, Berlakovich G, et al. Outcomes of liver transplantation for non-alcoholic steatohepatitis: A European Liver Transplant Registry study. J Hepatol. 2019;71:313–322. doi: 10.1016/j.jhep.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mikolasevic I, Filipec-Kanizaj T, Mijic M, Jakopcic I, Milic S, Hrstic I, et al. Nonalcoholic fatty liver disease and liver transplantation-Where do we stand? World J Gastroenterol. 2018;24:1491–1506. doi: 10.3748/wjg.v24.i14.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Dare AJ, Plank LD, Phillips AR, Gane EJ, Harrison B, Orr D, et al. Additive effect of pretransplant obesity, diabetes, and cardiovascular risk factors on outcomes after liver transplantation. Liver Transpl. 2014;20:281–290. doi: 10.1002/lt.23818. [DOI] [PubMed] [Google Scholar]
- 267.Cotter TG, Charlton M. Nonalcoholic steatohepatitis after liver transplantation. Liver Transpl. 2020;26:141–159. doi: 10.1002/lt.25657. [DOI] [PubMed] [Google Scholar]
- 268.Germani G, Laryea M, Rubbia-Brandt L, Egawa H, Burra P, O’Grady J, et al. Management of recurrent and de novo NAFLD/NASH after liver transplantation. Transplantation. 2019;103:57–67. doi: 10.1097/TP.0000000000002485. [DOI] [PubMed] [Google Scholar]
- 269.Vallin M, Guillaud O, Boillot O, Hervieu V, Scoazec JY, Dumortier J. Recurrent or de novo nonalcoholic fatty liver disease after liver transplantation:natural history based on liver biopsy analysis. Liver Transpl. 2014;20:1064–1071. doi: 10.1002/lt.23936. [DOI] [PubMed] [Google Scholar]
- 270.Galvin Z, Rajakumar R, Chen E, Adeyi O, Selzner M, Grant D, et al. Predictors of de novo nonalcoholic fatty liver disease after liver transplantationand associated fibrosis. Liver Transpl. 2019;25:56–67. doi: 10.1002/lt.25338. [DOI] [PubMed] [Google Scholar]
- 271.Younossi ZM, Loomba R, Rinella ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, et al. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2018;68:361–371. doi: 10.1002/hep.29724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Suraweera D, Saab EG, Choi G, Saab S. Bariatric surgery and liver transplantation. Gastroenterol Hepatol (NY) 2017;13:170–175. [PMC free article] [PubMed] [Google Scholar]
- 273.Diwan TS, Rice TC, Heimbach JK, Schauer DP. Liver transplantation and bariatric surgery: Timing and outcomes. Liver Transpl. 2018;24:1280–1287. doi: 10.1002/lt.25303. [DOI] [PubMed] [Google Scholar]
- 274.Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) J Pediatr Gastroenterol Nutr. 2017;64:319–334. doi: 10.1097/MPG.0000000000001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Mann JP, Valenti L, Scorletti E, Byrne CD, Nobili V. Nonalcoholic fatty liver disease in children. Semin Liver Dis. 2018;38:1–13. doi: 10.1055/s-0038-1627456. [DOI] [PubMed] [Google Scholar]
- 276.Vajro P, Lenta S, Socha P, Dhawan A, McKiernan P, Bauman U, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2012;54:700–713. doi: 10.1097/MPG.0b013e318252a13f. [DOI] [PubMed] [Google Scholar]
- 277.Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–649. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 278.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 279.A-Kader HH, Henderson J, Vanhoesen K, Ghishan F, Bhattacharyya A. Nonalcoholic fatty liver disease in children: a single center experience. Clin Gastroenterol Hepatol. 2008;6:799–802. doi: 10.1016/j.cgh.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 280.Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents:A systematic review and meta-analysis. PLoS One. 2015;10:e0140908. doi: 10.1371/journal.pone.0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Yu EL, Golshan S, Harlow KE, Angeles JE, Durelle J, Goyal NP, et al. Prevalence of nonalcoholic fatty liver disease in children with obesity. J Pediatr. 2019;207:64–70. doi: 10.1016/j.jpeds.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Braun HA, Faasse SA, Vos MB. Advances in pediatric fatty liver disease: pathogenesis, diagnosis, and treatment. Gastroenterol Clin North Am. 2018;47:949–968. doi: 10.1016/j.gtc.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 283.Barrera F, George J. The role of diet and nutritional intervention for the management of patients with NAFLD. Clin Liver Dis. 2014;18:91–112. doi: 10.1016/j.cld.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 284.Chiu S, Sievenpiper JL, de Souza RJ, Cozma AI, Mirrahimi A, Carleton AJ, et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD):a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr. 2014;68:416–423. doi: 10.1038/ejcn.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Rehm JL, Connor EL, Wolfgram PM, Eickhoff JC, Reeder SB, Allen DB. Predicting hepatic steatosis in a racially and ethnically diverse cohort of adolescent girls. J Pediatr. 2014;165:319–325.e1. doi: 10.1016/j.jpeds.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Wiegand S, Keller KM, Robl M, L’Allemand D, Reinehr T, Widhalm K, et al. APV-Study Group and the German Competence Network Adipositas Obese boys at increased risk for nonalcoholic liver disease: evaluation of 16,390 overweight or obese children and adolescents. Int J Obes (Lond) 2010;34:1468–1474. doi: 10.1038/ijo.2010.106. [DOI] [PubMed] [Google Scholar]
