Abstract
The roles of gamma interferon (IFN-γ) and interleukin-12 (IL-12) in mediating and/or enhancing the in vivo trypanosomicidal activity of the nitroheterocyclic derivative benznidazole (Bz) were evaluated during early stages of experimental Chagas’ disease. Our results show that treatment of Trypanosoma cruzi-infected mice with anti-cytokine monoclonal antibodies (MAbs) had no apparent effect when the optimal dose of Bz (100 mg/kg of body weight) was used. In contrast, treatment with anti-IL-12 or anti-IFN-γ MAbs enhanced the parasitemia and accelerated the mortality of mice treated with a suboptimal dose of Bz (25 mg/kg). Simultaneous treatment with a suboptimal dose of Bz and recombinant IL-12 (rIL-12) enhanced the efficacy of drug treatment in terms of parasitemia and mouse survival. Interestingly, we found that drug-resistant T. cruzi strains were found to be poor inducers of IL-12 both in vitro and in vivo compared to strains of T. cruzi which are susceptible or partially resistant to Bz treatment. These results suggest that early activation of the cellular compartment of the immune system by IL-12 may favor in vivo Bz activity against T. cruzi. In order to test this hypothesis mice infected with the drug-resistant Colombiana strain of T. cruzi were treated with 100 mg of Bz per kg plus different concentrations of rIL-12. By using the results of PCR and serological and parasitological methods as the criteria of a cure, our results indicate that a higher percentage of mice treated with Bz combined with rIL-12 than mice treated with Bz alone are cured.
Chagas’ disease, a long-lived infection caused by Trypanosoma cruzi, affects approximately 20 million people in Latin America (50). Among humans, patients with a wide clinical spectrum of disease are observed, ranging from patients in whom morbidity is apparently absent upon superficial clinical examination methods to patients with severe heart disease leading to heart failure and sudden death (10). It is clear from different epidemiological studies that a significant proportion of the chagasic population eventually develops the severe manifestations of chronic disease (49). Although nitroimidazole derivatives have low levels of efficacy, they have been used for the treatment of either acute or recent asymptomatic chronic infections (4). Such treatment is thought to prevent pathology during the late chronic stages of Chagas’ disease.
The specific chemotherapy for human Chagas’ disease has several limitations, such as the requirement for long-term administration of toxic nitroheterocyclic derivatives (19) and natural drug resistance even among parasite populations without previous exposure to these drugs (21). Nevertheless, a considerable percentage of patients treated at the early stages of T. cruzi infection are supposed to be cured on the basis of a combination of negative hemoculture, xenodiagnosis, and serological analysis (22, 27). In the murine model the efficacy of specific chemotherapy varies according to the T. cruzi strains, and in general, chemotherapy is more efficient during the acute phase of infection (21).
The host resistance developed during experimental acute Chagas’ disease is dependent on innate and specific immunities requiring the combined efforts of several lymphocyte subpopulations including natural killer (NK) (15), CD4+ T (28), and CD8+ T (40, 41) cells. Infection of macrophages with T. cruzi trypomastigotes or exposure of these cells to parasite products initiates the production of interleukin-12 (IL-12), which triggers the synthesis of gamma interferon (IFN-γ) by different cells from the lymphocytic lineage (2, 13). The latter cytokine has been closely associated with host resistance during acute infection with T. cruzi. Thus, while treatment with IFN-γ enhances resistance in mice (34, 44), treatment with neutralizing doses of anti-IFN-γ antibodies makes animals more susceptible to T. cruzi infection (37). In vivo and in vitro experiments suggest that activation of macrophages with IFN-γ and tumor necrosis factor alpha leads to the induction of nitric oxide synthesis and inhibition of parasite replication during acute infection with T. cruzi in mice (14, 23, 48).
In the study described here we evaluated the ability of different T. cruzi strains to induce the synthesis of IFN-γ and IL-12 both in vitro and in vivo. Because these cytokines are important mediators of resistance during acute infection with T. cruzi in mice, we evaluated their roles in mediating and/or enhancing the in vivo trypanosomicidal effects of the nitroheterocyclic derivative benznidazole (Bz) during the early stages of experimental Chagas’ disease. Mice were infected with T. cruzi strains showing different patterns of susceptibility and resistance to Bz therapy followed by chemotherapy and concomitant administration with either neutralizing antibodies against IL-12 or IFN-γ. Alternatively, the Bz therapy during acute infection with T. cruzi was associated with recombinant IL-12 (rIL-12). Our results suggest that Bz activity does not depend on IL-12 or IFN-γ. However, the activation of the cellular compartment from the immune system by IL-12 may favor the in vivo parasiticidal action of chemotherapy against T. cruzi.
MATERIALS AND METHODS
Animals.
Male C3H/HeJ or Swiss-Webster mice (age, 6 to 7 weeks) were obtained from the animal house of FIOCRUZ (Rio de Janeiro, Brazil) and were used as a source of inflammatory macrophages or for experiments of Bz therapy combined with either anti-cytokine monoclonal antibodies (MAbs) or rIL-12. T. cruzi strains were continuously maintained in Swiss-Webster mice.
Parasite strains.
The parasite strains used in the in vivo experiments were continuously maintained in irradiated (650 rads), immunosuppressed male outbred Swiss-Webster mice or in intact male outbred Swiss-Webster mice (weight, 18 to 20 g) acutely infected with the parasite. Strains CL (11), Y (38), and Colombiana (20) strains, which are susceptible, partially resistant, and resistant strains of T. cruzi, respectively, were used. For the in vitro experiments the parasites were maintained in murine tissue culture fibroblasts (L929; American Type Culture Collection, Rockville, Md.); and strains CL (11), Gilmar (21), and J (36) (susceptible strains), strain Y (38) (a partially resistant strain), and strains Colombiana (20) and VL-10 (36) (resistant strains) of T. cruzi were used.
Experimental infections and parasitemia assessment.
Swiss-Webster mice (males; weight, 20 to 25 g) were infected intraperitoneally with 5,000 blood trypomastigotes. The level of parasitemia in tail blood was assessed daily by the method of Brener (9). For mice infected with the Y, the CL, or the Colombiana strain, the parasitemia was followed daily from days 4 to 15, 8 to 24, or 8 to 24 postinfection, respectively. For each experiment mouse survival was monitored daily and cumulative mortality was determined.
Treatment with Bz.
Animals infected with different strains of T. cruzi received per oral treatment with either optimal (100 mg/kg of body weight/day) or suboptimal (25 mg/kg/day) dosages of Bz (Rochagan; Roche) for 7 days starting on the day after infection. Infected mice with no treatment were used as controls.
Hemoculture.
The animals were bled aseptically 30 to 40 days after the end of treatment. About 0.4 ml of blood was taken from each animal and was divided into two tubes containing 5 ml of LIT medium (12). The hemocultures were incubated at 27°C for 30 and 60 days. After the incubation period, an aliquot from each hemoculture tube was examined under an optical microscope for the detection of parasites.
PCR.
