Abstract
Objective
We aimed to compare the safety and efficacy of a doxycycline-based regimen against Cameroon National Standard Guidelines (hydroxychloroquine plus azithromycin) for the treatment of mild symptomatic COVID-19.
Methods
We conducted an open-label, randomized, non-inferiority trial in Cameroon comparing doxycycline 100 mg, twice daily for seven days versus hydroxychloroquine 400 mg daily for five days and azithromycin 500 mg at day 1 and 250 mg from day 2 through 5 in mild COVID-19 patients. Clinical recovery, biological parameters, and adverse events were assessed. The primary outcome was the proportion of clinical recovery on days 3, 10, and 30. Non-inferiority was determined by the clinical recovery rate between protocols with a 20-percentage points margin.
Results
One hundred and ninety-four participants underwent randomization and were treated either with doxycycline (n = 97) or hydroxychloroquine-azithromycin (n = 97). On day 3, 74/92 (80.4%) participants on doxycycline versus 77/95 (81.1%) on hydroxychloroquine-azithromycin-based protocols were asymptomatic (p = 0.91). On day 10, 88/92 (95.7%) participants on doxycycline versus 93/95 (97.9%) on hydroxychloroquine-azithromycin were asymptomatic (p = 0.44). On day 30, all participants were asymptomatic. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) polymerase chain reaction (PCR) test was negative on day 10 in 60/92 (65.2%) participants who were assigned to doxycycline and in 63/95 (66.3%) participants who were assigned to hydroxychloroquine-azithromycin. None of the participants were admitted for worsening of the disease after treatment initiation.
Conclusion
Doxycycline 100 mg twice daily for seven days proved to be safe and non-inferior in terms of efficacy when compared to hydroxychloroquine-azithromycin for preventing clinical worsening of mild symptomatic or asymptomatic COVID-19 and achieving virological suppression.
Keywords: sub-saharan africa, mild covid-19, azithromycin, hydroxychloroquine, doxycycline
Introduction
Since the outbreak of COVID-19, no confirmed successful targeted treatment has emerged [1] for mild or moderate forms of disease. Nonetheless, symptomatic treatments and several therapeutic agents have demonstrated some clinical efficacy in clinical trials [2], including remdesivir, tocilicizumab, and convalescent plasma. The combination of hydroxychloroquine and azithromycin initially gained widespread use appearing in many national therapeutic guidelines mostly in Africa [1], which has a past history of extensive use of chloroquine for malaria treatment and prophylaxis. On the basis of in vitro evaluation against the severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and SARS-CoV-2 and observational data [3], hydroxychloroquine and azithromycin remain the mainstay treatment of mild to moderate COVID-19 in many sub-Saharan African countries [4], despite concerns about possible adverse drug reactions when used in combination at high doses. In the absence of approved treatment for SARS-CoV-2, numerous alternative empirical treatments are being suggested. These comprise contemporary and traditional pharmacopeia with known anti-inflammatory and antiviral properties.
Doxycycline is a derivative of tetracycline that possesses broad antimicrobial and anti-inflammatory activities [5,6]. Doxycycline was FDA-approved as an antibiotic in 1967 and to this day remains in the antibiotic arsenal for diverse clinical use [7]. It has demonstrated antimicrobial, antiparasitic, and antiviral properties in several studies [8], including against some coronaviruses. Moreover, it is generally well accepted and tolerated in clinical settings [8].
The main objective of treating mild and moderate forms of COVID-19 is to prevent adverse progression to severe presentation requiring admission and/or intensive care. We thus aimed to compare the safety and efficacy of doxycycline to that of the national standard guidelines (hydroxychloroquine plus azithromycin) for the treatment of mild symptomatic and asymptomatic COVID-19 patients receiving ambulatory care in Cameroon to prevent the disease from evolving to more severe forms potentially requiring admission.
This article was previously posted to the medRxiv preprint server in July 2021.
Materials and methods
Study design
The trial was an open-label, randomized, non-inferiority trial to evaluate the safety and efficacy of doxycycline therapy versus Cameroon National Standard therapy (hydroxychloroquine + azithromycin) in ambulatory patients with mild symptomatic or asymptomatic COVID-19.