- 287.Malespin M, Sleesman B, Lau A, Wong SS, Cotler SJ. Prevalence and correlates of suspected nonalcoholic fatty liver disease in Chinese American children. J Clin Gastroenterol. 2015;49:345–349. doi: 10.1097/MCG.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 288.Kong L, Lu Y, Zhang S, Nan Y, Qiao L. Role of nutrition, gene polymorphism, and gut microbiota in non-alcoholic fatty liver disease. Discov Med. 2017;24:95–106. [PubMed] [Google Scholar]
- 289.Brumbaugh DE, Tearse P, Cree-Green M, Fenton LZ, Brown M, Scherzinger A, et al. Intrahepatic fat is increased in the neonatal offspring of obese women with gestational diabetes. J Pediatr. 2013;162:930–936.e1. doi: 10.1016/j.jpeds.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Brumbaugh DE, Friedman JE. Developmental origins of nonalcoholic fatty liver disease. Pediatr Res. 2014;75:140–147. doi: 10.1038/pr.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 292.Cioffi CE, Welsh JA, Cleeton RL, Caltharp SA, Romero R, Wulkan ML, et al. Natural history of NAFLD diagnosed in childhood: A single-center study. Children (Basel) 2017;4:34. doi: 10.3390/children4050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009;58:1538–1544. doi: 10.1136/gut.2008.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Xanthakos S, Lavine JE, Yates KP, Schwimmer JB, Molleston JP, Rosenthal P, et al. Natural history of nonalcoholic fatty liver disease (NAFLD) in children receiving standard lifestyle counseling and placebo in NASH Clinical Research Network (CRN) trials. Hepatology. 2017;66:31A–32A. [Google Scholar]
- 295.Schwimmer JB, Celedon MA, Lavine JE, Salem R, Campbell N, Schork NJ, et al. Heritability of nonalcoholic fatty liver disease. Gastroenterology. 2009;136:1585–1592. doi: 10.1053/j.gastro.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Molleston JP, Schwimmer JB, Yates KP, Murray KF, Cummings OW,Lavine JE, et al. Histological abnormalities in children with nonalcoholic fatty liver disease and normal or mildly elevated alanine aminotransferase levels. J Pediatr. 2014;164:707–3.e3. doi: 10.1016/j.jpeds.2013.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Schwimmer JB, Newton KP, Awai HI, Choi LJ, Garcia MA, Ellis LL, et al. Paediatric gastroenterology evaluation of overweight and obese children referred from primary care for suspected non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;38:1267–1277. doi: 10.1111/apt.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Awai HI, Newton KP, Sirlin CB, Behling C, Schwimmer JB. Evidence and recommendations for imaging liver fat in children, based on systematic review. Clin Gastroenterol Hepatol. 2014;12:765–773. doi: 10.1016/j.cgh.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Nobili V, Alisi A, Vania A, Tiribelli C, Pietrobattista A, Bedogni G. The pediatric NAFLD fibrosis index: a predictor of liver fibrosis in children with non-alcoholic fatty liver disease. BMC Med. 2009;7:21. doi: 10.1186/1741-7015-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Yang HR, Kim HR, Kim MJ, Ko JS, Seo JK. Noninvasive parameters and hepatic fibrosis scores in children with nonalcoholic fatty liver disease. World J Gastroenterol. 2012;18:1525–1530. doi: 10.3748/wjg.v18.i13.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Alkhouri N, Carter-Kent C, Lopez R, Rosenberg WM, Pinzani M, Bedogni G, et al. A combination of the pediatric NAFLD fibrosis index and enhanced liver fibrosis test identifies children with fibrosis. Clin Gastroenterol Hepatol. 2011;9:150–155. doi: 10.1016/j.cgh.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 302.Alkhouri N, Mansoor S, Giammaria P, Liccardo D, Lopez R, Nobili V. The development of the pediatric NAFLD fibrosis score (PNFS) to predict the presence of advanced fibrosis in children with nonalcoholic fatty liver disease. PLoS One. 2014;9:e104558. doi: 10.1371/journal.pone.0104558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Tutar E, Akkelle B, Kesici CB, Yılmaz Y, Celikel CA, et al. The correlation between histopathologic steatosis/fibrosis and various non-invasive imaging and blood fibrosis indicators in children with NAFLD. J Pediatr Gastroenterol Nutr. 2018;66(Suppl 2):646A. [Google Scholar]