After being bled for hemoculture, the animals were killed. The heart and 500 μl of blood were used as DNA sources for the detection of a T. cruzi-specific gene. Briefly, DNA was extracted from mouse tissue with phenol-chloroform-isoamyl alcohol, precipitated in isopropanol, and washed with 70% ethanol. The DNA preparation was resuspended in water, the concentration was adjusted to 10 and 100 ng/μl, and the DNA preparation was used as a template for PCR with primers specific for the T. cruzi guanine hypoxantine phosphoribosyltransferase (HGPRT) gene: primers HGPRT1 (forward; 5′-CTACAAGGGAAAGGGTCTGC-3′) and HGPRT2 (reverse; 5′-ACCGTAGCCAATCACAAAGG-3′). The primers were designed from the complete nucleotide sequence of the HGPRT gene (3). The size of the expected PCR product is 412 bp. Each amplification reaction was performed with a final volume of 10 μl containing 0.5 U of Taq DNA polymerase (CENBIOT, Porto Alegre, Brazil), each deoxynucleoside triphosphate at a concentration of 200 μM, 15 mM MgCl2, 50 mM KCl, 10 mM Tris-HCl (pH 8.5), 5 pmol of each primer, and 10 or 100 ng of DNA extracted from blood or cardiac tissue from the infected mice. After heat denaturation (5 min at 95°C), the samples were submitted to 30 cycles at three temperatures (95°C for 1 min, 55°C for 1 min, and 72°C for 1 min). In the final cycle the extension step was for 5 min. Following amplification, 3 μl of each reaction mixture was electrophoresed in a 6% polyacrylamide gel and silver stained (35).
Immunofluorescence.
Sera from infected mice were tested against the epimastigote forms of T. cruzi Y. Briefly, 2 μl of a suspension of epimastigotes in phosphate-buffered saline (PBS) containing 106 parasites/ml was used to coat each well from immunofluorescence glass slides, and the slides were dried at room temperature. Sera from mice infected with the Colombiana strain and treated with either Bz alone or Bz plus rIL-12 were diluted from 1:20 to 1:320. Samples of 10 μl from the different serum dilutions were added to different wells of the immunofluorescence slide, and the slide was incubated at 37°C for 30 min. The slides were washed twice, dried at room temperature, and incubated with anti-mouse immunoglobulin antibodies conjugated with fluorescein isothiocyanate (Sigma, St. Louis, Mo.) for 30 min at room temperature. The slides were then fixed with glycerol and were examined in an Ortholux (Leitz) fluorescence microscope.
Treatment with rIL-12 and anti-cytokine MAbs.
Murine rIL-12 was a generous gift from the Genetics Institute (Cambridge, Mass.). Groups of mice untreated or treated with different doses of Bz were injected intraperitoneally with 100 or 250 ng of rIL-12 in PBS containing 0.5% bovine serum albumin. rIL-12 doses were given every other day (e.o.d.) starting on the day after parasite inoculation, and the animals received a maximum of seven doses. In some experiments mice were also treated weekly, starting on the day before infection, with 1 mg of either neutralizing rat anti-IL-12 MAbs (MAbs C15.6.7.5 and C15.1.2.1) (25) or 1 mg of an anti-IFN-γ MAb (MAb XMG 1.6) (25) or a control anti-β-galactosidase MAb (MAb GL-113) (25). All MAbs were purified from ascites fluid by ammonium sulfate precipitation.
Macrophage preparation.
C3H/HeJ mice were used as a source of inflammatory macrophages. Mouse macrophages were harvested from the peritoneal cavities 5 days after injection with 1.5 ml of thioglycolate and were suspended at a concentration of 2 × 106 cells/ml in Dulbecco modified Eagle medium (Advanced Biotechnology, Inc., Columbia, Md.) containing 10% fetal calf serum, 1% l-glutamine, and 1% Pen Strep. After 4 h of incubation (37°C and 5% CO2) in a 96-well plate (Costar Cambridge, Mass.), the nonadherent cells were removed and live trypomastigotes were added to the macrophage preparations at different concentrations in the absence or presence of 75 U of IFN-γ/ml. The supernatants were harvested at 48 h after the addition of live trypomastigotes to the macrophage cultures for measurement of IL-12 concentrations (13).
Measurement of IL-12 (p40) in supernatant from macrophages cultures.
The IL-12 measurements were performed with a pair of MAbs against the p40 polypeptide of IL-12 [IL-12 (p40)], which is tightly regulated in macrophages. An enzyme-linked immunosorbent assay was performed with 5 μg of an anti-p40 MAb (MAb C17.15.10)/ml as the capture antibody and an biotinylated anti-IL-12 MAb (MAb C15.6.76), diluted 750-fold, as the detection antibody. The development was done with a streptavidin-peroxidase conjugate. The IL-12 (p40) concentration was calculated by reference to a standard curve for murine rIL-12 (13).
Statistical analysis.
An unpaired analysis of variance was performed with INSTAT software (GraphPad, San Diego, Calif.) in order to compare the levels of parasitemia for the animals in different groups. Differences were considered statistically significant when the P value was less than 0.05. The Kruskal-Wallis test was used to compare the mouse survival rates, and the differences were considered statistically significant when the P value was less than 0.05. Chi-square analysis was used to evaluate the differences among different groups of mice (see Table 2), and the differences were considered statistically significant when the P value was less than 0.05.
TABLE 2.
Cure rates for mice infected with T. cruzi Colombiana and treated with Bz and rIL-12a
| Experimental group | No. (%) of mice that survived (n = 15) | No. of mice in the
following groups with negative results by the indicated test:
|
|||
|---|---|---|---|---|---|
| Survivorsb
|
PCR
after negative hemoculture result
|
||||
| IIF | Hemo | Blood DNA | Heart DNA | ||
| Control (untreated) | 1 (6.6) | 0 | 0 | 0 | 0 |
| Bz | 15 (100.0)* | 2 | 3* | 3 | 2 |
| Bz + rIL-12 (100 ng/ml) | 13 (86.7)* | 9 | 9** | 9 | 7 |
| Bz + rIL-12 (250 ng/ml) | 5 (33.3)** | 5 | 5** | 5 | 5 |
* and **, results shown in the same column are statistically significant (P < 0.01).
IIF, indirect immunofluorescence (titer, ≥1:20); Hemo, hemoculture.
RESULTS
Strains of T. cruzi differentially induce IL-12 synthesis in vitro.
