Abstract
There is increasing evidence showing that microglia play a critical role in mediating synapse formation and spine growth, although the molecular mechanism remains elusive. Here, we demonstrate that the secreted morphogen WNT family member 5A (WNT5A) is the most abundant WNT expressed in microglia and that it promotes neuronal maturation. Co-culture of microglia with Thy1-YFP+ differentiated neurons significantly increased neuronal spine density and reduced dendritic spine turnover rate, which was diminished by silencing microglial Wnt5a in vitro. Co-cultured microglia increased post-synaptic marker PSD95 and synaptic density as determined by the co-localization of PSD95 with pre-synaptic marker VGLUT2 in vitro. The silencing of Wnt5a expression in microglia partially reduced both PSD95 and synaptic densities. Co-culture of differentiated neurons with microglia significantly enhanced neuronal firing rate as measured by multiple electrode array, which was significantly reduced by silencing microglial Wnt5a at 23 days differentiation in vitro. These findings demonstrate that microglia can mediate spine maturation and regulate neuronal excitability via WNT5A secretion indicating possible pathological roles of dysfunctional microglia in developmental disorders.
Keywords: Microglia, Neural development, Synaptic transmission, WNT, WNT5A
1. Introduction
During development, microglia emerge as key producers of cytokine and immune-related molecules in the brain and are involved in neurogenesis, synaptic maturation, and organization of neuronal circuits (Bilbo and Stevens, 2017; Kracht et al., 2020). meta-analysis studies reveal an increased prevalence of neurodevelopmental disorders, including autism spectrum disorders (ASD) and schizophrenia, in the offspring of pregnancies that coincided with infections such as rubella, influenza, measles, mumps, and polio, suggesting a strong link between maternal inflammation and risk of neurodevelopmental disorders in offspring (Minakova and Warner, 2018; Jiang et al., 2016; Han et al., 2021a). Additional studies also show that maternal inflammatory status, including gestational diabetes, maternal diet, maternal depression, chronic exposure to air pollution, and maternal autoimmune disorders, are also associated with an increased risk of neurodevelopmental disorders in offspring (Han et al., 2021b). Alterations in cytokine or chemokine levels in the fetal brain due to maternal inflammation or other environmental factors can disrupt synaptic function and perturb refinement of neural connectivity (Estes and McAllister, 2016). Recent studies suggest microglia can contribute to the pathogenesis of neurodevelopmental disorders through aberrant synaptic pruning and dysregulated secretion of inflammatory molecules (Han et al., 2021a). Postmortem human brain studies revealed that individuals with ASD have upregulation of genes involved in microglia-related and astrocyte-related inflammation and increased density of reactive microglia (Voineagu, 2011; Vargas et al., 2005; Morgan, 2010). Imaging studies show increased inflammation marker translocator protein in ASD brains, suggesting glial activation in the brain (Kumar et al., 2015; Suzuki et al., 2013) The underlying molecular mechanism of microglial regulation of neuronal development remains understudied (Yeh and Ikezu, 2019).
We have recently shown that immune perturbation during early pregnancy alters microglial phenotype in offspring of maternal immune activation (MIA) mouse model (Ikezu et al., 2020). Transcriptomic analysis of adult microglia from offspring of dams injected with poly (I: C) at embryonic day 9.5 to induce MIA showed upregulation of neuritogenic factors, including WNT family member 5A (WNT5A), along with enhanced growth of filopodia subgroup of dendritic spines (Ikezu et al., 2020), suggesting that microglia may contribute to altered neural network during development leading to social deficits in neurodevelopmental disorders (Ikezu et al., 2020; Kalavai and Ikezu, 2021).
Disruption of the canonical WNT pathway leads to altered neuronal migration, disturbed circuit development, and behavioral deficits, demonstrating that WNT molecules play critical roles in neuronal development (Bocchi et al., 2017). WNT signaling can be classified into canonical and noncanonical pathways whose activation depends on cellular and molecular composition and specificity (Kikuchi et al., 2012; Oliva et al., 2013). Most of the WNT family, including WNT1, WNT3A, WNT7A/B, and WNT8, mediate the canonical WNT/β-catenin pathway, whereas WNT4, WNT5A, WNT9B, and WNT11 mediate the noncanonical WNT/Ca2+ or WNT/planar cell polarity pathway (PCP)/ c-Jun N-terminal kinase (JNK) pathways independent of β-catenin (Oliva et al., 2013). In addition, activation of noncanonical WNT-Receptor like Tyrosine Kinase (RYK) pathway can lead to suppression of neurite and axonal growth, highlighting the complexity of WNT signaling in neurodevelopment (Lanoue et al., 2017).
WNT5A, a secreted glycoprotein and neurotrophic factor, acts primarily through a noncanonical WNT pathway (Mikels and Nusse, 2006). WNT5A binds Receptor Tyrosine Kinase Like Orphan Receptor 2 (ROR2) and activates the JNK and Transforming Growth Factor (TGF)-β signaling pathways (Bian et al, 2015; Nye et al., 2020; Shao et al., 2016; Calandria et al., 2022). Many studies have shown that WNT5A supports neurodevelopment by promoting neurite outgrowth and synapse formation in neurons (Halleskog et al., 2011; Chen et al., 2017). In hippocampal neurons, WNT5A stimulates calcium and cytoskeletal-mediated signaling pathways for transcription of the NMDA receptor subunit GluN1, suggesting that WNT5A acts primarily through post-synaptic mechanisms (Halleskog et al., 2011; Chen et al., 2017). WNT5A can enhance neural stem cell differentiation through the JNK signaling pathway, specifically via inhibiting activation of the RhoA/Rock signaling pathway (Garcia et al., 2018; Li et al., 2020). In addition, WNT5A can induce neurogenesis via Ca2+/calmodulin-dependent protein kinase II (CaMKII) signalling (Li et al., 2020; Arredondo et al., 2020; Park et al., 2018). WNT5A activation can increase neurite branching complexity in hippocampal neurons and stimulate PSD95 or GABAA receptor insertion on neuronal surface membranes CaMKII pathway (Arredondo et al., 2020; Cuitino et al., 2010; Paina et al., 2011; Ramírez et al., 2016). Several studies have shown that WNT5A acts primarily on post-synaptic sites of differentiated hippocampal neurons in vitro and in vivo, increasing spine density and clustering of PSD95 in dendritic spines through the WNT/JNK signaling pathway (Chen et al., 2017; Varela-Nallar et al., 2010; Farías et al., 2009) and formation of filamentous actin (F-actin) in dendritic spines through activation of Rho GTPases/cofilin axis (Vallejo et al., 2021). Indeed, WNT5A can increase spine head size and support synapse formation via stabilization of dendritic spines (Ramos-Fernández et al., 2021).
However, the extent of microglial WNT5A’s contribution to neurodevelopmental processes remains elusive. We hypothesize that microglial WNT5A can regulate dendritic spine development and maintenance and alter neural circuitry. Therefore, dysregulation of microglial WNT5A expression may lead to abnormal neuronal activity. Here, we show that WNT5A is expressed in microglia and can be silenced by siRNA transfection. Silencing microglial WNT5A alters dendritic spine maintenance and neuronal firing, indicating that microglial secretome can mediate neuronal development and maturation.
2. Methods
2.1. Primary cell culture of microglia and neural stem cell
2.1.1. Primary microglial culture:
Primary murine microglia were isolated from P0/1CD-1 pups (Charles River Laboratory) using CD11b+ magnetic beads (MicroBeads, Miltenyi) according to the manufacturer’s protocol as previously described (Ikezu et al., 2020) and cultured overnight in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with murine 20 ng/ml recombinant murine macrophage-colony stimulating factor (M–CSF, R&D System) and conditioned media supernatant in Neurobasal media were collected the following day. Microglia lysates were digested with QIAzol (Qiagen), and RNA was isolated using the miRNeasy Mini Kit (Qiagen).
