Abstract
The health-beneficial outcomes of doenjang, a Korean fermented food have been questioned due to its high salt content; moreover, the detailed underlying mechanisms of its health beneficial effects are not fully investigated. Thus, this study aimed to investigate doenjang’s anti-obesity effects, anti-hypertensive effects, and its underlying mechanisms in high-fat diet -induced obesity. Sprague-Dawley rats fed with normal diet (ND), high-fat diet (HD), HD with 8% additive salt (HDS), or HD with doenjang containing 8% salt (HDJ) for 13 weeks. Compared to HD and HDS groups, the HDJ group had lower body and epididymal fat tissue weight gain and showed hypotrophy and hypoplasia. The RAS-related mRNA levels in the adipose tissue, including Renin and Ace were downregulated in the HDJ group compared to HD and HDS groups. Additionally, HDJ groups had significant improvements in systolic blood pressure, serum RAS-associated parameters (e.g., angiotensin II and aldosterone), renal mRNA levels related to RAS (e.g., angiotensin II receptor type 1 and 2), and aldosterone-associated mRNA expressions (e.g., mineralocorticoid receptor) in the kidney of HD-induced obese rats. Most importantly, HDS and HDJ groups showed distinct outcomes regarding adipogenesis and electrolytes metabolism, even though both diets contain a high level of salt. HDS group showed a higher epididymal fat tissue weight, mass, and adipocyte size than HDJ group. In addition, compared with HDJ group, HDS group significantly decreased the release of Na+ and K+ throughout the urine and feces. The present study addresses that doenjang has anti-obesity effects and anti-hypertensive effects by activating RAS in the adipose tissue and kidney, respectively. Additionally, this study also demonstrates that salt in doenjang and the additive salt differently influences adipogenesis and electrolytes metabolism, supporting doenjang has health advantageous effects regardless of its high salt contents.
1. Introduction
The imbalance between energy intake and expenditure results in obesity, which is one of the huge burdens for global health [1]; and the prevalence of obesity and obesity-related diseases have been increasing continuously [2]. For example, obesity is one of the major risk factors for hypertension [3]. A significant weight gain is strongly associated with increased blood pressure. Approximately 70% of hypertensive adults have excessive adipose tissues compared to normal individuals and obesity causes hypertension treatment resistance [2, 4].
The renin-angiotensin system (RAS) plays a pivotal role in the control of blood pressure also fluid and electrolyte balance through blood vessel constriction and reabsorption of Na+ and water [5]. Angiotensinogen (Agt) is the precursor of the bioactive angiotensin peptides; and angiotensin I (Ang I) and angiotensin II (Ang II) are produced from the subsequent cleavages of angiotensinogen by two key enzymes, renin and angiotensin-converting enzymes (Ace), respectively [6]. In addition to the roles in blood pressure, earlier studies have reported the significant involvement of RAS in obesity and insulin resistance because of its role in adipogenesis [7]. The level of Agt is positively associated with adipose tissue mass; and the upregulations of renin, Ace, and angiotensin II receptor type 1 (Agtr1) mRNA expression in the adipose tissue are observed in obesity [8, 9]. Indeed, activated RAS is commonly observed in metabolic syndrome patients, including obese subjects [10].
Korean traditional soybean paste, doenjang is one of the representative fermented products in South Korea. Doenjang is made with brine and fermented Meju, then mixed with salt (Fig 1). During the fermentation, the enzymes secreted by Bacillus break down the soybean protein and produce various amino acids and/or peptides, providing its own savory taste. Due to the fermentation and its components (e.g., soybean), doenjang has multiple health-beneficial effects, including anti-obesity effects [11–15]. According to a clinical study, the consumption of doenjang led to the anti-obesity and antioxidant effects, but also improved the overall health-related index in overweight individuals [12]. However, the exact underlying mechanisms of the anti-obesity effects of doenjang are not fully explored.
Fig 1. A diagram of manufacturing process of Doenjang.
A recent study found that doenjang ameliorates high-salt diet-induced hypertension [16]. According to this study, the doenjang-fed group showed a significant reduction in blood pressure and a lower expression of sodium transferase-related genes compared to the group containing the same amount of salt in doenjang [16]. Both high salt diet and high fat diet have been considered as the major dietary factor for the hypertension; and interestingly, several publications presented that high-salt diet and high-fat diet have distinct impacts on the blood pressure, its related mechanisms, and the risk of hypertension [17, 18]. However, anti-hypertensive effects of doenjang in high-fat diet-induced hypertension are unexplored.
Although many previous studies reported the doenjang’s advantageous effects on the health, it is still controversial due to the high salt content in doenjang. This is because an excessive sodium intake increases the risk of hypertension via hypervolemia, promotes heart and kidney disease, and contributes to the development of gastric cancer [19, 20]. However, the addition of salt is essential in the manufacturing process of doenjang, as it inhibits harmful microbial growth, improves the survival of beneficial microbe, prolongs preservation, suppresses undesirable fermentation, and provides its unique tastes [14]. Therefore, it is critical to study the potential effects of high salt content in doenjang when the health beneficial effects of doenjang are investigated.
Therefore, the aims of this study were 1) to investigate whether doenjang influences RAS in adipose tissues to exert anti-obesity effects in HD-induced obesity, 2) to investigate the anti-hypertensive effects of doenjang in HD-induced obesity and its underlying mechanisms, and 3) to prove the high content of salt in doenjang does not have negative effects.
2. Materials and methods
2.1 Preparation of doenjang
Doenjang was produced by the Sunchang Sauce Corporation of South Korea (Sunchang-gun, Jeollabuk-do, Korea). This fermented food is prepared using soybean after maturing it for 6 months, by traditional Korean fermentation process (Fig 1). After steamed soybeans, it was made into blocks, and fermented with Aspergillus oryzae and Bacillus subtilis for one month. After this, it was mixed with brine (saltwater, 26%, w/v) in a 1:3 ratio and further fermented for 2 more months. Once matured, doenjang was dried using a freeze-dryer (FD12008, Ilshin Biobase, Gyeonggi-do, Korea), and its salinity was adjusted with NaCl (Samchun, Pyeongtaek-si, Gyeonggi-do, Korea) to 8%, using Mohr’s method [21].
For the cell treatment, 1 g of each freeze-dried doenjang was mixed with 10 mL of solvent (80% ethanol) at room temperature for 24 h on a shaker. The supernatants were collected and filtered through ADVANTEC No. 2 filter paper, and 1 mL of each filtrate was freeze-dried in a speed vacuum concentrator (FD12008, Ilshin Biobase, Gyeonggi-do, Korea).