Study site and period
The trial was conducted at the Yaoundé Central Hospital Coronavirus Treatment Center between March 16 and April 09, 2021. The Yaoundé Central Hospital is a second-category hospital in the Cameroon health pyramid. It is a decentralized structure of the Ministry of Public Health and has a capacity of about 650 beds for all specialties and a considerable human resource of more than 600 employees. During the COVID-19 pandemic, it was one of the main hospitals that took care of infected patients.
Study participants
Eligible participants were 18 years of age or older, who had COVID-19 infection confirmed by SARS-CoV-2 real-time polymerase chain reaction (RT-PCR) test and were asymptomatic or were mildly symptomatic with an SpO2 >94%. An asymptomatic patient was defined as a patient with a positive COVID-19 test with no symptoms [9]. A mild symptomatic patient was defined as a patient with a positive COVID-19 test with any of the following symptoms, including fever less than 103°F (39.4°C), fatigue, cough, sore throat, headache, muscle pain, malaise, nausea, vomiting, loss of taste and smell, lack of appetite, and nasal congestion [9]. The patients were excluded if they were pregnant, breastfeeding, had currently taken trial study medication, had any contraindication to study medication, had known severe cardiac disease, renal or liver insufficiency, respiratory rate ≥30/min, and BP ˂90/60 mmHg. All participants gave written informed consent before any trial procedures were performed.
Randomization, allocation concealment, and blinding
The participants were randomly assigned, in a 1:1 ratio, to receive hydroxychloroquine + azithromycin combination or doxycycline combination in a central procedure performed before the start of the trial. Randomization codes were computer-generated, using the Random allocation software (Randomization Main) with a one-block size. Randomly generated treatment allocation was given to clinical trial medical doctors within sealed opaque envelopes. Once a patient had consented to enter the trial, the corresponding envelope was opened, and the patient was then offered the allocated treatment regimen.
Study procedures and intervention
Patients assigned to the study arm received a fixed-dose combination of doxycycline (100 mg PO) twice daily for seven days. Patients assigned to the National guideline received hydroxychloroquine 400 mg daily for five days; azithromycin 500 mg on day 1 and 250 mg from day 2 to day 5. Concomitant medications included vitamin C 1000 mg once daily for five days, and oral zinc 20 mg once daily for five days in both arms.
Data collection and management
Trial visits were scheduled for the baseline, day 3, day 10, and day 30 to evaluate the safety of the trial drugs. RT-PCR control tests to evaluate the efficacy of the treatment were performed on day 10. Blood samples were collected following good laboratory practice by venipuncture of the brachial vein in a 5 ml dry tube without a tourniquet. These samples were collected at baseline and day 10 for full blood count and glycemia.
Study outcomes
The primary endpoint was the rate of clinical recovery determined by the percentage of participants who became or remained asymptomatic. Key secondary endpoints included the percentage of participants requiring hospitalization due to worsening symptoms, as well as the proportion of participants displaying a negative SARS-CoV-2 PCR test. Changes in full blood count and glycemia from the initial measurement were also evaluated. All endpoints were assessed on day 3, day 10, and day 30 in relation to the baseline. Safety assessments involved monitoring of adverse events and mortality, occurring from the first administration of the study treatment to the final follow-up visit on day 30 in accordance with the protocol.
Sample size and statistical analyses
The study was designed with 80% power to test the primary hypotheses, employing a non-inferiority margin of 20% and type 1 error of 0.05 giving a minimum sample size of 194 participants. We computed a two-sided 95% confidence interval (CI) to assess the disparity between treatments with regard to the proportion of patients achieving clinical recovery, using the unstratified approach outlined by Miettinen and Numinen [10].
The condition to define the non-inferiority between doxycycline and hydroxychloroquine-azithromycin-based protocols was established if the lower limit of the two-sided 95% CI for the treatment difference (doxycycline minus hydroxychloroquine-azithromycin) was greater than -20%. Additionally, a p-value was calculated for the corresponding one-sided non-inferiority hypothesis test. Efficacy, safety, and patient-reported outcomes were analyzed within the Intention-to-treat population. All statistical analyses were carried out using SPSS version 23.0 (IBM Corp, Armonk, NY).
