Abstract
According to the 2019 National Survey on Drug Use and Health, 14.5 million people ages 12 and older had alcohol abuse disorder. Alcohol withdrawal syndrome (AWS) can be defined as a collection of physical symptoms experienced due to abrupt cessation of alcohol after long-term dependence. In instances where regular inpatient management fails to control AWS symptoms, patients are shifted to intensive care units (ICUs) for closer monitoring and prevention of life-threatening complications like withdrawal seizures and delirium tremens (DTs), labeled as severe alcohol withdrawal syndrome (SAWS). Although this represents a significant healthcare burden, minimal studies have been conducted to determine objective predictors. In this study, we aim to determine the effect of patient demographics, socio-economic status, biochemical parameters, and clinical factors on the need for escalation to ICU level of care among admissions for AWS. Our study showed that factors such as a history of DTs or alcohol-related seizures, the initial protocol of management, degree of reported alcohol usage, activation of rapid response teams, mean corpuscular value, alcohol level on admission, highest Clinical Institute Withdrawal Assessment Alcohol Revised (CIWA-Ar) scored during the hospital stay, and the total amount of sedatives used were significantly associated with escalation to ICU level of care. Clinicians must use these objective parameters to identify high-risk patients and intervene early. We encourage further studies to establish a scoring algorithm incorporating biochemical parameters to tailor management algorithms that might better suit high-risk patients.
Keywords: CIWA-Ar protocol, Alcohol withdrawal, Alcohol abuse, Alcohol dependence, Benzodiazepine use
Summary box.
What is already known on this topic:
The current gold standard for the management of alcohol withdrawal syndrome (AWS) is the Clinical Institute Withdrawal Assessment Alcohol revised (CIWA-Ar) scale and treatment with benzodiazepines (BZDs).
CIWA-Ar scoring involves discussion with the patient, which negates the objectivity of the symptoms.
BZDs are administered either using a symptom triggered regimen (STR) or fixed dose taper regimen (FDTR). STRs are favored as studies have shown STRs to be associated with a shorter length of stay, decreased rates of hospital-acquired infection and lower cumulative dose administration.
Despite the wide acceptance and utilization of these management protocols, several patients with severe symptoms of AWS are transferred to the intensive care unit (ICU) for more aggressive care and closer monitoring.
What this study adds:
Studies evaluating associations between objective parameters such as demographic, clinical, and biochemical parameters and escalation to ICU care in AWS are lacking. Our study contributes in this aspect.
We attempt to validate the use of CIWA-Ar score and evaluate the outcomes of STR and FDTR in our institution.
We identified that parameters such as a history of delirium tremens or alcohol-related seizures, the initial protocol of management, degree of reported alcohol usage, activation of rapid response teams, mean corpuscular value, alcohol level on admission, single highest CIWA-Ar scored during the hospital stay, and the total amount of sedatives used are significantly associated with escalation to ICU level of care.
How this study might affect research, practice, or policy:
Objective cut-offs including a blood alcohol level on admission <13 mg/dl and a CIWA-Ar score >16.5 offer a good balance of sensitivity and specificity in reliably predicting patients at a high risk of requiring ICU level of care.
Clinicians can use these objective parameters to stratify patients and guide management.
Additionally, our results encourage further research to create an objective tool using the parameters explored and design management algorithms that might be better tailored to patients at higher risk.
