Abstract
Heme-copper oxygen reductases are membrane-bound oligomeric complexes that are integral to prokaryotic and eukaryotic aerobic respiratory chains. Biogenesis of these enzymes is complex and requires coordinated assembly of the subunits and their cofactors. Some of the components are involved in the acquisition and integration of different heme and copper (Cu) cofactors into these terminal oxygen reductases. As such, MFS-type transporters of the CalT family (e.g., CcoA) are required for Cu import and heme-CuB center biogenesis of the cbb3-type cytochrome c oxidases (cbb3-Cox). However, functionally homologous Cu transporters for similar heme-Cu containing bo3-type quinol oxidases (bo3-Qox) are unknown. Despite the occurrence of multiple MFS-type transporters, orthologs of CcoA are absent in bacteria like Escherichia coli that contain bo3-Qox. In this work, we identified a subset of uncharacterized MFS transporters, based on the presence of putative metal-binding residues, as likely candidates for the missing Cu transporter. Using a genetic approach, we tested whether these transporters are involved in the biogenesis of E. coli bo3-Qox. When respiratory growth is dependent on bo3-Qox, because of deletion of the bd-type Qox enzymes, three candidate genes, yhjE, ydiM, and yfcJ, were found to be critical for E. coli growth. Radioactive metal uptake assays showed that ΔydiM has a slower 64Cu uptake, whereas ΔyhjE accumulates reduced 55Fe in the cell, while no similar uptake defect is associated with ΔycfJ. Phylogenomic analyses suggest plausible roles for the YhjE, YdiM, and YfcJ transporters, and overall findings illustrate the diverse roles that the MFS-type transporters play in cellular metal homeostasis and production of active heme-Cu oxygen reductases.
Introduction
Respiratory complexes are oligomeric membrane proteins with multiple cofactors, which are widely distributed among prokaryotes and eukaryotes. Their biogenesis is an intricate process involving the insertion of appropriate cofactors into the subunits and assembly of mature subunits into functional enzymes [1–3]. The cytochrome c oxidases (Cox) and quinol oxidases (Qox) catalyze the terminal steps of aerobic respiration, which is a four-electron reduction of oxygen to water [4–6]. Both enzymes are multi-heme complexes containing different types of hemes (a, b, and c) [4–6]. However, the two types of oxidases are different with respect to their electron donors as substrates. Cox employs extra-cytoplasmic water-soluble or membrane-attached c-type cytochromes, whereas Qox uses lipid-soluble membrane-integral quinones. The aa3-type cytochrome c oxidase (aa3-Cox or mitochondrial complex IV) contains two a-type (a and a3) hemes and also two Cu centers (CuA with two Cu atoms and CuB with one Cu atom near of the Fe atom of heme a3) [7–9]. The cbb3-type cytochrome c oxidase (cbb3-Cox) is exclusively found in prokaryotes, and it contains three c-type (co, cp1 and cp2) hemes, two b-type (b and b3) hemes and only one Cu atom near heme b3 iron at the CuB center) [10–12]. Some Qox enzymes, like the Escherichia coli bo3-type Qox (bo3-Qox), also contain one Cu atom at their CuB center [13] like that of the aa3-Cox or the cbb3-Cox [14,15]. The nature of the cofactors, subunit structures, and electron donors vary among the heme-Cu oxygen reductases but their catalytic Fe-CuB centers remain conserved [15,16]. Besides bo3-Qox, E. coli has two other bd-type Qox enzymes (bd-Qox1 and bd-Qox2) involved in aerobic respiration, but they contain no Cu atom [6,17,18].
Earlier studies indicated that covalent insertion of the c-type hemes to apoproteins is carried out by the cytochrome c maturation (ccm) systems (e.g., cbb3-Cox) [19]. The Ccm systems operate independently from the insertion of axially coordinated a-, o-, and b-type hemes [2,12]. Coordination of the b-type hemes to the apoproteins may be spontaneous, like the soluble four-helical cytochrome b562 [20]. In other cases, the process might be chaperone-assisted, like the a-type hemes of aa3-Cox that rely on the Surf-like (Surf1 or Shy1) proteins [21–24]. In Paraccocus denitrificans Surf1 [23] and in Thermus thermophilus Surf1q and CbaX [25] are essential to produce active ba3-type quinol oxidases (ba3-Qox), possibly needed for the insertion of the a-type hemes. Similarly, the insertion of the b-type hemes to the facultative photosynthetic model organism Rhodobacter capsulatus cbb3-Cox requires the CcoS protein [26,27]. While the small protein CydX was proposed to position/stabilize the b-type hemes of the bd-type quinol oxidase [18,28,29], this process remains unknown in bo3-Qox.
In contrast to the heme groups, Cu insertion into the Cox enzymes has been studied in more detail. In Rhodobacter species, the mitochondrial Sco-like proteins [30] SenC or PrrC [31–34], PCuAC-like (PccA) [32,33,35], and Cox11 [36–39] chaperones are involved in this process [39–42]. In the case of cbb3-Cox, Cu is imported by a MFS-type transporter (CcoA) and reduced via a cupric reductase (CcoG) on its way to the cytoplasm [2,43]. Then, Cu is channeled through a specific chaperone (CopZ) and a P1B-type transporter (CcoI, CtpA or CopA2) to the periplasmic Sco-like and PCuAC-like chaperones [2,26,44,45], in its way to the CuB center of cbb3-Cox [2,46–48]. In R. capsulatus, ccoA mutants are cbb3-Cox Cu-deficient and unable to import radioactive 64Cu [46,47]. This cytoplasmic deficiency can be rescued either by exogenous Cu supplementation, or by deletion of the P1B-type Cu exporter CopA, involved in excretion of excess Cu out of the cytoplasm. Remarkably, similar studies in Rhodobacter sphaeroides indicated that CcoA is solely dedicated to Cu insertion into the cbb3-Cox and is not required for the similar heme-CuB center of aa3-Cox [49]. For the eukaryotic aa3-Cox, Cu located in the mitochondrial intermembrane space is conveyed to the CuA center via Cox17 [40–42]. Although no homologue of Cox17 exists in prokaryotes, recently, the Bradyrhizobium japonicum ScoI homologue and the thioredoxin TlpA were shown to metalate in vitro the CuA center of cognate aa3-Cox [50,51]. Apparently, distinct Cu routes for the biogenesis of similar centers occur in species containing different types of Cox enzymes.
The superfamily of MFS-type transporters belongs to one of the largest groups of secondary active transporters and are exceptionally diverse and ubiquitous to all three kingdoms of living organisms. They selectively transport a wide range of substrates, including sugars, amino acids, peptides, and antibiotics [52]. Despite their structural similarities, members of this superfamily are divided into many families and subfamilies, classified in the IUBMB-approved Transport Classification Database (TCDB, http://www.tcdb.org), based on the diversity of their substrates and their modes of transport (uniporters, symporters, and antiporters). To date, about 105 families of the MFS-type transporters are reported [53], and among them about 28 are classified as Uncharacterized Major Facilitators (UMFs). The CalT subfamily is defined based on their conserved MXXXM and HXXXM motifs [49] and phylogenetic relatedness. They also frequently co-occur with the Cox enzymes [48,49]. The R. capsulatus CcoA is the founding member of this subfamily as the first bacterial Cu uptake transporter involved in the biogenesis of the cbb3-Cox [46], and is also the first MFS-type transporter that uses Cu as a substrate [48,49]. Some CcoA-distant members (i.e., the RfnT-like proteins) of the CalT family are also Cu transporters but they do not provide Cu to the cbb3-Cox [48], suggesting that they might play a role in the biogenesis of other cupro-enzymes.
In this work, the role of MFS-type transporters of unknown function (UMFs) in E. coli bo3-Qox biogenesis was investigated employing a genetic approach. Using mutants lacking both the bd-Qox1 and bd-Qox2 enzymes, where the bo3-Qox was the only intact terminal oxidase, the uncharacterized MFS-type transporters YhjE, YdiM, and YfcJ were shown to be required to produce active bo3-Qox to support E. coli aerobic respiration. Of these UMFs, YhiE and YdiM affected cellular Fe and Cu homeostasis, respectively, suggesting that MFS-type transporters are required for the biogenesis of different heme-Cu oxygen reductases, possibly as metal or related compound transporters.
Materials and methods
Growth conditions, strains and plasmids used
The bacterial strains and plasmids used in this work are described in S1 Table in S1 File. All E. coli K-12 strains were grown at 37°C on Luria Bertani (LB) enriched or M9 minimal media, supplemented with ampicillin (Amp, 100 μg/ml) and kanamycin (Km, 50 μg/ml), as appropriate. For anaerobic growth, liquid cultures in filled vessels and plates placed in anaerobic jars with H2+CO2 generating gas-packs (Becton, Dickinson and Co., MD) were used. The optical density (OD600) of cells in liquid cultures were monitored at 600 nm.
KanS derivatives of the MFS-type transporter mutants
The putative MFS-type transporter mutants ΔsetC, ∆yhjE, ∆yhjX, ∆ynfM, ∆ydiM, ∆yebQ, ∆yfcJ, ∆araJ and the ∆cyoB mutant were obtained from the E. coli Keio library and were KanR [54]. In each case, the kanamycin cassette was removed by introduction of the Flp recombinase carried by the plasmid pEL8 (pCP20) [55], which is AmpR and temperature sensitive (Ts) for replication. After electroporation, AmpR mutants harboring pEL8 were grown at 30° C on LB containing ampicillin to allow excision of the kan cassette via its FRT sites located adjacent to it. Plates were transferred to 42° C to eliminate the AmpR provided by pEL18, and the genotypes of the KanS and AmpS colonies were confirmed by PCR using appropriate primers (S2 Table in S1 File).
Construction of the Δbd-Qox1and Δbd-Qox1+Δbd-Qox2 knockout derivatives of selected MFS-type transporter mutants
The KanS and AmpS derivatives of chosen MFS-type transporter mutants were used as recipients to knockout the bd-Qox1 and bd-Qox2 by P1 transduction. The Δbd-Qox1 derivatives of the MFS-type transporter mutants were obtained by using a P1 lysate grown on fresh cultures of the cydB:kan (Δbd-Qox1) strain, in LB medium supplemented with 0.2% glucose and 5 mM CaCl2. Before use, the P1 lysates were sterilized with a few drops of chloroform, and the recipient cells were mixed with the P1(cydB::kan) lysate (at 1:1 v/v ratio), incubated 20 min at 37°C, supplemented with one volume of 1M CaCl2 and further incubated for 40 min at 37°C in LB medium. The KanR (i.e., Δbd-Qox1) transductants were selected on kanamycin containing plates supplemented with 5 mM sodium citrate to chelate Ca++ required for P1 reinfection. Following extensive purification, the genotypes of the double (i.e., ΔMFS + Δbd-Qox1) mutants were confirmed by PCR using the primers listed in S2 Table in S1 File.