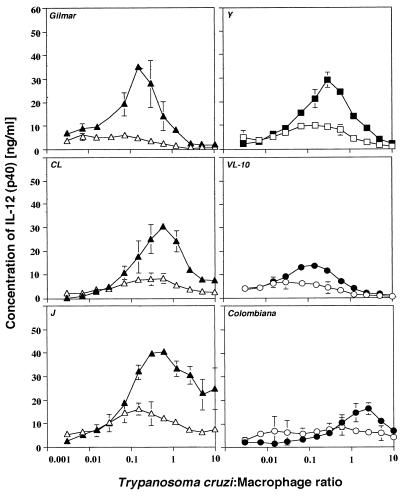
The abilities of different T. cruzi strains to induce the synthesis of IL-12 by macrophages were compared. The T. cruzi strains were divided into three groups according to their drug susceptibilities: (i) susceptible strains, strains CL, Gilmar, and J; (ii) partially resistant strain, strain Y; and (iii) resistant strains, strains Colombiana and VL-10 (Table 1). The strains were susceptible to Bz in vitro, with the 50% inhibitory concentrations varying from 0.9 to 12.0 μg/ml on the basis of the parasite strain and the developmental stage (data not shown), thus indicating an intrinsic parasite susceptibility to Bz. We found that all of the different strains of T. cruzi triggered the synthesis of IL-12 by inflammatory macrophages. As reported previously (13), we found that costimulation of macrophages with IFN-γ (75 U/ml) clearly enhanced the ability of the parasites to induce IL-12 (Fig. 1). Interestingly, drug-resistant strains VL-10 and Colombiana were poorer inducers of IL-12 synthesis by macrophages than either partially resistant and drug-susceptible strains of T. cruzi.
TABLE 1.
Susceptibilities of T. cruzi strains to in vivo treatment with Bz
| T. cruzi susceptibility group and strain | Cure rate for mice treated with the following Bz
dosage (mg/kg/day)a:
|
|
|---|---|---|
| 25 | 100 | |
| Susceptible | ||
| CL | R | S |
| J | R | S |
| Gilmar | R | S |
| Partially resistant, Y | R | MR |
| Resistant | ||
| Colombiana | R | R |
| VL-10 | R | R |
Percentage of infected mice cured after Bz treatment: 80 to 100% of the animals (S), 21 to 79% of the animals (MR), and 0 to 20% of the animals (R) (21).
FIG. 1.
Induction of IL-12 (p40) synthesis by inflammatory macrophages exposed to live trypomastigotes from different strains of T. cruzi. Macrophages were cultured in the presence (closed symbols) or absence (open symbols) of IFN-γ (75 U/ml) and were exposed to different ratios of live trypomastigotes from various T. cruzi strains. The levels of IL-12 (p40) in the supernatant were measured 48 h after macrophage exposure to parasite. The Gilmar, CL, and J strains were drug-susceptible parasites (▵, ▴); the Y strain was a partially resistant parasite (□, ■); and the VL-10 and Colombiana strains were drug-resistant parasites (○, •).
Strains of T. cruzi differentially induce the IL-12 synthesis in vivo.
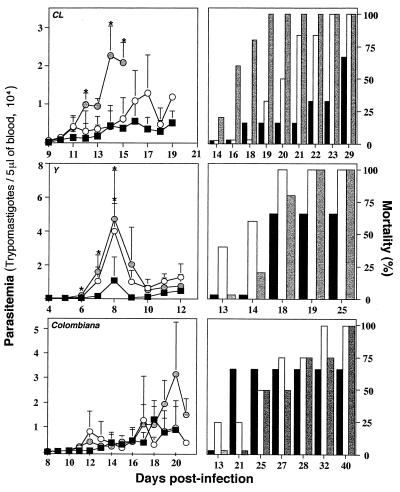
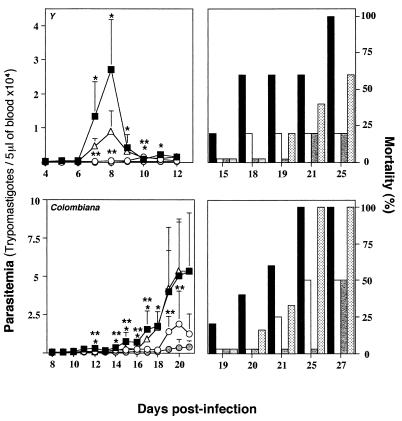
In order to evaluate the in vivo induction of IL-12 during acute infection with T. cruzi, one parasite strain was selected according to its susceptibility or resistance to Bz treatment (Table 1). Animals were infected with 5,000 blood trypomastigotes of T. cruzi CL, Y, or Colombiana. They received a weekly dose of 1 mg of either anti-IL-12 or anti-IFN-γ starting on the day before infection. A control group received a dose of 1 mg of an unrelated MAb (MAb GL-113) per week. The results presented in Fig. 2 indicate that treatment with neutralizing antibodies against IL-12 or IFN-γ greatly altered the natural course of infection with strain CL or Y, resulting in enhanced levels of parasitemia and an accelerated rate of mortality. The untreated control mice showed the same levels of parasitemia (data not shown). No statistically significant differences were observed when mice infected with strain Colombiana were treated with either the anti-IL-12 or the anti-IFN-γ MAb. The asterisks in Figure 2 indicate that the differences between experimental groups (i.e., treated with anti-cytokine MAbs) are statistically significant (P < 0.05) compared to the results for the control group (i.e., treated with the control MAb). The statistical analysis of the mouse survival rates indicates that (i) the group of animals infected with strain CL and treated with either anti-IL-12 or anti-IFN-γ had a higher rate of mortality than the group treated with the control MAb (P < 0.05) and (ii) the group of animals infected with strain Y and treated with anti-IFN-γ (but not with anti-IL-12) had a higher rate of mortality than the group treated with the control MAb (P < 0.05). No statistically significant differences in terms of levels of parasitemia or mortality rates were observed among different groups of animals infected with T. cruzi Colombiana. Thus, after treatment with anti-IL-12 the drug-susceptible strains of T. cruzi became much more virulent. In contrast, when anti-IL-12 was administered to mice infected with the Colombiana strain, parasite virulence increased only slightly. These findings indicate that the drug-resistant strain of T. cruzi is a poor inducer of IL-12 synthesis in vivo.
FIG. 2.
Effect of in vivo neutralization of IL-12 or IFN-γ on
resistance to acute infection with different strains of T.
cruzi. Swiss mice were infected with 5,000 trypomastigotes of
T. cruzi CL, Y, or Colombiana. Animals were treated with
either an unrelated MAb (■, black bars), an anti-IFN-γ MAb (○,
white bars), or an anti-IL-12 MAb
( , gray
bars) 1 day prior to infection and once a week afterward. The levels of
parasitemia (left panels) and the rates of mortality (right panels)
were monitored daily until the end of the experiment. For each datum
point presented in the parasitemia curve the average and positive
standard deviation are shown for three to six animals per group.
Similar numbers of animals were used for the mortality study. This
experiment was repeated once and yielded identical results. Note that
the scale indicating the level of parasitemia (left panels) is
different for each T. cruzi strain.
, gray
bars) 1 day prior to infection and once a week afterward. The levels of
parasitemia (left panels) and the rates of mortality (right panels)
were monitored daily until the end of the experiment. For each datum
point presented in the parasitemia curve the average and positive
standard deviation are shown for three to six animals per group.
Similar numbers of animals were used for the mortality study. This
experiment was repeated once and yielded identical results. Note that
the scale indicating the level of parasitemia (left panels) is
different for each T. cruzi strain.
Parasite-induced IL-12 and IFN-γ enhance trypanosomicidal effect of Bz.