Data Safety Monitoring Board
An independent data and safety monitoring committee revised safety and efficacy data during the trial. The data safety monitoring committee was able to pause the trial or give suggestions on potential safety issues to improve the study design and intervention.
Results
Trial population
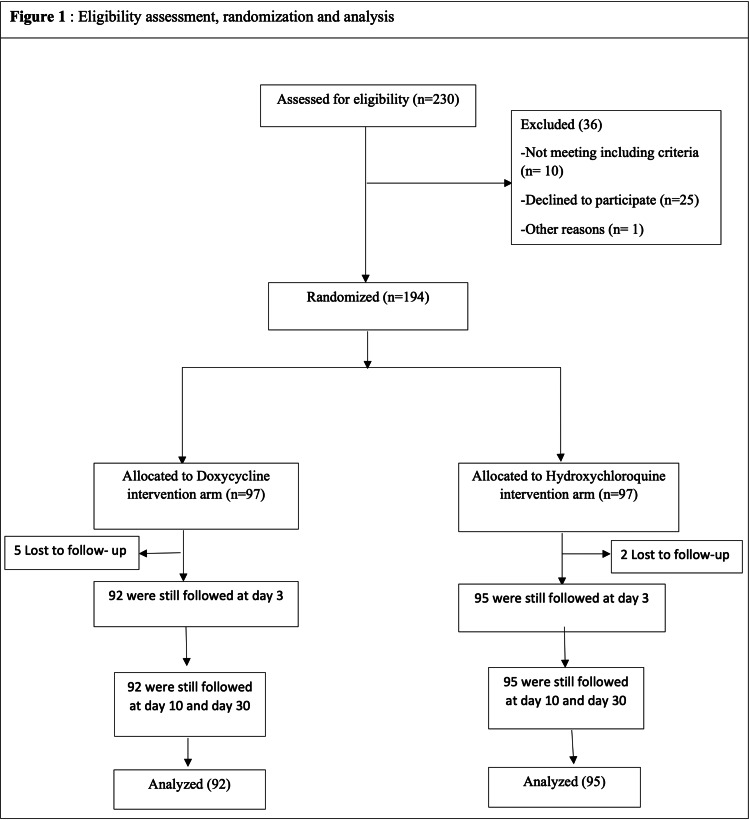
From the 194 participants who underwent randomization, at least one dose of the assigned treatment was administered (Figure 1). Baseline and disease characteristics of participants are shown in Table 1. The mean age was 39 years and 47.6% of participants were women. 42.2% (79/187) of the analyzed participants were mildly symptomatic at baseline (42/92 on the doxycycline baseline regimen versus 37/95 on the hydroxychloroquine-azithromycin arm treatment) (Table 1). The most common symptoms were fatigue, muscle or joint pain, anosmia, cough, headache, and chill.
Table 1. Baseline characteristics.
HTN: hypertension; SD: standard deviation; RBC: red blood cell; HGB: hemoglobin; HCT: hematocrit; MCV: mean corpuscular volume; WBC: white blood cell; LYM: lymphocyte; NEUT: neutrophil; PLT: platelet; DRx: doxycycline; HCTa: hydroxychloroquine + azithromycin; FBC: full blood count.
