Abstract
Rhino-cerebral aspergillosis is a rare phenomenon describing a contiguous spread of Aspergillus species from the paranasal sinuses to the intracranial space. In this case report, we describe a case of invasive rhino-cerebral aspergillosis arising in the setting of prolonged intranasal steroid use in an 81-year-old patient with chronic sinusitis. This case report emphasizes the importance of recognizing steroid use as a risk factor for invasive aspergillosis in otherwise immunocompetent individuals.
Keywords: Aspergillus, Invasive aspergillosis, Chronic sinusitis, Rhino-cerebral aspergillosis, Intranasal steroid
1. Introduction
The Aspergillus fungus is found ubiquitously in the environment in soils or organic debris and its airborne spores are inhaled regularly.1 Among the Aspergillus genus, Aspergillus fumigatus is known to be the most common pathological agent in human hosts and immunocompromised individuals are at a higher risk for developing invasive disease.1 The spores of the Aspergillus can colonize the paranasal sinuses and can cause a wide spectrum of disease which can be classified into four types: fulminant sinusitis, indolent chronic sinusitis, aspergilloma, and allergic aspergillosis of the sinuses.2 Contiguous spread of the infection invading into the intracranial space is a rare phenomenon termed rhino-cerebral aspergillosis that has been observed in immunocompromised and less commonly in immunocompetent individuals.2 In this paper, we present a case of an 81-year-old man with medical history significant for chronic sinusitis and prolong intranasal steroid use who presented with left-sided headaches, visual changes, and ambulatory dysfunction and was diagnosed with rhino-cerebral aspergillosis. This case highlights the importance of maintaining a high index of suspicion among immunocompetent individuals with less traditional risk factors including intranasal steroids use.
2. Case description
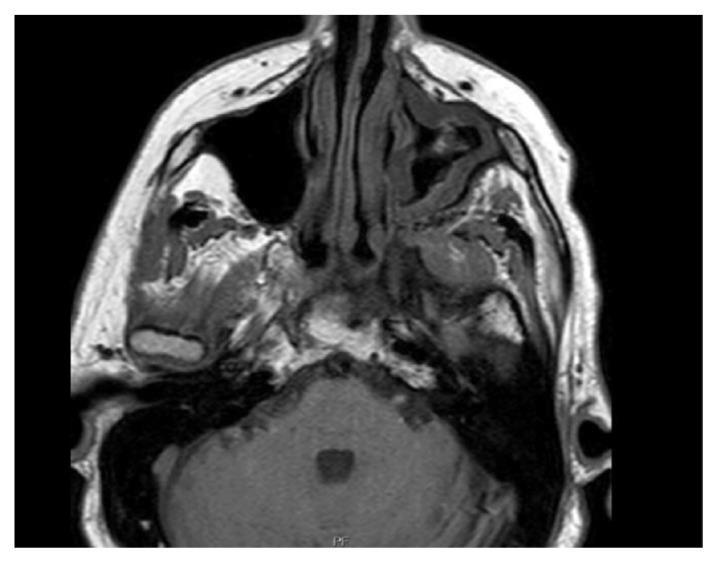
An 81-year-old male presented to the emergency department after suffering a ground-level fall at his workplace. His medical history is significant for hypertension, well-controlled type 2 diabetes, chronic kidney disease, chronic sinusitis on long-term fluticasone, and candida sinusitis treated with topical itraconazole, mupirocin, and budesonide irrigation for 3 months. Prior to the fall, the patient experienced a left-sided headache with left-sided ocular pain and diplopia. The initial physical exam was significant for bilateral proptosis and mild limitation of abduction in his left eye. CT scan of the head (Fig. 1) revealed mucosal disease in the left maxillary sinus and sphenoid sinus with abnormal soft tissue in the pterygopalatine fissure extending into the orbital apex. A follow-up MRI of orbit and brain (Fig. 2) was obtained which showed aggressive sinus inflammatory process within the posterior aspect of the left maxillary sinus with evidence of bone destruction and invasion through the left pterygopalatine fossa, left middle cranial fossa into epidural space, and left orbital floor into intraconal space. A small 8 X 5 × 3 mm left temporal epidural collection with brain edema was visualized as well.
Fig. 1.
Sinus CT without contrast showed moderate left maxillary sinusitis with narrowing of the left osteomeatal unit with mucosal disease.
Fig. 2.
MRI orbit with and without contrast showing aggressive sinus inflammatory process with evidence of bone destruction through the left pterygopalatine fossa.