2.2. Primary neural stem cell culture
Primary mouse neural stem cells (NSCs) were isolated from fetal brains isolated from E13.5 to E14 pregnant CD-1 mice (Charles River Laboratory). The cortical and hippocampal tissues were isolated following removal of the cerebellum and brainstem and placed in cold PBS with 2 % glucose on ice. Meninges were removed and discarded. Pooled brain tissue was resuspended in proliferation media [DMEM/F12 (Invitrogen) supplemented with 1 % penicillin/streptomycin, 2 % B27 supplement (Invitrogen), 20 ng/ml recombinant human EGF (Invitrogen) and 20 ng/ml recombinant human FGF2 (eBioscience), or NeuroCult medium with proliferation supplements (STEMCELL Technologies)] and triturated to obtain a single cell suspension. Cells were centrifuged for 5 min at 150 × g and resuspended in proliferation media. The cell suspension was filtered and seeded at least 12 × 106 cells in proliferation media in a T175 flask and incubated at 37 °C in 5 % CO2. Neurospheres were dissociated when they reached a diameter of ~ 100 μm (every 3–4 days) and re-plated at a density of 1.25 × 105 cells in 8–10 mL per T25 flask. Neurospheres were used until seven passages, at which point cell viability and proliferation rate began to decrease. For microglial conditioned media microarray analysis, neural stem cells were plated for 24 h with conditioned media from primary microglia culture or control neurobasal media. Neural stem cells were collected using Qiazol lysis reagent for RNA extraction and microarray profiling.
2.3. Primary Thy1-YFP + neuronal culture
Embryonic primary mouse cortical neurons were cultured from E16.5 pregnant Thy1-YFP mice (Feng et al., 2000) (Stock# 003782, Jackson Laboratory) as previously described (You et al., 2020). Embryonic cortices were dissected in Hibernate-E medium (Gibco Invitrogen) on ice, minced into 1 mm3 pieces, and dissociated into single cells using 0.25 % trypsin-EDTA solution (Gibco Invitrogen) for 15 min at 37 °C and gentle pipetting. The neurons were plated onto poly-d-lysine coated 24-well glass-bottom plates (CellVis) and 4 × 4 microelectrode array (MEA) 24-well plate (Axion Biosystems) with a neuronal density of 2.5 × 105 cells/well using 20 % FBS in DMEM (Gibco Invitrogen). The culture medium was replaced with Complete Neurobasal medium (Neurobasal, 2 % B-27, 2 mM Glutamax, Gibco Invitrogen). 50 % of media was changed every 3–4 days.
2.4. Genome-wide microarray profiling of microglia and neural stem cells
For neural stem cell gene expression profiling, total mRNA was extracted using miRNeasy Kit (Qiagen). Microarray kits include the Affymetrix Mouse Gene 1.0 ST Array (Affymetrix) for microglia expression profiling and Affymetrix Mouse Genome 430 2.0 ST array (Affymetrix) for neural stem cell profiling. Microarray data were generated and analyzed by Boston University School of Medicine Microarray Core facility. Neural stem cell gene expression data was analyzed using moderated t-test. Ingenuity pathway analysis (Qiagen) or Panther 16.0 https://pantherdb.org were used to identify biological pathways enriched by microglia-conditioned media. IPA core analysis based on log2 fold change for neural stem cells with microglial conditioned media group versus control NSCs group. Statistical analysis on microarray data was analyzed using moderated t-test in the case of pairwise comparisons with support from Boston University School of Medicine’s Microarray Core facility,
2.5. siRNA silencing of Wnt5a in microglia and qPCR validation
Accell SMARTPool small interfering RNA (siRNA) was purchased from Horizon Discovery. Lyophilized siRNA was reconstituted in RNAse-free 1x siRNA buffer at a stock concentration of 100uM according to provided manufacturer protocol. Product codes of siRNAs with target transcripts are E-065584–00-0005 (mouse Wnt5a) and K-005000-G1-02 (mouse Control siRNA). siRNA was resuspended in Accell Delivery Media (Horizon Discovery) supplemented with M–CSF 20 ng/mL to a final concentration of 1 μM Accell siRNA to 50,000 murine microglial cells.
Following isolation of microglia from CD-1 E17.5 embryos using CD11B MicroBeads (Miltenyi Biotec) according to the manufacturer’s protocol and previously described (Ikezu et al., 2020), microglial cells were plated in 48-well plates with DMEM supplemented with 10 % FBS and 25 ng/mL M–CSF (R&D Systems). At days in vitro (DIV) 2, cells were incubated with Accell siRNA for additional 3 days and dissociated with 25 % Accutase (STEMCELL Technologies) in PBS for quantitative polymerase chain reaction (qPCR) validation and co-culture with neurons at DIV14.
2.6. Incucyte live imaging for phagocytosis assay
Primary murine microglia cells were plated in a 48-well plate at 20,000 cells/well for phagocytosis assay. At DIV2, media was replaced with Accell siRNA Delivery Media containing 1 μM Accell siRNA (Horizon Discovery) supplemented with 20 ng/mL M–CSF (R&D Systems) for three days. Media was changed to 2 % FBS in DMEM supplemented with 20 ng/mL M–CSF (R&D Systems) at DIV5 to remove siRNA and delivery media. Before imaging, pHrodo-RED E Coli bioparticles (Sartorius) were immediately added to a final concentration of 10 μg/mL (Kapellos et al., 2016). Both phase and orange channels were captured for microglial confluence and fluorescence signal of internalized red-pHrodo bioparticles, respectively. Images were captured by 20x objective (IncuCyte S3, Sartorius) every 15 min for the first 3 h, then at 1-h intervals for a total of 72 h. Images were analyzed on IncuCyte software for the total fluorescent signal of pHrodo-RED signal per well and normalized to starting time at 0 h.
2.7. Live imaging and analysis at DIV17 and DIV22
At DIV17 and DIV22, live imaging was captured by confocal microscopy with an incubation system at 37 °C and 5 % CO2 (Leica SP8 with Resonance Imaging) using 40x water objective at a resolution of 1024 × 1024 pixels. Z-stack of neuronal dendrite and spine images were taken over 15 imaging sessions at 1 min intervals (z step = 0.4 μm, 8–10 μm) with adapted focus to maintain z-stacks over time. Images were processed and deconvoluted using Leica Lightning. Dendrite properties were quantified using IMARIS (Bitplane). Spine density was counted from a total dendritic length of ~ 50 μm using ImageJ software (n = 2–5 neurons per group, 3 embryonic pups per litter) at the beginning and end of the imaging period. The protrusion rates gained and lost were determined, respectively, as the fraction of spines that appeared and disappeared between two successive frames relative to the total spine number, as described previously (Cruz-Martín et al., 2010). The dendritic spine turnover rate was calculated as the total number of spines lost and gained divided by twice the total protrusion number (Cruz-Martín et al., 2010).
2.8. Immunocytochemistry
At DIV23, following the completion of live imaging, cells were fixed with 4 % paraformaldehyde for 15 min at room temperature. Cells were then washed with PBS and permeabilized by 0.1 % Triton-X in PBS. Following permeabilization, samples were blocked for 1 h using a blocking buffer of 5 % normal donkey serum in PBS. Primary antibodies Goat anti-PSD95 (1:500, Abcam), and Guinea pig anti-VGLUT2 (1:500, Synaptic Systems) were incubated overnight in staining buffer 5 % normal donkey serum + 0.1 % Triton-X 100 + 0.2 % BSA at 4 °C. The following day, cells were washed with PBS and incubated with secondary antibodies at 1:1000 dilution (Alexa 546 donkey-goat Invitrogen, 647-donkey guinea pig).
2.9. Imaging and analysis
Confocal imaging of fixed cells was captured by confocal microscopy (Leica SP8) using a 40x water objective at a resolution of 1024 × 1024, z-step = 0.3 μm. All images were deconvoluted and processed using Leica Lightning to enhance resolution and clarity. Following image processing, image analysis was completed by IMARIS (Bitplane) for unbiased and automated quantification of synaptic puncta VGLUT2 and PSD95, and the co-localization using the volume function. The threshold for co-localization was 0 μm distance between VGLUT2 and PSD95.
2.10. Multi-electrode array (MEA) recordings
Following 5 min equilibration, MEA recordings began after the MEA plates (16 electrodes per well, 24-well plate) were placed in the Maestro MEA system (Axion Biosystems) at 37 °C and 5 % CO2. Spontaneous extracellular action potential firing rate was recorded over 15 min using AxIS Navigator (Axion Biosystems) with 200–3000 Hz cut-off frequencies. Spontaneous neuronal action potential spikes were detected using an adaptive threshold set to 3 times the standard deviation. Neuronal bursts were automatically calculated using Inter-Spike Interval (ISI) threshold algorithm. Network bursts were computed using an Envelope algorithm with the default threshold. The mean firing rate was calculated based on the total number of action potential spike events over 15 min recording period and was analyzed using Neural Metrics Tool (Axion Biosystems) as previously described (You et al., 2020).