| Baseline characteristics | DRx (N = 92) | HCTa (N = 95) | Total (N = 187) | p-Value |
| Age - years, mean (SD) | 38 (±13) | 39 (±14) | 39 (±13) | 0.76 |
| Number (%) | ||||
| Sex female - n (%) | 49 (53.3) | 40 (42.1) | 89 (47.6) | 0.14 |
| Comorbidities | ||||
| Asthma (%) | 2 (2.2) | 1 (1.1) | 3 (1.6) | |
| HIV (%) | 0 (0.0) | 1 (1.1) | 1 (0.5) | 0.55 |
| Treated HTN (%) | 1 (1.1) | 1 (1.1) | 2 (1.1) | |
| No comorbidities (%) | 89 (96.7) | 92 (96.7) | 181 (96.8) | |
| Baseline symptoms | ||||
| Asymptomatic | 50 (54.3) | 58 (61.1) | 108 (57.8) | 0.55 |
| Mild illness | 42 (45.7) | 37 (38.9) | 79 (42.2) | |
| Baseline FBC | Mean (±SD) | |||
| RBC tera/l | 5.0 (±1.0) | 5.0 (±1.1) | 5.0 (±1.0) | 0.95 |
| HGB g/l | 13.1 (±2.9) | 13.1 (±3.6) | 13.1 (±3.3) | 0.93 |
| HCT % | 45.6 (±11.2) | 45.8 (±12.5) | 45.7 (±11.8) | 0.92 |
| MCV fl | 91.8 (±7.9) | 89.1 (±12.2) | 87.9 (±8.5) | 0.11 |
| WBC giga/l | 5.3 (±2.1) | 5.0 (±1.8) | 5.2 (±2.0) | 0.53 |
| LYM giga/l | 2.2 (±0.8) | 2.1 (±0.8) | 2.1 (±0.9) | 0.36 |
| NEUT giga/l | 3.0 (±1.6) | 2.6 (±1.1) | 2.8 (±1.4) | 0.08 |
| PLT giga/l | 204 (±75.7) | 202 (±72.9) | 203 (±74.1) | 0.95 |
| Baseline glycemia | ||||
| Glycemia | 0.94 (±0.2) | 1.0 (±0.6) | 0.98 (±0.3) | 0.32 |
Figure 1. Eligibility assessment, randomization, and analysis.
Clinical and patient-reported outcomes
Doxycycline was non-inferior to hydroxychloroquine-azithromycin for the primary endpoint (Table 2). On day 3, the clinical recovery rate was at 80.4% (74/92) for doxycycline and 81.1 % (77/95) for hydroxychloroquine-azithromycin [difference (95%) CI, 0.6% (-0.1, 0.1)]. On day 10, the clinical recovery rate was at 95.7% (88/92) for doxycycline and 97.9 % (93/95) for hydroxychloroquine-azithromycin [difference (95%) CI, 2.2% (-0.07, 0.03)]. On day 30, all the participants were asymptomatic.
Table 2. Clinical recovery rate by protocol visit.
DRx: doxycycline; HCTa: hydroxychloroquine + azithromycin.
| Clinical status | DRx n (%) | HCTa n (%) | CI | p-Value |
| Day3 | ||||
| Asymptomatic | 74 (80.4) | 77 (81.1) | [-0.1, 0.1] | 0.91 |
| Mild Illness | 18 (19.6) | 18 (18.9) | ||
| Day 10 | ||||
| Asymptomatic | 88 (95.7) | 93 (97.9) | [-0.07, 0.03] | 0.44 |
| Mild Illness | 4 (4.3) | 2 (2.1) | ||
| Day30 | ||||
| Asymptomatic | 92 (100) | 95 (100) |
SARS-CoV2 PCR testing
The rate of SARS-CoV2 PCR negative test on day 10 was 65.2% (60/92) in the doxycycline arm and 66.3% (63/95) in the hydroxychloroquine + azithromycin arm. The difference between treatment groups was 1.1 percentage points (95% CI, [-0.15, 0.13]; p > 0.05) (Table 3).
Table 3. Percentage of negative SARS-CoV-2 RT-PCR test on day 10 .
DRx: doxycycline; HCTa: hydroxychloroquine + azithromycin; SARS-CoV2, severe acute respiratory syndrome coronavirus 2; RT-PCR, real-time polymerase chain reaction.
| Assessment visit day 10 | DRx n (%) | HCTa n (%) | Difference, % (95% CI) | p-Value |
| Negative PCR-COVID-19 | 60 (65.2) | 63 (66.3) | 1.1 [-0.15, 0.13] | >0.05 |
Adverse events
No major adverse event was reported. None of our participants was admitted for worsening of the disease after treatment initiation. There was no difference between treatment arms regarding changes in full blood count and glycemia from baseline (Table 4).
Table 4. Biological parameters of participants on day 10.