- 304.Mansoor S, Collyer E, Alkhouri N. A comprehensive review of noninvasive liver fibrosis tests in pediatric nonalcoholic Fatty liver disease. Curr Gastroenterol Rep. 2015;17:23. doi: 10.1007/s11894-015-0447-z. [DOI] [PubMed] [Google Scholar]
- 305.Shah J, Okubote T, Alkhouri N. Overview of updated practice guidelines for pediatric nonalcoholic fatty liver disease. Gastroenterol Hepatol (N Y) 2018;14:407–414. [PMC free article] [PubMed] [Google Scholar]
- 306.Kleiner DE, Makhlouf HR. Histology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in adults and children. Clin Liver Dis. 2016;20:293–312. doi: 10.1016/j.cld.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Dezsofi A, Baumann U, Dhawan A, Durmaz O, Fischler B, Hadzig N, et al. Liver biopsy in children: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2015;60:408–420. doi: 10.1097/MPG.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 308.Crespo M, Lappe S, Feldstein AE, Alkhouri N. Similarities and differences between pediatric and adult nonalcoholic fatty liver disease. Metabolism. 2016;65:1161–1171. doi: 10.1016/j.metabol.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 309.DeVore S, Kohli R, Lake K, Nicholas L, Dietrich K, Balistreri WF, et al. A multidisciplinary clinical program is effective in stabilizing BMI and reducing transaminase levels in pediatric patients with NAFLD. J Pediatr Gastroenterol Nutr. 2013;57:119–123. doi: 10.1097/MPG.0b013e318290d138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 311.Nobili V, Alkhouri N, Bartuli A, Manco M, Lopez R, Alisi A, et al. Severity of liver injury and atherogenic lipid profile in children with nonalcoholic fatty liver disease. Pediatr Res. 2010;67:665–670. doi: 10.1203/PDR.0b013e3181da4798. [DOI] [PubMed] [Google Scholar]
- 312.Pacifico L, Anania C, Martino F, Cantisani V, Pascone R, Marcantonio A, et al. Functional and morphological vascular changes in pediatric nonalcoholic fatty liver disease. Hepatology. 2010;52:1643–1651. doi: 10.1002/hep.23890. [DOI] [PubMed] [Google Scholar]
- 313.Gokce S, Atbinici Z, Aycan Z, Cinar HG, Zorlu P. The relationship between pediatric nonalcoholic fatty liver disease and cardiovascular risk factors and increased risk of atherosclerosis in obese children. Pediatr Cardiol. 2013;34:308–315. doi: 10.1007/s00246-012-0447-9. [DOI] [PubMed] [Google Scholar]
- 314.Schwimmer JB, Zepeda A, Newton KP, Xanthakos SA, Behling C,Hallinan EK, et al. Longitudinal assessment of high blood pressure in children with nonalcoholic fatty liver disease. PLoS One. 2014;9:e112569. doi: 10.1371/journal.pone.0112569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Barlow SE, Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 316.Newton KP, Hou J, Crimmins NA, Lavine JE, Barlow SE, Xanthakos SA, et al. Nonalcoholic Steatohepatitis Clinical Research Network Prevalence of prediabetes and Type 2 Diabetes in children with nonalcoholic fatty liver disease. JAMA Pediatr. 2016;170:e161971. doi: 10.1001/jamapediatrics.2016.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Cali AM, De Oliveira AM, Kim H, Chen S, Reyes-Mugica M, Escalera S, et al. Glucose dysregulation and hepatic steatosis in obese adolescents: is there a link? Hepatology. 2009;49:1896–1903. doi: 10.1002/hep.22858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Kim G, Giannini C, Pierpont B, Feldstein AE, Santoro N, Kursawe R, et al. Longitudinal effects of MRI-measured hepatic steatosis on biomarkers of glucose homeostasis and hepatic apoptosis in obese youth. Diabetes Care. 2013;36:130–136. doi: 10.2337/dc12-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Allen AM, Van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare cost and utilization in nonalcoholic fatty liver disease: real-world data from a large US claims database. Hepatology. 2018;68:2230–2238. doi: 10.1002/hep.30094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Yonuossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 321.EASL (European Association for the Study of the Liver) High cost of advanced nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH) revealed but screening high-risk patients may not be cost effective EASL. 2019. Last Accessed Date: 18.04.2023. Available at: https://2019.ilc-congress.eu/press-release/high-cost-of-advanced-non-alcoholic-fatty-liver-disease-non-alcoholic-steatohepatitis-nafld-nash-revealed-but-screening-high-risk-patients-may-not-be-cost-effective/
- 322.WHO “COVID-19 significantly impacts health services for noncommunicable diseases” from COVID-19 significantly impacts health services for noncommunicable diseases. Last Accessed Date: 18.04.2023. Available at: https://www.who.int/news/item/01-06-2020-covid-19-significantly-impacts-health-services-for-noncommunicable-diseases.