Categories: Internal medicine, critical care medicine, behavioral health, psychiatry, substance abuse
1. Introduction
According to the 2019 National Survey on Drug Use and Health, 14.5 million people ages 12 and older had alcohol abuse disorder. Alcohol withdrawal syndrome (AWS) can be defined as a collection of physical symptoms experienced due to abrupt cessation of alcohol after long-term dependence.1 It is common in patients with alcohol use disorder (AUD), affecting up to 40% of patients being hospitalized. Critically ill patients with AWS are often associated with poor outcomes, such as higher rates of infection and sepsis.2 Medical consensus has classified the symptoms of withdrawal based on the time of onset or severity. Early withdrawal symptoms (such as diaphoresis, tremor, hyperactivity, insomnia, and headache) can start around 6 h after the last drink and last up to 48 h. Perceptual disturbances such as visual, auditory, and tactile hallucinations can be classified as moderate withdrawal and may last up to 6 days. However, the most severe consequence that we aim to avoid when treating patients for AWS includes withdrawal seizures and delirium tremens (DTs). This last for up to 2 weeks with anticipated mortality of 37% without treatment.3–5
Considering the fatality of AWS, clinical guidelines suggest a standardized instrument to measure the severity of AWS. The center of attention has shifted from the reactive management of AWS to the implementation of different screening and assessment tools. Clinical Institute Withdrawal Assessment Alcohol revised (CIWA-Ar) is a widely used and corroborated 10-item assessment tool developed over the last few decades to objectively quantify the severity of AWS with a score based on history from the patient and pertinent physical exam. In an inpatient setting, the CIWA-Ar score is often ordered preventatively in suspected cases of alcohol withdrawal.1,6 The 10 items used to monitor the progression of AWS include agitation, anxiety, auditory disturbances, clouding of the sensorium, headache, nausea/vomiting, paroxysmal sweating, tactile disturbances, tremor, and visual disturbances. 7 CIWA-Ar assigns scores of 0–8, 9–15, and 16 or more to indicate the severity of AWS (mild, moderate, and severe, respectively).
A pharmacotherapeutic regimen involving benzodiazepines (BZDs) that is either a symptom-triggered regimen (STR) or a fixed-dose taper regimen (FDTR) is generally used to manage patients.3,8 The STR is defined as the delivery of adequate pharmacotherapy on an as-needed basis while monitoring patients every 4–6 h based on symptom severity. In comparison, an FDTR involves administering a tapering dose of medication based on a predetermined schedule every 4–6 h. STRs are ordered concurrently with FDTRs to administer extra doses as needed.9 Previous studies have depicted STR dosing to be superior to FDTR, having a shorter length of stay and decreased rates of hospital-acquired infection in patients receiving a shorter course of BZDs with lower cumulative doses.10 However, no studies evaluate if STRs compared to FDTRs decrease escalation to ICU level of care. BZDs are regarded as the drug of choice in treating AWS. Diazepam and lorazepam are the most used drugs. However, in different inpatient settings, other agents such as anti-convulsants (such as haloperidol and olanzapine) and adrenergic drugs (dexmedetomidine and clonidine) are also utilized in case of failure of BZDs to control AWS symptoms.
The National Institute of Health and the National Institute for Health and Care Excellence guidelines continue to recommend CIWA-Ar as the gold standard tool for the management of AWS, with STR being the superior choice.3 One prominent drawback of the CIWA-Ar tool is its subjective nature. Scoring involves discussion with the patient, which negates the objectivity of the symptoms. Primary factors contributing to the unreliability of the assessed score include the language barrier, the need for frequent reassessment by different scorers, and altered mentation in patients.6
In instances where regular inpatient management fails to control AWS symptoms, patients are often shifted to intensive care units (ICUs) for closer monitoring and prevention of life-threatening complications like withdrawal seizures and DTs, labeled as severe alcohol withdrawal syndrome (SAWS). Several single-center studies were done in the past to determine predictors of SAWS. Higher baseline blood pressure, lower potassium level, lower platelet count, higher initial level of alanine aminotransferase (ALT), and high gamma-glutamyl transpeptidase were a few of the significant predictors. 11 Risk factors for developing DTs, the most severe form of AWS included prior history of SAWS, tachycardia, and higher systolic blood pressure.12 However, few studies have been conducted to further determine other objective parameters to predict admission to the intensive care unit (ICU). In this study, we aim to study the association of a wide range of patient characteristics such as demographics, biochemical parameters, and clinical factors on the need for ICU admission. Additionally, we establish significant cut-off values of clinical parameters such as the admission blood alcohol level, CIWA-Ar scores and the dosage of benzodiazepines administered in predicting ICU admissions.