It has previously been reported (6, 43) that treatment with nitroheterocyclic derivatives is less effective in immunodeficient murine hosts than mice with normal immune system infected with T. cruzi. In our next set of experiments, we investigated whether the endogenously produced IL-12 and IFN-γ are important mediators of the in vivo trypanosomicidal effect of Bz. Treatment with the optimal doses of Bz (100 mg/kg/day) decreases the level of parasitemia to undetectable levels during the acute phase of infection with the different T. cruzi strains used. While aborting the infection with strain CL (a drug-susceptible strain), treatment with the optimal dose of Bz results in the parasitological cure of 50% of the mice infected with T. cruzi Y (a partially drug-resistant strain) and less than 20% of the mice infected with T. cruzi Colombiana (a drug-resistant strain) (Table 1). In contrast, treatment with a suboptimal dose of Bz (25 mg/kg/day) lowers the level of parasitemia but does not eliminate the patent parasitemia caused by all different T. cruzi strains.
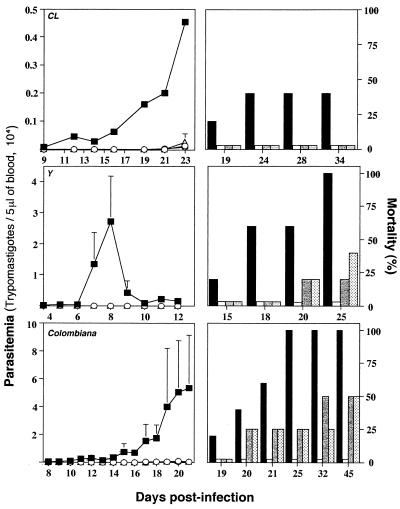
In order to study the role of endogenous IL-12 and IFN-γ in mediating the trypanosomicidal effects of Bz, mice were infected with T. cruzi CL, Y, or Colombiana and were treated with the optimal or a suboptimal dose of Bz. Different groups of mice treated with Bz also received simultaneous treatment with either an unrelated MAb, anti-IFN-γ, or anti-IL-12, and the levels of parasitemia and the rates of mortality were evaluated. The experiments whose results are presented in Fig. 3 indicate that for animals receiving 100 mg of Bz/day, treatment with either anti-IFN-γ or anti-IL-12 did not affect the parasitemia levels in animals infected with any of the three T. cruzi strains used, but it slightly enhanced the mortality rate for animals infected with strain Y or Colombiana. No statistically significant differences were observed in terms of the levels of parasitemia or the rates of mortality when different groups of animals infected with T. cruzi Colombiana, Y, or CL received the optimal dose of Bz and were treated with anti-cytokine MAbs.
FIG. 3.
Effect of in vivo neutralization of IL-12 or IFN-γ on trypanosomicidal activity of treatment with an optimal dose of Bz during acute phase of infection with different strains of T. cruzi. Swiss-Webster mice were infected with 5,000 trypomastigotes of T. cruzi CL, Y, or Colombiana and were treated with Bz at 100 mg/kg/day for 7 days. Different groups of animals receiving Bz alone (○, white bars) were simultaneously injected with either an anti-IFN-γ MAb (○, gray bars) or an anti-IL-12 MAb (▵, dotted bars) 1 day prior to infection and once a week afterward. The levels of parasitemia (left panels) and the rates of mortality (right panels) were monitored daily until the end of the experiment. Infected and nontreated animals were used as controls (■, black bars). For each datum point presented in the parasitemia curve the average and positive standard deviation are shown for four to five animals per group. Similar numbers of animals were used for the mortality study. This experiment was repeated once and yielded identical results. Some of the parasitemia curves ( , ▵) are not seen in this figure, because none of the treated groups showed an apparent parasitemia. Note that the scale indicating the level of parasitemia (left panels) is different for each T. cruzi strain.
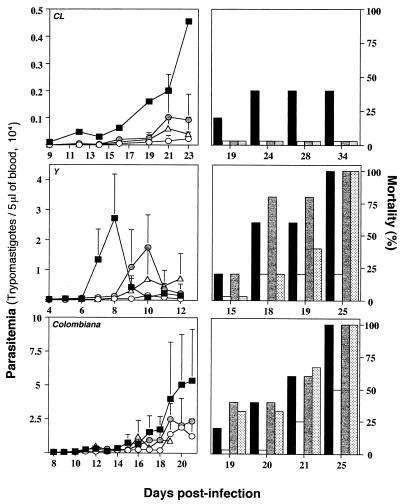
In animals that were infected with strain Y and that received a suboptimal dose of Bz, a more dramatic effect of either anti-IL-12 or anti-IFN-γ treatment was observed (Fig. 4). Thus, after stopping Bz treatment, animals treated with anti-IFN-γ MAbs had higher levels of parasitemia than animals treated with an unrelated MAb (data not shown) and higher rates of mortality than animals treated with Bz alone (P < 0.05). However, the enhancement of the level of parasitemia in animals treated with anticytokine MAbs was observed only after treatment was stopped at day 7 postinfection. In the case of animals infected with strain CL, treatment with either anti-IFN-γ or anti-IL-12 also slightly enhanced the levels of parasitemia after treatment with Bz at 25 mg/kg/day was stopped; however, no mortality was observed in this group of animals (Fig. 4). This observation may be explained in part by the fact that strain CL is highly susceptible to drug treatment. Thus, treatment with Bz even at a dosage of 25 mg/kg/day, although not curative, may be sufficient to reduce parasitemia levels in vivo. These results suggest that although the trypanosomicidal effect of Bz is dependent on IL-12 and IFN-γ, in some instances these cytokines may help Bz treatment to clear T. cruzi from the host.
FIG. 4.
Effect of in vivo neutralization of IL-12 or IFN-γ on
trypanosomicidal activity of treatment with a suboptimal dose of Bz
during acute phase of infection with different strains of T.
cruzi. Swiss-Webster mice were infected with 5,000 trypomastigotes
of T. cruzi CL, Y, or Colombiana and were treated with Bz at
25 mg/kg/day for 7 days. Different groups of animals receiving drug
alone (○, white bars) were simultaneously injected with either an
anti-IFN-γ MAb
( , gray
bars) or an anti-IL-12 MAb (▵, dotted bars) 1 day prior to infection
and once a week afterward. The levels of parasitemia (left panels) and
the rates of mortality (right panels) were monitored daily until the
end of the experiment. Infected and nontreated animals were used as
controls (■, black bars). For each datum point presented in the
parasitemia curve the average and positive standard deviation are shown
for three to five animals per group. Similar numbers of animals were
used for the mortality study. This experiment was repeated once and
yielded identical results. Note that the scale indicating the level of
parasitemia (left panels) is different for each T. cruzi
strain.
, gray
bars) or an anti-IL-12 MAb (▵, dotted bars) 1 day prior to infection
and once a week afterward. The levels of parasitemia (left panels) and
the rates of mortality (right panels) were monitored daily until the
end of the experiment. Infected and nontreated animals were used as
controls (■, black bars). For each datum point presented in the
parasitemia curve the average and positive standard deviation are shown
for three to five animals per group. Similar numbers of animals were
used for the mortality study. This experiment was repeated once and
yielded identical results. Note that the scale indicating the level of
parasitemia (left panels) is different for each T. cruzi
strain.
rIL-12 enhances the efficacy of Bz treatment in animals infected with either resistant and partially resistant strains of T. cruzi.