2.2 Animals and treatments
Three-week-old male Sprague-Dawley rats were purchased from Central Lab. Animal, Inc. (Seoul, Korea), and acclimated at 12 h light and 12 h dark cycles at 25 ± 2°C and humidity 50% ± 5% conditions. After 7 days adaptation, they were randomly (no diffrence in body weight and initial SBP) divided into 4 groups (n = 6); normal diet control (ND; AIN76A, Research Diets, Inc. New Brunswick, NJ, USA), high-fat diet control (HD; 60% fat by weight, D12492, Research Diets, New Brunswick, NJ, USA), high-fat diet with 8% table salt (HDS), and a high-fat diet with doenjang containing 8% table salt (HDJ). All rats were fed with their corresponding experimental diets (Table 1) for 13 weeks, with water supplied. In addition, the nutritional composition of doenjang is shown in S1 Fig.
Table 1. The composition of experimental diets.
| Ingredient | ND | HD | HDS | HDJ |
|---|---|---|---|---|
| Casein | 20 | 25.85 | 23.96 | 22.93 |
| L-Cystine | 0.3 | 0.39 | 0.36 | 0.34 |
| Corn Starch | 15 | 0 | 0 | 0 |
| Maltodextrin | 16.15 | 14.97 | 14.33 | |
| Sucrose | 50 | 8.89 | 8.24 | 7.89 |
| Cellulose | 5 | 6.46 | 5.99 | 5.73 |
| Corn/Soybean Oil | 5 | 3.23 | 2.99 | 2.87 |
| Lard | 31.66 | 29.35 | 28.08 | |
| Mineral Mix | 3.5 | 1.29 | 1.20 | 1.15 |
| Dicalcium Phosphate | 1.68 | 1.56 | 1.49 | |
| Calcium Carbonate | 0.71 | 0.66 | 0.63 | |
| Potassium Citrate | 2.13 | 1.98 | 1.89 | |
| Vitamin Mix | 1 | 1.29 | 1.20 | 1.15 |
| Choline Bitartrate | 0.2 | 0.26 | 0.24 | 0.23 |
| NaCl (Table salt) | 7.3 | 6.3 | ||
| Dried doenjang | 5 | |||
| total | 100 | 100 | 100 | 100 |
| kcal/g | 3.9 | 5.24 | 4.86 | 4.82 |
ND; AIN76A, Research Diets, Inc. New Brunswick, NJ, USA), (HD; 60% kcal% fat, D12492, Research Diets, New Brunswick, NJ, USA), (HDS; HD + 8% table salt), (HDJ; HD + Doenjang containing 8% salt)
Body weights were noted once a week and food intake were measured each day. Systolic blood pressure (SBP) was recorded weekly by the indirect tail-cuff method (BP-2000, Visitech Systems, Inc., Apex, NC, USA) 30 min after placing them at 37°C. The mean SBP was recorded after 7 measurements. After 10 weeks on each experimental diet, the rats were transferred to the metabolic cages (Med Associates Inc., VT, USA) for 3 days. Rats were kept under constant conditions and fed their respective diets. Urine and feces were collected daily from the metabolic cages into bottles. Additionally, food and water intake, and fecal and urine output were measured and collected for analyses. Mean value of each criterion was recorded for each rat. All animal procedures were approved by the Animal and Use Committee of Jeonbuk National University (JBNU 2018–052).
2.3 Tissue collection
At the end of 13 weeks, rats were fasted for 12 h before anesthetizing with 2 mg/kg BW alfaxalone (Alfaxan; Jurox, Australia) and 0.5 mL/kg BW xylazine (Rompun; Bayer, Seoul, Korea) through intramuscular injection to collect blood and tissues. Liver, epididymal adipose tissue, and one of the kidneys were rinsed with saline, weighed, and immediately frozen in liquid nitrogen and stored at -80°C for further analyses. The other kidney was fixed in 10% formaldehyde and embedded in paraffin. Blood was drawn by orbital vein puncture and centrifuged at 3,000 rpm for 15 min at 4°C to collect serum.
2.4 Biochemical analysis
A blood glucose test was conducted 10 weeks after rats were fed experimental diets. Blood samples were collected from the lateral tail vein after 12 hours of fasting. Blood glucose levels were measured by Accu Check Active Blood Glucose Meter Kit (Roche, Indianapolis, Indiana).
The serum levels of renin, angiotensin II and aldosterone were measured using assay kits (Rat Renin ELISA Kit, MyBioSource, San Diego, CA, USA; Angiotensin II ELISA Kit, and Aldosterone ELISA Kit, Enzo Life Sciences, Inc., Farmingdale, NY, USA), following the manufacturer’s protocols.
2.5 Histology of fat cryosections
Frozen epididymal adipose tissues were cryopreserved in OCT (Scigen Scientific Gardena, CA, USA), and frozen in liquid nitrogen. Sections of 10 μm thickness were cut with cryomicrotome (Shandon Cryotome FE, Thermo Scientific, MA, USA), transferred onto glass slide (Marienfeld, Germany) at -30°C. Sections were stained with hematoxylin and eosin (H&E) and mounted in glycerol gelatin. Cells were observed using an Axiophot Zeiss Z1 microscope (Carl Zeiss, Gottingen, Germany) at X200 magnification, and adipocytes were counted. Difference in cell size in each group were noted.
2.6 RNA extraction and real-time PCR
Total RNA was isolated after homogenizing the tissues in TRIzol reagent (Invitrogen, Grand Island, NY, USA), and the concentration of total RNA was equalized using quantifying on Biodrop Duo (Biochrom, Holliston, MA, USA). cDNA was synthesized using PrimeScript™ RT Master Mix (Takara Bio Inc., Shiga, Japan) according to the manufacturer’s instructions. RNA expression was measured by quantitative real-time polymerase chain reaction (qPCR) using the SYBR Green real-time PCR master mix (TOYOBO, Osaka, Japan), on a 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA), and quantitative analysis of PCR data were calculated through 2−ΔΔCt method, using beta-actin as an internal control. Primers used for the qPCR are listed in Table 2.
Table 2. Sequences of primers used for PCR.
| Gene | Forward | Reverse |
|---|---|---|
| Pparγ | ACCACTCGCATTCCTTTGAC | CCACAGACTCGGCACTCAAT |
| Leptin | TGACACCAAAACCCTCATCA | TCATTGGCTATCTGCAGCAC |
| Adiponectin | GCACTGGCAAGTTCTACTGCAA | GTAGGTGAAGAGAACGGCCTTGT |
| Agt | GATGCGCACAAGGTCCTG | CAGGGTGCTGTCCACACTGGCTCGC |
| renin | TTCTCTCCCAGAGGGTGCTA | CCCTCCTCACACAACAAGGT |
| Ace | GAGCCATCCTTCCCTTTTTC | GGCTGCAGCTCCTGGTATAG |
| Agtr1 | ACTCTTTCCTACCGCCCTTC | TTAGCCCAAATGGTCCTCTG |
| Agtr2 | GAAGGACAACTTCAGTTTTGC | CAAGGGGAACTACATAAGATGC |
| Star | GACCAGCCCATGGACAGACTC | AGGTCAATAGTGAGCAGCCA |
| Hsd3b1 | ATGCCCAGTACCTGAGGAGA | TTGAGGGCCGCAAGTATCA |
| Cyp11a1 | AGAAGCTGGGCAACATGGAGTCAG | TCACATCCCAGGCAGCTGCATGGT |
| Cyp21 | CATCGTGCAACTAAGGCTAG | TGGAAGGGAGGAATTAAGAG |
| MR | GCTTTGATGGTAGCTGCG | TGAGCACCAATCCGGTAG |
| Renalase | TGACCTTGTCATCCTCACCA | AACTCCAAATGGGACAGTGG |
| ß-actin | AGCCTTCCTTCTTGGGTATGG | CACTTGCGGTGCACGGTATGG |
Pparγ, Peroxisome proliferator-activated receptor-gamma; Agt, Angiotensinogen; Ace, Angiotensin-converting enzyme; Agtr1, Angiotensin II receptor type 1; Agtr2, Angiotensin II receptor type 2; Star, Steroidogenic acute regulatory protein; Hsd3b1, 3β-Hydroxysteroid dehydrogenase type 1; Cyp11a1, Cholesterol side-chain cleavage enzyme; Cyp21, 21-Hydroxylase; MR, Mineralocorticoid receptor.