SD: standard deviation; RBC: red blood cell; HGB: hemoglobin; HCT: hematocrit; MCV: mean corpuscular volume; WBC: white blood cell; LYM: lymphocyte; NEUT: neutrophil; PLT: platelet; DRx: doxycycline; HCTa: hydroxychloroquine + azithromycin.
| Biological parameters | Day 10 | CI | p-Value | |
| DRx | HCTa | |||
| Mean (SD) | ||||
| RBC tera/l (SD) | 4.6 (±0.8) | 4.8 (±0.5) | [-0.4, 0.1] | 0.21 |
| HGB g/l (SD) | 12.2 (±3.8) | 12.2 (±1.7) | [-1.1, 1.2] | 0.93 |
| HCT % (SD) | 41.9 (±6.8) | 41.3 (±8.8) | [-2.5, 3.7] | 0.69 |
| MCV fl (SD) | 90.3 (±7.3) | 88.9 (±7.8) | [-1.5, 4.4] | 0.33 |
| WBC giga/l (SD) | 5.2 (±2.4) | 5.0 (±1.3) | [-0.6, 0.9] | 0.64 |
| LYM giga/l (SD) | 2.1 (±0.8) | 2.2 (±0.7) | [-0.4, 0.1] | 0.32 |
| NEUT giga/l (SD) | 3.0 (±1.3) | 2.8 (±1.3) | [-0.3, 0.7] | 0.46 |
| PLT giga/l (SD) | 247 (±72.6) | 230 (±75.8) | [-12.0, 46.0] | 0.25 |
| Glycemia (SD) | 0.99 (±0.1) | 0.95 (±0.2) | [-0.06, 0.15] | 0.41 |
Discussion
Since the outbreak of COVID-19 in December 2019, the world has been going through waves of the pandemic of widely variable levels of clinical severity. Up to 80% of people affected develop an asymptomatic or mildly symptomatic disease. It remains unclear whether a specific treatment is required for mild COVID-19. However, taking into consideration the risk of adverse progression toward severe and critical forms of the disease, several therapeutic approaches are proposed worldwide to prevent the need for hospitalization and admission to critical care units.
In early 2020, a small series of COVID-19 patients treated in France with hydroxychloroquine showed a rapid decline in SARS-CoV-2 viral load compared with controls, which seemed further improved by the addition of azithromycin [11]. However, methodological flaws were reported, limiting the interpretation of the results [12], in addition to a few other conflicting trials on the use of hydroxychloroquine, and its possible cardiac adverse events [3]. Nevertheless, some countries, especially in sub-Saharan Africa, still recommend hydroxychloroquine-azithromycin as the reference treatment in the management of COVID-19 patients [13]. The development of vaccines is expected to drastically reduce hospital and intensive care admissions as well as deaths. In the meantime, many other treatment options for mild and moderate COVID-19 were investigated to mitigate progression toward severe disease, hospitalization, or death with variable efficacy, including mostly drugs with antiviral and anti-inflammatory effects [14,15].
In this randomized, open-label trial, we compared doxycycline with hydroxychloroquine-azithromycin for the treatment of ambulatory patients with mild symptomatic or asymptomatic COVID-19. None of the participants progressed to severe disease nor required hospital admission, and we showed the non-inferiority of a seven-day course of doxycycline to a five-day course of hydroxychloroquine-azithromycin in terms of clinical recovery rate, virological suppression, and safety profile. No major adverse event was recorded possibly due to the young age of the study population, the safe dosage used in our participant (400 mg hydroxychloroquine and 250 mg azithromycin), and prior exposures to the study drugs for malaria, and in accordance with our preliminary safety study [16].
Our results support the observation by Mohammud M Alam et al. in 2021 in the United States of America, where they evaluated the clinical outcomes of early treatment with doxycycline for 89 high-risk COVID-19 patients in long-term care facilities, and found that early treatment with doxycycline for high-risk patients with moderate-to-severe COVID-19 infections in non-hospital settings is associated with early clinical recovery, decreased hospitalization, and decreased mortality [17]. The present trial was not placebo-controlled, but the >95% cure rate on day 10 contrasts with the 70% reported in placebo groups on day 14 in another trial [18].