2.11. Statistical analysis
Data were analyzed using Student’s t-test for single means comparison. In the case of multiple mean comparisons, data were analyzed by one-way analysis of variance (ANOVA) and Tukey’s post hoc or otherwise noted using Prism 6.0 or 8.0 (GraphPad). Two-way ANOVA with Sidak’s or Tukey’s was performed for time × treatments comparison. P-values < 0.05 were regarded as a significant difference. Microarray data were analyzed with the aid of Boston University School of Medicine’s Microarray Core facility, using a Moderated t-test for pairwise comparisons.
3. Results
3.1. Microglia secretome can accelerate neuronal maturation
Microglia can communicate with neurons by secretory molecules throughout development. We performed genome-wide microarray analysis in murine embryonic neural stem cells (NSC) stimulated with control or microglia-conditioned media to elucidate the molecular mechanisms of neural stem cell differentiation influenced by microglial secretome. Gene Ontology pathway analyses were performed to determine the effects of microglial secretory molecules on neurodevelopment (Fig. 1a). The enriched genes by microglial conditioned media are associated with neuropeptide signaling pathways, interferon-γ (IFNγ) production, adenylate cyclase-modulated G-protein coupled receptor signaling pathway, and immune-related response pathways (Fig. 1b, top red). Downregulated genes by microglial conditioned media are associated with pathways involved in translational initiation, telomere maintenance, and mitotic/anaphase transition (Fig. 1b, bottom blue). Abnormal neuropeptide signaling, IFNγ production, and translational initiation pathways have been implicated in the pathophysiology of neurodevelopmental disorders, suggesting microglia can alter multiple neuronal signaling pathways through the secretion of molecules (Angelidou et al., 2010; Nelson, 2001; Kathuria et al., 2022; Chen et al., 2019). We identified top canonical pathways influenced by microglia-secreted factors, which include neural differentiation, axonal guidance, and stem cell pluripotency using Ingenuity Pathway Analysis (IPA) (Table 1). These pathway analysis results show that the microglia secretome can directly impact neural stem cell differentiation and physiology.
Fig. 1.

Neural stem cells treated with microglia-conditioned media show enrichment of genes associated with neuronal maturation a) Microarray of neural stem cells (NSCs) following 24 h treatment with control PBS or microglial conditioned media (MG-CM). b) Gene ontology analysis of 196 genes increased (top) and 208 decreased (bottom) by microglia-conditioned media treatment. (Genes with p < 0.05, FDR < 0.05, and fold change greater than/equal to 2 or less than/equal to −2). c) Relative expression fold change of Wnt pathway genes in MG-CM NSCs compared to control NSCs. Several genes are involved in both canonical and noncanonical pathways. Black represents the general Wnt pathway. Red represents the noncanonical Wnt-Ca2+ signaling pathway. Teal represents the noncanonical Wnt/planar cell polarity (PCP) signaling pathway. Navy blue represents the canonical Wnt/β-catenin pathway. Purple represents WNT antagonists, whereas light blue represents WNT agonist Ndp. For a-c, n = 3 replicates per group.
Table 1.
Top canonical pathways enriched in neural stem cells with microglial conditioned media as determined by Ingenuity Pathway Analysis.
| Ingenuity Canonical Pathways | −log (p-value) |
|---|---|
|
| |
| G-Protein Coupled Receptor Signaling | 10.70 |
| Axonal Guidance Signaling | 8.31 |
| Role of NANOG in Mammalian Embryonic Stem Cell Pluripotency | 6.43 |
| Nicotinate and Nicotinamide Metabolism | 6.02 |
| Inositol Phosphate Metabolism | 5.82 |
| Mouse Embryonic Stem Cell Pluripotency | 5.60 |
| Signaling by Rho Family GTPases | 5.36 |
| cAMP-mediated signaling | 5.19 |
| Wnt/β-catenin Signaling | 4.99 |
| NGF Signaling | 4.93 |
3.2. Microglia express WNT pathway genes, including Wnt5a
Wnt signaling was highly represented in neural stem cells by the Wnt/β-catenin pathway, axonal guidance signaling, and stem cell pluripotency (Table1). These results suggest that microglial secretome promoted neural stem cell differentiation. Furthermore, Ror1, Ror2, and several Wnt interacting Frizzled receptors were upregulated in NSCs with microglia-conditioned media relative to control media (Fig. 1c). In contrast, several putative Wnt intracellular signaling components in this pathway were down-regulated (Fig. 1c). We show the expression levels across all WNTs secreted from mouse microglia using microarray analysis (Fig. S1a). Wnt5a was the most abundantly expressed Wnt gene in microglia in vitro, followed by Wnt7a. We also show that WNT5A was more abundantly expressed in microglia than in astrocytes and oligodendrocytes (Fig. S1b–c). Previously, Halleskog et al. showed astrocytes to express higher levels of WNT5A (Halleskog et al., 2011). This inconsistency may be due to the age of cells since microglial WNT5A expression peaks during the embryonic stage and reduces with aging (Bennett et al., 2016). Expression of WNT5A peaks at the embryonic and early neonatal stage in mouse cerebellum (Zhang et al., 2014). On the other hand, human microglia had the highest expression of WNT5A, suggesting microglia may play a more significant role in WNT5A signaling in the human brain (Figure S1d–f) (Bennett et al., 2016; Zhang et al., 2014; Zhang et al., 2016). Wnt5a expression level is higher in immature microglia compared to adult microglia, suggesting microglial WNT5A involvement in mediating early neurodevelopment.
3.3. Silencing of microglial Wnt5a reduces a subset of microglia markers and Tnfa and increases Il1b expression
To determine if microglial WNT5A deletion can affect microglial phenotype or cytokine production, we reduced Wnt5a expression in microglia through siRNA transfection and performed qRT-PCR for microglial signature genes and cytokines (Fig. 2a). qRT-PCR was performed to confirm the knockdown of Wnt5a, and we found a 67 % reduction of Wnt5a mRNA expression in microglia (Fig. 2b). siRNA-mediated silencing of Wnt5a led to a reduction of complement-mediated phagocytic gene C1qa expression (Fig. 2c), consistent with previous findings of complement regulation by Wnt pathways in macrophages (Maiti et al., 2012). There were no significant changes in other microglial genes, such as the homeostatic gene P2ry12 or integrin gene Itgam (Fig. 2c). WNT5A is also known to activate microglia by increasing inflammation-related gene expression, including IL-1β, IL-6, and TNF-α, and is associated with activating immune cells in response to neural cell injury (Halleskog et al., 2011). Here we showed that silencing Wnt5a in microglia significantly enhanced Il1b expression and reduced Tnfa but did not change the expression level of other cytokines, including Il6 or Il10, suggesting that microglial WNT5A can modulate the immune response in a molecular-specific manner (Fig. 2d). The increase in Il1b in microglia may be a compensatory immune response to the suppression of WNT5A since IL-1β can increase WNT5A expression in neurons (Park et al., 2018). Changes in C1qa and Clec7a by silencing microglial Wnt5a suggest a possible alteration in phagocytosis function (Fig. 2c–d). However, phagocytosis assay using E. Coli pHrodo bioparticles showed that microglial Wnt5a-deficiency had no significant impact on their phagocytic function in vitro (Fig. 2e–f). Despite changes observed by qRT-PCR, microglial WNT5A is likely not the primary regulator of phagocytosis.
Fig. 2.