2.7 Urine and feces analyses
Urine and feces samples, collected from metabolic cages, were analyzed for their Na+ and K+ contents, by inductively coupled plasma-mass spectroscopy (ICP-MS; 7500A, Agilent Technologies, Germantown, MD, USA), the center for University-Wide Research Facilities (CURF) at Jeonbuk National University.
2.8 Analyses of serum ion levels
The Na+ and K+ concentrations in the serum were determined using Fuji Dri-Chem Slide Na-K-Cl (FUJIFILM, Tokyo, Japan) with FDC 3500i chemistry analyzer (Fuji Dri-Chem Analyzer, Tokyo, Japan).
2.9. Cell culture and treatments
The 3T3-L1 preadipocyte cell line (CL‐173, ATCC, VA, USA) was maintained in DMEM (Hyclone, USA) containing 10% bovine serum (Gibco, NY, USA) and 100 U/mL 1% penicillin‐streptomycin (Hyclone, USA) at 37°C under 5% CO2 in a humidified incubator. RNA was extracted from 3T3-L1 cells at different times after differentiation.
To observe the effect of RAS blockers, the 3T3-L1 cells were seeded in 6-well plates and upon reaching 100% confluence (day 0), they were continued in culture for 48 h. Then the growth medium was replaced with differentiation medium, containing DMEM, 10% fetal bovine serum (FBS), 0.5 μM isobutylmethylxanthine (IBMX), 1 μM dexamethasone (DEXA), and 10 μg/mL insulin (Sigma‐Aldrich Co., St. Louis, MO, USA) with or without Losartan (10−4 M), Captopril (10−4 M), or doenjang (0.4% salinity). All treatments chemicals were dissolved in 30% EtOH to match the stock concentrations. The media, with or without the treatment chemicals, were changed every day, and the cells were harvested on the day 4. Total RNA was extracted using TRIzol reagent, according to the manufacturer’s instructions (Invitrogen) and quantified by quantitative real-time PCR with gene-specific primers (Table 2).
2.10 Statistical analyses
The data are expressed as mean ± SEM. By utilizing GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA) or SPSS 17.0 (SPSS, Inc., Chicago, IL, USA), ANOVA and Tukey’s post hoc test, or unpaired t-test were conducted to detect significant differences between groups. The curve graph of body weight and systolic blood pressure were analyzed by two-way ANOVA with Tukey’s multiple comparisons. A p-value of less than 0.05 was considered significant.
3. Results
3.1 Doenjang improves body and fat tissue weight gains, and blood glucose levels in HD-induced obese rats
The initial body weight did not differ among different groups (Fig 2A and Table 3). The ND group had significantly lower body weight than HD group. Compared to HD group, HDJ group started to show a significant reduction of body weight from 8 weeks of the experimental period. On the final day of the experiment period, the HD group had significantly higher body weight compared to the ND group. Both HDS and HDJ groups showed a slightly elevated tendency of body weight compared to the ND group, but those elevations were markedly lower compared to the HD group (Fig 2A and Table 3). The diet intake of the ND group was significantly higher than all HD-fed groups (Table 3). Compared to the ND group, the blood glucose levels were significantly elevated in both HD and HDS groups, whereas the HDJ group had markedly attenuated blood glucose levels (Table 3).
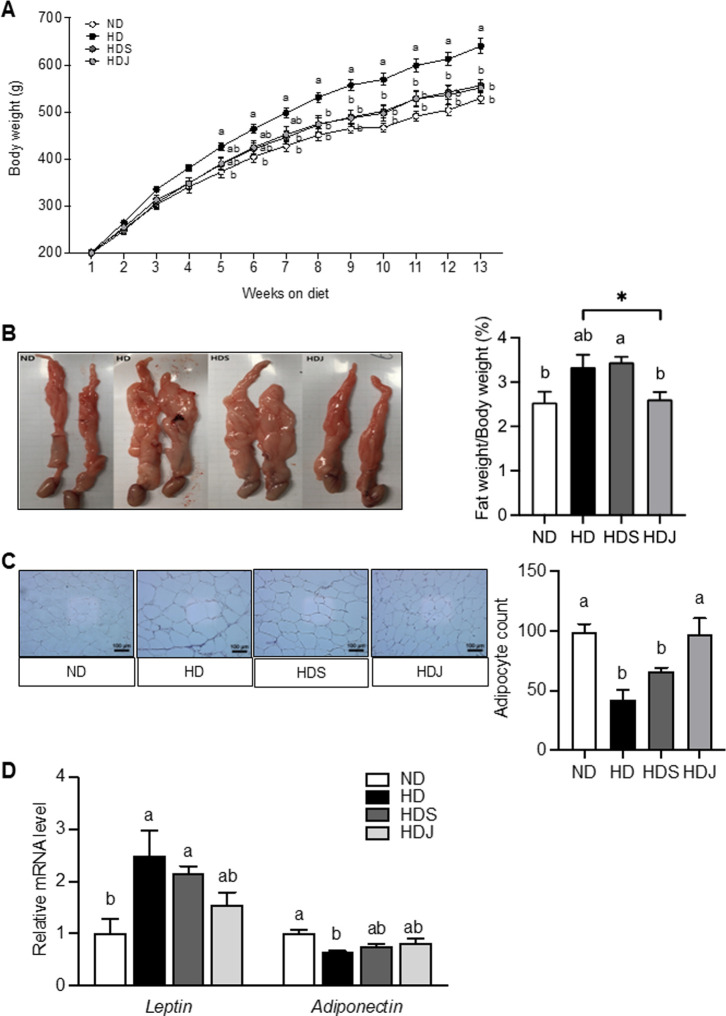
Fig 2. Doenjang reduces body and fat tissue weight gain in HD-induced obese rats.