2. Material and methods
2.1. Design and data source
In this retrospective cohort study, we included all adult hospitalizations with a discharge diagnosis of alcohol withdrawal, alcohol dependence with withdrawal, or alcohol use taken from the medical records department of Monmouth Medical Center, Long Branch, United States of America. We collected details of all inpatient adult admissions for alcohol withdrawal between January 1, 2021, and December 31, 2021. Patients were searched using the International Classification of Diseases, 10th revision, and Clinical Modification/Procedure Coding System (ICD-10-CM/PCS) codes. We used all subbranches of the ICD-10-CM/PCS codes - F10.1, F10.2, and F10.9 to identify patients who were admitted for alcohol withdrawal and its complications. After individual chart review, patients who were admitted to the inpatient medicine service for the management of alcohol withdrawal without other co-existing illnesses complicating the hospital course were included. Patients who left prematurely against medical advice, had polydrug intoxication, altered mental status attributable to other causes, and those admitted to the ICU directly from the emergency room or admitted to the ICU for reasons other than the management of AWS were excluded. Medical record numbers corresponding to the diagnoses were obtained from the medical records department. Non-identifiable data and details of hospitalization were extracted from the electronic health record system, Cerner, and entered into the REDCap survey software. Any dose of lorazepam in our collected data was converted to diazepam using a 1:5 ratio (1 mg of Lorazepam = 5 mg of Diazepam). Alcohol consumption was recorded as heavy drinking when >14 drinks were consumed per week and as binge drinking when 5 drinks for men or 4 drinks for women were consumed on one occasion as a recurring pattern. This ensured that outcomes were analyzed in a blinded fashion as all patient identifiers were removed. Data on patients' demographics, comorbidities, and biochemical and clinical parameters were collected and compared to look for factors with a positive association with the incidence of ICU admission. Any missing data were filled by using the patient's medical record number to revisit the patient chart and acquire the missing values.
2.2. Statistical analysis
All other benzodiazepines were also converted to diazepam equivalents when needed. Statistical analysis for the data was done using the IBM Statistical Package for the Social Science (SPSS) Statistics for Windows, Version 20.0. Armonk, New York. Numeric variables including age, alcohol level on admission, single highest CIWA-Ar scored by the patient during ward stay, the total amount of sedatives received during medical wards stay, along with biochemical parameters (platelets count, albumin, mean corpuscular volume (MCV), international normalized ratio (INR), alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP)) were compared between these two groups using a t-test. Categorical data such as gender, race, insurance status, prior ICU admissions for alcohol withdrawal symptoms, history of delirium tremens or alcohol-related seizures, initiation of CIWA-Ar protocol within the first 24 h of admission, the initial protocol of management, the degree of alcohol usage, psychiatric or substance abuse history, and rapid response team activation frequency were also compared using the chi-square test or a Fisher's exact test. Multiple regression analysis was then done on variables with significant association with ICU admission. Additionally, cutoff points with sensitivity and specificity levels were determined using ROC curves for significant variables. A p-value of <0.05 was considered significant in this study.
3. Results
A total of 123 patients were included in this study, with a mean age of 50.8 (±12.8) years. 70.7% (87) were males, and 74% (91) were insured. Only 13%16 had a history of prior ICU admission for alcohol withdrawal symptoms, with an average of one admission per patient. About one-third of the patients (31.7%) had a history of DTs or alcohol-related seizures.
CIWA-Ar evaluation was done for 95.9% (118) within the first 24 h of their presentation. Half of all patients (52.8%) received initial STR with lorazepam. The rest of the patients received either STR with diazepam (18.7%), an FDT of lorazepam (17.9%), or an FDT of diazepam (10.6%).
Most of the patients were either heavy drinkers with >14 drinks a week (43.9%) or binge drinkers (25.2%), while only 16.3% were moderate drinkers (7–14 drinks a week), and 14.6% with 1–7 drinks a week as defined by our study protocol. About 60% had a history of liver cirrhosis or steatosis, while 45% had a history of either psychiatric or other substance use disorders.
In this cohort, 16 patients (13%) of the total sample were admitted to the ICU during their admission. Most of these patients (62.5%) were admitted to the ICU on either hospital day one or two. The rapid response team (RRT) was activated at least once for 15 patients of the sample. To study the effect of different variables on the risk of ICU admission, the sample was divided into 2 groups; those who were admitted to the ICU,16 and those who did not need ICU management (107). Table 1 lists the various categorical variables analyzed along with the calculated odds ratio. Table 2 lists the p-values obtained from comparing continuous variables across the two groups with the associated mean values.