To construct the triple (i.e., ΔMFS + Δbd-Qox1 + Δbd-Qox2) mutants, the KanR marker in the cydB gene of the ΔMFS + Δbd-Qox1 double mutants was removed using pEL8 as described above. The KanS derivatives thus obtained were used to knock out the bd-Qox2 by transduction using a P1 lysate obtained by growth on the ∆appB::kan (bd-Qox2) mutant. The triple mutants lacking both the Δbd-Qox1, Δbd-Qox2 and the desired deletion of MFS-type transporter were selected on kanamycin containing plates, and their genotypes confirmed by PCR using the primers listed in S2 Table in S1 File.
RNA isolation and RT-PCR assays
The E. coli cells used for RNA isolation and subsequent RT-PCR analyses were grown aerobically at OD600 of 0.05, 0.1 (early growth) and 0.15 (late growth), as needed. Prior to RNA extraction, the cultures were washed with sterile water treated with two volumes of “RNAprotect Bacteria Reagent” (Qiagen). The total RNA was extracted using the Qiagen RNeasy mini kit according to the “Enzymatic Lysis of Bacteria” protocol of the manufacturer. 10 μg of total RNA was digested with RNAse-free Dnase I from Qiagen for 25 min at room temperature, followed by overnight precipitation using 20 μl of NaOAc (3M, pH 5.5), 3 μl of glycogen (5mg/ml), and 600 μl ethanol in a final volume of 800 μl. 2 ng of total RNA were used for RT-PCR analyses with OneStep RT-PCR kit from Qiagen using the CyoAQ-F/CyoAQ-R (327 bp amplicon), CyoBQ-F2/CyoBQ-R3 (322 bp amplicon), CyoCQ-F/CyoCQ-R (344 bp amplicon), and CyoDQ-F/CyoDQ-R (310 bp amplicon) primer pairs (S2 Table in S1 File) to reverse transcribe and amplify separately portions of mRNA specific of cyoA, cyoB, cyoC, and cyoD, respectively. The RrsA-F1 and RrsA-R1 primers were used as an internal control to reverse transcribe and amplify a 100 bp long portion of the 16S ribosomal mRNA. DNA contamination was checked using the master mix containing the heat-inactivated reverse transcriptase (95° C, 15 min) prior to the RT-PCR analyses. The amplified products were separated using 2% agarose gel, and their intensities estimated using ImageJ software (NIH).
Reduced-minus-oxidized optical difference spectra
To monitor the presence of bo3-Qox in appropriate E. coli mutants, optical spectra of n-dodecyl β-D-maltoside (DDM)-solubilized membranes from cells grown aerobically at OD600 of 0.1 were recorded at room temperature between the 500 and 600 nm using a Varian Cary 50 UV-visible spectrophotometer. DDM-solubilized membrane fractions (final concentration of 5 mg/mL) were prepared in 25 mM Tris-HCl pH 7.0, 150 mM NaCl and 1 mM 4-benzenesulfonyl fluoride hydrochloride (AEBSF). Reduced minus oxidized optical difference spectra were obtained by subtracting the spectra of samples fully reduced with sodium dithionite from the spectra of samples fully oxidized with potassium ferricyanide to detect the bo3-Qox.
Determination of the bo3-Qox enzyme activity
The oxygen consumption activity of bo3-Qox was monitored using a Clark-type oxygen electrode (INSTECH, Sys203 model). The cells were grown on LB medium to an OD600 of 0.1, washed with 0.1 M potassium phosphate buffer, pH 7.0 and resuspended in the same buffer to a total of OD600 of 0.5 per assay. 400 μM of ubiquinol-1 was used as an artificial electron donor in the presence of 5 mM of DTT, and the electrode chamber contained one ml of the assay buffer (0.1 M potassium phosphate, pH 7.0, and 0.05% of DDM) at 30°C. The enzymatic reaction was initiated by adding the cells. When tested for inhibitor sensitivity, cells were incubated with either 10 μM of sodium sulfide (Na2S) or 200 μM of potassium cyanide (KCN) for 2 min prior to addition to the reaction mixture. The μM of oxygen consumed/min/OD600 of cells were calculated using the formula: ∆mm-Hg x 236/140/min/OD600 of cells (140 mm-Hg corresponding to 236 μM of oxygen at 30° C was taken as the maximum of oxygen present in the electrode chamber).
Radioactive 64Cu and 55Fe uptake assays using whole cells
Whole cells radioactive 64Cu uptake assays were performed according to [47]. The radioactive 64Cu (1.84 x 104 mCi/μmol specific activity) was obtained from the Mallinckrodt Institute of Radiology, Washington University Medical School. E. coli strains were grown at an OD600 of 0.1 in 10 ml of LB supplemented with the appropriate antibiotics, centrifuged, washed with the assay buffer (50 mM sodium citrate, pH 6.5 and 5% glucose) and re-suspended in one ml of the same buffer. All cultures were normalized to the same number of total cells (7.5 X 108 cells) per 500 μl based on their OD600 values. Cells were pre-incubated at 35° C or 0° C for 10 min before the assay, and the uptake activity was initiated by addition of 107 cpm of 64Cu, determined immediately before use (half-life of 64Cu isotope is ~ 12 h). At each time point, 50 μl of 1 mM CuCl2 and 50 μl of 50 mM EDTA (pH 6.5) were added to an aliquot of 50 μl of assay mixture to stop the uptake reaction, and the samples were placed on ice. At the end of the assay, cells were pelleted, pellets washed twice with 100 μl of ice-cold 50 mM EDTA solution, re-suspended in 1 ml of scintillation liquid, and counted using a scintillation counter (Coulter-Beckman Inc.) with wide open window. The uptake assay with 55Fe (1 μmol correspond to 73 mCi/mg specific activity) was performed essentially as described for 64Cu, except that 1M sodium ascorbate was added to the 55Fe stock solution and incubated for 10 min at room temperature to reduce it prior to the assays. The assays were stopped using 1 mM of FeSO4 instead of CuCl2 and processed as described for the 64Cu uptake assays.
Sequence comparison analyses
The protein sequence similarity networks were constructed using the EFI-EST tool (https://efi.igb.illinois.edu/efi-est/) [56] with an alignment score of 70 (YdiM), 110 (YhjE) or 80 (YfcJ), and nodes were collapsed at a sequence identity of 95% (ydiM) or 100% (YhjE and YfcJ). The networks were visualized with Cytoscape (https://www.cytoscape.org) [57] using the Prefuse Force Directed OpenCL Layout. For phylogenetic analyses, protein sequences were aligned using the CIPRES web portal [58] with MAFFT on XSEDE (v. 7.490) [59], and the IQ-TREE web server for construction of phylogenetic trees under maximum likelihood [60]. Trees were visualized with iTOL [61], and branches with less than 50% bootstrap support were deleted. Gene neighborhoods (a window of 10 genes upstream and downstream of the ydiM, yhjE, or yfcJ homologues) were retrieved using the EFI-GNT tool (https://efi.igb.illinois.edu/efi-gnt/) [56]. The lists of proteins used for bioinformatic analyses can be found in S1 Dataset.
Statistical analyses
In all cases, at least three independent experiments were performed with at least three technical replicates. The error bars reflect the standard deviation with n indicating the number of independent repeats for each experiment. Statistical analyses were performed using the Student t-test with the wild-type activity as reference, and all p-values (when a phenotype is involved) were <0.05 as needed.
Results
Search for distant CcoA homologues among the E. coli MFS-type transporters
Homology searches were performed to identify putative CalT family members in E. coli that contains bo3-Qox, but lacks cbb3-Cox, to inquire whether the two similar heme-CuB center containing enzymes share analogous Cu-uptake pathways. Although CalT homologues are readily identified in species belonging to the Gammaproteobacteria [48,49], including Pseudomonas aeruginosa, Shewanella pealeana, and Vibrio species, none are found in the Enterobacteriaceae, including E. coli (EcoCyc, https://ecocyc.org). Currently there are about 70 ORFs annotated as an “MFS-type transporter” in the genomes of various E. coli strains, and about 28 of them have an unknown function (i.e., UMFs). None of these UMFs contains the conserved hallmark (membrane-integral Cu-binding motifs MXXXM and HXXXM) of the CalT family members [11]. This observation suggested that cytoplasmic import of Cu inserted to the E. coli bo3-Qox CuB center might be delivered by a CalT-unrelated transporter(s), like the R. sphaeroides aa3-Cox [49]. However, this suggestion did not exclude whether any one of the UMFs could be involved in bo3-Qox production. Consequently, these UMFs were scrutinized by aligning their amino acid sequences with that of the canonical CalT member (i.e., R. capsulatus CcoA) and the occurrence of potential metal binding amino acid residues, like Cys, Met and His [62] (Supplementary Materials, S1 Fig). This search yielded eight candidates, yfcJ, yhjX, yebQ, ynfM, ydiM, yhjE, araJ, and setC that were studied further.
MFS-type transporters that affect the bo3-Qox supported respiration in E. coli
E. coli contains three distinct terminal respiratory oxidases, the bo3-Qox, bd-Qox-1 and bd-Qox-2 that convert oxygen to water during respiration. The bo3-Qox is the major enzyme when oxygen concentration is high in the growth media, whereas bd-Qox-1 becomes predominant when the oxygen level is low [63,64]. Simultaneous absence of these enzymes renders E. coli defective for respiration. However, under certain conditions such as carbon and phosphate starvation, a third O2 reductase, the bd-Qox-2 encoded by appBCX, could be induced [65,66]. The occurrence of suppressor mutations that turn on the bd-Qox-2 is frequent, and this event readily overcomes the respiratory defect of a double mutant lacking both bo3-Cox and bd-Qox-1 [67]. Hence, assessing the role, if any, of the UMFs in the production of an active bo3-Cox requires an E. coli strain lacking both the bd-Qox-1 and bd-Qox-2 enzymes. Such a double mutant renders the aerobic respiratory growth of E. coli exclusively dependent on the activity of bo3-Qox. Thus, the double deletion ∆bd-Qox1 + ∆bd-Qox2 strain (strain BF24 with an active bo3-Qox) and the triple ∆bo3-Qox + ∆bd-Qox1 + ∆bd-Qox2 mutant (strain BF17 with an inactive bo3-Qox) were constructed as positive and negative controls for bo3-Qox activity, respectively, using the E. coli K-12 Keio collection library [54] (Materials and Methods). The deletion alleles of the desired UMFs, equally originating from the Keio library, were introduced under anaerobic growth conditions on minimal medium into the double deletion ∆bd-Qox1 + ∆bd-Qox2 background, and their aerobic respiratory growth phenotypes were determined in both minimal (M9) and enriched (LB) media. For the sake of simplicity, these mutants are referred to as bo3+ (double mutant ∆bd-Qox1 + ∆bd-Qox2 with active bo3-Qox), ∆bo3 (triple mutant ∆bo3-Qox + ∆bd-Qox1 + ∆bd-Qox2 with inactive bo3-Qox), and ∆mfs (triple mutant with a chosen ∆mfs + ∆bd-Qox1 + ∆bd-Qox2, where ∆mfs corresponds to ∆yfcJ, ∆yhjX, ∆yebQ, ∆nfM, ∆ydiM, ∆yhjE, ∆araJ, or ∆setC, as appropriate).