(A) A growth curve of body weight of each group is shown. The body weight was recorded weekly. (B) The pictures of representative epididymal fat tissues and a ratio of epididymal fat/body weight (%) are shown. (C) The histology sections of epididymal fat tissue and a ratio of the number of adipocytes to area are presented (D) The mRNA levels of leptin and adiponectin in epididymal fat tissue. Values are the mean ± SEM (n = 6) with different letters significantly different (p < 0.05) by ANOVA with Tukey’s post hoc test (a > b). Values with Asterisk present significant differences between the groups by unpaired t-test (*p < 0.05). ND; normal diet, HD; high-fat diet, HDS; high-fat diet with salt, and HDJ; high-fat diet with doenjang.
Table 3. Body weight, diet intake, and blood glucose in rat.
| Initial Body Weight (g) | Final Body Weight (g) | Diet Intake (g/day) | Blood Glucose Level (mg/dL) | |
|---|---|---|---|---|
| ND | 202.95 ± 5.47ns | 509.03 ± 10.88c | 19.99 ± 0.39a | 89.83 ± 2.36b |
| HD | 201.82 ± 3.51ns | 633.27 ± 17.20a | 17.73 ± 0.46b | 93.8 ± 4.19a |
| HDS | 195.43 ± 8.61ns | 558.65 ± 9.58b | 17.02 ± 0.46b | 90.33 ± 1.89a |
| HDJ | 200.9 ± 4.73ns | 548.48 ± 6.41bc | 17.37 ± 0.42b | 80.13 ± 2.86c |
Values are given as mean ± SEM (n = 6). Values with different superscripts in the same column are significantly different (p < 0.05) by Duncan’s multiple range test (a > b; ns, not significant). ND; normal diet, HD; high-fat diet, HDS; high-fat diet with salt, and HDJ; high-fat diet with doenjang.
The relative epididymal fat weight ratio of the HD and HDS groups was significantly higher than the ND groups, while the HDJ group had markedly lower epididymal fat mass and the levels of the relative epididymal fat weight ratio compared to both HD and HDS groups (Fig 2B). Moreover, the HDJ group showed a significantly reduced adipocyte size and an increased ratio of the number of adipocytes to the area compared to HD and HDS groups (Fig 2C). Consistent with these results, the HDJ group showed a downregulated tendency of leptin mRNA expression and an upregulated tendency of adiponectin mRNA level in epididymal adipose tissues compared to HD and HDS groups without statistical significance (Fig 2D). These observations suggest that the consumption of doenjang ameliorates HD-induced obesity by inhibiting body weight gain and adipogenesis, and by improving obesity-related gene expression in adipose tissue.
3.2 Doenjang changes RAS-related gene levels in the liver and adipose tissue in HD-induced obese rats
The elevations of RAS-related gene expression and RAS activation in the adipose tissues are associated with obesity; for example, Agtr1 and Agtr2 regulate adipogenesis [9, 22]. Previously, doenjang significantly reduces the relative adipose tissue weight ratio, adipocyte size, and increased ratio of the number of adipocytes to the area in HD-induced obese rats (Fig 2B and 2C). Therefore, hepatic Agt and RAS-associated gene expressions in the adipose tissues were analyzed to scrutinize whether doenjang influences adipogenesis via RAS.
Compared to the ND group, the hepatic Agt expression was significantly upregulated in HD and HDS groups; while the HDJ group had significantly decreased hepatic Agt level compared to the HD group (Fig 3A). In addition, all RAS-associated gene expressions, including Agt, Renin, Ace, Agtr1, and Agtr2 in the adipose tissue were higher in both HD and HDS groups compared to the ND group with and/or without statistical significance (Fig 3B). Consistent with the results in Fig 2B and 2C, the levels of the above genes in the HDJ group were lower than HD and HDS groups with and/or without statistical significance (Fig 3B).
Fig 3. Doenjang alters the mRNA expression related to RAS in the liver and white adipose tissue in HD-induced obese rats.
(A) mRNA level of hepatic Agt. (B) mRNA levels of RAS-related genes in white adipose tissue. Values are given as mean ± SEM (n = 6). Values with different superscripts are significantly different (p < 0.05) by ANOVA with Tukey’s post hoc test (a > b). Values with Asterisk present significant differences between the groups by unpaired t-test (*p < 0.05 and **p<0.01). ND; normal diet, HD; high-fat diet, HDS; high-fat diet with salt, and HDJ; high-fat diet with doenjang. Agt; Angiotensinogen, Ace; Angiotensin-converting enzyme, Agtr1; Angiotensin II receptor type 1, and Agtr2; Angiotensin II receptor type 2.
To further investigate the direct effects of doenjang on adipogenesis and RAS-related gene expressions, the differentiated 3T3L1 cells were treated with Losartan (Los, Agtr inhibitor), Captopril (Cap, Ace inhibitor), and doenjang extraction (0.4% salinity). Treatments of doenjang and Los significantly reduced the key adipogenic transcription factor, Pparγ in the differentiated 3T3L1 cells (Fig 4A). Moreover, doenjang and Cap treatments decreased Agt without statistical significance and significantly lowered Ace expressions in the differentiated 3T3L1 cells (Fig 4B and 4C). These findings indicate that doenjang downregulates the expressions of RAS-related gene and adipogenesis-associated gene in the adipose tissue, resulting in the reduction of adipogenesis and the improvements of obesity, subsequently.
Fig 4. The effects of doenjang in differentiated 3T3L1 cells.
Differentiated 3T3L1 cells were treated with losartan (Los, 10−4 M), captopril (Cap, 10−4 M), and Doenjang (DJ, 0.4% salinity) for 4 days. (A) The mRNA levels of Pparγ (B) The mRNA levels of Agt. (C) The mRNA levels of Ace. Values with different superscripts are significantly different (p < 0.05) by ANOVA with Tukey’s post hoc test (a > b). Pre; preadipocytes, Con; differentiated adipocyte, Pparγ; peroxisome proliferator-activated receptor gamma, Agt, Angiotensinogen, Ace; Angiotensin-converting enzyme.
3.3 Doenjang improves systolic blood pressure and serum RAS-related parameters in HD-induced obese rats
Next, the parameters involved in hypertension were studied to investigate the anti-hypertensive effects of doenjang in HD-induced obesity. The initial SBP of all groups was 125 ± 10 mmHg, which was continuously and significantly increased over the experimental period, especially in both HD and HDS groups (Fig 5A). After 2 weeks, HD the blood pressure of HD and HDS groups became significantly higher than HDJ group. At 8 week, HDS group had the highest blood pressure among all groups, while HDJ group showed the lowest level of blood pressure despite of the fact that HDJ diet contains the same amount of salt as the HDS diet. ND group had the highest serum renin level among the groups (Fig 5B). Compared to the ND group, both HD and HDS groups had lower serum renin levels with and/or without statistical significance, whereas the HDJ group had a significantly higher serum renin level than the HDS group (Fig 5B). Compared to the ND group, both HD and HDS groups showed a decreased tendency of serum angiotensin II levels, while the HDJ group had a significant reduction of serum angiotensin II levels (Fig 5C). The levels of serum aldosterone were significantly increased in both HD and HDS groups compared to the ND group, and the HDJ group had markedly reduced levels of serum aldosterone compared with HD and HDS groups (Fig 5D). These findings imply doenjang intake could improve hypertension in HD-induced obesity via the ameliorations of RAS-related serum indicators.