Table 1.
Categorical predictors with p values and odds ratio calculated using chi-square test.
| Variable | p-value (chi-square) | Odds ratio with 95% confidence interval |
|---|---|---|
| Gender | 0.687 | 0.78 (0.23–2.61) |
| Race | 0.395 | – |
| Insurance status | 0.262 | 1.87 (0.62–5.64) |
| Prior ICU admission for alcohol withdrawal symptoms | 0.126 | 2.64 (0.73–9.50) |
| History of delirium tremens or alcohol-related seizures | 0.032 | 3.13 (1.07–9.17) |
| Initiation of CIWA-Ar protocol within the first 24 h of admission | 0.635 | 0.58 (0.06–5.57) |
| Initial protocol of management (FDT with lorazepam or diazepam) | 0.008 | 4.01 (1.36–11.82) |
| Degree of alcohol usage (<7 drinks/week compared to 7 drinks a week or binge drinkers) | 0.059 | 3.23 (0.98–10.98) |
| Psychiatric or substance abuse history | 0.377 | – |
| History of liver diseases | 0.701 | – |
| Rapid response team activation once or more | 0.000 | 21.43 (5.92–77.55) |
ICU – Intensive care unit.
CIWA-Ar - Clinical institute withdrawal assessment alcohol revised.
FDT – Fixed dose taper.
Table 2.
Continuous predictors with p-value calculated using t-test. Mean values of significant parameters listed for patients who did not require ICU level of care and those that did require ICU level of care.
| Variable | Mean | ||
|---|---|---|---|
|
| |||
| p-value (t-test) | Patients that did not require ICU level of care | Patients that required ICU level of care | |
| Age | 0.305 | – | – |
| Platelets count | 0.102 | – | – |
| Mean corpuscular volume (fL) | 0.015 | 94.43 | 100.06 |
| International normalized ratio | 0.424 | – | – |
| Albumin | 0.531 | – | – |
| Alanine transaminase | 0.673 | – | – |
| Aspartate transaminase | 0.666 | – | – |
| Alkaline phosphatase | 0.651 | – | – |
| Alcohol level on admission (mg/dL) | 0.000 | 151.66 | 32.30 |
| Highest CIWA-Ar scored during ward stay | 0.031 | 14.14 | 22.56 |
| Total amount of sedatives received during ward stay (mg) | 0.000 | 113.37 | 383.37 |
ICU – Intensive care unit.
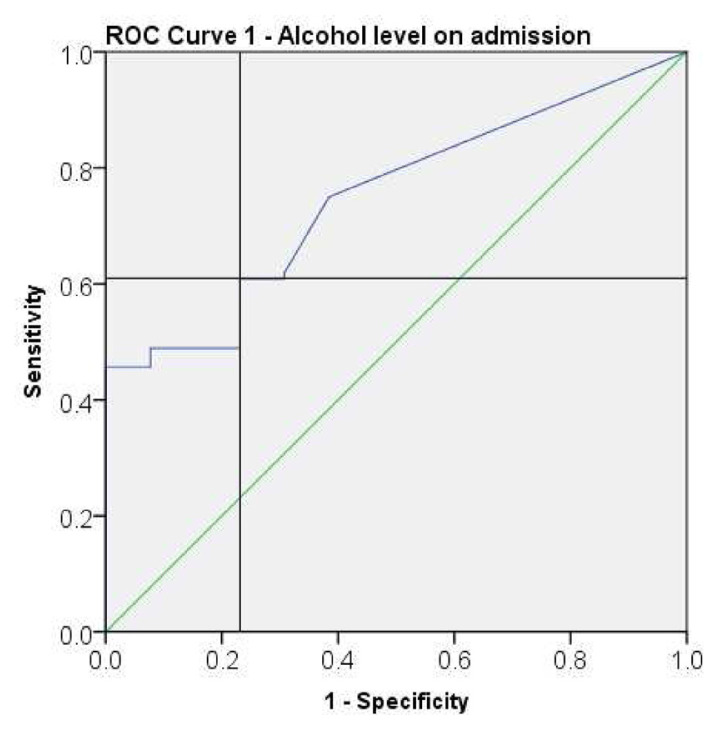
Receiver operator curves (ROC) were plotted for the following parameters - alcohol level on admission (Fig. 1), maximum CIWA-Ar score (Fig. 2), and total sedatives (diazepam) received (Fig. 2) to determine cut-offs significant for ICU admission. Table 3 describes cut-off values with associated sensitivity and specificity obtained from the ROCs.