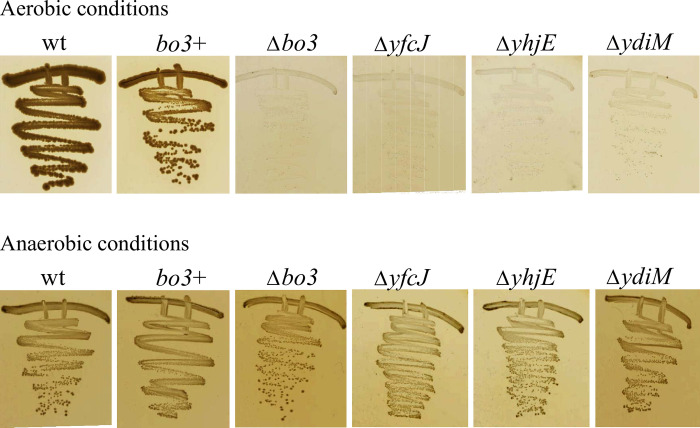
As expected, the bo3+ (∆bd-Qox1 and ∆bd-Qox2) strain grew aerobically, though less vigorously than the wild-type parental E. coli K-12 (BW25113) strain (S1 Table in S1 File), whereas the ∆bo3 strain showed no appreciable respiratory growth (Fig 1, top row). When respiratory growth was dependent solely on bo3-Qox, the MFS-type transporter mutants ∆setC, ∆yhjX, ∆ynfM, ∆yebQ, and ∆araJ were respiration proficient like the bo3+ (∆bd-Qox1 and ∆bd-Qox2) strain. In contrast, the ∆yhjE (BF22), ∆ydiM (BF23) and ∆yfcJ (BF21) mutants exhibited aerobic growth defect like the ∆bo3 strain while their anaerobic growth were fine (Fig 1, top and bottom rows). On aerobic-enriched medium, unlike the remaining ∆mfs derivatives or the bo3+ (∆bd-Qox1 and ∆bd-Qox2) strain that can attain an OD600 of ~ 4 (with 1 h doubling time), the ∆yhjE, ∆ydiM, and ∆yfcJ mutants and the ∆bo3 strain can reach a maximum OD600 of only ~ 0.15 (with ~ 4 h doubling time), indicating that their biomass yields were very low. The growth defect of the ∆yhjE, ∆ydiM and ∆yfcJ mutants in the absence of bd-Qox1 and bd-Qox2 suggested that these UMFs might be required to produce an active bo3-Qox under aerobic growth conditions.
Fig 1. Growth phenotype of ∆yfcJ, ∆yhjE, and ∆ydiM mutants.
The strains were grown on plates under either aerobic (top row) or anaerobic (bottow row) conditions. The parental strain is the E. coli K-12 (BW25113) used to generate the Keo library (Materials and Methods). In the bo3+ strain bo3-Qox is the only functional quinol oxidase, whereas all quinol oxidases are absent in ∆bo3 strain, which does not grow under aerobic conditions. The MFS-type transporters mutants ∆yfcJ, ∆yhjE, and ∆ydiM lack bd-Qox1 and bd-Qox2 quinol oxidases, and unlike the bo3+ strain show poor aerobic growth phenotype like the ∆bo3 mutant bo3-Qox.
Effects of YhjE, YdiM, and YfcJ on the transcription of bo3-Qox
Whether the aerobic growth defect seen in the ∆yhjE, ∆ydiM and ∆yfcJ mutants reflected the lack of transcription of the cyoABCD operon encoding the bo3-Qox subunits was tested. As the aerobic growth is needed to produce the bo3-Qox, transcription of cyoB gene by RT-PCR was used as a proxy for the cyoABCDE operon. Mutant cells grown under aerobic conditions at an OD600 of ~ 0.05 and ~ 0.1 (early stage of growth) showed that the cyoB transcript was detectable in the ∆yhjE, ∆ydiM, and ∆yfcJ mutants (Fig 2A). However, at later growth stages (OD600 of ~ 0.15 or above) where cell growth was arrested, the amounts of cyoB mRNA greatly decreased, possibly reflecting compromised mRNA transcription or stability upon growth stagnation (Fig 2B). Hence, the data indicated that at least at the early stage of growth the absence of yhjE, ydiM or yfcJ did not abolish the transcription of cyoB. Similar data were also obtained for the cyoA, cyoC and cyoD genes (S2 Fig, left lanes). Note that when these mutants were complemented with the multicopy plasmid pJRHisA overexpressing a wild type bo3-Qox, the cyoA, cyoB, cyoC and cyoD transcripts were detectable at all growth stages (S2 Fig, right lanes).
Fig 2. Effects of the absence of YfcJ, YhjE, and YdiM on the transcription of bo3-Qox.
One step RT-PCR was performed on total RNA extract from bo3+ strain as well as ∆bo3 (cyoB), ∆yfcJ, ∆yhjE, and ∆ydiM mutants using the cyoB primers to amplify a 322 bp DNA fragment, and the rrsA primers to amplify a 100 bp region of the 16S ribosomal mRNA as a control (Materials and Methods). (A) Early stage of growth. The cells were grown under aerobic conditions at OD600 of 0.05 (top panel) and 0.1 (bottom panel) where cell division continues. The transcription of cyoB was readily detected and showed no difference at both OD600. (B) Late stage of growth. The cells were grown under aerobic conditions at OD600 of 0.15 (maximum OD600 reached). The transcripts of cyoB gene were barely detectable when the bo3-Qox or the MFS-type transporters YfcJ, YhjE, and YdiM are absent. The numbers below each panel indicate the intensities of the corresponding bands, normalized to that of rrsA then compared to that seen with the bo3+ strain (taken as 100%). These intensities were determined using ImageJ software (NIH). A control PCR where the reverse transcriptase enzyme was inactivated at 95°C was performed for each total RNA extract to check for DNA contamination. Each experiment is repeated at least three times, and a representative sample is shown for each case.
Absence of YhjE or YdiM or YfcJ affects the enzymatic activity of bo3-Qox
The bo3-Qox activities of the ∆yhjE, ∆ydiM and ∆yfcJ mutants (in the ∆bd-Qox1 ∆bd-Qox2 background) were monitored using whole cells at their early stage of growth (at OD600 = 0.1, i.e., cyoABCDE transcript is like the parent), using a Clark-type oxygen electrode and ubiquinol-1 (UQ1) as an electron donor (Materials and Methods). Under these conditions, the bo3+ (∆bd-Qox1 and ∆bd-Qox2) strain exhibited ~ 34 μmoles of O2 consumed/min/OD600 of cells (referred to as 100%). This activity was inhibited by the addition of 10 μM of the bo3-Qox specific inhibitor Na2S (to ~ 15%) or 200 μM of Cox or Qox inhibitor KCN (to ~ 17%) (Table 1), indicating that the measured activity was specific to bo3-Qox. A mutant lacking bo3-Qox (Δbo3) had ~ 2% of O2 consumption activity that decreased by one half upon addition of either Na2S or KCN.
Table 1. The bo3-Qox activity of various strains.
| Strains | UQ1 | UQ1+ Na2S | UQ1+ KCN |
|---|---|---|---|
| a bo 3 + | a100 ± 3.5 | c14.7 ± 2.8 |
c17.0 ± 2.9 |
| ∆bo3 | 2.2 ± 0.2 | 0.7 ± 0.2 | 0.5 ± 2.3 |
| ∆yfcJ | 7.9 ± 3.1 | 1.1 ± 2.3 | 1.5 ± 1.1 |
| ∆yhjE | 4.3 ± 1.1 | 1.8 ± 0.2 | 1.4 ± 0.6 |
| ∆ydiM | 5.0 ± 0.7 | 0.5 ± 0.6 | 2.7 ± 0.7 |
| bbo3+ + pJRhisA | b132.3 ± 0.3 | 22.7 ± 0.7 | 26.3 ± 1.7 |
| ∆bo3 + pJRhisA | 118.5 ± 6.1 | 13.9 ± 1.8 | 19.0 ± 0.5 |
| ∆yfcJ + pJRhisA | 91.1 ± 7.0 | 11.5 ± 1.0 | 21. 8 ± 4.3 |
| ∆yhjE + pJRhisA | 87.0 ± 5.0 | 6.6 ± 2.5 | 15.3 ± 4.4 |
| ∆ydiM +pJRhisA | 49.1 ± 2.4 | 3.8 ± 0.9 | 8.3 ± 3.7 |
The bo3-Cox activities were measured by monitoring the oxygen consumption activities of whole cells using a Clark-type oxygen electrode. The activities were measured by incubating ubiquinol-1 (UQ1) with sodium dithionate at 30° C prior to adding the cells (see Materials and Methods) and all the assays were performed at least three times, with the p values being <0.05 for all mutants.
aThe parental bo3+ strain exhibited ~ 34 mmoles of O2 consumed/min/OD600 of cells and taken as 100%.
b+ pJRhisA refers to the complementation of various mutants with a plasmid harboring bo3-Qox operon [14].
c10 μM of sodium disulfide (Na2S) or 200 μM of potassium cyanide (KCN) were used to inhibit the bo3-Qox activity, as needed.