As indicated above, endogenous IL-12 and IFN-γ may help to control T. cruzi infection in animals treated with Bz and drug-resistant strains of T. cruzi appear to be poor inducers of IL-12 synthesis both in vitro and in vivo. Therefore, we decided to treat the mice infected with T. cruzi Y or Colombiana with rIL-12 and Bz concomitantly. In our initial experiments (Fig. 5), animals were infected with either T. cruzi Y or Colombiana and treated with a suboptimal dosage of Bz (25 mg/kg/day) plus five consecutive doses of 250 ng of rIL-12 (per mouse) e.o.d. As shown in Fig. 5, animals that received treatment with rIL-12 plus Bz had lower levels of parasitemia as well as delayed mortality compared to the levels of parasitemia and times to mortality for untreated animals or animals treated with rIL-12 alone or Bz alone. The group of animals infected with strain Y and treated with Bz presented with higher levels of parasitemia (days 7, 8, and 10) than animals treated with Bz plus rIL-12 (Fig. 5). The differences in the levels of parasitemia were also statistically significant when T. cruzi Colombiana-infected animals receiving Bz alone were compared with those treated with Bz plus rIL-12 (days 12, 14, 15, 16, 17, 19, and 20) (P < 0.05) (Fig. 5). The group of animals infected with strain Colombiana and treated with Bz plus rIL-12 (but not Bz alone) had lower rates of mortality than the untreated control group (P < 0.05).
FIG. 5.
Administration of rIL-12 enhances in vivo
trypanosomicidal activity of treatment with a suboptimal dose of Bz
during acute infection with T. cruzi Colombiana.
Swiss-Webster mice were infected with 5,000 trypomastigotes of T.
cruzi Y or Colombiana and were treated with Bz alone (25
mg/kg/day; ○, white bars), Bz (25 mg/kg/day) plus rIL-12 (250
ng/mouse every other day)
( , gray
bars), or rIL-12 alone (250 ng/mouse every other day; ▵, dotted
bars). The levels of parasitemia (left panels) and the rates of
mortality (right panels) were monitored daily until the end of the
experiment. Infected and nontreated animals were used as controls (■,
black bars). For each datum point presented in the parasitemia curve
the average and positive standard deviation are shown for four to six
animals per group. Similar numbers of animals were used for the
mortality study. This experiment was repeated once and yielded
identical results. Note that the scale indicating the level of
parasitemia (left panels) is different for each T. cruzi
strain. One asterisk indicates that the differences between
experimental groups (i.e., treated with Bz or Bz plus rIL-12) are
statistically significant (P < 0.05) compared to the
results for the control group (i.e., untreated mice). Two asterisks
indicate that the differences were statistically significant when
infected animals treated with Bz and infected animals treated with Bz
plus rIL-12 are compared.
, gray
bars), or rIL-12 alone (250 ng/mouse every other day; ▵, dotted
bars). The levels of parasitemia (left panels) and the rates of
mortality (right panels) were monitored daily until the end of the
experiment. Infected and nontreated animals were used as controls (■,
black bars). For each datum point presented in the parasitemia curve
the average and positive standard deviation are shown for four to six
animals per group. Similar numbers of animals were used for the
mortality study. This experiment was repeated once and yielded
identical results. Note that the scale indicating the level of
parasitemia (left panels) is different for each T. cruzi
strain. One asterisk indicates that the differences between
experimental groups (i.e., treated with Bz or Bz plus rIL-12) are
statistically significant (P < 0.05) compared to the
results for the control group (i.e., untreated mice). Two asterisks
indicate that the differences were statistically significant when
infected animals treated with Bz and infected animals treated with Bz
plus rIL-12 are compared.
Our next step was to investigate whether the combined therapy with rIL-12 and Bz would enhance the rate of parasitological cure in mice infected with strain Colombiana, the highly resistant strain of T. cruzi. Animals were infected with 10,000 trypomastigotes of strain Colombiana and were treated with the optimal dosage of Bz (100 mg/kg/day) for 20 days. At the same time animals from different groups received seven doses of PBS or rIL-12 at either 100 or 250 ng/mouse every other day. The parasitological cure was evaluated by hemoculture, serology, and PCR at 2, 4, and 4 months, respectively, after stopping treatment. The results of hemoculture (Table 2) indicate that the combination of rIL-12 and Bz enhanced the levels of parasitological cure (9 of 13 and 5 of 5 mice treated with 100 and 250 ng of rIL-12, respectively) compared to that for animals treated with Bz only (3 of 15 mice). These results were also confirmed by serology. These differences were statistically significant (P < 0.01). Among the animals negative by hemoculture, only one was positive for specific antibodies to epimastigotes in an immunofluorescence assay (Table 2). This mouse belonged to the group of animals treated with Bz alone. All other animals, which belonged to the group of animals treated with Bz alone, Bz plus rIL-12 (100 ng/mouse/e.o.d.), or Bz plus rIL-12 (250 ng/mouse/e.o.d.), had negative results by hemoculture and negative results by an immunofluorescence assay. Concomitant treatment with Bz and rIL-12 increased the rate of cure (with 100 ng/mouse, 9 of 13 [69.2%] mice; with 250 ng/mouse, 5 of 5 [100%] mice) but also enhanced the rate of mortality among the mice when the higher dose of rIL-12 was used (P < 0.01). Although two mice from the group of animals treated with Bz plus rIL-12 (100 ng/mouse) died, the number of survivors was not statistically significant compared with the numbers of survivors among the animals treated with Bz alone.
Samples from mice with negative hemoculture results were subjected to PCR with DNA extracted from blood and heart tissue used as a template. All PCRs with DNA from the blood of mice in the experimental groups (i.e., treated with Bz alone or Bz plus rIL-12 at 100 and 250 ng/mouse) were negative (Table 2). Nevertheless, the PCRs with DNA from heart tissue detected parasite DNA in 3 of 17 mice with negative hemoculture results. Figure 6 shows representative results of a T. cruzi-specific PCR with the HGPRT primers and DNA extracted from the heart tissue of mice infected with T. cruzi Colombiana and treated with Bz or Bz plus rIL-12. The PCR was positive for the mice chronically infected and untreated (lanes 2 and 3), one mouse treated with Bz alone (lane 4), and two mice treated with Bz plus rIL-12 (lanes 5 and 6). The mouse that was treated with Bz alone and that had a positive PCR result was the same one that was indirect immunofluorescence positive. The negative results of the parasite-specific PCR with DNA from heart tissue from mice receiving combined therapy are shown in lanes 7 to 11. Lanes 12 and 13 present the results for two uninfected mice, and lanes 14 and 15 are the positive (T. cruzi DNA) and negative (without DNA) controls of the PCR, respectively.
FIG. 6.