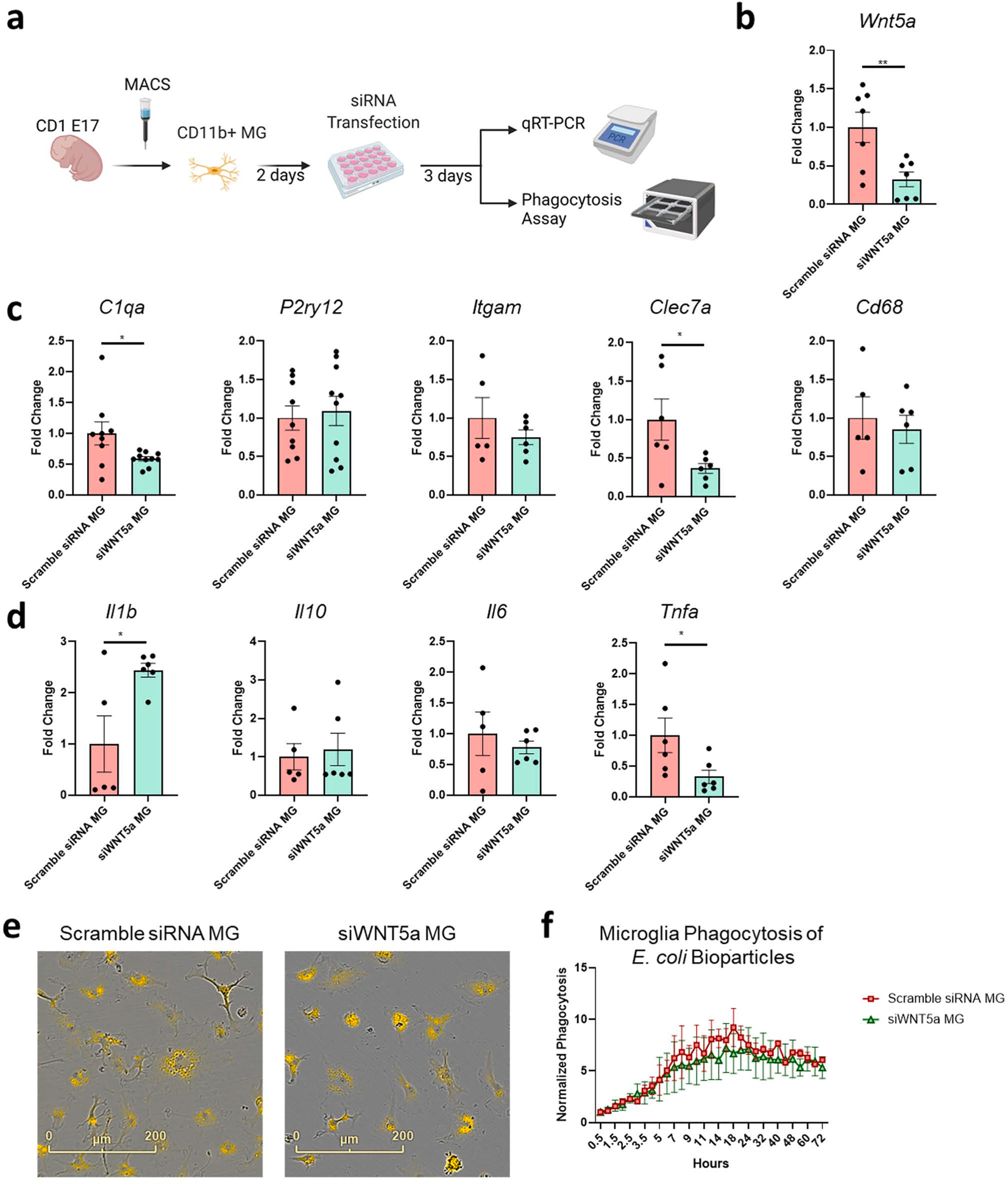
Silencing of microglial WNT5A reduced C1qa and increased IL1β but had no effect on phagocytosis of E. coli bioparticles a) Experimental scheme of primary microglia culture and siRNA transfection treatment to silence Wnt5a. Primary mouse microglia were isolated from E17 CD-1 embryonic brains and cultured for 2 days before transfection. Microglia were transfected with scramble siRNA or siWNT5a. mRNA was extracted from microglia at DIV7, 4 days following the complete removal of siRNA-containing transfection media. b) Wnt5a silencing efficacy was quantified by qPCR comparing scramble siRNA versus siWnt5a-treated microglia. (n = 7 wells from 2 independent batches, n = 10–12 CD-1 embryonic pups per batch). c) qPCR quantification of microglial markers C1qa, P2ry12, Itgam, Clec7a, and Cd68. Silencing microglia Wnt5a reduced phagocytosis marker C1qa and Clec7a. (n = 6–10 wells from 3 independent batches, n = 10–12 CD-1 embryonic pups per batch). d) Microglial cytokine expression was largely unchanged by silencing of Wnt5a except for proinflammatory cytokine Il1b, Tnfa. Unpaired t-test for statistical analysis. e) Representative images of E17.5 primary microglia phagocytosing pHrodo E. coli bioparticles at 72 h of incubation. f) IncuCyte quantification of microglial phagocytosis following transfection with scramble siRNA or siWNT5a. Normalized phagocytosis is quantified by the number of pHrodo+ microglia relative to the number of microglia cells per well (n = 4 wells, two independent batches isolated from 12 E17.5 CD-1 pups). Statistical analysis by Two-way ANOVA with Sidak’s multiple comparison test, *p < 0.05, **p < 0.01, bars show mean ± SEM.
3.4. Microglia increase spine density and stabilize spine turnover
To determine if microglial WNT5A affects neuronal maturation and maintenance, we isolated cortical neurons from Thy1-YFP embryonic mouse brains, which were co-cultured with murine primary microglia transfected with siRNA targeting Wnt5a or scrambled non-targeted siRNA as control (Fig. 3a). Thy1-YFP+ neurons were tested as they express Ctip2, a marker for subcortical projection neurons in Layer V cortex (Feng et al., 2000), whose survival is microglia dependent (Ueno et al., 2013). At DIV17, the addition of microglia increased dendritic length and the number of Sholl intersections, suggesting that microglia can promote dendrite growth and complexity (Fig. S2a–b). Silencing of microglial Wnt5a has no significant differences compared to the control scramble siRNA-treated microglia, suggesting a minimal impact of microglial WNT5A on neurite development (Fig. S2a). Thy1-YFP+ dendritic spines became visible and more mature, forming mushroom-like spines at DIV22-23 when the live imaging was performed to evaluate dendritic spine density and turnover rate (Fig. 3a–b). Dendritic spine density of Thy1-YFP+ neurons was significantly increased by the co-culture with scramble siRNA-treated microglia, which was completely reversed by siRNA-mediated silencing of microglial Wnt5a, indicating pivotal support by microglial WNT5A on dendritic spine formation (Fig. 3b–c). Previous in vivo studies confirmed microglial depletion increased spine turnover rate and pre-synaptic filopodia, suggesting spine turnover depends on the presence of microglia (Garcia et al., 2018). Thus, we examined whether silencing microglial Wnt5a can modulate the spine turnover rate of Thy1-YFP+ neurons in vitro. The appearance and elimination of spines were quantified over 15-minute intervals to determine spine stability over time. Immature spines have a high turnover rate, defined by the sum of the protrusions gained and lost divided by twice the total protrusion number previously described (Cruz-Martín et al., 2010). Co-culture of Thy1-YFP+ neurons with scramble siRNA-treated microglia reduced the turnover rate of YFP+ spines, suggesting that microglia stabilize spine formation (Fig. 3d). Interestingly, silencing of Wnt5a in microglia significantly increased the turnover rate of spines compared to scramble siRNA-treated microglia, which may explain the reduction of spine density observed by silencing microglial Wnt5a. In summary, our results demonstrated that microglial WNT5A is important in promoting dendritic spine stability and maintenance.
Fig. 3.