In comparison, the ∆yhjE, ∆ydiM and ∆yfcJ mutants (in the bd- bo3+ background) exhibited highly decreased activities corresponding to ~ 8%, 4% and 5% compared to the parental strain, and similarly, these activities were inhibited drastically by the addition of Na2S or KCN (Table 1). The data showed that in the absence of the MFS-type transporters YhjE, YdiM, or YfcJ the bo3-Qox activity was drastically reduced, consequently impairing aerobic growth in the absence of the two bd-Qox enzymes. As expected, when the ∆bo3 strain carried a plasmid born copy of cyoABCDE (pJRhisA) (14, 68) its bo3-Qox activity was restored (~ 118.5% ± 6.12), and the bo3+ strain carrying the same plasmid overproduced (~ 132.3% ± 0.31) bo3-Qox activity compared to the bo3+ parental wild-type strain [14,68]. Increased bo3-Qox activity was observed in the ∆yhjE (87.0% ± 5.01) or ∆ydiM (49.11% ± 2.4) or ∆yfcJ (~ 91.17% ± 6.96) mutants when they carried the plasmid pJRHisA, and their aerobic growth defects were at least partially palliated, yielding increased enzymatic activities in all cases (Table 1). In agreement with the earlier transcription profiles, RT-PCR data also indicated that that the plasmid-borne cyoABCDE sustained transcription at later stages of growth (OD600 of 0.15 or above) (S2 Fig, right side).
Absence of YhjE, YdiM, or YfcJ affects the heme composition of bo3-Qox
The dithionite-reduced minus ferricyanide-oxidized optical difference spectra was obtained using membrane fractions of appropriate E. coli mutants grown at an early stage of growth to monitor their b- and o-type heme compositions. The data indicated that the membranes of the bo3+ strain or its derivative overproducing bo3-Qox (bo3+ + pJRhisA), showed a broad band centered at 560 nm with a shoulder at ~ 563 nm (Fig 3), characteristic of the presence of the b and o3 hemes of bo3-Qox [69]. In the ∆bo3 strain this band was drastically reduced, consistent with the absence of the bo3-Qox. The remaining small amounts of absorbance likely reflected possible contamination from the abundant periplasmic cyt b562. Similarly, the membranes of ∆yfcJ, ∆yhjE, or ∆ydiM mutants exhibited trace amount of heme b and o3 spectra (Fig 3), confirming that in the absence of either YfcJ, YhjE, or YdiM the b- and o-type hemes of bo3-Qox were undetectable despite the presence of cyoABCD mRNA, and consistent with the absence of the enzyme activity and defective aerobic growth (Table 1). The overall data indicate that the absence of either YhjE, YdiM, or YfcJ abolishes the production of an active bo3-Qox enzyme when this enzyme is expressed from a chromosomal copy, whereas the effect(s) of these UMFs was still apparent but less pronounced when the cyoABCDE operon was overexpressed from the multicopy plasmid pJRHisA. Combined with the RT-PCR assays, these results suggest that the negative impact of the ∆yhjE, ∆ydiM, and ∆yfcJ mutants on bo3-Qox gene expression does not fully explain the complete loss of activity of bo3-Qox.
Fig 3. b-type heme compositions of the ∆yfcJ, ∆yhjE, and ∆ydiM mutants.
The reduced minus oxidized spectra of the membranes prepared from the bo3+ strain as well as the ∆bo3, ∆yfcJ, ∆yhjE, and ∆ydiM mutants grown under aerobic condition at an OD600 of 0.1. The bd-Qox1 and bd-Qox2 being absent, the observed broad peak at 560 nm in bo3+ and the strain overproducing bo3-Qox (bo3+ + pJRHisA) was taken as corresponding to the hemes b and o3 of bo3-Qox. This peak is drastically reduced in ∆bo3 as well as the ∆yfcJ, ∆yhjE, and ∆ydiM strains. Each experiment is repeated at least three times, and a representative sample is shown for each case.
Cellular 64Cu or 55Fe uptake by mutants lacking either YhjE or YdiM or YcfJ
The E. coli bo3-Qox is a heme-Cu containing enzyme, and some members of MFS-type transporters transport Cu (e.g., CalT family members) [46,47,49] or Fe [70] or siderophores [71,72]. Thus, the ∆yhjE, ∆ydiM and ∆yfcJ derivatives of an otherwise wild-type strain (BW25113) were assessed for their abilities to take up radioactive 64Cu or 55Fe using whole cells at an early stage of their growth (OD600 of 0.1).
In the case of Cu, E. coli wild-type cells (BW25113) showed robust, time dependent and temperature sensitive (35° C versus 4° C) 64Cu uptake kinetics (Fig 4A). Under the same conditions, the ΔyfcJ and ΔyhjE mutants behaved like a wild-type strain in respect to 64Cu uptake kinetics, indicating that the absence of YfcJ or YhjE had no effect on cellular Cu accumulation. The ΔcyoB strain exhibited reduced 64Cu uptake kinetics, suggesting that in the absence of bo3-Qox, the main cupro-enzyme present in E. coli, cellular Cu accumulation decreased, possibly due to Cu homeostasis. Remarkably, the ΔydiM mutant also exhibited slow 64Cu uptake kinetics (Fig 4A) compared with it parental strain, indicating that cellular Cu accumulation decreased. This behavior was reminiscent to that observed with the R. capsulatus ccoA mutant that is defective in 64Cu uptake [47]. As controls, when the assays were performed at 4° C, all strains showed greatly reduced rates of 64Cu uptake.
Fig 4. Whole cell radioactive 64Cu and 55Fe uptake kinetics of the ∆cyo, ∆yfcJ, ∆yhjE, and ∆ydiM mutants.
64Cu (top panel) and 55Fe (bottom panel) uptake kinetics (Materials and Methods) were carried out at 35°C using whole cells grown aerobically until an OD600 of 0.1. In each case, the uptake assays were repeated at least three times using at least three independently grown cells, and the p values were > 0.05 The ∆ydiM and ∆yhjE mutants shows lower 64Cu (p < 0.05) and higher 55Fe (p < 0.05) accumulations in cells, respectively.
In the case of Fe, the uptake of 55Fe-sodium ascorbate (i.e., reduced iron) followed similar kinetics for the wild type and the DycfJ and DydiM mutants, except the ΔyhjE strain that accumulated higher amounts of cellular 55Fe (Fig 4B). Since the assays report the net accumulation of the radioisotope used (i.e., total import minus total export during a given incubation period), the data suggested that the ΔyhjE was either overactive for import, or deficient for export, of cellular Fe leading to gradual accumulation over the time (Fig 4B). As in the case of Cu, when the Fe uptake assays were performed at 4° C, very reduced 55Fe uptake rates were observed. Further, when uptake assays were performed without prior incubation of 55Fe with sodium ascorbate (i.e., with oxidized form of 55Fe), then all strains including the ΔyhjE exhibited comparable 55Fe uptake activities. Thus, YhjE affected the transport of reduced, but not oxidized, form of Fe. Overall, whole cells uptake kinetics indicated that the absence of YdiM and YhjE perturbs Cu and Fe homeostasis, respectively, in E. coli cells. How the cellular imbalance of Cu or Fe in mutants lacking these two MFS-type transporters is linked to the observed bo3-Qox deficiency and aerobic growth defect, requires further studies.
YhjE is related to the putative hydroxy-ethyl-thiazol (HET) and other transporters that cluster with bo3-Qox
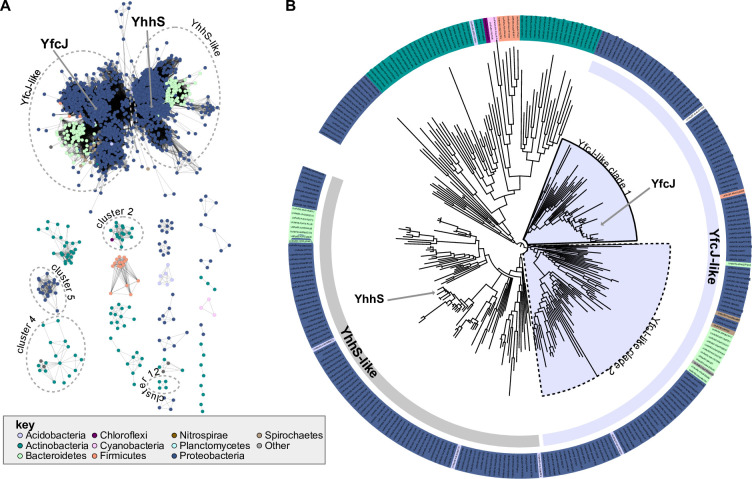
YhjE (TC: 2.A.1.6.10) belongs to a large subfamily of the MFS-type transporters with homologues in most major bacterial phyla. In TCDB, YhjE is listed as a metabolite:H+ symporter (MHS) family member, but its metabolite cargo is unknown. YhjE homologues are identified in beta- and alpha-proteobacteria in addition to gamma-proteobacteria, with sequence similarity hits (based on top 1000 blastP hits against UniProt reference proteomes) being predominately related to proteins from the Proteobacteria (49%) and Actinobacteria (43%). A sequence similarity network analysis indicates that of the previously published MFS-type transporters, YhjE is most similar to ThiU [73] (TC 2.A.1.6.12; putative thiazol transporter according to TCDB) (Fig 5A) (S4 Fig). Based on previous gene clustering and phylogenetic profiling analyses, ThiU is predicted to be a hydroxy-ethyl-thiazole (HET) transporter required for thiamin biosynthesis [73], although this hypothesis has not been tested experimentally. Based on phylogenetic reconstruction, ThiU and YhjE may be paralogs, suggesting that ThiU and YhjE could have separate functions (Fig 5A). As an example, the Haemophilus influenzae genome encodes a ThiU ortholog (HI_0418) located in the thiamine-related genes cluster, and a YhjE ortholog (HI_0281) next to two genes encoding enzymes involved in menaquinone biosynthesis (2-succinyl-6-hydroxy-2, 4-cyclohexadiene-1-carboxylate synthase/2-oxoglutarate decarboxylase (MenD; HI_0283) and menaquinone-specific isochorismate synthase (MenF; HI_0285). Intriguingly, MenD is a thiamine-dependent protein. (Fig 5A). However, this proximity between a gene encoding YhjE-like proteins and a gene encoding MenD is only observed in Haemophilus species.
Fig 5. Sequence similarity analysis of YhjE-like transporters and gene neighborhoods containing its homologues.
(A) Sequence similarity network using an alignment score of 110. Sequences for the network were collected by searching against UniRef50 (500 hits) with YhjE and mapping to UniRef90 for network construction. Experimentally characterized proteins or proteins with predicted functions (in italics) based on bioinformatic analyses are labeled. The taxonomic classification of each node is colored according to the key shown on top left. (B) iqTREE using edited MAFFT alignment based on UniRef50 sequences, and (C) examples of gene neighborhoods containing a YhjE-like MFS transporter with the key defining them located at the bottom right.
