Abstract
The use of cardiac point-of-care ultrasound (P.O.C.U.S.) is underutilized in the field of internal medicine for the assessment of patients with cardiac complaints. Numerous studies in emergency medicine, anesthesia, and critical care have demonstrated the successful application of cardiac P.O.C.U.S. in resident and attending physicians with limited prior exposure. This article review overviews the practical implementation of cardiac P.O.C.U.S. for hospitalists by discussing proper technique and assessment for common pathology seen in the medical ward setting. We describe how to assess for left ventricular (LV) systolic function, right ventricular (RV) systolic function, suspected acute coronary syndrome (ACS), post-myocardial infarction (MI) complications, suspected pulmonary embolus, and assessment of intravascular volume status. In each section, we overview the pertinent literature to show how cardiac P.O.C.U.S. has been used to directly impact patient care.
Keywords: Cardiac point-of-care ultrasound, P.O.C.U.S., Internal medicine, Left ventricular systolic function, Right ventricular systolic function, Post-myocardial infarction complication, Acute coronary syndrome, Intravascular volume status, Pulmonary embolus, Parasternal long-axis view, Parasternal short-axis view, Apical four chamber view, Subcostal view, Cardiopulmonary arrest, Portable ultrasonography, Emergency medicine, Anesthesia
1. Introduction
The utilization of point-of-care ultrasound (P.O.C.U.S.) as an adjunct to the physical exam is becoming ubiquitous within internal medicine. A primary driver in the increased utilization of P.O.C.U.S. is the advent of portable ultrasound machines, with smaller footprints and user-friendly interfaces which make practical ultrasound exams more accessible to hospitalists.1 Additionally, internal medicine has invested significant resources in equipment procurement and educational opportunities for developing dedicated P.O.C.U.S. curriculums. 2 The investment in education and utilization of P.O.C.U.S. specific to cardiac exams has not increased at the same rate as other P.O.C.U.S. applications.3 There are several key barriers to implementing basic bedside echocardiography, namely a need for more confidence in educator and trainee probe handling, need for understanding of how to assess for specific cardiac pathophysiology, and connecting ultrasound physics and measurements to practical application.4
Traditionally, a robust history and physical exam, assessment of cardiac risk factors, analysis of an electrocardiogram tracing, and clinician judgment have been the cornerstone of assessing patients with cardiac complaints on medical wards for years. With advancements in P.O.C.U.S. technology and education, P.O.C.U.S. as an adjunct to the history and physical is becoming increasingly popular. Cardiac P.O.C.U.S., when compared with traditional auscultation of the heart, can be utilized to provide the physician with more information during a cardiac examination. Cardiac P.O.C.U.S. can be used to assess left and right ventricular function, assess for wall motion abnormalities in patients with suspected acute coronary syndrome (ACS), evaluate for post-myocardial infarction (MI) complications, evaluation for hemodynamically significant pulmonary embolus, and assessment of intravascular volume status.5 The scope of this review is to provide an overview of the practical application of cardiac P.O.C.U.S. for hospitalists and to review the existing evidence supporting the use of cardiac P.O.C.U.S.