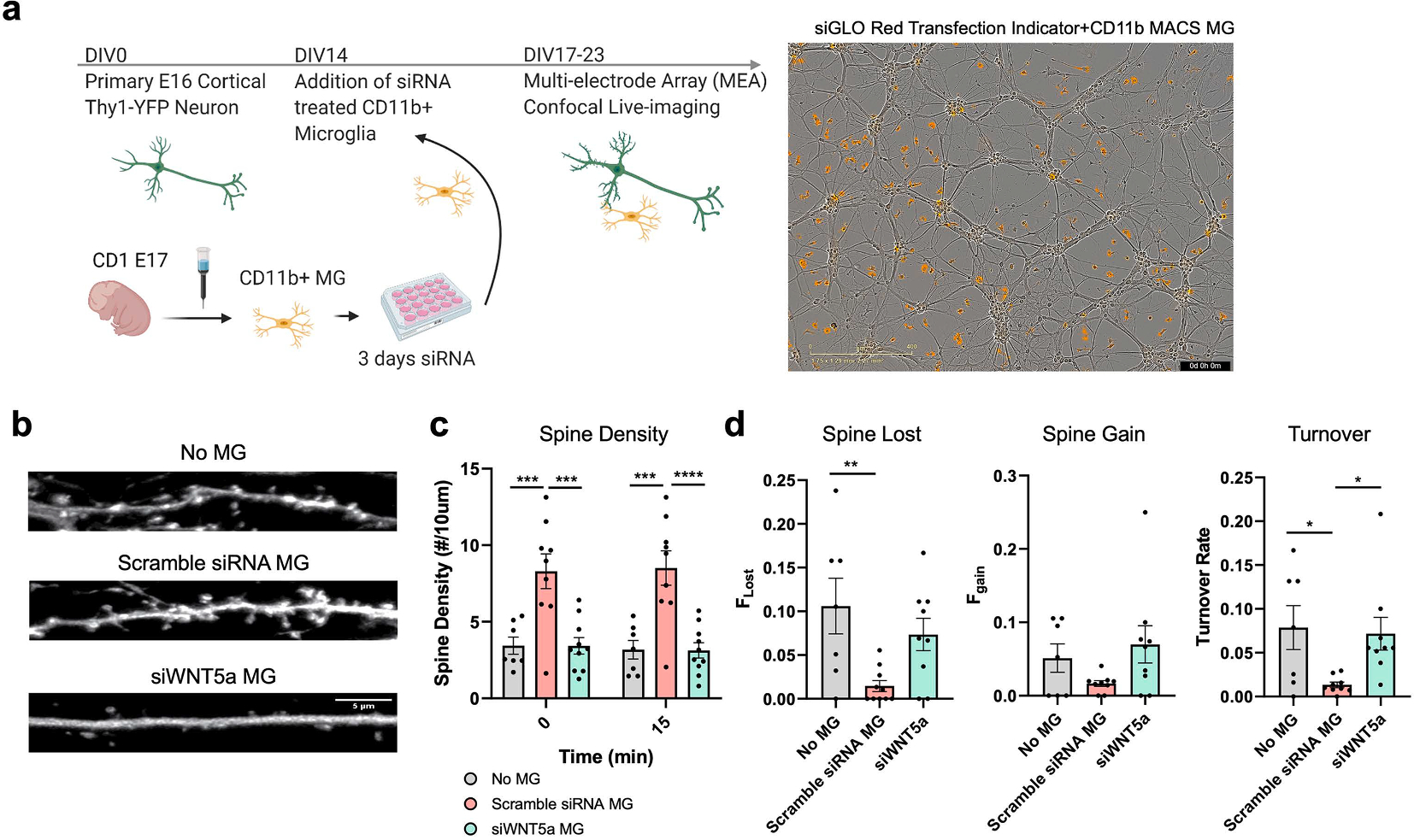
Microglia increases spine density and stabilizes spine maintenance at DIV22-23 a) Timeline for Thy1-YFP primary neuron and siRNA-treated microglia experiment indicator. Representative images showed the cells were plated at an approximate ratio of 1 siGLO red transfection indicator/CD11b+ microglial cells: 5 mixed cortical neuronal cells b) Representative images of Thy1-YFP+ neurons co-cultured with microglia treated with scramble siRNA or siWNT5a. scale bar: 5 μm. c) Spine density over 10 μm dendrite. Silencing of microglia Wnt5a diminished the effect of increased spine numbers by co-culturing with microglia. Analysis was performed by two-way ANOVA and Sidak’s multiple comparisons to determine the time and microglial treatment interaction (n = 7–9 dendrites from 3 to 4 neurons from two independent batches). d) Rate of spine numbers which are lost and gained during 15 min intervals (Left and Middle) and spine turnover rate (Right). Microglia presence reduced spine turnover rate and silencing of microglia Wnt5a increased spine turnover rate. Fgain and Flost were respectively defined as the ratio of spines that appeared and disappeared between two successive frames over the 15 min imaging period relative to the total spine number. Spine turnover rate is the sum of the protrusions lost and gained divided by twice the total spine number over 15 min intervals to measure the stability of Thy1-YFP + dendritic spines. Statistical analysis was performed using one-way ANOVA with Tukey’s multiple comparison test. n = 6–9 dendrites from 3 to 4 neurons from two independent batches; 1 outlier was removed from siWNT5a MG for Fgain, Flost, turnover rate, and 1 outlier removed from scramble siRNA MG group for Fgain and turnover rate based on 1 % ROUT outlier test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, bars show mean ± SEM.
3.5. Microglia co-culture with neurons enhances synaptic densities and neuronal firing
Previous studies show that exogenous WNT5A treatment increased PSD-95 clustering and induced mature dendritic protrusions via Ca2+-dependent intracellular pathways (Varela-Nallar et al., 2010; Farías et al., 2009). To determine pre-and post-synaptic changes by microglia, we performed immunocytochemistry for pre-synaptic VGLUT2 and post-synaptic PSD95 (Fig. 4a). Co-culture of neurons with microglia significantly increased the number of PSD95+ puncta (Fig. 4b) without affecting the number of VGLUT2+ puncta at DIV23 (Fig. 4c), while silencing microglial Wnt5a showed tendency of reduction in both measures (Fig. 4b, 4c). Synaptic densities determined by colocalized puncta of PSD95 and VGLUT2 in vitro were significantly increased by co-culture with microglia (Fig. 4d), which was significantly reduced by silencing microglial Wnt5a (Fig. 4d). Microglial Wnt5a-deficiency led to a reduced number of synapses, corresponding to the reduced dendritic spine density at DIV22-23 (Fig. 3c). Previous studies have shown that WNT5A decreases the number of pre-synaptic inputs in immature hippocampal neurons in vitro, suggesting a possible antagonistic effect of WNT5A on canonical WNT signalling (Davis et al., 2008). Here we show that microglial WNT5A did not affect pre-synaptic VGLUT2 but rather the formation of synapses. These results suggest that microglia can secrete WNT5A and may regulate neuronal excitability by affecting synapse formation in Thy1-YFP+ neurons.
Fig. 4.

DIV22-23 Postsynaptic and synapse co-localization density increased by microglia a) Immunocytochemistry representative images of primary neurons co-cultured with microglia for pre-synaptic VGLUT2 (Red) and post-synaptic PSD95 (Green) markers. Scale bar: 5 μm b) Total number of PSD95 normalized by volume increased by the addition of microglia. c) Total number of VGLUT2 normalized by total volume showed no significant differences between no microglia compared to the addition of microglia with or without siRNA WNT5a. d) Total amount of co-localization of PSD95 and VLGUT2 normalized synapse density was significantly increased by microglia co-culture and reduced by silencing microglia Wnt5a. For b-d, n = 31, 29, 23 fields from 3 independent batches of neuron and microglia culture isolated from 9 embryonic Thy1-YFP+ pups, 36 embryonic CD-1 pups. Statistical analysis using one-way ANOVA with Dunnett’s multiple comparison test. *p < 0.05, ****p < 0.0001. Bars show mean ± SEM.
Lastly, we recorded neuronal spikes and burst using the multi-electrode array to test if microglial WNT5A can affect neuronal activity, starting at DIV15 one day after the addition of microglia to primary neurons (Fig. 5a). Despite the significant effect of microglia treatment on dendrite growth, neuronal spikes were not significantly altered at DIV17 (Fig. 5b). This discrepancy could suggest that microglia could alter intrinsic excitability by increasing dendritic branching length and complexity but not firing rate since neuronal firing is more dependent on synapses than its dendritic length at DIV17. At DIV22, we observed that microglia significantly enhanced neuronal action potential firing rate. siRNA silencing of Wnt5a in microglia significantly diminished the increase in firing rate mediated by microglia, suggesting that microglia may regulate neuronal firing via WNT5A (Fig. 5b). WNT5A increases the efficacy of glutamatergic synaptic transmission via increased intracellular Ca2+ level and NMDAR trafficking (Varela-Nallar et al., 2010; McQuate et al., 2017). Thus, microglia WNT5A may promote neuronal firing by upregulating post-synaptic PSD95 or inducing Ca2+ influx. There was no significant effect of microglia on neuronal burst or neuronal synchrony, suggesting microglia act differently from astrocytes, which enhances neuronal synchrony (Fellin et al., 2004). However, glutamate signaling mechanisms are complex and influenced by many factors. Examining structural components of glutamatergic synapses, including α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)/ N-methyl-d-aspartic acid (NMDA) receptors, can determine the microglial effect on intrinsic excitability. Whole-cell patch clamp can be performed in vitro to record membrane resistance, action potential properties, and excitatory post-synaptic currents in future studies.