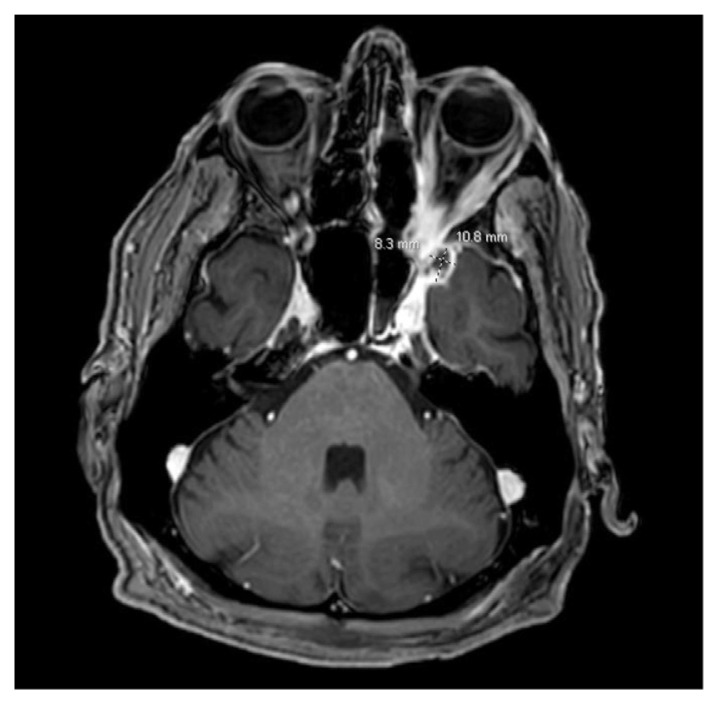
The patient was empirically started on IV liposomal amphotericin B and broad-spectrum antibiotics on first day of hospital admission. He underwent endoscopic sinus surgery, which revealed a fungal ball along the maxillary sinus floor and sphenoid rostrum. Erosion with necrotic tissue was seen along the posterior wall of the maxillary sinus extending into the pterygopalatine fossa and floor of the orbit. The procedure involved the removal of the fungal ball, mucosal stripping of the maxillary sinus, orbital wall decompression, ethmoidectomy, clipping of the distal maxillary artery, and sphenoidotomy. After the procedure, an MRI of the head was repeated which showed post-operative changes with a redemonstration of the epidural abscess in the left middle cranial fossa (Fig. 3). However, it additionally showed a new peripherally enhancing lesion in the left anterior temporal lobe measuring 4 X 7 × 6 mm. Given the extent of the disease, the patient was again taken for a second endoscopic operation for resection of the skull base lesions on hospital day 10. Necrotic debris was removed from the middle cranial fossa and infratemporal fossa with Amphotericin irrigation. Cultures from the endoscopic surgery was consistent with invasive fungal sinusitis and grew Stenotrophomonas maltophilia with Aspergillus fumigatus. Trimethoprim-sulfamethoxazole was added to his antibiotic regimen, and IV liposomal amphotericin B was continued which was then later on replaced by IV voriconazole. However, on hospital day 12, the patient developed auditory and visual hallucinations. Voriconazole was discontinued, and the patient was restarted on liposomal amphotericin B, but the patient developed an acute kidney injury on amphotericin B. The antifungal therapy agent was then changed to isavuconazole. The patient was recommended for a lifelong course of isavuconazole therapy and was discharged to a rehabilitation center on hospital day 34.
Fig. 3.
MRI brain with contrast demonstrating peripheral enhancing collection in left anteromedial temporal lobe measuring 11 x 8 × 11 mm appearing more organized and well-defined. Although not seen in this view, an additional peripherally enhancing lesion measuring 4 × 7 x 6 mm was visualized along the posterior lateral margin of the collection.
3. Discussion
Invasive aspergillosis is a highly lethal infection that must be recognized and treated early to avoid significant mortality and morbidity. The spectrum of paranasal fungal infections includes non-invasive variants and indolent and aggressively invasive forms.3 Invasive aspergillosis is a highly lethal infection that may rapidly extend to adjacent tissues, including the orbit and skull base. The spectrum of paranasal fungal infections includes non-invasive variants and indolent and aggressively invasive forms.3 Based on clinical presentation, the two most common forms of aspergillosis are rhino-cerebral (44%–49%) and cutaneous (10%–16%). The disseminated form is rare and only represents 6%–11.6%.4
The first case of rhino-cerebral mycosis was reported by Paltauf in 1885.5 Traditional risk factors for invasive aspergillosis include prolonged neutropenia, stem cell transplants or solid-organ transplants, AIDS, chronic systemic steroids, and genetic immunodeficiency. Few cases of rhino-cerebral aspergillosis in immunocompetent patients have been reported.6,7 In the case described here, our patient was immunocompetent but presented with pre-existing chronic sinusitis, candida sinusitis, and chronic nasal steroid use.