Noticeably, YhjE-like proteins are identified in some Proteobacterial genomes that are encoded by genes next to the cyoABCD operons, encoding the structural subunits of bo3-Qox (Fig 5B). Except for the gene clusters from Thiotrichales, Chromatiales, and Hyphomicrobiales, where the yhjE-like gene is in the same operon with cyoABCD, most yhjE-like genes are found in the opposite orientation, suggesting that although yhjE and cyoABCD may not form an operon, still they might be co-regulated (Fig 5C). These YhjE-like proteins, although not connected to the main sequence similarity network cluster (i.e., have an Evalue > 1E-70 with any other protein in the main cluster), might be closely related to YhjE in the phylogenetic tree (Fig 5B). This observation suggests that the role of YhjE in bo3-Qox function is likely conserved outside of E. coli.
The ydiM-like genes are linked to the shikimate pathway
In the E. coli K-12 genome, ydiM (TC: 2.A.1.15.12) is located next to its paralog ydiN (TC: 2.A.1.15.13), and two other genes encoding two enzymes in the shikimate pathway, aroD and ydiB, encoding 3-dehydroquinate dehydratase and quinate/shikimate dehydrogenase enzymes, respectively. The shikimate pathway is a major link between carbohydrate metabolism and the biosynthesis of aromatic compounds via chorismate, a precursor of aromatic amino acids phenylalanine, tyrosine, and tryptophan. The cargo of YdiN is not listed in TCDB, but it was previously hypothesized to transport a shikimate by-product [74] based on the genomic context and co-expression data of the ydiN, aroD, and ydiB genes. YdiM is listed as a putative isoprenol exporter due to increase susceptibility that it provides to E. coli upon its deletion [75].
Phylogenetic and genomic context analyses of YdiM and YdiN homologues further defined their relationship to the shikimate pathway. The YdiM and the YdiN orthologous group are largely limited to Enterobacterales genomes and not widespread in Proteobacteria. YdiM/YdiN-like proteins are frequently found in Firmicute genomes, represented by YfkL in Bacillus subtilis (Fig 6). Accordingly, among YdiM homologues (top 1000 blastP hits) ~ 57% are from Firmicutes and ~ 23% are from Proteobacteria. Remarkably, ~ 75% of YdiM/YdiN homologues analyzed here are encoded by a gene that is adjacent (on either the 5’ or 3’ side) to a gene encoding an enzyme in the shikimate pathway (Fig 6). Moreover, this frequency increases to ~ 86% when a larger (20 instead of the usual 10) genes window is used, showing a clear link between the YdiM and YdiN subfamily of the MFS-type transporters and the enzymes of the shikimate pathway (Fig 7). In addition to E. coli, other Enterobacteriaceae genomes including Shigella flexneri, Salmonella typhimurium, and Citrobacter tructae also encode both YdiM and YdiN located next to the shikimate pathway genes ydiB and aroD (Figs 6 and 7). However, Klebsiella pneumoniae encodes only YdiM, and in such Enterobacterial genomes that lack a YdiN ortholog, ydiM is found between aroD and ydiB in a putative operon (Fig 7A), further linking YdiM with the shikimate pathway. Moreover, in multiple Firmicutes ydiM genes encoding YdiM orthologs are often physically located next to genes encoding shikimate pathway enzymes (Fig 7B), and even in a handful of cases, the YdiM orthologs are fused to chorismate mutase (Fig 7C and 7D). Chorismate mutase is one of the seven enzymes that form the shikimate pathway (Fig 7E) and is often found fused to other enzymes of this pathway and thought to serve regulatory purposes [76]. Of the genes analyzed here, ydiM and ydiN homologues are most often near the enzymes catalyzing the early steps of the shikimate pathway, starting from quinate (i.e., ydiB>aroD>aroK) compared to others operating in the pathway (Fig 7E). The overall findings indicated that the YdiM-like proteins are closely associated with the shikimate pathway, and consistent with YdiM and YdiN performing different transport function(s) related to the aromatic acid synthesis pathway.
Fig 6. Phylogenomic analysis of the YdiM/YdiN subfamily.
(A) Phylogenetic tree of YdiM, YdiN, and their YdmiM-like and YdiN-like homologues. The taxonomic classification of each leaf, presence of the chorismite mutase fusion, and whether the corresponding gene is next to a gene encoding a shikimate pathway enzyme are indicated with inner rings according to the key shown at the bottom of the figure. Lines colored by taxonomic classification connecting two leaves are used to indicate that those two proteins are encoded by the same genome. The innermost grey ring corresponds to the clusters depicted in panel B, and YfkL indicates the homologue present in Bacillus subtilus (Bs). Gene neighborhoods from clades with background shading are shown in Fig 6. Sequences are the 250 best hit from blastp against UniProt reference proteomes. The 10 most similar proteins to YdiM in E. coli, Clostridioides difficile, Klebsiella pneumoniae, Bacillus subtilis were used as an outgroup to root the tree. (B) Sequence similarity network (SSN) of YdiM/YdiN homologues. Nodes are colored by taxonomy according to the key shown at the bottom of the figure, and clusters are labeled as in the innermost grey ring of panel A. Edge-weighted Spring Embedded Layouts using % id for clustering. (C) SSN as in panel B but colored based on presence of neighbor gene(s) encoding enzyme(s) in the shikimate pathway, and (D) shows the nodes in red representing the YdiM orthologs with a chorismate mutase fusion.
Fig 7. Gene neighborhoods containing ydiM/ydiN homologues.
(A) Gene neighborhoods of the YdiM and (B) gene neighborhoods of the YdiN clades. (C) Representatives from Cluster 4 shown in Fig 5. (D) Alphafold prediction depicting the YdiM-chorismate mutase fusion from Fructobacillus durionis, showing its two distinct domains. (E) Shikimate pathway from quinate to 4-hydroxy-phenylpyruvate and the structural genes of the enzymes involved. A star indicates the step catalyzed by chorismite mutase (CM) that is sometimes found fused to YdiM as shown in (D). The number of times a YdiM/YdiN homologue is encoded in a gene neighborhood with a gene encoding the indicated enzyme is shown as a heatmap (176 red to 35 blue).
Bioinformatic analysis of YfcJ-like proteins
Currently little is known about YfcJ (TC: 2.A.1.46.6) and its homologues. The closest related protein with some associated experimental data is YhhS (TC: 2.A.1.46.7), a paralog of YfcJ in E. coli, which was previously linked to cellular arabinose levels [77] and glyophosate (inhibitor of 5-enolpyruvylshikimate-3-phosphate synthase) resistance [78] based on loss-of-function and gain-of-function experiments, respectively. The sequence similarity network and phylogenetic reconstruction analyses were able to distinguish the YfcJ-like homologues from the closest subfamily composed of YhhS homologues. (Fig 8A and 8B). The YfcJ-like subfamily was mainly identified in Proteobacteria and Bacteroidetes (89% and 8.5% of the homologs, respectively), and within the Proteobacteria, there was a roughly equal split between gamma- (30%), alpha- (30%), and beta- (27%) proteobacteria. Analysis of conserved gene proximity revealed multiple putative operons encoding YfcJ- and YhhS-like transporters. Although defined biochemical functions could readily predicted for proteins encoded by genes neighboring the yfcJ homologues, such as amidohydrolases or tautomerases, no specific pathway or process that may be associated with YfcJ could be predicted (Fig 9, upper part). Note that the clusters 4, 5, and 12 of YhhS homologues are in putative operons with nucleotide metabolism and tRNA-related proteins, linking the YhhS family to nucleotide metabolism and tRNA modification processes based on conserved gene proximity (Fig 9, lower part). Of these clusters, the genes in cluster 4, which is dominated by Actinobacteria, are often in a putative operon with a YacP-like endoribonuclease, and a protein resembling an epoxyqueuosine reductase responsible for a synthesis of queuosine found in some tRNAs. Cluster 5 from Proteobacteria is found in a putative operon with proteins involved in nucleotide metabolism, including a putative hydrolase from the YjjG superfamily involved in cleaving nucleotides with non-canonical nucleotide bases. In cluster 12 the YfcJ- and YhhS-like homologues are in a putative operon with glutamyl-Q tRNA (Asp) synthetase, which is a protein that functions immediately downstream of epoxyqueuosine reductase involved in the synthesis of the hypermodified base glutamyl-queuosine [79]. For cluster 2 in the network, which is largely confined to Actinobacteria, all YfcJ- and YhhS-like homologues are encoded by a gene that is potentially in a biosynthetic gene cluster for an unknown secondary metabolite, suggesting that they may be metabolite transporters.
Fig 8. Sequence similarity analysis of YfcJ homologues.
(A) Sequence similarity network using an alignment score of 80. Sequences for the network were collected by searching against UniProt using YfcJ as a query. Clusters with examples of gene proximity in Fig 9 are circled and labeled. The taxonomic classification of each node is colored according to the key shown at the bottom left of the figure. (B) Protein sequences from the network were mapped to UniRef50 and representative nodes were used to build a phylogenetic tree. The background color of each leaf is colored according to the key. In addition to clear separation from the YhhS-like clade, the YfcJ group can be distinguished into two major clades, indicated as YfcL-like clade 1 and clade 2.
Fig 9. Conserved gene proximity analysis of YfcJ homologues.
Examples of conserved gene neighborhoods encoding proteins from the YfcJ sequence similarity network are shown. Clusters corresponding to genes involved in nucleotide metabolism and tRNA modification (YhhS-like). tRNA modification (cluster 4), nucleotide metabolism (cluster 5) and tRNA modification (cluster 12) are shown with the related enzymes found shown on the right.