2. Steps in cardiac P.O.C.U.S. Exam
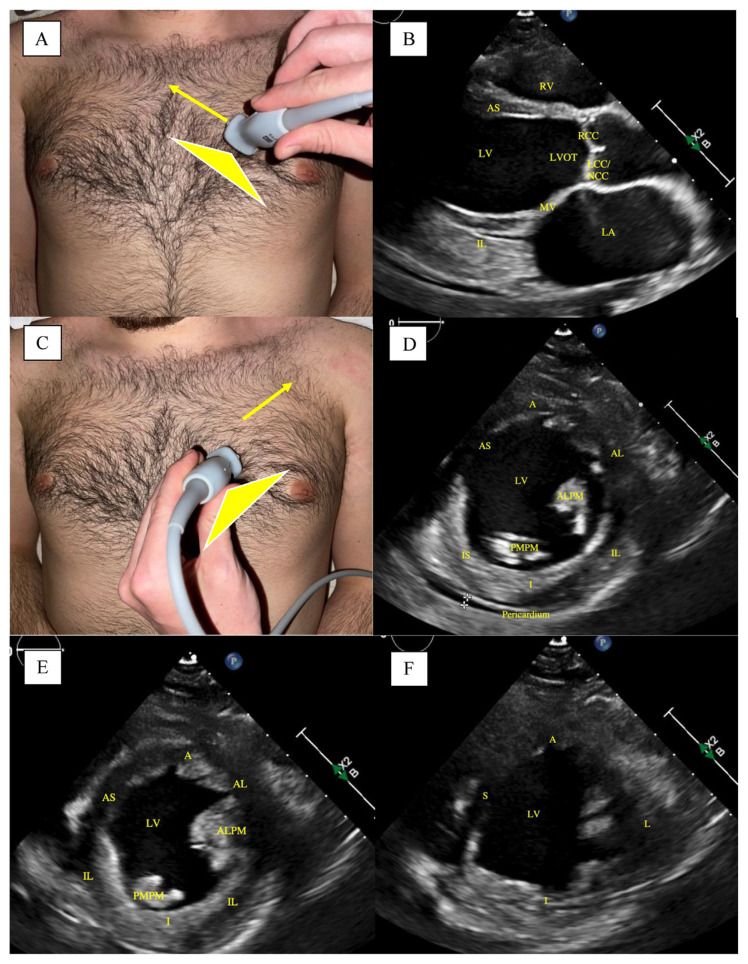
There are five main views in the bedside cardiac P.O.C.U.S. exam; Parasternal long axis (PLAX) view, parasternal short axis (PSAX) view, apical four chamber (A4C) view, subcostal cardiac view, and subcostal inferior vena cava (IVC) view. The PLAX view (Image 1A) is best obtained with the patient's left arm raised above their head, if possible, to maximize spacing between the ribs to allow for better probe positioning and less interference. The probe should be positioned at the left sternal border, third or fourth intercostal space, with the indicator pointed to the right shoulder. To optimize the image, consider moving an intercostal space higher or lower and tilting the transducer further away from the sternum. The inferolateral and anteroseptal walls of the left ventricle (LV) can be assessed for wall thickness and motion. Typically, the LV apex cannot be visualized in this view. Color Doppler can visualize evidence of aortic and mitral valve stenosis and regurgitation, however, a formal echocardiogram can provide advanced metrics to grade the severity.
The PSAX view is obtained by rotating the transducer 90° such that the probe indicator is now facing the patient's left shoulder (Fig. 1C). The probe's position will be the same as the PLAX view, at the left sternal border, third or fourth intercostal space. In the PSAX view, the right ventricle (RV), LV chamber size, and LV wall motion can be assessed. By changing the angle of the probe, one can assess all LV wall aspects, including the basal (Image 1D), mid-cavity (Image 1E), and apical LV (Image 1F). The basal short-axis views can visualize the short axis of the AV, and long axis of the PV and TV.
Fig. 1.
Parasternal Long-Axis (PLAX) and Parasternal Short-Axis (PSAX)-Basal, Mid-cavitary, and Apical Views. (A): Probe placement for PLAX view. Arrow showing the direction of the probe indicator. (B): PLAX view on ultrasound. (C): Probe placement for PSAX view. Arrow showing the direction of the probe indicator. (D): PSAX-Basal view on ultrasound. (E): PSAX-Mid-cavitary view on ultrasound. (F): PSAX-Apical-view on ultrasound. LA, left atrium; MV, mitral valve; LV, left ventricle, RV, right ventricle; LVOT, left ventricular outflow tract; RCC, right coronary cusp of aortic valve; LCC/NCC, left coronary cusp and non-coronary cusp of aortic valve; IL, inferior lateral wall; AS, anterior septal wall; PMPM, posteromedial papillary muscle; ALPM, anterolateral papillary muscle; A, anterior wall; AL, anterolateral wall; IL, inferolateral wall; I, inferior wall; IS, inferoseptal wall; AS, anteroseptal wall; S, septal wall; L, lateral wall Note, the crosshairs in figure (1D) at the pericardium show a trivial pericardial effusion. Images are obtained as deidentified patient chart information at our institution.