PCR detection of T. cruzi DNA in heart tissue from mice infected with strain Colombiana and treated with Bz alone or Bz combined with rIL-12. The lanes contain molecular size markers (lane 1) and PCR products from DNA (50 ng/reaction) extracted from the heart tissue of chronically infected and untreated mice (lanes 2 and 3), mice treated with Bz alone (lane 4), mice treated with Bz plus rIL-12 (lane 5–11), and uninfected mice (lanes 12 and 13). Lane 14, PCR product from a reaction with 10 pg of T. cruzi DNA; lane 15, negative result by PCR without DNA.
Because one mouse from the group treated with Bz alone and two mice from the group treated with Bz plus rIL-12 (100 ng/mouse) were positive by PCR with DNA from heart tissue, the statistical analysis was repeated. The difference for the cured animals remained statistically significant (P < 0.05) compared the results for the groups treated with Bz alone or Bz plus rIL-12 (100 ng/mouse).
DISCUSSION
The natural transmission of T. cruzi has been controlled in different countries of South America (17, 49, 50). However, Chagas’ disease is still a major public health problem even in those countries where natural transmission has been controlled due to the low level of efficacy of specific chemotherapy against this protozoan. Approximately 20 million persons in Latin America have long-term T. cruzi infection (50), and 20 to 30% of these individuals will eventually develop one of the symptomatic forms of disease (i.e., cardiac and/or digestive forms of disease) (10). Thus, at the moment a major goal of research on Chagas’ disease is the development of specific chemotherapy that can eliminate the infection from individuals who are acutely or chronically infected but who have not yet developed cardiac and/or digestive forms of disease.
One of the strategies is to search for new drugs which are more effective for the treatment of the acute and chronic forms of Chagas’ disease (47). An alternative approach that we have taken in our laboratories is to use the combination of different drugs known to have trypanosomicidal activities in vivo. Under different circumstances, the use of combinations of drugs leads to a higher level of efficacy in eliminating parasitic infection and requires lower doses during chemotherapy (7). In the study described here we decided to evaluate how the efficacy of Bz is affected by the cytokines IL-12 and IFN-γ, which are known to promote resistance during the acute phase of experimental Chagas’ disease (2, 15, 26, 37). Several studies have shown that treatment with rIL-12 is partially protective against different parasitic (25, 39), bacterial (46), fungal (51), or viral (32) infections. Most of those studies indicate that the protective effects of rIL-12 are mediated by IFN-γ (8, 45). More importantly, the combination of rIL-12 with specific chemotherapy has been shown to enhance the efficacies of drugs in animals infected with either Cryptococcus spp. (16), Leishmania major (29), or Histoplasma capsulatum (52).
Among the several drugs that are used for the treatment of experimental Chagas’ disease, the nitroheterocyclic derivatives have been the most investigated and have been used to treat human disease. The mechanism of action of such drugs is largely unknown. However, some studies suggest that Bz may inhibit T. cruzi respiration (18).
Different studies performed with either humans and murine models indicate that the efficacy of specific chemotherapy against Chagas’ disease varies according to the T. cruzi strains. On the basis of their susceptibility or resistance to Bz treatment, T. cruzi strains can be divided into the following three groups: (i) resistant, (ii) partially resistant, and (iii) susceptible to treatment (21). Other studies have also demonstrated that in both humans and mice, chemotherapy with Bz is much more efficient during the acute stages than the chronic stages of infection with T. cruzi (4, 21).
In fact, it is known that during the acute phase of Chagas’ disease a high level of activation of cells from the immune system, including macrophages, NK cells, B cells, and T lymphocytes, occurs (15, 28, 33, 40). It is noteworthy that recent studies indicate that blood trypomastigote and freshly released intracellular amastigotes have the ability to initiate the synthesis of IL-12 and other cytokines by macrophages (2, 13, 26). The strong activation of the immune system observed during the acute phase of Chagas’ disease is due at least in part to the release of proinflammatory cytokines induced by T. cruzi parasites. For instance, IL-12 is a potent activator of NK cells and T lymphocytes, which trigger the synthesis of IFN-γ (8, 45), which is a potent macrophage activator and which enhances the synthesis of IL-12. Different studies indicate the importance of macrophage activation by IFN-γ as a major mechanism responsible for controlling parasite replication via the generation of free radicals such as reactive oxygen intermediates and reactive nitrogen intermediates during the acute phase of infection in the murine model (1, 23, 24, 30, 31, 48).
Considering that the efficacy of treatment with Bz is much higher during the acute stage of infection, when the cellular compartment of the immune system is highly activated and armed to fight T. cruzi infection, we propose that therapy with IL-12 may enhance the parasiticidal effects of Bz through the induction of IFN-γ by NK cells and T lymphocytes, culminating in macrophage activation. Interestingly, the results presented here indicate a higher degree of efficacy of in vivo treatment with Bz when mice are experimentally infected with parasite strains, such as strains CL and Y, which are stronger inducers of IL-12 synthesis by macrophages. Moreover, we demonstrate that mice infected with strain Colombiana, a drug-resistant T. cruzi strain, develop a severe and fatal cardiac disease, and parasitologic cure can be achieved in these mice by providing combined treatment with the trypanosomicidal drug Bz and rIL-12. Thus, our data indicate that IL-12 enhances the efficacy of Bz therapy against T. cruzi. The enhancement of the effects of Bz in animals infected with a naturally resistant strain of T. cruzi by coadministration of rIL-12 was evaluated by hemoculture, serology, and PCR. Most of the animals which were negative by hemoculture were also negative by serology as well as PCR. Among nine animals that were treated with Bz and rIL-12 and that were negative by hemoculture, two had negative PCR results when DNA extracted from blood was used but positive PCR results when DNA extracted from heart tissue was used. Although the results conflict, we considered these animals to be cured, since their serology was clearly negative. It is possible that residual parasite DNA may remain in the cardiac tissue even after the elimination of infection, as reported previously for parasite antigens (5), or that T. cruzi DNA may integrate into the genome of the host cell (42).
Although the efficacy of Bz was enhanced by rIL-12 and treatment with Bz has a higher degree of efficacy in animals infected with T. cruzi strains which are better inducers of IFN-γ, it is important to mention that we were unable to block the effects of Bz therapy with neutralizing antibodies against either IFN-γ or IL-12. Considering that in many different systems it has been demonstrated that the antimicrobial effects of IL-12 are blocked by simultaneous treatment with neutralizing anti-IFN-γ antibodies (8, 45), we believe that the mechanisms of action of Bz and IL-12 are distinct. Although rather speculative, the enhancement of the action of Bz by IL-12 may be related to the ability of IL-12 to induce IFN-γ, which, in return, further enhances the synthesis of free radicals by macrophages exposed to T. cruzi products (1, 23, 24, 30, 31, 48).
Finally, our studies suggest that the ability of T. cruzi strains to evade early induction of IL-12 and IFN-γ may be an important characteristic of highly virulent parasites and may be responsible at least in part for parasite resistance to specific chemotherapeutic agents during the acute phase of Chagas’ disease.