Fig 5. Doenjang ameliorates systolic blood pressure and serum RAS-associated indicators in HD-induced obese rats.
(A) A periodical changes of systolic blood pressure during the experimental period are shown. Values are given as mean ± SEM (n = 4) (B) The level of serum renin. (C) Serum angiotensin II level. (D) Serum aldosterone level. Values are given as mean ± SEM (n = 6). Values with different superscripts are significantly different (p < 0.05) by ANOVA with Tukey’s post hoc test (a > b). Values with Asterisk present significant differences between the groups by unpaired t-test (*p < 0.05). ND; normal diet, HD; high-fat diet, HDS; high-fat diet with salt, and HDJ; high-fat diet with doenjang.
3.4 Doenjang alters RAS-related gene expressions in the kidney in HD-induced obese rats
As doenjang improves SBP and serum RAS-associated parameters (Fig 5), the expressions of RAS-involved genes in the kidney were measured. The Renin mRNA expression in the kidney, a feedback controller of Agt was decreased in both HD and HDS groups compared to ND groups without statistical significance (Fig 6A). Compared to the HD groups, the HDS group had a significant reduction of Renin mRNA level, while the HDJ group showed a significantly higher expression of Renin mRNA compared to the HDS groups (Fig 6A). Compared to the ND group, all HD groups showed a significantly elevated level of renal Ace, but the HDJ group had a slightly reduced Ace level compared to HD and HDS groups (Fig 6A). The expression of renal Agtr1 was increased in both HD and HDS groups with and/or without statistical significance, but it was reduced in the HDJ group compared to the HD and HDS groups with and/or without statistical significance (Fig 6A). The level of Agtr2 in the kidney did not significantly change among the groups; however, both HD and HDS groups had a lower tendency than ND group, while HDJ group had an elevated trend compared with HD and HDS groups (Fig 6A).
Fig 6. Doenjang changes renal mRNA levels related to RAS in HD-induced obese rats.
(A) mRNA levels of RAS-related genes in the kidney. (B) Aldosterone releasing factors-related mRNA levels in the kidney. (C) The levels of Mr and Renalase mRNA in the kidney. Values are given as mean ± SEM (n = 6). Values with different superscripts are significantly different (p < 0.05) by ANOVA with Tukey’s post hoc test (a > b). Values with Asterisk present significant differences between the groups by unpaired t-test (*p < 0.05 and **p<0.01). ND; normal diet, HD; high-fat diet, HDS; high-fat diet with salt, and HDJ; high-fat diet with doenjang. Ace; Angiotensin-converting enzyme, Agtr1; Angiotensin II receptor type 1, Agtr2; Angiotensin II receptor type 2, Star; Steroidogenic Acute Regulatory Protein, Hsd3b1; 3β-hydroxysteroid dehydrogenase type 1, Cyp11a1; cholesterol side-chain cleavage enzyme, Cyp21; 21-hydroxylase, and Mr; Mineralocorticoid receptor.
Additionally, aldosterone-associated genes in the kidney, including Star, Hsd3b1, Cyp11a1, and Cyp21 were analyzed. Compared to the ND group, HD and HDS groups had significant upregulation of Star, Hsd3b1, Cyp11a1, and Cyp21 levels (Fig 6B). HDJ group showed a slight downregulated tendency of Star, Hsd3b1, and Cyp11a1 expressions compared to both HD and HDS groups without statistical significance; however, the level of Cyp21 was significantly downregulated in the HDJ group compared to HD and HDS groups (Fig 6B).
A deficiency of renalase is linked with the elevated blood pressure; and mineralocorticoid receptor (MR) plays an essential role in aldosterone functions [23, 24]. Both HD and HDS groups had significantly downregulated renalase mRNA levels compared to the ND group, whereas the HDJ group showed markedly upregulated Renalase mRNA expression compared to HDS groups (Fig 6C). The mRNA expression of Mr was significantly elevated in both HD and HDS groups, while the HDJ group had significantly lower Mr mRNA expression compared to HD and HDS groups (Fig 6C). These results address that doenjang changes the renal gene expressions related to RAS and aldosterone in HD-induced obese rats, which explains how doenjang has anti-hypertensive effects against hypertension coming from HD-induced obesity.
3.5 Doenjang modifies urine excretion, water intake, and electrolyte ratio in HD-induced obese rats
As doenjang changes serum RAS-associated parameters (Fig 5B–5D) and the renal mRNA expression related to RAS and aldosterone (Fig 6), the effects of doenjang on fluid and electrolyte balance, urine excretion, and water intake were analyzed. Additionally, Na+ and K+ levels in urine, feces, and serum were also measured.
As expected, urine output and water intake were significantly higher in both HDS and HDJ groups compared to ND and HD groups (Fig 7). HDS and HDJ groups had a significantly higher level of urinary Na+ compared to ND and HD groups; however, the urinary K+ level was significantly higher in the HDJ group than in the HDS group (Table 4). As a result, the HDS group had the highest urinary Na+/K+ ratio among all groups, whereas HDJ group had a markedly lower urinary Na+/K+ ratio compared to the HDS group. Additionally, the HDJ group had an elevated tendency of the fecal Na+ level compared to both HD and HDS groups, but there were no statistically significant differences among all groups (Table 4). The level of fecal K+ was markedly higher in the HDJ group compared with the HDS group. Consequently, the HDS group showed the highest fecal Na+/K+ ratio among the groups, while the HDJ group had a significantly reduced fecal Na+/K+ ratio compared to the HDS group (Table 4). All HD groups had an increased tendency of the serum Na+ level compared to ND group, but HDJ group showed a slight reduction of serum Na+ level compared to HD and HDS groups (Table 4). The serum K+ level of HD and HDS groups was significantly higher than ND groups, whereas HDJ group had a markedly decreased serum level of K+ compared to HD and HDS groups. These observations suggest that doenjang improves hypertension in HD-induced obese rats by increasing water excretion and elimination of Na+ and K+ through urine and feces.
Fig 7. Doenjang increases urine output and water intake in HD-induced obese rats.