The A4C view (Fig. 2A and B) is best obtained with the patient in the left lateral decubitus position. The position of the probe is inferior to the left nipple around the point of maximal impulse (PMI), with the indicator pointed to the left flank. In this view, all four chambers and atrioventricular valves can be visualized. Furthermore, the direction of blood flow aligns well with the ultrasound beams making this view ideal for Doppler-based interrogation of stenosis or regurgitation of the mitral valve (MV) and tricuspid valve (TV).
Fig. 2. Apical Four-Chamber (A4C) View, Subcostal, and Subcostal-IVC Views.
(A): Probe placement for A4C View. Arrow showing the direction of the probe indicator. (B): A4C view on ultrasound. (C): Probe placement for SC View. (D): SC view on ultrasound. Arrow showing the direction of the probe indicator. (E): SC-IVC view on ultrasound. LV, left ventricle; LA, left atrium; RV, right ventricle; RA, right atrium; TV, tricuspid valve; MV, mitral valve; BL, basolateral wall; ML, mid-lateral wall; Al, anterolateral wall; AS, anteroseptal wall; MS, mid-septal wall; BS, basal septal wall; IVC, inferior vena cava. Images are obtained as deidentified patient chart information at our institution.
The subcostal view (Fig. 4B) allows the visualization of all the cardiac chambers. The probe should be subxiphoid with the probe indicator pointed to the left flank. This view is beneficial during ongoing advanced cardiac life support (ACLS) to look for signs of RV strain, cardiac motion, and pericardial effusion, as the probe is inferior to the xiphoid process and out of the way of compressions. A rightward angulation of the transducer in this location will allow for visualization of all cardiac structures (Fig. 2C and D). If the probe indicator is pointed towards the head, this is considered the subcostal IVC view, which should show the IVC in the long axis emptying into the right atrium (RA) (Fig. 2E).
Fig. 4.
Cardiac Tamponade and Mitral Regurgitation on Cardiac P.O.C.U.S. (A): Arrow pointing to a large pericardial effusion with evidence of collapse of RV. These findings are consistent with tamponade physiology. (B): Severe mitral regurgitation (MR) RV, right ventricle; RA, right atrium; LV, left ventricle; LA, left atrium. Images are obtained as deidentified patient chart information at our institution.
2.1. Evaluation of left ventricular (LV) and right ventricular (RV) systolic function
When examining a patient with suspected clinical heart failure, visual estimation of LVEF can be utilized to both grade severity of LV/RV function and triage patients with new-onset heart failure. The estimation of LVEF can be accomplished though assessing how well the LV walls move in systole and how the anterior mitral valve leaflet (AMVL) moves during diastole. The visual estimation of LVEF is the simplest method to perform given how quick it is to perform and how accurate it tends to be when compared to calculation estimates using Simpson's Biplane method of disks.6 A reduced LVEF (LVEF <50%) is associated with poor contraction of the LV walls during systole and an AMVL that moves <50% of the way to the intraventricular septum during diastole. Evaluation of LVEF by visual estimation requires some degree of practice to appreciate normal from abnormal. Operators can also qualitatively assess LVEF using endpoint septal separation (EPSS) (Fig. 3A). EPSS is calculated in the PLAX view with the ultrasound machine in M-mode with the indicator line placed over the distal aspect of the AMVL in early diastole. Within M-mode, calculate the difference in centimeters between wave peak that corresponds to the mitral valve early in diastole (E-point) and the wave that corresponds to the contraction of the left atrium (LA) in end-diastole (A-point). An abnormal EPSS (more than 7 mm) was found to be 87% sensitive and 75% specific in detecting individuals with reduced ejection fraction (<50%).7
Fig. 3.
End-Point Septal Separation (EPSS) and Tricuspid Annular Plane Systolic Excursion (TAPSE). (A): Calculation of EPSS in PLAX View. (B): Calculation of TAPSE in A4C View. Images are obtained as deidentified patient chart information at our institution.