ACKNOWLEDGMENTS
We gratefully acknowledge Ivan Barbosa Machado Sampaio and Jaqueline Alvarez Leite for suggestions with the statistical analysis. We are indebted to the Mammalian and Microbial Cell Sciences and Process Biochemistry groups of the Genetics Institute for production and purification of rIL-12. We also acknowledge Giovani Gazzinelli and Antoniana U. Krettli for critical reading of the manuscript.
This work was partially supported by PAPES (#2) and PRONEX (#2704-FINEP). R.T.G. is the recipient of a Biotechnology Career Fellowship from the Rockefeller Foundation. R.T.G., A.J.R., and Z.B. are research fellows of CNPq.
REFERENCES
- 1.Adams L B, Hibbs J B, Jr, Taintor R R, Krahenbuhl J L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990;144:2725–2729. [PubMed] [Google Scholar]
- 2.Aliberti J C S, Cardoso M A G, Martins G A, Gazzinelli R T, Vieira L Q, Silva J S. IL-12 mediates resistance to Trypanosoma cruziin mice and is produced by murine macrophages in response to live trypomastigotes. Infect Immun. 1996;64:1961–1967. doi: 10.1128/iai.64.6.1961-1967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen J, Ullman B. Molecular characterization and overexpression of hypoxantine guanine phosphoribosyl transferase gene from Trypanosoma cruzi. Mol Biochem Parasitol. 1994;65:233–245. doi: 10.1016/0166-6851(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 4.Andrade A L S S, Zicker F, Oliveira R M, Almeida S, Luquetti A, Travassos L R, Almeida I C, Andrade S S, Andrade J G, Martelli C M T. Randomized trial of efficacy of benznidazole in treatment of early Trypanosoma cruziinfection. Lancet. 1996;348:1407–1413. doi: 10.1016/s0140-6736(96)04128-1. [DOI] [PubMed] [Google Scholar]
- 5.Andrade S G, Freitas L A R, Peyrol S, Pimentel A R, Sadigursky M. Experimental chemotherapy of Trypanosoma cruziinfection: persistence of parasite antigens and positive serology in parasitologically cured mice. Bull W H O. 1991;69:191–197. [PMC free article] [PubMed] [Google Scholar]
- 6.Andrade S G, Filho A C, de Souza A J M, de Lima E S, Andrade Z. Influence of treatment with immunosuppressive drugs in mice chronically infected with Trypanosoma cruzi. Int J Exp Pathol. 1997;78:391–399. doi: 10.1046/j.1365-2613.1997.390370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Araújo M S S, Molina J, Pereira M E S, Brener Z. Combination of drugs in the treatment of experimental Trypanosoma cruziinfection. Mem Inst Oswaldo Cruz. 1996;91:315. doi: 10.1590/s0074-02761996000100011. [DOI] [PubMed] [Google Scholar]
- 8.Biron A C, Gazzinelli R T. Effects of IL-12 on immune responses to microbial infections: a key mediator in regulating disease outcome. Curr Opin Immunol. 1995;7:485–493. doi: 10.1016/0952-7915(95)80093-x. [DOI] [PubMed] [Google Scholar]
- 9.Brener Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev Inst Med Trop. 1962;4:389–396. [PubMed] [Google Scholar]
- 10.Brener Z. Chagas disease—clinical features. Chagas disease (American trypanosomiasis): its impact on transfusion and clinical medicine. Int Soc Blood Transfusion Cap. 1992;6:81–101. [Google Scholar]
- 11.Brener Z, Chiari E. Variacões morfológicas observadas em diferentes amostras de Trypanosoma cruzi. Rev Inst Med Trop. 1963;5:220–224. [PubMed] [Google Scholar]
- 12.Camargo E P. Growth and differentiation in Trypanosoma cruzi. Origin of metacyclic trypanosomas in liquid media. Rev Inst Med Trop. 1964;6:93–100. [PubMed] [Google Scholar]
- 13.Camargo M M, Almeida I C, Pereira M E S, Ferguson M A J, Travassos L R, Gazzinelli R T. GPI-anchored mucin-like glycoproteins isolated from Trypanosoma cruzitrypomastigotes initiate the synthesis of pro-inflammatory cytokines by macrophages. J Immunol. 1997;158:5890–5901. [PubMed] [Google Scholar]
- 14.Camargo M M, Andrade A C, Almeida I C, Travassos L R, Gazzinelli R T. Glycoconjugates isolated from Trypanosoma cruzi but not from Leishmania sp.parasite membranes trigger nitric oxide synthesis as well as microbicidal activity by IFN-γ-primed macrophages. J Immunol. 1997;159:6131–6139. [PubMed] [Google Scholar]
- 15.Cardillo F, Voltarelli J C, Reed S G, Silva J S. Regulation of Trypanosoma cruziinfection in mice by gamma interferon and interleukin-10: the role of NK cells. Infect Immun. 1996;64:128–134. doi: 10.1128/iai.64.1.128-134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clemons K V, Brummer E, Stevens D A. Cytokine treatment of central nervous system infection: efficacy of IL-12 alone and synergy with conventional antifungal therapy in experimental cryptococcoses. Antimicrob Agents Chemother. 1994;38:460–464. doi: 10.1128/aac.38.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dias J P C. Control of Chagas’ disease in Brazil. Parasitol Today. 1987;3:336–341. doi: 10.1016/0169-4758(87)90117-7. [DOI] [PubMed] [Google Scholar]
- 18.Diaz De Toranzo E G, Castro J, Frankle De Cazzulo J J. Interaction of benznidazole reactive metabolites with nuclear and kinetoplastic DNA, proteins and lipids from Trypanosoma cruzi. Experientia. 1988;44:880–881. doi: 10.1007/BF01941187. [DOI] [PubMed] [Google Scholar]
- 19.Docampo R, Moreno S N J. Biochemical toxicology of antiparasitic compounds used in the chemotherapy and chemoprophylaxis of American trypanosomiasis (Chagas’ disease) Rev Biochem Toxicol. 1985;7:159–204. [Google Scholar]
- 20.Federici E E, Albemann W H, Neva F A. Chronic and progressive myocarditis and myositis in C3H mice infected with Trypanosoma cruzi. Am J Trop Med Hyg. 1964;13:272–280. doi: 10.4269/ajtmh.1964.13.272. [DOI] [PubMed] [Google Scholar]
- 21.Filardi L S, Brener Z. Susceptibility and natural resistance of Trypanosoma cruzistrains to drugs used clinically in Chagas disease. Trans R Soc Trop Med Hyg. 1987;81:755–759. doi: 10.1016/0035-9203(87)90020-4. [DOI] [PubMed] [Google Scholar]
- 22.Galvão L M C, Nunes M B, Cançado J R, Brener Z, Kretteli A U. Lytic antibody titre as a means of assessing cure after treatment of Chagas’ disease: a 10 years follow-up study. Trans R Soc Trop Med Hyg. 1992;86:1–4. doi: 10.1016/0035-9203(93)90501-g. [DOI] [PubMed] [Google Scholar]
- 23.Gazzinelli R T, Oswald I P, Hieny S, James S, Sher A. The microbicidal activity of interferon-γ treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-β. Eur J Immunol. 1992;22:2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- 24.Gazzinelli R T, Oswald I P, James S, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-γ activated macrophages. J Immunol. 1992;148:1792–1796. [PubMed] [Google Scholar]