Fig. 1.
Receiver operating characteristic (ROC) curve for alcohol level on admission.
Fig. 2.
Receiver operating characteristic (ROC) curve for maximum CIWA-Ar score and total sedatives (diazepam) received.
Table 3.
Cutoffs with sensitivity and specificity determined using ROC curves.
| Variable | Cut-off point | Higher risk patients | Sensitivity | Specificity |
|---|---|---|---|---|
| Alcohol level on admission | 13.00 | <13.00 | 61% | 77% |
| Maximum CIWA-Ar scored during ward stay | 16.50 | >16.50 | 62.5% | 70% |
| Total sedatives (diazepam equivalents) received | 185.00 | >185.00 | 68.8% | 83.5% |
CIWA-Ar - Clinical institute withdrawal assessment alcohol revised.
4. Discussion
Studies of hospitalized patients have shown the incidence of alcohol withdrawal is between 11% and 32%.13,14 Among those with alcohol use disorder, 50% of patients have been noted to undergo withdrawal at some point in their lifetime.15 Patients withdrawing from alcohol are often agitated, significantly burdening physicians, nurses, and other hospital staff. It is when their agitation cannot be managed on the medicine floors that they are transferred to the ICU where they may necessitate the need for a dexmedetomidine drip. Through our study, we hope to identify patients at risk for progression to severe withdrawal and intervene early thereby decreasing ICU burden.
Our study found that men were most often the predominant sex for AUD admissions. The mean age was around 50 ± 12.8 years pointing towards a wide population sample. Among the patients who came into our hospital, 16 (13%) were transferred to the ICU. It is commonly observed clinically that it is often the same patients who come back in for treatment of alcohol withdrawal and its complications.
Fortunately, however, most patients (95.9%) admitted for alcohol withdrawal were identified early and started on medication. Most patients were started on lorazepam, rather than diazepam at admission. Lorazepam is chosen by most clinicians for its better hepatic tolerability and shorter half-life compared to diazepam. Despite early medication initiation, 13% of patients were admitted to the ICU.
Factors significantly associated with escalation to ICU level of care were a history of delirium tremens or alcohol-related seizures, the initial protocol of management, degree of reported alcohol usage, activation of rapid response teams, mean corpuscular value, alcohol level on admission, single highest CIWA-Ar scored during the hospital stay, and the total amount of sedatives used.
A history of DTs and alcohol-related seizures is expected to be associated with higher ICU admissions as a prior history increases subsequent risk. However, a history of prior ICU admissions did not carry a similar correlation. This may be because a history of DTs might convey a more severe disease burden. Additionally, patients with a history of prior ICU admissions possibly received more intensive care right from their admission as it might be readily apparent to clinicians based on chart review of their electronic medical records. Among patients that were transferred to the ICU, only 16 patients (13%) had a previous history of ICU admission for alcohol withdrawal whereas 31.7% had a previous history of DTs or alcohol-related seizures. The positive correlation with the activation of rapid response teams and ICU admissions points to an opportunity for early intervention in high-risk patients.