In assessing RV systolic function, the standard of practice is visual analysis and calculating the tricuspid annular plane systolic excursion (TAPSE). The TAPSE (Fig. 3B) is assessed in the A4C view and is calculated by determining the descent of the tricuspid valve (TV) annulus toward the RV apex. After obtaining the A4C view, M-mode should be selected, and the cursor should be positioned through the TV annulus and the RV apex. The TAPSE is the excursion difference (in cm) between the nadir and the peak. A TAPSE <1.7 cm indicates RV systolic dysfunction, and >1.7 cm indicates a preserved RV systolic function. Patients with RV dysfunction may be more preload dependent and require more advanced hemodynamic status assessment. It is essential to mention that TAPSE should be used in conjunction with visual analysis. An RV with normal function can have a falsely low TAPSE if the free wall base or apex does not move well. Similarly, an RV with reduced function can have a falsely low TAPSE if the free wall base does not move particularly well. Conversely, one can have a high TAPSE in an akinetic RV if the RV is being “pulled” but is not contracting well.
2.2. Evaluation of patients with suspected acute coronary syndrome (ACS)
Cardiac P.O.C.U.S. is an integral part in the assessment of patients with suspected ACS, as ischemic changes may be evident on an echo as regional wall motion abnormalities (RWMA) prior to the appearance of ischemic changes on electrocardiogram (EKG). The applicability within the field of internal medicine is nuanced from the cardiac-focused assessment with sonography for trauma, which emergency medicine physicians use to quickly triage life-threatening conditions such as cardiac tamponade, myocardial free-wall ruptures, and penetrating cardiac injuries.8–11 Cardiac P.O.C.U.S. can be used to localize areas of ischemia in specific coronary distributions by assessment for areas of hypokinetic myocardium. Through repetition, hospitalists can become familiar with the walls that are supplied blood by the left anterior descending artery (LAD), left circumflex artery (LCx), and the right coronary artery (RCA). In a study of 466 patients, early echocardiography in patients with NSTEMI or unstable angina could predict outcomes such as heart failure, arrhythmias, and recurrent myocardial ischemia.12
The LAD supplies cardiac regions visualized in the PLAX, PSAX, and A4C views. In the PLAX view, RWMA can be seen in the mid-anteroseptal and basal anteroseptal walls. In the PSAX view, RWMA can be seen in the anterior, anteroseptal, and anterolateral walls. In the A4C view, RWMA can be seen in the apex, anterolateral, and inferoseptal walls. The RCA supplies areas are best seen in the PSAX and A4C view. In the PSAX-Basal and Mid-cavitary views, RWMA can be seen in the inferior, inferoseptal, and occasionally in the inferolateral walls. In the A4C view, the operator may see RWMA in the basal inferoseptal wall. The LCx supplies areas seen in the PLAX, A4C, and PSAX views, though there is considerable vascular overlap with the LAD and RCA. Dual LCx and LAD distribution exists in the PLAX in the apical lateral wall, in the PSAX in the anterolateral walls, and in the A4C in the apical lateral, mid-anterolateral, and basal anterolateral walls. Dual LCx and RCA distribution can be seen in the mid-cavity PSAX and PLAX view as RWMA in the mid-inferolateral and basal inferolateral walls.
2.3. Evaluation for post-myocardial infarction (MI) complications
Cardiac P.O.C.U.S. is integral for assessment of post–MI complications which often contribute to significant morbidity and mortality. This includes assessment for pericardial effusions, cardiac tamponade, left ventricular thrombus, acute mitral regurgitation (MR), acute ventricular septum rupture, and left ventricular aneurysm as well as pseudoaneurysm.
Pericardial effusions, which may occur as a mechanical complication of MI, typically occur within one to three days post–MI. Echocardiographic findings consistent with pericardial effusions are represented by hypoechoic fluid surrounding the heart in any view. In one study, a cardiac P.O.C.U.S. was 100% sensitive for the diagnosis of pericardial effusion.13 The immediate concern for a large effusion would be myocardial rupture, coronary dissection, or cardiac tamponade. Cardiac tamponade classically presents with pulses paradoxus, hypotension, jugular venous distension, and distant heart sounds. Echocardiographic findings consistent with cardiac tamponade include the collapse of the right atrium (RA) for more than 1/3rd of the cardiac cycle and the RV/LV collapse during diastole, and IVC dilation without collapsibility during inspiration. Fig. 4 shows an example of tamponade physiology. Right-sided chambers are typically affected prior to the left-sided chambers as the filling pressures of the right chambers are less than that of the left-sided chambers. However, this depends upon the distribution of effusion within the pericardium.