- 25.Gazzinelli R T, Wysocka M, Hayashi S, Denkers E Y, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite induced IL-12 stimulates early IFNγ synthesis and resistance during infection with Toxoplasma gondii. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 26.Hunter C A, Slifer T, Araujo F. Interleukin-12-mediated resistance to Trypanosoma cruziis dependent on tumor necrosis factor alpha and gamma interferon. Infect Immun. 1996;64:2381–2386. doi: 10.1128/iai.64.7.2381-2386.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krettli A U, Cançado J R, Brener Z. Criterion of cure human Chagas’ disease after specific chemotherapy: recent advances. Mem Inst Oswaldo Cruz. 1984;79:157–164. [Google Scholar]
- 28.Minoprio P, Eisen H, Joskowicz M, Pereira P, Coutinho A. Suppression of polyclonal antibody production in murine Trypanosoma cruziinfected mice by treatment with anti-L3T4 antibodies. J Immunol. 1987;139:545–550. [PubMed] [Google Scholar]
- 29.Nabors G S, Afonso L C C, Farrell J P, Scott P. A switch from a Th2 to Th1 type response and cure of established Leishmania majorinfection in mice is induced by combined therapy with interleukin 12 and pentostam. Proc Natl Acad Sci USA. 1995;92:3142–3146. doi: 10.1073/pnas.92.8.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nathan C, Nogueira N, Juangbhanich C, Ellis J, Cohn Z. Activation of macrophages: in vivo and in vitro correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J Exp Med. 1979;149:1056. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nathan C F, Hibbs J B. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3:65. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- 32.Orange C A, Wolf S F, Biron C A. Effects of IL-12 on the response and susceptibility to experimental viral infections. J Immunol. 1994;152:1253–1264. [PubMed] [Google Scholar]
- 33.Ortiz-Ortiz L, Ortega T, Capin R, Martinez T. Enhanced mononuclear phagocytic activity during Trypanosoma cruziinfection in mice. Int Arch Allergy Appl Immunol. 1976;50:232–242. doi: 10.1159/000231501. [DOI] [PubMed] [Google Scholar]
- 34.Reed S G. In vivo administration of recombinant IFNγ induces macrophage activation, and prevents acute disease, immune suppression, and death in experimental Trypanosoma cruziinfections. J Immunol. 1988;140:4342–4347. [PubMed] [Google Scholar]
- 35.Santos F R, Pena S D J, Epplen J T. Genetic and population study of a Y-linked tetranucleotide repeat DNA polymorphism. Hum Genet. 1993;90:655–656. doi: 10.1007/BF00202486. [DOI] [PubMed] [Google Scholar]
- 36.Schlemper B R, Jr, Avila C M, Coura J R, Brener Z. Course of infection and histopathological lesions in mice infected with seventeen Trypanosoma cruzistrains isolated from chronic patients. Rev Soc Bras Med Trop. 1983;16:23–30. [Google Scholar]
- 37.Silva J S, Morrissey P J, Grabstein K H, Mohler K M, Anderson D, Reed S G. Interleukin-10 and interferon-γ regulation of experimental Trypanosoma cruziinfection. J Exp Med. 1992;175:169–174. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva L H P, Nussenzweig V. Sobre uma cepa de Trypanosoma cruzialtamente virulenta para camundongo branco. Folia Clin Biol. 1953;20:191–207. [Google Scholar]
- 39.Sypeck T, Chung C L, Mayor S E H, Subramanyam J M, Goldman S J, Sieburth D S, Wolf S F. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective type 1 immune response. J Exp Med. 1993;177:1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarleton R L. Depletion of CD8+ T cells increases susceptibility and reverses vaccine-induced immunity in mice infected with Trypanosoma cruzi. J Immunol. 1990;144:717–724. [PubMed] [Google Scholar]
- 41.Tarleton R L, Koller B H, Latour A, Postan M. Susceptibility of β2-microglobulin-deficient mice to Trypanosoma cruziinfection. Nature. 1992;356:338–340. doi: 10.1038/356338a0. [DOI] [PubMed] [Google Scholar]
- 42.Texeira A R, Arganaraz E R, Freitas L H, Jr, Lacava Z G, Santana J M, Luna H. Possible integration of Trypanosoma cruzikDNA minicircles into the host cell genome by infection. Mutat Res. 1994;305:197–209. doi: 10.1016/0027-5107(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 43.Toledo M J O, Machado G B N, Pereira M E S, Brener Z. Results of treatment in mice immunosuppressed inoculated with different Trypanosoma cruzistrains. Mem Inst Oswaldo Cruz. 1991;86:237. [Google Scholar]
- 44.Torrico F, Heremans H, Rivera M T, Van Mark E, Billiau A, Carlier Y. Endogenous IFNγ is required for resistance to acute Trypanosoma cruziinfection in mice. J Immunol. 1991;146:3626–3632. [PubMed] [Google Scholar]
- 45.Trinchieri G. Interleukin 12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 46.Tripp C S, Gately M K, Hakini J, Ling P, Unanue E R. Neutralization of IL-12 decreases resistance to Listeria in SCID and CB-17 mice, reversal by IFNγ. J Immunol. 1994;152:1883–1887. [PubMed] [Google Scholar]
- 47.Urbina J A, Payares G, Molina J, Sanoja C, Liendo A, Lazarde K, Piras M, Piras R, Peres N, Wincker P, Ryley J. Cure of a short- and long-term experimental Chagas’ disease using DO870. Science. 1996;273:969–971. doi: 10.1126/science.273.5277.969. [DOI] [PubMed] [Google Scholar]
- 48.Vespa G N R, Cunha F Q, Silva J S. Nitric oxide is involved in the control of Trypanosoma cruziinduced parasitemia and directly kills parasite in vitro. Infect Immun. 1994;62:5177–5182. doi: 10.1128/iai.62.11.5177-5182.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization. Chagas disease-frequency and geographical distribution. Weekly Epidemiol Rec W H O. 1990;65:125–135. , 257–261. [PubMed] [Google Scholar]
- 50.World Health Organization. Tropical disease research. Twelfth Programme of UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR). Geneva, Switzerland: World Health Organization; 1995. Chagas disease; pp. 125–135. [Google Scholar]
- 51.Zhou P, Sieve M C, Bennett J, Kwon-Chung K J, Tewari R P, Gazzinelli R T, Sher A, Seder R A. IL-12 prevents mortality in mice infected with Histoplasma capsulatumthrough induction IFNγ. J Immunol. 1995;155:785–795. [PubMed] [Google Scholar]
- 52.Zhou P, Sieve M C, Tewari R P, Seder R A. Interleukin-12 modulates the protective immune response in SCID mice infected with Histoplasma capsulatum. Infect Immun. 1997;65:1–7. doi: 10.1128/iai.65.3.936-942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]