Discussion
The MFS-type transporter CcoA (TC: 2.A.1.81.1) of the CalT subfamily is a well-established Cu importer identified in bacteria and required for biogenesis of the cbb3-Cox CuB center [11,46,49]. However, although CcoA is widespread among alpha-proteobacterial species and it frequently co-occurs with the genes encoding aa3-Cox and cbb3-Cox [48,49], it is only required for cbb3-Cox and not the quasi-identical CuB center containing aa3-Cox, as seen with R. sphaeroides [49]. Moreover, no functional ortholog of R. capsulatus CcoA is found among the ~ 70 MFS-type transporter genes of E. coli, suggesting a different mechanism for Cu import and biogenesis for the bo3-Cox CuB center. These observations point out the specificity of the CalT members among the MFS-type transporters and indicate the possible occurrence of different routes for the biogenesis of CuB centers of heme-Cu enzymes (e.g., E. coli bo3-Cox). Indeed, bacterial cbb3-Cox and aa3-Cox require specific transporters and chaperones for the biogenesis of their CuB centers assembly, including the periplasmic Sco-like [31,35] and PCuAC-like chaperones [2,32,33,38]. Moreover, cbb3-Cox requires in addition to the Cu importer CcoA [46] the cupric reductase CcoG [43], and the P1B-type transporter CcoI/CtpA [26,44,45]. Remarkably, none of the latter proteins are involved in the case of the aa3-Cox, which instead uses the Cu chaperone Cox11 [36–38]. How the CuB center insertion occurs in E. coli bo3-Qox is not known, and as a true CalT homologue does not seem to exist in this species, raising the issue of whether any other type of MFS-transporter might accomplish this function.
A survey of the E. coli genome indicated that among the ~ 70 MFS-type transporters, ~ 28 of them (i.e., UMFs) had no identified cargo, and at least eight of them were richly endowed with plausible metal-coordinating amino acid residues. This enticed us to examine the role of these UMFs in bo3-Cox biogenesis, using a genetic screen based on the essentiality for aerobic respiratory growth sustained by this enzyme in the absence the bd-Qox1 and bd-Qox2. This screen identified YhjE, YdiM, and YfcJ as required MFS-type transporters for bo3-Cox dependent aerobic respiratory growth of E. coli. In the absence of any one of these proteins, the bo3-Qox activity and its b- and o-type hemes were absent, even though at low cell-densities detectable amounts of cyoABCD mRNA transcripts were produced. Remarkably, a multicopy plasmid carrying these genes and overproducing the bo3-Qox could bypass at least partially the need for these UMFs. These findings suggested that some regulatory event(s) (e.g., titrating out a regulator) controlling the transcription or destabilizing the transcript(s) might occur in the absence of these UMFs. Alternatively, although these mutants might produce the structural constituents of the bo3-Qox, they could not assemble an active enzyme in the absence of the imported/exported cargo(s). Thus, the specific nature(s) of currently unidentified cargos transported by these MFS-type transporters seem important for bo3-Qox biogenesis. Earlier genetic studies have suggested that yhjE, ydiM, and ycfJ may be involved in transporting an unknown metabolite (see TCDB), isoprenol [75] and arabinose [77], respectively. Here, the whole-cell transport assays further showed that cells without YdiM accumulated less 64Cu, and those without YhjE contained more reduced 55Fe (Fig 4), while no such difference was seen in the absence of YfcJ. Note that currently no conclusive data exist for any of these transporters, as none of them has been purified and shown to bind and transport their putative substrates.
Bioinformatics analysis have been performed to unravel the function of YfcJ, YhjE and YdiM. No link between bo3-Qox and metal transport were found for YfcJ. YhjE was referred to as a member of the metabolite: H+ symporter (MHS) Family (see TCDB), and phylogenomic analyses show that in many bacterial genomes, yhjE gene clusters with the bo3-Qox structural genes cyoABCD, suggesting that the role of YhjE-like transporters in bo3-Qox function could be widely conserved (Fig 7). Based on an analysis of previously published high-throughput (HTP) interaction data [80], YhjE was found to physically interact with FhuA, a ferrichrome outer membrane transporter [81]. Out of 331 identified genetic interactions in a separate study, a positive genetic interaction was identified with fhuA (i.e., the double yhjE fhuA mutant grew better in rich medium than the single mutants), and negative genetic interactions with other Fe transporters (fecA, fecB, fecC, fecD, fepA, fepB, febD, fes, and fhuC) [80]. If these results obtained with HTP studies are not misleading false positives, they could potentially explain the Fe-homeostasis defect in the ∆yhjE strain. A negative genetic interaction was also observed between yhjE and cyoA or cyoB and a positive genetic interaction with cyoC. Such results may suggest that YhjE could have functional roles beyond bo3-Qox biogenesis. Overall, the available experimental and bioinformatic data support that this transporter is required to produce an active bo3-Qox, but the underlying molecular link(s) remains unknown.
YdiM was initially selected as a candidate metal transporter based on the presence of M21XXXXM26 and M76XXM79XXXM83 motifs in its predicted TM1 and TM3, and other motifs in the TM4, and TM6 (S5 Fig). The 3D structural model of YdiM is reminiscent of that of CcoA since the Met residues are positioned in a similar fashion throughout the TMs of both proteins. However, the putative transmembrane metal-binding motif(s) are different from the CalT subfamily members (S5 Fig) [11]. These putative metal binding residues combined with the experimental data presented here suggest that YdiM could be a plausible candidate for Cu transport. In the E. coli K-12 genome ydiM gene and its paralog ydiN are clustered together with several genes involved in the shikimate pathway, which is the metabolic pathway governing biosynthesis of aromatic amino acids, like phenylalanine, tyrosine, and tryptophan [82,83]. Phylogenetic analyses of bacterial species other than E. coli also indicate that ydiM and ydiN cluster frequently with the shikimate pathway genes (Figs 5 and 6). An earlier study indicated that the 3-deoxy-D-arabino-hepulosonate-7-phosphate synthase (DAHP synthase) catalyzing the first step of this pathway binds Cu, suggesting that the DAHP synthase may be a cuproenzyme [84]. However, no conclusive study has been conducted, leaving the identity of the metal of DAHPS contested [85], and the link between Cu, the shikimate biosynthetic pathway, and bo3-Qox remains unclear, deserving future studies.
In summary, this study unexpectedly implicated three MFS-type transporters, YhjE, YdiM and YfcJ in the production of an active bo3-Qox in E. coli. Available data showing impaired Cu and Fe uptake kinetics suggest that YdiM and YhjE are involved in cellular metal homeostasis, which may be essential for the biogenesis of the heme-Cu enzyme bo3-Cox. However, the cargo of these transporters being currently unknown, and their role(s) in specific metabolic pathway(s) undefined, a direct mechanistic link between them and the expression or assembly of the bo3-type Qox remains hypothetical until such data become available. Nonetheless, the overall findings increased the arsenal of the different gene products that cells use to produce heme-Cu enzymes, including the bo3-Qox. These studies also illustrated how broad a biological function the MFS-type transporters may play in cells and spur future investigations to identify the transported substrates and shed light to the mechanistic link(s) between these MFS-type transporters and the biogenesis of heme-Cu containing metalloproteins.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(DOCX)
(XLSX)
Acknowledgments
We thank Drs. R. B. Gennis for the plasmid pJRHisA(cyoABCD), A. Dancis for help with performing Fe uptake kinetics, and M. Goulian for providing E. coli strains, phage, and plasmids.
Abbreviations
- Cu
copper
- cbb 3
Cox
- cbb 3
type cytochrome c oxidase
- bo 3
quinol oxidase
- bo 3
Qox
- HCO
heme-copper oxidases
- TM
transmembrane
- MFS
major facilitator superfamily; reverse transcription-PCR
- RT-PCR
DDM, n-Dodecyl β-D-maltoside
- UQ1
ubiquinol-1
- Na2S
sodium disulfide
- KCN
potassium cyanide
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by DOE grant DE-FG02-91ER20052 (FD). Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231 (CEB-H). Work at the U.S. Department of Energy Joint Genome Institute (https://ror.org/04xm1d337), a DOE Office of Science User Facility, is supported by the Office of Science of the U.S. Department of Energy operated under Contract No. DE-AC02-05CH11231 (CEB-H). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ekici S, Pawlik G, Lohmeyer E, Koch HG, Daldal F (2012) Biogenesis of cbb(3)-type cytochrome c oxidase in Rhodobacter capsulatus. Biochim Biophys Acta 1817:898–910. doi: 10.1016/j.bbabio.2011.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrei A, Ozturk Y, Khalfaoui-Hassani B, Rauch J, Marckmann D, Trasnea PI, Daldal F, Koch HG (2020) Cu Homeostasis in Bacteria: The Ins and Outs. Membranes (Basel) 10. doi: 10.3390/membranes10090242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedrich T, Wohlwend D, Borisov VB (2022) Recent Advances in Structural Studies of Cytochrome bd and Its Potential Application as a Drug Target. Int J Mol Sci 23. doi: 10.3390/ijms23063166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Horsman JA, Barquera B, Rumbley J, Ma J, Gennis RB (1994) The superfamily of heme-copper respiratory oxidases. J Bacteriol 176:5587–600. doi: 10.1128/jb.176.18.5587-5600.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereira MM, Teixeira M (2004) Proton pathways, ligand binding and dynamics of the catalytic site in haem-copper oxygen reductases: a comparison between the three families. Biochim Biophys Acta 1655:340–6. doi: 10.1016/j.bbabio.2003.06.003 [DOI] [PubMed] [Google Scholar]