Echocardiographic evidence of left ventricular rupture, which can occur three to five days post–MI, includes a hypoechoic space in the pericardium and Doppler flow evidence of a jet visible through the ventricular wall. These patients will often present with shock-like symptoms with circulatory collapse. It is essential to mention that hypoechoic layering in the pericardium may suggest an associated thrombus. A pseudoaneurysm may be present if this rupture is contained within the pericardium and is visualized by an outpouching of the LV wall with a narrow neck. An aneurysm would demonstrate an outpouching with a wide neck adjacent to a hypokinetic or akinetic wall during systole.14
Papillary muscle rupture typically occurs secondary to infarction in the posteromedial papillary muscle due to a single blood supply from the posterior descending artery (PDA) branch of a dominant RCA or as a branch from the dominant LCx. Typically, papillary muscle rupture is seen between four to seven days post–MI. Patients in that scenario present with symptoms of acute heart failure and often cardiogenic shock. The physical exam can reveal signs of pulmonary edema which include crackles on lung auscultation and a new holosystolic murmur in the mitral location. However, they may not always be present. Papillary muscle rupture can cause significant severe mitral regurgitation (MR). On bedside echo, the operator may see a regurgitant jet (Fig. 4B) that is new or worsened from prior examinations and possibly evidence of a flail mitral leaflet. Echo is essential in evaluating papillary muscle rupture as auscultation may only reveal a soft murmur due to equalization of the systolic pressures between the LV/LA.
2.4. Assessment of intravascular volume status
The assessment of body volume status is performed in the subcostal IVC view. This is very important for clinicians assessing patients with possible heart failure exacerbation and assessing the response to intravenous diuresis. Anesthesiologists commonly utilize cardiac P.O.C.U.S. to assess patients' vascular volume status postoperatively and evaluate for fluid shifts that may have happened during procedures.15
The IVC can be best localized as the longitudinal vascular structure in the long axis empties into the RA. Notably, the IVC must be differentiated from the aorta by color doppler or visually in a more experienced operator. Position the probe 1–2 cm from the junction with RA and calculate the diameter of the IVC at its widest point. An IVC diameter of <2.1 cm with collapsibility of >50% upon inspiration indicates a normal RA pressure (typically around 3 mmHg). An IVC diameter >2.1 cm with collapsibility of <50% indicates a high RA pressure (typically 15 mmHg) and a more hypervolemic state. Note that this latter assumption is not valid if the patient is mechanically ventilated due to altered intrathoracic pressure.
2.5. Assessment for pulmonary embolus
Utilization of cardiac P.O.C.U.S. can also be utilized to assess for acute pulmonary embolus, especially in patients that are too unstable to be formally evaluated in a computed tomography (CT) machine with pulmonary artery contrasted protocol. Pulmonary embolism typically presents with clinical signs and symptoms of hypoxia, tachycardia, and pleuritic chest pain. Although it is not used to diagnose a pulmonary embolism, an echocardiogram can help guide diagnosis. Echocardiographic findings include visualization of a dilated right ventricle with septal flattening and a dilated pulmonary artery. The inferior vena cava can also be dilated. The sparing of the right ventricular apex with hypokinesis of the right ventricular free wall is known as McConnell's sign. In the appropriate setting, it can have a high positive predictive value for pulmonary embolism.16
Familiarity with cardiac P.O.C.U.S. during a cardiopulmonary arrest could also be helpful. In the setting where pulmonary embolism was suspected secondary to right ventricular overload and other echocardiographic evidence supporting the diagnosis, thrombolysis administration showed increased survival rates to hospital discharge.17 Post-resuscitation echocardiogram can also aid in identifying reversible causes of the arrest. These include regional wall abnormalities that can signify a myocardial injury. Other findings include the detection of global wall stunning or other structural dysfunctions that could have led to cardiopulmonary arrest.18