Fig. 5.

Microglia enhanced neuronal firing rate at DIV23-4 but diminished by silencing of microglia WNT5A a) Scheme of microglia co-cultured with primary cortical neurons isolated from Thy1-YFP+ E16 mice at DIV16-23. b) Mean firing rate was measured over DIV16-23, with the most significant increase at DIV23-24. Two-way ANOVA with Tukey’s multiple comparison test was performed to determine the time and treatment interaction effect. (n = 3–6 replicates, 3 independent batches of neuron and microglia culture (Three Thy1-YFP+ pups for neuronal culture and 12 CD-1 pups for microglial culture per independent experiment), one outlier removed at DIV15 in siWNT5a, one outlier removed each at DIV16, DIV22, DIV23 in no MG group) *p < 0.05, **p < 0.01, ***p < 0.001. Bars show mean ± SEM. multiple comparison test. *p < 0.05; bars show mean ± SEM.
4. Discussion
In the current study, we showed that the microglia secretome regulates neural stem cell signaling involved in the pathophysiology of neurodevelopmental disorders and Wnt pathway gene expressions. We found that Wnt5a is most highly expressed among Wnt family genes in microglia. Silencing microglial Wnt5a abolishes the supportive effect of microglia on spine density and partially diminishes their impact on the synaptic density or neuronal firing of co-cultured Thy1-YFP+ primary neurons. Dysregulation of neuropeptides, such as elevated levels of neurotensin and vasoactive intestinal peptide, is observed in ASD individuals (Angelidou et al., 2010; Nelson, 2001), suggesting that microglia may contribute to the pathogenesis of ASD by increasing neuropeptide signaling in neurons. In addition to the neuropeptide pathway, we found that the microglial secretome can increase the expression of IFNγ signaling pathway genes involved in neuronal differentiation and neurite outgrowth (Warre-Cornish et al., 2020). In a mouse model, IFNγ is an important molecular pathway for promoting social behavior (Filiano et al., 2016). Furthermore, IFNγ stimulation can reduce dendritic spine density in human induced pluripotent stem cell (iPSC)-derived cortical neurons (Kathuria et al., 2022). Interestingly, a previous study reported that IFNγ disproportionately altered the expression of genes associated with schizophrenia and ASD in iPSC-derived neurons, suggesting that microglia can further contribute to the pathogenesis of neurodevelopmental disorders via IFNγ signaling (Warre-Cornish et al., 2020). The microarray results also indicate that the microglial secretome reduces the translational initiation signaling pathway, a key molecular pathway in neurodevelopmental disorders. Loss of translational repressor 4E-BP2, linked to ASD, increased protein synthesis in microglia and induced autistic-like behavior deficits in mice (Gkogkas et al., 2013; Xu et al., 2020). Thus, our NSC transcriptome results corroborate the idea that microglial secretome, consisting of an array of cytokines and molecules, can promote neuronal development, and its dysregulation can contribute to neurodevelopmental disorders.
The WNT pathway is implicated in several psychiatric disorders, including schizophrenia and bipolar disorder (Hoseth et al., 2018). Suppression of noncanonical WNT pathways reversed hippocampal-dependent deficits in a mouse model of Fragile-X syndrome (Oliva et al., 2013; Li, 2011; Chacón et al., 2008; Guo et al., 2012). The non-canonical WNT5A signaling pathway is one of the most significantly dysregulated pathways in schizophrenia, and several WNT5A-associated genes have de novo mutations in schizophrenia and ASD (Evgrafov et al., 2020). WNT5A regulates neuronal differentiation and specialization, proper axon guidance, and glial progenitor cell proliferation in the cerebellum (Keeble et al., 2006; Blakely et al., 2011; Subashini et al., 2017). Wnt5a deficient mice show impaired developmental processes, including the inability to extend the anterior-posterior and proximal–distal axes of the embryo (Kikuchi et al., 2012; Yamaguchi et al., 1999). Wnt5a deficient mice also show enhanced axon defects of dopaminergic neurons in the dorsal-lateral striatum (Blakely et al., 2011). In contrast, Chen et al. reported embryonic deletion of Wnt5a in neurons did not result in any structural abnormalities in CA1 pyramidal neurons during development, suggesting that neuronal WNT5A mediates synaptic plasticity rather than maturation (Chen et al., 2017).
The role of glial WNT5A on neuronal development remains elusive. One recent study showed that astrocyte-derived WNT5A could mediate microglial calcium signaling and alter microglia proliferation (Halleskog et al., 2012). WNT5A can also stimulate noncanonical WNT signaling in microglia by binding to ROR/FZD receptors (Halleskog, 2011; Halleskog, 2012; Yang and Zhang, 2020). Glia-derived WNT5A can induce microglial secretion of inflammatory cytokines and chemokines, including IL-6, IL-1β, and CCL2 (Shao et al., 2016; Halleskog, 2012). Our study shows that silencing of microglial Wnt5a suppressed the expression of inflammatory cytokine Tnfα and neurodegenerative microglial genes Clec7a and C1q, suggesting that microglial WNT5A positively regulates aspects of microglial inflammatory signals. However, we observed enhanced microglial Il1b expression. While this could be due to the compensatory upregulation of Il1b to restore Wnt5a expression, we cannot rule out another compensatory mechanism by other canonical WNT pathways to promote Il1b expression. Our data thus show that microglial WNT5A can modulate autonomous and non-autonomous WNT signaling in microglia and NSC.
We have recently reported a pathological role of microglial neuritogenic factors, including WNT5A, in the MIA mouse model (Ikezu et al., 2020). We demonstrated that enhanced microglia-neuron contact is associated with increased microglial WNT5A expression in MIA microglia (Ikezu et al., 2020), suggesting that inflammation-induced upregulation of WNT5A may alter microglia-mediated pathways in neuronal development. Here, we show that silencing Wnt5a diminished the microglial effect on increasing dendritic spine density and decreased the number of PSD95+/ VGLUT2+ synapse, suggesting that microglia derived WNT5A enhanced spine maturity and synaptogenesis corresponding with increased neuronal activity. Our results are consistent with previous studies showing a significant effect of WNT5A on spine densities and stabilizing dendritic spines via a post-synaptic mechanism (Ramos-Fernández et al., 2021). WNT5A increases calcium entry and increases F-actin mobility to enhance spine maturation through increasing expression of NMDAR subunit GluN2B (Ramos-Fernández et al., 2021). Our results reveal a lesser role of microglial WNT5A on neurite growth since silencing microglial WNT5A had no significant effect on reducing neurite extension or branching. This discrepancy could suggest that other microglial molecules may be involved in regulating neurite growth or branching. Another explanation is that siRNA transfection-based silencing was insufficient, and it may require complete silencing, such as using CRISPR-based gene editing for the effect of microglial WNT5A on neurite outgrowth. Another possibility is that our experimental design consisted of co-culturing microglia at a later time point of neural differentiation, which potentially is after the critical period for microglial WNT5A to have a robust effect on regulating neurite outgrowth.
Our study also identified several other WNT family in microglia, including Wnt2a/b, Wnt3a, Wnt4, Wnt5b, Wnt6, Wnt7a/b, Wnt9a/b, Wnt10a/b, Wnt11 and Wnt16. WNT3 is also involved in the regulation of patterning and neurogenesis in the dorsal cortex (Lie et al., 2005; Munji et al., 2011). WNT3 induces neurogenesis by stimulating WNT/β-catenin signaling in neural progenitor cells (Lie et al., 2005). In contrast to WNT5A, WNT3A suppresses dendrite maturation of subventricular zone-derived neurons in vitro (Pino et al., 2011). Canonical WNT3A and WNT7A mediate recycling and exocytosis of synaptic vesicles in mature hippocampal neurons and enhance synaptic transmission in adult hippocampal brain slices (Cerpa, 2010). WNT7A is another highly expressed WNT molecule in microglia and can alter phagocytosis and immune responses in macrophages (Wallace, 2018). WNT7A can promote axonal branching and pre-synaptic protein synapse I, which is involved in synapse formation and function (Lucas and Salinas, 1997). One study demonstrated that TGFβ-induced phagocytic microglial secretion of WNT7a may promote canonical WNT/β-catenin signaling associated with oligodendrogenesis of neural stem cells (Mecha, 2020). Noncanonical WNT7B has been associated with dendritic complexity by activating Rac and JNK pathways (Rosso et al., 2005). Further investigation is necessary for the role of other microglial WNT molecules on neural development and maturation.