- 6.Borisov VB, Siletsky SA (2019) Features of Organization and Mechanism of Catalysis of Two Families of Terminal Oxidases: Heme-Copper and bd-Type. Biochemistry (Mosc) 84:1390–1402. doi: 10.1134/S0006297919110130 [DOI] [PubMed] [Google Scholar]
- 7.Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaono R, Yoshikawa S (1996) The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science 272:1136–44. doi: 10.1126/science.272.5265.1136 [DOI] [PubMed] [Google Scholar]
- 8.Ostermeier C, Harrenga A, Ermler U, Michel H (1997) Structure at 2.7 A resolution of the Paracoccus denitrificans two-subunit cytochrome c oxidase complexed with an antibody FV fragment. Proc Natl Acad Sci U S A 94:10547–53. doi: 10.1073/pnas.94.20.10547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zong S, Wu M, Gu J, Liu T, Guo R, Yang M (2018) Structure of the intact 14-subunit human cytochrome c oxidase. Cell Res 28:1026–1034. doi: 10.1038/s41422-018-0071-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buschmann S, Warkentin E, Xie H, Langer JD, Ermler U, Michel H (2010) The structure of cbb3 cytochrome oxidase provides insights into proton pumping. Science 329:327–30. doi: 10.1126/science.1187303 [DOI] [PubMed] [Google Scholar]
- 11.Khalfaoui-Hassani B, Verissimo AF, Koch HG, Daldal F (2016). Uncovering the Transmembrane Metal Binding Site of the Novel Bacterial Major Facilitator Superfamily-Type Copper Importer CcoA. mBio 7:e01981–15. doi: 10.1128/mBio.01981-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khalfaoui-Hassani B, Verissimo AF, Shroff NP, Ekici S, Trasnea P-I, Utz M, Koch H-G, Daldal F (2016) Biogenesis of Cytochrome c complexes: From Insertion of redox cofactors to Assembly of different subunits. Springer, Dordrecht, Netherlands. [Google Scholar]
- 13.Abramson J, Riistama S, Larsson G, Jasaitis A, Svensson-Ek M, Laakkonen L, Puustinen A, Iwata S, Wikstrom M (2000) The structure of the ubiquinol oxidase from Escherichia coli and its ubiquinone binding site. Nat Struct Biol 7:910–7. doi: 10.1038/82824 [DOI] [PubMed] [Google Scholar]
- 14.Chepuri V, Lemieux L, Au DC, Gennis RB (1990) The sequence of the cyo operon indicates substantial structural similarities between the cytochrome o ubiquinol oxidase of Escherichia coli and the aa3-type family of cytochrome c oxidases. J Biol Chem 265:11185–92. [PubMed] [Google Scholar]
- 15.Sousa FL, Alves RJ, Ribeiro MA, Pereira-Leal JB, Teixeira M, Pereira MM (2012) The superfamily of heme-copper oxygen reductases: types and evolutionary considerations. Biochim Biophys Acta 1817:629–37. doi: 10.1016/j.bbabio.2011.09.020 [DOI] [PubMed] [Google Scholar]
- 16.Murali R, Hemp J, Gennis RB (2022) Evolution of quinol oxidation within the heme‑copper oxidoreductase superfamily. Biochim Biophys Acta Bioenerg 1863:148907. [DOI] [PubMed] [Google Scholar]
- 17.Borisov VB, Gennis RB, Hemp J, Verkhovsky MI (2011) The cytochrome bd respiratory oxygen reductases. Biochim Biophys Acta 1807:1398–413. doi: 10.1016/j.bbabio.2011.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safarian S, Rajendran C, Muller H, Preu J, Langer JD, Ovchinnikov S, Hirose T, Kusumoto T, Sakamoto J, Michel H (2016) Structure of a bd oxidase indicates similar mechanisms for membrane-integrated oxygen reductases. Science 352:583–6. doi: 10.1126/science.aaf2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanders C, Turkarslan S, Lee DW, Daldal F (2010) Cytochrome c biogenesis: the Ccm system. Trends Microbiol 18:266–74. doi: 10.1016/j.tim.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch H-G, Schneider D (2016) Assembly of transmembrane b-type cytochromes amnd cytochrome complexes, p 555–584. In Cramer WA, Kallas T (ed), Cytochrome complexes: evolution, structures, energy transduction and signaling. Springer. [Google Scholar]
- 21.Smith D, Gray J, Mitchell L, Antholine WE, Hosler JP (2005) Assembly of cytochrome-c oxidase in the absence of assembly protein Surf1p leads to loss of the active site heme. J Biol Chem 280:17652–6. doi: 10.1074/jbc.C500061200 [DOI] [PubMed] [Google Scholar]
- 22.Bundschuh FA, Hoffmeier K, Ludwig B (2008) Two variants of the assembly factor Surf1 target specific terminal oxidases in Paracoccus denitrificans. Biochim Biophys Acta 1777:1336–43. doi: 10.1016/j.bbabio.2008.05.448 [DOI] [PubMed] [Google Scholar]
- 23.Bundschuh FA, Hannappel A, Anderka O, Ludwig B (2009) Surf1, associated with Leigh syndrome in humans, is a heme-binding protein in bacterial oxidase biogenesis. J Biol Chem 284:25735–41. doi: 10.1074/jbc.M109.040295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davoudi CF, Ramp P, Baumgart M, Bott M (2019) Identification of Surf1 as an assembly factor of the cytochrome bc(1)-aa(3) supercomplex of Actinobacteria. Biochim Biophys Acta Bioenerg 1860:148033. doi: 10.1016/j.bbabio.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 25.Werner C, Richter OM, Ludwig B (2010) A novel heme a insertion factor gene cotranscribes with the Thermus thermophilus cytochrome ba3 oxidase locus. J Bacteriol 192:4712–9. doi: 10.1128/JB.00548-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch HG, Winterstein C, Saribas AS, Alben JO, Daldal F (2000) Roles of the ccoGHIS gene products in the biogenesis of the cbb(3)-type cytochrome c oxidase. J Mol Biol 297:49–65. doi: 10.1006/jmbi.2000.3555 [DOI] [PubMed] [Google Scholar]
- 27.Kulajta C, Thumfart JO, Haid S, Daldal F, Koch HG (2006) Multi-step assembly pathway of the cbb3-type cytochrome c oxidase complex. J Mol Biol 355:989–1004. doi: 10.1016/j.jmb.2005.11.039 [DOI] [PubMed] [Google Scholar]
- 28.Hoeser J, Hong S, Gehmann G, Gennis RB, Friedrich T (2014) Subunit CydX of Escherichia coli cytochrome bd ubiquinol oxidase is essential for assembly and stability of the di-heme active site. FEBS Lett 588:1537–41. doi: 10.1016/j.febslet.2014.03.036 [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Luo Q, Yin J, Gao T, Gao H (2015) Evidence for the requirement of CydX in function but not assembly of the cytochrome bd oxidase in Shewanella oneidensis. Biochim Biophys Acta 1850:318–28. doi: 10.1016/j.bbagen.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 30.Ekim Kocabey A, Kost L, Gehlhar M, Rodel G, Gey U (2019) Mitochondrial Sco proteins are involved in oxidative stress defense. Redox Biol 21:101079. doi: 10.1016/j.redox.2018.101079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lohmeyer E, Schroder S, Pawlik G, Trasnea PI, Peters A, Daldal F, Koch HG (2012) The ScoI homologue SenC is a copper binding protein that interacts directly with the cbb(3)-type cytochrome oxidase in Rhodobacter capsulatus. Biochim Biophys Acta 1817:2005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trasnea PI, Utz M, Khalfaoui-Hassani B, Lagies S, Daldal F, Koch HG (2016) Cooperation between two periplasmic copper chaperones is required for full activity of the cbb3 -type cytochrome c oxidase and copper homeostasis in Rhodobacter capsulatus. Mol Microbiol 100:345–61. doi: 10.1111/mmi.13321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trasnea PI, Andrei A, Marckmann D, Utz M, Khalfaoui-Hassani B, Selamoglu N, Daldal F, Koch HG (2018) A Copper Relay System Involving Two Periplasmic Chaperones Drives cbb(3)-Type Cytochrome c Oxidase Biogenesis in Rhodobacter capsulatus. ACS Chem Biol 13:1388–1397. doi: 10.1021/acschembio.8b00293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canonica F, Klose D, Ledermann R, Sauer MM, Abicht HK, Quade N, Gossert AD, Chesnov S, Fischer HM, Jeschke G, Hennecke H, Glockshuber R (2019) Structural basis and mechanism for metallochaperone-assisted assembly of the Cu(A) center in cytochrome oxidase. Sci Adv 5:eaaw8478. doi: 10.1126/sciadv.aaw8478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banci L, Bertini I, Ciofi-Baffoni S, Katsari E, Katsaros N, Kubicek K, Mangani S (2005) A copper(I) protein possibly involved in the assembly of CuA center of bacterial cytochrome c oxidase. Proc Natl Acad Sci U S A 102:3994–9. doi: 10.1073/pnas.0406150102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hiser L, Di Valentin M, Hamer AG, Hosler JP (2000) Cox11p is required for stable formation of the Cu(B) and magnesium centers of cytochrome c oxidase. J Biol Chem 275:619–23. doi: 10.1074/jbc.275.1.619 [DOI] [PubMed] [Google Scholar]
- 37.Carr HS, George GN, Winge DR (2002) Yeast Cox11, a protein essential for cytochrome c oxidase assembly, is a Cu(I)-binding protein. J Biol Chem 277:31237–42. doi: 10.1074/jbc.M204854200 [DOI] [PubMed] [Google Scholar]
- 38.Thompson AK, Gray J, Liu A, Hosler JP (2012) The roles of Rhodobacter sphaeroides copper chaperones PCu(A)C and Sco (PrrC) in the assembly of the copper centers of the aa(3)-type and the cbb(3)-type cytochrome c oxidases. Biochim Biophys Acta 1817:955–64. doi: 10.1016/j.bbabio.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radin I, Kost L, Gey U, Steinebrunner I, Rodel G (2021) The mitochondrial copper chaperone COX11 has an additional role in cellular redox homeostasis. PLoS One 16:e0261465. doi: 10.1371/journal.pone.0261465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horng YC, Cobine PA, Maxfield AB, Carr HS, Winge DR (2004) Specific copper transfer from the Cox17 metallochaperone to both Sco1 and Cox11 in the assembly of yeast cytochrome C oxidase. J Biol Chem 279:35334–40. doi: 10.1074/jbc.M404747200 [DOI] [PubMed] [Google Scholar]
- 41.Banci L, Bertini I, Ciofi-Baffoni S, Hadjiloi T, Martinelli M, Palumaa P (2008) Mitochondrial copper(I) transfer from Cox17 to Sco1 is coupled to electron transfer. Proc Natl Acad Sci U S A 105:6803–8. doi: 10.1073/pnas.0800019105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi H, Jiang Y, Yang Y, Peng Y, Li C (2021) Copper metabolism in Saccharomyces cerevisiae: an update. Biometals 34:3–14. doi: 10.1007/s10534-020-00264-y [DOI] [PubMed] [Google Scholar]
- 43.Marckmann D, Trasnea PI, Schimpf J, Winterstein C, Andrei A, Schmollinger S, Blaby-Haas CE, Friedrich T, Daldal F, Koch HG (2019) The cbb(3)-type cytochrome oxidase assembly factor CcoG is a widely distributed cupric reductase. Proc Natl Acad Sci U S A 116:21166–21175. doi: 10.1073/pnas.1913803116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonzalez-Guerrero M, Raimunda D, Cheng X, Arguello JM (2010) Distinct functional roles of homologous Cu+ efflux ATPases in Pseudomonas aeruginosa. Mol Microbiol 78:1246–58. doi: 10.1111/j.1365-2958.2010.07402.x [DOI] [PubMed] [Google Scholar]