3. Discussion
Cardiac P.O.C.U.S. can be used to accurately assess basic cardiac pathophysiology with minor training of hospitalists.19–21 The utilization of cardiac P.O.C.U.S. when used as an adjunct to the physical exam can increase diagnostic sensitivity, though to varying degrees, when assessing patients with cardiac complaints. In the assessment of patients with congestive heart failure, the use of cardiac P.O.C.U.S. can significantly increase the sensitivity of diagnosis and decrease hospitalization duration by guiding diuretics.22–25 In the assessment of pulmonary embolus, cardiac P.O.C.U.S. as a diagnostic tool has a sensitivity of 32.7% and specificity of 90.9%.26 For the evaluation of cardiac tamponade, cardiac P.O.C.U.S. has a sensitivity of 48–60% and a specificity of 75–90%.27 Finally, in the assessment of RV dysfunction, indicated by abnormal TAPSE has a sensitivity of 87% and a specificity of 91%.28
There are several considerations when we discuss the increased use of cardiac P.O.C.U.S. First and foremost, hospital systems must invest resources in procuring enough ultrasound equipment (traditional machines, portable, etc.) to ensure that timely examinations can be made on patients. A study showed that large-sized hospitals, typically in urban areas, have more access to ultrasonography than medium-sized and small-sized hospitals, especially in rural hospitals.29 The advent of handheld ultrasound machines offers a cheaper option than traditional ultrasound machines, with smaller footprints and more user-friendly interfaces. These portable devices may also play a vital role in the daily assessment of hemodynamics and volume status on the medicine floors. In a study of experts who analyzed four common handheld ultrasonography devices, no single device was perceived to be superior, though the Lumify™ was deemed highest in image quality, and the Vscan Air™ was deemed highest for ease of use.30 A vital limitation of handheld ultrasound devices is advanced echocardiographic applications. Focused cardiac P.O.C.U.S. can still be performed, though few devices currently offer spectral Doppler capabilities.
Additionally, the increased use of cardiac P.O.C.U.S. could lead to a paradoxical increase in TTE referrals due to a rise in the number of incidental findings.31 This could disproportionately affect healthcare systems in rural or underserved communities who may be unable to keep up with the increased demand of formal echocardiograms. Ultimately, cardiac P.O.C.U.S. exam findings should be confirmed with a dedicated echocardiogram read by a board-certified cardiologist. A dedicated echocardiogram can provide metrics to quantify the severity of valvopathies, assessment for left ventricular thrombi, assessment for infiltrative cardiomyopathies, grading of diastolic dysfunction, atrial myopathies, and calculation of right ventricular systolic pressures in the setting of pulmonary hypertension.
4. Conclusion
Hospitalists should incorporate cardiac P.O.C.U.S. to improve the assessment of patients with cardiac complaints. Cardiac P.O.C.U.S. education has been taught with relatively low time investment in emergency medicine and anesthesia, which could serve as an example to model during a dedicated curriculum within internal medicine. Cardiac P.O.C.U.S. is more accessible than ever with the improvement of ultrasonography technology and the portability of new handheld devices. Uncertain findings on a cardiac P.O.C.U.S. exam should be confirmed with a dedicated complete echocardiography study.
Supplementary Information
Footnotes
Disclaimers
There is no source(s) of support such as grants, drug(s), equipment, and/or other support that facilitated conduct of the work described in the article or in the writing of the article.
Conflict of interest
There exists no relevant financial or non-financial competing interests to report.