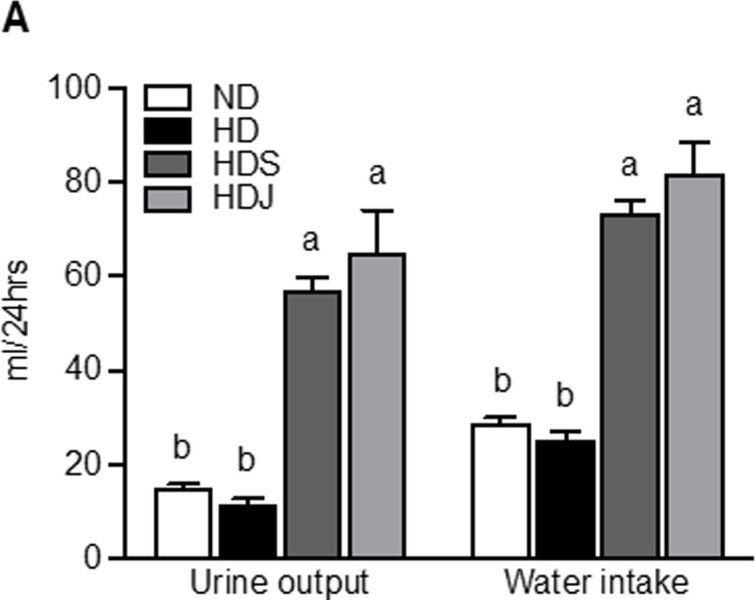
The output of urine and water intake were measured in the metabolic cages for 24 hours. Values are given as mean ± SEM (n = 6). Values with different superscripts are significantly different (p < 0.05) by ANOVA with Tukey’s post hoc test (a > b). ND; normal diet, HD; high-fat diet, HDS; high-fat diet with salt, and HDJ; high-fat diet with doenjang.
Table 4. Na+ and K+ levels of urine, feces, and serum in rats.
| Urine (ppm) | Feces (ppm) | Serum (mEq/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Na+/K+ | Na+ | K+ | Na+/K+ | Na+ | K+ | |
| ND | 1845.86 ± 134.35d | 4929.48 ± 461.13b | 0.42 ± 0.02c | 504.08 ± 41.96ns | 1098.18 ± 100.55a | 0.48 ± 0.37c | 134.7 ± 0.85b | 4.34 ± 0.11b |
| HD | 2431.53 ± 158.41c | 12571.03 ± 469.02a | 0.19 ± 0.01d | 548.99 ± 98.73ns | 728.30 ± 68.08b | 0.43 ± 0.42c | 138.3 ± 1.31a | 5.43 ± 0.17a |
| HDS | 9315.33 ± 32.78a | 1870.31 ± 67.18d | 4.46 ± 0.00a | 568.70 ± 2.70ns | 462.66 ± 39.11c | 1.59 ± 0.60a | 137.5 ± 0.43ab | 5.62 ± 0.12a |
| HDJ | 9426.76 ± 36.69a | 2183.75 ± 64.67c | 3.83 ± 0.00b | 614.53 ± 2.47ns | 828.92 ± 36.06b | 0.84 ± 0.77b | 136.8 ± 1.05ab | 4.72 ± 0.21b |
Values are given as mean ± SEM (n = 6). Values with different superscripts in the same column are significantly different (p < 0.05) by Duncan’s multiple range test (a > b; ns, not significant). ND; normal diet, HD; high-fat diet, HDS; high-fat diet with salt, and HDJ; high-fat diet with doenjang.
4. Discussion
Doenjang, a Korean traditional soybean paste has diverse health-beneficial effects [11–13], although it has been contentious due to the high salt content in doenjang. Moreover, most of the previous studies regarding the health-advantageous effects of doenjang (e.g., anti-obesity and anti-hypertensive effects) did not fully present its underlying mechanisms. The present study found that doenjang reduces adipogenesis in HD-induced obese rats via the downregulation of RAS-associated gene levels in adipose tissue. Additionally, doenjang elicits anti-hypertensive effects by ameliorating renal RAS in HD-induced obese rats. Most importantly, doenjang shows these beneficial effects nevertheless its high salt contents.
It has been reported that the dietary sodium in the HD diet lowers body weight gain due to the reduction of digestive efficiency coming from RAS inhibition by sodium [25]. Even though high sodium intake did not elevate body weight gain, it markedly increases fat accumulation [26]. Consistent with the earlier reports, the present study found that the supplementations of additive salt in the HD diet do not increase the body weight gain but, significantly elevate the relative epididymal fat weight ratio, epididymal fat mass, and adipocyte size. In contrast, doenjang significantly improved those indicators but also the mRNA levels of leptin and adiponectin in HD-induced obese rats, suggesting the anti-obesity effects of doenjang against HD-induced obesity. Many previous studies have been suggesting that soybean-based Korean fermented elicits its health beneficial outcomes due to its components, although the exact compound is not fully established. As a major component of doenjang, soybean contains multiple bioactive compounds, such as saponin, isoflavones, and peptides [27, 28]. Therefore, future studies should investigate the potential and/or exact compounds in doenjang that play role in its anti-obesity effects. Moreover, like many previous studies [29, 30], the current study did not have doenjang-only supplemented group to investigate the effects of doenjang itself. Thus, it will be also essential to set up doenjang-only supplemented group in the future studies to concrete understanding of its health beneficial effects.
Obesity activates RAS and increases RAS-related gene expressions in the adipose tissue, including Agt, Renin, Ace, and Agtr1 [8, 9]. Ang II increases adipogenesis by activating Agtr1 and Agtr2, resulting in lipolysis reduction and lipogenesis elevation, respectively [22]. This study reported that the supplementation of doenjang downregulates hepatic Agt and RAS-associated genes in the adipose tissue in HD-induced obese rats. Moreover, doenjang directly decreased the adipogenesis-related gene expression (e.g., Pparγ) and RAS-related gene levels (e.g., Agt and Ace) in differentiated 3T3L1 cells. In summary, these findings address that the hypotrophy and hypoplasia of adipocytes by doenjang came from the reductions of RAS-related gene expression in the adipose tissue. According to the earlier findings, Ang II activates phosphoinositide 3-kinase (PI3K)/Akt and mitogen-activated protein kinase (MAPK) signaling pathways in the adipose tissue to increase adipocyte proliferation and adipogenic differentiation, respectively [31]. Thus, future studies regarding the activation of RAS-adipogenesis-related signaling pathways by doenjang are essentially required to understand its underlying molecular and cellular mechanisms.
Excessive fat accumulation is strongly associated with blood pressure elevation and the risk of hypertension [32, 33]. For example, adipocyte-derived angiotensin activates Ang II, leading to the vasoconstriction and activation of aldosterone [34]. As aldosterone stimulates the reabsorption of Na+ and K+ release, activation of aldosterone contributes to the elevation of blood pressure [34, 35]. In the present study, as expected, the HD diet and the additive salt supplemented HD diet significantly increased SBP, while doenjang did not elevate SBP in the HD-induced obese rats. Moreover, doenjang improved the levels of serum RAS-related indicators (e.g., Ang II and aldosterone), mRNA expressions involved in renal RAS (e.g., Ace and Agtr1) and the functions of aldosterone (e.g., Cyp21 and Mr). A previous study reported that soybean bioactive peptide strongly improved kidney structure, antioxidant defense system, and inflammation in hypertensive rats [36]. Hence, the effects of doenjang consumption on overall kidney structure, renal antioxidant defense system, and renal inflammatory response are needed in the future to study another possible key mechanism of doenjang’s anti-hypertensive outcomes.