- 45.Hassani BK, Astier C, Nitschke W, Ouchane S (2010) CtpA, a copper-translocating P-type ATPase involved in the biogenesis of multiple copper-requiring enzymes. J Biol Chem 285:19330–7. doi: 10.1074/jbc.M110.116020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ekici S, Yang H, Koch HG, Daldal F (2012) Novel transporter required for biogenesis of cbb3-type cytochrome c oxidase in Rhodobacter capsulatus. mBio 3. doi: 10.1128/mBio.00293-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ekici S, Turkarslan S, Pawlik G, Dancis A, Baliga NS, Koch HG, Daldal F (2014) Intracytoplasmic copper homeostasis controls cytochrome c oxidase production. mBio 5:e01055–13. doi: 10.1128/mBio.01055-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Blaby-Haas CE, Steimle S, Verissimo AF, Garcia-Angulo VA, Koch HG, Daldal F, Khalfaoui-Hassani B (2019) Cu Transport by the Extended Family of CcoA-like Transporters (CalT) in Proteobacteria. Sci Rep 9:1208. doi: 10.1038/s41598-018-37988-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khalfaoui-Hassani B, Wu HJ, Blaby-Haas CE, Zhang Y, Sandri F, Verissimo AF, Koch HG, Daldal F (2018) Widespread Distribution and Functional Specificity of the Copper Importer CcoA: Distinct Cu Uptake Routes for Bacterial Cytochrome c Oxidases. Mbio 9. doi: 10.1128/mBio.00065-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abicht HK, Scharer MA, Quade N, Ledermann R, Mohorko E, Capitani G, Hennecke H, Glockshuber R (2014) How periplasmic thioredoxin TlpA reduces bacterial copper chaperone ScoI and cytochrome oxidase subunit II (CoxB) prior to metallation. J Biol Chem 289:32431–44. doi: 10.1074/jbc.M114.607127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canonica F, Hennecke H, Glockshuber R (2019) Biochemical pathway for the biosynthesis of the Cu(A) center in bacterial cytochrome c oxidase. FEBS Lett 593:2977–2989. doi: 10.1002/1873-3468.13587 [DOI] [PubMed] [Google Scholar]
- 52.Quistgaard EM, Low C, Guettou F, Nordlund P (2016) Understanding transport by the major facilitator superfamily (MFS): structures pave the way. Nat Rev Mol Cell Biol 17:123–32. doi: 10.1038/nrm.2015.25 [DOI] [PubMed] [Google Scholar]
- 53.Wang SC, Davejan P, Hendargo KJ, Javadi-Razaz I, Chou A, Yee DC, Ghazi F, Lam KJK, Conn AM, Madrigal A, Medrano-Soto A, Saier MH Jr (2020) Expansion of the Major Facilitator Superfamily (MFS) to include novel transporters as well as transmembrane-acting enzymes. Biochim Biophys Acta Biomembr 1862:183277. doi: 10.1016/j.bbamem.2020.183277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006 0008. doi: 10.1038/msb4100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Libby EA, Ekici S, Goulian M (2010) Imaging OmpR binding to native chromosomal loci in Escherichia coli. J Bacteriol 192:4045–53. doi: 10.1128/JB.00344-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zallot R, Oberg N, Gerlt JA (2019) The EFI Web Resource for Genomic Enzymology Tools: Leveraging Protein, Genome, and Metagenome Databases to Discover Novel Enzymes and Metabolic Pathways. Biochemistry 58:4169–4182. doi: 10.1021/acs.biochem.9b00735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–504. doi: 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop:1–8. [Google Scholar]
- 59.Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–80. doi: 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trifinopoulos J, Nguyen LT, von Haeseler A, Minh BQ (2016) W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res 44:W232–5. doi: 10.1093/nar/gkw256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Letunic I, Bork P (2021) Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res 49:W293–W296. doi: 10.1093/nar/gkab301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rubino JT, Franz KJ (2012) Coordination chemistry of copper proteins: how nature handles a toxic cargo for essential function. J Inorg Biochem 107:129–43. doi: 10.1016/j.jinorgbio.2011.11.024 [DOI] [PubMed] [Google Scholar]
- 63.Anraku Y, Gennis RB (1987) The Aerobic Respiratory-Chain of Escherichia-Coli. Trends in Biochemical Sciences 12:262–266. [Google Scholar]
- 64.Puustinen A, Finel M, Haltia T, Gennis RB, Wikstrom M (1991) Properties of the two terminal oxidases of Escherichia coli. Biochemistry 30:3936–42. doi: 10.1021/bi00230a019 [DOI] [PubMed] [Google Scholar]
- 65.Dassa J, Fsihi H, Marck C, Dion M, Kieffer-Bontemps M, Boquet PL (1991) A new oxygen-regulated operon in Escherichia coli comprises the genes for a putative third cytochrome oxidase and for pH 2.5 acid phosphatase (appA). Mol Gen Genet 229:341–52. doi: 10.1007/BF00267454 [DOI] [PubMed] [Google Scholar]
- 66.Borisov VB, Murali R, Verkhovskaya ML, Bloch DA, Han H, Gennis RB, Verkhovsky MI (2011) Aerobic respiratory chain of Escherichia coli is not allowed to work in fully uncoupled mode. Proc Natl Acad Sci U S A 108:17320–4. doi: 10.1073/pnas.1108217108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sturr MG, Krulwich TA, Hicks DB (1996) Purification of a cytochrome bd terminal oxidase encoded by the Escherichia coli app locus from a delta cyo delta cyd strain complemented by genes from Bacillus firmus OF4. J Bacteriol 178:1742–9. doi: 10.1128/jb.178.6.1742-1749.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rumbley JN, Furlong Nickels E, Gennis RB (1997) One-step purification of histidine-tagged cytochrome bo3 from Escherichia coli and demonstration that associated quinone is not required for the structural integrity of the oxidase. Biochim Biophys Acta 1340:131–42. doi: 10.1016/s0167-4838(97)00036-8 [DOI] [PubMed] [Google Scholar]
- 69.Puustinen A, Wikstrom M (1991) The heme groups of cytochrome o from Escherichia coli. Proc Natl Acad Sci U S A 88:6122–6. doi: 10.1073/pnas.88.14.6122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laranjeira-Silva MF, Wang W, Samuel TK, Maeda FY, Michailowsky V, Hamza I, Liu Z, Andrews NW (2018) A MFS-like plasma membrane transporter required for Leishmania virulence protects the parasites from iron toxicity. PLoS Pathog 14:e1007140. doi: 10.1371/journal.ppat.1007140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Furrer JL, Sanders DN, Hook-Barnard IG, McIntosh MA (2002) Export of the siderophore enterobactin in Escherichia coli: involvement of a 43 kDa membrane exporter. Mol Microbiol 44:1225–34. doi: 10.1046/j.1365-2958.2002.02885.x [DOI] [PubMed] [Google Scholar]
- 72.Miethke M, Schmidt S, Marahiel MA (2008) The major facilitator superfamily-type transporter YmfE and the multidrug-efflux activator Mta mediate bacillibactin secretion in Bacillus subtilis. J Bacteriol 190:5143–52. doi: 10.1128/JB.00464-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS (2002) Comparative genomics of thiamin biosynthesis in procaryotes. New genes and regulatory mechanisms. J Biol Chem 277:48949–59. doi: 10.1074/jbc.M208965200 [DOI] [PubMed] [Google Scholar]
- 74.Johansson L, Liden G (2006) Transcriptome analysis of a shikimic acid producing strain of Escherichia coli W3110 grown under carbon- and phosphate-limited conditions. J Biotechnol 126:528–45. doi: 10.1016/j.jbiotec.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 75.Wang C, Yang L, Shah AA, Choi ES, Kim SW (2015) Dynamic interplay of multidrug transporters with TolC for isoprenol tolerance in Escherichia coli. Sci Rep 5:16505. doi: 10.1038/srep16505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fan Y, Cross PJ, Jameson GB, Parker EJ (2018) Exploring modular allostery via interchangeable regulatory domains. Proc Natl Acad Sci U S A 115:3006–3011. doi: 10.1073/pnas.1717621115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koita K, Rao CV (2012) Identification and analysis of the putative pentose sugar efflux transporters in Escherichia coli. PLoS One 7:e43700. doi: 10.1371/journal.pone.0043700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Staub JM, Brand L, Tran M, Kong Y, Rogers SG (2012) Bacterial glyphosate resistance conferred by overexpression of an E. coli membrane efflux transporter. J Ind Microbiol Biotechnol 39:641–7. doi: 10.1007/s10295-011-1057-x [DOI] [PubMed] [Google Scholar]
- 79.Blaise M, Becker HD, Lapointe J, Cambillau C, Giege R, Kern D (2005) Glu-Q-tRNA(Asp) synthetase coded by the yadB gene, a new paralog of aminoacyl-tRNA synthetase that glutamylates tRNA(Asp) anticodon. Biochimie 87:847–61. doi: 10.1016/j.biochi.2005.03.007 [DOI] [PubMed] [Google Scholar]
- 80.Babu M, Bundalovic-Torma C, Calmettes C, Phanse S, Zhang Q, Jiang Y, Minic Z, Kim S, Mehla J, Gagarinova A, Rodionova I, Kumar A, Guo H, Kagan O, Pogoutse O, Aoki H, Deineko V, Caufield JH, Holtzapple E, Zhang Z, Vastermark A, Pandya Y, Lai CC, El Bakkouri M, Hooda Y, Shah M, Burnside D, Hooshyar M, Vlasblom J, Rajagopala SV, Golshani A, Wuchty S, J FG, Saier M, Uetz P, T FM, Parkinson J, Emili A (2018) Global landscape of cell envelope protein complexes in Escherichia coli. Nat Biotechnol 36:103–112. doi: 10.1038/nbt.4024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coulton JW, Mason P, DuBow MS (1983) Molecular cloning of the ferrichrome-iron receptor of Escherichia coli K-12. J Bacteriol 156:1315–21. doi: 10.1128/jb.156.3.1315-1321.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herrmann KM (1995) The Shikimate Pathway: Early Steps in the Biosynthesis of Aromatic Compounds. Plant Cell 7:907–919. doi: 10.1105/tpc.7.7.907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mir R, Jallu S, Singh TP (2015) The shikimate pathway: review of amino acid sequence, function and three-dimensional structures of the enzymes. Crit Rev Microbiol 41:172–89. doi: 10.3109/1040841X.2013.813901 [DOI] [PubMed] [Google Scholar]
- 84.Baasov T, Knowles JR (1989) Is the first enzyme of the shikimate pathway, 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase (tyrosine sensitive), a copper metalloenzyme? J Bacteriol 171:6155–60. doi: 10.1128/jb.171.11.6155-6160.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Imlay JA (2014) The mismetallation of enzymes during oxidative stress. J Biol Chem 289:28121–8. doi: 10.1074/jbc.R114.588814 [DOI] [PMC free article] [PubMed] [Google Scholar]