References
- 1. Kelly N, Esteve R, Papadimos TJ, et al. Clinician-performed ultrasound in hemodynamic and cardiac assessment: a synopsis of current indications and limitations. Eur J Trauma Emerg Surg. 2015;41:469–480. doi: 10.1007/s00068-014-0492-6. [DOI] [PubMed] [Google Scholar]
- 2. LoPresti CM, Schnobrich DJ, Dversdal RK, Schembri F. A road map for point-of-care ultrasound training in internal medicine residency. Ultrasound J. 2019;11:10. doi: 10.1186/s13089-019-0124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williams JP, Nathanson R, LoPresti CM, et al. Current use, training, and barriers in point-of-care ultrasound in hospital medicine: a national survey of VA hospitals. J Hosp Med. 2022;17:601–608. doi: 10.1002/jhm.12911. [DOI] [PubMed] [Google Scholar]
- 4. Dieden A, Carlson E, Gudmundsson P. Learning echocardiography-what are the challenges and what may favour learning? A qualitative study. BMC Med Educ. 2019;19:212. doi: 10.1186/s12909-019-1656-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omerovic S, Jain A. StatPearls. Treasure island (FL): StatPearls Publishing Copyright © 2022 StatPearls Publishing LLC; 2022. Echocardiogram. https://www.ncbi.nlm.nih.gov/books/NBK558940/#_NBK558940_pubdet_ . [Google Scholar]
- 6. Gudmundsson P, Rydberg E, Winter R, Willenheimer R. Visually estimated left ventricular ejection fraction by echocardiography is closely correlated with formal quantitative methods. Int J Cardiol. 2005;101:209–212. doi: 10.1016/j.ijcard.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 7. Ahmadpour H, Shah AA, Allen JW, Edmiston WA, Kim SJ, Haywood LJ. Mitral E point septal separation: a reliable index of left ventricular performance in coronary artery disease. Am Heart J. 1983;106:21–28. doi: 10.1016/0002-8703(83)90433-7. [DOI] [PubMed] [Google Scholar]
- 8. Ghafil C, Matsushima K, Guzman R, et al. Performance of focused assessment with sonography for trauma following resuscitative thoracotomy for traumatic cardiac arrest. World J Surg. 2022;46:91–97. doi: 10.1007/s00268-021-06317-8. [DOI] [PubMed] [Google Scholar]
- 9. Tayal VS, Beatty MA, Marx JA, Tomaszewski CA, Thomason MH. FAST (focused assessment with sonography in trauma) accurate for cardiac and intraperitoneal injury in penetrating anterior chest trauma. J Ultrasound Med. 2004;23:467–472. doi: 10.7863/jum.2004.23.4.467. [DOI] [PubMed] [Google Scholar]
- 10. Offenbacher J, Liu R, Venitelli Z, et al. Hemopericardium and cardiac tamponade after blunt thoracic trauma: a case series and the essential role of cardiac ultrasound. J Emerg Med. 2021;61:e40–e45. doi: 10.1016/j.jemermed.2021.05.013. [DOI] [PubMed] [Google Scholar]
- 11. Hegde R, Lafayette N, Sywak M, et al. Isolated right atrial appendage rupture following blunt chest trauma. Trauma Case Rep. 2018;13:26–29. doi: 10.1016/j.tcr.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fleischmann KE, Lee TH, Come PC, et al. Echocardiographic prediction of complications in patients with chest pain. Am J Cardiol. 1997;79:292–298. doi: 10.1016/s0002-9149(96)00750-3. [DOI] [PubMed] [Google Scholar]
- 13. Riera A, Weeks B, Emerson BL, Chen L. Evaluation of a focused cardiac ultrasound protocol in a pediatric emergency department. Pediatr Emerg Care. 2021;37:191–198. doi: 10.1097/pec.0000000000001495. [DOI] [PubMed] [Google Scholar]
- 14. Okabe T, Julien HM, Kaliyadan AG, Siu H, Marhefka GD. Prompt recognition of left ventricular free-wall rupture aided by the use of contrast echocardiography. Tex Heart Inst J. 2015;42:474–478. doi: 10.14503/THIJ-14-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haskins SC, Tanaka CY, Boublik J, Wu CL, Sloth E. Focused cardiac ultrasound for the regional anesthesiologist and pain specialist. Reg Anesth Pain Med. 2017;42:632–644. doi: 10.1097/aap.0000000000000650. [DOI] [PubMed] [Google Scholar]