While the study population was relatively young with limited comorbidities, it reflects the general population of Cameroon and most sub-Saharan countries. In 2019, the Cameroon Demographic Health Survey estimated that 60% of the population is aged 20-40 years, and only 14% of the population is above 40 [19]. In the earlier stages of the COVID-19 pandemic, older age was considered among the important factors associated with clinical severity [20]; however, recent reports indicated, in more than 3000 adults ages 18-34 who contracted COVID-19 and became sick enough [20], to require hospital care, 21% ended up in intensive care, 10% were placed on a breathing machine, and 2.7% died. Young adults are therefore a major target population for interventions that would reduce progression to severity to mitigate the public health and economic effects of the pandemic.
There are several limitations to this study. Primarily, the absence of a placebo arm within the study to assess the significance of the two treatments being investigated for COVID-19 management. However, it is important to acknowledge that during the period of conducting this research, obtaining ethical approval from the Cameroon National Ethics Committee to conduct a study wherein certain participants would not receive any treatment after testing positive for COVID-19 would have presented significant ethical challenges and constraints. Other limitations of this study include the absence of follow-up of participants at the end of the trial to find out long-term adverse drug effects. Finally, although the study recorded a manageable rate of loss to follow-up, a comprehensible outcome assessment for all initially enrolled participants could not be achieved.
Conclusions
In conclusion, we did not observe any significant difference in terms of safety and efficacy between doxycycline and hydroxychloroquine-azithromycin for the management of mild COVID-19. Future research should consider including a cohort of participants who do not receive any treatment. The group would be monitored for the same study timeline, with a focus on assessing identical outcomes, and would help evaluate the necessity of these two regimens in the management of COVID-19 in a sub-Saharan population. A post-study follow-up might also be necessary for all the participants to evaluate the impact of COVID long and drug adverse effects.
Acknowledgments
We gratefully acknowledge the funding from the French Embassy in Cameroon, the RSD-Institute for logistic support, the personnel of the COVID-19 unit of the Yaoundé Central Hospital, and all the participants who voluntarily accepted to be enrolled in this study. The trial was registered on ClinicalTrials.gov under number NCT04715295. ES conceived and coordinated the study. SZ participated in the design of the study, data collection, data analysis and coordination. MGF participated in the design of the study. JCK participated in the design of the study. CK participated in the design of the study. LMK participated in the design of the study. AZ participated in the design of the study and data analysis. YW participated in the data collection. AAN participated in the data collection. ANM participated in the data collection. JST participated in the design, WN participated in the design, CMO participated in the design, JCM participated in the design, POZ participated in the design, and PJF participated in the design. All authors read and approved the final manuscript. Correspondence concerning this article should be addressed to Professor Eugene Sobngwi, Department of Internal Medicine and Specialties, Faculty of Medicine and Biomedical Sciences, University of Yaoundé 1, Yaoundé, Cameroon. Email: eugene.sobngwi@fmsb-uy1.cm.
The authors have declared that no competing interests exist.
Funding Statement
We gratefully acknowledge the funding from the French Embassy in Cameroon, the RSD-Institute for logistic support.
Human Ethics
Consent was obtained or waived by all participants in this study. Cameroon National Ethics Committee issued approval No. 2021/03/1336/CE/CNERSH/SP. The Cameroon National Ethics Committee approves for a period of one year the implementation of this version of the protocol.