Among biochemical parameters, interestingly, a lower blood alcohol level was associated with a higher risk. This might be because a lower alcohol level at presentation would indicate that the patient is further along their abstinence from alcohol and closer to withdrawal. Clinically we administer similar amounts of benzodiazepines to patients irrespective of their admission blood alcohol levels. This possibly underdoses patients further into their abstinence with lower blood alcohol levels than those who had their last drink more recently and presented with higher blood alcohol levels. A higher MCV was also noted to be associated with a higher risk for ICU admission possibly because this represents more chronic alcohol use and hepatic function impairment. Prior studies have established that MCV is elevated in chronic alcohol use, however, a relationship between higher MCV values with higher levels of drinking is lacking.16
Among clinical parameters, a higher maximum CIWA-Ar score during ward stay was associated with ICU admission. This helps us further validate the CIWA-Ar scale which is widely used. As a corollary, higher sedatives administered to patients were also found to be significantly associated with ICU admission. This can be expected as higher CIWA-Ar scores call for higher doses of sedatives. Also, before ICU admissions, large quantities of sedatives are administered to control agitation during rapid response team activations for agitated patients. We also found that patients on an FDTR compared to STR were associated with an increased risk. This is likely because patients on a fixed dose taper had high-risk features such as a history of ICU admission for withdrawal or DTs which prompted the clinician to choose this regimen in the first place. It is unclear if this points to the possibility that the FDTR at our institution is not adequate to prevent impending withdrawal among high-risk patients. The FDTR and STR at our institution are detailed in Appendix 1.
Statistical analyses using ROCs showed us that an alcohol level of <13 g/dl and a CIWA-Ar score of >16.5 significantly predicted ICU admission. Both these values are objective parameters clinicians may use to identify such high-risk patients during their management. We attempted to find a dose of sedative that prevented ICU admissions and a linear response with CIWA-Ar score was noted. Through our study, it is not possible to suggest a threshold dose of benzodiazepine that might reliably prevent an ICU admission.
5. Conclusion
We found a significant association between ICU admissions and parameters such as a history of delirium tremens or alcohol-related seizures, the initial protocol of management, activation of rapid response teams, mean corpuscular value, alcohol level on admission, single highest CIWA-Ar scored during the hospital stay, and the total amount of sedatives used. Clinicians must be aware of these biochemical and clinical parameters and monitor patients at high risk closely. Objective cut-offs including a blood alcohol level on admission <13 mg/dl and CIWA-Ar score >16.5 offer a good balance of sensitivity and specificity in reliably predicting patients at a high risk of requiring ICU level of care. Our results encourage further research to create an objective tool using the parameters explored and explore management algorithms that might be better tailored to patients at higher risk.
Acknowledgments
None
Abbreviations
- AWS
alcohol withdrawal syndrome
- AUD
alcohol use disorder
- DTs
delirium tremens
- STR
symptom-triggered regimen
- FDTR
fixed-dose taper regimen
- CIWA-Ar
clinical institute withdrawal assessment alcohol revised
- BZD
benzodiazepine
- ICU
intensive care unit
- ROC
receiver operator curve
- SAWS
severe alcohol withdrawal syndrome
- RRT
rapid response team
- MCV
mean corpuscular volume
- INR
international normalized ratio
- ALT
alanine transaminase
- AST
aspartate transaminase
- ALP
alkaline phosphatase
- ICD-10-CM/PCS
international classification of diseases, 10th revision, and clinical modification/procedure coding system
Appendix 1. Clinical Institute Withdrawal Assessment Alcohol Revised (CIWA-Ar) regimen
Fixed dose taper regimen
| 0 h–24 h | Diazepam 10 mg every 6 h |
| 24 h–48 h | Diazepam 10 mg every 8 h |
| 48 h–72 h | Diazepam 5 mg every 6 h |
| 72 h–96 h | Diazepam 5 mg every 8 h |
| 96 h–120 h | Diazepam 5 mg every 12 h |
| 120 h onwards | Diazepam 5 mg every 24 h |