Although Korean consumes high salt from their traditional fermented foods, including doenjang, the prevalence of obesity and hypertension is relatively lower than in other countries [37]; and this phenomenon is defined as the “Korean Paradox” [38, 39]. Korean Paradox proposes the possibility that the metabolism and functions of the salt in doenjang and the additive salt may differ. The present study found that the additive salt induces hypertrophy and hyperplasia, while doenjang shows opposite outcomes in HD-induced obese rats, even though both diets contain the same amounts of salt. Due to the high salt contents, doenjang and the additive salt supplementation significantly increased water intake and urine output; however, doenjang markedly elevated the excretions of Na+ and K+ via urine and feces; therefore, the ratio of Na+/K+ was markedly lowered by doenjang than the additive salt supplementation. As a lower Na+/K+ ratio is associated with a lesser risk of hypertension [40], the findings in this study address that doenjang ameliorates hypertension in HD-induced obesity by altering electrolyte metabolism and its excretion. Moreover, these observations support the potential differences in salt metabolism that is contained in doenjang and the additive salt. Thus, in the future, more detailed underlying mechanisms regarding the metabolism of salt in doenjang should be investigated.
Supporting information
(TIF)
Acknowledgments
Authors thank Yu-Jung Chang, PhD, Center for University-wide Research Facilities (CURF) at Jeonbuk National University, for helping in cryomicrotome.
Abbreviations
- Ace
Angiotensin-converting enzymes
- Agt
Angiotensinogen
- Agtr1
Angiotensin II receptor type 1
- Agtr2
Angiotensin II receptor type 2
- Ang I
Angiotensin I
- Ang II
Angiotensin II
- Cyp11a1
cholesterol side-chain cleavage enzyme
- Cyp21
21-hydroxylase genes
- Hsd3b1
3β-hydroxysteroid dehydrogenase type 1
- Mr
Mineralocorticoid receptor
- Pparγ
Peroxisome proliferator-activated receptor gamma
- RAS
Renin-angiotensin system
- SBP
Systolic blood pressure
- Star
Steroidogenic acute regulatory protein
Data Availability
All data presented in the study are publicly available at: DOI: https://doi.org/10.21203/rs.3.rs-320019/v1
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT). (No. 2018R1A2B6006477)
References
- 1.Jung Y-H, editor The life expectancy and health-adjusted life expectancy of Koreans. Health and Welfare Policy Forum; 2012. [Google Scholar]
- 2.Kotchen TA. Obesity-related hypertension: epidemiology, pathophysiology, and clinical management. American journal of hypertension. 2010;23(11):1170–8. doi: 10.1038/ajh.2010.172 [DOI] [PubMed] [Google Scholar]
- 3.Kawarazaki W, Fujita T. The role of aldosterone in obesity-related hypertension. American journal of hypertension. 2016;29(4):415–23. doi: 10.1093/ajh/hpw003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circulation research. 2015;116(6):991–1006. doi: 10.1161/CIRCRESAHA.116.305697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schunkert H, Ingelfinger JR, Hirsch AT, Pinto Y, Remme WJ, Jacob H, et al. Feedback regulation of angiotensin converting enzyme activity and mRNA levels by angiotensin II. Circulation research. 1993;72(2):312–8. doi: 10.1161/01.res.72.2.312 [DOI] [PubMed] [Google Scholar]
- 6.Rüster C, Wolf G, editors. The role of the renin-angiotensin-aldosterone system in obesity-related renal diseases. Seminars in nephrology; 2013: Elsevier. [DOI] [PubMed] [Google Scholar]
- 7.Frigolet ME, Torres N, Tovar AR. The renin–angiotensin system in adipose tissue and its metabolic consequences during obesity. The Journal of nutritional biochemistry. 2013;24(12):2003–15. doi: 10.1016/j.jnutbio.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Soltani-Bejnood M, Quignard-Boulange A, Massiera F, Teboul M, Ailhaud G, et al. The adipose renin-angiotensin system modulates systemic markers of insulin sensitivity and activates the intrarenal renin-angiotensin system. Journal of Biomedicine and Biotechnology. 2006;2006. doi: 10.1155/JBB/2006/27012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalupahana NS, Moustaid‐Moussa N. The renin‐angiotensin system: a link between obesity, inflammation and insulin resistance. Obesity reviews. 2012;13(2):136–49. doi: 10.1111/j.1467-789X.2011.00942.x [DOI] [PubMed] [Google Scholar]
- 10.Lu H, Boustany-Kari CM, Daugherty A, Cassis LA. Angiotensin II increases adipose angiotensinogen expression. American Journal of Physiology-Endocrinology and Metabolism. 2007;292(5):E1280–E7. doi: 10.1152/ajpendo.00277.2006 [DOI] [PubMed] [Google Scholar]
- 11.Kwak CS, Park SC, Song KY. Doenjang, a fermented soybean paste, decreased visceral fat accumulation and adipocyte size in rats fed with high fat diet more effectively than nonfermented soybeans. Journal of medicinal food. 2012;15(1):1–9. doi: 10.1089/jmf.2010.1224 [DOI] [PubMed] [Google Scholar]
- 12.Cha Y-S, Park Y, Lee M, Chae S-W, Park K, Kim Y, et al. Doenjang, a Korean fermented soy food, exerts antiobesity and antioxidative activities in overweight subjects with the PPAR-γ2 C1431T polymorphism: 12-week, double-blind randomized clinical trial. Journal of Medicinal Food. 2014;17(1):119–27. [DOI] [PubMed] [Google Scholar]
- 13.Jang S-E, Kim K-A, Han MJ, Kim D-H. Doenjang, a fermented Korean soybean paste, inhibits lipopolysaccharide production of gut microbiota in mice. Journal of medicinal food. 2014;17(1):67–75. doi: 10.1089/jmf.2013.3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin D, Jeong D. Korean traditional fermented soybean products: Jang. Journal of Ethnic Foods. 2015;2(1):2–7. [Google Scholar]
- 15.Chai C, Ju HK, Kim SC, Park JH, Lim J, Kwon SW, et al. Determination of bioactive compounds in fermented soybean products using GC/MS and further investigation of correlation of their bioactivities. Journal of Chromatography B. 2012;880:42–9. doi: 10.1016/j.jchromb.2011.11.013 [DOI] [PubMed] [Google Scholar]