Our study has a few technical limitations. One major limitation of this study is the reliance on siRNA transfection. Based on qPCR results, microglial Wnt5a expression is reduced rather than eliminated in mouse microglia. For complete silencing of Wnt5a in microglia, gene editing tools such as CRISPR/Cas9, antisense oligonucleotides, short hairpin RNA (shRNA), microRNAs, or over-expression of dominant negative Wnt5a may be applied in future studies in both human and mouse microglia. The importance of microglial-derived WNT5A can also be interrogated by depleting WNT5A in cell culture media by anti-WNT5A and determining whether cytokine secretion can be affected by blocking autocrine signaling pathways.
Another limitation of this study is the limited capacity to address microglial WNT5A in vitro. Previous studies have shown that microglia is highly dependent on the environment (Bohlen et al., 2017; Gosselin et al., 2017). Neuronal and glial co-culture promotes a homeostatic microglia phenotype that resembles more in vivo microglia phenotypes. Further investigation requires determining whether the microglial phenotype is maintained when co-cultured with neurons and other glial cells such as astrocytes. In addition, our study is limited to mainly assessing excitatory neurons in vitro. Previously, Cuitino et al. showed that WNT5A could induce the recycling of GABAAR and increase the amplitude of inhibitory currents in hippocampal neurons via the non-canonical WNT/Ca2+ pathway (Cuitino et al., 2010). Thus, microglial WNT5A may also affect inhibitory synapse maturity and inhibitory synaptic signaling. Future studies can determine whether microglial WNT5A can affect GABAergic synapse formation and regulate neuronal physiology through inhibitory synapses. In this study, we based the quantification of synapses on immunochemistry, which is limited in resolution and cannot directly indicate functionality at specific synapses. Thus, examining neuronal firing by whole cell patch clamp to monitor spontaneous action potential firing and excitatory/inhibitory postsynaptic potential, followed by analyzing dendritic spines of the biocytin-filled neurons, will enable us to perform a more direct correction assessment of neuronal firing and synaptic alterations.
In summary, we show that microglia can secrete factors that can modulate neuronal development and physiology. Microglial WNT5A can support dendritic spine development and maintenance without direct microglia-neuron contact. We found that silencing microglial WNT5A can diminish the effect of microglia co-culture on dendritic spine density, turnover, and spontaneous neuronal firing in vitro. Future studies can investigate rodent models to examine the effects of microglial WNT5A on specific brain circuits across developmental ages.
5. Institutional review board statement
All animal procedures followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Boston University Institutional Animal Care and Use Committee.
Supplementary Material
Acknowledgments
We would like to thank Clarence Schutt at the Nancy Lurie Marks Family Foundation (NLMFF), Robert Landreth at the Landreth Family Fund and Susan Leeman at Boston University for supporting this study, Microarray Center at Boston University School of Medicine for microarray service and analysis help. We would also like to thank Robert Freilich for preliminary data collection and analysis.
Funding
This work was funded in part by NLMFF (T.I., S.I.), Robert E. Landreth, and Dona Landreth Family Foundation (T.I., S.I.), NIH 5T32GM008541 (H.Y.), PhRMA Foundation Fellowship (M.W.).
Abbreviations:
- PSD95
Post-synaptic density protein 95
- WNT5A
WNT family member 5A
- VGLUT2
Vesicular-glutamate transporter 2
- ASD
Autism spectrum disorders
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Hana Yeh: Conceptualization, Methodology, Formal analysis, Writing – original draft, Visualization. Maya E. Woodbury: Conceptualization, Formal analysis, Writing – original draft. Kaitlin L. Ingraham Dixie: Methodology. Tsuneya Ikezu: Conceptualization, Writing – review & editing, Supervision, Funding acquisition. Seiko Ikezu: Conceptualization, Writing – review & editing, Supervision, Funding acquisition.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2022.11.003.
Data availability
Data will be made available on request.
References
- Angelidou A, et al. , 2010. Neurotensin is increased in serum of young children with autistic disorder. J. Neuroinflamm. 7, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredondo SB, et al. , 2020. Wnt5a promotes differentiation and development of adult-born neurons in the hippocampus by noncanonical Wnt signaling. Stem Cells 38, 422–436. [DOI] [PubMed] [Google Scholar]
- Bennett ML, et al. , 2016. New tools for studying microglia in the mouse and human CNS. PNAS 113. E1738–E1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian W-J, et al. , 2015. A novel Wnt5a-Frizzled4 signaling pathway mediates activity-independent dendrite morphogenesis via the distal PDZ motif of Frizzled 4. Dev. Neurobiol. 75, 805–822. [DOI] [PubMed] [Google Scholar]
- Bilbo S & Stevens B Microglia: The Brain’s First Responders. Cerebrum 2017, cer-14–17 (2017). [PMC free article] [PubMed] [Google Scholar]
- Blakely BD, et al. , 2011. Wnt5a Regulates Midbrain Dopaminergic Axon Growth and Guidance. PLoS ONE 6, e18373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchi R, et al. , 2017. Perturbed Wnt signaling leads to neuronal migration delay, altered interhemispheric connections and impaired social behavior. Nat. Commun. 8, 1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen CJ, et al. , 2017. Diverse requirements for microglial survival, specification, and function revealed by defined-medium cultures. Neuron 94, 759–773.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandria JM, et al. , 2022. cRel and Wnt5a/Frizzled 5 receptor-mediated inflammatory regulation reveal novel neuroprotectin D1 targets for neuroprotection. Cell. Mol. Neurobiol. 10.1007/s10571-022-01231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerpa W, et al. , 2010. Wnt-5aoccludes Aβ oligomer-induced depression of glutamatergic transmission in hippocampal neurons. Mol. Neurodegener. 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacón MA, Varela-Nallar L, Inestrosa NC, 2008. Frizzled-1 is involved in the neuroprotective effect of Wnt3a against Aβ oligomers. J. Cell. Physiol. 217, 215–227. [DOI] [PubMed] [Google Scholar]
- Chen C-M, et al. , 2017. Wnt5a is essential for hippocampal dendritic maintenance and spatial learning and memory in adult mice. PNAS. 10.1073/pnas.1615792114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-C, Chang Y-W, Huang Y-S, 2019. Dysregulated translation in neurodevelopmental disorders: an overview of autism-risk genes involved in translation. Dev. Neurobiol. 79, 60–74. [DOI] [PubMed] [Google Scholar]
- Cruz-Martín A, Crespo M, Portera-Cailliau C, 2010. Delayed stabilization of dendritic spines in fragile X mice. J. Neurosci. 30, 7793–7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuitino L, et al. , 2010. Wnt-5a modulates recycling of functional GABAA receptors on hippocampal neurons. J. Neurosci. 30, 8411–8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EK, Zou Y, Ghosh A, 2008. Wnts acting through canonical and noncanonical signaling pathways exert opposite effects on hippocampal synapse formation. Neural Dev. 3, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes ML, McAllister AK, 2016. Maternal immune activation: Implications for neuropsychiatric disorders. Science 353, 772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evgrafov OV, et al. , 2020. Gene expression in patient-derived neural progenitors implicates WNT5A signaling in the etiology of schizophrenia. Biol. Psychiatry 88, 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farías GG, et al. , 2009. Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J. Biol. Chem. 284, 15857–15866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, et al. , 2004. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43, 729–743. [DOI] [PubMed] [Google Scholar]
- Feng G, et al. , 2000. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51. [DOI] [PubMed] [Google Scholar]
- Filiano AJ, et al. , 2016. Unexpected role of interferon-γ in regulating neuronal connectivity and social behavior. Nature 535, 425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AL, Udeh A, Kalahasty K, Hackam AS, 2018. A growing field: The regulation of axonal regeneration by Wnt signaling. Neural Regen Res 13, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkogkas CG, et al. , 2013. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature 493, 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin D, et al. , 2017. An environment-dependent transcriptional network specifies human microglia identity. Science 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, et al. , 2012. Inhibition of GSK3β improves hippocampus-dependent learning and rescues neurogenesis in a mouse model of fragile X syndrome. Hum. Mol. Genet. 21, 681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halleskog C, et al. , 2011. WNT signaling in activated microglia is pro-inflammatory. Glia 59, 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halleskog C, et al. , 2012. Heterotrimeric G protein-dependent WNT-5A signaling to ERK1/2 mediates distinct aspects of microglia proinflammatory transformation. J. Neuroinflamm. 9, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han VX, et al. , 2021b. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review. Transl. Psychiatry 11, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han VX, Patel S, Jones HF, Dale RC, 2021a. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 17, 564–579. [DOI] [PubMed] [Google Scholar]