- 16. McConnell MV, Solomon SD, Rayan ME, Come PC, Goldhaber SZ, Lee RT. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78:469–473. doi: 10.1016/s0002-9149(96)00339-6. [DOI] [PubMed] [Google Scholar]
- 17. Kürkciyan I, Meron G, Sterz F, et al. Pulmonary embolism as a cause of cardiac arrest: presentation and outcome. Arch Intern Med. 2000;160:1529–1535. doi: 10.1001/archinte.160.10.1529. [DOI] [PubMed] [Google Scholar]
- 18. Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: postcardiac arrest care: 2010 American heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S768–S786. doi: 10.1161/circulationaha.110.971002. [DOI] [PubMed] [Google Scholar]
- 19. Secko MA, Lazar JM, Salciccioli LA, Stone MB. Can junior emergency physicians use E-point septal separation to accurately estimate left ventricular function in acutely dyspneic patients? Acad Emerg Med. 2011;18:1223–1226. doi: 10.1111/j.1553-2712.2011.01196.x. [DOI] [PubMed] [Google Scholar]
- 20. Unlüer EE, Karagöz A, Akoğlu H, Bayata S. Visual estimation of bedside echocardiographic ejection fraction by emergency physicians. West J Emerg Med. 2014;15:221–226. doi: 10.5811/westjem.2013.9.16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kimura BJ. Point-of-care cardiac ultrasound techniques in the physical examination: better at the bedside. Heart. 2017;103:987–994. doi: 10.1136/heartjnl-2016-309915. [DOI] [PubMed] [Google Scholar]
- 22. Mozzini C, Di Dio Perna M, Pesce G, et al. Lung ultrasound in internal medicine efficiently drives the management of patients with heart failure and speeds up the discharge time. Intern Emerg Med. 2018;13:27–33. doi: 10.1007/s11739-017-1738-1. [DOI] [PubMed] [Google Scholar]
- 23. Zanobetti M, Scorpiniti M, Gigli C, et al. Point-of-Care ultrasonography for evaluation of acute dyspnea in the. Chest. 2017;151:1295–1301. doi: 10.1016/j.chest.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 24. Gartlehner G, Wagner G, Affengruber L, et al. Point-of-Care ultrasonography in patients with acute dyspnea: an evidence report for a clinical practice guideline by the American college of physicians. Ann Intern Med. 2021;174:967–976. doi: 10.7326/m20-5504. [DOI] [PubMed] [Google Scholar]
- 25. Qaseem A, Etxeandia-Ikobaltzeta I, Mustafa RA, et al. Appropriate use of point-of-care ultrasonography in patients with acute dyspnea in emergency department or inpatient settings: a clinical guideline from the American college of physicians. Ann Intern Med. 2021;174:985–993. doi: 10.7326/m20-7844. [DOI] [PubMed] [Google Scholar]
- 26. Nazerian P, Vanni S, Volpicelli G, et al. Accuracy of point-of-care multiorgan ultrasonography for the diagnosis of pulmonary embolism. Chest. 2014;145:950–957. doi: 10.1378/chest.13-1087. [DOI] [PubMed] [Google Scholar]
- 27. Pérez-Casares A, Cesar S, Brunet-Garcia L, Sanchez-de-Toledo J. Echocardiographic evaluation of pericardial effusion and cardiac tamponade. Front Pediatr. 2017;5:79. doi: 10.3389/fped.2017.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fields JM, Davis J, Girson L, et al. Transthoracic echocardiography for diagnosing pulmonary embolism: a systematic review and meta-analysis. J Am Soc Echocardiogr. 2017;30:714–723e714. doi: 10.1016/j.echo.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 29. Mengarelli M, Nepusz A, Kondrashova T. A comparison of point-of-care ultrasonography use in rural versus urban emergency departments throughout Missouri. Mo Med. 2018;115:56–60. [PMC free article] [PubMed] [Google Scholar]
- 30. Le MT, Voigt L, Nathanson R, et al. Comparison of four handheld point-of-care ultrasound devices by expert users. Ultrasound J. 2022;14:27. doi: 10.1186/s13089-022-00274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jenkins S, Shiha MG, Yones E, et al. Cardiovascular examination using hand-held cardiac ultrasound. J Echocardiogr. 2022;20:1–9. doi: 10.1007/s12574-021-00540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.