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Evaluation of the efficacy and safety of intravenous remdesivir in adult patients with severe COVID-19: study protocol for a phase 3 randomized, double-blind, placebo-controlled, multicentre trial. Wang Y, Zhou F, Zhang D, et al. Trials. 2020;21:422. doi: 10.1186/s13063-020-04352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.A review of potential suggested drugs for coronavirus disease (COVID-19) treatment. Tarighi P, Eftekhari S, Chizari M, Sabernavaei M, Jafari D, Mirzabeigi P. Eur J Pharmacol. 2021;895:173890. doi: 10.1016/j.ejphar.2021.173890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, Gold HS. JAMA Cardiol. 2020;5:1036–1041. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Effective treatment of urethritis. A practical guide. Bowie WR. Drugs. 1992;44:207–215. doi: 10.2165/00003495-199244020-00005. [DOI] [PubMed] [Google Scholar]
- 5.The history of the tetracyclines. Nelson ML, Levy SB. Ann N Y Acad Sci. 2011;1241:17–32. doi: 10.1111/j.1749-6632.2011.06354.x. [DOI] [PubMed] [Google Scholar]
- 6.Doxycycline as a potential partner of COVID-19 therapies. Malek AE, Granwehr BP, Kontoyiannis DP. IDCases. 2020;21:0. doi: 10.1016/j.idcr.2020.e00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Gautret P, Lagier JC, Parola P, et al. Int J Antimicrob Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Chloroquine and hydroxychloroquine for the prevention or treatment of COVID-19 in Africa: caution for inappropriate off-label use in healthcare settings. Abena PM, Decloedt EH, Bottieau E, et al. Am J Trop Med Hyg. 2020;102:1184–1188. doi: 10.4269/ajtmh.20-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inhibitory effect of doxycycline against dengue virus replication in vitro. Rothan HA, Mohamed Z, Paydar M, Rahman NA, Yusof R. Arch Virol. 2014;159:711–718. doi: 10.1007/s00705-013-1880-7. [DOI] [PubMed] [Google Scholar]
- 10.Lu K. Cochran-Mantel-Haenszel Weighted Miettinen & Nurminen Method for Confidence Intervals of the Difference in Binomial Proportions from Stratified 2x2 Samples. Henehan M, Montuno M, De Benedetto A. https://www.markstat.net/en/images/stories/lu_asa_2008.pdf J Eur Acad Dermatol Venereol. 2008;31:1800–1808. [Google Scholar]
- 11.Clinical outcomes of early treatment with doxycycline for 89 high-risk COVID-19 patients in long-term care facilities in New York. Alam MM, Mahmud S, Rahman MM, Simpson J, Aggarwal S, Ahmed Z. Cureus. 2020;12:0. doi: 10.7759/cureus.9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigorous randomized controlled trial implementation in the era of COVID-19. Oldenburg CE, Doan T. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7253099/ Am J Trop Med Hyg. 2020;102:1154–1155. doi: 10.4269/ajtmh.20-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Molina JM, Delaugerre C, Le Goff J, Mela-Lima B, Ponscarme D, Goldwirt L, de Castro N. Med Mal Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doxycycline as a modulator of inflammation in chronic wounds. Wilcox JR, Covington DS, Paez N. https://europepmc.org/article/med/25876218. Wounds. 2012;24:339–349. [PubMed] [Google Scholar]
- 15.National Institute of Health. COVID-19 Treatment Guidelines. Clinical Spectrum of SARS-CoV2 Infection. [ Mar; 2023 ]. 2023. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
- 16.COVID-19: In Cameroon, the Raoult Method Established as a State Protocol. [ Aug; 2023 ];https://www.france24.com/fr/20200502-covid-19-au-cameroun-la-m%C3%A9thode-raoult-%C3%A9rig%C3%A9e-en-protocole-d-%C3%A9tat 2020 15:2021. [Google Scholar]
- 17.Electrocardiographic safety of daily hydroxychloroquine 400 mg plus azithromycin 250 mg as an ambulatory treatment for COVID-19 patients in Cameroon. Mfeukeu-Kuate L, Ngatchou WD, Temgoua MN, et al. World J Cardiovasc Dis. 2021;11:106–112. [Google Scholar]
- 18.Hydroxychloroquine in nonhospitalized adults with early COVID-19: A randomized trial. Skipper CP, Pastick KA, Engen NW, et al. Ann Intern Med. 2020;173:623–631. doi: 10.7326/M20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID‐19 patients. Zhang J, Cao Y, Tan G, et al. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7404752/ Allergy. 2021;76:533–550. doi: 10.1111/all.14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johns Hopkins Medecine. Coronavirus and COVID- 19: Younger Adults Are at Risk, Too. [ Aug; 2023 ]. 2020. https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/coronavirus-and-covid-19-younger-adults-are-at-risk-too https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/coronavirus-and-covid-19-younger-adults-are-at-risk-too