- Hoseth EZ, et al. , 2018. Exploring the Wnt signaling pathway in schizophrenia and bipolar disorder. Transl. Psychiatry 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezu S, et al. , 2020. Inhibition of colony stimulating factor 1 receptor corrects maternal inflammation-induced microglial and synaptic dysfunction and behavioral abnormalities. Mol. Psychiatry. 10.1038/s41380-020-0671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H-Y, et al. , 2016. Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis. Brain Behav. Immun. 58, 165–172. [DOI] [PubMed] [Google Scholar]
- Kalavai SV, Ikezu S, 2021. Neuritogenic function of microglia in maternal immune activation and autism spectrum disorders. Neural Regener. Res. 16, 1436–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapellos TS, et al. , 2016. A novel real time imaging platform to quantify macrophage phagocytosis. Biochem. Pharmacol. 116, 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria A, Lopez-Lengowski K, Roffman JL, Karmacharya R, 2022. Distinct effects of interleukin-6 and interferon-γ on differentiating human cortical neurons. Brain Behav. Immun. 103, 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeble TR, et al. , 2006. The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J. Neurosci. 26, 5840–5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H, Sato A, Matsumoto S, 2012. Wnt5a: its signalling, functions and implication in diseases. Acta Physiol (Oxf) 204, 17–33. [DOI] [PubMed] [Google Scholar]
- Kracht L, et al. , 2020. Human fetal microglia acquire homeostatic immune-sensing properties early in development. Science 369, 530–537. [DOI] [PubMed] [Google Scholar]
- Kumar A, Williams MT, Chugani HT, 2015. Evaluation of basal ganglia and thalamic inflammation in children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection and tourette syndrome: a positron emission tomographic (PET) study using 11C-[R]-PK11195. J. Child Neurol. 30, 749–756. [DOI] [PubMed] [Google Scholar]
- Lanoue V, et al. , 2017. The Wnt receptor Ryk is a negative regulator of mammalian dendrite morphogenesis. Sci. Rep. 7, 5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, et al. , 2011. WNT5A signaling contributes to Aβ-induced neuroinflammation and neurotoxicity. PLoS ONE 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, et al. , 2020. Transplantation of Wnt5a-modified NSCs promotes tissue repair and locomotor functional recovery after spinal cord injury. Exp. Mol. Med. 52, 2020–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie D-C, et al. , 2005. Wnt signalling regulates adult hippocampal neurogenesis. Nature 437, 1370–1375. [DOI] [PubMed] [Google Scholar]
- Lucas FR, Salinas PC, 1997. WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Dev. Biol. 192, 31–44. [DOI] [PubMed] [Google Scholar]
- Maiti G, Naskar D, Sen M, 2012. The wingless homolog Wnt5a stimulates phagocytosis but not bacterial killing. PNAS 109, 16600–16605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuate A, Latorre-Esteves E, Barria A, 2017. A Wnt/calcium signaling cascade regulates neuronal excitability and trafficking of NMDARs. Cell Rep. 21, 60–69. [DOI] [PubMed] [Google Scholar]
- Mecha M, et al. , 2020. Involvement of Wnt7a in the role of M2c microglia in neural stem cell oligodendrogenesis. J. Neuroinflamm. 17, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R, 2006. Purified Wnt5a protein activates or inhibits β-catenin–TCF signaling depending on receptor context. PLoS Biol. 4, e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakova E, Warner BB, 2018. Maternal immune activation, central nervous system development and behavioral phenotypes. Birth Defects Res. 110, 1539–1550. [DOI] [PubMed] [Google Scholar]
- Morgan JT, et al. , 2010. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol. Psychiatry 68, 368–376. [DOI] [PubMed] [Google Scholar]
- Munji RN, Choe Y, Li G, Siegenthaler JA, Pleasure SJ, 2011. Wnt signaling regulates neuronal differentiation of cortical intermediate progenitors. J. Neurosci. 31, 1676–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KB, et al. , 2001. Neuropeptides and neurotrophins in neonatal blood of children with autism or mental retardation. Ann. Neurol. 49, 597–606. [PubMed] [Google Scholar]
- Nye DMR, et al. , 2020. The receptor tyrosine kinase Ror is required for dendrite regeneration in Drosophila neurons. PLoS Biol. 18, e3000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva CA, Vargas JY, Inestrosa NC, 2013. Wnts in adult brain: from synaptic plasticity to cognitive deficiencies. Front. Cell. Neurosci. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paina S, et al. , 2011. Wnt5a Is a Transcriptional Target of Dlx Homeogenes and Promotes Differentiation of Interneuron Progenitors In Vitro and In Vivo. J. Neurosci. 31, 2675–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-Y, Kang M-J, Han J-S, 2018. Interleukin-1 beta promotes neuronal differentiation through the Wnt5a/RhoA/JNK pathway in cortical neural precursor cells. Mol. Brain 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino D, Choe Y, Pleasure SJ, 2011. Wnt5a controls neurite development in olfactory bulb interneurons. ASN Neuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez VT, Ramos-Fernández E, Henríquez JP, Lorenzo A, Inestrosa NC, 2016. Wnt-5a/Frizzled9 receptor signaling through the gαo-gβγ complex regulates dendritic spine formation *. J. Biol. Chem. 291, 19092–19107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Fernández E, et al. , 2021. Wnt5a promotes hippocampal postsynaptic development and GluN2B-induced expression via the eIF2α HRI kinase. Sci. Rep. 11, 7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC, 2005. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat. Neurosci. 8, 34–42. [DOI] [PubMed] [Google Scholar]
- Shao Y, et al. , 2016. Biological functions of macrophage-derived Wnt5a, and its roles in human diseases. Oncotarget 7, 67674–67684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subashini C, et al. , 2017. Wnt5a is a crucial regulator of neurogenesis during cerebellum development. Sci. Rep. 7, 42523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, et al. , 2013. Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry 70, 49–58. [DOI] [PubMed] [Google Scholar]
- Ueno M, et al. , 2013. Layer V cortical neurons require microglial support for survival during postnatal development. Nat. Neurosci. 16, 543–551. [DOI] [PubMed] [Google Scholar]
- Vallejo D, Lindsay CB, González-Billault C, Inestrosa NC, 2021. Wnt5a modulates dendritic spine dynamics through the regulation of Cofilin via small Rho GTPase activity in hippocampal neurons. J. Neurochem. 158, 673–693. [DOI] [PubMed] [Google Scholar]
- Varela-Nallar L, Alfaro IE, Serrano FG, Parodi J, Inestrosa NC, 2010. Wingless-type family member 5A (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses. PNAS 107, 21164–21169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA, 2005. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 57, 67–81. [DOI] [PubMed] [Google Scholar]
- Voineagu I, et al. , 2011. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474, 380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J, et al. , 2018. Wnt7a induces a unique phenotype of monocyte-derived macrophages with lower phagocytic capacity and differential expression of pro- and anti-inflammatory cytokines. Immunology 153, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warre-Cornish K, et al. , 2020. Interferon-γ signaling in human iPSC–derived neurons recapitulates neurodevelopmental disorder phenotypes. Sci. Adv. 6, eaay9506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z-X, et al. , 2020. Elevated protein synthesis in microglia causes autism-like synaptic and behavioral aberrations. Nat. Commun. 11, 1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S, 1999. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126, 1211–1223. [DOI] [PubMed] [Google Scholar]
- Yang Y, Zhang Z, 2020. Microglia and Wnt pathways: prospects for inflammation in alzheimer’s disease. Front. Aging Neurosci. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H, Ikezu T, 2019. Transcriptional and epigenetic regulation of microglia in health and disease. Trends Mol. Med. 25, 96–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y et al. Activated human astrocyte-derived extracellular vesicles modulate neuronal uptake, differentiation and firing. J Extracell Vesicles 9, 1706801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, et al. , 2014. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, et al. , 2016. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89, 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