Symptom-triggered regimen
Diazepam 20 mg intramuscular stat as needed every 1-h when CIWA-Ar score >/ = 15
Diazepam 20 mg intravenous stat as needed every 1-h when CIWA-Ar score >/ = 15
Diazepam 10 mg intravenous stat as needed every 1-h when CIWA-Ar score 11–14
Diazepam 5 mg intravenous stat as needed every 2-h when CIWA-Ar score 8–11
Footnotes
Conflict of interest
Gaurav Mohan as the corresponding author declares no competing interests on behalf of all the coauthors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1. Seshadri A, Appelbaum R, Carmichael SP, et al. Prevention of alcohol withdrawal syndrome in the surgical ICU: an American association for the surgery of trauma critical care committee clinical consensus document. Trauma Surg Acute Care Open. 2022;7(1):e001010. doi: 10.1136/tsaco-2022-001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Wit M, Jones DG, Sessler CN, Zilberberg MD, Weaver MF. Alcohol-use disorders in the critically ill patient. Chest. 2010;138(4):994–1003. doi: 10.1378/chest.09-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pribék IK, Kovács I, Kádár BK, et al. Evaluation of the course and treatment of alcohol withdrawal syndrome with the clinical institute withdrawal assessment for alcohol - revised: A systematic review-based meta-analysis. Drug Alcohol Depend. 2021;220:108536. doi: 10.1016/j.drugalcdep.2021.108536. [DOI] [PubMed] [Google Scholar]
- 4. Jesse S, Bråthen G, Ferrara M, et al. Alcohol withdrawal syndrome: mechanisms, manifestations, and management. Acta Neurol Scand. 2017;135(1):4–16. doi: 10.1111/ane.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahman A, Paul M. StatPearls. Treasure Island (FL): StatPearls publishing; 2022. Delirium tremens. copyright © 2022 StatPearls Publishing LLC. [PubMed] [Google Scholar]
- 6. Knight E, Lappalainen L. Clinical Institute Withdrawal Assessment for Alcohol-Revised might be an unreliable tool in the management of alcohol withdrawal. Can Fam Physician. 2017;63(9):691–695. [PMC free article] [PubMed] [Google Scholar]
- 7. Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84(11):1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 8. Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American society of addiction medicine working group on pharmacological management of alcohol withdrawal. JAMA. 1997;278(2):144–151. doi: 10.1001/jama.278.2.144. [DOI] [PubMed] [Google Scholar]
- 9. The ASAM clinical practice guideline on alcohol withdrawal management. J Addiction Med. 2020;14(3S Suppl 1):1–72. doi: 10.1097/ADM.0000000000000668. [DOI] [PubMed] [Google Scholar]
- 10. Duby JJ, Berry AJ, Ghayyem P, Wilson MD, Cocanour CS. Alcohol withdrawal syndrome in critically ill patients: protocolized versus nonprotocolized management. J Trauma Acute Care Surg. 2014;77(6):938–943. doi: 10.1097/TA.0000000000000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goodson CM, Clark BJ, Douglas IS. Predictors of severe alcohol withdrawal syndrome: a systematic review and meta-analysis. Alcohol Clin Exp Res. 2014;38(10):2664–2677. doi: 10.1111/acer.12529. [DOI] [PubMed] [Google Scholar]
- 12. Eyer F, Schuster T, Felgenhauer N, et al. Risk assessment of moderate to severe alcohol withdrawal–predictors for seizures and delirium tremens in the course of withdrawal. Alcohol Alcohol. 2011;46(4):427–433. doi: 10.1093/alcalc/agr053. [DOI] [PubMed] [Google Scholar]
- 13. Doering-Silveira J, Fidalgo TM, Nascimento CL, et al. Assessing alcohol dependence in hospitalized patients. Int J Environ Res Publ Health. 2014;11(6):5783–5791. doi: 10.3390/ijerph110605783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dolman JM, Hawkes ND. Combining the audit questionnaire and biochemical markers to assess alcohol use and risk of alcohol withdrawal in medical inpatients. Alcohol Alcohol. 2005;40(6):515–519. doi: 10.1093/alcalc/agh189. [DOI] [PubMed] [Google Scholar]
- 15. Schuckit MA, Danko GP, Smith TL, Hesselbrock V, Kramer J, Bucholz K. A 5-year prospective evaluation of DSM-IV alcohol dependence with and without a physiological component. Alcohol Clin Exp Res. 2003;27(5):818–825. doi: 10.1097/01.ALC.0000067980.18461.33. [DOI] [PubMed] [Google Scholar]
- 16. Addolorato G, Vassallo GA, Mirijello A, Gasbarrini A. Diagnosis and management of alcohol use disorder in patients with liver disease: lights and shadows. Neurotherapeutics. 2020;17(1):127–141. doi: 10.1007/s13311-019-00802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]