Patients with rhino-cerebral aspergillosis most commonly present with unilateral retro-orbital or periorbital pain associated with fever, nasal congestion, rhinorrhea, epistaxis, facial pain, and numbness. Dizziness, altered mental status, focal neurological deficit, and convulsions suggest central nervous involvement. Epistaxis and proptosis are associated with advanced local disease. Individuals with cerebral vascular involvement may show signs of a stroke.8
Definitive diagnosis of aspergillosis is based on histopathological, cytopathological, or direct microscopic examination of the affected tissue.9 Blood cultures are rarely positive, and sputum cultures are only positive in pulmonary aspergillosis. Classical histopathological findings include septated hyaline hyphae with acute angle (45°) dichotomous branching visible with hematoxylin and eosin stain or methenamine silver staining. Galactomannan (GMA) and β-(1,3)-d-glucan (BDG) testing may be useful, particularly in immunocompromised patients and in invasive aspergillosis infection.10 Finally, the treatment of rhino-cerebral aspergillosis is highly complex and require a multidisciplinary team that often involve otorhinolaryngology, ophthalmology, infectious disease and neurosurgery. The patient on our case initially presented to a tertiary medical center and was transferred to an affiliated quaternary care center for better continuity of care.
4. Conclusion
Rhino-cerebral aspergillosis is commonly restricted to immunocompromised or immunologically immature individuals. This case emphasizes the importance of a high index of suspicion for diagnosis in patients with less common risk factors for invasive aspergillosis, including nasal steroids, chronic sinusitis, and prior antibiotic use.
Footnotes
Disclaimers
This article has not been submitted to other publications or presented at a conference or a meeting.
Conflicts of interest
There are no conflicts of interest.
Source(s) of support
This case report was not supported by grants, drug(s), or equipment.
References
- 1. Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev. 2009 Jul;22(3):447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Latgé JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999 Apr;12(2):310–350. doi: 10.1128/CMR.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siddiqui AA, Shah AA, Bashir SH. Craniocerebral aspergillosis of sinonasal origin in immunocompetent patients: clinical spectrum and outcome in 25 cases. Neurosurgery. 2004 Sep;55(3):602–611. doi: 10.1227/01.neu.0000134597.94269.48. [DOI] [PubMed] [Google Scholar]
- 4. Prabhu RM, Patel R. Mucormycosis and entomophthoramycosis: a review of the clinical manifestations, diagnosis and treatment. Clin Microbiol Infect. 2004 Mar;10(Suppl 1):31–47. doi: 10.1111/j.1470-9465.2004.00843.x. [DOI] [PubMed] [Google Scholar]
- 5. Paltauf A. Mycosis mucorina. Virchows Arch. 1885;102(3):543–564. [Google Scholar]
- 6. Hussain S, Salahuddin N, Ahmad I, Salahuddin I, Jooma R. Rhinocerebral invasive mycosis: occurrence in immunocompetent individuals. Eur J Radiol. 1995 Jul;20(2):151–155. doi: 10.1016/0720-048x(95)00644-6. [DOI] [PubMed] [Google Scholar]
- 7. Venkatesh D, Dandagi S, Chandrappa PR, Hema KN. Mucormycosis in immunocompetent patient resulting in extensive maxillary sequestration. J Oral Maxillofac Pathol. 2018 Jan;22(Suppl 1):S112–S116. doi: 10.4103/jomfp.-JOMFP_163_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neville WB, Damm D, Allen CM, Bouquot JE. Text Book of Oral & Maxillofacial Pathology. second ed. Philadelphia: WB: Saunders; 2001. [Google Scholar]
- 9. Lamoth F, Alexander BD. Nonmolecular methods for the diagnosis of respiratory fungal infections. Clin Lab Med. 2014 Jun;34(2):315–336. doi: 10.1016/j.cll.2014. . 02.006. [DOI] [PubMed] [Google Scholar]
- 10. Kameswaran M, al-Wadei A, Khurana P, Okafor BC. Rhinocerebral aspergillosis. J Laryngol Otol. 1992 Nov;106(11):981–985. doi: 10.1017/s0022215100121528. [DOI] [PubMed] [Google Scholar]