- 16.Mun E-G, Park JE, Cha Y-S. Effects of Doenjang, a traditional Korean soybean paste, with high-salt diet on blood pressure in Sprague–Dawley rats. Nutrients. 2019;11(11):2745. doi: 10.3390/nu11112745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segovia SA, Vickers MH, Harrison CJ, Patel R, Gray C, Reynolds CM. Maternal high-fat and high-salt diets have differential programming effects on metabolism in adult male rat offspring. Frontiers in nutrition. 2018;5:1. doi: 10.3389/fnut.2018.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Q, Larson DF, Slayback D, Lundeen TF, Baxter JH, Watson RR. Characterization of high-salt and high-fat diets on cardiac and vascular function in mice. Cardiovasc Toxicol. 2004;4(1):37–46. doi: 10.1385/ct:4:1:37 [DOI] [PubMed] [Google Scholar]
- 19.Kochanek KD, Murphy SL, Xu J, Arias E. Deaths: final data for 2017. 2019. [PubMed] [Google Scholar]
- 20.Bae C-R, Kwon DY, Cha Y-S. Anti-obesity effects of salted and unsalted Doenjang supplementation in C57BL/6J mice fed with high fat diet. Journal of the Korean Society of Food Science and Nutrition. 2013;42(7):1036–42. [Google Scholar]
- 21.Meija J, Michaowska-kaczmarczyk AM, Michaowski T. Mohr’s method challenge. Analytical and bioanalytical chemistry. 2016;408(7):1721. doi: 10.1007/s00216-015-9273-2 [DOI] [PubMed] [Google Scholar]
- 22.Frantz EDC, Prodel E, Braz ID, Giori IG, Bargut TCL, Magliano DAC, et al. Modulation of the renin–angiotensin system in white adipose tissue and skeletal muscle: focus on exercise training. Clinical science. 2018;132(14):1487–507. doi: 10.1042/CS20180276 [DOI] [PubMed] [Google Scholar]
- 23.Desir GV. Role of renalase in the regulation of blood pressure and the renal dopamine system. Curr Opin Nephrol Hypertens. 2011;20(1):31–6. doi: 10.1097/MNH.0b013e3283412721 [DOI] [PubMed] [Google Scholar]
- 24.Le Menuet D, Isnard R, Bichara M, Viengchareun S, Muffat-Joly M, Walker F, et al. Alteration of cardiac and renal functions in transgenic mice overexpressing human mineralocorticoid receptor. Journal of Biological Chemistry. 2001;276(42):38911–20. doi: 10.1074/jbc.M103984200 [DOI] [PubMed] [Google Scholar]
- 25.Weidemann BJ, Voong S, Morales-Santiago FI, Kahn MZ, Ni J, Littlejohn NK, et al. Dietary sodium suppresses digestive efficiency via the renin-angiotensin system. Scientific reports. 2015;5(1):1–10. doi: 10.1038/srep11123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fonseca‐Alaniz MH, Brito LC, Borges‐Silva CN, Takada J, Andreotti S, Lima FB. High dietary sodium intake increases white adipose tissue mass and plasma leptin in rats. Obesity. 2007;15(9):2200–8. doi: 10.1038/oby.2007.261 [DOI] [PubMed] [Google Scholar]
- 27.Moriyama T, Kishimoto K, Nagai K, Urade R, Ogawa T, Utsumi S, et al. Soybean β-conglycinin diet suppresses serum triglyceride levels in normal and genetically obese mice by induction of β-oxidation, downregulation of fatty acid synthase, and inhibition of triglyceride absorption. Bioscience, biotechnology, and biochemistry. 2004;68(2):352–9. [DOI] [PubMed] [Google Scholar]
- 28.Kim I-S. Current perspectives on the beneficial effects of soybean isoflavones and their metabolites for humans. Antioxidants. 2021;10(7):1064. doi: 10.3390/antiox10071064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko JW, Chung YS, Kwak CS, Kwon YH. Doenjang, A Korean Traditional Fermented Soybean Paste, Ameliorates Neuroinflammation and Neurodegeneration in Mice Fed a High-Fat Diet. Nutrients. 2019;11(8). doi: 10.3390/nu11081702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang HJ, Jeong SJ, Ryu MS, Ha G, Jeong DY, Park YM, et al. Protective effect of traditional Korean fermented soybean foods (doenjang) on a dextran sulfate sodium-induced colitis mouse model. Food Funct. 2022;13(16):8616–26. doi: 10.1039/d2fo01347a [DOI] [PubMed] [Google Scholar]
- 31.Tyurin‐Kuzmin PA, Kalinina NI, Kulebyakin KY, Balatskiy AV, Sysoeva VY, Tkachuk VA. Angiotensin receptor subtypes regulate adipose tissue renewal and remodelling. The FEBS Journal. 2020;287(6):1076–87. doi: 10.1111/febs.15200 [DOI] [PubMed] [Google Scholar]
- 32.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity, kidney dysfunction and hypertension: mechanistic links. Nature reviews nephrology. 2019;15(6):367–85. doi: 10.1038/s41581-019-0145-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendoza MF, Kachur SM, Lavie CJ. Hypertension in obesity. Current Opinion in Cardiology. 2020;35(4):389–96. doi: 10.1097/HCO.0000000000000749 [DOI] [PubMed] [Google Scholar]
- 34.Denton KM, Fennessy PA, Alcorn D, Anderson WP. Morphometric analysis of the actions of angiotensin II on renal arterioles and glomeruli. American Journal of Physiology-Renal Physiology. 1992;262(3):F367–F72. doi: 10.1152/ajprenal.1992.262.3.F367 [DOI] [PubMed] [Google Scholar]
- 35.Yim HE, Yoo KH. Renin-Angiotensin system-considerations for hypertension and kidney. Electrolytes & Blood Pressure: E & BP. 2008;6(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai BC-K, Kuo W-W, Day CH, Hsieh DJ-Y, Kuo C-H, Daddam J, et al. The soybean bioactive peptide VHVV alleviates hypertension-induced renal damage in hypertensive rats via the SIRT1-PGC1α/Nrf2 pathway. Journal of Functional Foods. 2020;75:104255. [Google Scholar]
- 37.Park J, Kwock CK. Sodium intake and prevalence of hypertension, coronary heart disease, and stroke in Korean adults. Journal of Ethnic Foods. 2015;2(3):92–6. [Google Scholar]
- 38.Mun E-G, Cha Y-S. Korean Traditional Fermented Foods (KTFFs): Antiobesity Effects and Salt Paradox. Chemistry of Korean Foods and Beverages. 2019:121–34. [Google Scholar]
- 39.Song JW, Edward OC, Han A, Mun EG, Lee EJ, Cha YS. Traditional Sunchang Gochujang Attenuates Obesity in High-Fat Diet Induced Obese Mice. Current Developments in Nutrition. 2022;6(Supplement_1):1087-. [Google Scholar]
- 40.Mirmiran P, Gaeini Z, Bahadoran Z, Ghasemi A, Norouzirad R, Tohidi M, et al. Urinary sodium-to-potassium ratio: a simple and useful indicator of diet quality in population-based studies. European Journal of Medical Research. 2021;26(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Data Availability Statement
All data presented in the study are publicly available at: DOI: https://doi.org/10.21203/rs.3.rs-320019/v1